Abstract
We have previously developed a cost-effective, chemically defined medium formula for the weekend-free culture of human induced pluripotent stem cells costing ~3% of commercial media. This medium, we termed B8, is specifically optimized for robust and fast growth of hiPSC and to allow a weekend-free media change regimen. We demonstrated that this medium is suitable for the reprogramming of somatic cells to hiPSC and for differentiation to a variety of lineages. Here, we provide a protocol for the simple generation of the most cost-effective variant of this medium (Basic Protocol 1), along with protocols for making Matrigel-coated plates, culture of hiPSC, passaging, cryopreservation, and thawing (Basic Protocol 2).
Basic Protocol 1: Preparation of a highly optimized, robust, and cost effective human induced pluripotent stem cell culture medium.
Basic Protocol 2: Weekend-free maintenance and passaging of human pluripotent stem cells in b8 medium
Keywords: human induced pluripotent stem cells, culture medium, chemically defined, cost-effective, weekend-free
INTRODUCTION
Human induced pluripotent stem cells (hiPSCs), with their unlimited expansion and differentiation potential, serve as a unique source of cells for disease modeling, precision medicine, drug assays, and pharmacogenomics. All these aforementioned applications require the representation of the variability existent in human population and therefore cohorts with large number of hiPSC lines are required. The traditional approaches to culture hiPSCs using mouse embryonic fibroblasts (MEFs) as feeder layer and media containing Knockout Serum Replacement (KSR) results in variable outcome, which lacks the required consistency for high-throughput assays [1]. To resolve this issue, efforts were made to generate more defined culture conditions. MEFs were replaced by extracellular matrices such as Matrigel and complex media components like KSR were replaced with defined components such as in the TeSR formula, which is a mixture of 19 components [2-4]. Further efforts then led to the generation of the first robust chemically defined medium named E8 with only 8 components [5, 6]. Recently, we developed a new cost effective, chemically defined culture medium called B8 which supports the high growth rate of hiPSCs yet with minimal cost [7]. Cells grown in this media maintain normal expression pattern of undifferentiated cell markers. Moreover, no chromosomal abnormality was observed after long-term culture of the cells in B8 media as shown by normal G-banding karyotype analysis [7]. In this protocol we present a detailed guide for the generation of the B8 medium (Basic Protocol 1). We also describe approaches for the preparation of Matrigel-coated plates, passaging hiPSCs, cryopreservation, and thawing in B8 medium (Basic Protocol 2).
BASIC PROTOCOL 1
PREPARATION OF A HIGHLY OPTIMIZED, ROBUST, AND COST EFFECTIVE HUMAN INDUCED PLURIPOTENT STEM CELL CULTURE MEDIUM
In this approach, hiPSCs are grown in chemically defined B8 medium [7]. In B8, each component has been individually optimized for fast growth and reduced costs, with higher L-ascorbic acid 2-phosphate, lower transferrin and insulin, no additional sodium bicarbonate, in-house generated, codon-optimized FGF2-G3 with multiple point mutations to improve thermostability [8], replacement of TGFß1 with TGFß3, and the addition of NRG1. The plasmids for generating these growth factors are available via Addgene. The specific variant of B8 described here has lower levels of insulin and transferrin, as with the optimizations described, these have become the most expensive components and lowering their concentration has only minimal negative effect on hiPSCs proliferation. In this variant we also use commercial recombinant TGFß3 and NRG1 as these components are used at very low concentrations and the effort required to make them is not cost-efficient.
