Abstract
Background
Nonalcoholic fatty liver disease (NAFLD) is now considered as a hepatic manifestation of metabolic syndrome and elevated alanine aminotransferase (ALT) is commonly related to NAFLD in the absence of viral hepatitis or alcohol abuse. Previous studies have indicated that elevated ALT is associated with diabetes or metabolic syndrome in adults, but the clinical significance of ALT or NAFLD in pregnancy has not been well determined. The objective of this study was to determine the association between elevated ALT in early pregnancy and the development of gestational diabetes or preeclampsia in late pregnancy.
Methods
In this retrospective cohort study, pregnant women who met the following inclusion criteria were included: 1) singleton pregnancy; 2) ALT levels were measured in antenatal outpatient clinic at 4–20 weeks of gestation; 3) patients were screened for gestational diabetes and delivered in Cheil General Hospital and Women's Healthcare Center. Cases with viral hepatitis or other liver diseases were excluded. The early ALT levels were divided into two groups (normal ALT [≤ 95th percentile] and elevated ALT [> 95th percentile]), and the frequency of gestational diabetes and preeclampsia was compared between the two groups of cases. Gestational diabetes was screened and diagnosed by two-step procedure (50 g oral glucose challenge test and 75 g glucose challenge test with World Health Organization [WHO] criteria).
Results
A total of 2,322 women met the inclusion criteria. Cases with elevated early ALT levels (> 95th percentile) had a higher risk of subsequent gestational diabetes and preeclampsia (gestational diabetes by WHO criteria, 2.1% in normal ALT vs. 6.5% in elevated ALT, P < 0.01; preeclampsia, 1.0% in normal ALT vs. 4.1% in elevated ALT, P < 0.05). This relationship between elevated ALT and increased risk of gestational diabetes/preeclampsia remained significant after adjustment for maternal age and pre-pregnancy body mass index.
Conclusion
Elevated unexplained ALT in early pregnancy is associated with the risk of subsequent development of gestational diabetes and preeclampsia in late pregnancy.
Keywords: Alanine Aminotransferase, Nonalcoholic Fatty Liver Disease, Gestational Diabetes, Preeclampsia, Pregnancy
Graphical Abstract
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), defined as lipid infiltration inside the hepatocytes in the absence of severe alcohol consumption or viral hepatitis, is one of the most common liver diseases. The prevalence of NAFLD is estimated to be 20% to 30% in western countries1 and 12% to 33% in Asia.2,3,4,5 In recent decades, a number of studies have suggested an association between NAFLD and metabolic disease, such as insulin resistance, cardiovascular disease, and obesity in non-pregnant subjects.6,7,8,9 Based on this evidence, NAFLD is now considered a hepatic manifestation of metabolic syndrome.
The enzyme alanine aminotransferase (ALT) is commonly related to NAFLD in the absence of viral hepatitis or alcohol abuse,7 and NALFD can be firstly detected by elevated liver enzymes in the general population.10,11 Considering this relationship between NAFLD and elevated ALT levels, previous studies have reported that elevated ALT is associated with diabetes, high blood pressure, or cardiovascular disease in adults.7,12,13,14
Until now, the clinical significance of NAFLD or elevated ALT levels in pregnancy has not been well determined, although a few studies have reported the possibility of increased risk for adverse pregnancy outcomes with NAFLD or elevated ALT levels.15,16,17,18 The objective of this study was to determine the association between elevated ALT in early pregnancy and the development of gestational diabetes or preeclampsia in late pregnancy.
METHODS
Study design
A retrospective cohort study was conducted including pregnant women who met the following inclusion criteria: 1) singleton pregnancy; 2) ALT levels were measured in antenatal outpatient clinic at 4–20 weeks of gestation; 3) patients were screened for gestational diabetes and delivered in Cheil General Hospital and Women's Healthcare Center between January 2014 and December 2014. Women with pregestational diabetes, chronic hypertension, and those with known chronic viral hepatitis B, hepatitis C, or other liver diseases were excluded.
Measurement of ALT
Measurement of ALT at the time of initial laboratory tests in early pregnancy is the routine practice in Cheil General Hospital and Women's Healthcare Center. Patients were divided into two groups according to their ALT levels (normal ALT [≤ 95th percentile] and elevated ALT [> 95th percentile]).
