Abstract
Background
Pulsed field ablation (PFA) is a nonthermal energy that may provide safety advantages over radiofrequency ablation (RFA). One-shot PFA catheters have been developed for pulmonary vein isolation, but they do not permit flexible lesion sets. This study investigated a novel lattice-tip catheter designed for focal RFA or PFA ablation.
Methods
The effects of PFA (biphasic, 24 amperes) were investigated in 25 swine using a lattice-tip catheter and system (Affera Inc). Step 1 (n=14) examined the feasibility to create atrial line of block and described its acute effects on the phrenic nerve and esophagus. Step 2 (n=7) examined the subacute effects of PFA on block durability, phrenic nerve and esophagus ≥2 weeks. Step 3 compared the effects of PFA and RFA on the esophagus using a mechanical deviation model approximating the esophagus to the right atrium (n=4) and by direct ablation within its lumen (n=4). The effects of endocardial PFA and RFA on the phrenic nerve were also compared (n=10). Histological analysis was performed.
Results
PFA produced acute block in 100% of lines, achieved with 2.1 (1.3-3.2) applications/cm-line. Histological analysis following [35 (18-37)] days showed 100% transmurality (thickness range 0.4-3.4 mm) with a lesion width of 19.4 (10.9-27.4 mm). PFA selectively affected cardiomyocytes but spared blood vessels and nervous tissue. PFA applied from the posterior atria [23 (21-25) applications] to the approximated esophagus [6 (4.5-14) mm] produced transmural lesions without esophageal injury. PFA [16.5 (15-18) applications)] applied inside the esophageal lumen produced mild edema compared to RFA [13 (12-14) applications] which produced epithelial ulcerations. PFA resulted in no or transient stunning of the phrenic nerve (<5min) without histological changes while RFA produced paralysis.
Conclusions
PFA using a lattice-tip ablation catheter for focal ablation produced durable atrial lesions and showed lower vulnerability to esophageal or phrenic nerve damage compared to RFA.
Keywords: atrial fibrillation, ablation, catheter ablation, pulmonary vein isolation, cardiac arrhythmia, pulsed field, radiofrequency, Arrhythmias, Implantable Cardioverter-Defibrillator, Treatment, Translational Studies
Graphical Abstract
Introduction
Radiofrequency (RF) and cryothermy are the most common energies used for ablation of cardiac arrhythmias.1 While generally effective, both can result in thermal-induced injury to neighboring structures including the esophagus, phrenic nerve and coronary arteries.1, 2 Although the frequency of these complications is relatively low, they might be devastating. In recognition of these safety concerns, significant effort has been placed in exploring alternative energy sources with greater selectivity for cardiac tissue. Among these, pulsed field energy produced by delivery of short high-voltage pulses applied to cells/tissue, can induce cellular death by destabilizing cell membranes instead of by heat production.3 Pioneering work by Wittkampf and colleagues found that pulsed field energy can produce transmural and durable atrial lesions with minimal or no effect to the esophagus, phrenic nerve and the coronary arteries.3–6 More recently, Koruth, Reddy and colleagues reported that pulsed field produced rapid and durable pulmonary vein isolation (PVI) in humans without causing esophageal injury, inspiring the use of pulsed field for the treatment of human arrhythmias. 7, 8
These initial reports were performed using one-shot technologies designed for stand-alone PVI. While PVI is effective in most patients with paroxysmal atrial fibrillation (PAF), a substantial subset of patients requires ablation of non-PV triggers. This is particularly relevant to patients with persistent AF in whom PVI alone may be insufficient, and additional ablation is often performed despite the lack of consensus regarding the optimal additional lesion set. It is therefore not surprising that one-shot technologies constitute ~25% of all AF ablation procedures performed worldwide while the remainder employs point-by-point ablation catheters. The latter are also suitable for mapping and ablation of concurrent arrhythmias such as atrial flutter.
To this end, a novel catheter technology incorporating an expandable spheroid-shaped tip has been designed for high-current RF ablation (RFA) in a point-by-point fashion, allowing tailored ablation. In preclinical studies, it produced rapid and durable atrial lesions, and ventricular lesions with a maximal depth that was twice of standard irrigated catheters.9, 10 In humans, this technology produced rapid and durable PVI with a similar safety profile to other RF technologies.11, 12 However, given the potential safety benefits of PFA, we sought to develop a system that can deliver either focal RFA or PFA using the same catheter.
The aims of this preclinical study were to investigate the effects of PFA delivered from a novel focal catheter on atrial lesion durability, and to compare the effects of PFA and RFA on the esophagus and phrenic nerve.
Methods
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Experimental Design
This study included three experimental components: The first component investigated the feasibility of PFA to create an atrial line of block and examined the acute effects of PFA on extracardiac tissue including the phrenic nerve and the esophagus. The second component examined the durability of atrial lines created by PFA including the subacute effects of PFA on extracardiac tissue after >2 weeks of survival period. The third component compared the effects of PFA and RFA on the esophagus and phrenic nerve using the following methods: 1) esophageal deviation model in which the esophagus was mechanically deviated closer to the posterior atrium with ablation performed from the posterior atrium opposing the esophagus, and 2) direct ablation within the esophageal lumen. The effects of PFA and RFA on the phrenic nerve were examined by applying ablation lesions at the lateral endocardial right atrium opposing the phrenic nerve. A detailed description is provided below. In order to maximize animal usage, often more than one study objective was evaluated per animal. Supplemental Table 1 details the different experimental components performed in each animal
Animals and Protocols
The study included 25 Yorkshire swine (65-90kg) studied under general anesthesia with isoflurane inhalation and mechanical ventilation. The research protocol was approved by the Institutional Animal Care and Use Committee and conformed to the Position of the American Heart Association on Research Animal Use. The study was performed at the Beth Israel Deaconess Medical Center Experimental Electrophysiology Laboratory in Boston, MA.
