Abstract
Sex differences have been clinically documented in numerous neurodegenerative diseases and yet the reasons for these sex differences are not well understood. Recent studies have found that microglia, the innate immune cells of the central nervous system, are a key cell type involved in neurodegenerative diseases. Interestingly, this cell type displays sex differences in their expression profiles and function. Could these sex differences in microglia explain the sex differences seen in neurodegenerative diseases? How can we further probe these differences to better understand disease mechanisms? In this opinion piece, we highlight the recent evidence that microglia have sex differences, factors that contribute to these differences, and how these differences could shed new light on the pathophysiology of neurological diseases.
Keywords: Microglia, neurodegenerative disease, sex differences
Why study microglial sex differences?
For decades, the scientific community has recognized the differences between male and female patients and the sex differences in clinical manifestations of disease. One of the most well-understood sex differences are documented in autoimmune diseases including those affecting the central nervous system (CNS) such as Multiple Sclerosis (MS; See Glossary). In MS, women are three times more affected by relapsing-remitting multiple sclerosis than men and have a higher relapse rate, making sex one of the top risk factors of MS [1–3]. Genome-wide association studies (GWAS) have found polymorphisms in the HLA region and in close proximity to genes involved in T-helper cell activation, suggesting MS is likely a Th cell-driven autoimmune disease [4]. Hence many studies elucidating the mechanism for sex-differences in MS have focused on sex-differences in T cell immune responses [5].
Non-autoimmune neurodegenerative diseases are also found to be sexually dimorphic in their prevalence, incidence, symptomology, and neuropathology (Figure 1), but the reasons for these sex differences are not well understood. The field of neurodegenerative disease research, including in MS, has recently focused on the importance of innate immune pathways, especially on the contributions of microglia, the resident innate immune cells of CNS. Similar to the studies outlining the cellular contributions to sex-differences in T cells in MS, we believe a focus on microglia may yield better understanding of the sex-differences of neurodegenerative diseases. This opinion piece was prompted by recent studies outlining the differences between microglia isolated from male and female mice. We propose these sex differences in microglia could lead to maladaptive responses during disease and non-homeostatic conditions in a sex-specific manner and ultimately contribute to the sex-differences seen in non-autoimmune neurological diseases.
Figure 1. Examples of non-autoimmune neurodegenerative diseases with sex differences.
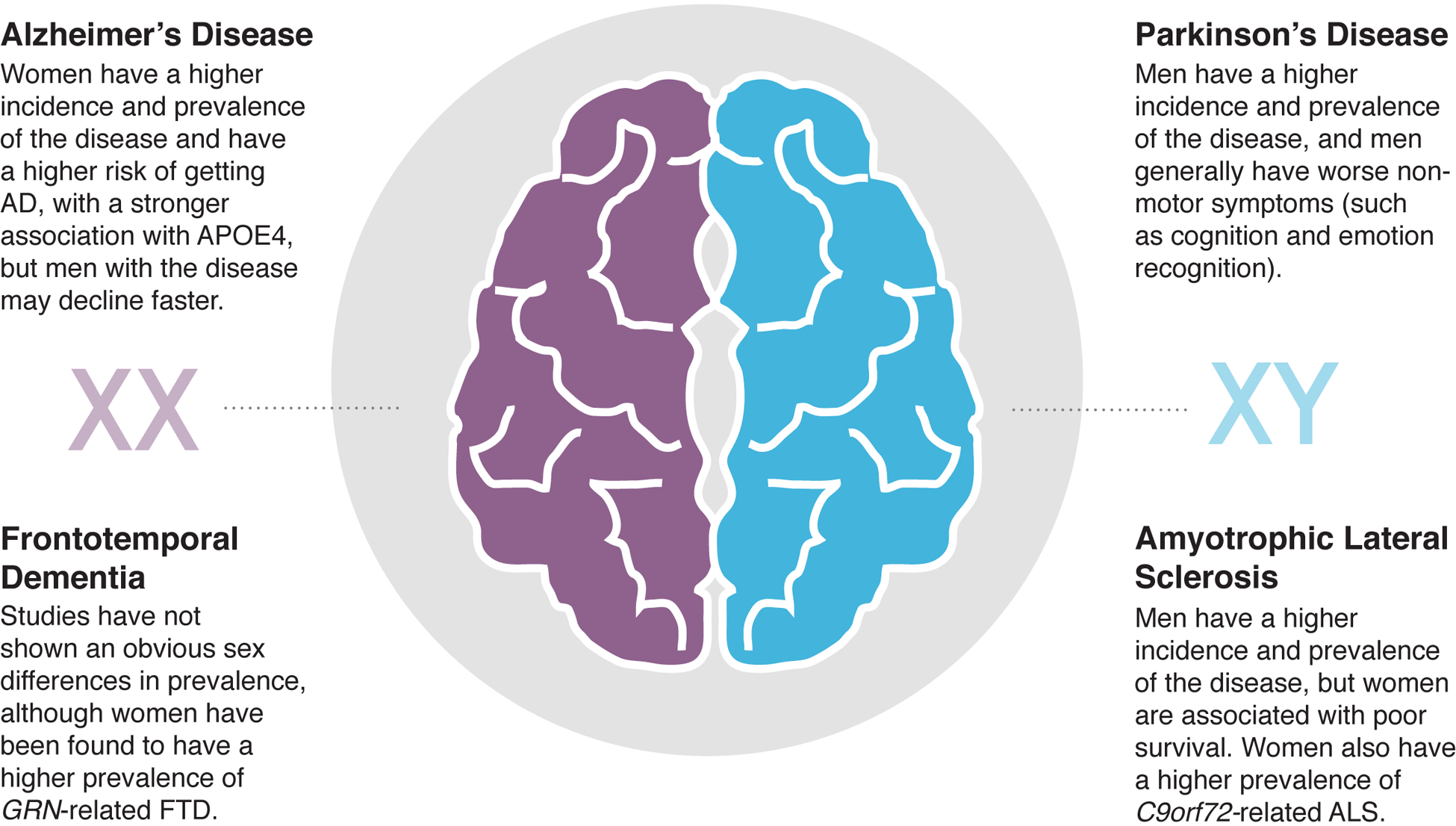
The incidence, prevalence, and disease progression of common human neurodegenerative diseases broken down by biological sex (purple XX represents female, blue XY represents male). References: AD [65,68,76–79], FTD [80,81], PD [82–87], ALS [88–91].
