Abstract
A novel phosphine-catalyzed intermolecular cyclization between 2-sulfonamidobenzaldehyes and ynones is reported. This methodology serves as a conduit for the construction of benzo[b]azepin-3-ones in good to excellent yields under mild conditions. The resulting 2-benzylidene moieties are formed exclusively in the E-configuration. Mechanistically, this unusual annulation occurs through a phosphine-catalyzed α-umpolung addition, followed by an aldol reaction. One of the benzo[b]azepin-3-one products was converted to the core structure of 3-amino-[a]benzazepin-2-one-1-alkanoic acids, many of which function as angiotensin-converting enzyme inhibitors.
Graphical Abstract

Benzoazepines are structural motifs within a wide variety of biologically and pharmacologically significant compounds.1 In particular, a benzo[b]azepine moiety is a core unit of several marketed tricyclic antidepressant drugs, including tienopramine, anafranil, amezepine, and imipramine (Figure 1).2 Lotensin, which features a benzoazepine nucleus as the key subunit, is used in the treatment of hypertension, congestive heart failure, and heart attacks, and also to prevent the renal and retinal complications of diabetes.3 Mozavaptan, which also possesses a benzoazepine skeleton, is an effective vasopressin V-2 receptor antagonist.4 Despite their promising biological properties, benzo[b]azepines have drawn relatively little synthetic attention. Nevertheless, several methods have been described for the construction of benzo[b]azepines, including approaches through intramolecular Heck coupling,5 iridium-catalyzed asymmetric allylic amination,6 cyclizations of suitably substituted Morita–Baylis–Hillman adducts,7 FeCl3− and Au-catalyzed cyclizations of alkynes and alkenes,8 Pd-catalyzed oxidative annulations of ortho-alkenylanilines and allenes,9 and Pd- and Au-catalyzed ring-expansions of alkylidenecyclopropanes.10 Despite the great potential of these methods, they require harsh reaction conditions and/or starting materials that are synthesized in several steps. In this regard, we became interested in developing new pathways for the synthesis of benzoazepines from readily available starting materials under benign reaction conditions.
Figure 1.

Representative marketed drugs containing benzo[b]azepine core structures.
Nucleophilic phosphine-catalyzed cyclizations have expanded chemical space by allowing access to a variety of carbo- and heterocycles.11 While the use of electron-deficient alkenes, allenes, and alkynoates in those reactions has been explored widely,12 the application of α,β-ynones in phosphine-catalyzed annulations has drawn scant attention until quite recently. The products of ynone annulations can be synthetically useful and biologically important compounds, including oxazolidines,13a thiazolidines,13a pyrrolidines,13a dihydropyrrolopyridines,13b benzimidazoline,13b benzomorpholines,13b tetrahydroquinolines, 3-oxanones, 3-oxepanones, spirooxazolines, spirooxindoles,13e spirocyclopentanones,13f spiro-cyclopentanone pyrazolones,13g cyclopent[b]annulated heteroarenes,13h hydropyridazines,13i cyclopentanone-fused benzosultams,13j and spirobarbiturate-cyclopentanones.13k In almost all of these examples, the α,β-ynone provides two- or three-carbon atoms for the formation ofcommon five- or six-membered rings; to the best of our knowledge, there has been only one report of seven-membered ring formation.14
Considering these precedents and based on our previously described tandem γ-umpolung addition–Wittig olefination,15 we envisioned the possibility of synthesizing the dihydroquinoline 3′ through α-umpolung addition–Wittig alkenylation (Scheme 1). We found, however, that a solution of o-(p-toluenesulfonamido)benzaldehyde (1a) and the α,β-ynone 2a in toluene produced, in the presence of 1 equiv of PPh3, the benzo[b]azepin-3-one 3a in 38% yield. We identified the E-configuration of this benzylidene moiety through NOESY analysis of the product 3a.
Scheme 1.

