Abstract
Background
Supplemental oxygen administration to critically ill patients is ubiquitous in the intensive care unit (ICU). Uncertainty persists as to whether hyperoxia is benign in patients with traumatic brain injury (TBI), particularly in regard to their long-term functional neurological outcomes.
Methods
We conducted a retrospective multicenter cohort study of invasively ventilated patients with TBI admitted to the ICU. A database linkage between the Australian and New Zealand Intensive Care Society Adult Patient Database (ANZICS-APD) and the Victorian State Trauma Registry (VSTR) was utilized. The primary exposure variable was minimum acute physiology and chronic health evaluation (APACHE) III PaO2 in the first 24 h of ICU. We defined hypoxia as PaO2 < 60 mmHg, normoxia as 60–299 mmHg, and hyperoxia as ≥ 300 mmHg. The primary outcome was a Glasgow Outcome Scale-Extended (GOSE) < 5 at 6 months while secondary outcomes included 12 and 24 months GOSE and mortality at each of these timepoints. Additional sensitivity analyses were undertaken in the following subgroups: isolated head injury, patients with operative intervention, head injury severity, and PaO2 either subcategorized by increments of 60 mmHg or treated as a continuous variable.
Results
A total of 3699 patients met the inclusion criteria. The mean age was 42.8 years, 77.7% were male and the mean acute physiology and chronic health evaluation (APACHE) III score was 60.1 (26.3). 2842 patients experienced normoxia, and 783 hyperoxia. The primary outcome occurred in 1470 (47.1%) of patients overall with 1123 (47.1%) from the normoxia group and 312 (45.9%) from the hyperoxia group—odds ratio 0.99 (0.78–1.25). No significant differences in outcomes between groups at 6, 12, and 24 months were observed. Sensitivity analyses did not identify subgroups that were adversely affected by exposure to hyperoxia.
Conclusions
No associations were observed between hyperoxia in ICU during the first 24 h and adverse neurological outcome at 6 months in ventilated TBI patients.
Electronic supplementary material
The online version of this article (10.1007/s12028-020-01033-y) contains supplementary material, which is available to authorized users.
Keywords: Hyperoxia, Critical care, Traumatic brain injury, Functional neurological outcome, Glasgow Outcome Score-Extended, Oxygen toxicity
Introduction
Supplementation of oxygen in critically ill patients is ubiquitous [1–3]. However, increasing recognition that hyperoxia may not be benign, has led to re-examination of liberal oxygen administration in this setting [4, 5]. Indeed, retrospective studies have raised concern about greater mortality with hyperoxia [6–8], albeit a recent large, multicenter randomized controlled trial (ICU-ROX) demonstrated no effect of liberal oxygen exposure on ventilator-free days or mortality in a mixed intensive care unit (ICU) population [9].
In traumatic brain injury (TBI) specifically, the impact of hyperoxia on patient-centered outcomes remains confused. A retrospective cohort study of 1547 patients with TBI from North America, found hyperoxia was associated with both increased mortality and a worse Glasgow Coma Scale (GCS) score at hospital discharge [10]. In contrast, others have shown no association between oxygen exposure and mortality [11, 12], including a large retrospective bi-national multicenter study of over 20,000 patients [13].
Clinical concern about the deleterious effects of hyperoxia persist however, because functional outcome may be more sensitive than crude mortality to any potential exposure related injury. Currently, there is a paucity of data exploring the association between hyperoxia and functional outcome in TBI, an endpoint that has critical implications for patients, caregivers, and the community.
In view of this knowledge gap, we designed a retrospective cohort study to explore the association between early hyperoxia (as measured by the partial arterial pressure of oxygen [PaO2]) and 6, 12, and 24 months functional outcomes in ventilated TBI patients admitted to the ICU. We hypothesized that early hyperoxia would be associated with a higher proportion of adverse functional outcomes.
Methods
Design
We undertook a retrospective multicenter observational cohort study of mechanically ventilated TBI patients admitted to ICU in Victoria, Australia. This utilized linked data, from the Australian and New Zealand Intensive Care Society (ANZICS) Centre for Outcome and Resource Evaluation (CORE) Adult Patient Database (APD), and the Victoria State Trauma Registry (VSTR). The APD is a binational voluntary database containing records on over 2 million ICU admissions. De-identified data are entered on a quarterly basis, and are primarily used for quality assurance and benchmarking activities. ICUs at major tertiary trauma centers in Australia and New Zealand contribute data.
The VSTR receives data from 138 health services in the Australian state of Victoria, capturing all major trauma patients from a population of approximately 6.3 million people, and aims to improve the delivery of trauma care by reducing preventable deaths and permanent disability from major trauma. The VSTR includes the follow-up of major trauma patients who survive to hospital discharge and utilizes a dedicated call center, with trained staff to undertake the 6-month GOSE assessment [14, 15]. Individual patient records in the ANZICS-APD and VSTR were linked for the period January 1, 2005, through to December 31, 2017. This was performed using a probabilistic merge using site, admission date, discharge date, age, gender, mortality status, and ICU length of stay. All data included were de-identified.
Case Selection and Data Extraction
All patient records in the linked database were adult (age > 17 years) non-transferred index trauma admissions to Victorian hospitals for the study period. From this cohort, we then selected patients who had a primary APACHE III-J code of head injury ± multitrauma. We excluded patients who were not mechanically ventilated, and those with missing ICU PaO2 data. These criteria match that of our previous study concerning in-hospital mortality [13].