Materials
Cell culture water (Corning, 25-055-CV), store at RT
150 mL PES 0.2 μm bottle top filters (Corning, 431153)
L-ascorbic acid 2-phosphate tri sodium salt (Wako, 321-44823), powder, store at RT
Recombinant human insulin (Life Technologies, A11382IJ), powder, store at −20 °C
Sodium hydroxide (1 N) (Sigma, S2770), store at RT
Hydrochloric acid (1 N) (Sigma, H9892), store at RT
Recombinant human transferrin (InVitria Optiferrin, 777TRF029-10G), powder, store at −20 °C
Sodium selenite (See recipe; Sigma, S5261-10G), store at RT
Recombinant human FGF2-G3 (made by a Recombinant Protein Production Core), commonly results in 80 mg per L of E. coli at ~5 mg/mL, store at −20 °C
Recombinant human NRG1 (See recipe, Peprotech, 100-03-250UG), store at −20 °C
Recombinant human TGFß3 (250 μg/mL; Cell Guidance Systems GFH109, 100 μg), store at 4 °C
DMEM/F12 (1 L, Corning, 10-092-CM), store at 4 °C
50 mL polystyrene conical tubes (Corning Falcon, 352098)
1.7 mL sterile microtubes (Axygen, MCT-175-C-S)
Analytical balance such as Mettler Toledo ML204
pH meter such as Mettler Toledo SevenCompact S220
GENERATING B8 SUPPLEMENTS
NOTE: All the mentioned values here are for 100 L of B8 media. Smaller volumes could be achieved by reducing the components accordingly.
Tube A:
Add 47 mL of room temperature cell culture water to a 50 mL conical tube.
Weight 20 g of L-ascorbic acid 2-phosphate.
Slowly add L-ascorbic acid 2-phosphate to the water.
Mix by inverting until the solution becomes clear.
Tube B:
Place 49 mL of room temperature cell culture water in 50 mL conical tube.
-
Add 0.5 g of insulin.
NOTE: Insulin dissolves in acidic solutions (~pH 3).
Using a pH meter, measure the pH of the solution.
Slowly add 1 N HCl until the pH reaches to 3. Roughly 400 μL is needed.
Mix by inverting until the solution becomes clear.
-
Bring the pH to 7.1 by slowly adding 1 N NaOH. Roughly 500 μL is needed.
NOTE: By increasing the pH the solution becomes cloudy again.
Mix by inverting until the solution becomes clear.
Tube C:
Place 47 mL of room temperature cell culture water in 50 mL conical tube.
Add 0.5 g of Optiferrin.
Add 1 mL of 2 mg/mL sodium selenite.
Add 1 mL of 4 mg/mL FGF2-G3.
Add 40 μL of 250 μg/mL TGFß3.
Add 100 μL of 100 μg/mL NRG1.
Mix by inverting until the solution becomes clear.
Combining:
In the cell culture hood, transfer the contents of Tube A, Tube B, and Tube C to the top of a 150 mL Corning PES 0.2 μm bottle top filter. Filter sterilize the solution.
Mix until a homogenous solution is achieved.
Make 100 x 1.5 mL aliquots in sterile 1.7 mL microtubes.
Store the aliquots in −20 °C freezer, use aliquots within 6 months.
PREPARATION OF B8 MEDIA
Thaw one B8 aliquot.
Add the aliquot to 1 liter of cold DMEM/F12 aseptically.
Invert the bottle to mix the reagents.
Keep the media at 4 °C and avoid exposure to light.
Media should be used within 4 weeks.
BASIC PROTOCOL 2
WEEKEND-FREE MAINTENANCE AND PASSAGING OF HUMAN PLURIPOTENT STEM CELLS IN B8 MEDIUM
hiPSCs are grown on low-concentration (1:800) Matrigel which we have optimized for cost-effectiveness without sacrificing cell growth. We have shown that a dilution of 1:1000 is suitable for hiPSCs culture yet 1:800 is chosen to allow for variation in supplier concentrations. We have previously shown that Matrigel performs similarly to Geltrex or Cultrex at the same dilutions, irrelevant of supplier’s stated concentration [7].
We recommend that cells are cultured in B8 using a 3.5+3.5 day splitting schedule, at a 1:20 split ratio. This schedule avoids media changes at the weekends and reduces labor by ~50% whilst maintaining differentiation capacity (Figure 1). We grow cells to ~70-80% confluence; critically, we avoid overgrowing cells (>90% confluence) which results in cells adopting a compact morphology, becoming contact inhibited, resulting in slower grow for subsequent passages and in poor differentiation efficiency.
Figure 1.
Weekend-free hiPSC culture schedule. Time-course of pluripotent growth, Δ, media change.
