Abstract
Background
The HIV epidemic in the US is a collection of diverse local microepidemics. Targeted strategies have been proposed to reduce HIV incidence by 90% within 10 years. We aimed to identify optimal combination implementation strategies of evidence-based interventions to reach these targets in six cities, comprising 24.1% of people living with HIV/AIDS in the US.
Methods
In this economic modelling study, we used a dynamic HIV transmission model calibrated with the best available evidence on epidemiological and structural conditions for six US cities: Atlanta (GA), Baltimore (MD), Los Angeles (CA), Miami (FL), New York City (NY), and Seattle (WA). We assessed 23 040 combinations of 16 evidence-based interventions (ie, HIV prevention, testing, treatment, engagement, and re-engagement) to identify combination strategies providing the greatest health benefit while remaining cost-effective. Main outcomes included averted HIV infections, quality-adjusted life-years (QALYs), total cost (in 2018 US$), and incremental cost-effectiveness ratio (ICER; from the health-care sector perspective, 3% annual discount rate). Interventions were implemented at previously documented and ideal (90% coverage or adoption) scale-up, and sustained from 2020 to 2030, with outcomes evaluated until 2040.
Findings
Optimal combination strategies providing health benefit and cost-effectiveness contained between nine (Seattle) and 13 (Miami) individual interventions. If implemented at previously documented scale-up, these strategies could reduce incidence by between 30·7% (95% credible interval 19·1–43·7; Seattle) and 50·1% (41·5–58·0; New York City) by 2030, at ICERs ranging from cost-saving in Atlanta, Baltimore, and Miami, to $95 416 per QALY in Seattle. Incidence reductions reached between 39·5% (26·3–53·8) in Seattle and 83·6% (70·8–87·0) in Baltimore at ideal implementation. Total costs of implementing strategies across the cities at previously documented scale-up reached $559 million per year in 2024; however, costs were offset by long-term reductions in new infections and delayed disease progression, with Atlanta, Baltimore, and Miami projecting cost savings over the 20 year study period.
Interpretation
Evidence-based interventions can deliver substantial public health and economic value; however, complementary strategies to overcome social and structural barriers to HIV care will be required to reach national targets of the ending the HIV epidemic initiative by 2030.
Introduction
Concerted efforts and substantial investments in HIV prevention and care in the USA have resulted in a 69% reduction in mortality and a 48% reduction in new diagnoses since the mid-1990s.1, 2 Nonetheless, 38 000 new cases were diagnosed in 2017, a reduction of only 7% from 2012. In 2017, adolescent and adult males accounted for 81% of new diagnoses,2 and 67% of all new diagnoses were attributed to male-to-male sexual contact.2 Overall, 1·1 million people in the USA are currently living with HIV infection, including an estimated 15% who are unaware of their status.
Ethnic, racial, and sexual minorities have not benefited from advances in treatment and prevention of HIV/AIDS in the USA.3 Of new infections reported in US men in 2017, 56% were in black and Hispanic men who have sex with men (MSM), a group representing less than 1% of the US population.3 If current rates of HIV infection persist, 41% of black MSM and 22% of Hispanic MSM in the US will be diagnosed with HIV during their lifetimes.4 Black and Hispanic women are also disproportionately affected,3 with lifetime risk of HIV diagnosis nearly 17 times higher in black women and four times higher in Hispanic women than in white women.4 Furthermore, 2015 was the first time in 20 years that infections attributed to drug injection increased.5
Rather than a homogeneous national epidemic, the US HIV epidemic is a collection of diverse local microepidemics, concentrated primarily in the southern, so-called hotspot counties5 and large urban centres with fundamental differences in health system infrastructure, funding, and HIV-related laws and policies between the regions.6 Health literacy deficits, stigma, and challenges in navigating the complex US health system further undermine epidemic responses.7, 8 These issues have resulted in disparate rates of new HIV diagnoses;6 for instance, in 2017, Miami had 49 new diagnoses per 100 000 residents annually, representing the highest rate among US cities, while Seattle had ten new diagnoses per 100 000, ranking 75th overall.
On Feb 5, 2019, at the State of the Union Address, the US President Donald Trump announced an intention to end the US HIV epidemic by reducing new infections by 75% within 5 years and by 90% within 10 years. This announcement marked a departure from the 2015 National HIV/AIDS Strategy, which called for 90–90–90 goals (90% of people with HIV diagnosed, 90% of those diagnosed treated with antiretroviral therapy, and 90% of those treated achieving viral suppression) to be reached by 2020. The US Department of Health and Human Services proposed initially to target 48 counties plus Washington, DC, San Juan (Puerto Rico), and seven southern states, which comprise approximately 50% of new diagnoses in the USA.9 New funding allocations have been proposed alongside parallel cuts to existing social safety net programmes. Affected programmes include those that provide access to affordable medical insurance and reasonably priced drugs, with the cuts threatening to undermine benefits of the 2019 strategy in states with uneven access to health care, as medications are foundational to many combination HIV prevention strategies. Furthermore, as a result of limitations at each stage of implementation, previously documented scale-ups of delivery of evidence-based interventions have been shown to have limited population effects.10
A data-driven public health approach, embracing evidence-based interventions and engaging communities in ways that mitigate stigma and discrimination, has been proposed as a means of reaching the ambitious 2019 targets.3, 5 Focusing resources on those at greatest risk of infection and mortality has long been a central theme in guidance issued by the President’s Emergency Fund for AIDS Research, WHO, and UNAIDS, with data-driven programming informed by a so-called know your epidemic assessment framework.11 Simulation models can quantify the potential public health and economic effects of multiple health interventions over the long term within specific geographical regions, accounting for synergistic effects of different interventions and local context.12 Model-based cost-effectiveness analyses, guided by national and international practice standards13, 14 and supported with the best available data, can ensure that the ending the HIV epidemic strategy is executed efficiently and on the principle of health equity. This approach can also weigh opportunity costs against competing priorities elsewhere in the health sector.15
In this report, we aimed to identify combinations of evidence-based interventions with the highest value in terms of reducing the public health burden of HIV/AIDS in disparate geographical regions. In the context of the ambitious goals of the national strategy to end the HIV epidemic, we focus on six US cities comprising 24·1% of all people living with HIV in the USA.
Study design
In this economic modelling study, we used a computer simulation model based on our previous synthesis of the best available data on city-level HIV microepidemics16 and evidence-based interventions to diagnose, treat, and prevent HIV.10 We simulated HIV microepidemics in Atlanta (GA), Baltimore (MD), Los Angeles (CA), Miami (FL), New York City (NY), and Seattle (WA), capturing current availability of prevention, testing, and treatment services.17 These cities were selected to show the breadth of differences in local demographics, structural features, and available services to address the HIV/AIDS epidemic in the USA. We assessed alternative strategies by defining a set of optimal strategies that provide the greatest value across a range of investment levels, termed the health production function. The health-maximising strategy that was also cost-effective was determined by calculating the incremental cost-effectiveness ratio (ICER), defined as the additional cost of a specific combination implementation strategy divided by its additional health benefit, as compared with the next most costly strategy in the health production function. The numerator represented the total increment in health-care costs (in 2018 US$) for the adult population (aged 15–64 years) in a given city, and the denominator represented the total gain in quality-adjusted life-years (QALYs) for this group. ICERs were categorised as cost-saving (greater health benefits and lower costs versus comparator), cost-effective (ICER ≤$100 000 per QALY gained), or not cost-effective (ICER >$100 000 per QALY gained).