Discovery of α-Umpolung–Aldol Reaction
As displayed in Scheme 2, we suspect that the mechanism begins with nucleophilic addition of PPh3 to the ynone 2a to generate the zwitterion I. The intermediate I subsequently deprotonates the sulfonamide 1a, with α-umpolung addition of the resulting amide anion onto the vinylphosphonium species producing the ylide II. Proton transfer delivers the enolate III, which is ready for the aldol reaction with the aldehydic unit to construct the benzoazapin-3-one ring IV. Alkoxide-to-enolate proton transfer and subsequent β-elimination of the catalyst (PPh3) furnishes the product 3a. The exclusive formation of the E-benzylidene moiety is due, we believe, to steric repulsion between the p-toluenesulfonyl group and the phenyl ring when forming the alternative Z-isomer. Although there have been reported α-umpolung additions16 and tandem reactions involving α-umpolung addition,17 to the best of our knowledge there are no previous reports of phosphine-catalyzed α-umpolung-aldol processes. Herein, we describe our study of this cascade process for the synthesis of benzo[b]azapin-3-ones through a phosphine-catalyzed α-umpolung-aldol reaction.
Scheme 2.

Proposed Mechanism for the Formation of the Benzo[b]azepin-3-one 3a
Recognizing that the α-umpolung–aldol reaction is catalytic in PPh3, we tested various catalysis conditions for the α-umpolung–aldol reaction, engaging ethyl o-(p-toluenesulfon-amido)benzaldehyde (1a) and the ynone 2a as model substrates and employing various tertiary phosphines as catalysts at 20 mol%. We first screened various solvents in the presence of 20 mol % of PPh3 at room temperature (Table 1). The product benzo[b]azepin-3-one 3a was obtained in 10–42% yields in toluene, CH2Cl2, CHCl3, and benzene (entries 1–4), but no product formed in tetrahydrofuran (THF), MeCN, or dioxane (entries 5–7). Noting that CHCl3, with its acidic proton, was a particularly efficacious medium, we tested other protic solvents—alcohols, which could be deprotonated by the zwitterionic phosphonium enolate intermediates to promote the aldol reaction—for this reaction.18 Interestingly, the Michael addition product 3” was generated in 83% yield when using MeOH as the solvent (entry 8).19 In contrast, EtOH and n-propanol enhanced the yield of the α-umpolung–aldol reaction to 58 and 69%, respectively (entries 9 and 10). n-Butanol could be used as the solvent without increasing the yield, but no significant product formed when running the reaction in isopropanol or tert-butanol (entries 11 and 13). Furthermore, we examined the effects of other phosphine catalysts (entries 14–17). Performing the reaction in the presence of tributylphosphine, a more nucleophilic catalyst, did not provide any product, but the yield increased to 74% when employing ethyldiphenylphosphine as the catalyst. Attempts to modulate the electronic properties of PPh3 by using tris(p-fluorophenyl)-phosphine or tris(p-tolyl)phosphine had no significant benefits, supplying the benzo[b]azapin-3-one in yields of 69 and 54%, respectively.
Table 1.
optimization of the Reaction Conditionsa
 | ||||
|---|---|---|---|---|
| entry | phosphine | solvent | temp. (°C) | yield (%)b |
| 1 | Ph3P | toluene | rt | 36 |
| 2 | Ph3P | CH2Cl2 | rt | 10 |
| 3 | Ph3P | CHCl3 | rt | 42 |
| 4 | Ph3P | benzene | rt | 14 |
| 5 | Ph3P | THF | rt | NR |
| 6 | Ph3P | MeCN | rt | NR |
| 7 | Ph3P | dioxane | rt | NR |
| 8 | Ph3P | MeOH | rt | 3″(83%)c |
| 9 | Ph3P | EtOH | rt | 58 |
| 10 | Ph3P | n-propanol | rt | 69 |
| 11 | Ph3P | isopropanol | rt | NR |
| 12 | Ph3P | n-butanol | rt | 40 |
| 13 | Ph3P | tert-butanol | rt | NR |
| 14 | n-Bu3P | n-propanol | rt | NR |
| 15 | EtPh2P | n-propanol | rt | 74 |
| 16 | (p-FC6H4)3P | n-propanol | rt | 69 |
| 17 | (p-MeC6H4)3P | n-propanol | rt | 54 |
Reaction conditions: 1a (0.2 mmol), 2a (0.3 mmol), phosphine (0.04 mmol), and solvent (2 mL) at room temperature.