Diagnosis of gestational diabetes and preeclampsia
The frequency of gestational diabetes and preeclampsia was compared between the groups. According to the recommendations of the American College of Obstetricians and Gynecologists (ACOG), two-step approach with universal screening was used to screen and diagnose gestational diabetes.19 For the 50 g screening, the plasma glucose level was measured one hour after 50 g glucose loading, and women with glucose levels of 140 mg/dL or higher were defined as screening positive. For women with positive screening results, a 75 g 2-hour oral glucose tolerance test (OGTT) was conducted. In our institution, the World Health Organization (WHO) criteria is usually adopted for the diagnosis of gestational diabetes (95 mg/dL for fasting glucose, 180 mg/dL for 1-hour glucose, 155 mg/dL for 2-hour glucose; two or more of these must be met or exceeded for diagnosis).20 In the analysis of the current study, the frequency of gestational diabetes according to the newly suggested criteria by the International Association of Diabetes and Pregnancy Study Groups (IADPSG) was also evaluated (92 mg/dL for fasting glucose, 180 mg/dL for 1-hour glucose, 153 mg/dL for 2-hour glucose; at least one of these must be met or exceeded for diagnosis).21
For the diagnosis of pregnancy-associated hypertension, the criteria suggested by ACOG were adopted.22 Pregnancy hypertension included gestational hypertension, preeclampsia, and eclampsia. The frequency of preeclampsia (including preeclampsia and eclampsia) was also evaluated.
Statistical methods
Proportions were compared using Fisher's exact test and comparisons of continuous variables between groups were performed with the Mann-Whitney U test. Logistic regression was conducted for multivariate analysis. The association between elevated levels and the risk of preeclampsia was assessed by logistic regression with the Firth personalized likelihood to avoid bias due to small number of cases with preeclampsia. Statistical analysis was performed by SPSS software (version 22.0; IBM Corp., Chicago, IL, USA) and R (version 3.6.2; R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org). P value < 0.05 was considered as significant.
Ethics statement
This study was approved by the Institutional Review Board of Cheil General Hospital and Women's Healthcare Center (approval No. CGH-IRB-2013-9). The requirements for informed consent were waived due to the retrospective study design.
RESULTS
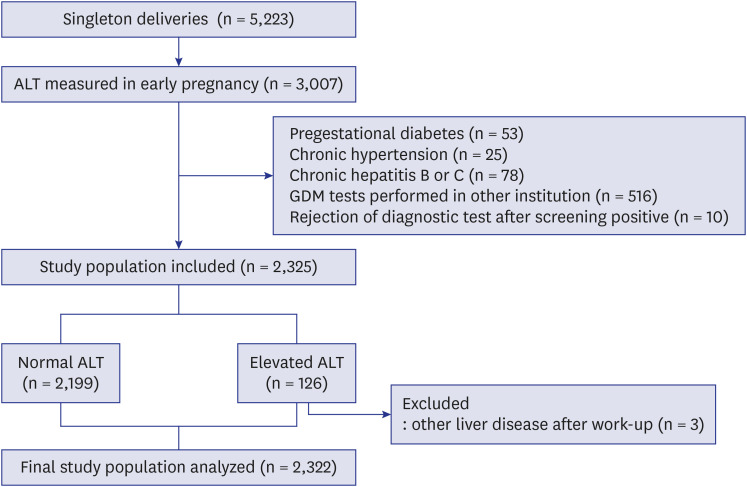
During the study period, there were a total of 5,223 singleton deliveries. Among these, 3,007 women visited our institution in early pregnancy and received initial laboratory tests, including ALT. After excluding cases with pregestational diabetes (n = 53), chronic hypertension (n = 25), chronic hepatitis B or C (n = 78), those in which screening and diagnostic testing for gestational diabetes were performed in other institutions (n = 516), and those in which patient rejected 75 g OGTT after a positive result in 50 g OGTT (n = 10), 2,325 women were included in the analysis (Fig. 1).
Fig. 1.
Study population.
ALT = alanine aminotransferase, GDM = gestational diabetes mellitus.
Women with ALT levels > 30 IU/L were designated as the elevated ALT group (> 95th percentile values). According to this cut-off value, 2,199 women had normal ALT levels, and 126 women had elevated ALT levels. After excluding 3 women who were diagnosed with other liver diseases after work-up for elevated ALT levels, a total of 2,322 women were included in the final analysis.