Ablation System
This proprietary technology (Affera Inc.) includes a mapping and ablation catheter (Sphere-9™, “lattice”), a generator designed for PFA (HexaPULSE™) and RFA (HexaGEN™), an electroanatomical mapping system (EAM; Prism-1™) and a peristaltic pump (HexaFLOW™).9 At the core of this technology is the lattice catheter, a 7.5Fr bidirectional deflectable catheter with an expandable conductive lattice electrode. The catheter is inserted into the sheath in a collapsed form, but once in the heart, the lattice expands to a 9 mm diameter spherical configuration. The lattice contains 9 mini-electrodes/temperature sensors (0.7mm diameter) that are uniformly distributed on its surface. An additional electrode within its center serves as a local indifferent electrode embedded in blood. Bipolar electrograms can be configured between any of the electrodes on the catheter. In particular, bipolar electrograms between each mini-electrode and the center electrode can be used to assess the local response to ablation without contamination of far-field activity. The high and low pass filters for unipolar and bipolar electrogram were set to 1-300Hz and 30-300Hz, respectively. The EAM system (Prism-1™) uses a magnetic sensor placed in the lattice catheter tip for tracking and building a 3-dimensional map of the chamber. Anatomy acquisition is performed at a pre-defined respiratory phase (respiratory gating) for accuracy. The system also provides an indication of proximity between the catheter and the tissue by continuously evaluating impedance between each of the mini-electrodes and the center electrode, highlighting electrodes with elevated local impedance.9, 10 The center of the lattice contains a protected irrigation nozzle with micropores that provide homogeneous cooling unaffected by pressure applied to the catheter or the angle of tissue engagement.
When the catheter is used for RFA, the lattice struts act as a solid electrode generating a uniform cloud of current, allowing high-current output at a relatively low density. Ablation is then performed in a temperature-controlled mode.13 Ablation using pulsed field relies on generating an electric field to cause irreversible electroporation. The applied electric field is proportional to current density, which in turn depends on the applied current and the surface area over which that current is delivered (Figure 1). PFA was applied from the entire lattice struts using a symmetric, microsecond scale, biphasic waveform with a current of ~24 amperes for a duration of 3-5 seconds. Although pulsed field is considered nonthermal energy, it produces small rise in tissue temperature as a result of current passing through the tissue. This small temperature rise is recognized by the temperature sensors surrounding the lattice, providing real-time indication of tissue engagement. The procedures were performed under unfractionated heparin with activated clotting time target of 300-400 seconds.
Figure 1:
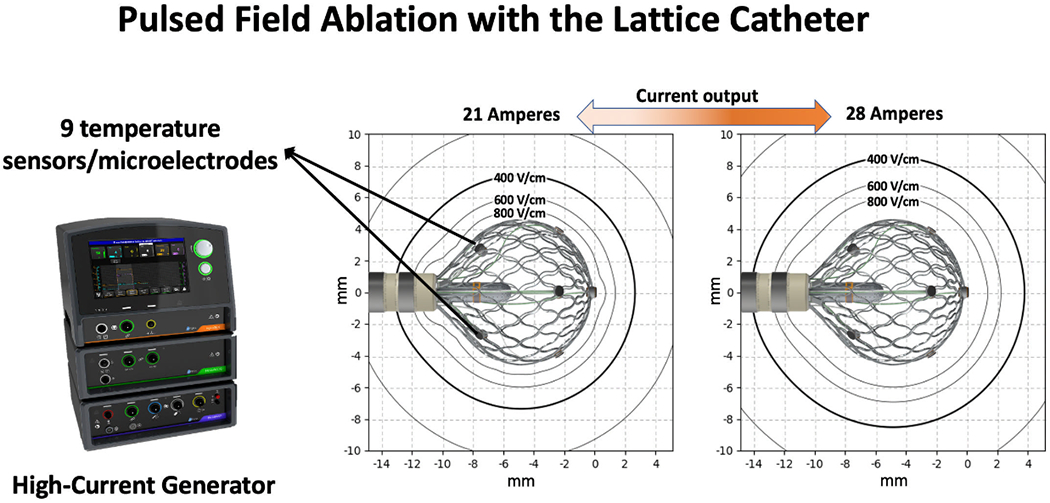
Description of the Catheter and Mapping/Ablation System. The system includes a lattice-tip electrode mapping/ablation catheter, high-current ablation system that can be used for either radiofrequency or pulsed field ablation, a mapping system and an irrigation pump (not shown here). The expanded lattice electrode has a diameter of 9 mm and contains 9 thermocouples / mini electrodes. The generator delivers a biphasic current to the large surface area of the lattice, producing a uniform electric field in the adjacent tissue. The current output influences the strength of the electric field. In this example, a current of 21 Amperes creates an electric field of 400 Volts/cm approximately 3mm from the lattice. Increasing the current to 28 Amperes results in an electric field of 400 Volts/cm approximately 4mm from the lattice.
Atrial Lines in the Beating Heart
Acute model
In 14 animals, right atrial lines were created with PFA. First, an electroanatomical map of the right atrium was created with the investigational catheter and the compatible mapping system. A posterior ablation line extending from the superior vena cava (SVC) to the inferior vena cava (IVC) was then created with PFA in point-by-point fashion using the investigational catheter. Ablation parameters included a biphasic pulsed field of 24 amperes (18-24) delivered with approximately 6mm distance between applications. Upon completion of the line, presence or absence of conduction block was assessed by the following criteria: (1) activation map during pacing from the septal and/or lateral sides of the line and 2) pacing the entire length of the line at 10mA@2ms to determine excitability. Animals were euthanized upon completion of the procedures, and tissue was excised for analysis in order to determine the acute effect of PFA on cardiac and extracardiac tissue.
Chronic model
In 7 additional animals, an ablation line with acute block was created as described above. However, animals were then survived for a minimum period of 2 weeks (2-5 weeks) before they underwent a second mapping study to investigate the subacute effect of PFA on cardiac and extracardiac tissue. EAM was performed as described to determine presence or absence of block. Animals were then sacrificed for histological analysis.
