Abstract
Background
Spinal nerve ligation (SNL) model is one of the representative models of the neuropathic pain model. Neuropathic pain in a chronic post-ischemic pain (CPIP) mimics the symptoms of complex regional pain syndrome (CRPS). The administration of polydeoxyribonucleotide (PDRN), which has regenerative and anti-inflammatory effects, has been studied and is used in clinical practice treating various diseases. However, the analgesic effect of PDRN in a neuropathic pain or CRPS model remains unknown.
Methods
PDRN (3.3, 10, and 20 mg/kg) was administered into the subcutaneous (SC) layer of the hind paws of SNL and CPIP models. Mechanical anti-allodynic effects were then investigated using the von Frey test. In the immunohistochemical examination, dorsal root ganglia (DRG) and the spinal cord were harvested and examined for the expression of glial fibrillary acidic protein (GFAP) after the 20 mg PDRN injection.
Results
Mechanical allodynia was significantly alleviated by administration of PDRN in SNL and CPIP mice at all of the time point. As the dose of PDRN increased, the effect was greater. The 20 mg PDRN injection was found to have the most effective anti-allodynic effect. The increased expression of GFAP in DRG and the spinal cord of SNL and CPIP model decreased following the administration of PDRN than vehicle.
Conclusion
SC administration of PDRN results in the attenuation of allodynia and activation of astrocytes in neuropathic pain or CRPS models. We propose that PDRN can have significant potential advantages in neuropathic pain treatment.
Keywords: Polydeoxyribonucleotide, Neuropathic Pain, Complex Regional Pain Syndrome, Spinal Nerve Ligation Model, Chronic Post-ischemic Pain Model
Graphical Abstract
INTRODUCTION
Neuropathic pain is generally defined as pain resulted from nerve damage or dysfunction of the somatosensory system and, can present in the form of hyperalgesia, allodynia, and paroxysmal or spontaneous pain, which are ordinarily intractable.1 Complex regional pain syndrome (CRPS) is a condition associated with neuropathic pain characterized by intractable pain, multiple system dysfunction, and motor and autonomic dysfunction. CRPS often causes serious impairment of activities and functions of daily living.2,3 The obvious pathophysiology of neuropathic pain or CRPS has not yet been fully understood. However, various studies have proposed several consistent pathophysiological mechanisms that are related to neurogenic inflammatory responses and central sensitization. Studies have shown that astrocytes in the spinal cord are activated through the increased expression of glial fibrillary acidic protein (GFAP) observed by immunostaining of the gray matter of spinal cord in the neuropathic pain model. Studies have shown that astrocytes in the spinal cord were activated that was proven as increased GFAP immunostaining was observed in the gray matter of spinal cord of neuropathic pain model. These results suggested that astrocytes could facilitate central sensitization.4,5,6 One of the important factors for astrocyte activation is various inflammatory agents. The increased levels of pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 and delayed expression levels of anti-inflammatory cytokines such as of IL-10 are closely related to activation of astrocyte. The activation of astrocyte brings out increasing intracellular Ca2+ which stimulate a calcium-dependent glutamate release, resulting in altering the polarization characteristics of the afferent neurons and modulating the transmission of painful stimuli. In this process, astrocytes are involved in function to sustain neuropathic pain.7,8
Polydeoxyribonucleotide (PDRN) is the active fraction from the sperm of trout bred containing polymers of deoxyribonucleotides with chain lengths ranging between 50 and 2,000 base pairs. PDRN is a mixture of nucleotides, acting through adenosine receptors, which represent the source of purine and pyrimidine deoxynucleosides/deoxyribonucleotides and bases. In previous studies, PDRN stimulated the expression of vascular endothelial growth factor by activating adenosine receptors. Through this mechanism, PDRN stimulates angiogenesis and wound healing in the diabetic mouse model and hind limb ischemia the rat model.9,10,11 Additionally, clinical research showed the similar effects after skin graft.12 PDRN promotes the growth rate of fibroblasts and osteoblasts.13,14,15 PDRN has an anti-inflammatory effect through the activation of adenosine receptors by decreasing inflammatory cytokines such as TNF-α and IL-6. It also increases the anti-inflammatory cytokine IL-10. These mechanisms of action of PDRN reduce the severity of arthritis, periodontitis, inflammatory bowel disease, and various other inflammatory diseases.16,17,18,19
PDRN, which is associated with regenerative and anti-inflammatory mechanisms, may be considered appropriate in the management of neuropathic pain or CRPS. Its application has not been documented in previous research. The present study was performed to evaluate the therapeutic effectiveness of PDRN in two representative neuropathic animal models, chronic post-ischemic pain (CPIP) and spinal nerve ligation (SNL).
METHODS
Animals
Male adult C57/Bl6 mice (weighing, 25–30 g each) were used in this study, were housed in groups of five, and allowed free access to food and water under a 12:12-hour light:dark cycle. All animals were allowed to adapt to their environmental conditions for 7 days prior to the experiment.
SNL model
The surgical procedure for SNL was performed as described in several previous studies.20,21,22 Under general anesthesia with isoflurane, left paraspinal muscles were dissected from the spinous processes at the L4-S2 levels. Under magnification, the transverse process of L5 was then carefully removed to identify the L4-L6 spinal nerves. The L5 spinal nerve was identified and carefully dissected from the other adjacent tissues after which it was tightly ligated using a silk suture. The wound was irrigated with antiseptic solution, and the muscle layer was sutured. Mice in the control group were placed under general anesthesia and the same procedure was carried out on them with the exception of ligation of the spinal nerves.
