Abstract
Moderate intensity sounds can reduce pain sensitivity (i.e., audio-analgesia) whereas intense sounds can induce aural pain, evidence of multisensory interaction between auditory and pain pathways. To explore auditory-pain pathway interactions, we used the tail-flick (TF) test to assess thermal tail-pain sensitivity by measuring the latency of a rat to remove its tail from 52 °C water. In Experiment 1, TF latencies were measured in ambient noise and broadband noise (BBN) presented from 80 to 120 dB SPL. TF latencies gradually increased from ambient to 90 dB SPL (audio-analgesia), but then declined. At 120 dB, TF latencies were significantly shorter than normal, evidence for audio-hyperalgesia near the aural threshold for pain. In Experiment II, the opioid pain pathway was modified by treating rats with a high dose of fentanyl known to induce post-treatment hyperalgesia. TF latencies in ambient noise were normal 10-days post-fentanyl. However, TF latencies became shorter than normal from 90 to 110 dB indicating that fentanyl pre-treatment had converted audio-analgesia to audio-hyperalgesia. In Experiment III, we tested the hypothesis that hearing loss could alter pain sensitivity by unilaterally exposing rats to an intense noise that induced a significant hearing loss. TF latencies in ambient noise gradually declined from 1- to 4-weeks post-exposure indicating that noise-induced hearing loss had increased pain sensitivity. Our results suggest that auditory and pain pathways interact in ways that depend on intensity, hearing loss and opioid pain signaling, results potentially relevant to pain hyperacusis.
Keywords: audio-hyperalgesia, hypoalgesia, noise-induced hearing loss, opioid, pain, multi-sensory integration, hyperacusis
1. Introduction
As the intensity of a sound increases, normal listeners experience an increase in loudness, but for levels above 120 dB SPL, listeners not only perceive the sound as extremely loud, but also painful, the aural threshold of pain (Franks et al., 1996; Gierke et al., 1953). Some individuals with hearing loss and other neurological disorders (Fournier et al., 2014; Ison et al., 2007; Knipper et al., 2013; Moller, 2007; Ramos Macias et al., 2018) perceive even moderate-intensity sounds as both painful and loud, a condition known as pain hyperacusis (Tyler et al., 2014). Pain hyperacusis evoked by moderate intensity sounds could be triggered by lower auditory pain thresholds and/or heightened pain sensitivity at sites within the central nervous system where auditory and pain signals interact (Bellgowan and Helmstetter, 1996; Bellgowan and Helmstetter, 1998; Benison et al., 2011). Evidence for such interactions comes from studies showing a high prevalence of hyperacusis among individuals with complex regional pain syndrome, migraine and fibromyalgia, conditions believed to be mediated in part by aberrant central pain signaling (de Klaver et al., 2007; Irimia et al., 2008; Suhnan et al., 2017). Similar links have been reported between vestibular and pain mechanisms (Balaban, 2011).