The Effects of PFA on the Esophagus
Eight animals were used in 2 experimental models designed to examine the effects of PFA on the esophagus. The first model (n=4) consisted of mechanical deviation of the esophagus into close proximity with the posterior right atrium using a steerable sheath (Agilis™ NxT, Abbott, St. Paul, MN). The right atrium and esophagus were mapped using the EAM and the esophagus was deviated into ≤10mm from the endocardial posterior right atrium using the steerable sheath as measured by a geolocation tool available in the mapping system. In this model, RFA delivered from the approximated right atrium produces acute and subacute esophageal injury, serving as a reference to examine the effect of PFA on the esophagus (Supplemental Figure 1). RFA settings were temperature-controlled ablation at Tmax of 73°C applied for 5 seconds, as previously reported with this technology.14 Using this esophageal deviation model, PFA applications were delivered from the approximated right atrium, and animals were survived for ≥2 weeks before histopathological analysis of the esophagus. In these animals RFA was not performed because its effect on the esophagus is known and to minimize unnecessary suffering to the animals.
The second experimental model involved direct ablation in the lumen of the esophagus with RFA (Tmax 73°C; 5 seconds) and PFA (24 amperes) from the lattice catheter. In 4 swine, the esophagus was mapped using the EAM system, and alternating RFA and PFA applications with 1-2cm spacing between applications were applied along the esophagus after it was filled with saline. Animals were survived for 20-24 hours before histopathological analysis of the esophagus.
The Effects of PFA on the Phrenic Nerve
The effects of PFA on the right phrenic nerve were examined in a total of 10 swine. The course of the right phrenic nerve was first identified by pacing the lateral right atrium from the SVC to the IVC with tagging of all sites exhibiting diaphragmatic capture. In 6 animals, PFA (24 amperes) was applied directly on the atrial course of the right phrenic nerve and the effect of ablation on phrenic nerve function was evaluated by pacing before ablation, immediately after completion of ablation, and again during the repeat mapping procedure after the survival period.
In 4 additional animals, we examined the effect of PFA at a supratherapeutic dose (range 28 – 34A). This was done to evaluate the safety margin of PFA for ablation near the phrenic nerve. The effect of each application was examined by pacing from the ablation catheter (10mA@2ms) immediately before and after each application. In the case of phrenic nerve palsy as determined by reduced or absent diaphragmatic contraction, further ablation was held, and pacing was repeated after a waiting period of 5 minutes to differentiate between transient or persistent injury. In these experiments, RFA was also applied as a positive control for phrenic nerve paralysis (Tmax 73°C; 5 seconds, in 2 animals). In order to avoid a cumulative ablation effect, RFA applications were placed >2cm from PFA applications, and because RFA was more likely to result in phrenic nerve paralysis, RFA lesions were delivered after PFA. At the end of the study, animals were euthanized, and the phrenic nerve was examined with histopathology.
Histopathological Analysis
Following euthanasia, the heart-lungs and esophagus including the intact phrenic nerve were explanted en bloc. The right atrium was dissected along the antero-lateral wall from the SVC to the IVC. Tissues collected at necropsy were fixed in 10% neutral buffered formalin, routinely processed and sectioned every 5 mm perpendicularly to the ablation lines and embedded in paraffin according to accepted histologic technique, and tissue cassettes were sized according to tissue samples size. Five-micron-thick sections were stained with hematoxylin and eosin (H&E), and Masson’s trichrome (MT) stain. Ablation line continuity, transmurality, width as well as the effect of PFA on blood vessels and nerves were determined by a board-certified veterinary pathologist blinded to the intervention procedure (RM). All extracardiac tissue was carefully examined for damage. The esophagus and phrenic nerve were also submitted for histological evaluation with hematoxylin-eosin and Masson’s Trichrome staining. Another objective of this study was to determine the bipolar voltage amplitude cutoff corresponding to transmural PFA performed with this technology. To do that, voltage amplitude was correlated with the histological findings ≥2 weeks after ablation (in the chronic model).
Statistical Analysis
Normality of continuous variables was assessed by graphic measures due to the small sample size which precludes robust statistical tests of normality. Given non-normal distribution in all variables, continuous variables were summarized by median (range). Categorical variables were summarized by frequency and percentage. To compare paired data (such as in the acute versus chronic phases of the survival atrial study), a signed rank test was performed. Comparison of non-paired data was performed with a Wilcoxon rank-sum test. P value <0.05 was considered statistically significant. In order to identify the bipolar voltage cutoff value that corresponds to the ablation lesion width, a Spearman correlation analysis was performed between the lesion width determined at several empiric voltage cutoff values (0.25 and 0.5mV) and gross pathology (gold standard) at 5mm intervals along the ablation line. Statistical analyses were performed with Stata/SE, version 16 (StataCorp, College Station, TX).
Results
Table 1 details the biophysical parameters of ablation, including the width and length of the line at the acute and chronic stages. Supplemental Table 1 provides experimental details for each animal.
Table 1:
Biophysical Experimental of Ablation
| Acute Study (n=14) | Survival Study (n=7) | ||
|---|---|---|---|
| Immediate post ablation | Immediate post ablation | Chronic | |
| Survival Time | - | - | 35 (18-37) |
| Application/cm line | 2.0 (1.3-3.2)* | 2.0 (1.7-2.5)* | - |
| PFA time (min) | 1.5 (0.9-3.8)† | 2.1 (1.5-3)† | - |
| Current (Amperes) | 24 (18-28)* | 24 (24-24)* | - |
| Length of Line (mm)§ | 74.9 (24.8-105.2)* | 90.2 (73.1-98.8)*‡ | 99.4 (83-112.7)‡ |
| Width of Line (mm)§ | 12.5 (10.3-26) | 16.9 (12.7-21.9)*† | 19.4 (10.9-27.4)† |
| Lesion depth (mm)|| | - | - | 1.2 (0.4-3.4) |
| Lesion width (mm)|| | - | - | 21.5 (0-41.5) |
| % Transmurality | - | - | 100% (72/72) |
indicates p=nonsignificant comparison between acute and immediate post ablation in the survival study
indicates p=significant comparison between acute and immediate post ablation in the survival study
indicates p=significant comparison between immediate post ablation and chronic in the survival study
measured using voltage amplitude cutoff of 0.25mV
measured using histology
Atrial line with PFA
PFA resulted in immediate attenuation of local electrograms, loss of tissue capture with pacing, and formation of a continuous line of block in all 14 animals. Figure 2 shows an example of a right atrial voltage and activation maps before and after PFA. Note the significant attenuation of electrogram amplitude, lack of tissue capture with pacing and evidence of conduction block. The median length of the line was 74.9 (24.8-105.2) mm, requiring 2.1 (1.3-3.2) applications/cm-line (Table 1). There were no instances of muscle capture. Ablation near the phrenic nerve caused occasional transient diaphragmatic capture that stopped upon completion of the application as discussed later.