Isolated yield of 3a.
3″ = 4-methoxy-4-phenyl-3-buten-2-one.
Hereafter, employing ethyldiphenylphosphine as the catalyst in n-propanol as the solvent, our focus shifted to applying various other o-sulfonamidobenzaldehydes 1 and ynones 2 for the synthesis of a range of benzoazapinones 3 (Table 2). Initially, we studied the effects of varying the substituents on the benzene ring of the o-sulfonamidobenzaldehydes in reactions with the ynone 2a. Electron-donating 5-methoxy and 5-methyl substituents on the benzene ring generated the desired products 3b and 3c in yields of 82 and 77%, respectively, while N-(3-formylnaphth-2-yl)-4-methylbenzenesulfonamide provided the benzo[b]azapin-3-one 3d in 70% yield (entries 2–4). Electron-withdrawing groups, namely, 5-iodo, 5-bromo, and 5-chloro units, on the benzene ring induced the formation of the desired products 3e–g in yields of 70–75% (entries 5–7). When we employed the 3-methyl-substituted 2-sulfonamidobenzaldehyde as the pronucleophile, no reaction occurred, presumably because of steric effects (entry 8). Performing the reaction with 4-methyl-2-sulfonamidobenzaldehyde delivered the benzoazapin-3-one 3h in 72% yield (entry 9). Next, we probed the effects of various protecting groups of the aniline functionality. N-(2-Formylphenyl)benzenesulfonamide, N-(2-formylphenyl)-4-methoxybenzenesulfonamide, and N-(2-formylphenyl)-4-bromobenzenesulfonamide produced their desired products 3i–k, respectively, in moderate yields (59–63%, entries 10–12).
Table 2.
Synthesis of Benzo[b]azapin-3-onesa
 | ||||
|---|---|---|---|---|
| entry | R1 | R2 | R3 | yield (%)b |
| 1 | H | Ts | H | 3a, 74 |
| 2 | 5-MeO | Ts | H | 3b, 82 |
| 3 | 5-Me | Ts | H | 3c, 77 |
| 4 | 1dc | Ts | H | 3d, 70 |
| 5 | 5-I | Ts | H | 3e, 75 |
| 6 | 5-Br | Ts | H | 3f, 73 |
| 7 | 5-Cl | Ts | H | 3g, 70 |
| 8 | 3-Me | Ts | H | NR |
| 9 | 4-Me | Ts | H | 3h, 72 |
| 10 | H | PhSO2 | H | 3i, 63 |
| 11 | H | (4-MeOC6H4)SO2 | H | 3j, 65 |
| 12 | H | (4-BrC6H4)SO2 | H | 3k, 59 |
| 13 | H | Ts | 4-MeO | 3l, 94 (83)d |
| 14 | 5-MeO | Ts | 4-MeO | 3m, 99 |
| 15 | 5-Me | Ts | 4-MeO | 3n, 95 |
| 16 | H | Ts | 4-Me | 3o, 91 |
| 17 | 5-MeO | Ts | 4-Me | 3p, 93 |
| 18 | H | Ts | 3-Me | 3q, 85 |
| 19 | 5-MeO | Ts | 3-Me | 3r, 81 |
| 20 | H | Ts | 2-Me | NR |
| 21 | H | Ts | 4-Cl | 3s, 75 |
Reaction conditions: 1 (0.2 mmol), 2 (0.3 mmol), phosphine (0.04 mmol), and n-propanol (2 mL) at room temperature.
Isolated yields.
1d = N-(3-formylnaphth-2-yl)-4-methylbenzenesulfbnamide.
Reaction run on 1 mmol scale.