Sound stimulation can induce analgesia (hypoalgesia) in humans and other mammals (Benedek and Szikszay, 1985; Gardner and Licklider, 1959; Helmstetter and Bellgowan, 1994; Marone, 1968; Ramar et al., 2016; Shankar et al., 1999; Stevens and Domer, 1973). These findings have led to the use of white noise and music to induce audio-analgesia in some medical settings (Baghdadi, 2000; Blass, 1975; Gardner et al., 1959; Handley, 1970; Horowitz, 1992; Marone, 1968; Mitchell et al., 2006; Ramar et al., 2016; Simkin and Bolding, 2004). A recent review found that 70% experienced a 50% decrease in pain with music-analgesia as well as a reduction in the amount of opioid medication (Cepeda et al., 2006). Interestingly, audio-analgesia can be suppressed by naltrexone, an opioid antagonist. These results suggest that pain suppression evoked by moderate intensity sounds and music may be mediated in part by endogenous opioid signaling (Flor et al., 2002; Goldstein, 2013; Helmstetter et al., 1994; Yilmaz et al., 2010). However, other signaling pathways related to anxiety and stress could also be involved (Helmstetter et al., 1994)
It has long been known that very intense sounds around 130 dB SPL can evoke aural pain (Franks et al., 1996; Gierke et al., 1953). Based on studies of crossmodal binding through neural coherences, we speculated that aural pain evoked by intense sounds might exacerbate the pain evoked by thermal stimulation (Pomper et al., 2013; Senkowski et al., 2008). However, we are unaware of any studies that have investigated changes in pain sensitivity involving intense sounds (Helmstetter et al., 1994; Shankar et al., 1999) near the aural threshold of pain (Franks et al., 1996; Gierke et al., 1953). Therefore, in Experiment I, we tested the hypothesis that intense sounds near the aural threshold of pain might exacerbate thermal pain sensitivity. To accomplish this, we measured thermal TF latencies under ambient sound conditions and in the presence of broadband noise (BBN) presented at intensities from 80 to 120 dB SPL. Because audio-analgesia is thought to be mediated in part through the opioid-pain pathway, (Winters et al., 2017), we attempted to modify the sensitivity of the opioid pathway by administering fentanyl, a μ-opioid agonist (Hauser et al., 2014; Painter and Crofford, 2013; Stricker et al., 2017). Fentanyl normally reduces pain (analgesia); however, when the drug is administered as a single high dose, fentanyl induces a post-treatment state of heightened pain sensitivity (i.e., hyperalgesia) (Celerier et al., 2006; Celerier et al., 2000; Rivat et al., 2002; Van Elstraete et al., 2008). Therefore, in Experiment II, we tested for opioid-induced changes in audio-analgesia after administering a high-dose of fentanyl. Because pain hyperacusis is often associated with hearing loss, we speculated that cochlear hearing loss might lead to a change in thermal tail-pain sensitivity due to neuroplastic changes in the central nervous system involving auditory and/or pain pathways (Eggermont, 2017; Kraus et al., 2010; Meredith and Allman, 2012; N’Gouemo and Faingold, 1999; Segerdahl et al., 2015). Therefore, in Experiment III, we tested for changes in pain sensitivity after unilaterally exposing rats to an intense noise that induced a severe hearing loss.
2. Materials and methods
2.1. Animals
Thirty-six male SASCO Sprague-Dawley rats (3 to 4 months old, mean body weight of 325 ± 50 g, Charles River Laboratories, Wilmington, MA) were used in this study. Twelve rats were used in Experiment I, Experiment II and Experiment III. All procedures in this study were reviewed and approved by the University at Buffalo Institutional Animal Care and Use Committee in accordance with NIH guidelines for the use and care of animals in research.
2.2. Thermal TF latency
TF latencies were used to assess thermal pain sensitivity as described previously by others (Bellgowan et al., 1996; Bellgowan et al., 1998; Foo and Helmstetter, 1999). Rats were first acclimated to the testing procedure in which their tails were immersed in 37 °C water several times per session over multiple days during a two-week acclimation period. Acclimation sessions were conducted four times per week during the two-week acclimation period. Tails were submerged three times per session. Rats were habituated to the test environment and remained calm in the restrainer. Acclimation to the test environment was similar across animals.
During TF testing, the rat was placed in a restrainer (#FB-L, Braintree Scientific, Inc., Braintree, MA) located 46 cm above the floor of the test apparatus. The rat’s tail protruded through a hole at the back of the restrainer allowing it to naturally descended downward. A 2-liter temperature-controlled bath filled with 52 ± 0.2 °C water was placed below the back of the rat restrainer. The height of the water bath was adjusted so that the last 2.25 cm of the rat’s tail was immersed in the water of the bath. Prior to TF testing, the water bath was covered with a sheet of plastic to prevent the rat’s tail from entering the water. During TF testing, the plastic sheet was removed and the tip of the rat’s tail was placed into the 52 °C water. The experimenter, blind to the experimental treatment, used a stop watch to record the time it took for the rat to withdraw its tail from the water. A second experimenter, blind to the testing stimuli, performed the test. TF latency was used to assess the level of thermal nociception (Bohn et al., 2000; Janssen et al., 1963; Kovelowski et al., 1999; Mogil et al., 1999).