CPIP model
The CPIP model was induced in mice under general anesthesia with isoflurane by placing a tight-fitting O-ring (O-Rings West, Seattle, WA, USA) with a 5/64 inch internal diameter around the left ankle for 3 hours, as described by Coderre et al.23 and Ryu et al.,24 The O-rings were removed while the mice were still under general anesthesia, allowing for reperfusion. Mice in the control group were also placed under general anesthesia. However, they were provided with cut O-rings which were fitted loosely rather than tightly around their ankles.
Measurement of tactile allodynia
The plantar surfaces of the ipsilateral and contralateral hind paws of CPIP, SNL and the control mice were tested for tactile allodynia 1 day and 30 days after hind paw ischemia/reperfusion (I/R) injury. To examine the threshold of withdrawal response, the floors of the cages for all three groups of mice were replaced with mesh floors that allowed easy access to the plantar surfaces of their hind paws with von Frey filaments. After a 30-minute adaptation period, tactile hyperalgesia of the hind leg was assessed by performing up and down strokes using a von Frey filament (Stoelting Co., Wood Dale, IL, USA) ranging from 2.44 to 4.31 (0.03–2.00 g).25,26 This behavioral testing was performed in a blinded manner. The 50% response threshold (grams) was calculated based on the response pattern and the value (in log units) of the final von Frey filament used.
Drug administration
Both SNL and CPIP models that exhibited tactile allodynia were subjected to PDRN injections for 8 days, which is the appropriate duration of therapy for allodynia after I/R injury. Prior to the administration of PDRN, SNL, and CPIP mice were accommodated in an observation cage for 30 minutes, after which mechanical allodynia was measured using von Frey filaments hairs. All SNL and CPIP mice injected with the drug and who showed distinct mechanical allodynia were selected. Saline or PDRN (Placentex Integro®; Mastelli SRL, Sanremo, Italy) was administered subcutaneously into the dorsum of the ipsilateral hind paw that showed an allodynic response in the von Frey test. Placentex Integro® consists of PDRN 5.625 mg/3 mL. In the SNL model, 3.3 mg/kg, 10 mg/kg and 20 mg/kg of PDRN (n = 6 per groups) or a vehicle (NaCl 0.9%, n = 4) were administered. Because the mice's weight is 23.5–25.5 g, it was injected according to the volume according to the highest weight, 0.51 cc. In the CPIP model, 3.3 mg/kg, 10 mg/kg and 20 mg/kg of PDRN (n = 6 per groups) or a vehicle (NaCl 0.9%, n = 4) were administered at different doses. After injection, mechanical allodynia was assessed in the same process 30, 60, 90,120, 180, 240 minutes, and 24 hours after PDRN administration.
Assessment of GFAP expression in dorsal root ganglia (DRG) and spinal cord
All the mice in each of the groups were sacrificed, and DRG and lumbar 4–6 spinal cord were collected 60 minutes after PDRN and vehicle injection, according to the mechanical allodynia results. Mice injected with saline and mice treated with PDRN were anesthetized and perfused transcardially with 50 mL of 4% paraformaldehyde dissolved in 0.01 M phosphate-buffered saline with pH 7.2–7.4. The DRG and spinal cord of the mice were then dissected. All obtained tissues were postfixed, and immersed in a 30% sucrose solution overnight. DRG and spinal cord segments were cut into 10 µm thick slices on a freezing microtome. The slices were incubated with mouse anti-GFAP (1:150; Millipore, Burlington, MA, USA) overnight at 4°C. After the sections were washed with buffer, they were exposed for 1 hour at 37°C to secondary antibodies, goat anti-mouse IgG conjugated to fluorescein isothiocyanate (1:200, FITC; EarthOx, Millbrae, CA, USA).
The stained sections were cover-slipped and examined using a Zeiss LSM 510 Meta confocal microscope (Zeiss, Oberkochen, Germany), and the mean intensity was measured using Image-Pro Plus v. 6.0 (Media Cybernetics, Inc., Rockville, MD, USA).
Statistics
The data are presented as the mean ± standard error of the mean. Statistical analyses were performed using the GraphPad Prism 5.0 (GraphPad Software, Inc., San Diego, CA, USA). A repeated measures 2-way analysis of variance (ANOVA) was performed to identify overall differences in the 50% von Frey threshold at each time point under different conditions, followed by Bonferroni post hoc tests. Comparisons between the control group and treated group values were made at each time point using Student's t-tests by Bonferroni post hoc tests. A two-tailed P test was conducted and a P value < 0.05 was considered to indicate statistical significance. A Kruskal-Wallis test was also used for the comparison of the immunohistochemical expression of GFAP among the SNL, CPIP, and treatment groups.
Ethics statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee of the College of Medicine, Catholic University of Korea (CUMC-2017-0059). All mouse studies were performed in accordance with the guidelines and regulations governing these animals.