Model description
We adapted and calibrated our previous dynamic compartmental HIV transmission model16, 18 to replicate city-level HIV microepidemics in each of our US cities (table 1). Administrative regions (counties) were the main unit of analysis because they correspond to both the lowest level of resource allocation decisions and, in many cases, the finest resolution of available input data. Of the cities above, three are contained within a single county each (Los Angeles, Miami, and Seattle), and three span multiple counties (Atlanta, Baltimore, and New York City), with full details on city boundary selection described previously.16
Table 1.
Demographic and HIV-related characteristics of the focal US cities
| Atlanta, GA | Baltimore, MD | Los Angeles, CA | Miami, FL | New York, NY | Seattle, WA | |
|---|---|---|---|---|---|---|
| Total adult 15–64 Population (% projected change to 2040) | ||||||
| Total population (2016) | 3,812,143 (37%) | 1,874,601 (−1%) | 6,964,983 (−2%) | 1,821,311 (16%) | 5,865,683 (3%) | 1,503,497 (15%) |
| Adult 15–64 Population by race/ethnicity (% projected change in proportion by 2040) | ||||||
| Black / African American | 1,336,469 (−1%) | 553,665 (5%) | 568,815 (−1%) | 296,354 (−2%) | 1,304,687 (−1%) | 95,550 (1%) |
| Hispanic / Latinx | 391,265 (10%) | 102,495 (3%) | 3,385,948 (4%) | 1,246,583 (7%) | 1,703,286 (4%) | 137,818 (7%) |
| Non-Hispanic White and others | 2,084,409 (−9%) | 1,218,441 (−8%) | 3,010,220 (−3%) | 278,374 (−5%) | 2,857,710 (−3%) | 1,270,129 (−8%) |
| People Living with HIV (rate/100,000)† | ||||||
| Prevalence | 31,961 (670) | 16,931 (718) | 48,100 (564) | 26,128 (1,120) | 117,260 (959) | 7,768 (312) |
| New diagnoses | 1,618 (33) | 441 (19) | 1,720 (20) | 1,150 (49) | 2,608 (21) | 248 (10) |
| National Rank Δ | 2 | 25 | 27* | 1 | 21* | 75* |
Data reported is from the Centers for Disease Control and Prevention’s Surveillance HIV Surveillance Supplemental Report. Metropolitan statistical areas may differ from our city boundaries. Counties included in city boundaries for Atlanta, Baltimore, Los Angeles, and Miami match those included in the definition of Ryan White Eligible Metropolitan Area (EMA) or Transitional Grant Area (TGA) while New York City and Seattle boundaries are restricted to a subset of counties. Counties included in each city are found in brackets: Atlanta (Barrow, Bartow, Carroll, Cherokee, Clayton, Cobb, Coweta, DeKalb, Douglas, Fayette, Forsyth, Fulton, Gwinnett, Henry, Newton, Paulding, Pickens, Rockdale, Spalding, Walton); Baltimore (Anne Arundel, Baltimore City, Baltimore County, Carroll, Harford, Howard, Queen Anne’s); Los Angeles (Los Angeles county); Miami (Miami-Dade county); New York City (county with borough in brackets: New York [Manhattan], Kings [Brooklyn], Queens [Queens], Bronx [Bronx], Richmond [Staten Island]); Seattle (King county). Excluded counties for New York City compared to the Ryan White EMA definition included Westchester, Rockland and Putnam, and excluded counties for Seattle compared to Ryan White TGA definition included Snohomish and Island.
Ranking based on rate of new diagnoses.
Note that ranking was for the metropolitan statistical area and may not reflect city boundaries as defined.
Percentages do not always equal 100% due to rounding. Metropolitan statistical areas might differ from our city boundaries. Counties containing the city boundaries of Atlanta, Baltimore, Los Angeles, and Miami match those included in the definition of Ryan White Eligible Metropolitan Area or Transitional Grant Area; the New York City and Seattle boundaries were restricted to a subset of counties within the Ryan White definitions. Counties containing each city are listed in parentheses: Atlanta (Barrow, Bartow, Carroll, Cherokee, Clayton, Cobb, Coweta, DeKalb, Douglas, Fayette, Forsyth, Fulton, Gwinnett, Henry, Newton, Paulding, Pickens, Rockdale, Spalding, and Walton); Baltimore (Anne Arundel, Baltimore City, Baltimore County, Carroll, Harford, Howard, and Queen Anne’s); Los Angeles (Los Angeles County); Miami (Miami-Dade County); New York City (New York [Manhattan borough], Kings [Brooklyn borough], Queens [Queens borough], Bronx [Bronx borough], and Richmond [Staten Island borough]); and Seattle (King County). Excluded counties for New York City compared with the Ryan White Eligible Metropolitan Area definition were Westchester, Rockland, and Putnam, and excluded counties for Seattle compared with the Ryan White Transitional Grant Area definition were Snohomish and Island.
For each city, the model tracked individuals susceptible to HIV over the course of infection, diagnosis, and treatment with antiretroviral therapy (ART; accounting for ART dropout). In each city, the adult population was partitioned by biological sex (male or female), HIV risk group (MSM, people who inject drugs, MSM who inject drugs, and heterosexual individuals), race and ethnicity (black or African American, Hispanic or Latino, and non-Hispanic white or other) and sexual risk behaviours (high risk vs low risk). The model captured heterogeneity in the risk of HIV transmission, maturation, and mortality, and the disparities in accessing health, prevention, and treatment services, including HIV testing, ART, syringe service programmes, medication for opioid use disorder, and targeted pre-exposure prophylaxis (PrEP) for MSM at high risk of infection. The model was populated with 1667 parameters, 1517 (91%) of which were city-specific and 150 (9%) of which were common for all cities. We used a mixed-method evidence synthesis strategy to populate model parameters in six categories: (1) initial HIV-negative and HIV-infected populations; (2) parameters used to calculate the probability of HIV transmission; (3) screening, diagnosis, treatment, and HIV disease progression; (4) HIV prevention programmes; (5) the costs of medical care; and (6) health utility weights. We synthesised evidence from 11 primary database analyses, 59 peer-reviewed publications, and 24 public health and surveillance reports to generate the parameters needed to populate the model for each city.16 Parameters ranked as best-quality to moderate-quality evidence, based on clasifications of the Oxford Centre for Evidence-based Medicine—Levels of Evidence, comprised 58 (39%) of the common parameters, and ranged from 56% (848 parameters in Baltimore) to 60% (909 in New York City) of the city-specific parameters in each city. We calibrated the model to match the the total number of diagnosed cases (11 calibration targets), new diagnoses (3 targets), and deaths (3 targets) across race and ethnicity groups and HIV risk groups (17 calibration targets in total) and validated against external incidence estimates (overall and among MSM) from 2012–15 for each city.18 The projected model outcomes showed a good fit to calibration targets according to an overall goodness of fit metric (defined as the weighted sum of the goodness of fit of the individual calibration targets), and model-derived incidence estimates for the years 2012–2015 corresponded with externally estimated uncertainty ranges, showing good external validity.18 Our calibration and validation process18 has been documented in detail previously. With our model we projected HIV microepidemic trajectories, accounting for official population growth estimates and demographic shifts for each city up to 2040 (table 1), to serve as the so-called status quo scenario for comparison.17
Evidence-based interventions
Into our model, we incorporated 16 evidence-based interventions with established efficacy or effectiveness data and promising scalability according to three pillars of the ending the HIV epidemic strategy: protect (via HIV prevention programmes such as syringe service programmes, medication for opioid use disorder, and targeted PrEP); diagnose (via HIV testing); and treat (via ART initiation and retention and ART re-initiation; table 2). These interventions were selected from the US Centers for Disease Control and Prevention compendium of evidence-based interventions and best practices for HIV prevention and from the published literature,10 with evidence for efficacy and scale-up based on the Reach, Effectiveness, Adoption, Implementation, and Maintenance (RE-AIM) framework for health interventions.