Cells are passaged non-enzymatically using 0.5 mM EDTA in DPBS−/−, to maintain small clumps of cells rather than making single cells as is typically achieved using TrypLE. For 24 hours after splitting, B8 is supplemented with a Rho-associated protein kinase inhibitor (thiazovivin) to improve cell survival and split ratio reliability, and to reduce single cell survival selective pressure.
ADDITIONAL MATERIALS (also see Basic Protocol 1)
6-well tissue culture treated plates (Greiner, 657165)
Matrigel Reduced Growth Factor Basement Membrane Matrix (Corning, 354230)
500 mL DMEM with L-glutamine and 4.5 g/L D-glucose (Corning, 10-017-CV)
Thiazovivin (See recipe; LC Labs)
DMSO (Fisher BioReagents, BP231-100)
EDTA, 0.5 mM (See recipe; Gibco, 15575020)
Dulbecco’s phosphate-buffered saline without Ca2+ or Mg2+ (DPBS−/− ; Corning, 21-031-CV)
15 and 50 mL polystyrene conical tubes (Corning Falcon, cat. no. 352097 and 352098, respectively)
Microscope such as Nikon Eclipse Ts2
CoolCell LX (Corning, 432002)
Cryovials (Greiner, 122261)
5, 10, 25 mL pipettes sterile, individually wrapped (Corning Falcon, 357543, 357551, 35752)
2 mL aspiration pipettes sterile, individually wrapped (Corning Falcon, 357558)
Cell culture incubators with 5% CO2/5% O2 such as Thermo Scientific Heracell VIOS 160i
Class II, Type A2 or better biosafety cabinet such as Labconco Purifier Cell Logic+
Liquid nitrogen (liquid N2)
Centrifuge (Thermo Sorvall ST40)
Aspirator vacuum
PREPARING MATRIGEL-COATED PLATES
-
Thaw a bottle of growth factor reduced Matrigel overnight at 4 °C.
NOTE: Matrigel can be stored at 4 °C while being used. There is no need to aliquot or leave on ice. The bottle of thawed Matrigel will be stable at 4 °C for > 6 months assuming it is placed in a high-performance refrigerator that maintains constant temperature such as a Thermo TSG or TSX sliding door laboratory refrigerator.
Set out 42 x 6-well plates in the hood.
Have the P1000 tip and the pipette set to 625 μL ready.
-
Take the Matrigel stock out and add 625 μL of Matrigel to 500 mL of 4 °C DMEM (1:800 dilution).
NOTE: Matrigel is supplied at 8-12 mg/mL (see product insert) all of which are suitable for use at 1:800.
-
Return the Matrigel bottle to 4 °C very quickly to prevent gelling.
NOTE: Try to minimize the time Matrigel is outside the fridge. Optionally, to keep Matrigel for more than 1 minute outside the fridge it can be put it on ice.
NOTE: While transferring the Matrigel stock avoid keeping the bottle in your hand as the heat from your hand could cause the Matrigel to gel.
Mix by inverting.
-
Add 2 mL of the solution per each well of a 6-well plate.
NOTE: Addition of 2 mL Matrigel solution per well (rather than 1 mL) helps with long term storage of coated plates in the incubator at 37 °C.
-
Place plates in the incubator at 37 °C for at least 1 h before use. Plates may be stored in the incubator for up to 4 weeks without risk of wells drying out and being unusable.
NOTE: Do not use the Matrigel-coated plates if they have dried out. Check the Matrigel plates frequently for significant reduction in the volume of the media and top them up with 1 mL of DMEM if necessary.
THAWING AND INITIAL PLATING OF hiPSCs
NOTE: All the volumes here are for one well of a 6-well plate. For other types of plates, the volumes need to be changed accordingly.
Take one Matrigel-coated 6-well plate out of 37 °C incubator. Aspirate the Matrigel from 3 wells and add 2 mL of B8T in each well.
Remove the vial from liquid nitrogen and place it in a 37 °C water bath immediately.
Warm the cells until only a sliver of ice remains.