2.3. Effects of Sound Stimulation on TF Latency
TF latencies were assessed in ambient noise (no stimulus, NS) and in the presence of BBN presented at 80, 90, 100, 110 or 120 dB SPL. Each NS and BBN trial lasted 60 seconds. During the last 15 seconds of each trial, the rat’s tail was immersed in the warm water. TF latency was measured from the time it took for the rat to abruptly remove its tail from the warm water (Helmstetter et al., 1994). The BBN (1–20 kHz) was synthesized using a personal computer and Tucker-Davis Technology hardware (RM1). The electrical signal was routed to an amplifier (Crown XLS202) and then to a loudspeaker (D-59; GMI Sound Corp., NY, USA) located 10 cm above the restrainers. Sound levels were calibrated with a sound level meter coupled to a half-inch condenser microphone (Model 824, Larson Davis). TF latencies were measured in ambient room noise (~45 dB SPL) and during the presentation of 80, 90, 100, 110 or 120 dB SPL BBN. On each test day, a rat was evaluated on only one of the six conditions (ambient noise or 80, 90, 100, 110 or 120 dB SPL BBN); the order of presentation of the six conditions was randomized. Tests were carried out once every third day. On each test day, three trials were run; each trial was separated by approximately 30 seconds and the average of the three trials comprised a data point.
2.4. Noise exposure
The noise exposure used in Experiment III causes a severe unilateral hearing loss and massive loss of hair cell loss and auditory nerve fibers as described in our previous publications (Baizer et al., 2015; Kraus et al., 2011; Kraus et al., 2010; Manohar et al., 2019). The electrical signal used for the noise exposure was generated by a D/A converter (Tucker-Davis Technology, RM1). The electrical signal was amplified (Crown XLS202) and delivered to a loudspeaker (D-59; GMI Sound Corp., NY, USA). The noise was calibrated using a sound level meter (Model 824, Larson Davis equipped with a ½ inch condenser microphone) as described in our earlier publications (Baizer et al., 2015; Kraus et al., 2011; Kraus et al., 2010; Manohar et al., 2019). The rats used in Experiment III were anesthetized with a combination of ketamine (100 mg/kg, i.p.) and xylazine (20 mg/kg, intraperitoneal, i.p.). The left ear of the rat was exposed for 2 h to a 126 dB SPL narrow band noise (100 Hz bandwidth) centered at 12 kHz. The speaker was placed approximately 10 mm from the opening of the left ear canal. A foam earplug was inserted into the contralateral ear canal and covered with petroleum jelly. Sham control rats underwent the same procedure, but without the noise exposure.
2.5. Hearing assessment
Our auditory brainstem response (ABR) procedures have been described previously (Manohar et al., 2017). Approximately 4 week following the noise exposure, the rats were anesthetized with ketamine (50 mg/kg, i.p.) and xylazine (6 mg/kg, i.p.) and placed on a heating pad (FHC, model 40-90-2) to maintain body temperature at 37 °C. Subdural electrodes were placed at the vertex (non-inverting), ipsilateral mastoid (inverting) and hind limb (ground). Stimuli were delivered to each ear through the sound delivery tube connected to a loudspeaker of a commercial ABR test system (Intelligent Hearing Systems, IHC, Miami Florida). ABRs were collected using a computerized stimulus presentation and data acquisition protocol (IHC, Miami Florida) with Smart-EP 10 version software. Neural responses were amplified, filtered (30–3000 Hz) and digitized (1024 presentations, 40 kHz sampling rate) in response to 4, 12, 16 and 32 kHz tone bursts (1 ms rise/fall, cosine gated, 5 ms duration, 21/s). Stimulus presentations began at a high intensity where a clear and reproducible ABR waveform was present and then the intensity was lowered in 10 dB steps until the response disappeared. The ABR threshold was defined as the lowest intensity at which the ABR waveform was consistently detectable. ABR threshold measurements were obtained from both the left and right ear of the noise-exposed rats and in the left ear of the sham control rats. During ABR testing, a foam earplug was inserted securely into the contralateral, non-test ear and covered with petroleum jelly while tone-bursts were presented to the ipsilateral test ear. After completing the ABR testing in ipsilateral test ear, the earplug material was removed and placed in the opposite ear and tone bursts presented to the unplugged test ear to determine the ABR thresholds in that ear.