Data from the ANZICS-APD included: age, gender, date of admission, GCS, hospital and ICU admission source, hospital and ICU length of stay, discharge location, level of admitting hospital, APACHE II and III scores (as well as their predicted mortality), and the Australian and New Zealand Risk of Death (ANZROD) (including the related component physiological data required for this model). All physiological data recorded in the ANZICS-APD represent the ‘worst’ values recorded in the first 24-h of ICU admission. In the case of GCS, this is the lowest value recorded at the time of, or just prior to institution of sedation and/or neuromuscular blockade.
Data from the VSTR included: initial ambulance GCS, Injury Severity Scores (ISS), and functional assessment at 6, 12, and 24 months. The latter were undertaken using the Glasgow Outcome Scale-Extended (GOSE) [16]. An adverse neurological outcome was defined as a GOSE < 5 at 6 months, and was used as the study’s primary outcome.
Oxygen exposure in the first 24-h was determined using the ‘worst’ PaO2 values from the ANZICS-APD [17]. For ABGs where the patient is intubated and the FiO2 values are ≥ 0.5, the A-a gradient is used to determine the APACHE III-J score. For ABGs where the patient is not intubated, or for intubated patients with FiO2 values < 0.5, the PaO2 value is used to determine the APACHE III-J score. For the purposes of analysis, we defined hypoxia as a PaO2 < 60 mmHg, normoxia as 60–299 mmHg, and hyperoxia as ≥ 300 mmHg. This is similar to our previous work concerning in-hospital mortality [13] and is based on prior work by Bellomo et al. [18]. To provide greater granularity, we also considered six categories of PaO2, e.g. < 60, 60–120, 120–180, 180–240, 240–300, and > 300 mmHg, and additionally examined PaO2 as a continuous variable.
Statistical Analysis
The primary exposure variable of interest was PaO2 as defined above. The primary outcome was GOSE at 6 months, with a value < 5 being considered as unfavorable. This dichotomy is consistent with previous large TBI trials conducted in our region [19–21]. Group comparisons between those with and without an adverse outcome, were performed using Chi square tests for equal proportion, analysis of variance for normally distributed data and Kruskal–Wallis tests otherwise, with results presented as counts (%), means (standard deviations) or medians [interquartile range (IQR)], respectively. GOSE at 12 and 24 months were similarly examined as secondary outcomes.
To explore the relationship between oxygen exposure and outcome, hierarchical logistic regression models were used with patients nested within sites and sites treated as a random variable adjusting for patient severity and utilizing three categories of PaO2: hypoxia, normoxia, and hyperoxia. Patient severity was measured by ANZROD [22] with the oxygen component removed to avoid confounding with PaO2. Of note, this methodology includes treatment limitations on ICU admission as a covariate. Results are presented as odds ratios (95% CI) referenced against a normal range (60–299). Additional sensitivity was performed using the six categories of PaO2, as described above.
Subgroup analysis included those coded as having an isolated head injury, non-operative versus postoperative admissions, and on the basis of TBI severity (GCS < 9, 9–12, and > 12). Based on the observed standard deviation in PaO2 of 133, this study has 90% power (2 sided p value of 0.05) to detect a 15 mmHg difference in PaO2 between patients with good and bad outcomes in the primary analysis. No imputation was made for missing data, and all proportions were reported on the basis of available data. SAS version 14.3 (SAS Institute Inc., Cary, NC, USA) and Stata® version 14 (StataCorp LLC, College Station, TX USA) were employed for statistical analysis, with a p value < 0.05 deemed as statistically significant. No adjustment has been made for multiple comparisons.
Results
Baseline Characteristics
Linking the ANZICS-APD and VSTR for the period 1 January 2005 to 31 December 2017 yielded 13,596 patients. 4456 of these were identified as having head injury ± multitrauma based on APACHE III-J diagnostic codes. Patients not requiring intubation were excluded from this group, as were 16 patients for whom PaO2 data was missing, leaving 3699 in the final dataset for analysis (Fig. 1).
Fig. 1.