We examine a range of ynones for the ethyldiphenylphosphine-catalyzed α-umpolung–aldol reaction. An electron-donating group on the benzene ring of the ynone 2 seemed crucial for a highly efficient reaction. When 4-(4-methoxyphenyl)but-3-yn-2-one was involved in the annulation with 1a, the desired product was obtained in 94% yield (entry 13). When run on 1 mmol scale, the reaction produced the product 3l in 83% yield. The combination of electron-rich o-sulfonamidobenzaldehydes and 4-(4-methoxyphenyl)but-3-yn-2-one produced the desired benzo[b]azapin-3-ones 3m and 3n in the highest yields of this study: 95 and 99%, respectively (entries 14 and 15). Following this trend, 4-(4-methylphenyl)but-3-yn-2-one, when reacted with N-(2-formylphenyl)-4-methylbenzenesulfonamide and N-(2-formyl-4-methoxyphenyl)-4-methylbenzenesulfonamide, also provided high yields of the benzoazapin-3-ones 3o and 3p (91 and 93%, respectively; entries 16 and 17). Conducting the reactions of 4-(3-methylphenyl)but-3-yn-2-one with N-(2-formylphenyl)-4-methylbenzenesulfonamide and N-(2-formyl-4-methoxyphenyl)-4-methylbenzenesulfonamide gave the desired products 3q and 3r in slightly lower yields (85 and 81%, respectively; entries 18 and 19). When we employed 4-(2-methylphenyl)but-3-yn-2-one, however, no reaction occurred, presumably because the o-methyl group on the benzene ring of the alkynone blocked the nucleophilic addition of ethyldiphenylphosphine to the β-carbon atom of the α,β-ynone (entry 20). When an electron-withdrawing chlorine atom was positioned on the phenyl ring of the ynone, the corresponding product 3s was furnished in 75% yield (entry 21). Interestingly, when 1,4-diphenylbut-3-yn-2-one was subjected to the standard reaction conditions, we obtained the 1,4-dihydroquinoline 4 instead of the benzo[b]azapin-3-one (eq 1).20
 |
(1) |
Applying this α-umpolung–aldol technology, we realized the rapid synthesis of the 3-amino-benzo[b]-azepin-2-one skeleton–the core unit of 3-amino-[a]benzazepin-2-one-1-alkanoic acids that are known angiotensin-converting enzyme (ACE) inhibitors.21 Starting from the benzo[b]azapin-3-one 3a, diastereoselective reductive amination generated the syn-aminoalcohol 5, whose benzylidene motif was readily converted to a C=O group through ozonolysis to obtain the 3-amino-[a]benzazepin-2-one 6 (Scheme 3).
Scheme 3.
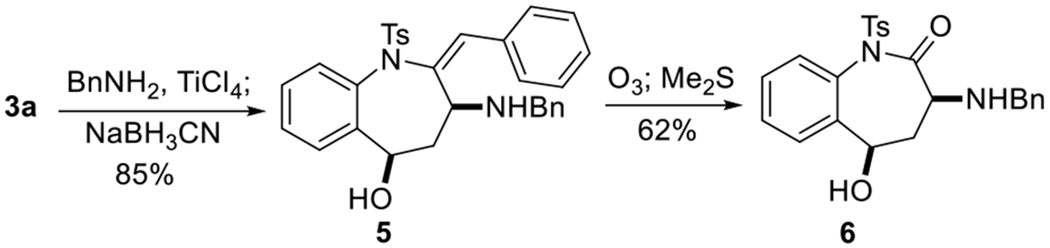
Synthetic Application of the α-Umpolung–Aldol Reaction
In conclusion, we have developed a phosphine-catalyzed intermolecular cyclization of o-sulfonamidobenzaldehydes and ynones to yield highly functionalized (E)-benzo[b]azapin-3-ones under mild reaction conditions at room temperature. This α-umpolung–aldol process provides a range of benzo[b]azapin-3-ones 3 in synthetically useful yields and with exclusive E-selectivity. In the further functionalization of 3a, we found that compound 6 could be constructed rapidly through reductive amination and ozonolysis, providing an alternative means toward the skeleton of 3-amino-[a]benzazepin-2-one-1-alkanoic acids that are useful ACE inhibitors.
Supplementary Material
ACKNOWLEDGMENTS
We thank the NIH (GM071779) for financial support.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.orglett.9b01749.