2.6. Fentanyl administration
The rats treated with fentanyl received four consecutive subcutaneous injections of the drug delivered every 15 min; each 2-ml injection contained 100 μg/kg of fentanyl for a total of 400 μg/kg. Rats in the sham control group received four 2-ml subcutaneous injections of 0.9% saline separated by 15-minutes.
2.7. Experimental Design
In Experiment I, rats were evaluated on the TF latency test with no-stimulus (NS) except ambient background noise (~45 dB SPL) or BBN presented at 80, 90, 100, 110 and 120 dB SPL. The purpose of the study was to identify the sound levels that induced audio analgesia (hypoalgesia) and hyperalgesia. In Experiment II, rats in the fentanyl group and sham fentanyl control group were evaluated on the TF latency tests during the NS condition before and then 3-hours, 1-day, 3-days, 6-days and 10-days post-treatment. The goal of these measurements was to confirm that fentanyl reduced thermal pain sensitivity (analgesia) shortly after it was administered and to determine if it induced post-treatment hyperalgesia and when thermal pain sensitivity returned to normal. In Experiment II, the fentanyl group and sham control group were also evaluated 10-d post-treatment to determine if fentanyl pre-treatment disrupted sound-evoked audio analgesia and multisensory interactions between auditory and opioid pain pathways. TF latencies were evaluated in the NS ambient condition and when BBN was presented at 80, 90, 100, 110 and 120 dB SPL. Because cochlear hearing loss has been shown to alter multisensory integration, including changes in somatosensory and pain processing regions, we speculated that noise-induced hearing loss might alter thermal pain sensitivity (Allman et al., 2009; Meredith et al., 2012; Meredith et al., 2016; Moshourab et al., 2017; Shore et al., 2008; Zeng et al., 2012). Therefore, in Experiment III, we unilaterally exposed rats to intense noise and measured the hearing in noise-exposed rats and sham control rats 4-weeks post-treatment. To test for noise-induced changes in pain sensitivity, we measured TF latencies in the NS ambient condition in noise-exposed rats and sham control rats before and 1-, 2-, 3- and 4-weeks post-exposure.
2.8. Statistics
The statistical analyses were carried out with GraphPad Prism (version 5.01) or SigmaStat (version 3.5) as described detail below.