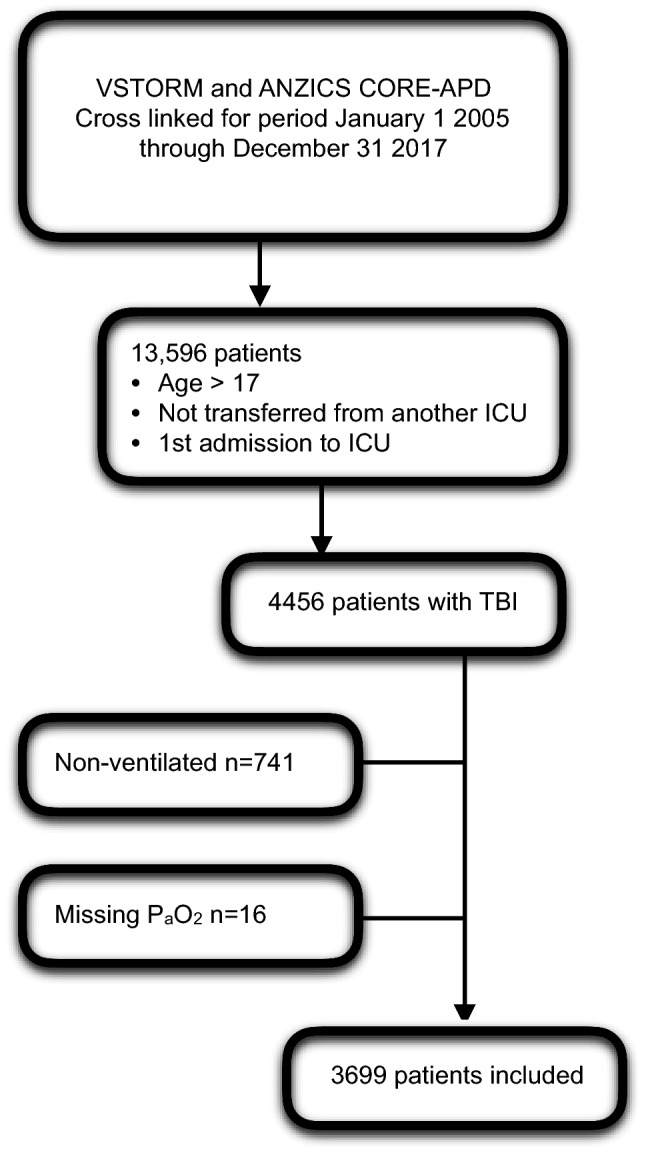
Patient enrollment flow diagram
The mean patient age was 42.8 (19.9) years, 77.7% of patients were male, and the initial median ambulance GCS was 9 [4–14]. The mean APACHE III score was 60.1 (26.3) and the mean ISS was 26.5 (12.7) in keeping with a cohort of patients suffering ‘major trauma’ [23]. The median worst PaO2 in the overall cohort was 150 [95–278] mmHg. The median PaO2 for the hypoxia, normoxia, and hyperoxia groups was 52 [45–57 mmHg], 126 [90–191] mmHg, and 401 [335–478] mmHg, respectively. Baseline characteristics are presented in Table 1.
Table 1.
Baseline patient characteristics
| n | All patients | PaO2 < 60 mmHg | PaO2 60–300 mmHg | PaO2 > 300 | |
|---|---|---|---|---|---|
| Age | 3699 | 42.8 (19.9) | 44.5 (22.3) | 43.2 (20.1) | 41 (19.1) |
| Male gender | 3699 | 2873 (77.7%) | 63 (85.1%) | 2215 (78%) | 595 (75.8%) |
| Isolated head injury | 3699 | 682 (18.4%) | 16 (21.6%) | 491 (17.3%) | 175 (22.3%) |
| Operative care prior to ICU | 3699 | 1402 (37.9%) | 32 (43.2%) | 1036 (36.5%) | 334 (42.5%) |
| Operative care prior to or during ICU admission | 3699 | 2181 (59%) | 39 (52.7%) | 1635 (57.6%) | 507 (64.6%) |
| ANZROD probability of death: median [IQR] | 3699 | 6.4 [2.2–21.2]% | 18.6 [3.3–43.6]% | 6.2 [2.1–20.7]% | 6.6 [2.4–21.2]% |
| Mean (std) | 3699 | 16.8 (22.4)% | 27.6 (28.4)% | 16.6 (22.3)% | 16.4 (22.0)% |
| ANZROD with O2 component removed: median [IQR] | 3699 | 7.0 [2.3–22.1]% | 16.1 [2.9–36.1]% | 6.9 [2.3–22.1]% | 6.9 [2.4–21.4]% |
| Mean (std) | 3699 | 17.0 (22.2)% | 25.2 (27.5)% | 17.0 (22.2)% | 16.3 (21.5)% |
| Proceeded to organ donation | 3699 | 44 (1.2%) | 4 (5.4%) | 34 (1.2%) | 6 (0.8%) |
| Injury Severity Score (ISS) | 3698 | 26 [17–34] | 28 [21–41] | 25 [17–34] | 26 [18–34] |
| Ambulance initial GCS | 3699 | 8 [3–13] | 8 [4–14] | 8 [3–13] | 8 [3–13] |
| GCS in ICU (APACHE II) | 3691 | 8 [4–13] | 8 [5–13] | 8 [4–13] | 8 [5–12] |
| Worst PaO2 in first 24 h (APACHE II) | 3698 | 150 [95–278] | 52 [45–57] | 126 [90–191] | 401 [335–478] |
| Highest FiIO2 in first 24 h (APACHE II) | 3697 | 0.5 [0.35–1] | 0.85 [0.4–1] | 0.5 [0.3–0.6] | 1 [0.8–1] |
| Highest PaCO2 in first 24 h (APACHE II) | 3698 | 39.8 (7.1) | 49.1 (15.9) | 39.8 (6.7) | 38.9 (6.5) |
| Highest respiratory rate in first 24 h (APACHE II) | 3365 | 15.9 (6.9) | 19.5 (8.6) | 16 (7.0) | 15.5 (6.2) |
| APACHE II score | 3699 | 17.6 (7.6) | 22.2 (8.8) | 17.3 (7.6) | 18 (7.3) |
| APACHE III risk of death | 3699 | 15.4 [6.3–37.7]% | 23.5 [13.1–55.2]% | 14.8 [6.0–37.2]% | 16.0 [7.1–38.6]% |
| APACHE III 3 score | 3699 | 60.1 (26.3) | 75.2 (28.9) | 59.3 (26.4) | 61.7 (24.9) |
| Hospital admission source: home | 3699 | 2702 (73%) | 52 (70.3%) | 2041 (71.9%) | 609 (77.6%) |
| Hospital admission source: other hospital | 3699 | 803 (21.7%) | 16 (21.6%) | 625 (22%) | 162 (20.6%) |
| Hospital admission source: other hospital ED | 3699 | 43 (1.2%) | 3 (4.1%) | 34 (1.2%) | 6 (0.8%) |