-
Fill a 10 mL pipette with B8T medium, and use this to remove contents of vial, transfer ~5 mL to a 15 mL conical tube, wash out vial with ~1 mL of medium, and transfer the remainder to the conical tube.
NOTE: Addition of thiazovivin in the B8T medium improves cell survival after dissociation which enhances the consistency of plating.
NOTE: There is no need to warm up the media to 37 °C. No negative effect on the growth of the cells has been observed using 4 °C media in our hands.
Centrifuge at 200 × g for 3 min. Aspirate supernatant.
-
Resuspend the pellet in 2 mL of B8T and transfer to 3 wells of a Matrigel-coated 6-well plate at a 1:2, 1:3, and 1:6 ratio.
NOTE: For 1:2 ratio add 1 mL, 1:3 add 670 μL and 1:6 add 330 μL of the cell suspension to one well of the 6-well plate.
-
Change media every 24 h with B8 until wells become ~70-80% confluent.
NOTE: We grow hiPSCs in 5% O2 (hypoxic) incubators. 5% O2 is not essential for the success of this protocol although has been demonstrated to improve genome stability and maintenance of the pluripotent state.
PASSAGE OF hiPSCs WITH EDTA
NOTE: All the volumes here are for one well of a 6-well plate. For other types of plates, the volumes need to be changed accordingly.
NOTE: Ideally, cells should have reached 70-80% confluence in 3.5 days. To achieve this, cells may need to be plated at split ratios of 1:12, 1:15, 1:20, 1:24 and assess to see which ratio is suitably confluent in 3.5 days. This can be repeated for multiple passages until the cells are adapted to a ≥1:20 split which is the most efficient for differentiations.
NOTE: It is essential that the number of days of culture is kept consistent. In this protocol, it is aimed to keep the pluripotent cells in the logarithmic growth phase. Cells should not be allowed to become more than 90% confluent (i.e., 90% of the culture surface covered with cells). More than 90% confluence results in the cells becoming contact inhibited, which results in a slow lag phase growth after passaging.
Aspirate culture medium.
-
Add 1 mL of 0.5 mM EDTA per well and incubate for 6 min at RT (in the hood)
NOTE: The length of incubation time may need to be optimized from ~3-8 min. Enough time should be used so that all cells in the well come off easily after ~5 times pipetting but not so long that cells begin to float freely.
While EDTA is working, aspirate Matrigel from two new 6-well plates and replaced with 1 mL of B8T.
Aspirate EDTA from well.
-
With a P1000 tip, add 1 mL of B8T medium to the well, and blast medium against cell surface to dissociate cells. Cells should come off easily after ~5 times pipetting.
NOTE: Avoid pipetting cells too much as it decreases the cell survival after passaging.
For 1:12 split ratio, top up well to 12 mL of B8T. For 1:15, top up well to 15 mL of B8T. For 1:20, remove 250 μL, then top up well to 15 mL of B8T. For 1:24 remove 400 μL. then top up well to 15 mL of B8T.
Plate out cells at 1 mL per well into two new Matrigel-coated 6-well plates so the final volume is 2 mL per well.
FREEZING OF hiPSCs
NOTE: All the volumes here are for one well of a 6-well plate. For other types of plates, the volumes need to be changed accordingly.
Start with cells at 70-80% confluency.
Aspirate culture medium.
Add 1 mL of 0.5 mM EDTA per well and incubate for 6 min at RT (in the hood).
Aspirate EDTA from well.
-
With a P1000 tip, add 1 mL of B8T medium to the well, and blast medium against cell surface to dissociate cells. Cells should come off easily after ~5 times pipetting.
NOTE: Avoid pipetting cells too much as it decreases the cell survival after passaging.
Centrifuge at 200 × g for 3 min. and aspirate supernatant.
Resuspend the cells in 1 mL freezing solution and transfer to a cryovial.
-
Place the cryovial in a Coolcell and move it to −80 °C freezer.
NOTE: After addition of freezing media to the cells, move the cells to −80 °C freezer quickly. Keeping the cells in freezing medium for a long time at RT decreases the viability of the cells.