Why focus on microglia?
In healthy brains, microglia play critical roles during development as well as during the aging process of the brain. They serve many functions in the CNS including surveillance of the environment through dynamic movements of their processes documented in vivo [6,7]; phagocytosis and clearance of debris such as protein aggregates in disease models [8–11], synapses during development and neurodegeneration to modulate neuronal circuits [12–15], and apoptotic cells in neurogenic niches [16,17]; and signaling to other microglia and other cell types through cytokine release [10,18,19]. Several groups have also published studies to transcriptomically and proteomically characterize microglia and their heterogeneity in normal adult mice and human brain tissues [20–23].
The observation that microglia play a role in non-autoimmune neurodegenerative diseases was made by Alois Alzheimer in 1911, when he documented glial changes in post-mortem brain tissue in the first descriptions of Alzheimer’s Disease (AD) patients [24]. Similar findings were further shown in a study published in 1987 that described microglia with amoeboid morphologies surrounding amyloid plaques in AD patient brains [24,25]. GWAS and co-expression gene network studies also pointed to involvement of the innate immune system in late-onset AD, such as BIN1, TYROBP, CD33, and GRN [26–28]. The role of microglia in AD was further strengthened through whole genome sequencing studies revealing that mutations in a gene only expressed by microglia in the CNS, Triggering Receptor Expressed on Myeloid Cells 2 (TREM2), increased the risk of late-onset AD by two- to four-fold [29,30]. Microglia’s involvement in other non-autoimmune neurodegenerative diseases have also been elucidated through GWAS and other genetic studies. For instance, mutations in GRN, which are highly expressed in microglia, cause Frontotemporal Dementia (FTD), although the protein is expressed in non-microglial cells as well [31,32], and a GWAS meta-analysis found enrichment of immune genes specifically in FTD [33]. More recently, mouse models of these neurodegenerative diseases show microglial dysfunction and manipulation of microglial pathways alter disease pathology, including in mouse models of Amyotrophic Lateral Sclerosis (ALS) [34–36], and Parkinson’s Disease (PD) [17,37,38]. Furthermore, microglia share common transcriptional profiles in many neurodegenerative diseases that distinguish them from their non-disease counterparts [39–41], suggesting common pathways of microglial response in the diseased brain.
These studies have pushed the field to better understand microglial biology, but how microglia contribute to disease pathogenesis in neurodegeneration is still debated. Though many studies have illustrated the importance of the neuroimmune pathway in neurodegeneration, broad anti-inflammatory therapies have not been shown to be efficacious for non-autoimmune diseases such as AD, ALS, and PD [42]. Therefore, there is a need to focus on the disease-specific mechanisms of microglial function and their heterogeneity in order to better target these cells for therapeutic purposes. One intriguing aspect of microglia biology that may underlie some of the heterogeneity of these cells is their differences in male and female brains.
Are microglia from males and females different?
Previous studies highlighting sex differences in microglia show that microglia number, morphology, and immune molecule load is dependent on the age of the animal, brain region, as well as hormonal and environmental factors (Figure 2). For instance, sex differences in number and morphology do not emerge until the testosterone surge at postnatal day 4 (P4) when males have more microglia with activated morphology, while this pattern shifts at P30 prior to the onset of adult circulating hormones [43]. These findings seem to vary by brain region, and the sex differences is especially prominent in the hippocampus and amygdala [43]. Bulk microglia number through flow cytometry analysis, however, did not show differences in microglia number due to age or sex [44]. Microglia can also influence sex-specific maturation of the brain, as seen in Lenz et al. (2013), where inactivation of microglia through minocycline prevents masculinization of the Preoptic Are (a region responsible for reproductive behaviors) assessed through microglia number, the masculine pattern of dendritic spines, and adult sexual behavior [45].
Figure 2. Overview of sex differences in microglia.
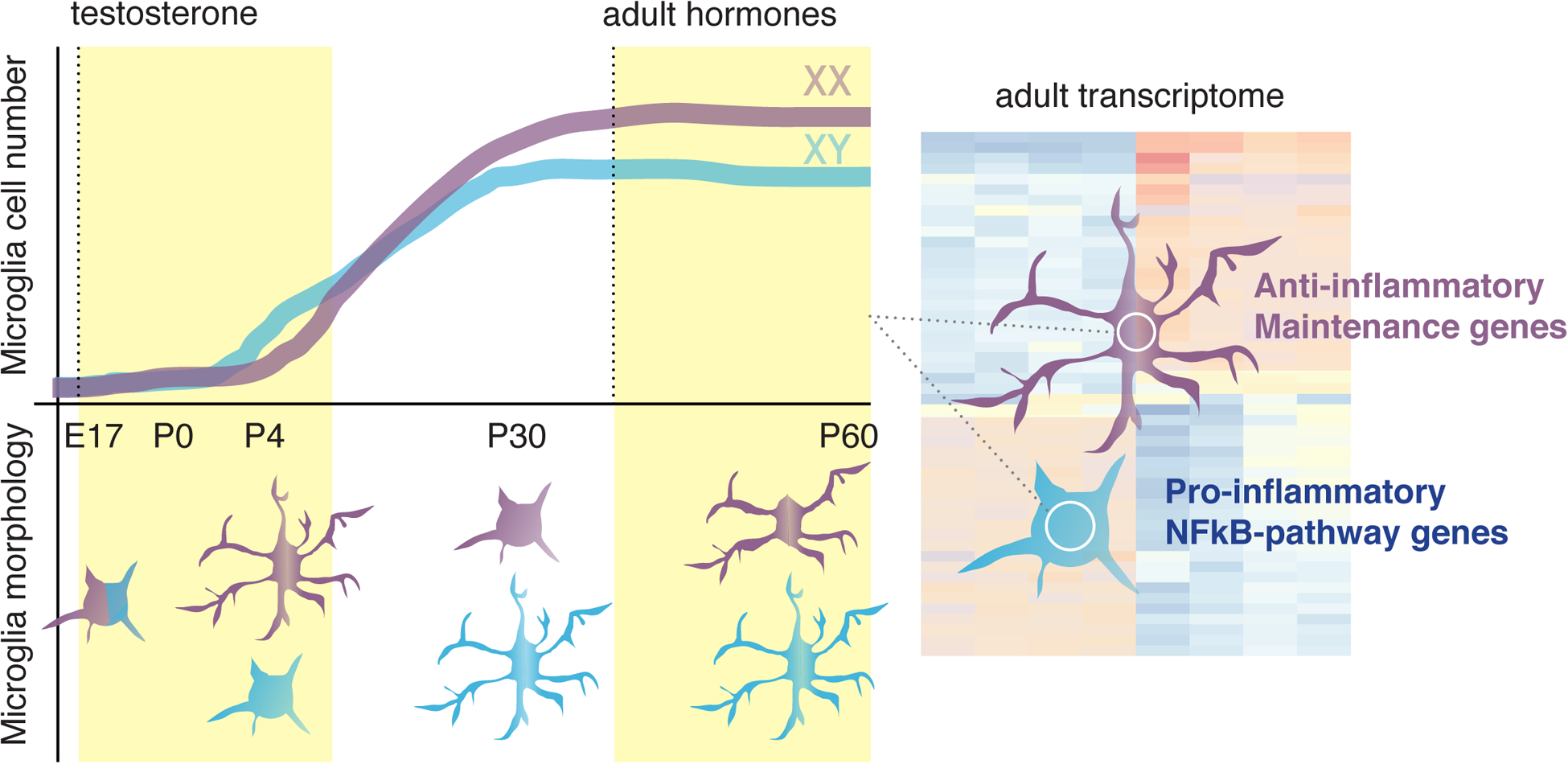
An overview of differences in male and female microglia in rodents, highlighting differences in cell number (upper half of graph) and morphology (bottom half of graph) during development and in the adult brain [43], as well as transcriptional differences in the adult rodent [49,50]. Purple and blue microglia represent those from female and male animals, respectively.