Table 1 shows the clinical characteristics and laboratory results according to the ALT levels. Cases with elevated AST levels had higher median pre-pregnancy body mass index (BMI) and a higher proportion of nulliparity. In addition, cases with elevated ALT levels had higher median concentrations of AST, cholesterol, and alkaline phosphatase.
Table 1. Characteristics and laboratory results according to the early ALT levels.
| Characteristics | Normal (ALT ≤ 95th percentile) (n = 2,199) | Elevated (ALT > 95th percentile) (n = 123) | P value |
|---|---|---|---|
| Maternal age, yr | 35 (32–37) | 35 (33–38) | NS |
| Old maternal age, > 35 yr | 1,023 (47) | 67 (55) | 0.095 |
| Nulliparity | 1,363 (62) | 104 (85) | < 0.001 |
| Pre-pregnancy BMI, kg/m2 | 20.5 (19.1–22.3) | 21.6 (19.4–24.9) | < 0.001 |
| Gestational age at laboratory test, wk | 11.7 (8.6–12.4) | 9.3 (8.1–12.4) | NS |
| AST, IU/L | 17 (15–19) | 31 (25–41) | < 0.001 |
| ALT, IU/L | 11 (9–15) | 41 (33–52) | < 0.001 |
| Cholesterol | 170 (151–189) | 178 (160–200) | < 0.005 |
| Total protein | 6.9 (6.6–7.1) | 6.9 (6.7–7.2) | NS |
| Albumin | 4.2 (4.0–4.3) | 4.1 (4.0–4.3) | NS |
| Globulin | 2.7 (2.6–2.9) | 2.8 (2.6–2.9) | NS |
| Total bilirubin | 0.1 (0.1–0.2) | 0.1 (0.1–0.1) | NS |
| Alkaline phosphatase | 37 (33–43) | 43 (36–51) | < 0.001 |
Data are presented as median (interquartile range) or number (%).
BMI = body mass index, AST = aspartate aminotransferase, ALT = alanine aminotransferase.
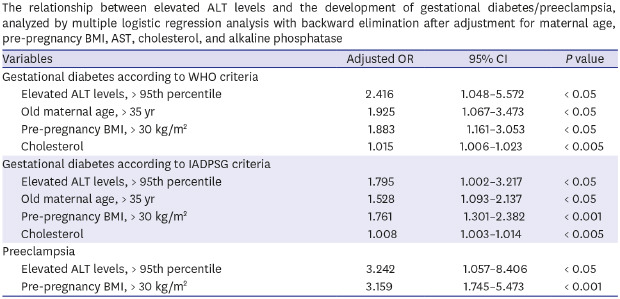
Table 2 compares the pregnancy outcomes between the two groups of cases. Cases with elevated ALT levels had a higher risk for the subsequent development of gestational diabetes mellitus (GDM) by both WHO and IADPSG criteria. In addition, cases with elevated ALT levels also had higher risks for both pregnancy hypertension and preeclampsia. Supplementary Tables 1 and 2 shows that the frequency of women with elevated ALT level were also higher in cases with gestational diabetes or preeclampsia. This relationship between elevated ALT levels and the development of GDM and preeclampsia remained significant after adjustment of confounding variables (Table 3).
Table 2. Pregnancy outcomes according to the early ALT levels.
| Characteristics | Normal (ALT ≤ 95th percentile) (n = 2,199) | Elevated (ALT > 95th percentile) (n = 123) | P value |
|---|---|---|---|
| GDM, WHO criteria | 46 (2.1) | 8 (6.5) | < 0.01 |
| GDM, IADPSG criteria | 144 (6.5) | 16 (13.0) | < 0.05 |
| Pregnancy hypertension | 22 (1.0) | 5 (4.1) | < 0.05 |
| Preeclampsia | 21 (1.0) | 5 (4.1) | < 0.05 |
| Gestational age at delivery, wk | 39.3 (38.4–40.2) | 39.3 (38.4–40.2) | NS |
| PTB < 37 wk | 107 (4.8) | 8 (6.3) | NS |
| Fetal birth weight, kg | 3,235 (2,980–3,495) | 3,230 (2,980–3,525) | NS |
| Macrosomia, > 4 kg | 65 (3.0) | 2 (1.6) | NS |
| Low 1-minute AS, < 7 | 57 (2.6) | 7 (5.7) | 0.079 |
| Low 5-minute AS, < 7 | 8 (0.4) | 1 (0.8) | NS |
Data are presented as number (%) or median (interquartile range).