RESULTS
SNL and CPIP mice exhibited prominent mechano-allodynia
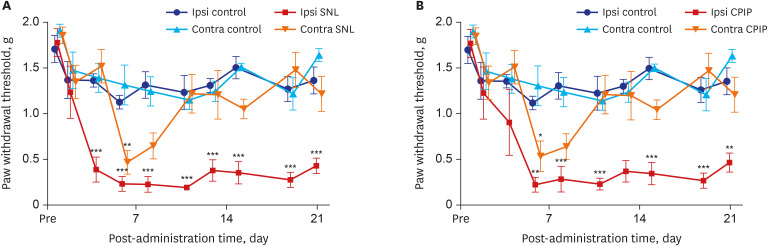
The tight ligation of the L5 spinal nerve produced significant ipsilateral mechano-allodynia (group effect: F [3, 15] = 27.46, P < 0.001; time effect: F [9, 45] = 24.75, P < 0.001; interaction: F [27, 135] = 3.881, P < 0.001; two-way ANOVA followed by Bonferroni's post hoc test). Ipsilateral mechano-allodynia initially occurred 4 days after the procedure and peaked 8 days post-operatively. This difference was statistically significant until at least 21 days after ligation. Contralateral mechano-allodynia was not maintained prolonged as long as ipsilateral mechano-allodynia in SNL mice as it did in CPIP mice. On the 6th day after operation contralateral mechano-allodynia occurred for the first time, and lasted until the 11th day (Fig. 1A).
Fig. 1. Time course of mechanical allodynia in the ipsilateral and contralateral hind paw of SNL and control mice, CPIP and control mice as shown via von Frey testing. (A) The withdrawal thresholds of control mice were not significantly changed throughout the 21 days of testing. The withdrawal thresholds of ipsilateral SNL mice paws significantly increased on the fourth day and continued for 21 days after reperfusion. (B) The withdrawal thresholds of control mice were not significantly altered throughout the 21 days of testing. The withdrawal thresholds of ipsilateral CPIP mice were significantly reduced and lasted for 21 days after reperfusion.
SNL = spinal nerve ligation, CPIP = chronic post-ischemic pain.
*P < 0.05, **P < 0.005, ***P < 0.001 at each time point between control and CPIP mice.
CPIP mice developed mechano-allodynia over a prolonged period in their ipsilateral hind paws (group effect: F [3, 20] = 19.22, P < 0.001; time effect: F [9, 180] = 16.02, P < 0.001; interaction: F [27, 180] = 2.723, P < 0.001; two-way ANOVA followed by Bonferroni's post hoc test). Ipsilateral mechano-allodynia was exhibited within 4 days following a 3-hour period of ischemia and reperfusion; it peaked at 6 days and was maintained for at least 21 days after reperfusion. Contralateral mechano-allodynia was not as prolonged as ipsilateral mechano-allodynia. Contralateral mechano-allodynia occurred within 6 days following reperfusion, it peaked at 6 days and persisted for a maximum of 11 days after perfusion (Fig. 1B).
PDRN attenuated mechanical allodynia in SNL mice
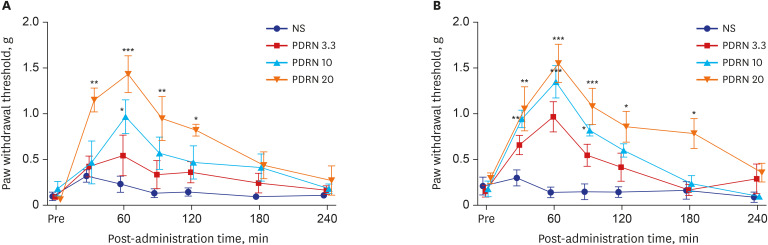
Intra-paw PDRN injections dose-dependently reduced mechanical allodynia in SNL mice (interaction: F [18, 126] = 1.85, P = 0.026 time: F [6, 126] = 10.8, P < 0.001; group: F [3, 126] = 19.2, P < 0.001). The 20 mg PDRN injection group experienced the most significant decrease in mechanical allodynia and its anti-allodynic effect lasted longest. The effect occurred within 30 minutes after injection and peaked at 60 minutes and lasted for 120 minutes (P values at 30, 60, and 120 minutes were P < 0.001, P < 0.001, and P < 0.05 respectively). In the 10 mg PDRN injection group, the anti-allodynic effect was significant at 60 minutes (P < 0.001). The effectiveness of the 3.3 mg PDRN injection was not found to be statistically significant at any time point (Fig. 2A).
Fig. 2. The effect of the administration of PDRN on the tactile threshold in SNL and CPIP mice. (A) PDRN injections dose-dependently reduced mechanical allodynia in SNL mice when compared with that in the control group. PDRN 20 mg/kg injection group showed the most effective attenuation of mechanical allodynia. (B) Mechanical allodynia was attenuated in all groups of PDRN injections. The PDRN 20 mg/kg group showed the greatest decrease in mechanical allodynia and remained effective.
NS = not significant, PDRN = polydeoxyribonucleotide, SNL = spinal nerve ligation, CPIP = chronic post-ischemic pain.
*P < 0.05, **P < 0.005, ***P < 0.001 at each time point compared to that in the vehicle.
PDRN attenuated mechanical allodynia in CPIP mice
Intra-paw PDRN injections dose-dependently decreased mechanical allodynia in CPIP mice (interaction: F [18, 126] = 2.401, P = 0.026; time: F [6, 126] = 19.36, P < 0.001; group: F [3, 126] = 29.08, P < 0.001). Upon comparing with that in the control group at each point, all three PDRN injection groups showed the largest anti-allodynic effect at 60 minutes (PDRN 3.3, P < 0.001; PDRN 10, P < 0.001; PDRN 20, P < 0.001). In the 3.3 mg PDRN injection group, only one point at 60 minutes showed a significant anti-allodynic effect. In the 10 mg PDRN injection group, mechanical allodynia significantly decreased from 30 minutes to 90 minutes, while in the 20 mg PDRN injection group, mechanical allodynia significantly decreased from 30 minutes to 180 minutes (Fig. 2B). The 20 mg PDRN injection was found to have the most effective anti-allodynic effect.