Microglia also have sex differences in their transcriptome. In Thion et al. (2018) and Hanamsagar et al. (2017), the authors show that during development, microglia isolated from male and female embryos and pups as well as during puberty do not display dramatic transcriptomic differences between the sexes [46,47]. Both studies profiled microglia isolated from rodents over the course of development up to P60 and found that only in adulthood do microglia show sex-divergence in their gene expression [46,47]. In Kang et al. (2018), the authors isolated microglia from 3-, 12-, and 24-month-old male and female mice and found sex differences at all time points, but the transcriptome was especially divergent at 24-months of age [48]. This suggests sex differences could emerge more prominently in aging-related diseases, such as neurodegenerative diseases, as the microglia also become more sexually-divergent.
Although it is agreed upon that microglia have transcriptional sex-differences in adulthood, which sex has a more proinflammatory phenotype is debated. In Hanamsagar et al., microglia from female mice were found to express more proinflammatory genes, especially genes involved in lipopolysaccharide (LPS) stimulation [46]. An earlier study by Schwarz et al. (2012) also found that in bulk tissue, immune factors analyzed, including interleukin-1 beta, were more highly expressed in females compared to males [43]. However, two studies published in 2018 by Villa et al. and Guneykaya et al. found that microglia isolated from 12-week-old male mice were more reactive and responsive to stimuli than those isolated from female mice [49,50]. These disparate findings could be due to technical differences in the isolation protocol, the age of microglial isolation, microbiome and environmental factors, and/or the brain regions studied. Nevertheless, these studies suggest microglia from male and female animals exhibit transcriptome differences that become more distinct with aging.
How are these sex differences established?
Sexual differentiation of the brain is achieved through the coordinated influence of the sex chromosomes and hormonal environment. The incomplete process of X-inactivation in females leading to approximately 15% of X-chromosome genes escaping inactivation [51] and the expression of genes on the Y-chromosome would suggest that potentially all brain cells are distinct between males and females, not just in traditionally sexually-dimorphic brain regions involved in reproduction and mating behaviors. How hormones contribute to brain sexualization on top of, and independently of, this differential genetic background is a major open question in the field of sex biology. Similarly, a characterization of the influence of sex hormones and sex chromosomes on microglia function needs to be further dissected to better understand how sex differences in microglia are established (Figure 3).
Figure 3. Model of how microglial sex differences are established.
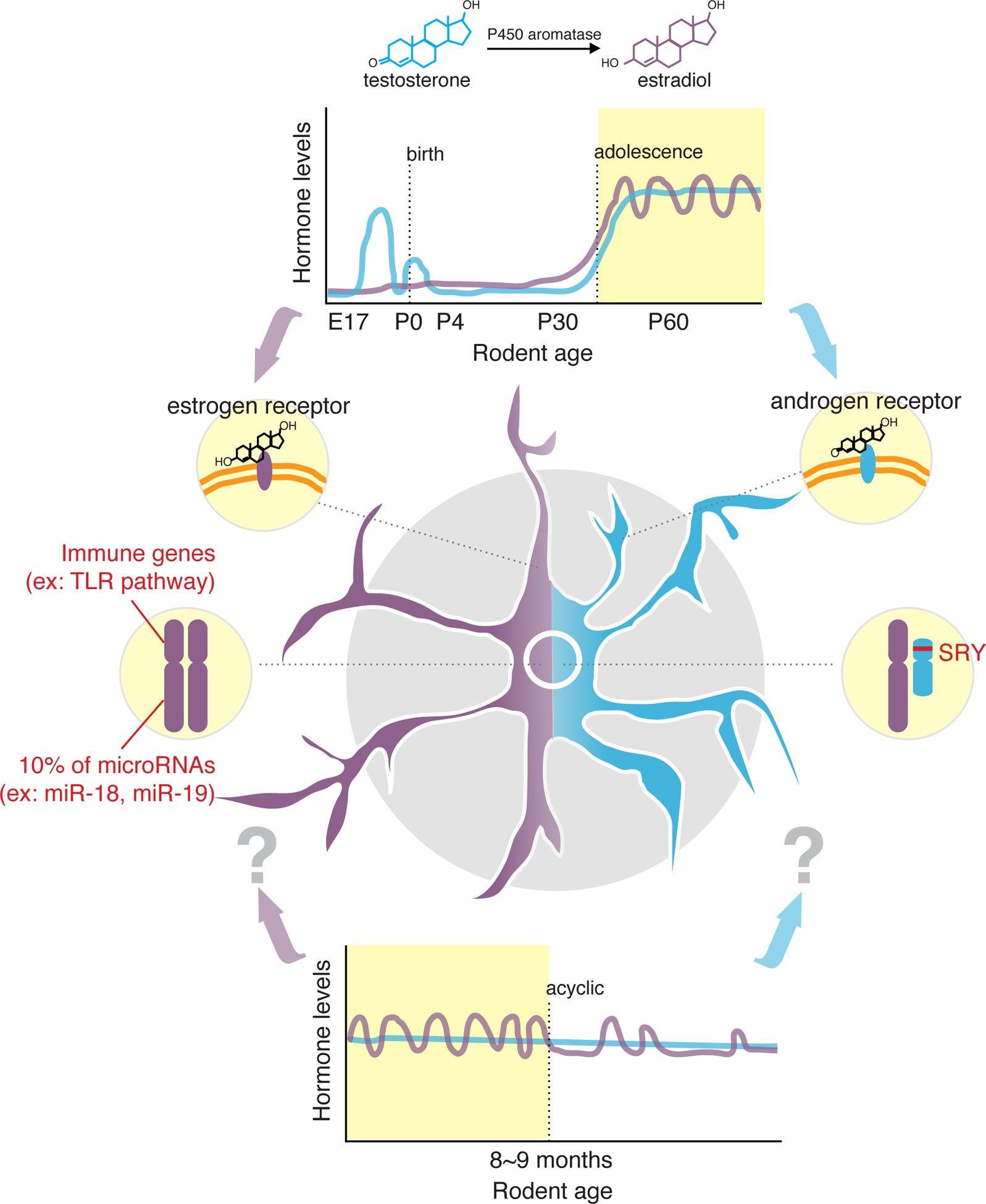
Potential mechanisms for how sex differences are achieved in microglia, applying the classical linear model of brain sexualization [92,93] and contributions of sex chromosomes [49,58–60] as well as introducing the idea of how irregular hormonal cycling seen in aged female mice may modulate microglial function [94]. Purple and blue represent female- and male-associated features, respectively.