Figure 2:
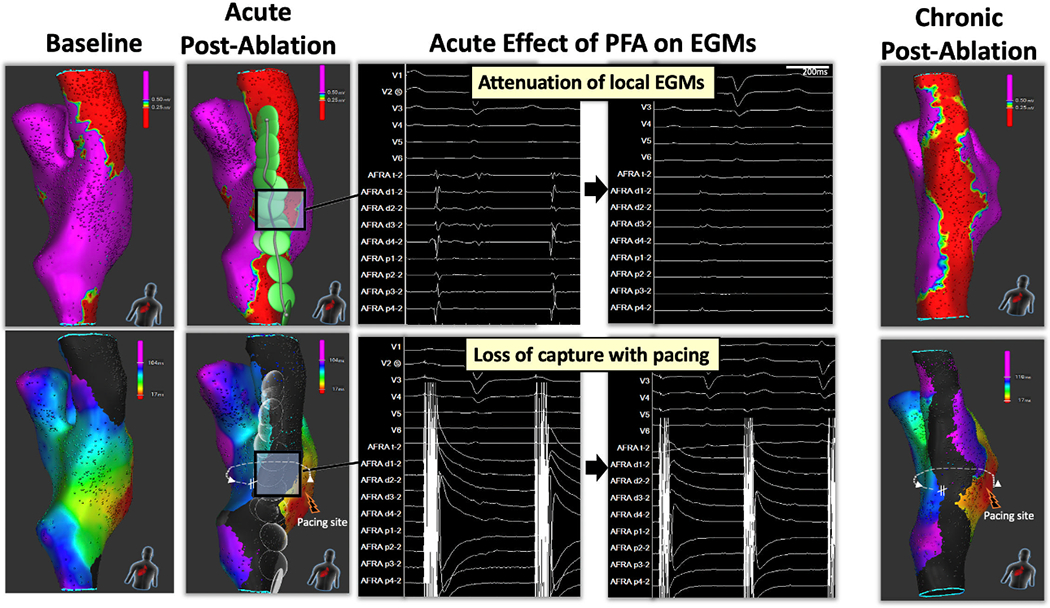
Atrial Line of Block Created with Pulsed Field Ablation. Electroanatomical map of the right atrium was performed using the investigational mapping catheter and compatible system during pacing from the lateral tricuspid annulus. The left panel shows the voltage and corresponding activation naps before ablation. The second panel from the left shows the voltage and activation maps obtained immediately after completion of a posterior line created with pulsed field (PF) energy. Note the presence of conduction block across the line. PFA resulted significant attenuation of electrograms amplitude and the lack of capture with pacing (10mA@2ms; middle panel). Following a survival period of 35 days, persistent conduction block was present (right panel).
The durability of atrial lines created with PFA was evaluated in 7 swine after a survival period of 35 (18–37) days. All lines were blocked acutely. In 6/7 swine (85.7%), the ablation line remained blocked after the survival period, while in 1 swine mapping revealed focal reconnection at the right atrium-SVC junction. Figure 2 shows the voltage and activation maps at baseline, immediately post ablation, and 35 days after ablation in the same swine. Gross pathological examination showed contiguous lines in 6/7 animals. In the one animal with electrical reconnection, there was a ~3mm gap at the upper part of the line. Histological analysis showed transmural fibrosis in all 72/72 (100%) tissue sections with a median wall thickness was 1.2mm (ranged from 0.4 mm at the higher right atrium to 3.4 mm at the lower right atrium). In the one animal with gap, all histological slides showed transmurality. However, since sections were made every 5mm, it is possible that the gap was caught in-between slicing intervals or that there was an epicardial connection. Figure 3 shows the effects of PFA on cardiomyocytes, blood vessels and nerves. PFA resulted in replacement of cardiomyocytes by collagen, elastin and fat; however, blood vessels (red arrows) and nerve fibers (black arrows) within the ablation lesion remained unaffected. A voltage amplitude of 0.25mV showed the best correlation to lesion width measured from the fresh pathology with a correlation coefficient of 0.50 (p<0.001). Figures 3 and 4 show voltage maps of ablation lines from two different animals with their corresponding fresh pathology. Note, the correspondence between the width of the line on fresh pathology and a voltage amplitude cutoff of 0.25mV.