PDRN reduced GFAP expression in SNL & CPIP mice
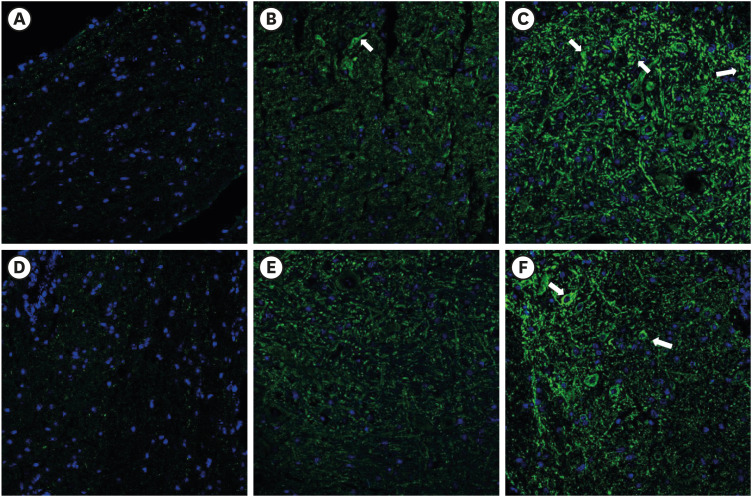
In the 20 mg PDRN-injected group of the SNL model, the expression of GFAP in DRG (Fig. 3B) was significantly reduced, as exhibited by the lower optical densities measured in the PDRN-injected group compared to the vehicle-injected group (Fig. 3C) (P = 0.004). In the 20 mg PDRN-injected group of the SNL model, the expression of GFAP in spinal cord (Fig. 3E) was also significantly reduced, comparing with vehicle-injected group (P = 0.032) (Fig. 3F). There was no difference between the sham group (Fig. 3A and D) and 20 mg PDRN-injected group (P values of the DRG and spinal cord were P = 0.78, P = 0.82), and sham group and vehicle-injected group are statistically different (P values of DRG and spinal cord were P = 0.003, P = 0.008, respectively) (Fig. 4).
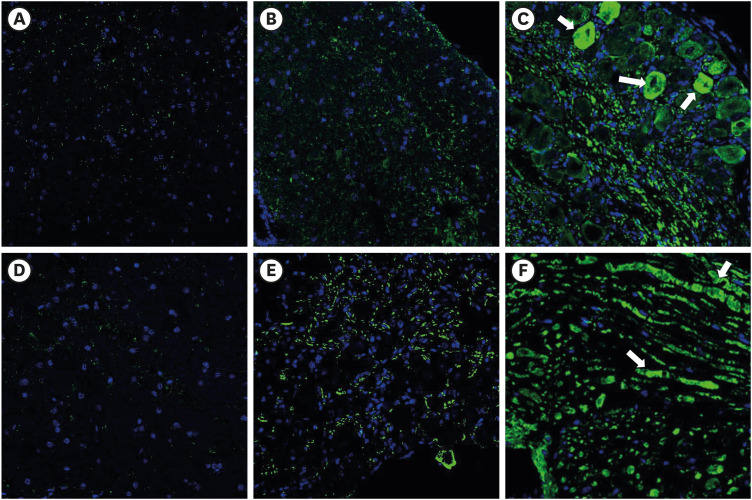
Fig. 3. The effect of subcutaneous PDRN on GFAP expression (luminous green) in DRG and spinal cord of SNL model. Original magnification: ×200. (A) Immunostaining for GFAP in DRG of sham mouse. (B) PDRN-injected SNL mouse. (C) Vehicle-injected SNL mouse. (D) Immunostaining for GFAP in spinal cord of sham mouse. (E) PDRN-injected SNL mouse. (F) Vehicle-injected SNL mouse.
PDRN = polydeoxyribonucleotide, GFAP = glial fibrillary acidic protein, DRG = dorsal root ganglia, SNL = spinal nerve ligation.
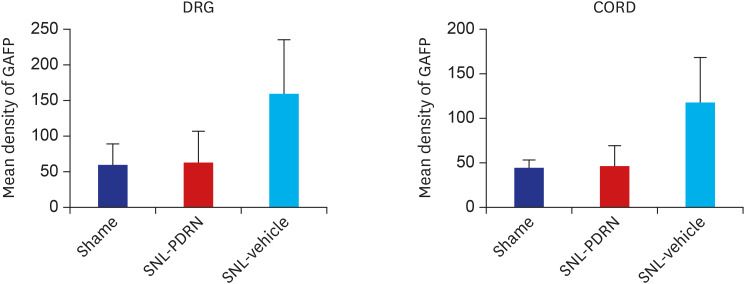
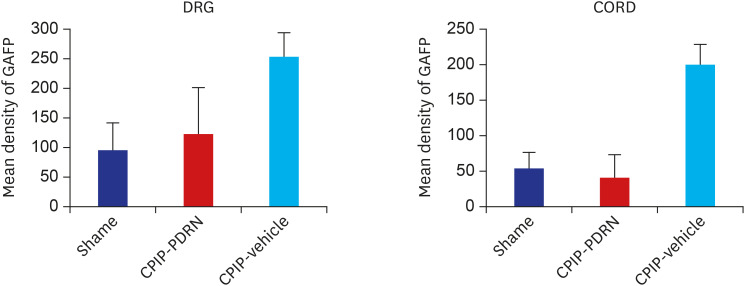
Fig. 4. Histogram representing the optical density of GFAP in DRG, spinal cord from sham (n = 4), PDRN-treated SNL mice (n = 6), and vehicle-treated SNL mice (n = 4). The expression of GFAP in the PDRN-treated group (DRG, 64.19 ± 46.21 optical density; spinal cord, 47.35 ± 22.12 optical density) was significantly decreased compared to the vehicle treated group (DRG, 169.13 ± 71.01 optical density; spinal cord, 118.01 ± 50.82 optical density).