ALT = alanine aminotransferase, GDM = gestational diabetes mellitus, WHO = World Health Organization, IADPSG = International Association of Diabetes and Pregnancy Study Groups, PTB = preterm birth, AS = Apgar score.
Table 3. The relationship between elevated ALT levels and the development of gestational diabetes/preeclampsia, analyzed by multiple logistic regression analysis with backward elimination after adjustment for maternal age, pre-pregnancy BMI, AST, cholesterol, and alkaline phosphatase.
| Variables | Adjusted OR | 95% CI | P value | |
|---|---|---|---|---|
| Gestational diabetes according to WHO criteria | ||||
| Elevated ALT levels, > 95th percentile | 2.416 | 1.048–5.572 | < 0.05 | |
| Old maternal age, > 35 yr | 1.925 | 1.067–3.473 | < 0.05 | |
| Pre-pregnancy BMI, > 30 kg/m2 | 1.883 | 1.161–3.053 | < 0.05 | |
| Cholesterol | 1.015 | 1.006–1.023 | < 0.005 | |
| Gestational diabetes according to IADPSG criteria | ||||
| Elevated ALT levels, > 95th percentile | 1.795 | 1.002–3.217 | < 0.05 | |
| Old maternal age, > 35 yr | 1.528 | 1.093–2.137 | < 0.05 | |
| Pre-pregnancy BMI, > 30 kg/m2 | 1.761 | 1.301–2.382 | < 0.001 | |
| Cholesterol | 1.008 | 1.003–1.014 | < 0.005 | |
| Preeclampsia | ||||
| Elevated ALT levels, > 95th percentile | 3.242 | 1.057–8.406 | < 0.05 | |
| Pre-pregnancy BMI, > 30 kg/m2 | 3.159 | 1.745–5.473 | < 0.001 | |
OR = odds ratio, CI = confidence interval, ALT = alanine aminotransferase, WHO = World Health Organization, BMI = body mass index, IADPSG = International Association of Diabetes and Pregnancy Study Groups, AST = aspartate aminotransferase.
Among the study population, 2,312 women had the result for ALT levels during late pregnancy (measured after 20 weeks of gestation, median gestational age at measurement 36.7 weeks). Women with elevated ALT levels during late pregnancy also had increased risk for the development of GDM and preeclampsia (Supplementary Table 3). This increased risk of GDM and preeclampsia in women with elevated ALT levels during late pregnancy remained significant after adjustment of confounding variables.
DISCUSSION
The principal findings of this study were: 1) cases with elevated early ALT levels (> 95th percentile) had a higher risk of subsequent gestational diabetes or preeclampsia; 2) this relationship between elevated ALT and an increased risk of gestational diabetes/preeclampsia remained significant after adjustment.
In spite of firm evidence on the association between NAFLD and metabolic syndrome, the clinical significance of NAFLD or elevated ALT levels in pregnancy is not well determined. Recently, a large Swedish national cohort reported that women with a diagnosis of NALFD are at high risk for adverse pregnancy outcomes, such as gestational diabetes, preeclampsia, preterm birth, and cesarean delivery.15 In this study 110 women were identified with a pre-pregnancy International Classification of Diseases, 9th Revision diagnosis of NAFLD among 1,960,416 births, although the prevalence of NAFLD is known to be about 20%–30% in western countries.1 The fact that this population was not routinely screened for NAFLD has been attributed to the lower detection of NAFLD in this study. Recently, in another nested case-control study, 83 women with gestational diabetes had elevated ALT levels when compared to 247 controls in early gestation.16 This study also reported that elevated ALT levels in early pregnancy increased the risk of large for gestational age birth weight even in the absence of clinical glucose tolerance.23 In a more recent study, De Souza et al.24 followed up the cohort of NAFLD diagnosed by ultrasound, and have shown that they are at risk of a composite outcome of mid-pregnancy dysglycemia, which was defined as impaired fasting glucose, impaired glucose tolerance, or gestational diabetes in 75 g OGTT.