| Hospital admission source: unknown | 3699 | 108 (2.9%) | 3 (4.1%) | 103 (3.6%) | 2 (0.3%) |
| ICU admission source: operating theatre | 3699 | 1332 (36%) | 30 (40.5%) | 985 (34.7%) | 317 (40.4%) |
| ICU admission source: emergency | 3699 | 2232 (60.3%) | 40 (54.1%) | 1765 (62.1%) | 427 (54.4%) |
| ICU admission source: ward | 3699 | 31 (0.8%) | 2 (2.7%) | 20 (0.7%) | 9 (1.1%) |
| ICU admission source: other ICU or hosp | 3699 | 104 (2.8%) | 2 (2.7%) | 70 (2.5%) | 32 (4.1%) |
| Hospital type: rural | 3699 | 17 (0.5%) | 1 (1.4%) | 14 (0.5%) | 2 (0.3%) |
| Hospital type: metropolitan | 3699 | 20 (0.5%) | 1 (1.4%) | 11 (0.4%) | 8 (1%) |
| Hospital type: tertiary | 3699 | 3662 (99%) | 72 (97.3%) | 2815 (99.1%) | 775 (98.7%) |
With (standard deviation) for means and [interquartile ranges] for medians
APACHE acute physiology and chronic health evaluation, ANZROD Australian and New Zealand Risk of Death, ED emergency department, GCS Glasgow Coma Scale, ICU intensive care unit, IQR interquartile range
Primary Analysis
A GOSE < 5 at 6 months occurred in 47.1% (1470/3123) of patients overall, 47.1% (1123/2384) in the normoxia group and 45.9% (312/680) in the hyperoxia group (Fig. 2). The odd’s ratio (OR) with 95% confidence intervals (CI) for the primary outcome in the hyperoxia group was 0.99 (0.78–1.25). Secondary outcomes by PaO2 subgroups are presented in Table 2. The ICU mortality was 14.2% (525/3699), while the overall in-hospital mortality for the cohort was 17.7% (656/3699). At 6 months 22.8% (713/3123) had died.
Fig. 2.
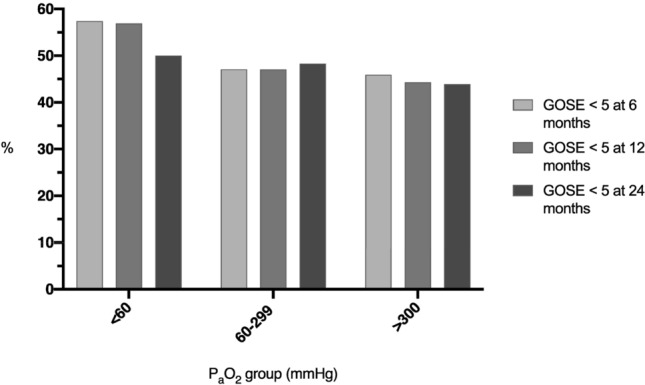
Percentage of patients with GOSE < 5 at 6, 12 and 24 months by PaO2 category (mmHg)
Table 2.
Patient outcome characteristics
| n | All patients | PaO2 < 60 mmHg | PaO2 60–300 mmHg | PaO2 > 300 | p | |
|---|---|---|---|---|---|---|
| Died in ICU | 3699 | 525 (14.2%) | 19 (25.7%) | 398 (14%) | 108 (13.8%) | 0.017 |
| ICU LOS (days) | 3699 | 5.7 [2.6–11.7] | 7.6 [2.1–14.3] | 5.7 [2.6–11.5] | 5.9 [2.4–11.7] | 0.67 |
| ICU LOS for survivors (days) | 3174 | 6.4 [2.9–12.3] | 10.3 [4.0–16.5] | 6.2 [2.9–12.2] | 6.5 [2.7–12.4] | 0.033 |
| ICU LOS for deaths (days) | 525 | 3.2 [1.2–7.2] | 1.5 [0.4–6.0] | 3.2 [1.3–7.0] | 3.9 [1.1–7.5] | 0.1 |
| Hospital outcome: death | 3699 | 656 (17.7%) | 21 (28.4%) | 498 (17.5%) | 137 (17.5%) | 0.05 |
| Hospital LOS in days | 3699 | 13.2 [6.6–23.9] | 13 [5.9–27] | 13 [6.6–23.7] | 13.8 [6.6–24.1] | 0.82 |
| Hospital LOS for survivors (days) | 3043 | 15.8 [9.0–25.9] | 19.6 [11.7–28.4] | 15.5 [9–25.8] | 16.3 [9.3–25.6] | 0.14 |
| Hospital LOS for deaths (days) | 656 | 4.3 [1.4–9.4] | 1.6 [0.7–8.2] | 4.3 [1.5–9.7] | 4.5 [1.7–8.9] | 0.15 |
| Hospital outcome: home | 3699 | 814 (22%) | 15 (20.3%) | 643 (22.6%) | 156 (19.9%) | 0.24 |
| Nursing home/chronic care/palliative | 3699 | 1760 (47.6%) | 32 (43.2%) | 1324 (46.6%) | 404 (51.5%) | 0.042 |
| Hospital outcome: other hospital | 3699 | 337 (9.1%) | 4 (5.4%) | 270 (9.5%) | 63 (8%) | 0.24 |
| Hospital outcome: rehab | 3699 | 129 (3.5%) | 2 (2.7%) | 102 (3.6%) | 25 (3.2%) | 0.8 |