Figure 3:
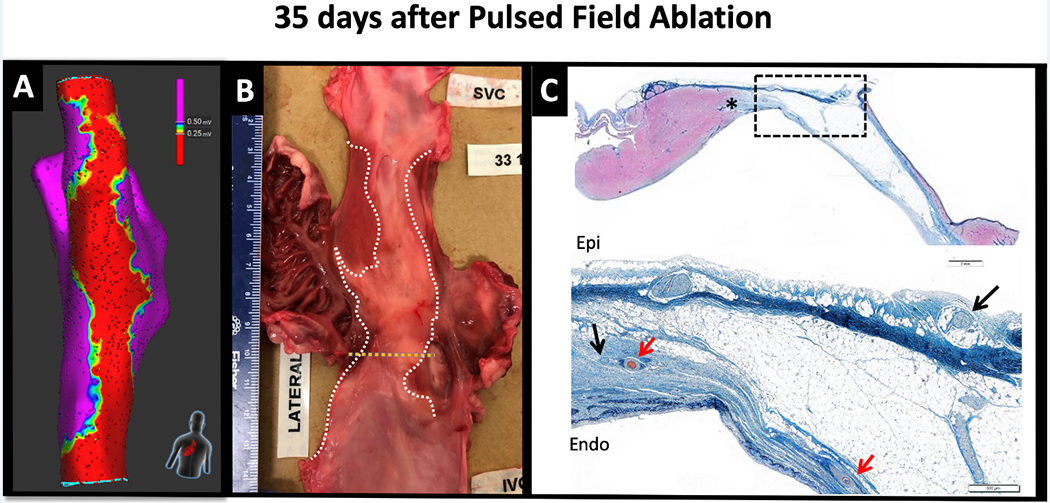
Effect of Pulsed Field Ablation on Cardiomyocytes, Blood Vessels and Nerves. Following a 35-day survival period after pulsed field ablation (PFA), a voltage amplitude cutoff of 0.25mV corresponded with the territory of ablation marked on the fresh gross pathological specimen (right and middle panels, respectively). The right panel shows the histological slide stained with Masson’s trichrome at the level marked by the dotted yellow line of the gross pathology. The upper panel is a lower magnification image highlighting the demarcation between the ablation lesion and the viable myocardium (marked by the asterisk) . The lower panel is a higher magnification of the zone marked by the dotted rectangle. Note that cardiomyocytes have been replaced by collagen (blue) and elastin (black). The red arrows indicate intact blood vessels within the site of ablation lesion and the black arrows indicate viable nerve fibers. Also note the correlation in lesion width between the gross pathology and a bipolar voltage amplitude of 0.25mV.
Figure 4:
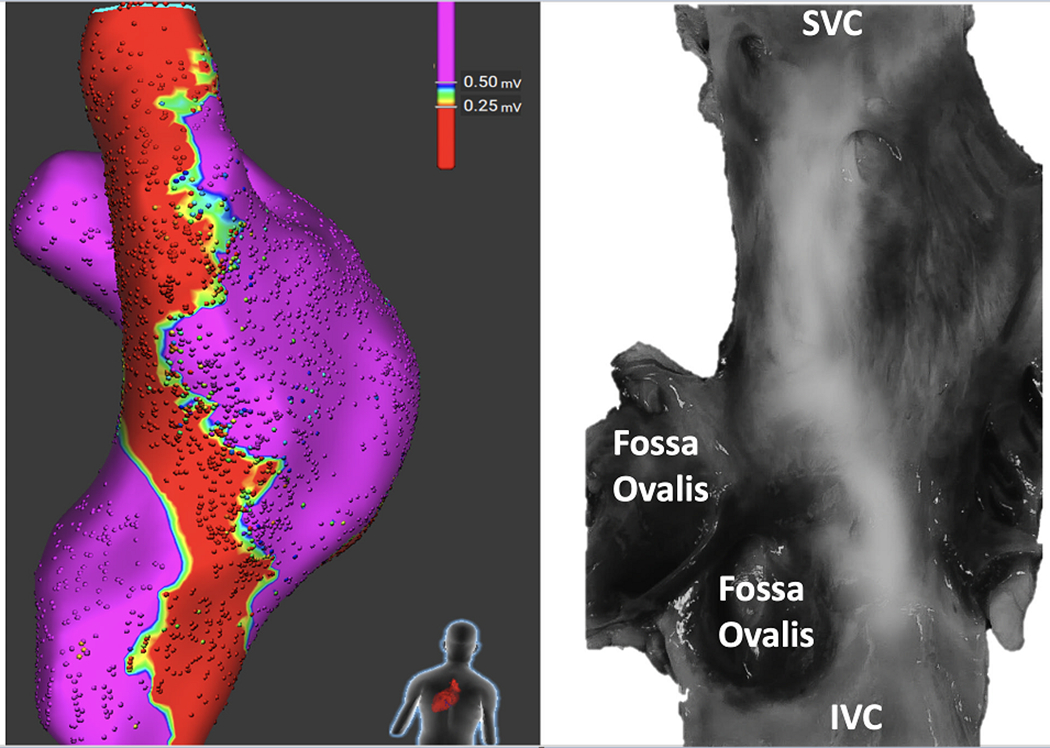
Correlation Between Bipolar Voltage and Gross Pathology. The bipolar voltage amplitude that best corresponds to the gross pathology in terms of lesion width was determined by comparing the width of the chronic ablation line measured at 5mm intervals from the superior to the inferior vena cava on the fresh gross pathology to several empiric voltage cutoff values (0.1, 0.25 and 0.5mV). A voltage amplitude of 0.25mV showed the best correlation to lesion width measured from the fresh pathology with a correlation coefficient of 0.50. In this figure, the gross pathological specimen was scanned in high resolution and digitally processed to enhance infrared light. Note, the reasonable correlation between bipolar voltage amplitude ≤0.25mV and the scar.
The Effect of PFA on the Esophagus
In 4 animals, the esophagus was mechanically deviated toward the posterior right atrium. Figure 5, Panel A shows an example of the approximated esophagus into contact with the posterior right atrium visualized by fluoroscopy and the electroanatomical mapping. PFA (23; 21-25) applications were delivered 6mm (4.5-14) from the esophagus. During a median survival period of 35 (18-37) days, animals consumed food regularly without noticeable gastrointestinal symptoms. Histopathological examination of the posterior right atria and corresponding esophagi showed transmural right atrial lines without injury to the adventitial layers, surrounding fat, or esophagi (Figure 5, Panel A). In 4 additional swine, RFA 6[13 (12-14)] and PFA [16.5 (14-18)] applications were delivered directly to the esophageal lumen. Animals were then survived for one day before histopathological analysis of the esophagus. Gross pathological analysis of the esophagi showed well-demarcated epithelial damage produced by all RFA applications; however not with any of the PFA applications (Figure 5, Panel B). Histologically, PFA resulted in mild edema restricted to the superficial muscularis layer, inflammation and mild focal necrosis in the superficial epithelium. In contrast, RFA resulted in moderate to severe edema, inflammation, necrosis and hemorrhage spanning from the superficial epithelium down to the deep muscularis layer.