The result of the current study on the association between elevated ALT levels and gestational diabetes/preeclampsia is consistent with these previous studies. The strengths of the current study, when compared to prior studies, are: 1) in this consecutive retrospective cohort study, we have evaluated ALT levels in all the women who visited our clinic in early pregnancy; 2) we have evaluated other metabolic markers, such as cholesterol and alkaline phosphatase, and have shown that elevated ALT is associated with the risk of gestational diabetes/preeclampsia even after adjustment of these markers; 3) we have also shown that elevated ALT levels are associated with the risk of preeclampsia development. To the best of our knowledge, this is the first study which has shown the association between elevated ALT levels and the subsequent risk of preeclampsia.
In addition to firm evidence on the association between NAFLD and diabetes, several reports have documented NAFLD as a risk factor for hypertension and cardiovascular disease in non-pregnant adults.25,26,27 The current study's finding that elevated ALT in early pregnancy is associated with the risk of preeclampsia in late pregnancy will enhance our understanding on the pathophysiology underlying NAFLD and hypertension.
Then why are the elevated ALT levels in early pregnancy associated with subsequent risk of gestational diabetes/preeclampsia? There are accumulating evidences that NAFLD is a hepatic manifestation of metabolic syndrome,6,7,8,9 which can result in the hypertension or insulin resistance. Considering that pregnancy itself is a window period of future metabolic risk,28 the association between NAFLD and metabolic disease can be clearly depicted during gestational period. The underlying mechanism between NAFLD and diabetes/hypertension is not clear, but several mechanisms have been suggested. First, the NAFLD and diabetes/hypertension may share common underlying metabolic dysfunction such as obesity.15,24 However, the relationship between elevated AST levels and the risk of gestational diabetes/preeclampsia remained significant even after adjustment for pre-pregnancy BMI in the current study. This suggest that other mechanisms may be likely to be involved. Second, proinflammatory cytokines from adipose tissues may be transferred to the liver resulting in fat accumulation (NAFLD), and make adipose tissues more metabolically adverse, resulting in gestational diabetes and hypertension. Third, the fat accumulation can also happen in the maternal spiral artery suppling the placenta, leading to atherosclerosis and impaired vascular remodeling, which is a hallmark of preeclampsia.29
There are several points to be considered. First, we used elevated ALT levels as a crude marker of NAFLD, although the confirmative diagnosis of NAFLD is made by liver biopsy, which is hard to perform in pregnant women. Other diagnostic modalities, such as liver ultrasound or transient elastography may be needed to confirm the association between NAFLD and adverse pregnancy outcomes. And the cut-off value for elevated ALT was lower than that in non-pregnant adult (> 30 IU/L). It is well known that serum ALT levels are slightly lower compared with non-pregnant values.30 In addition, the AST was not elevated in cases with gestational diabetes in the current study population. Although AST was elevated in cases with preeclampsia, this relationship between elevated AST and preeclampsia did not remain significant after adjustment (Table 3). However, other liver enzymes such as γ-glutamyltransferase (γ-GT), which was not evaluated in the current study, should be evaluated in the context of NAFLD and gestational diabetes/preeclampsia. The γ-GT is a well-known biomarker for liver fat accumulation, and γ-GT also predicted well the risk of diabetes in non-pregnant population.31 Second, we could not evaluate alcohol consumption during pregnancy, but women with heavy alcohol consumption during pregnancy were reported to be nearly absent in Korea.32 Next, in our institution, we adopted a two-step approach for gestational diabetes (50 g OGTT with 140 mg/dL as a cut-off value, and 75 g OGTT with the WHO criteria for the diagnosis of gestational diabetes).20 There are controversies regarding the screening and diagnostic strategies in the diagnosis of gestational diabetes.19,21,33 In the current study, we also analyzed the frequency of gestational diabetes according to the IADPSG criteria,21 and have shown that gestational diabetes also increased in women with elevated ALT levels according to this criteria. Third, the incidence of pregnancy hypertension in the current study is 1.2%. This incidence is relatively lower than those reported in previous studies. However, Korean pregnant women have been reported to have relatively low prevalence of preeclampsia in the literature.34 This lower prevalence of preeclampsia may be attributed to lower BMI, lifestyle factors or different ethnicity.35,36,37,38,39 Further studies in other races/ethnicities are needed to confirm the finding of the current study. Lastly, other pregnancy complications also need to be evaluated in pregnant women with elevated ALT or NAFLD. In the current study, the risk of preterm birth or small for gestational age was not increased with cases with elevated ALT.