| Tracheostomy indicator | 190 | 39 (20.5%) | 1 (25%) | 34 (22.2%) | 4 (12.1%) | 0.42 |
| Death at 6 months | 3123 | 713 (22.8%) | 21 (34.4%) | 548 (23%) | 144 (21.2%) | 0.06 |
| Death at 12 months | 2815 | 746 (26.5%) | 21 (36.2%) | 577 (27.2%) | 148 (23.3%) | 0.036 |
| Death at 24 months | 2577 | 774 (30%) | 21 (38.9%) | 597 (31%) | 156 (26.1%) | 0.027 |
| GOSE at 6 months | 3123 | 5 [3–6] | 4 [1–6] | 5 [3–6] | 5 [3–6] | 0.16 |
| GOSE at 12 months | 2815 | 5 [1–6] | 3.5 [1–6] | 5 [1–6] | 5 [3–6] | 0.13 |
| GOSE at 24 months | 2577 | 5 [1–6] | 4.5 [1–6] | 5 [1–6] | 5 [1–6] | 0.04 |
| GOSE < 5 at 6 months | 3123 | 1470 (47.1%) | 35 (57.4%) | 1123 (47.1%) | 312 (45.9%) | 0.23 |
| GOSE < 5 at 12 months | 2815 | 1313 (46.6%) | 33 (56.9%) | 999 (47.1%) | 281 (44.3%) | 0.13 |
| GOSE < 5 at 24 months | 2577 | 1220 (47.3%) | 27 (50%) | 931 (48.3%) | 262 (43.9%) | 0.15 |
With (standard deviation) for means and [interquartile ranges] for medians
GOSE Glasgow Outcome Scale-Extended, ICU intensive care unit, LOS length of stay
Adjusted and Sensitivity Analysis
Odds ratios (95% CI) for an adverse functional outcome at 6, 12, and 24 months, based on PaO2 category (hypoxia, normoxia, or hyperoxia) are presented in Fig. 3. Sensitivity analyses for patients with an isolated head injury, who required operative care, and according to TBI severity (GCS < 9; GCS 9–12; GCS > 12) are presented in the supplementary material, along with the isolated mortality data for each analysis. Hyperoxia was not associated with a greater likelihood of adverse functional outcomes overall, nor in any of the pre-defined subgroups. Moreover, subgrouping PaO2 values by 60 mmHg increments, did not result in identification of subgroups with an increased OR of adverse functional neurological outcome or death. These data are presented in Fig. 4. Finally, as a further sensitivity analysis, we treated PaO2 as a continuous variable, and generated a Locally Weighted Scatterplot Smoothing (LOESS) of predicted risk of a GOSE < 5 at 6 months, versus worst PaO2 in the first 24 h of ICU admission (see supplementary material). When treated as a continuous variable (including splines), there was no evidence of a significant relationship between PaO2 and worse outcomes.
Fig. 3.
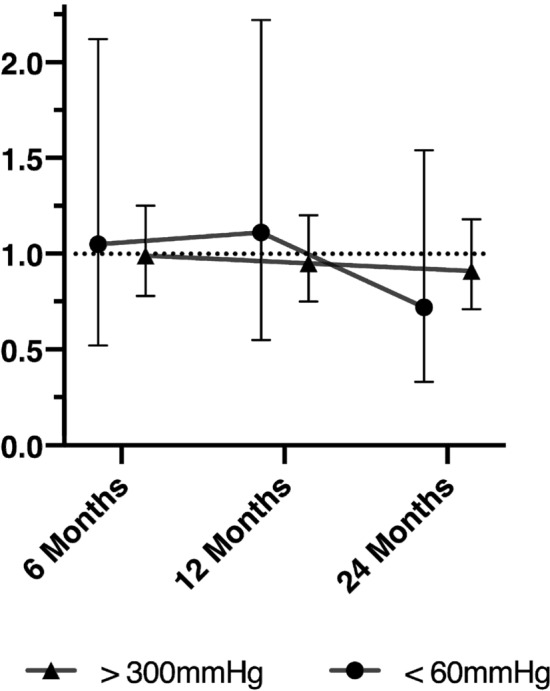
Adjusted OR of GOSE < 5 relative to normoxia group
Fig. 4.
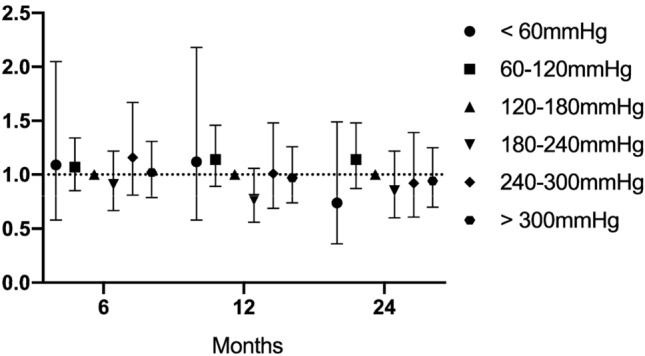
Adjusted OR of GOSE < 5 by PaO2 by 60 mmHg increments relative to 120–180 mmHg group
Discussion
Key Findings
In a large cohort of mechanically ventilated TBI patients, hyperoxia in the first 24 h of ICU admission was not associated with greater neurological morbidity at 6 months. This finding was consistent regardless of TBI severity, the need for operative care, and whether or not the head injury was isolated, or part of multisystem trauma. These findings were consistent out to 24 months of follow-up and persisted regardless of how PaO2 was subcategorized.