NOTE: It is important to reduce the temperature of the cells slowly, that is the reason Coolcell is used here to avoid temperature shock to the cells.
-
After one day transfer the cells to liquid nitrogen storage.
NOTE: Make sure not to keep in the −80 °C freezer for long time as it will decreases the viability of the cells after thawing.
WEEKEND-FREE CULTURE OF hiPSCs
-
Start on Monday morning and passage the cells with 1:20 ratio in B8T media.
NOTE: The ratio mentioned here could be optimized for each cell line in order to have cells ready for passage after 3.5 days.
Change the media to B8 on Tuesday morning.
Skip media change on Wednesday.
-
On Thursday afternoon split the cells with 1:20 ratio in B8T media.
NOTE: The ratio mentioned here should be optimized for each cell line in order to have cells ready for passage after 3.5 days.
Change media to B8 on Friday afternoon.
Skip media change on Saturday and Sunday.
Passage the cells again on Monday morning with 1:20 ratio in B8T media.
REAGENTS PREPARATION
Sodium selenite: Dissolve 100 mg of sodium selenite in 50 mL of cell culture water (final concentration of 2 mg/mL). Filter sterilizes. Make 1 mL aliquots and store at −20 °C.
Recombinant human FGF2-G3: (made by Recombinant Protein Production Core), commonly results in 80 mg per L of E. coli at ~5 mg/mL, store at −20 °C.
Recombinant human NRG1: Dissolve 250 μg of Recombinant human NRG1 in 2.5 mL of sterile cell culture water (final concentration of 100 μg/mL). Make 100 μL aliquots and store at −20 °C.
Thiazovivin: Dissolve in DMSO at the final concentration of 10 mM and make 200 μL aliquots. Store at −20 °C.
SOLUTIONS PREPARATION
EDTA:
Add 500 μL of 0.5 M EDTA to 500 mL of DPBS−/− (final concentration 0.5 mM) and invert to mix. Store at RT indefinitely.
B8 media:
Add one thawed aliquot of B8 to 1 L of DMEM/F12 and mix well. There is no need to filter sterilize. The medium is stable at 4 °C for 4 weeks.
B8T media:
Add one thawed aliquot of thiazovivin to 1 L of B8 media (final concentration 2 μM) invert to mix. There is no need to filter sterilize. B8T is stable at 4 °C for 4 weeks.
Freezing solution:
For 50 mL of freezing solution, add 5 mL of DMSO to 45 mL of B8T (final concentration 10%) and mix well. Make fresh each time.
ANTICIPATED RESULTS
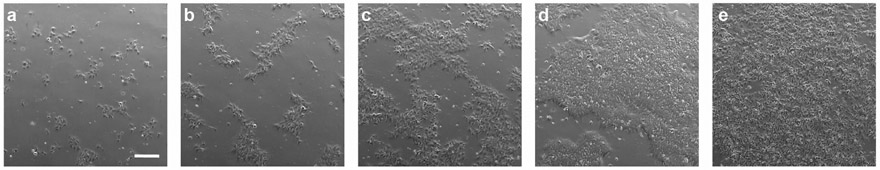
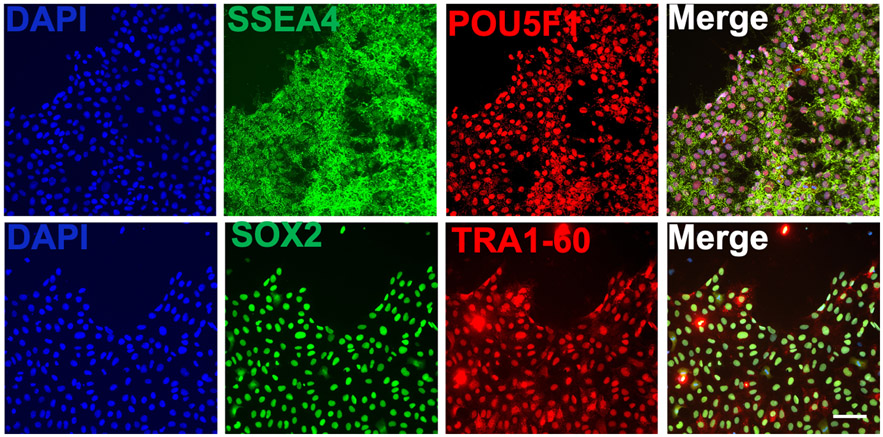
Following the protocols described here, hiPSCs could be generated and maintained at high quality. Moreover, this approach prevents spontaneous differentiation of hiPSCs. In an established cell line, after 1 to 2 hours post passaging, a significant number of the cells are attached and have formed small clusters (Figure 2a). After 24 hours, the clusters start to grow (Figure 2b). By day 2, the clusters start coming together (Figure 2c). By day 3 the cells have reached ~70% confluency and are ready for passaging (Figure 2d). If the cells are not passaged on time, they will reach ~90% confluency by day 4 (Figure 2e). It is highly recommended to avoid overgrowing the cells as the quality of the culture will drop dramatically. Additionally, it will affect the cell survival after passaging. Immunostaining of the hiPSCs using our protocol demonstrate the quality of cultured cells as marked by the expression of pluripotency markers OCT4, SSEA4, SOX2 and TRA1-60 (Figure 3).