In conclusion, unexplained elevated ALT in early pregnancy was associated with the risk of subsequent development of gestational diabetes and preeclampsia in late pregnancy.
ACKNOWLEDGMENTS
The authors would like to thank Sohee Oh, PhD of the Department of Biostatistics in Seoul Metropolitan Government-Seoul National University Boramae Medical Center for statistical advice.
Footnotes
Funding: This study was supported by a clinical research grant-in-aid from the Seoul Metropolitan Government-Seoul National University (SMG-SNU) Boramae Medical Center (03-2014-4) and from Seoul National University Hospital research fund (03-2018-0400).
Disclosure: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Lee SM, Kim W.
- Data curation: Lee SM, Park JS, Han YJ, Kim W, Kim MY.
- Formal analysis: Lee SM, Han YJ, Kim W, Kim MY.
- Investigation: Lee SM, Park JS, Han YJ, Kim W, Bang SH, Kim BJ, Park CW, Kim MY.
- Validation: Lee SM, Park JS, Han YJ, Kim W, Kim MY.
- Writing - original draft: Lee SM, Park JS, Han YJ, Kim W, Kim MY.
- Writing - review & editing: Lee SM, Park JS, Han YJ, Kim W, Bang SH, Kim BJ, Park CW, Kim MY.
SUPPLEMENTARY MATERIALS
Characteristics and laboratory results according to the development of gestational diabetes (WHO criteria)
Characteristics and laboratory results according to the preeclampsia
Pregnancy outcomes according to the late ALT levels
References
- 1.Vernon G, Baranova A, Younossi ZM. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 2011;34(3):274–285. doi: 10.1111/j.1365-2036.2011.04724.x. [DOI] [PubMed] [Google Scholar]
- 2.Cho HC. Prevalence and factors associated with nonalcoholic fatty liver disease in a nonobese Korean population. Gut Liver. 2016;10(1):117–125. doi: 10.5009/gnl14444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi SY, Kim D, Kim HJ, Kang JH, Chung SJ, Park MJ, et al. The relation between non-alcoholic fatty liver disease and the risk of coronary heart disease in Koreans. Am J Gastroenterol. 2009;104(8):1953–1960. doi: 10.1038/ajg.2009.238. [DOI] [PubMed] [Google Scholar]
- 4.Zhang T, Zhang C, Zhang Y, Tang F, Li H, Zhang Q, et al. Metabolic syndrome and its components as predictors of nonalcoholic fatty liver disease in a northern urban Han Chinese population: a prospective cohort study. Atherosclerosis. 2015;240(1):144–148. doi: 10.1016/j.atherosclerosis.2015.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Jung JY, Park SK, Oh CM, Chung PW, Ryoo JH. Non-alcoholic fatty liver disease and its association with depression in Korean general population. J Korean Med Sci. 2019;34(30):e199. doi: 10.3346/jkms.2019.34.e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ramesh S, Sanyal AJ. Evaluation and management of non-alcoholic steatohepatitis. J Hepatol. 2005;42 Suppl(1):S2–S12. doi: 10.1016/j.jhep.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 7.Schindhelm RK, Diamant M, Dekker JM, Tushuizen ME, Teerlink T, Heine RJ. Alanine aminotransferase as a marker of non-alcoholic fatty liver disease in relation to type 2 diabetes mellitus and cardiovascular disease. Diabetes Metab Res Rev. 2006;22(6):437–443. doi: 10.1002/dmrr.666. [DOI] [PubMed] [Google Scholar]
- 8.Utzschneider KM, Kahn SE. Review: the role of insulin resistance in nonalcoholic fatty liver disease. J Clin Endocrinol Metab. 2006;91(12):4753–4761. doi: 10.1210/jc.2006-0587. [DOI] [PubMed] [Google Scholar]
- 9.Kim JY, Cho J, Yang HR. Biochemical predictors of early onset non-alcoholic fatty liver disease in young children with obesity. J Korean Med Sci. 2018;33(16):e122. doi: 10.3346/jkms.2018.33.e122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radcke S, Dillon JF, Murray AL. A systematic review of the prevalence of mildly abnormal liver function tests and associated health outcomes. Eur J Gastroenterol Hepatol. 2015;27(1):1–7. doi: 10.1097/MEG.0000000000000233. [DOI] [PubMed] [Google Scholar]