Previous studies have shown that microglia are likely modulated by male and female sex hormones. Villa et al. (2018) demonstrated that hormones are necessary to initially establish microglial sex differences, as masculinizing the female brain by treating embryonic day 2 pups with benzoate resulted in transcriptionally “male” microglia in adult female mice [49]. Hormones not only serve to establish microglia identity during development but also continue to modulate microglial function in adulthood. For example, microglia are known to express steroid hormone receptors in normal and injured brains, and both estrogen and testosterone have anti-inflammatory effects on microglial cytokine production [52–55]. In line with these findings, ovariectomized aged mice show more microglia with inflammatory phenotypes and upregulation of inflammatory microglial and macrophage-associated genes [56,57].
Interestingly, young adult microglia are able to maintain their sex differences in the absence of hormones, as ovariectomized female mice did not drastically alter the microglial transcriptome [49]. These findings seem to be age-dependent, as Sarvari et al. found that ovariectomizing 2-month-old mice did not change the expression of inflammatory genes, while a similar treatment of 13-month-old mice had dramatic changes [57]. Additionally, female microglia transplanted into male brains after microglia-depletion, using a colony-stimulating factor 1 receptor inhibitor, were found to retain their “female” transcriptomic profile [49]. These experiments suggest microglia are able to maintain some sex-specific signatures during young adulthood even in different hormonal environments. These sex-specific profiles could, therefore, be a result of genomic (i.e. sex chromosomal) and/or epigenetic regulation. This explanation is especially compelling since the X chromosome is enriched in many genes involved in the immune system, including Toll-like receptor pathways (BTK, IRAK1, IKKγ) and microRNAs (miRNA-18 and miRNA-19) involved in immune regulation [58–60], suggesting sex chromosomes are an important sex-specific regulator of immune function (Figure 3).
Further studies are needed to establish how endocrine signaling changes microglial phenotypes specifically during aging when the estrous cycle becomes acyclic in female rodents and in post-menopause women. Changes in circulating hormone levels could also explain why the microglial transcriptome becomes more divergent as the mice age. Characterizing this would be especially important for understanding neurodegenerative diseases, as these diseases manifest in humans in the context of low circulating hormones [61].
Do microglial sex differences matter?
Despite genetic and hormonal differences between males and females leading to sex differences in microglia, it is possible these sex differences are established to achieve equivalent cellular functionality [62,63]. For instance, microglia from adult male and female mice show no difference in the uptake of synthetic beads [50]. How can microglia function similarly in sex-specific environments given differences in underlying genetics, hormone responsiveness and gene expression patterns? One possibility is that each sex uses different mechanisms to achieve similar baseline functional states i.e. aim to make the sexes functionally similar in sex-specific environments. This idea has been seen in other CNS cells. For example, knocking down expression of the Y chromosome gene, Sry, resulted in a decrease in Tyrosine Hydroxylase (TH) expression in the substantia nigra, leading to functional motor deficits in male mice [64]. Notably, even though females have lower numbers of TH-positive neurons due to the lack of Sry, they have comparable motor function to healthy males. This suggests compensatory pathways may be at play in females to maintain motor function at a similar level to healthy males. In this way, sex differences could be sex-specific mechanisms for obtaining the same function.
In response to non-homeostatic disease states, sex differences in microglia may have important functional consequences for disease progression. In a model of ischemic stroke (permanent middle cerebral artery occlusion model), transplanting female microglia into male mice reduced the progression of ischemic damage compared to transplanting male microglia, suggesting a protective role of female microglia in ischemia [49]. Interestingly, altering the maternal microbiome using a germ-free environment resulted in sex-specific effects on microglia after birth and later in adulthood [46]. The unmasking of functional sex differences in non-homeostatic states suggests that differences in disease susceptibility may partially result from sex-specific maladaptive responses, and therefore, studying sex differences could provide novel insights into disease mechanisms.
One interesting gene with a sex-specific phenotype in disease is Apolipoprotein E (Apoe). Humans with the ε4 variant of the Apoe gene have a higher risk of developing late-onset AD, and this risk factor is correlated more strongly in females compared to males [65–68] (Figure 1). Emerging evidence suggests that ApoE is a modulator of the CNS immune system and can have differential outcomes on microglial function depending on the variant [53,69,70]. A recent study also showed microglia are a major source of plaque-associated ApoE and that Apoe expression is modulated by TREM2 function in AD mouse models [71]. Interestingly, Apoe is highly upregulated in disease-associated microglia and has a differential expression in male versus female SOD1 mice, a mouse model of ALS [39,40,72]. Transcripts driven by the APOE network also seem to be upregulated in microglia isolated from female 24-month-old mice compared to those isolated from male mice [48]. Therefore, Apoe is a potential gene that could tie microglia to the sex differences of AD and perhaps other neurodegenerative diseases. We advise other microglia-associated genetic risk factors of neurodegeneration be further assessed in a sex-specific manner.