Figure 2.
Time course of hiPSC proliferation post seeding: (a) 1 hour, (b) 1 day, (c) 2 days, (d) 3 days, and (e) 4 days. Scale bar 200 μm.
Figure 3.
Example expression of undifferentiated cell markers in hiPSCs maintained in B8 medium. Scale bar 100 μm.
DISCUSSION
In this protocol we present a simple, efficient and cost-effective approach for high quality culture of hiPSCs. Using this protocol more than 100 hiPSC lines have been generated and maintained. To achieve the simplest approach, we have eliminated the unnecessary procedures during hiPSCs culture.
One common misconception is that the media should be warmed up before use. Although it is important to avoid any heat shock to the cells, it is important to consider limiting or avoiding keeping the media at 37 °C for long term due to the limited stability of the growth factors in the media [6]. Moreover, the water bath could be a contamination source in the tissue culture lab. It could provide an environment for the growth of bacteria and fungi if not cleaned regularly. Hence, in this protocol, it is recommended to use media as soon as it is removed from the fridge. This protocol has been tested on several hiPSCs in long-term cultures and, thus far, no negative effect on the survival, growth, and differentiation of the cells was observed.
One other approach to simplify the hiPSCs culture is our method for the preparation of Matrigel-coated plates. The Matrigel bottle is thawed overnight in the fridge. However, unlike the common procedure, it is not aliquoted and kept in the −20 °C freezer. The Matrigel bottle could be kept at 4 °C until used. This is particularly beneficial for labs that use Matrigel routinely and go through a bottle quickly. Extra care is required while handling the bottle here. Therefore, in this protocol, we recommend preparing everything for the coating before bringing the bottle out of the fridge. Immediately after its use, the bottle should be moved back to the fridge to avoid gelation. Moreover, we recommend the preparation of Matrigel-coated plates for the 2-3 weeks of usage at once to save time. The coated plates could be kept at 37 °C degrees for up to a month and will keep their quality as long as they do not dry out.
This efficient and cost-effective approach could be used in high throughput generation and maintenance of hiPSCs. Additionally, it could pave the way for the application of hiPSCs in personalized medicine as well as the pharmaceutical application of hiPSCs in drug toxicity assays.
TROUBLE SHOOTING
-
L-ascorbic acid 2-phosphate does not dissolve in the water with the concentrations mentioned in the protocol. Potential causes:
Make sure to use the L-ascorbic acid 2-phosphate tri sodium salt as only the trisodium salt of L-ascorbic acid 2-phosphate can be used at this high concentration.
- Cells do not survive after thawing. Potential causes:
- Make sure that the frozen cells were of high quality before freezing. This will affect the survival rate after thawing of the cells.
- When freezing the cells, avoid keeping the cells in freezing solution at RT for long time. Cells need to be transferred to −80 °C after addition of freezing media as soon as possible.