3. Results
3.1. Experiment I: Effect of sound intensity on thermal pain sensitivity
Very high intensity sounds can induce aural pain (Franks et al., 1996; Gierke et al., 1953), which could combine with and exacerbate thermal pain sensitivity. To test for sound-induced changes in thermal pain sensitivity, TF latencies were measured in the ambient NS condition and in the presence of BBN presented at 80, 90, 100, 110 or 120 dB SPL. The mean (n=12, +/− SEM) TF latency was 4.6 sec in the NS condition, but gradually increased with intensity to a mean latency of 6.4 sec when the BBN was presented at 90 dB SPL (Fig. 1). Further increases in intensity caused a gradual decline in latency from 6.4 sec at 90 dB SPL to 2.7 sec at 120 dB SPL. A one-way repeated measure ANOVA revealed a statistically significant effect of intensity (F (5,66 df) =8.76, p<0.0001). The TF-latency at 100 dB SPL was significantly longer than in the NS condition (Tukey’s post-hoc, p<0.05) consistent with earlier studies showing that moderate intensity sounds can reduced thermal pain sensitivity, i.e., audio-analgesia (Bellgowan et al., 1998; Helmstetter et al., 1994). In contrast, the TF-latency at 120 dB SPL was significantly shorter (Tukey’s post-hoc, p<0.05) than in ambient NS condition. These results suggest that as sound levels rise above ~100 dB SPL, pain sensitivity begins to increase and eventually gives way to sound-induced hyperalgesia at 120 dB SPL, near the aural threshold of pain (Franks et al., 1996; Gierke et al., 1953).
Figure 1:
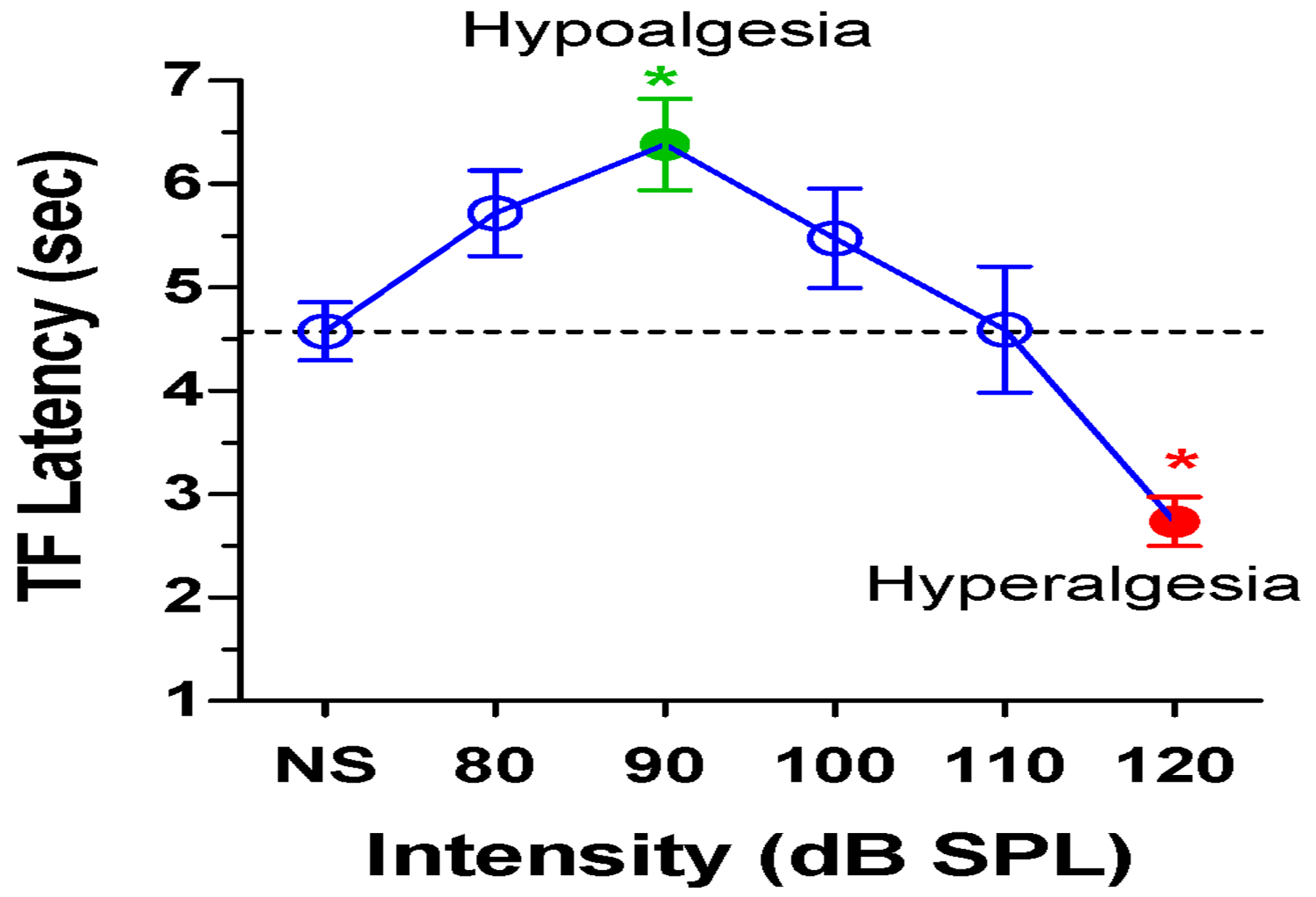
Mean (n=12, +/− SEM). TF-latencies with no stimulus (NS) or with 80 to 120 dB SPL BBN. TF-latency at 90 dB SPL is significantly longer (green filled circle, p<0.05) than with NS. TF-latency at 120 dB SPL (red filled circle) is significantly shorter than with NS. (red circle, p<0.05).
3.2. Experiment II: Fentanyl disrupts audio analgesia
Fentanyl is a potent analgesic that sometimes triggers a post-treatment period of enhanced pain sensitivity or hyperalgesia (Celerier et al., 2006; Celerier et al., 2000; Rivat et al., 2002). Therefore, rats were pre-treated with a high dose of fentanyl to assess its effects on tail pain sensitivity by measuring TF latencies in the ambient NS condition before and after fentanyl