How to study microglial sex differences?
Though deep and detailed characterization of microglia have been made at the transcriptomic, proteomic, and functional levels, most studies have either isolated microglia from one sex or pooled samples from both sexes. It is, therefore, essential to study microglia from male and female brains separately to avoid missing key functional differences and addressing fundamental questions such as: How are microglial sex differences maintained during aging? How do these differences change during disease and could they alter disease susceptibility? These and other basic questions (see Outstanding Questions) need to be addressed to further understand the contribution of sex differences to neurological health and disease.
One of the overarching and persisting challenges of the field of sex biology is to parse out the contribution of hormonal and non-hormonal (including sex chromosomal) effects. This dissection is further complicated by aging. Hormonal fluctuations during the aging process (especially during menopause) can have profound effects on neurological disease outcomes [61]. Mouse models can be used to study the effects of menopause, such as the use of 4-vinylcyclohexene diepoxide (VCD) to induce follicle depletion and ovary atrophy, leading to a gradual decline in hormones [73]. Mimicking these hormonal fluctuations in mice as well as using models to segregate hormonal from non-hormonal mechanisms of sex differentiation are crucial for understanding sexually dimorphic phenotypes.
One way to segregate the hormonal from the sex chromosomal contribution to microglial sex differences is to use the “Four Core Genotype” (FCG) mouse model. In these mice, the testis-determining Sry gene is deleted from the Y chromosome and inserted into an autosome [74]. Thus, XX and XY mice can develop ovaries or testis depending on whether they have the Sry gene. The effect of this model on microglia has yet to be studied and could be used to better understand the hormonal verses the sex chromosome effects on microglial function.
In addition, when crossed to mouse models of disease, the FCG model can be used as a powerful tool for understanding the mechanism of sex differential phenotypes in disease. For example, Smith-Bouvier et al. (2008) crossed FCG mice with experimental autoimmune encephalomyelitis (EAE) mice, a mouse model of MS, then gonadectomized them to remove sex hormones. They found that the disease was more severe in mice with the XX sex chromosomes than the XY group [75], suggesting differential effects of the X and Y chromosome on disease progression outside the context of sex hormones. Such a model could have powerful implications in other models of neurodegeneration such as AD, where the sex differences in disease progression are not well-understood.
Concluding remarks
Microglia from male and female rodents exhibit sex-differences, likely owing to their ability to sense different hormonal environments and their differential sex chromosome composition. As the field of neurodegenerative diseases has come to appreciate the role of microglia in a myriad of diseases, we should also appreciate how these cells may be contributing to sex differences in the diseased brain. Further studies need to be conducted to answer the questions outlined above as well as in the Outstanding Questions section, with the mindset that sex differences in cells may be an adaptation to normalize the differences of the sexes. Moreover, these sex differences may be maladaptive in disease states and lead to differences in disease vulnerability and progression. Therefore, studying sex differences in microglia could shed light onto why sex differences exist in human neurological diseases and potentially lead to sex-specific therapies.
Clinician’s Corner:
Many neurodegenerative diseases have sexually-dimorphic incidence, prevalence, and disease severity (Figure 1). The reasons for these sex-effects are not well-understood, but sex hormones and sex chromosomes as well as environmental factors have been extensively studied to explain this phenomenon.
Microglia are implicated in many neurological diseases, including these sex-stratified diseases. Furthermore, microglia from male and female rodents show sex differences in their baseline and response to stimuli and disease (Figure 2). Whether human microglia also show sex differences is not known and should be assessed. Understanding how human microglia respond differentially to disease based on sex may lead to sex-specific dosing of current therapies and/or development of new therapies.
Broad anti-inflammatory therapies have not been clinically efficacious against non-autoimmune neurodegenerative diseases, suggesting a need to focus on non-inflammatory mechanisms of microglial function and a better understanding of the heterogeneity of these cells. Sex differences in microglial cells may be one avenue of exploration.
Acknowledgements:
We would like to thank Claire D. Clelland for the helpful discussion and editing and Kathryn Claiborn for proof-reading.
Glossary
- Alzheimer’s Disease (AD)
The leading cause of dementia, characterized pathologically by accumulation of protein aggregates (amyloid plaques and tau tangles), gliosis, and brain atrophy.
- Amyotrophic Lateral Sclerosis (ALS)
A neurodegenerative disease that mainly affects motor neurons involved in voluntary muscle movements, leading to paralysis.
- Flow cytometry
A high-throughput technique to detect fluorescent antibodies against cellular structures of interest and optical properties of cells for analysis.
- Frontotemporal Dementia (FTD)
A heterogeneous collection of diseases leading to dementia and is the second leading cause of dementia, with symptoms typically manifesting during mid-adulthood.
- Genome-wide association study (GWAS)
A large-scale study to find associations between genetic variants with a trait, such as a major human disease, in different individuals.
- Lipopolysaccharide (LPS)
An endotoxin found in the outer membrane of Gram-negative bacteria. It is recognized by receptors on immune cells, leading to secretion of pro-inflammatory cytokines and immune activation.
- MicroRNAs
Small, noncoding RNAs that target the three prime untranslated region (3’UTR) of an mRNA in order to degrade the transcript or inhibit its translation. One microRNA can target many downstream mRNAs and control networks of protein expression.
- Multiple Sclerosis (MS)
An autoimmune, demyelinating neurodegenerative disease.
- Ovariectomy
A surgical procedure to remove the ovaries. This can be done in order to study female mice without hormonal circulation.
- Parkinson’s Disease (PD)
A neurodegenerative disease that mainly affects dopaminergic neurons in the substantia nigra, leading to motor symptoms such as slowed movement, stiffness, tremors, and loss of balance.
- Toll-like receptor
A class of proteins expressed on innate immune cells that recognize molecules broadly-shared by microbes, such as LPS, in order to mount an innate immune response.
- X-inactivation
The genetic process of silencing and inactivating one of the X chromosomes in female mammals through the X-chromosome inactivation center (Xic), initiated by the X-inactive specific transcript (XIST). This process is random and incomplete in cells, leading to mosaicism of gene expression.