Experimental procedures and characterization data for all new compounds (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Albright JD; Reich MF; Delos Santos EG; Dusza JP; Sum FW; Venkatesan AM; Coupet J; Chan PS; Ru X; Mazandarani H; Bailey TJ Med. Chem 2000, 43, 4388. [DOI] [PubMed] [Google Scholar]; (b) Le Diguarher T; Ortuno JC; Shanks D; Guilbaud N; Pierre A; Raimbaud E; Fauchere JL; Hickman JA; Tucker GC; Casara P Bioorg. Med. Chem. Lett 2004, 14, 767. [DOI] [PubMed] [Google Scholar]; (c) Failli AA; Shumsky JS; Steffan RJ; Caggiano TJ; Willoams DK; Trybulski EJ; Ning X; Lock Y; Tanikella T; Hartmann D; Chan PS; Park CH Bioorg. Med. Chem. Lett 2006, 16, 954. [DOI] [PubMed] [Google Scholar]
- (2).Singh H; Gupta N; Kumar P; Dubey SK; Sharma PK Org. Process Res. Dev 2009, 13, 870. [Google Scholar]
- (3).(a) Hou F; Zhang X; Zhang G; Xie D; Chen P; Zhang W; Jiang J; Liang M; Wang G; Liu Z; Geng RN N. Engl. J. Med 2006, 354, 131. [DOI] [PubMed] [Google Scholar]; (b) O’Grady MR; O’Sullivan ML; Minors SL; Horne RJ Vet. Intern. Med 2009, 23, 977. [DOI] [PubMed] [Google Scholar]
- (4).(a) Decaux G; Soupart A; Vassart G Lancet 2008, 371, 1624. [DOI] [PubMed] [Google Scholar]; (b) Robertson GL Nat. Rev. Endocrinol 2011, 7, 151. [DOI] [PubMed] [Google Scholar]; (c) Ghose AK; Herbertz T; Hudkins RL; Dorsey BD; Mallamo JP ACS Chem. Neurosci 2012, 3, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Qadir M; Cobb J; Sheldrake WP; Whittall N; White AJP; Hill KK; Horton PN; Hursthouse MB J. Org. Chem 2005, 70, 1545. [DOI] [PubMed] [Google Scholar]
- (6).(a) He H; Liu W; Dai L; You S Angew. Chem. Int. Ed 2010, 49,1496. [DOI] [PubMed] [Google Scholar]; (b) Ye K; Dai L; You S Org. Biomol Chem 2012,10, 5932. [DOI] [PubMed] [Google Scholar]
- (7).(a) Singh V; Batra S Eur. J. Org. Chem. 2007, 2007, 2970 [Google Scholar]; (b) Zhan G; Shi M; He Q; Du W; Chen Y Org. Lett 2015, 17, 4750. [DOI] [PubMed] [Google Scholar]
- (8).(a) Cui L; Zhang G; Peng Y; Zhang L Org. Lett 2009, 11, 1225. [DOI] [PubMed] [Google Scholar]; (b) Jalal S; Bera K; Sarkar S; Paul K; Jana U Org. Biomol. Chem 2014, 12, 1759. [DOI] [PubMed] [Google Scholar]; (c) Undeela S; Ravikumar G; Nanubolu JB; Singarapu KK; Menon RS Chem. Commun 2016, 52, 4824. [DOI] [PubMed] [Google Scholar]
- (9).(a) Wu L; Meng Y; Ferguson J; Wang L; Zeng FJ Org. Chem 2017, 82, 4121. [DOI] [PubMed] [Google Scholar]; (b) Dhandabani GK; Mutra MR; Wang J Adv. Synth. Catal 2018, 360, 4754. [Google Scholar]
- (10).(a) Yu L; Zhu Z; Hu X; Tang X; Shi M Chem. Commun 2016, 52,6581. [DOI] [PubMed] [Google Scholar]; (b) Yu L; Xu Q; Tang X; Shi M ACS Catal. 2016, 6, 526. [Google Scholar]