GFAP = glial fibrillary acidic protein, DRG = dorsal root ganglia, PDRN = polydeoxyribonucleotide, SNL = spinal nerve ligation.
In the 20 mg PDRN-injected group of the CPIP model, the expression of GFAP in DRG (Fig. 5B) and the spinal cord (Fig. 5E) was significantly reduced as exhibited by the lower optical densities measured in the PDRN-injected group compared to the vehicle-injected group (Fig. 5C and F) (the P values of the DRG and spinal cord were P = 0.001 and P = 0.003 respectively). Between the sham and 20 mg PDRN-injected group, the expression of GFAP in the DRG and spinal cord was not statistically different (P values of the DRG and spinal cord was P = 0.67, P = 0.79). On the other hand, there was a significant difference between the sham and vehicle-injected groups (P vales of the DRG and spinal cord were respectively, P = 0.001, P < 0.001) (Fig. 6).
Fig. 5. The effect of subcutaneous PDRN on GFAP expression (luminous green) in DRG and spinal cord of CPIP model. Original magnification: ×200. (A) Immunostaining for GFAP in DRG of sham mouse. (B) PDRN-injected CPIP mouse. (C) Vehicle-injected CPIP mouse. (D) Immunostaining for GFAP in spinal cord of sham mouse. (E) PDRN-injected CPIP mouse. (F) Vehicle-injected CPIP mouse.
PDRN = polydeoxyribonucleotide, GFAP = glial fibrillary acidic protein, DRG = dorsal root ganglia, CPIP = chronic post-ischemic pain.
Fig. 6. Histogram representing the optical density of GFAP in DRG, spinal cord from sham (n = 4), PDRN-treated CPIP mice (n = 6), and vehicle-treated CPIP mice (n = 4). The expression of GFAP in the PDRN-treated group (DRG, 122.24 ± 78.00 optical density; spinal cord, 39.98 ± 32.34 optical density) was significantly lower than that in the vehicle-treated group (DRG, 253.35 ± 39.23 optical density; spinal cord, 200.23 ± 27.78 optical density).
GFAP = glial fibrillary acidic protein, DRG = dorsal root ganglia, CPIP = chronic post-ischemic pain, PDRN = polydeoxyribonucleotide.
DISCUSSION
Our findings revealed that SNL and CPIP models as neuropathic pain models yielded mechanical allodynia, which was then attenuated by the administration of PDRN. The increased GFAP in both types of neuropathic pain models was histologically proven by reduction of expression after PDRN administration.
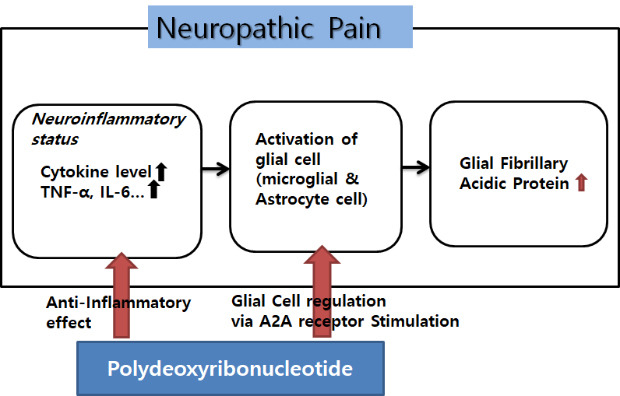
From previous studies, neuropathic pain or CRPS is associated predominantly with a neuro-inflammatory state. The inflammatory cytokine levels are up-regulated in CRPS. TNF-α, IL-1β, IL-6, and nerve growth factor levels are elevated.27,28 Cytokines are very potent proteins that function as cellular communicators. Not only are inflammatory cytokines increased in a neuropathic pain state, but also they present the ability to increase neural activity. This is a cascade of events following an inflammatory response, resulting in an activation of glial cells especially including microglia and astrocytes located in the spinal cord and brain, which demonstrate a prominent role in nociception.28,29,30 Upon activation, astrocytes proliferate, and increase expression of intermediate filaments such as GFAP.4,31,32,33 In a vicious cycle, the activated astrocytes release inflammatory stimulants such as cytokines, neurotrophic factors. These inflammatory materials result in the development and maintenance of central sensitization by altering the polarization characteristics of the afferent neurons and thus alter the pain transmission pathway. Astrocytes also directly alter neuronal connection because they completely encapsulate synapses and are in close contact with neuronal cells. The close astrocyte–neuron contact allows for astrocyte activation by neurotransmitters and neuromodulators.34,35,36 Furthermore, the activated astrocytes release inflammatory mediators such as TNF-α, which in turn activate other glial cells. Inflammatory pain begins with a peripheral inflammatory reaction at the site of injury, while neuropathic pain starts with a nerve injury or abnormal cascade that could be secondary to an inflammatory reaction.37,38,39 During the development of a neuropathic pain pathway, glial cells including astrocytes and inflammatory factors play a critical role.40,41 Therefore, treatment methods for neuropathic pain have been attempted to reduce the abnormal activation of inflammatory factors and glial cells.42 In this context, PDRN can be applied to CRPS, which shows the pattern of neuropathic pain.