Table 2.
Selected evidence-based interventions, their effectiveness and previously-documented scale-up of delivery based on the RE-AIM framework
| Population Coverage† |
|||
|---|---|---|---|
| Effectiveness and target population¶ [Evidence Level*] | Previously-documented Implementation | Ideal Implementation** | |
| Protect: HIV prevention programs | |||
| Syringe service program (SSP) | Clean injection equipment reduces parenteral HIV transmission by 58% among PWID. [2a] | Additional scale-up of 11% | 90% |
| MOUD with buprenorphine | Office-based MOUD for PWID reduces the number of injections by 54%. [2a] | Additional scale-up of 14% | 90% |
| MOUD with methadone | OTP-based MOUD for PWID reduces the number of injections by 54%. [2a] | Additional scale-up of 12% | 90% |
| Targeted PrEP for high-risk MSM | PrEP reduces the risk of HIV infection by 60%⍑ among high-risk MSM and MWID.§ [1b] | Additional scale-up of 68% | 90% |
| Diagnose: HIV Testing | |||
| Opt-out testing in ER | Routine HIV testing increases by 28% among individuals visiting the ER. [1b] | 3%–6% | 10%–26% |
| Opt-out testing in primary care | Routine HIV testing increases by 28% during primary care visits. [1b] | 25%–40% | 53%–85% |
| EMR testing offer reminder | HIV testing increases by 178% among individuals visiting the ER. [2b] | 11%–29% | 10%–26% |
| Nurse-initiated rapid testing | HIV testing increases by 73% during health care visits.^ [2b] | 25%–40% | 53%–85% |
| MOUD integrated rapid testing | On-site HIV testing increases by 352% among PWID receiving MOUD. [1b] | 17% | 49% |
| Treat: ART engagement and re-engagement | |||
| Case management (ARTAS) | ART initiation increases by 41% among PLHIV linked to care. [1b] | 57% | 77% |
| Care coordination | ART retention increases by 10% among PLHIV. [2b] | 10%–20% | 34%–68% |
| Targeted care coordination | ART retention increases by 32% among PLHIV with CD4<200 cells per μL. [2b] | 30%–46% | 41%–63% |
| EMR ART engagement reminder | ART drop-out is reduced by 31% among PLHIV on ART. [1b] | 42%–78% | 54%–82% |
| RAPID ART initiation | Immediate ART initiation increases by 32% among newly diagnosed PLHIV. [3b] | 30%–61% | 41%–84% |
| Enhanced personal contact | ART re-initiation increases by 22% among PLHIV having dropped-out of ART. [1b] | 45% | 62% |
| Re-linkage program | ART re-initiation increases by 70% among PLHIV who are out-of-care. [2b] | 8% | 22% |
PWID: People who inject drugs; MOUD: Medication for opioid use disorder; OTP: Opioid treatment program; PrEP: Pre-exposure prophylaxis; MSM: Men who have sex with men; MWID: MSM PWID; ER: Hospital emergency room; EMR: Electronic medical records; PLHIV: People living with HIV; ART: antiretroviral therapy.
Interventions target the general adult population 15–64 unless noted otherwise.
Where applicable, scale ranges indicate evidence stratified by sex/gender and/or race/ethnicity and/or city/region.
Adapted from the Oxford Centre for Evidence-based Medicine – Levels of Evidence: 1a - Systematic review of RCTs; 1b - Individual high-quality RCT; 2a - Systematic review of cohort studies; 2b - Individual cohort study or quasi-experimental study; 3a - Systematic review of case-control studies; 3b - Individual case-control study; 4 - Case series; 5-Expert opinion.
Ideal implementation defined as 90% adoption.
Effectiveness: efficacy for 4 doses/week [96% (90%, 99%)]19 X protective level adherence [62.5% (≥4 doses/week)]20.
We assumed that 25% of MSM are high-risk and indicated for PrEP in accordance with CDC guidelines21.
With either a physician or health care professional.
Initially, we estimated ranges on the costs and level of scale-up according to publicly available evidence,10 which constituted the previously documented level of implementation. We have documented our methods for estimating the effectiveness, scale, and costs of each individual intervention in a separate manuscript.10 Scale-up from existing service levels was implemented proportionally across risk and ethnic groups, implying an increased scale-up of delivery after implementation for groups receiving high service levels at baseline. Based on real-world evidence, the scenarios were designed to represent an estimate of the expected level of scale-up that can be achieved within current social and structural constraints on access to care.
We assessed all combinations of the 16 interventions (excluding combinations that would not practically be implemented jointly, such as two HIV testing interventions delivered in primary care) for a total of 23 040 unique combinations including the status quo scenario. We then reassessed these combinations in an ideal implementation scenario in which we assumed each intervention would achieve 90% coverage (protect programmes) or 90% adoption (for diagnose and treat interventions) within the target population of each intervention.
Cost-effectiveness analysis
We estimated health production functions that represented combination implementation strategies providing the greatest health benefits for a range of investment levels incremental to the status quo at both previously documented and ideal scale-up. Combination strategies were sustained for a period of 10 years (2020–30) to match the goals of the ending the HIV epidemic initiative5 and with outcomes evaluated over 20 years (2020–40) to capture long-term individual health benefits and second-order transmission effects (ie, prevented cases beyond those directly reached by the interventions).