- When freezing the cells avoid keeping the cells in −80 °C for long time. Survival rate decreases significantly after one week in −80 °C. Cells should be transferred to liquid nitrogen storage as soon as possible.
- Check the quality of the Matrigel-coated plates. If the Matrigel is dried out, do not use the plates.
- Check the B8 and B8T media batch. If the media is more than one month old, do not use it.
- Cells do not attach after passaging. Potential causes:
- Cells need to be around 70-80% confluent at the time of passaging. Overgrowing the cells causes contact inhibition and reduces the growth rate of the cells. This can lead to slow growth after passaging.
- Cells were left in EDTA for more than 6 min, leading some cells to start floating free, being aspirated, and reducing the number of cells plated after passaging.
- Check the quality of the Matrigel-coated plates. If the Matrigel is dried out, do not use the plates.
- Check the B8 and B8T media batch. If the media is more than one month old, do not use it.
- Cells growing slowly. Potential causes:
- Cells have been overgrown (>90% confluent) due to passaging too late (d5) or plating at too low of a split ratio (1:8 or 1:12) when cells are growing well.
- Cells have not been fed on the correct schedule.
- Cells have been passaged too early (<60% confluent) due to passaging too early (d3) or were plated at too high of a split ratio (1:15 or 1:20) when cells are growing poorly.
- Cells were left in EDTA for more than 6 min, leading some cells to start floating free, being aspirated, and reducing the number of cells plated after passaging.
- Check the quality of the Matrigel-coated plates. If the Matrigel is dried out, do not use the plates.
- Check the B8 and B8T media batch. If the media is more than one month old, do not use it.
Table 1.
shows the components and concentrations in B8 media.
| REAGENT | CONCENTRATION |
|---|---|
| DMEM/F12 | |
| L-ascorbic acid 2-phosphate | 200 μg/mL |
| Insulin | 5 μg/mL |
| Transferrin | 5 μg/mL |
| Sodium Selenite | 20 ng/mL |
| Fibroblast Growth Factor 2-G3 (FGF2-G3) | 40 ng/mL |
| Neuregulin 1 (NRG1) | 0.1 ng/mL |
| Transforming Growth Factor Beta-3 (TGFß3) | 0.1 ng/mL |
Acknowledgements
This work was supported by NIH NCI grant R01 CA220002, American Heart Association Transformational Project Award 18TPA34230105, and the Foundation Leducq (P.W.B.).
Literature Cited
- 1.Dakhore S, Nayer B, and Hasegawa K, Human Pluripotent Stem Cell Culture: Current Status, Challenges, and Advancement. Stem Cells Int, 2018. 2018: p. 7396905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ludwig T and J AT, Defined, feeder-independent medium for human embryonic stem cell culture. Curr Protoc Stem Cell Biol, 2007. Chapter 1: p. Unit 1C 2. [DOI] [PubMed] [Google Scholar]
- 3.Ludwig TE, et al. , Feeder-independent culture of human embryonic stem cells. Nat Methods, 2006. 3(8): p. 637–46. [DOI] [PubMed] [Google Scholar]
- 4.Ludwig TE, et al. , Derivation of human embryonic stem cells in defined conditions. Nat Biotechnol, 2006. 24(2): p. 185–7. [DOI] [PubMed] [Google Scholar]
- 5.Beers J, et al. , Passaging and colony expansion of human pluripotent stem cells by enzyme-free dissociation in chemically defined culture conditions. Nat Protoc, 2012. 7(11): p. 2029–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen G, et al. , Chemically defined conditions for human iPSC derivation and culture. Nat Methods, 2011. 8(5): p. 424–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuo HH, et al. , Negligible-Cost and Weekend-Free Chemically Defined Human iPSC Culture. Stem Cell Reports, 2020. 14(2): p. 256–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen G, et al. , Thermal stability of fibroblast growth factor protein is a determinant factor in regulating self-renewal, differentiation, and reprogramming in human pluripotent stem cells. Stem Cells, 2012. 30(4): p. 623–30. [DOI] [PMC free article] [PubMed] [Google Scholar]