It has been proposed that PDRN has the following main activities; tissue repairing, anti-ischemic, and anti-inflammatory. These positive effects of PDRN have been shown to be mainly due to a decrease in pro-inflammatory cytokines such as TNF-α and IL-6 and an increase in anti-inflammatory cytokines, IL-10. PDRN activities occur through binding of adenosine receptors, especially the A2A receptor, which plays a core role in modulating inflammation, oxygen consumption, ischemia/perfusion, cell regeneration, and angiogenesis.11,12,13,14,15,43 These actions did not only occur at the periphery, but at the spinal level, which can be found based on the changes in DRG and spinal cord in this experiment. Previous studies show that the concomitant application with an adenosine A2 receptor antagonist, 3,7-dimethyl-1-propargylxanthine abolish the effect of PDRN. These results prove that PDRN acts on via A2A receptor.11,16,44 Focusing on the fact that PDRN stimulates the A2A receptor, it could be presumed that PDRN has an effect on glial cells in the nervous system directly as well as cytokines. In previous studies, a spinal anti-nociceptive action of A2AR agonists occurred in nerve injury models. ATL313 (A2AR agonist) decreased the nerve injury-induced upregulation of markers for microglia and astrocytes in the spinal cord. ATL313 attenuated allodynia in nerve injury models such as SNL and sciatic inflammatory neuropathy and reduced it in PKA and PKC, as well as the TNF-α production by microglia and astrocytes.45,46,47 These results implied that A2AR agonists suppressed astrocyte and microglia activation. PDRN, exerting their pharmacological effect by interacting with the A2A receptor, is also assumed to reduce glial activation in the spinal cord and attenuates pro-inflammatory cytokine production induced in microglial cells at neuropathic pain sites. The immunohistochemical result that PDRN attenuated GFAP expression is proven by its action on glial cells, especially astrocytes.
This study still had some limitations. Inflammatory cytokine measurement was not performed in the present study, although the cause of GFAP reduction was thought to be based on anti-inflammatory cytokines. Based on the previous studies, it was described that the main mechanism of PDRN was performed by acting on the A2A receptor, but if concomitant application with an adenosine A2 receptor antagonist was performed, the mechanism of action would be more clearly clarified. We only observed the effect of PDRN on mice, the anti-allodynic effect on human should be further studied.
PDRN produces an anti-allodynic effect in neuropathic pain and CRPS models through behavior tests and an inhibitory action on astrocytes which could be activated in neuropathic pain states through immunohistochemical experiments. These results confirm the analgesic effect of PDRN in neuropathic pain and CRPS models, indicating that this compound might represent a new therapeutic approach to manage and reduce neuropathic pain.
Footnotes
Funding: This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (grant No. 2017R1D1A1B03028360).
Disclosure: All authors disclosed no conflicts of interest.
- Conceptualization: Park HJ.
- Data curation: Lee J, Lee SH.
- Formal analysis: Park HJ, Han D.
- Investigation: Cho EA, Jeon SH.
- Methodology: Lee SH, Yoo SH.
- Software: Jeon SH.
- Validation: Park HJ, Lee HJ.
- Writing - original draft: Lee SH.
- Writing - review & editing: Park HJ.