Footnotes
Conflict of Interest:
All authors confirm that there are no known conflicts of interest associated with this publication.
References:
- 1.Kalincik T et al. Sex as a determinant of relapse incidence and progressive course of multiple sclerosis. Brain : a journal of neurology 136, 3609–3617, doi: 10.1093/brain/awt281 (2019). [DOI] [PubMed] [Google Scholar]
- 2.Koch-Henriksen N & Sorensen PS The changing demographic pattern of multiple sclerosis epidemiology. The Lancet. Neurology 9, 520–532, doi: 10.1016/s1474-4422(10)70064-8 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Orton SM et al. Sex ratio of multiple sclerosis in Canada: a longitudinal study. The Lancet. Neurology 5, 932–936, doi: 10.1016/s1474-4422(06)70581-6 (2006). [DOI] [PubMed] [Google Scholar]
- 4.Sawcer S et al. Genetic risk and a primary role for cell-mediated immune mechanisms in multiple sclerosis. Nature 476, 214–219, doi: 10.1038/nature10251 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunn SE, Lee H, Pavri FR & Zhang MA Sex-Based Differences in Multiple Sclerosis (Part I): Biology of Disease Incidence. Curr Top Behav Neurosci 26, 29–56, doi: 10.1007/7854_2015_371 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Nimmerjahn A, Kirchhoff F & Helmchen F Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science (New York, N.Y.) 308, 1314–1318, doi: 10.1126/science.1110647 (2005). [DOI] [PubMed] [Google Scholar]
- 7.Davalos D et al. ATP mediates rapid microglial response to local brain injury in vivo. Nature neuroscience 8, 752–758, doi: 10.1038/nn1472 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Asai H et al. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nature neuroscience 18, 1584–1593, doi: 10.1038/nn.4132 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bolos M et al. Absence of CX3CR1 impairs the internalization of Tau by microglia. Mol Neurodegener 12, 59, doi: 10.1186/s13024-017-0200-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y et al. TREM2 Is a Receptor for beta-Amyloid that Mediates Microglial Function. Neuron 97, 1023–1031.e1027, doi: 10.1016/j.neuron.2018.01.031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yuan P et al. TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy. Neuron 90, 724–739, doi: 10.1016/j.neuron.2016.05.003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kettenmann H, Kirchhoff F & Verkhratsky A Microglia: new roles for the synaptic stripper. Neuron 77, 10–18, doi: 10.1016/j.neuron.2012.12.023 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Hong S et al. Complement and microglia mediate early synapse loss in Alzheimer mouse models. doi: 10.1126/science.aad8373 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dejanovic B et al. Changes in the Synaptic Proteome in Tauopathy and Rescue of Tau-Induced Synapse Loss by C1q Antibodies. Neuron 100, 1322–1336.e1327, doi: 10.1016/j.neuron.2018.10.014 (2018). [DOI] [PubMed] [Google Scholar]
- 15.Lui H et al. Progranulin Deficiency Promotes Circuit-Specific Synaptic Pruning by Microglia via Complement Activation. Cell 165, 921–935, doi: 10.1016/j.cell.2016.04.001 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sierra A et al. Microglia shape adult hippocampal neurogenesis through apoptosis-coupled phagocytosis. Cell Stem Cell 7, 483–495, doi: 10.1016/j.stem.2010.08.014 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fourgeaud L et al. TAM receptors regulate multiple features of microglial physiology. Nature 532, 240–244, doi: 10.1038/nature17630 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liddelow SA et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 541, 481–487, doi: 10.1038/nature21029 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krabbe G et al. Microglial NFκB-TNFα hyperactivation induces obsessive–compulsive behavior in mouse models of progranulin-deficient frontotemporal dementia. doi: 10.1073/pnas.1700477114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li Q et al. Developmental Heterogeneity of Microglia and Brain Myeloid Cells Revealed by Deep Single-Cell RNA Sequencing. Neuron 101, 207–223.e210, doi: 10.1016/j.neuron.2018.12.006 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matcovitch-Natan O et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science (New York, N.Y.) 353, aad8670, doi: 10.1126/science.aad8670 (2016). [DOI] [PubMed] [Google Scholar]
- 22.Hickman SE et al. The microglial sensome revealed by direct RNA sequencing. Nature neuroscience 16, 1896–1905, doi: 10.1038/nn.3554 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grabert K et al. Microglial brain region-dependent diversity and selective regional sensitivities to aging. Nature neuroscience 19, 504–516, doi: 10.1038/nn.4222 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moller HJ & Graeber MB The case described by Alois Alzheimer in 1911. Historical and conceptual perspectives based on the clinical record and neurohistological sections. European archives of psychiatry and clinical neuroscience 248, 111–122 (1998). [DOI] [PubMed] [Google Scholar]
- 25.McGeer PL, Itagaki S, Tago H & McGeer EG Reactive microglia in patients with senile dementia of the Alzheimer type are positive for the histocompatibility glycoprotein HLA-DR. Neuroscience letters 79, 195–200 (1987). [DOI] [PubMed] [Google Scholar]
- 26.Zhang B et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer’s disease. Cell 153, 707–720, doi: 10.1016/j.cell.2013.03.030 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones L et al. Genetic evidence implicates the immune system and cholesterol metabolism in the aetiology of Alzheimer’s disease. PloS one 5, e13950, doi: 10.1371/journal.pone.0013950 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wes PD, Sayed FA, Bard F & Gan L Targeting microglia for the treatment of Alzheimer’s Disease. Glia 64, 1710–1732, doi: 10.1002/glia.22988 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Guerreiro R et al. TREM2 variants in Alzheimer’s disease. The New England journal of medicine 368, 117–127, doi: 10.1056/NEJMoa1211851 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jonsson T et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. The New England journal of medicine 368, 107–116, doi: 10.1056/NEJMoa1211103 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cruts M et al. Null mutations in progranulin cause ubiquitin-positive frontotemporal dementia linked to chromosome 17q21. Nature 442, 920, doi:doi: 10.1038/nature05017 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Baker M et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 442, 916, doi:doi: 10.1038/nature05016 (2006). [DOI] [PubMed] [Google Scholar]