Figure 5:
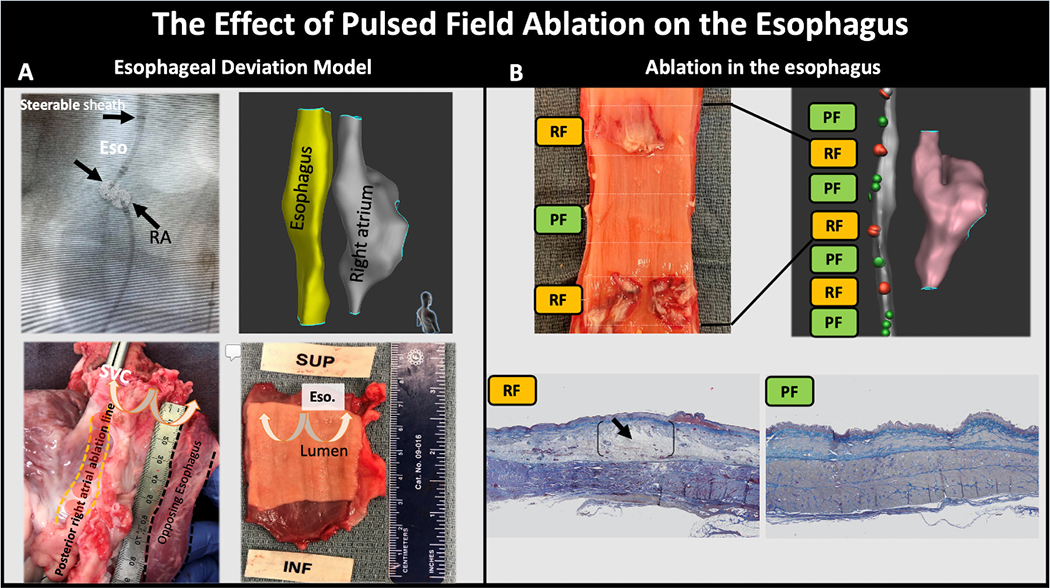
The Effect of Pulsed Field Ablation on the Esophagus. In panel A, the esophagus was mechanically deviated into contact with the posterior right atrium as shown by fluoroscopy (antero-posterior projection) and electroanatomical mapping (right posterior oblique projection). In the fluoroscopic image, note the steerable sheath in the esophagus with a lattice catheter (Eso) in contact with a second lattice catheter positioned in the posterior right atrium (images of the lattice electrodes were digitally enhanced to help in their visualization). The left lower panel shows the epicardial aspect of the posterior ablation line created with pulsed field (PF) energy and the opposing unaffected adventitial layer of the esophagus. The right lower panel shows the intact esophageal lumen. Panel B examined the effect of PF and radiofrequency (RF) ablation delivered directly in the esophagus. The gross pathological specimen of the esophagus shows epithelial ulceration at sites ablated using RF, but no injury created by PF. Histologically, PF resulted in mild edema in the superficial muscularis layer and mild focal necrosis and inflammation to the superficial epithelium. In contrast, RF resulted in severe edema, necrosis, inflammation and hemorrhage spanning from the superficial epithelium down to the deep muscularis layer (black arrows).
The Effect of PFA on the Phrenic Nerve
The chronic effect of PFA on the phrenic nerve was evaluated in 6 animals. A median of 5.5 (1-8) PFA applications were placed on the endocardial right atrium opposing the phrenic nerve in each animal. These did not result in acute phrenic paralysis, and a repeat mapping procedure after 35.5 (19-37) days showed intact phrenic nerve function. The gross pathology specimens of the phrenic nerves from these animals showed intact phrenic nerves without a visible damage.
In 4 additional animals, we examined the effect of PFA at a supratherapeutic dose. This was done to evaluate the safety margin of PFA for ablation near the phrenic nerve. PFA applications [4.5 (3-8)] were delivered from the endocardial right atrium opposing the right phrenic nerve at a supratherapeutic dose, ranging from 28 to 34A. PFA did not result in acute phrenic nerve paralysis. However, ablation at the highest current output (~32-34A) resulted in transiently reduced or absent diaphragmatic contraction for <5minutes before returning to baseline function. In contrast, RFA (4 applications per animal, n=2) resulted in phrenic nerve paralysis in both animals that lasted >5 minutes and until euthanasia. Figure 6, Panel A shows the EAM of the right atrium with tags marking the course phrenic nerve and the sites of PFA and RFA applications. Figure 6, panel B shows an in-situ section of the heart, lung and pericardium with visible injury over phrenic nerve sites corresponding to RFA but not to PFA. However, despite phrenic nerve paralysis and presence of visual injury with RFA, histological analysis of the phrenic nerve bundle showed intact structure with both RFA and PFA (Figure 6, panel D). This discrepancy between functional presence of nerve palsy and structural integrity may be related to the limited time interval between ablation and histological analysis, a duration that may be insufficient to result in necrosis of the encapsulated nerve fibers. However, this observation requires further investigation.