- 11.Rafiq N, Younossi ZM. Nonalcoholic fatty liver disease: a practical approach to evaluation and management. Clin Liver Dis. 2009;13(2):249–266. doi: 10.1016/j.cld.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Fraser A, Ebrahim S, Smith GD, Lawlor DA. A comparison of associations of alanine aminotransferase and gamma-glutamyltransferase with fasting glucose, fasting insulin, and glycated hemoglobin in women with and without diabetes. Hepatology. 2007;46(1):158–165. doi: 10.1002/hep.21667. [DOI] [PubMed] [Google Scholar]
- 13.Zhang Y, Lu X, Hong J, Chao M, Gu W, Wang W, et al. Positive correlations of liver enzymes with metabolic syndrome including insulin resistance in newly diagnosed type 2 diabetes mellitus. Endocrine. 2010;38(2):181–187. doi: 10.1007/s12020-010-9369-6. [DOI] [PubMed] [Google Scholar]
- 14.Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51(6):1889–1895. doi: 10.2337/diabetes.51.6.1889. [DOI] [PubMed] [Google Scholar]
- 15.Hagström H, Höijer J, Ludvigsson JF, Bottai M, Ekbom A, Hultcrantz R, et al. Adverse outcomes of pregnancy in women with non-alcoholic fatty liver disease. Liver Int. 2016;36(2):268–274. doi: 10.1111/liv.12902. [DOI] [PubMed] [Google Scholar]
- 16.Yarrington CD, Cantonwine DE, Seely EW, McElrath TF, Zera CA. The association of alanine aminotransferase in early pregnancy with gestational diabetes. Metab Syndr Relat Disord. 2016;14(5):254–258. doi: 10.1089/met.2015.0106. [DOI] [PubMed] [Google Scholar]
- 17.Lee SM, Kwak SH, Koo JN, Oh IH, Kwon JE, Kim BJ, et al. Non-alcoholic fatty liver disease in the first trimester and subsequent development of gestational diabetes mellitus. Diabetologia. 2019;62(2):238–248. doi: 10.1007/s00125-018-4779-8. [DOI] [PubMed] [Google Scholar]
- 18.Lee SM, Kim BJ, Koo JN, Norwitz ER, Oh IH, Kim SM, et al. Nonalcoholic fatty liver disease is a risk factor for large-for-gestational-age birthweight. PLoS One. 2019;14(8):e0221400. doi: 10.1371/journal.pone.0221400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Committee on Practice Bulletins--Obstetrics. Practice bulletin No. 137: gestational diabetes mellitus. Obstet Gynecol. 2013;122(2 Pt 1):406–416. doi: 10.1097/01.AOG.0000433006.09219.f1. [DOI] [PubMed] [Google Scholar]
- 20.Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36(Suppl 1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.American College of Obstetricians and Gynecologists; Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists' Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 23.Yarrington CD, Cantonwine DE, Seely EW, Mcelrath TF, Zera CA. The association of early unexplained elevated alanine aminotransferase with large-for-gestational-age birthweight. Am J Obstet Gynecol. 2016;215(4):474.e1–474.e5. doi: 10.1016/j.ajog.2016.04.051. [DOI] [PubMed] [Google Scholar]
- 24.De Souza LR, Berger H, Retnakaran R, Vlachou PA, Maguire JL, Nathens AB, et al. Non-alcoholic fatty liver disease in early pregnancy predicts dysglycemia in mid-pregnancy: prospective study. Am J Gastroenterol. 2016;111(5):665–670. doi: 10.1038/ajg.2016.43. [DOI] [PubMed] [Google Scholar]
- 25.Wang Y, Zeng Y, Lin C, Chen Z. Hypertension and non-alcoholic fatty liver disease proven by transient elastography. Hepatol Res. 2016;46(13):1304–1310. doi: 10.1111/hepr.12688. [DOI] [PubMed] [Google Scholar]
- 26.Ghamar-Chehreh ME, Khedmat H, Amini M, Taheri S. Predictive value of having positive family history of cardiovascular disorders, diabetes mellitus, dyslipidemia, and hypertension in non-alcoholic fatty liver disease patients. Acta Med Iran. 2013;51(5):307–313. [PubMed] [Google Scholar]