Model-projected outcomes included QALYs, total costs (in 2018 US$), and new HIV infections. Costs were disaggregated by type, as those for ART, PrEP, and medication for opioid use disorder, other medical costs, and other intervention costs. The cost-effectiveness analysis conformed to best practice guidelines of the Second Panel on Cost-Effectiveness in Health and Medicine, and was done from the health-care sector perspective, including government, employer-paid, and out-of-pocket health-care expenditures.14 Both costs and QALYs were reported with a 3% annual discount rate.13 We followed conventional incremental cost-effectiveness analysis rules22 to estimate ICERs as the incremental cost per QALY gained for successive optimal combination implementation strategies along the health production function, compared with the next most costly strategy. We identified the strategy producing the greatest health benefits while remaining cost-effective. Although no explicit threshold exists in the USA, we defined cost-effective interventions as those with an ICER below $100 000 per QALY, consistent with efforts in 2016 to approximate the threshold according to the opportunity costs of displacing existing services.23. We did probabilistic sensitivity analysis on optimal strategies and other strategies producing the most proximal value to quantify decision uncertainty in the recommended strategy (appendix p 3). We also did deterministic sensitivity analyses (appendix pp 3–4). In one scenario we considered the potential effects of the donation of PrEP medications to the USA from Gilead, first reported in 2019,24 by setting medication costs to zero but maintaining associated implementation and monitoring costs across our focal cities. In another scenario, we examined changes in the epidemiology of injection drug use, accounting for increased prevalence from the escalation of opioid prescribing, and increased mortality risk resulting from the influx of fentanyl in the illicit drug supply (appendix pp 3–4). We projected the effect of these changes by adjusting model parameters to increase entry into injection drug use over time, and increase mortality for people who inject drugs not receiving medication for opioid use disorder (appendix pp 3–4, 17)
Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
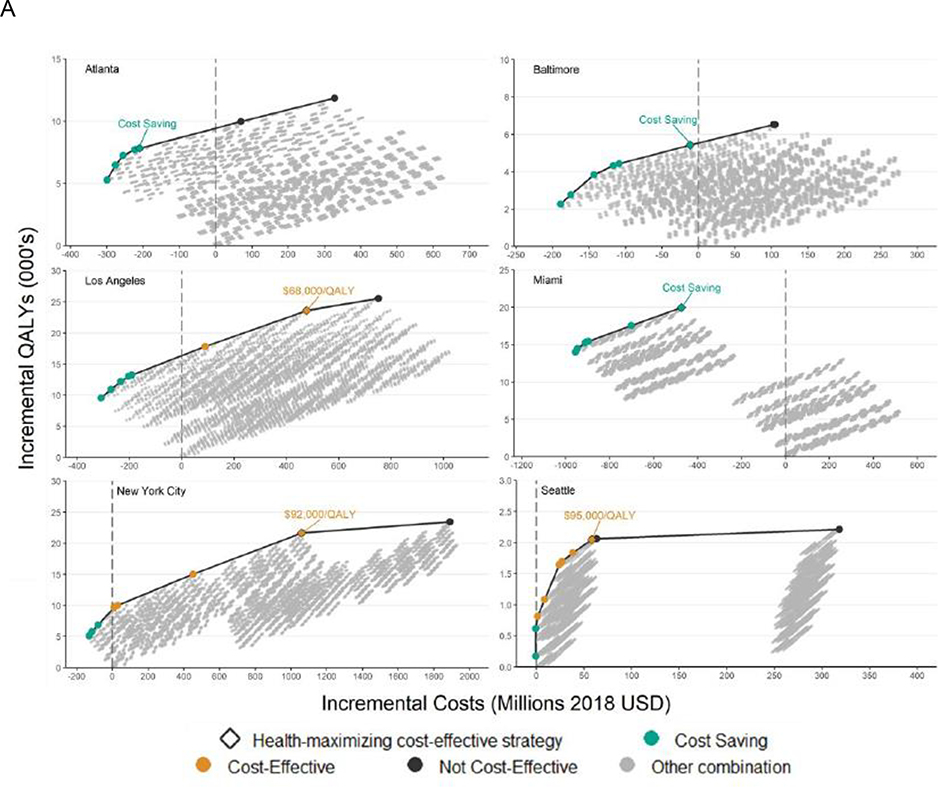
Results from the first step of our analysis, which was to calculate city-level health production functions at previously documented scale-up, showed production functions with the expected form (figure 1A). For each city, substantial gains in health were coupled with small increments in cost at low overall total costs, corresponding to steep rises at the start of the curve. The production functions then showed diminishing returns, whereby greater health effects were possible, but at much greater cost.
Figure 1. Panel A: City-level health production functions comprising optimal combinations of interventions for different investment levels; Panel B: Composition of optimal combination implementation strategies delivered at previously-documented scale-up.
Panel A) ICER – Incremental cost-effectiveness ratio; CS – Cost-saving; QALY – Quality adjusted life year.
All costs and benefits in present value and accrued over a 20-year study time horizon with interventions implemented for a 10-year period. Health production functions were created by plotting the cost and effects of all combinations of interventions, with each curve displaying the maximum possible health benefits (number of QALYs gained, 2020–2040) at a given cost, for each city. The incremental cost-effectiveness of each strategy along the curve was compared to the next-most costly strategy on the health production function, and ICERs were categorized as: cost-saving (greater health benefits and lower costs versus comparator); cost-effective (ICER < $100,000 per QALY gained; or not cost-effective (ICER > $100,000 per QALY gained). See supplementary material for full details.
Panel B) MOUD – Medication for opioid use disorder; PrEP – Pre-exposure prophylaxis; MSM – Men who have sex with men; ER – Emergency room; EMR – Electronic medical records; ARTAS – Antiretroviral treatment and access to services; ART – Antiretroviral therapy; RAPID – Rapid ART Program for Individuals with an HIV Diagnosis.
Each row represents one intervention and each column represents a city. The shading indicates whether an intervention was selected for a given city. Green indicates an intervention should be implemented or scaled up, yellow indicates it should not be implemented or increased beyond existing service levels.
When assessed at previously documented scale-up, strategies producing the greatest health benefits while remaining cost-effective included between nine (Seattle) and 13 (Miami) individual interventions (figure 1B). Opt-out testing in emergency departments and primary care settings, and non-targeted care coordination to improve ART retention were not included in the optimal strategy for any city, whereas expanded access to medication for opioid use disorder (with buprenorphine and methadone), offer reminders for testing of electronic medical records, nurse-initiated rapid HIV testing, medication for opioid use disorder-integrated rapid testing, and six ART engagement or re-engagement interventions (except care coordination, which would conflict with targeted care coordination) were included across all cities. Additional scale-up of syringe service programmes was only recommended in cities with insufficient syringe distribution (Atlanta, Los Angeles, and Miami), similar to targeted PrEP for high-risk MSM, which was included only in the optimal strategies for Atlanta, Baltimore, Los Angeles, and Miami.
At previously documented scale-up, these optimal combination strategies were estimated to produce QALY gains of between 2046 (95% credible interval [CrI] 1496–2656) in Seattle and 23 591 (17 930–31 118) in Los Angeles, over the 20 year time horizon. ICERs ranged from cost-saving in Atlanta, Baltimore, and Miami, to $95 416 per QALY in Seattle (figure 1A). Furthermore, at previously documented scale-up, we estimated the selected combination strategies could reduce HIV incidence by between 30·7% (19·1–43·7; Seattle) and 50·1% (41·5–58·0; New York City) by 2030 (figure 2), with a weighted average of 37·9% (95% CrI 27·5–46·0) across cities.
Figure 2. Projected reductions in HIV incidence at status quo service levels compared to implementation of optimal strategies at previously-documented scale-up and ideal implementation.
The 5- and 10-year targets correspond to 75% and 90% reductions in the number of new HIV infections in each city in 2025 and 2030, compared to 2020. The 2020 projections were constructed by holding all health services at their 2015 levels except for PrEP which was held at 2017 levels while accounting for externally reported population growth and demographic shifts in each city. More details regarding the construction of status quo scenarios can be found in a previous study.26 The blue shaded band surrounding the ideal implementation scenario incidence estimates represented the 95% credible interval on the optimal combination implementation scenario, derived from probabilistic sensitivity analysis. Credible intervals on the status quo and previously-documented scale-up estimates are suppressed for clarity, and presented in the supplementary appendix.
The costliest combination strategy in the health production function was not optimal according to our cost-effectiveness decision rules for any city (figure 1 and appendix pp 5–16). Furthermore, only in Seattle did the absolute costliest strategy produce greater health benefits than the optimal strategy. In that case, health gains were minimal (168 additional QALYs over 2020–40, 8·2% greater than the optimal strategy) and came at high incremental cost ($260 million; appendix p 15). In Miami, the costliest combination strategy was estimated to produce just 30·1% of the health benefits (13 955 less QALYs) estimated for the optimal strategy, at an incremental cost of $0·99 billion (figure 1).