Figure 6:
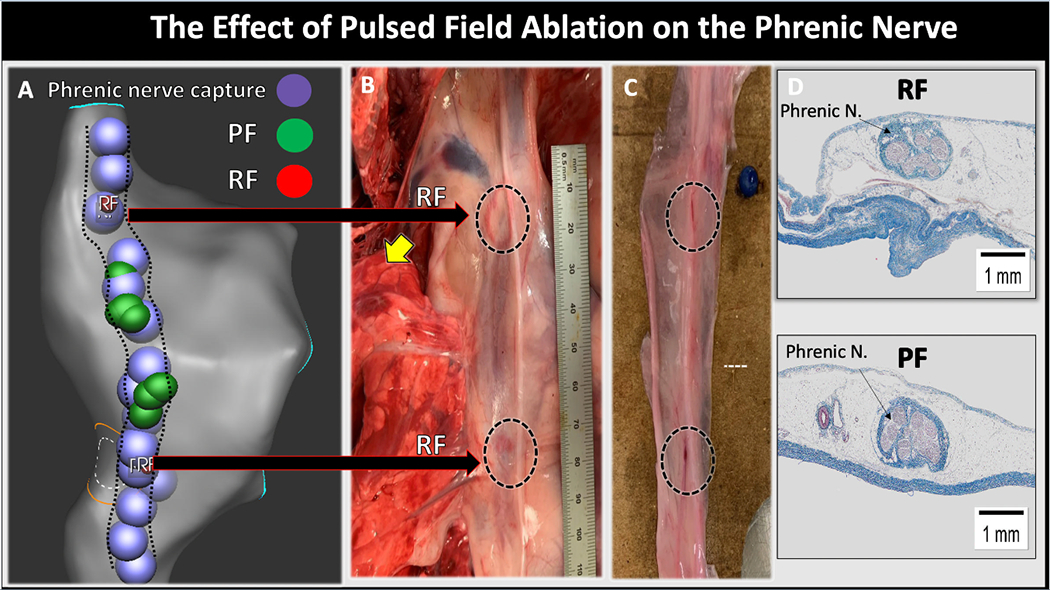
The Effect of Pulsed Field Ablation on the Phrenic Nerve. Panel A shows the electroanatomical shell of the right atrium with the course of the right phrenic nerve (dotted black lines) as identified by diaphragmatic capture with pacing (purple circles). The green circles represent pulsed field applications and the small red circles (total of 4 circles, 2 at each location) represent radiofrequency applications. Panel B shows the phrenic nerve in-situ with clear visual injury at sites of RFA (dotted black circles). Panel C shows the gross pathological specimen of the phrenic nerve after its removal from the heart/pericardium complex. Panel D shows histological slides of the phrenic nerve stained with Masson’s trichrome from sites of RF (upper) and PF (lower) ablation. The nerve bundle appeared normal in sites of both RFA and PFA.
Discussion
Pulsed field is a form of nonthermal energy with a presumed selective effect on cardiomyocytes. It differs significantly from thermal sources of ablation (i.e., radiofrequency and cryothermy) which create lesions by destruction of all structures achieving lethal temperatures, irrespective of tissue type. This premise has stimulated the development of catheter technologies for percutaneous delivery of PFA, with early catheter designs included one-shot catheters with multiple electrodes surrounding the PV ostium, intended for PVI.8, 15, 16 While initial clinical results appear promising, encouraging the use of PFA as a safer alternative to RFA, one-shot technologies do not permit the flexibility of tailored ablation as often desired for focal ablation and lines.
In this preclinical study, we examined the safety and efficacy of a novel technology utilizing a catheter designed for focal (point-by-point) PFA. PFA with this catheter was able to create a contiguous and transmural atrial line of block. The effect of PFA was predominantly selective to cardiomyocytes, sparing blood vessels and nerves embedded within the ablation lesion. PFA did not have an effect on esophageal tissue and similarly did not result in phrenic nerve paralysis, validating the cardiac specific effect of PFA. These findings are consistent with a previous study by Wittkampf et al which showed lower vulnerability of esophageal and phrenic nerve tissues to PFA6 and a more recent study by Jacob Koruth using an esophageal balloon deviation model to compare the effects of PFA and RFA on the esophagus.17 The combination of cardiac tissue selectivity and rapid energy delivery contribute to the increased efficacy and safety of PFA compared to RFA. However, unlike RFA in which the relationship between power, duration and lesion formation is fairly straightforward, PFA involves complex waveforms of multiple parameters. Thus, PFA should not treated as generic term, but each waveform/technology may require independent evaluation.
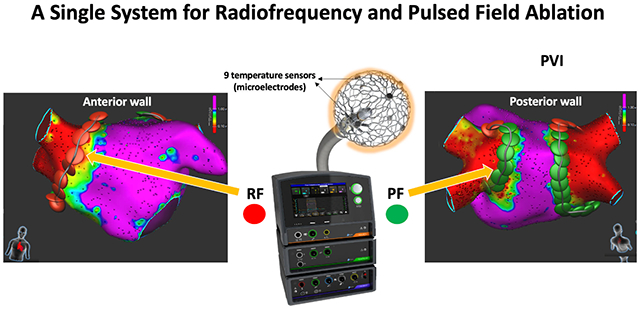
In this study, mapping and ablation was performed using the lattice catheter. The lattice is equipped with 9 mini-electrodes and a magnetic sensor allowing construction of anatomy with simultaneous acquisition of high-resolution electrograms.9 Clinically, the combination of multi-electrode mapping and ablation into one catheter simplifies the procedural workflow and may shorten the procedural duration and potentially reduce its cost. Two recent studies described the use of this technology for PVI and linear ablation (mitral and cavotricuspid isthmus lines as well as roof lines) in humans.11, 12 In these studies, ablation was performed using the lattice catheter and an RFA generator. The generator used in this study combines the ability to perform PFA and RFA using a single system. Thus, the operator can decide when and where to apply RFA vs PFA using a single catheter, ablation system and mapping system (Graphic Abstract). This is particularly relevant during this time when PFA has been optimized only for atrial ablation.
Limitations
The major limitation of this investigation is inherent to a preclinical animal model with unknown correlation to humans. Although the principle differences between RFA and PFA reported in this study are unlikely to differ significantly between swine and humans, specific dosing is often optimized following initial experience in humans. In addition, the lack of esophageal endoscopic and pathological assessments at fixed intervals during the survival period limits our conclusions regarding the full effect of PFA on the esophagus to observations taken acutely and after ≥ 2 weeks.
Conclusions
PFA using a lattice-tip ablation catheter for focal ablation produced durable atrial lesions and showed lower vulnerability to esophageal or phrenic nerve damage compared to RFA. The integration of this catheter with magnetic-based mapping technology and ablation system capable of RF and PF ablation provides a versatile platform for mapping and ablation of different arrhythmias.
Supplementary Material
What is known
Pulsed field ablation is a form of nonthermal energy that induces cellular death by destabilizing cell membranes and may therefore has safety advantages over radiofrequency ablation.
Current pulsed field ablation technologies include one-shot catheters designed for pulmonary vein isolation, but they do not permit flexible lesion sets (e.g., linear lesions).
What the study adds
A novel lattice-tip ablation catheter permits focal ablation for tailored pulsed field ablation.
In a preclinical animal model, it produced a durable atrial line of block with preferential effect on cardiomyocytes, but not on blood vessels or nerve fibers embedded in the lesion.