References
- 1.Zilliox LA. Neuropathic pain. Continuum (Minneap Minn) 2017;23(2):512–532. doi: 10.1212/CON.0000000000000462. [DOI] [PubMed] [Google Scholar]
- 2.Taha R, Blaise GA. Update on the pathogenesis of complex regional pain syndrome: role of oxidative stress. Can J Anaesth. 2012;59(9):875–881. doi: 10.1007/s12630-012-9748-y. [DOI] [PubMed] [Google Scholar]
- 3.Mrabet D, Khemiri C, Ben Mrad I, Mrabet H, Essaddem H, Amel M, et al. Pathophysiology of complex regional pain syndrome (CRPS) type 1. Tunis Med. 2012;90(4):278–281. [PubMed] [Google Scholar]
- 4.Yang L, Wang SH, Hu Y, Sui YF, Peng T, Guo TC. Effects of repetitive transcranial magnetic stimulation on astrocytes proliferation and nNOS expression in neuropathic pain rats. Curr Med Sci. 2018;38(3):482–490. doi: 10.1007/s11596-018-1904-3. [DOI] [PubMed] [Google Scholar]
- 5.Garrison CJ, Dougherty PM, Kajander KC, Carlton SM. Staining of glial fibrillary acidic protein (GFAP) in lumbar spinal cord increases following a sciatic nerve constriction injury. Brain Res. 1991;565(1):1–7. doi: 10.1016/0006-8993(91)91729-k. [DOI] [PubMed] [Google Scholar]
- 6.Shen J, Huo BB, Zheng MX, Hua XY, Shen H, Lu YC, et al. Evaluation of neuropathic pain in a rat model of total brachial plexus avulsion from behavior to brain metabolism. Pain Physician. 2019;22(3):E215–24. [PubMed] [Google Scholar]
- 7.Vallejo R, Tilley DM, Vogel L, Benyamin R. The role of glia and the immune system in the development and maintenance of neuropathic pain. Pain Pract. 2010;10(3):167–184. doi: 10.1111/j.1533-2500.2010.00367.x. [DOI] [PubMed] [Google Scholar]
- 8.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306(2):624–630. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 9.Bitto A, Galeano M, Squadrito F, Minutoli L, Polito F, Dye JF, et al. Polydeoxyribonucleotide improves angiogenesis and wound healing in experimental thermal injury. Crit Care Med. 2008;36(5):1594–1602. doi: 10.1097/CCM.0b013e318170ab5c. [DOI] [PubMed] [Google Scholar]
- 10.Galeano M, Bitto A, Altavilla D, Minutoli L, Polito F, Calò M, et al. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008;16(2):208–217. doi: 10.1111/j.1524-475X.2008.00361.x. [DOI] [PubMed] [Google Scholar]
- 11.Bitto A, Polito F, Altavilla D, Minutoli L, Migliorato A, Squadrito F. Polydeoxyribonucleotide (PDRN) restores blood flow in an experimental model of peripheral artery occlusive disease. J Vasc Surg. 2008;48(5):1292–1300. doi: 10.1016/j.jvs.2008.06.041. [DOI] [PubMed] [Google Scholar]
- 12.Valdatta L, Thione A, Mortarino C, Buoro M, Tuinder S. Evaluation of the efficacy of polydeoxyribonucleotides in the healing process of autologous skin graft donor sites: a pilot study. Curr Med Res Opin. 2004;20(3):403–408. doi: 10.1185/030079904125003116. [DOI] [PubMed] [Google Scholar]
- 13.Koo Y, Yun Y. Effects of polydeoxyribonucleotides (PDRN) on wound healing: Electric cell-substrate impedance sensing (ECIS) Mater Sci Eng C. 2016;69:554–560. doi: 10.1016/j.msec.2016.06.094. [DOI] [PubMed] [Google Scholar]
- 14.Vanelli R, Costa P, Rossi SM, Benazzo F. Efficacy of intra-articular polynucleotides in the treatment of knee osteoarthritis: a randomized, double-blind clinical trial. Knee Surg Sports Traumatol Arthrosc. 2010;18(7):901–907. doi: 10.1007/s00167-009-1039-y. [DOI] [PubMed] [Google Scholar]
- 15.Guizzardi S, Galli C, Govoni P, Boratto R, Cattarini G, Martini D, et al. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: a new proposal for bone tissue repair. Life Sci. 2003;73(15):1973–1983. doi: 10.1016/s0024-3205(03)00547-2. [DOI] [PubMed] [Google Scholar]
- 16.Bitto A, Polito F, Irrera N, D'Ascola A, Avenoso A, Nastasi G, et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A(2A) receptor. Arthritis Rheum. 2011;63(11):3364–3371. doi: 10.1002/art.30538. [DOI] [PubMed] [Google Scholar]
- 17.Bitto A, Oteri G, Pisano M, Polito F, Irrera N, Minutoli L, et al. Adenosine receptor stimulation by polynucleotides (PDRN) reduces inflammation in experimental periodontitis. J Clin Periodontol. 2013;40(1):26–32. doi: 10.1111/jcpe.12010. [DOI] [PubMed] [Google Scholar]
- 18.Pallio G, Bitto A, Pizzino G, Galfo F, Irrera N, Squadrito F, et al. Adenosine receptor stimulation by polydeoxyribonucleotide improves tissue repair and symptomology in experimental colitis. Front Pharmacol. 2016;7:273. doi: 10.3389/fphar.2016.00273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ko IG, Kim SE, Jin JJ, Hwang L, Ji ES, Kim CJ, et al. Combination therapy with polydeoxyribonucleotide and proton pump inhibitor enhances therapeutic effectiveness for gastric ulcer in rats. Life Sci. 2018;203:12–19. doi: 10.1016/j.lfs.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 20.Ye GL, Savelieva KV, Vogel P, Baker KB, Mason S, Lanthorn TH, et al. Ligation of mouse L4 and L5 spinal nerves produces robust allodynia without major motor function deficit. Behav Brain Res. 2015;276:99–110. doi: 10.1016/j.bbr.2014.04.039. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50(3):355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- 22.Choi BM, Lee SH, An SM, Park DY, Lee GW, Noh GJ. The time-course and RNA interference of TNF-α, IL-6, and IL-1β expression on neuropathic pain induced by L5 spinal nerve transection in rats. Korean J Anesthesiol. 2015;68(2):159–169. doi: 10.4097/kjae.2015.68.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004;112(1-2):94–105. doi: 10.1016/j.pain.