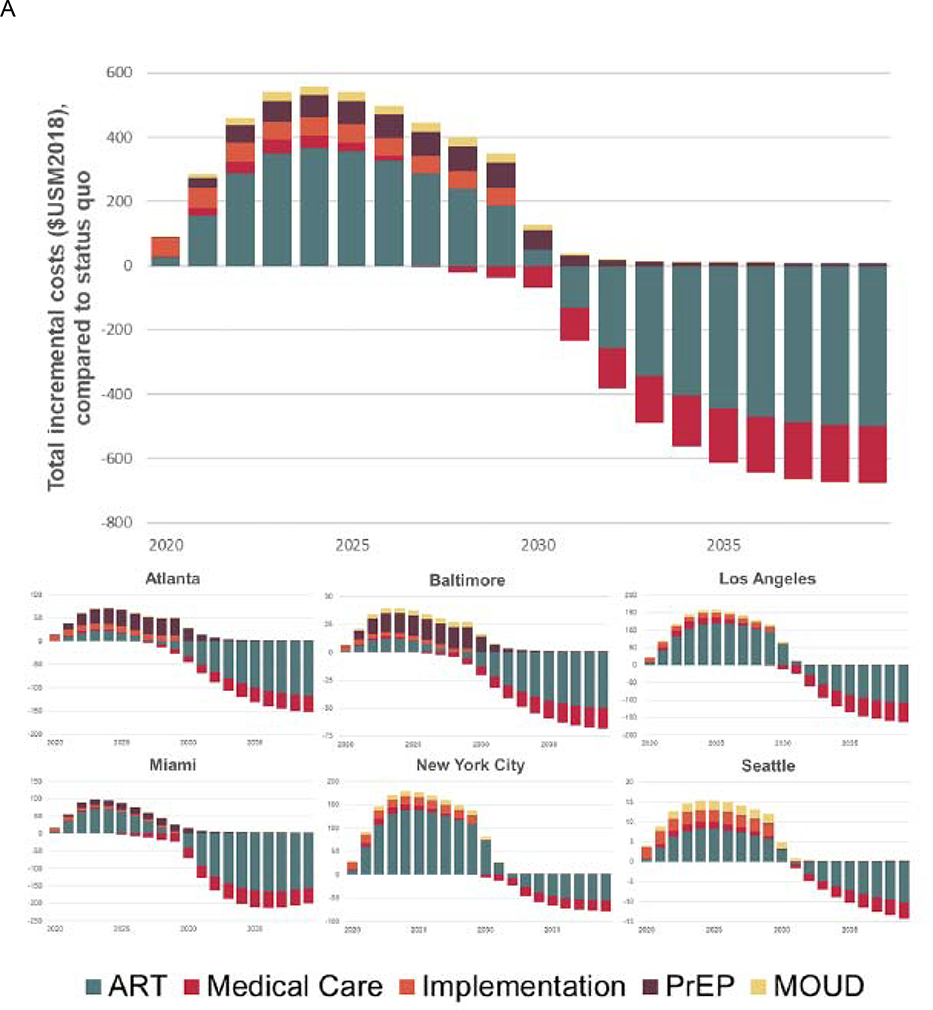
Implementing the optimal combination strategies at previously documented scale-up would entail savings at 2018 value of $474 million (95% CrI 214–895) in Miami, to incremental expenditures of $1·06 billion (0·56–1·51) in New York City over the 20 year time horizon. These expenditures would peak in 2023–25, with a peak annual overall annual expenditure of $559 million in 2024 (comprising an additional $15 million in Seattle, to $179 million in New York City; figure 3A). Overall costs over the 10 year implementation period would total $3·51 billion at 2018 value, with 63% of these costs attributable to expanding access to ART medications (figure 3B).
Figure 3. Panel A: Estimated annual incremental costs of implementing optimal combination implementation strategies, delivered at previously-documented scale-up, by source: 2020–2040; Panel B: Total incremental costs of implementing optimal combination implementation strategies, delivered at previously-documented scale-up, by source over the 10-year sustainment period*.
ART – Antiretroviral therapy; PrEP – Pre-exposure prophylaxis; MOUD – Medication for opioid use disorder. Costs are shown incrementally for each year, relative to projected status quo spending levels in a given year.
* We note that the impact on medical care costs of the highest-value combination implementation strategies in Atlanta, Baltimore and Miami was cost-saving and offset medical care costs in other cities. Total discounted costs are presented in present value using a 3% annual discount rate.
Implementing the combination strategies at ideal implementation levels would result in a weighted average incidence reduction of 63·5% across cities. Atlanta, Baltimore, and Miami would approach national incidence reduction targets: 74·4% (95% CrI 67·0–80·7), 83·6% (70·8–87·0), and 78·3% (51·5%-86·9%), respectively), whereas Los Angeles, New York City, and Seattle would reach reductions of 41·5% (30·5–56·1), 58·1% (48·1–66·9), and 39·5% (26·3–53·8).
The results of probabilistic sensitivity analyses showed that the selected strategies had a high probability of providing the greatest health gains, compared with competing strategies providing the next closest value, with probabilities ranging from 35·7% (Seattle) to 94·9% (Baltimore; appendix pp 19–20)..
When incorporating potential changes in injection drug use initiation and mortality rates to capture the opioid syndemic, we found the optimal set of interventions maintained the same composition in each city, but were estimated to increase QALY gains, which were between 2267 in Seattle and 24 199 in Los Angeles. The strategies also elicited savings of $475 million in Miami, to incremental expenditures of $1·08 billion in New York City, over the 20 year time horizon (appendix pp 24–25). Alternatively, if PrEP medication costs were eliminated, PrEP would be included in the optimal set of interventions for all cities, with QALY gains of 1993 in Seattle and 23 442 in New York City, with incremental costs of $15 million and $869 million, where additional PrEP scale-up was not recommended at baseline PrEP costs (appendix pp 21–22). With free PrEP, incidence would be reduced further by the resulting optimal strategies, reaching a 69·1% reduction by 2030 in New York City and a 49·1% reduction in Seattle at ideal levels of implementation (appendix p 23).
Discussion
In this simulation study of six US cities with substantial HIV disease burdens, each city required distinct combination implementation strategies to address their heterogeneous microepidemics. In all cities, the targets for ending the HIV epidemic were only approached by implementing interventions at scales of delivewry that have not previously been recorded. Nonetheless, we found city-specific combination strategies implemented at previously documented scale-up could reduce incidence by between 30·7–50·1% (with a weighted average of 37·9% across cities), at an overall estimated cost of $3·51 billion by 2030. This investment would be front-loaded, peaking at an annual expenditure of $559 million in 2024, equating to 2·7% of all federal domestic expenditures on the care and prevention of HIV/AIDS in 2018,26 with the timing of positive incremental costs varying by city and cost component. Our conservative annual incremental spending projections for the focal cities at previously documented implementation would thus require 1·9 times the proposed allotment of the US national budget ($291 million in fiscal year 2020) to the ending the HIV epidemic initiative, not accounting for potential budget cuts to existing services, which could make this discrepancy considerably larger. These investments would nonetheless provide long-term value in each setting, with upfront investments offset by downstream reductions in health-care costs as a result of averted infections and delayed disease progression. At previously documented scale-up, these health-care gains outweighed the costs of implementing and delivering health services in some settings, resulting in net cost savings in Atlanta, Baltimore and, most notably, Miami, where the highest rate of new HIV diagnoses in the USA was reported in 2017.25
The combination implementation strategies we assessed and recommended should not be considered exhaustive. The ending the HIV epidemic plan explicitly defines identification and rapid responses to clusters and outbreaks of new HIV infections as one of the four pillars of HIV prevention, and both HIV partner services and the testing of high-risk populations are long-standing core components of HIV prevention that could be expanded in some areas. If sufficient scale can be reached, partner services, in particular, might be effective.27, 28 Other interventions with a more limited evidence base include testing in non-health-care settings,29 particularly self-testing;30, 31 PrEP for people who inject drugs32 and heterosexuals at high risk of infection;33 testing in correctional settings34 and transitional care support,35 linkage to care programmes,36 and low-barrier care delivery;37 and e-health solutions to improve ART persistence.38, 39 Furthermore, we focused on evidence-based interventions, while remaining agnostic about the capacity or specific circumstances of public health departments. As new evidence on demographics, service delivery, and intervention effectiveness and scale of delivery emerges, the current modelling platform could assess the incremental value and fit of innovative interventions within a combination implementation strategy to maximise health.