Relationship to Previous Studies
Our study is unique in its investigation of long-term functional outcomes in a large cohort of TBI patients. In regard to mortality, the three largest randomized controlled trials of liberal versus conservative oxygen exposure in general ICU patients have shown mixed results. The hyperoxia intervention arm of the French HYPERS2S study received an FiO2 of 1.0 and was stopped early for safety after recruitment of 442 patients, with strong mortality and morbidity signals for harm [24]. The OXYGEN-ICU trial enrolled 434 patients from a single Italian centre and compared target oxygen saturations of 94–98% with > 97% [25]. They observed statistically significant associations between hyperoxia and shock, liver failure, and new bacteraemia. ICU-ROX, the largest of these studies to date, with 1000 patients, found no difference between outcomes with a targeted SpO2 of < 97% versus usual care. This study included only a small number of patients with TBI (17 patients) [9] limiting its comparability to our study’s population.
Studies focused on TBI have, to date, had mixed results and varied in quality and setting. A retrospective registry study of 3420 patients in the US found an association between extreme hyperoxia on the initial hospital blood gas and mortality—however, this was only seen with a PaO2 above 487 mmHg, not lower, limiting generalizability [26]. They observed a similar association using discharge destination as a proxy for functional outcome status at discharge from hospital. These results primarily reflected pre-hospital care, rather than oxygen exposure in the ICU. In contrast, a US prospective single-center cohort study of 653 patients with TBI found no association between hyperoxia in the first 24 h of ICU and excess mortality [12]. A small Iranian randomized controlled trial of 68 patients with severe TBI exposed patients to either 50% or 80% oxygen during the first 6 h of their ICU admission. They measured functional outcomes including the Glasgow Outcome Score at 6 months and reported significant improvements with higher FiO2 exposure [27]. However, given the small sample and large putative effect size, this result must be viewed cautiously. Finally, a small Finnish pilot randomized controlled trial of 65 severe TBI patients did not show differences in biochemical markers of neurological injury when patients were treated with either an FiO2 of 0.4 or 0.7, providing mechanistic evidence that hyperoxia is not injurious to this population [28].
Study Implications
In this study, no relationship was observed between hyperoxia in the first 24-h in ICU, and greater adverse functional outcomes in mechanically ventilated TBI patients. Given the lack of high-quality data to guide practice otherwise, our results imply that rigorous clinical avoidance of hyperoxia in TBI patients may not be necessary, and should reinforce clinical equipoise for future randomized controlled trials in this area. Specifically, given our findings, and those of previous clinical trials [9, 29], it may be that optimization of cerebral oxygenation represents the most logical study intervention, as opposed to simply avoiding hyperoxia.
Strengths
The key strength of our study and it is unique contribution to the TBI literature is our examination of long-term neurological outcomes. Functional neurological outcome after TBI may potentially be differentially and subtly more sensitive to exposure to hyperoxia in the hours following injury. Studies focused on mortality may be insensitive to long-term neurological morbidity and disability, and as such, our work provides critically needed insights.
Linkage between the ANZICS-APD and VSTR has also meant we have captured a large cohort of patients, managed within a standardized comprehensive state-wide trauma system, with relatively homogenous ventilation and/or oxygenation strategies. Data were collected by trained staff for the purposes of audit and quality assurance, and for this reason, are unlikely to be subject to bias. Moreover, use of centralized VSTR follow-up has ensured consistency in applying the GOSE. Finally, we have used statistically robust techniques, using validated markers of illness severity, to control for known confounders.
Limitations
As with any registry-based project, data were missing in some cases. Importantly, our secondary analysis suggested no differential effect of hyperoxia according to TBI severity, and given that hyperoxia may arguably be more harmful with more severe brain injury, it is unlikely that excluding these patients has concealed any signal. In a similar fashion, follow-up at 6, 12, and 24 months was not complete in all cases, albeit our cohort still represents one of the largest published to date regarding longer-term functional outcomes following TBI.
As our data demonstrate, TBI patients typically have a lengthy stay in both the ICU and hospital, and our ability to quantify oxygen exposure over this entire period is limited. Although the hyperoxia group manifest very high PaO2 values during the first 24 h in ICU—their nominated PaO2 represents the minimum PaO2 in this period—we were unable to quantify this over any other time frame (either before ICU admission or after 24-h). As such, our study does not examine the effect of pre-hospital and Emergency Department hyperoxia, which may be more prevalent.
Finally, we acknowledge that our cohort includes patients with varying degrees of multitrauma, and different subtypes of TBI (e.g., subdural hematoma versus diffuse axonal injury). Albeit such heterogeneity may have weakened the signal overall, we did not observe any significant association between hyperoxia and adverse functional outcomes in isolated head injury, operative versus non-operative diagnosis, nor on the basis of TBI severity.