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 24.Ryu TH, Jung KY, Ha MJ, Kwak KH, Lim DG, Hong JG. Superoxide and nitric oxide involvement in enhancing of N-methyl-D-aspartate receptor-mediated central sensitization in the chronic post-ischemia pain model. Korean J Pain. 2010;23(1):1–10. doi: 10.3344/kjp.2010.23.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 26.Bonin RP, Bories C, De Koninck Y. A simplified up-down method (SUDO) for measuring mechanical nociception in rodents using von Frey filaments. Mol Pain. 2014;10:26. doi: 10.1186/1744-8069-10-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.David Clark J, Tawfik VL, Tajerian M, Kingery WS. Autoinflammatory and autoimmune contributions to complex regional pain syndrome. Mol Pain. 2018;14:1744806918799127. doi: 10.1177/1744806918799127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlereth T, Drummond PD, Birklein F. Inflammation in CRPS: role of the sympathetic supply. Auton Neurosci. 2014;182:102–107. doi: 10.1016/j.autneu.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 29.Martínez-Lavín M, Vargas A, Silveira LH, Amezcua-Guerra LM, Martínez-Martínez LA, Pineda C. Complex regional pain syndrome evolving to full-blown fibromyalgia: a proposal of common mechanisms. J Clin Rheumatol. 2020 doi: 10.1097/RHU.0000000000001304. Forthcoming. [DOI] [PubMed] [Google Scholar]
- 30.Russo M, Georgius P, Santarelli DM. A new hypothesis for the pathophysiology of complex regional pain syndrome. Med Hypotheses. 2018;119:41–53. doi: 10.1016/j.mehy.2018.07.026. [DOI] [PubMed] [Google Scholar]
- 31.Hansson E. Could chronic pain and spread of pain sensation be induced and maintained by glial activation? Acta Physiol (Oxf) 2006;187(1-2):321–327. doi: 10.1111/j.1748-1716.2006.01568.x. [DOI] [PubMed] [Google Scholar]
- 32.Milligan ED, Watkins LR. Pathological and protective roles of glia in chronic pain. Nat Rev Neurosci. 2009;10(1):23–36. doi: 10.1038/nrn2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32(5):972–983. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 34.Broadhead MJ, Miles GB. Bi-directional communication between neurons and astrocytes modulates spinal motor circuits. Front Cell Neurosci. 2020;14:30. doi: 10.3389/fncel.2020.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ma Z, Stork T, Bergles DE, Freeman MR. Neuromodulators signal through astrocytes to alter neural circuit activity and behaviour. Nature. 2016;539(7629):428–432. doi: 10.1038/nature20145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ben Achour S, Pascual O. Glia: the many ways to modulate synaptic plasticity. Neurochem Int. 2010;57(4):440–445. doi: 10.1016/j.neuint.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 37.Kwilasz AJ, Ellis A, Wieseler J, Loram L, Favret J, McFadden A, et al. Sustained reversal of central neuropathic pain induced by a single intrathecal injection of adenosine A2A receptor agonists. Brain Behav Immun. 2018;69:470–479. doi: 10.1016/j.bbi.2018.01.005. [DOI] [PubMed] [Google Scholar]
- 38.Luongo L, Salvemini D. Targeting metabotropic adenosine receptors for neuropathic pain: focus on A2A. Brain Behav Immun. 2018;69:60–61. doi: 10.1016/j.bbi.2018.02.014. [DOI] [PubMed] [Google Scholar]
- 39.Sawynok J. Adenosine receptor targets for pain. Neuroscience. 2016;338:1–18. doi: 10.1016/j.neuroscience.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 40.Zhang ZJ, Jiang BC, Gao YJ. Chemokines in neuron-glial cell interaction and pathogenesis of neuropathic pain. Cell Mol Life Sci. 2017;74(18):3275–3291. doi: 10.1007/s00018-017-2513-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Old EA, Malcangio M. Chemokine mediated neuron-glia communication and aberrant signalling in neuropathic pain states. Curr Opin Pharmacol. 2012;12(1):67–73. doi: 10.1016/j.coph.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Ren K, Dubner R. Neuron-glia crosstalk gets serious: role in pain hypersensitivity. Curr Opin Anaesthesiol. 2008;21(5):570–579. doi: 10.1097/ACO.0b013e32830edbdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polito F, Bitto A, Galeano M, Irrera N, Marini H, Calò M, et al. Polydeoxyribonucleotide restores blood flow in an experimental model of ischemic skin flaps. J Vasc Surg. 2012;55(2):479–488. doi: 10.1016/j.jvs.2011.07.083. [DOI] [PubMed] [Google Scholar]
- 44.Thellung S, Florio T, Maragliano A, Cattarini G, Schettini G. Polydeoxyribonucleotides enhance the proliferation of human skin fibroblasts: involvement of A2 purinergic receptor subtypes. Life Sci. 1999;64(18):1661–1674. doi: 10.1016/s0024-3205(99)00104-6. [DOI] [PubMed] [Google Scholar]
- 45.Loram LC, Taylor FR, Strand KA, Harrison JA, Rzasalynn R, Sholar P, et al. Intrathecal injection of adenosine 2A receptor agonists reversed neuropathic allodynia through protein kinase (PK)A/PKC signaling. Brain Behav Immun. 2013;33:112–122. doi: 10.1016/j.bbi.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Puerto A, Wandosell F, Garrido JJ. Neuronal and glial purinergic receptors functions in neuron development and brain disease. Front Cell Neurosci. 2013;7:197. doi: 10.3389/fncel.2013.00197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kucher BM, Neary JT. Bi-functional effects of ATP/P2 receptor activation on tumor necrosis factor-alpha release in lipopolysaccharide-stimulated astrocytes. J Neurochem. 2005;92(3):525–535. doi: 10.1111/j.1471-4159.2004.02885.x. [DOI] [PubMed] [Google Scholar]