- 33.Broce I et al. in PLoS Med Vol. 15 (2018). [Google Scholar]
- 34.Boillee S et al. Onset and progression in inherited ALS determined by motor neurons and microglia. Science (New York, N.Y.) 312, 1389–1392, doi: 10.1126/science.1123511 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Frakes AE et al. Microglia induce motor neuron death via the classical NF-kappaB pathway in amyotrophic lateral sclerosis. Neuron 81, 1009–1023, doi: 10.1016/j.neuron.2014.01.013 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spiller KJ et al. Microglia-mediated recovery from ALS-relevant motor neuron degeneration in a mouse model of TDP-43 proteinopathy. Nature neuroscience 21, 329–340, doi: 10.1038/s41593-018-0083-7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghosh A et al. Selective inhibition of NF-kappaB activation prevents dopaminergic neuronal loss in a mouse model of Parkinson’s disease. Proceedings of the National Academy of Sciences of the United States of America 104, 18754–18759, doi: 10.1073/pnas.0704908104 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim C et al. Neuron-released oligomeric alpha-synuclein is an endogenous agonist of TLR2 for paracrine activation of microglia. Nature communications 4, 1562, doi: 10.1038/ncomms2534 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Keren-Shaul H et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 169, 1276–1290.e1217, doi: 10.1016/j.cell.2017.05.018 (2017). [DOI] [PubMed] [Google Scholar]
- 40.Krasemann S et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 47, 566–581.e569, doi: 10.1016/j.immuni.2017.08.008 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mathys H et al. Temporal Tracking of Microglia Activation in Neurodegeneration at Single-Cell Resolution. Cell Rep 21, 366–380, doi: 10.1016/j.celrep.2017.09.039 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Masgrau R, Guaza C, Ransohoff RM & Galea E Should We Stop Saying ‘Glia’ and ‘Neuroinflammation’? Trends in molecular medicine 23, 486–500, doi: 10.1016/j.molmed.2017.04.005 (2017). [DOI] [PubMed] [Google Scholar]
- 43.Schwarz JM, Sholar PW & Bilbo SD Sex differences in microglial colonization of the developing rat brain. Journal of neurochemistry 120, 948–963, doi: 10.1111/j.1471-4159.2011.07630.x (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manwani B et al. Differential effects of aging and sex on stroke induced inflammation across the lifespan. Experimental neurology 249, doi: 10.1016/j.expneurol.2013.08.011 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lenz KM, Nugent BM, Haliyur R & McCarthy MM Microglia are essential to masculinization of brain and behavior. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 2761–2772, doi: 10.1523/jneurosci.1268-12.2013 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thion MS et al. Microbiome Influences Prenatal and Adult Microglia in a Sex-Specific Manner. Cell 172, 500–516.e516, doi: 10.1016/j.cell.2017.11.042 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hanamsagar R et al. Generation of a microglial developmental index in mice and in humans reveals a sex difference in maturation and immune reactivity. Glia 65, 1504–1520, doi: 10.1002/glia.23176 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang SS et al. Microglial translational profiling reveals a convergent APOE pathway from aging, amyloid, and tau. The Journal of experimental medicine 215, 2235–2245, doi: 10.1084/jem.20180653 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Villa A et al. Sex-Specific Features of Microglia from Adult Mice. Cell Rep 23, 3501–3511, doi: 10.1016/j.celrep.2018.05.048 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guneykaya D et al. Transcriptional and Translational Differences of Microglia from Male and Female Brains. Cell Rep 24, 2773–2783.e2776, doi: 10.1016/j.celrep.2018.08.001 (2018). [DOI] [PubMed] [Google Scholar]
- 51.Carrel L & Willard HF X-inactivation profile reveals extensive variability in X-linked gene expression in females. Nature 434, 400–404, doi: 10.1038/nature03479 (2005). [DOI] [PubMed] [Google Scholar]
- 52.Sierra A, Gottfried-Blackmore A, Milner TA, McEwen BS & Bulloch K Steroid hormone receptor expression and function in microglia. Glia 56, 659–674, doi: 10.1002/glia.20644 (2008). [DOI] [PubMed] [Google Scholar]
- 53.Colton CA, Brown CM & Vitek MP Sex steroids, APOE genotype and the innate immune system. Neurobiol Aging 26, 363–372, doi: 10.1016/j.neurobiolaging.2004.08.001 (2005). [DOI] [PubMed] [Google Scholar]
- 54.Vegeto E et al. Estrogen prevents the lipopolysaccharide-induced inflammatory response in microglia. The Journal of neuroscience : the official journal of the Society for Neuroscience 21, 1809–1818 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Ovejero D, Veiga S, Garcia-Segura LM & Doncarlos LL Glial expression of estrogen and androgen receptors after rat brain injury. The Journal of comparative neurology 450, 256–271, doi: 10.1002/cne.10325 (2002). [DOI] [PubMed] [Google Scholar]
- 56.Benedusi V et al. A lack of ovarian function increases neuroinflammation in aged mice. Endocrinology 153, 2777–2788, doi: 10.1210/en.2011-1925 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sarvari M et al. Menopause leads to elevated expression of macrophage-associated genes in the aging frontal cortex: rat and human studies identify strikingly similar changes. J Neuroinflammation 9, 264, doi: 10.1186/1742-2094-9-264 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pinheiro I, Dejager L & Libert C X-chromosome-located microRNAs in immunity: might they explain male/female differences? The X chromosome-genomic context may affect X-located miRNAs and downstream signaling, thereby contributing to the enhanced immune response of females. BioEssays : news and reviews in molecular, cellular and developmental biology 33, 791–802, doi: 10.1002/bies.201100047 (2011). [DOI] [PubMed] [Google Scholar]
- 59.Fish EN The X-files in immunity: sex-based differences predispose immune responses. Nature Reviews Immunology 8, 737, doi:doi: 10.1038/nri2394 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klein SL & Flanagan KL Sex differences in immune responses. Nature reviews. Immunology 16, 626–638, doi: 10.1038/nri.2016.90 (2016). [DOI] [PubMed] [Google Scholar]