Conclusions
In a large cohort study of TBI patients, managed in a comprehensive state-wide trauma system, we found no association between hyperoxia in the first 24-h of ICU admission, and adverse long-term functional outcomes. This finding was consistent, regardless of need for surgery, presence of multitrauma, severity of head injury, or degree of hyperoxia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The Victorian State Trauma Registry (VSTR) is a Department of Health and Human Services, State Government of Victoria and Transport Accident Commission funded project. The Victorian State Trauma Outcome Registry and Monitoring (VSTORM) group is thanked for the provision of VSTR data. We would like to acknowledge the work done by each of the ICUs who contributed data to the ANZICS-APD: Albury Base Hospital ICU, Box Hill Hospital ICU, Austin Hospital ICU, Dandenong Hospital ICU, Ballarat Health Services ICU, Epworth Hospital (Richmond) ICU, Bendigo Health Care Group ICU, Goulburn Valley Health ICU, Northeast Health Wangaratta ICU, St John Of God Hospital (Ballarat) ICU, Alfred Hospital ICU, Monash Medical Centre-Clayton Campus ICU, The Northern Hospital ICU, Geelong Hospital ICU, Maroondah Hospital ICU, St Vincent’s Hospital (Melbourne) ICU, Latrobe Regional Hospital ICU, Frankston Hospital ICU, Royal Melbourne Hospital ICU, Footscray Hospital ICU, Mildura Base Hospital ICU, Central Gippsland Health Service ICU, Wimmera Health Care Group (Horsham) ICU, Western District Health Service (Hamilton) ICU, South West Healthcare (Warrnambool) ICU, Sunshine Hospital ICU.
Author Contributions
MW/AU designed the study. BG/DP/MB had full access to the raw data. Statistical analysis was performed by MB. MW/AU drafted the initial manuscript. All authors critically revised the manuscript for important intellectual content and read and approved the final version.
Source of Support
Professor Andrew Udy gratefully acknowledges salary support from the National Health and Medical Research Council of Australia (Early Career Fellowship; GNT1124532). ANZICS CORE is funded by the State and Territory Health Departments of Australia and the New Zealand Ministry of Health to monitor performance and provide benchmarking services to ICUs and Health Departments throughout both countries. The VSTR is a Department of Health and Human Services, State Government of Victoria and Transport Accident Commission funded project.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval/Informed consent
Access to the data was granted by the ANZICS CORE management committee and the VSTR data custodians. Ethics approval was obtained from The Alfred Health Human Research Ethics Committee (Ref: 243/19), with a waiver of individual patient informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Suzuki S, Eastwood GM, Peck L, Glassford NJ, Bellomo R. Current oxygen management in mechanically ventilated patients: a prospective observational cohort study. J Crit Care. 2013;28(5):647–654. doi: 10.1016/j.jcrc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 2.Young PJ, Beasley RW, Capellier G, Eastwood GM, Webb SA, ANZICS Clinical Trials Group and the George Institute for Global Health Oxygenation targets, monitoring in the critically ill: a point prevalence study of clinical practice in Australia and New Zealand. Crit Care Resusc. 2015;17(3):202–207. [PubMed] [Google Scholar]
- 3.Panwar R, Capellier G, Schmutz N, Davies A, Cooper DJ, Bailey M, Baguley D, Pilcher V, Bellomo R. Current oxygenation practice in ventilated patients-an observational cohort study. Anaesth Intensive Care. 2013;41(4):505–514. doi: 10.1177/0310057X1304100412. [DOI] [PubMed] [Google Scholar]
- 4.Jeong JH, Kim DH, Kim TY, Kang C, Lee SH, Lee SB, Kim SC, Park YJ. Harmful effects of early hyperoxaemia in patients admitted to general wards: an observational cohort study in South Korea. BMJ Open. 2018;8(10):e021758. doi: 10.1136/bmjopen-2018-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asfar P, Singer M, Radermacher P. Understanding the benefits and harms of oxygen therapy. Intensive Care Med. 2015;41(6):1118–1121. doi: 10.1007/s00134-015-3670-z. [DOI] [PubMed] [Google Scholar]
- 6.de Jonge E, Peelen L, Keijzers PJ, Joore H, de Lange D, van der Voort PHJ, Bosman RJ, de Waal RAL, Wesselink R, de Keizer NF. Association between administered oxygen, arterial partial oxygen pressure and mortality in mechanically ventilated intensive care unit patients. Crit Care (Lond, Engl) 2008;12(6):R156. doi: 10.1186/cc7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu DK, Kim LHY, Young PJ, Zamiri N, Almenawer SA, Jaeschke R, Szczeklik W, Schünemann HJ, Neary JD, Alhazzani W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. The Lancet. 2018;391(10131):1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
- 8.Palmer E, Post B, Klapaukh R, Marra G, MacCallum NS, Brealey D, Ercole A, Jones A, Ashworth S, Watkinson P, et al. The association between supra-physiologic arterial oxygen levels and mortality in critically ill patients: a Multi-Centre Observational Cohort Study. Am J Respir Crit Care Med. 2019;200(11):1373–1380. doi: 10.1164/rccm.201904-0849OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.ICU-ROX Investigators and the Australian and New Zealand Intensive Care Society Clinical Trials Group Conservative oxygen therapy during mechanical ventilation in the ICU. N Engl J Med. 2019;382(11):989–998. doi: 10.1056/NEJMoa1903297. [DOI] [PubMed] [Google Scholar]
- 10.Brenner M, Stein D, Hu P, Kufera J, Wooford M, Scalea T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch Surg. 2012;147(11):1042–1046. doi: 10.1001/archsurg.2012.1560. [DOI] [PubMed] [Google Scholar]
- 11.Raj R, Bendel S, Reinikainen M, Kivisaari R, Siironen J, Lang M, Skrifvars M. Hyperoxemia and long-term outcome after traumatic brain injury. Crit Care. 2013;17(4):R177. doi: 10.1186/cc12856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Russell DW, Janz DR, Emerson WL, May AK, Bernard GR, Zhao Z, Koyama T, Ware LB. Early exposure to hyperoxia and mortality in critically ill patients with severe traumatic injuries. BMC Pulm Med. 2017;17(1):29. doi: 10.1186/s12890-017-0370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ó Briain D, Nickson C, Pilcher DV, Udy AA. Early hyperoxia in patients with traumatic brain injury admitted to intensive care in Australia and New Zealand: a Retrospective Multicenter Cohort Study. Neurocrit Care. 2018;29(3):443–451. doi: 10.1007/s12028-018-0553-5. [DOI] [PubMed] [Google Scholar]
- 14.Ekegren CL, Hart MJ, Brown A, Gabbe BJ. Inter-rater agreement on assessment of outcome within a trauma registry. Injury. 2016;47(1):130–134. doi: 10.1016/j.injury.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 15.Gabbe BJ, Sutherland AM, Hart MJ, Cameron PA. Population-based capture of long-term functional and quality of life outcomes after major trauma: the experiences of the Victorian State Trauma Registry. J Trauma. 2010;69(3):532–536. doi: 10.1097/TA.0b013e3181e5125b. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- 17.https://www.anzics.com.au/wp-content/uploads/2018/08/ANZICS-APD-Programmers-Data-Dictionary.pdf. Accessed Jan 2020.