- 27.Latea L, Negrea S, Bolboaca S. Primary non-alcoholic fatty liver disease in hypertensive patients. Australas Med J. 2013;6(6):325–330. doi: 10.4066/AMJ.2013.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paauw ND, van Rijn BB, Lely AT, Joles JA. Pregnancy as a critical window for blood pressure regulation in mother and child: programming and reprogramming. Acta Physiol (Oxf) 2017;219(1):241–259. doi: 10.1111/apha.12702. [DOI] [PubMed] [Google Scholar]
- 29.Staff AC, Dechend R, Pijnenborg R. Learning from the placenta: acute atherosis and vascular remodeling in preeclampsia-novel aspects for atherosclerosis and future cardiovascular health. Hypertension. 2010;56(6):1026–1034. doi: 10.1161/HYPERTENSIONAHA.110.157743. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Extremera A, López-Garrido MA, Barranco E, Quintero MD, Ocete-Hita E, Muñoz de Rueda P, et al. Activity of hepatic enzymes from week sixteen of pregnancy. Am J Obstet Gynecol. 2005;193(6):2010–2016. doi: 10.1016/j.ajog.2005.04.045. [DOI] [PubMed] [Google Scholar]
- 31.Fraser A, Harris R, Sattar N, Ebrahim S, Davey Smith G, Lawlor DA. Alanine aminotransferase, gamma-glutamyltransferase, and incident diabetes: the British Women's Heart and Health Study and meta-analysis. Diabetes Care. 2009;32(4):741–750. doi: 10.2337/dc08-1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim O, Park K. Prenatal alcohol consumption and knowledge about alcohol consumption and fetal alcohol syndrome in Korean women. Nurs Health Sci. 2011;13(3):303–308. doi: 10.1111/j.1442-2018.2011.00618.x. [DOI] [PubMed] [Google Scholar]
- 33.Jung YJ, Kwon JY, Cho HY, Park YW, Kim YH. Comparison of the performance of screening test for gestational diabetes in singleton versus twin pregnancies. Obstet Gynecol Sci. 2015;58(6):439–445. doi: 10.5468/ogs.2015.58.6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cho GJ, Kim HY, Park JH, Ahn KH, Hong SC, Oh MJ, et al. Prepregnancy liver enzyme levels and risk of preeclampsia in a subsequent pregnancy: a population-based cohort study. Liver Int. 2018;38(5):949–954. doi: 10.1111/liv.13617. [DOI] [PubMed] [Google Scholar]
- 35.Yu CK, Teoh TG, Robinson S. Obesity in pregnancy. BJOG. 2006;113(10):1117–1125. doi: 10.1111/j.1471-0528.2006.00991.x. [DOI] [PubMed] [Google Scholar]
- 36.Bodnar LM, Ness RB, Markovic N, Roberts JM. The risk of preeclampsia rises with increasing prepregnancy body mass index. Ann Epidemiol. 2005;15(7):475–482. doi: 10.1016/j.annepidem.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Caughey AB, Stotland NE, Washington AE, Escobar GJ. Maternal ethnicity, paternal ethnicity, and parental ethnic discordance: predictors of preeclampsia. Obstet Gynecol. 2005;106(1):156–161. doi: 10.1097/01.AOG.0000164478.91731.06. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka M, Jaamaa G, Kaiser M, Hills E, Soim A, Zhu M, et al. Racial disparity in hypertensive disorders of pregnancy in New York State: a 10-year longitudinal population-based study. Am J Public Health. 2007;97(1):163–170. doi: 10.2105/AJPH.2005.068577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knuist M, Bonsel GJ, Zondervan HA, Treffers PE. Risk factors for preeclampsia in nulliparous women in distinct ethnic groups: a prospective cohort study. Obstet Gynecol. 1998;92(2):174–178. doi: 10.1016/s0029-7844(98)00143-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics and laboratory results according to the development of gestational diabetes (WHO criteria)
Characteristics and laboratory results according to the preeclampsia
Pregnancy outcomes according to the late ALT levels