The ability to deliver focal radiofrequency and pulsed field energy from a single catheter/system may increase the versatility of current mapping and ablation technologies.
Acknowledgments
Sources of Funding: This study was supported by a research grant from Affera Inc.
Nonstandard Abbreviations and Acronyms
- AF
atrial fibrillation
- EAM
Electroanatomical mapping
- H&E
hematoxylin and eosin
- MT
Masson’s trichrome
- RFA
Radiofrequency ablation
- PFA
Pulsed Field Ablation
- PVI
Pulmonary vein isolation
Footnotes
Disclosures: Elad Anter has stock options at Affera, Inc and is also a consultant to Biosense Webster and Boston Scientific and has equity in Itamar Medical. All other report none.
References:
- 1.Calkins H, Hindricks G, Cappato R, Kim YH, Saad EB, Aguinaga L, Akar JG, Badhwar V, Brugada J, Camm J, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Europace. 2018;20:e1–e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muthalaly RG, John RM, Schaeffer B, Tanigawa S, Nakamura T, Kapur S, Zei PC, Epstein LM, Tedrow UB, Michaud GF, et al. Temporal trends in safety and complication rates of catheter ablation for atrial fibrillation. J Cardiovasc Electrophysiol. 2018;29:854–860. [DOI] [PubMed] [Google Scholar]
- 3.Chang DC, Reese TS. Changes in membrane structure induced by electroporation as revealed by rapid-freezing electron microscopy. Biophys J. 1990;58:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neven K, van Driel V, van Wessel H, van Es R, du Pre B, Doevendans PA, Wittkampf F. Safety and feasibility of closed chest epicardial catheter ablation using electroporation. Circ Arrhythm Electrophysiol. 2014;7:913–9. [DOI] [PubMed] [Google Scholar]
- 5.van Driel VJ, Neven K, van Wessel H, Vink A, Doevendans PA, Wittkampf FH. Low vulnerability of the right phrenic nerve to electroporation ablation. Heart Rhythm. 2015;12:1838–44. [DOI] [PubMed] [Google Scholar]
- 6.Neven K, van Es R, van Driel V, van Wessel H, Fidder H, Vink A, Doevendans P, Wittkampf F. Acute and Long-Term Effects of Full-Power Electroporation Ablation Directly on the Porcine Esophagus. Circ Arrhythm Electrophysiol. 2017;10 pii: e004672. [DOI] [PubMed] [Google Scholar]
- 7.Koruth JS, Kuroki K, Iwasawa J, Viswanathan R, Brose R, Buck ED, Donskoy E, Dukkipati SR, Reddy VY. Endocardial ventricular pulsed field ablation: a proof-of-concept preclinical evaluation. Europace.2020;22:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy VY, Neuzil P, Koruth JS, Petru J, Funosako M, Cochet H, Sediva L, Chovanec M, Dukkipati SR, Jais P. Pulsed Field Ablation for Pulmonary Vein Isolation in Atrial Fibrillation. J Am Coll Cardiol. 2019;74:315–326. [DOI] [PubMed] [Google Scholar]
- 9.Barkagan M, Leshem E, Rottmann M, Sroubek J, Shapira-Daniels A, Anter E. Expandable Lattice Electrode Ablation Catheter. Circ Arrhythm Electrophysiol. 2019;12:e007090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shapira-Daniels A, Barkagan M, Yavin H, Sroubek J, Reddy VY, Neuzil P, Anter E. Novel Irrigated Temperature-Controlled Lattice Ablation Catheter for Ventricular Ablation: A Preclinical Multimodality Biophysical Characterization. Circ Arrhythm Electrophysiol. 2019;12:e007661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Anter E, Rackauskas G, Peichl P, Aidietis A, Kautzner J, Nakagawa H, Jackman WM, Natale A, Reddy VY. An Expandable Lattice Electrode Catheter for Rapid and Titratable Temperature-Controlled Radiofrequency Ablation: A First-in-Human Multicenter Trial. JACC Clin Electrophysiol. 2020; Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 12.Reddy VY NP, Peichl P, Rackauskas G, Anter E, Petru J, Funasako M, Minami K, Aidietis A, Marinskis G, Natale A, et al. A Lattice-Tip Temperature-Controlled Radiofrequency Ablation Catheter: Durability of Pulmonary Vein Isolation and Linear Lesion Block. JACC Clin Electrophysiol. 2020. [DOI] [PubMed] [Google Scholar]
- 13.Haines DE. Can an Expanding Lattice Electrode Catheter Expand Our Success in Catheter Ablation? Circ Arrhythm Electrophysiol. 2019;12:e007306. [DOI] [PubMed] [Google Scholar]
- 14.Barkagan M, Leshem E, Rottmann M, Sroubek J, Shapira-Daniels A, Anter E. Expandable Lattice Electrode Ablation Catheter: A Novel Radiofrequency Platform Allowing High Current at Low Density for Rapid, Titratable, and Durable Lesions. Circ Arrhythm Electrophysiol. 2019;12:e007090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koruth J, Kuroki K, Iwasawa J, Enomoto Y, Viswanathan R, Brose R, Buck ED, Speltz M, Dukkipati SR, Reddy VY. Preclinical Evaluation of Pulsed Field Ablation: Electrophysiological and Histological Assessment of Thoracic Vein Isolation. Circ Arrhythm Electrophysiol. 2019;12:e007781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy VY, Koruth J, Jais P, Petru J, Timko F, Skalsky I, Hebeler R, Labrousse L, Barandon L, Kralovec S, et al. Ablation of Atrial Fibrillation With Pulsed Electric Fields: An Ultra-Rapid, Tissue-Selective Modality for Cardiac Ablation. JACC Clin Electrophysiol. 2018;4:987–995. [DOI] [PubMed] [Google Scholar]
- 17.Koruth JS, Kuroki K, Kawamura I, Brose R, Viswanathan R, Buck ED, Donskoy E, Neuzil P, Dukkipati SR, Reddy VY. Pulsed Field Ablation vs Radiofrequency Ablation: Esophageal Injury in a Novel Porcine Model. Circ Arrhythm Electrophysiol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