In this study, we only considered costs of delivering interventions that directly affect HIV-related outcomes. People who are most likely to be living with or acquire HIV are frequently living in poverty, without stable housing or reliable health insurance, hindering access to care.3 The limited scale-up of delivery for interventions incorporated in this study10 reflects these realities. To bridge the implementation gap, interventions will need to be augmented with efforts to reduce stigma (particularly for syringe service programmes given the potential for HIV outbreaks among people who inject drugs),40, 41 improve health literacy, and address capacity constraints in health-care delivery and other social and structural barriers to health-care access.
To combat stigma, the U=U (undetectable equals untransmittable) concept, which acknowledges that people living with HIV who are treated effectively cannot transmit HIV to their partners, will need to be embraced in health-care settings and by the most affected communities.42 A systematic review of health literacy interventions suggested that future interventions should evaluate approaches to increase motivation, deliver information in formats other than writing, and utilise patient advocates.43 Peer navigation reduces stigma and increases access to services in Africa,3 and is acceptable to HIV-negative MSM for establishing linkage to PrEP.44, 45 Ending the HIV epidemic in southern US cities will require substantial efforts to overcome constraints in health-care access, including lower ratios of primary care providers to populations46 and higher proportions of people living in poverty than in other US regions.
More than half of Americans diagnosed with HIV (>500 000) receive services through the Ryan White HIV/AIDS Program, which reports achieving viral suppression in 85·9% of its clients with ART, exceeding the national average of 59·8%. Notably, the Ryan White HIV/AIDS Program has substantially increased the rate of viral suppression among key populations, including women, black or African American individuals, and people with unstable housing, by funding grants for supportive services targeted to the needs of local populations of patients, and by developing care provider networks to further enhance quality of care.47 These successes, and the best practices that produced them, will need to be expanded to approach the new national targets. Removing social safety programmes will undermine the strategy of the US Government, and any policy that substantially increases the number of uninsured people would lead to increased difficulty in accessing care and make achieving zero new cases nearly impossible.48
The ending the HIV epidemic initiative also recognises the need for a dedicated HIV workforce, or reallocation of existing human resources to ensure implementation of the plans to end the HIV epidemic. Our recommended combination implementation strategies would require substantial staffing to ensure adequate implementation, particularly for the labour-intensive interventions such as case management for ART initiation and retention.49, 50 As such, our cost estimates should be considered conservative, assuming both constant returns to scale and constant effort levels to reach and retain progressively more marginalised and hard-to-reach populations. These prerequisites further emphasise the need for scarce human and financial resources to be delivered to the right places and the right people at the right time.51
To reach national targets would be a staggering victory over racial HIV-related disparities and inequities in health-care access in the USA. In particular, these goals are simply not attainable without large reductions in new infections among black and Hispanic MSM. At our ideal implementation, incidence in 2030 in Miami among black MSM would be reduced by 78·8% and among Hispanic MSM by 84·7% (data not shown), nearly eliminating disparities relative to white MSM. A previous meta-analysis showed that HIV-related disparities in US black MSM, relative to MSM of other ethnicities, reflect disparities in HIV clinical care access and use, and structural (eg, unemployment and incarceration) and sexual partner characteristics, rather than sexual or substance use risk behaviours.52 Structural factors affecting availability and choice of sexual partners (eg, low income, unemployment, incarceration, and poor education) are also associated with isolation in neighbourhoods with high HIV prevalence and community viral load.52 Importantly, we imposed proportional scale-up of interventions across ethnic groups. An intervention approach focused on black and Hispanic populations could provide even greater value than our proposed combination strategies, as shown by modelling studies considering past national targets.53
We previously outlined limitations in the structure of the model,18 the evidence base on which it was built,16 and uncertainty in the previously documented scale-up of delivery and the attributable costs of implementation, delivery, and sustainment for each intervention.10 We note that the status quo scenario should be considered a reflection of the expected trajectory of the HIV epidemic in each city, conditional on data available up to 2015. As we were unable to capture more recent advances in service delivery, we felt our status quo scenario represented the most objective course of action and the most relevant comparator for the interventions considered. The model can be recalibrated as new evidence emerges and otherwise tailored to local capacity constraints. Otherwise, we assumed PrEP could be perfectly targeted to individuals at high risk of infection, potentially making our results more favourable compared with routinely implemented PrEP. We also used a 20 year time horizon for our analyses; longer time horizons could have favoured strategies averting more HIV-related deaths, although costs and health effects in future years would be discounted. To this end, a value of information analysis, focusing on the uncertainty surrounding recommended strategies, can identify the highest-valued targets for increased data collection.15 Furthermore, precise targeting of interventions, improved retention and adherence, and reduced medication costs, could substantially increase the cost-effectiveness of these interventions. In addition to high-quality surveillance data, improved data collection is required to evaluate existing interventions, and identify promising but less-studied interventions than those addressed in our scenarios.54 Our modelling platform is generalisable and updatable as new evidence is generated over the spatiotemporal course of the epidemic, and on emerging interventions. Furthermore, cost-effectiveness is only one consideration in developing disease control priorities among many, such as equity, ethics, and political factors, which might be weighted differently depending on circumstances.
Our projections suggest that implementing combinations of evidence-based interventions can provide public health and economic value and approach national incidence reduction targets in some settings; however, complementary strategies to overcome social and structural barriers to HIV care will be required for these strategies to bridge the implementation gap.
Supplementary Material
Research in context.
Evidence before this study
We conducted a search of PubMed for articles published in English up to June 30, 2019, with the terms (“HIV”) AND (“incidence” OR “end HIV epidemic”) AND ((“combination”) OR (“local*” OR “focus*” OR “target”)) AND (“model*” OR “cost-effectiveness”). We did not identify any multi-city dynamic transmission HIV models used in the United States. We broadened our search and identified a study modeling the relative effects of geographically focused HIV interventions compared to national interventions in Kenya. Other studies in the United States have modeled combination strategies for HIV among specific HIV risk groups or the general epidemic at a national level, however, none followed the unique approach undertaken in Kenya, which was a defining feature of this research. A focused approach was found to be more cost-effective in Kenya, while studies evaluating combination strategies found that selected intervention bundles were cost-effective and could avert more new HIV infections than single interventions. One national-level modeling study concluded that reaching the new “Ending the HIV Epidemic” targets in the US would require resources and implementation of HIV interventions at unprecedented levels.