- (11).For selective examples, see:; (a) Andrews IP; Kwon O Chem. Sci 2012, 3, 2510. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Cai L; Zhang K; Kwon O J. Am. Chem. Soc 2016, 138, 3298. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Li J; Zhang W; Zhang F; Chen Y; Li A J. Am. Chem. Soc 2017,139,14893. [DOI] [PubMed] [Google Scholar]; (d) Chen X; Zhang H; Yang X; Lv H; Shao X; Tao C; Wang H; Cheng B; Li Y; Guo J; Zhang J; Zhai H Angew. Chem. Int. Ed 2018, 57, 947. [DOI] [PubMed] [Google Scholar]
- (12).For selected reviews, see:; (a) Lu X; Zhang C; Xu Z Acc. Chem. Res 2001, 34, 535. [DOI] [PubMed] [Google Scholar]; (b) Valentine DH Jr.; Hillhouse JH Synthesis 2003, 2003, 317 [Google Scholar]; (c) Methot JL; Roush WR Adv. Synth. Catal 2004, 346,1035 [Google Scholar]; (d) Ye L-W; Zhou J; Tang Y Chem. Soc. Rev 2008, 37, 1140. [DOI] [PubMed] [Google Scholar]; (e) Cowen BJ; Miller SJ Chem. Soc. Rev 2009, 38, 3102. [DOI] [PubMed] [Google Scholar]; (f) Lopez F; Mascarenas JL Chem. - Eur. J 2011, 17, 418. [DOI] [PubMed] [Google Scholar]; (g) Zhao Q-Y; Lian Z; Wei Y; Shi M Chem. Commun 2012, 48, 1724. [DOI] [PubMed] [Google Scholar]; (h) Fan YC; Kwon O Chem. Commun 2013, 49, 11588. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Xu S; He Z RSC Adv. 2013, 3, 16885 [Google Scholar]; (j) Wang Z; Xu X; Kwon O Chem. Soc. Rev 2014, 43, 2927. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Li W; Zhang J Chem. Soc. Rev 2016, 45, 1657. [DOI] [PubMed] [Google Scholar]; (l) Wei Y; Shi M Org. Chem. Front 2017, 4, 1876 [Google Scholar]; (m) Li H; Lu Y Asian J. Org. Chem 2017, 6, 1130 [Google Scholar]; (n) Ni H; Chan W; Lu Y Chem. Rev 2018, 118, 9344. [DOI] [PubMed] [Google Scholar]; (o) Guo H; Fan YC; Sun Z; Wu Y; Kwon O Chem. Rev 2018, 118, 10049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).(a) Sriramurthy V; Kwon O J. Am. Chem. Soc 2007, 129, 12928. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Sriramurthy V; Kwon O Org. Lett 2010, 12, 1084. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Saha J; Lorenc C; Surana B; Peczuh MW J. Org. Chem 2012, 77, 3846. [DOI] [PubMed] [Google Scholar]; (d) Yang L; Xie P; Li E; Li X; Huang Y; Chen R Org. Biomol. Chem 2012, 10, 7628. [DOI] [PubMed] [Google Scholar]; (e) Ramachary DB; Venkaiah C; Krishna PM Org. Lett 2013, 15, 4714. [DOI] [PubMed] [Google Scholar]; (f) Liang L; Li E; Xie P; Huang Y Chem. -Asian J 2014, 9, 1270. [DOI] [PubMed] [Google Scholar]; (g) Li J; Du D Adv. Synth. Catal 2015, 357, 3986. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Raghu M; Grover J; Ramasastry SSV Chem. -Eur. J 2016, 22, 18316. [DOI] [PubMed] [Google Scholar]; (i) Liang L; Huang Y Org. Lett 2016, 18, 2604. [DOI] [PubMed] [Google Scholar]; (j) Ramachary DB; Krishna PM; Reddy TP Org. Biomol. Chem 2016, 14, 6413. [DOI] [PubMed] [Google Scholar]; (k) Gao X; Li Z; Yang W; Liu Y; Chen W; Zhang C; Zheng L; Guo H Org. Biomol. Chem 2017, 15, 5298. [DOI] [PubMed] [Google Scholar]
- (14).Sun L; Wei Y; Shi M Adv. Synth. Catal 2017, 359, 3176. [Google Scholar]