- 18.Bellomo R, Bailey M, Eastwood GM, Nichol A, Pilcher D, Hart GK, Reade MC, Egi M, Cooper DJ. Arterial hyperoxia and in-hospital mortality after resuscitation from cardiac arrest. Crit Care. 2011;15(2):R90. doi: 10.1186/cc10090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper DJ, Nichol AD, Bailey M, Bernard S, Cameron PA, Pili-Floury S, Forbes A, Gantner D, Higgins AM, Huet O, et al. Effect of early sustained prophylactic hypothermia on neurologic outcomes among patients with severe traumatic brain injury: the POLAR Randomized Clinical Trial. JAMA. 2018;320(21):2211–2220. doi: 10.1001/jama.2018.17075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, Kossmann T, Ponsford J, Seppelt I, Reilly P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364(16):1493–1502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 21.Nichol A, French C, Little L, Haddad S, Presneill J, Arabi Y, Bailey M, Cooper DJ, Duranteau J, Huet O, et al. Erythropoietin in traumatic brain injury (EPO-TBI): a double-blind randomised controlled trial. Lancet. 2015;386(10012):2499–2506. doi: 10.1016/S0140-6736(15)00386-4. [DOI] [PubMed] [Google Scholar]
- 22.Paul E, Bailey M, Pilcher D. Risk prediction of hospital mortality for adult patients admitted to Australian and New Zealand intensive care units: development and validation of the Australian and New Zealand Risk of Death model. J Crit Care. 2013;28(6):935–941. doi: 10.1016/j.jcrc.2013.07.058. [DOI] [PubMed] [Google Scholar]
- 23.Palmer CS, Gabbe BJ, Cameron PA. Defining major trauma using the 2008 Abbreviated Injury Scale. Injury. 2016;47(1):109–115. doi: 10.1016/j.injury.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 24.Asfar P, Schortgen F, Boisrame-Helms J, Charpentier J, Guerot E, Megarbane B, Grimaldi D, Grelon F, Anguel N, Lasocki S, et al. Hyperoxia and hypertonic saline in patients with septic shock (HYPERS2S): a two-by-two factorial, multicentre, randomised, clinical trial. Lancet Respir Med. 2017;5(3):180–190. doi: 10.1016/S2213-2600(17)30046-2. [DOI] [PubMed] [Google Scholar]
- 25.Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, Morelli A, Antonelli M, Singer M. Effect of conservative vs conventional oxygen therapy on mortality among patients in an intensive care unit: the Oxygen-ICU Randomized Clinical Trial. JAMA. 2016;316(15):1583–1589. doi: 10.1001/jama.2016.11993. [DOI] [PubMed] [Google Scholar]
- 26.Davis DP, Meade W, Sise MJ, Kennedy F, Simon F, Tominaga G, Steele J, Coimbra R. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J Neurotrauma. 2009;26(12):2217–2223. doi: 10.1089/neu.2009.0940. [DOI] [PubMed] [Google Scholar]
- 27.Taher A, Pilehvari Z, Poorolajal J, Aghajanloo M. Effects of normobaric hyperoxia in traumatic brain injury: a Randomized Controlled Clinical Trial. Trauma Mon. 2016;21(1):e26772. doi: 10.5812/traumamon.26772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lång M, Skrifvars MB, Siironen J, Tanskanen P, Ala-Peijari M, Koivisto T, Djafarzadeh S, Bendel S. A pilot study of hyperoxemia on neurological injury, inflammation and oxidative stress. Acta Anaesthesiol Scand. 2018;62(6):801–810. doi: 10.1111/aas.13093. [DOI] [PubMed] [Google Scholar]
- 29.BOOST-II Australia and United Kingdom Collaborative Groups Outcomes of Two trials of oxygen-saturation targets in preterm infants. N Engl J Med. 2016;374(8):749–760. doi: 10.1056/NEJMoa1514212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