Added value of this study
We identified the highest-valued combination implementation strategies of evidence-based interventions to reach new ‘Ending the HIV Epidemic’ targets in six US cities, evaluating averted HIV infections, quality-adjusted life-years, total costs and incremental cost-effectiveness ratios. Interventions were implemented at previously-documented and ideal levels of implementation or scale-up, and sustained from 2020 to 2030. In no city did we determine that targets could be approached without implementation of interventions at scales of delivery not previously recorded. Despite requiring higher levels of funding than what has been proposed in the ‘Ending the HIV Epidemic’ initiative, these strategies would be cost-effective, and cost-saving in some settings. Meeting national targets would also reduce racial HIV-related disparities and inequities in healthcare access.
Implications of all the available evidence
Within these six cities, implementing combinations of evidence-based interventions can provide good value and approach ‘Ending the HIV Epidemic’ targets in some settings, however, complementary strategies to overcome social and structural barriers to HIV care will be required to bridge the implementation gap.
Acknowledgments
This study was funded by the National Institutes on Drug Abuse (NIDA grant no. R01DA041747). Dr. Schackman received additional support from the Center for Health Economics of Treatment Interventions for Substance Use Disorder, HCV, and HIV (NIDA grant P30DA040500). Dr. Shoptaw is supported by UCLA Center for HIV Identification, Prevention and Treatment Services (NIH grant no. P30 MH058107). Dr. Strathdee is supported by a NIDA Method to Extend Research in Time (MERIT) award (R37DA019829).
Funding: US NIH-NIDA Grant No. R01-DA041747
Declaration of interests
BN, XZ, EK, BE, JM, CNB, CDR, DJF, MG, BDLM, LRM, SHM, AP, BRS, SS and SAS declare no competing interests. JCD has participated in research supported by grants to the University of Washington from Hologic.
Footnotes
The Localized HIV Modeling Study Group is composed of
Czarina N Behrends, PhD, Department of Healthcare Policy and Research, Weill Cornell Medical College
Carlos Del Rio, MD, Hubert Department of Global Health, Emory Center for AIDS Research, Rollins School of Public Health, Emory University
Julia C Dombrowski, MD, Department of Medicine, Division of Allergy & Infectious Disease, adjunct in Epidemiology, University of Washington and Deputy Director, HIV/STD Program, Public Health – Seattle & King County
Daniel J Feaster, PhD, Department of Public Health Sciences, Leonard M. Miller School of Medicine, University of Miami
Kelly A Gebo, MD, Bloomberg School of Public Health, Johns Hopkins University
Matthew Golden, MD, primary with Department of Medicine, Division of Allergy & Infectious Disease, University of Washington. Director, HIV/STD Program, Public Health – Seattle & King County.
Gregory Kirk, MD, Bloomberg School of Public Health, Johns Hopkins University
Brandon DL Marshall, PhD, Department of Epidemiology, Brown School of Public Health, Rhode Island, United States
Shruti H Mehta, PhD, Bloomberg School of Public Health, Johns Hopkins University
Lisa R Metsch, PhD, Department of Sociomedical Sciences, Mailman School of Public Health, Columbia University
Julio Montaner, MD, BC Centre for Excellence in HIV/AIDS; Faculty of Medicine, University of British Columbia
Bohdan Nosyk, PhD, BC Centre for Excellence in HIV/AIDS; Faculty of Health Sciences, Simon Fraser University
Ankur Pandya, PhD, T.H. Chan School of Public Health, Harvard University
Bruce R Schackman, PhD, Department of Healthcare Policy and Research, Weill Cornell Medical College
Steven Shoptaw, PhD, Centre for HIV Identification, Prevention and Treatment Services, School of Medicine, University of California Los Angeles
Steffanie A Strathdee, PhD, School of Medicine, University of California San Diego
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Centers for Disease Control and Prevention (CDC). HIV surveillance--United States, 1981–2008. MMWR Morbidity and mortality weekly report 2011; 60(21): 689. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC). HIV Surveillance Report, 2017; vol. 29 Atlanta, GA: U.S. Department of Health and Human Services, 2018. [Google Scholar]
- 3.El-Sadr WM, Mayer KH, Rabkin M, Hodder SL. AIDS in America - Back in the Headlines at Long Last. N Engl J Med 2019; 380(21): 1985–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hess KL, Hu X, Lansky A, Mermin J, Hall HI. Lifetime risk of a diagnosis of HIV infection in the United States. Ann Epidemiol 2017; 27(4): 238–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fauci AS, Redfield RR, Sigounas G, Weahkee MD, Giroir BP. Ending the HIV epidemic: a plan for the United States. Journal of the American Medical Association 2019; 321(9): 844–5. [DOI] [PubMed] [Google Scholar]
- 6.Panagiotoglou D, Olding M, Enns B, et al. Building the case for localized approaches to HIV: structural conditions and health system capacity to address the HIV/AIDS epidemic in six US cities. AIDS and behavior 2018; 22(9): 3071–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geter A, Herron AR, Sutton MY. HIV-Related Stigma by Healthcare Providers in the United States: A Systematic Review. AIDS Patient Care STDS 2018; 32(10): 418–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palumbo R Discussing the Effects of Poor Health Literacy on Patients Facing HIV: A Narrative Literature Review. Int J Health Policy Manag 2015; 4(7): 417–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Department of Health and Human Services. Ending the HIV Epidemic: A Plan for America. 2019. https://www.hhs.gov/blog/2019/02/05/ending-the-hiv-epidemic-a-plan-for-america.html] (accessed April 4, 2019.
- 10.Joint United Nations Programme on HIV/AIDS. UNAIDS practical guidelines for intensifying HIV prevention: towards universal access: World Health Organization; 2007. [Google Scholar]
- 11.Garnett GP, Cousens S, Hallett TB, Steketee R, Walker N. Mathematical models in the evaluation of health programmes. Lancet 2011; 378(9790): 515–25. [DOI] [PubMed] [Google Scholar]
- 12.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS)—explanation and alaboration: A report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value in Health 2013; 16: 231–50. [DOI] [PubMed] [Google Scholar]
- 13.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: Second Panel on Cost-Effectiveness in Health and Medicine. Journal of the American Medical Association 2016; 316(10): 1093. [DOI] [PubMed] [Google Scholar]
- 14.Shepherd K, Hubbard D, Fenton N, Claxton K, Luedeling E, de Leeuw J. Policy: Development goals should enable decision-making. Nature 2015; 523(7559): 152–4. [DOI] [PubMed] [Google Scholar]
- 15.Krebs E, Enns B, Wang L, et al. Developing a dynamic HIV transmission model for 6 U.S. cities: An evidence synthesis. PLOS ONE 2019; 14(5): e0217559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krebs E, Zang X, Enns B, et al. The impact of localized implementation: determining the cost-effectiveness of HIV prevention and care interventions across six U.S. cities. AIDS 2019; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nosyk B, Zang X, Krebs E, et al. Ending the epidemic in America will not happen if the status quo continues: modeled projections for HIV incidence in 6 US cities. Clin Infect Dis 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zang X, Krebs E, Min J, et al. Development and calibration of a dynamic HIV transmission model for 6 US cities. Medical Decision Making 2019; In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anderson PL, Glidden DV, Liu A, et al. Emtricitabine-Tenofovir Concentrations and Pre-Exposure Prophylaxis Efficacy in Men Who Have Sex with Men. Science Translational Medicine 2012; 4(151): 151ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu AY, Cohen SE, Vittinghoff E, et al. Preexposure Prophylaxis for HIV Infection Integrated With Municipal- and Community-Based Sexual Health Services. JAMA Intern Med 2016; 176(1): 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Estimated percentages and number of adults with indications for preexposure prophylaxis to prevent HIV acquisition–United States, 2015. MMWR 2015; 64: 1–6. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention (CDC). Compendium of Evidence-Based Interventions and Best Practices for HIV Prevention. 2018. https://www.cdc.gov/hiv/research/interventionresearch/compendium/index.html].