- 61.Dubal DB, Broestl L & Worden K Sex and gonadal hormones in mouse models of Alzheimer’s disease: what is relevant to the human condition? Biology of sex differences 3, 24, doi: 10.1186/2042-6410-3-24 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.De Vries GJ & Center for Neuroendocrine Studies, U. o. M., Amherst, Massachusetts 01003. Minireview: Sex Differences in Adult and Developing Brains: Compensation, Compensation, Compensation. Endocrinology 145, 1063–1068, doi: 10.1210/en.2003-1504 (2019). [DOI] [PubMed] [Google Scholar]
- 63.McCarthy MM & Arnold AP Reframing sexual differentiation of the brain. Nature neuroscience 14, 677, doi:doi: 10.1038/nn.2834 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dewing P et al. Direct regulation of adult brain function by the male-specific factor SRY. Current biology : CB 16, 415–420, doi: 10.1016/j.cub.2006.01.017 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Seshadri S et al. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology 49, 1498–1504 (1997). [DOI] [PubMed] [Google Scholar]
- 66.Hohman TJ et al. Sex-Specific Association of Apolipoprotein E With Cerebrospinal Fluid Levels of Tau. JAMA neurology 75, 989–998, doi: 10.1001/jamaneurol.2018.0821 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Altmann A, Tian L, Henderson VW & Greicius MD Sex modifies the APOE-related risk of developing Alzheimer disease. Annals of neurology 75, 563–573, doi: 10.1002/ana.24135 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Farrer LA et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. Jama 278, 1349–1356 (1997). [PubMed] [Google Scholar]
- 69.Lynch JR et al. APOE genotype and an ApoE-mimetic peptide modify the systemic and central nervous system inflammatory response. The Journal of biological chemistry 278, 48529–48533, doi: 10.1074/jbc.M306923200 (2003). [DOI] [PubMed] [Google Scholar]
- 70.Laskowitz DT et al. Downregulation of microglial activation by apolipoprotein E and apoE-mimetic peptides. Experimental neurology 167, 74–85, doi: 10.1006/exnr.2001.7541 (2001). [DOI] [PubMed] [Google Scholar]
- 71.Parhizkar S et al. Loss of TREM2 function increases amyloid seeding but reduces plaque-associated ApoE. Nature neuroscience 22, 191–204, doi: 10.1038/s41593-018-0296-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Butovsky O et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Annals of neurology 77, 75–99, doi: 10.1002/ana.24304 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mayer LP, Devine PJ, Dyer CA & Hoyer PB The follicle-deplete mouse ovary produces androgen. Biology of reproduction 71, 130–138, doi: 10.1095/biolreprod.103.016113 (2004). [DOI] [PubMed] [Google Scholar]
- 74.Lovell-Badge R & Robertson E XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development (Cambridge, England) 109, 635–646 (1990). [DOI] [PubMed] [Google Scholar]
- 75.Smith-Bouvier DL et al. A role for sex chromosome complement in the female bias in autoimmune disease. The Journal of experimental medicine 205, 1099–1108, doi: 10.1084/jem.20070850 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Oveisgharan S et al. Sex differences in Alzheimer’s disease and common neuropathologies of aging. Acta neuropathologica 136, 887–900, doi: 10.1007/s00401-018-1920-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kawas C, Gray S, Brookmeyer R, Fozard J & Zonderman A Age-specific incidence rates of Alzheimer’s disease: the Baltimore Longitudinal Study of Aging. Neurology 54, 2072–2077 (2000). [DOI] [PubMed] [Google Scholar]
- 78.Hua X et al. Sex and age differences in atrophic rates: an ADNI study with N=1368 MRI scans. Neurobiol Aging 31, 1463–1480, doi: 10.1016/j.neurobiolaging.2010.04.033 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Henderson VW & Buckwalter JG Cognitive deficits of men and women with Alzheimer’s disease. Neurology 44, 90–96 (1994). [DOI] [PubMed] [Google Scholar]
- 80.Mercy L, Hodges JR, Dawson K, Barker RA & Brayne C Incidence of early-onset dementias in Cambridgeshire, United Kingdom. doi: 10.1212/01.wnl.0000334277.16896.fa (2008). [DOI] [PubMed] [Google Scholar]
- 81.Curtis AF et al. Sex differences in the prevalence of genetic mutations in FTD and ALS. doi: 10.1212/WNL.0000000000004494 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Baldereschi M et al. Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology 55, 1358–1363 (2000). [DOI] [PubMed] [Google Scholar]
- 83.Augustine EF et al. Sex Differences in Clinical Features of Early, Treated Parkinson’s Disease. PloS one 10, e0133002, doi: 10.1371/journal.pone.0133002 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Taylor KS, Cook JA & Counsell CE in Journal of neurology, neurosurgery, and psychiatry Vol. 78 905–906 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heller J et al. Impact of gender and genetics on emotion processing in Parkinson’s disease - A multimodal study. NeuroImage. Clinical 18, 305–314, doi: 10.1016/j.nicl.2018.01.034 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Heller J, Dogan I, Schulz JB & Reetz K in Aging Dis Vol. 5 63–75 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haaxma CA et al. in Journal of neurology, neurosurgery, and psychiatry Vol. 78 819–824 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Manjaly ZR et al. The sex ratio in amyotrophic lateral sclerosis: A population based study. Amyotrophic lateral sclerosis : official publication of the World Federation of Neurology Research Group on Motor Neuron Diseases 11, 439–442, doi: 10.3109/17482961003610853 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.del Aguila MA, Longstreth WT Jr., McGuire V, Koepsell TD & van Belle G Prognosis in amyotrophic lateral sclerosis: a population-based study. Neurology 60, 813–819 (2003). [DOI] [PubMed] [Google Scholar]
- 90.McCombe PA & Henderson RD Effects of gender in amyotrophic lateral sclerosis. Gender medicine 7, 557–570, doi: 10.1016/j.genm.2010.11.010 (2010). [DOI] [PubMed] [Google Scholar]
- 91.Curtis AF et al. Sex differences in the prevalence of genetic mutations in FTD and ALS: A meta-analysis. Neurology 89, 1633–1642, doi: 10.1212/wnl.0000000000004494 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arnold AP The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian tissues. Hormones and behavior 55, 570–578, doi: 10.1016/j.yhbeh.2009.03.011 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Phoenix CH, Goy RW, Gerall AA & Young WC Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382, doi: 10.1210/endo-65-3-369 (1959). [DOI] [PubMed] [Google Scholar]
- 94.Finch CE, Felicio LS, Mobbs CV & Nelson JF Ovarian and steroidal influences on neuroendocrine aging processes in female rodents. - PubMed - NCBI. Endocrine reviews 5, 467–497, doi: 10.1210/edrv-5-4-467 (1984). [DOI] [PubMed] [Google Scholar]