- (15).Zhang K; Cai L; Yang Z; Houk KN; Kwon O Chem. Sci 2018, 9, 1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Trost BM; Dake GR J. Am. Chem. Soc 1997, 119, 7595 [Google Scholar]; (b) Zheng F; Leung T-F; Chan K-W; Sung HHY; Williams ID; Xie Z; Jia G Chem. Commun 2016, 52, 10767. [DOI] [PubMed] [Google Scholar]
- (17).(a) Liu B; Davis R; Joshi B; Reynolds DW J. Org. Chem 2002, 67, 4595. [DOI] [PubMed] [Google Scholar]; (b) Lu C; Lu X Org. Lett 2002, 4, 4677. [DOI] [PubMed] [Google Scholar]; (c) Kuroda H; Tomita I; Endo T Org. Lett 2003,5,129. [DOI] [PubMed] [Google Scholar]; (d) Yavari I; Moradi L Tetrahedron Lett. 2006, 47, 1627 [Google Scholar]; (e) Yavari I; Souri S; Sirouspour M; Djahaniani H Synthesis 2006, 2006,3243 [Google Scholar]; (f) Gabillet S; LecercK D ; Loreau O; Carboni M; Dezard S; Gomis J-M; Taran F Synthesis 2007, 2007, 515 [Google Scholar]; (g) Gabillet S; Lecercle D; Loreau O; Carboni M; Dezard S; Gomis J-M; Taran F Org. Lett 2007, 9, 3925. [DOI] [PubMed] [Google Scholar]; (h) Alizadeh A; Sheikhi E Tetrahedron Lett. 2007, 48, 4887 [Google Scholar]; (i) Carboni M; Gomis J-M; Loreau O; Taran F Synthesis 2008, 2008, 417 [Google Scholar]; (j) Wilson JE; Sun J; Fu GC Angew. Chem., Int. Ed 2010, 49, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Nasiri F; Bayzidi M; Zolali A Mol. Diversity 2012, 16, 619. [DOI] [PubMed] [Google Scholar]; (l) Lian Z; Shi M Eur. J. Org. Chem 2012, 2012, 581. [Google Scholar]; (m) Anary-Abbasinejad M; Farashah HD; Hassanabadi A; Anaraki-Ardakani H; Shams N Synth. Commun 2012, 42, 1877. [Google Scholar]; (n) Zhou Q-F; Chu X-P; Ge F-F; Li C; Lu T Mol. Diversity 2013, 16, 563. [DOI] [PubMed] [Google Scholar]; (o) Gabillet S; Loreau O; Specklin S; Rasalofonjatovo E; Taran F J. Org. Chem 2014, 79, 9894. [DOI] [PubMed] [Google Scholar]; (p) Li J; Du D Adv. Synth. Catal 2015,357, 3986. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Zhang J; Zhang M; Li Y; Liu S; Miao Z RSC Adv. 2016, 6, 107984 [Google Scholar]; (r) Ametovski J; Dutta U; Burchill L; Maiti D; Lupton DW; Hooper JF Chem. Commun 2017, 53, 13071. [DOI] [PubMed] [Google Scholar]
- (18).Thalji RK; Roush WR J. Am. Chem. Soc 2005, 127, 16778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).(a) Inanaga J; Baba Y; Hanamoto T Chem. Lett 1993,22,241 [Google Scholar]; (b) Stoddard RL; Luo J; van der Wal N; O’Rourke NF; Wulff JD; McIndoe JS New J. Chem 2014, 38, 5382 [Google Scholar]; (c) Wende M; Meier R; Gladysz JA J. Am. Chem. Soc 2001, 123, 11490. [DOI] [PubMed] [Google Scholar]
- (20).(a) Khong SN; Kwon O J. Org. Chem 2012, 77, 8257. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Khong SN; Kwon O Asian J. Org. Chem 2014, 3, 453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Watthey JWH 3-Amino-[1]-benzazepin-3-one-1-alkanoic acids. US 4575503 A, 1986.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