- 23.Hunink MM, Weinstein MC, Wittenberg E, et al. Decision making in health and medicine: integrating evidence and values: Cambridge University Press; 2014. [Google Scholar]
- 24.Woods B, Revill P, Sculpher M, Claxton K. Country-Level Cost-Effectiveness Thresholds: Initial Estimates and the Need for Further Research. Value in health : the journal of the International Society for Pharmacoeconomics and Outcomes Research 2016; 19(8): 929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaiser Family Foundation. U.S. Federal Funding for HIV/AIDS: Trends Over Time. 2019. https://www.kff.org/hivaids/fact-sheet/u-s-federal-funding-for-hivaids-trends-over-time/ [Accessed November 18, 2019].
- 26.Escudero DJ, Bennett B, Suarez S, Darrow WW, Mayer KH, Seage GR 3rd. Progress and Challenges in “Getting to Zero” New HIV Infections in Miami, Florida. J Int Assoc Provid AIDS Care 2019; 18(2325958219852122): 2325958219852122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalal S, Johnson C, Fonner V, et al. Improving HIV test uptake and case finding with assisted partner notification services. AIDS 2017; 31(13): 1867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sharma M, Smith JA, Farquhar C, et al. Assisted partner notification services are cost-effective for decreasing HIV burden in western Kenya. AIDS 2018; 32(2): 233–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thornton AC, Delpech V, Kall MM, Nardone A. HIV testing in community settings in resource-rich countries: a systematic review of the evidence. HIV Med 2012; 13(7): 416–26. [DOI] [PubMed] [Google Scholar]
- 30.Johnson CC, Kennedy C, Fonner V, et al. Examining the effects of HIV self-testing compared to standard HIV testing services: a systematic review and meta-analysis. J Int AIDS Soc 2017; 20(1): 21594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C, Li X, Brecht ML, Koniak-Griffin D. Can self-testing increase HIV testing among men who have sex with men: A systematic review and meta-analysis. PLOS ONE 2017; 12(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bernard CL, Brandeau ML, Humphreys K, et al. Cost-Effectiveness of HIV Preexposure Prophylaxis for People Who Inject Drugs in the United States. Ann Intern Med 2016; 26(2517406): M15–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Traynor SM, Rosen-Metsch L, Feaster DJ. Missed Opportunities for HIV Testing Among STD Clinic Patients. J Community Health 2018; 43(6): 1128–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beckwith CG, Nunn A, Baucom S, et al. Rapid HIV testing in large urban jails. Am J Public Health 2012; 102(S2): S184–S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunningham WE, Weiss RE, Nakazono T, et al. Effectiveness of a Peer Navigation Intervention to Sustain Viral Suppression Among HIV-Positive Men and Transgender Women Released From Jail: The LINK LA Randomized Clinical Trial. JAMA Intern Med 2018; 178(4): 542–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perelman J, Rosado R, Ferro A, Aguiar P. Linkage to HIV care and its determinants in the late HAART era: a systematic review and meta-analysis. AIDS Care 2018; 30(6): 672–87. [DOI] [PubMed] [Google Scholar]
- 37.Dombrowski JC, Galagan SR, Ramchandani M, et al. HIV Care for Patients With Complex Needs: A Controlled Evaluation of a Walk-In, Incentivized Care Model. Open Forum Infectious Diseases 2019; 6(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henny KD, Wilkes AL, McDonald CM, Denson DJ, Neumann MS. A Rapid Review of eHealth Interventions Addressing the Continuum of HIV Care (2007–2017). AIDS Behav 2018; 22(1): 43–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rana AI, van den Berg JJ, Lamy E, Beckwith CG. Using a Mobile Health Intervention to Support HIV Treatment Adherence and Retention Among Patients at Risk for Disengaging with Care. AIDS Patient Care STDS 2016; 30(4): 178–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golden MR, Lechtenberg R, Glick SN, et al. Outbreak of Human Immunodeficiency Virus Infection Among Heterosexual Persons Who Are Living Homeless and Inject Drugs—Seattle, Washington, 2018. Morbidity and Mortality Weekly Report 2019; 68(15): 344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peters PJ, Pontones P, Hoover KW, et al. HIV Infection Linked to Injection Use of Oxymorphone in Indiana, 2014–2015. N Engl J Med 2016; 375(3): 229–39. [DOI] [PubMed] [Google Scholar]
- 42.Eisinger RW, Dieffenbach CW, Fauci AS. HIV Viral Load and Transmissibility of HIV Infection: Undetectable Equals Untransmittable. JAMA 2019; 321(5): 451–2. [DOI] [PubMed] [Google Scholar]
- 43.Berkman ND, Sheridan SL, Donahue KE, et al. Health literacy interventions and outcomes: an updated systematic review. Evid Rep Technol Assess 2011; 199: 1–941. [PMC free article] [PubMed] [Google Scholar]
- 44.Arnold T, Brinkley-Rubinstein L, Chan PA, et al. Social, structural, behavioral and clinical factors influencing retention in Pre-Exposure Prophylaxis (PrEP) care in Mississippi. PLOS ONE 2017; 12(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jaramillo J Perceptions of Pre-Exposure Prophylaxis (PrEP) and Acceptability of Peer Navigation Among HIV-Negative Latinx and Black Men Who Have Sex with Men (MSM) in Western Washington: University of Washington; 2018. [Google Scholar]
- 46.Hing E, Hsiao C-J. State Variability in Supply of Office-based Primary Care Providers, United States, 2012: US Department of Health and Human Services, Centers for Disease Control and; …; 2014. [PubMed] [Google Scholar]
- 47.Emory University. State-level data on poverty in national data sets from AIDSVu.org 2019.
- 48.Mandsager P, Marier A, Cohen S, Fanning M, Hauck H, Cheever LW. Reducing HIV-Related Health Disparities in the Health Resources and Services Administration’s Ryan White HIV/AIDS Program. Am J Public Health 2018; 108(S4): S246–s50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz I, Jha AK. HIV in the United States: Getting to Zero Transmissions by 2030Getting to Zero US HIV Transmissions by 2030Getting to Zero US HIV Transmissions by 2030. JAMA 2019; 321(12): 1153–4. [DOI] [PubMed] [Google Scholar]
- 50.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS 2005; 19(4): 423–31. [DOI] [PubMed] [Google Scholar]
- 51.Irvine MK, Chamberlin SA, Robbins RS, et al. Improvements in HIV care engagement and viral load suppression following enrollment in a comprehensive HIV care coordination program. Clin Infect Dis 2015; 60(2): 298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das P Deborah L Birx: on a mission to end the HIV/AIDS epidemic. The Lancet 2016; 388(10060): 2583. [DOI] [PubMed] [Google Scholar]
- 53.Millett GA, Peterson JL, Flores SA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet 2012; 380(9839): 341–8. [DOI] [PubMed] [Google Scholar]
- 54.Borre ED, Hyle EP, Paltiel AD, et al. The Clinical and Economic Impact of Attaining National HIV/AIDS Strategy Treatment Targets in the United States. J Infect Dis 2017; 216(7): 798–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mayer KH. NextGen HIV prevention: new possibilities and questions. The Lancet 2016; 387(10023): 1036–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.