treatment. In the sham control group, TF latencies remained stable (~4.5 to 5 sec) from pre-treatment out to 10-days post-treatment (Fig. 2). In contrast, TF latencies in the fentanyl group increased significantly from approximately 4.5 sec pre-treatment to 14 sec 3-h post-treatment evidence of the drug’s potent analgesic properties. TF latencies then declined to 7-sec 1-day post-treatment and then to approximately 4.5 sec from 3- to 10-days post-treatment, values similar to those obtained pre-treatment and in sham controls. In contrast to our prediction based on earlier studies in rats and mice using different pain-assessment methods, we did not observe an enhancement in thermal tail pain sensitivity (Celerier et al., 2006; Celerier et al., 2000; Chang et al., 2018; Rivat et al., 2002). A two-way repeated measure ANOVA revealed a significant effect of treatment (F (1, 10 df) = 10.43, p<0.0011). TF-latencies at 3-h post-treatment were significantly longer in the fentanyl group than the sham control group (Bonferroni post-hoc, p<0.001). Thus, the large single dose of fentanyl induced a potent analgesic 3-h post-treatment (i.e., hypoalgesia); however, there was no evidence of post-treatment thermal hypoalgesia or hyperalgesia between 3-d and 10-d post-fentanyl.
Figure 2:

Mean (+/− SEM, n=6/group). TF latencies in the sham control group and fentanyl group are measured pre-treatment and 3 h, 1-day, and 3, 6 and 10 days after fentanyl treatment. At 3-d post-treatment, TF latencies in the fentanyl group are significantly longer than in the sham control group (Bonferroni post-hoc test, p<0.0011).
To determine if fentanyl pre-treatment in Experiment II exerted any long-term effects on audio analgesia, TF latencies were measured 10-d following fentanyl treatment. Mean (+/−SEM, n=6) TF latencies in the sham control group increased from approximately 4.4 sec in the ambient NS condition to roughly 6.7 sec at 90 dB SPL, evidence of audio hypoalgesia. Further increases in intensity caused TF latencies to decline to approximately 2.9 sec at 120 dB SPL, demonstrating once again that intensities near the aural threshold of pain induce hyperalgesia consistent with the results of Experiment I. However, mean TF latencies in the fentanyl group followed a completely different pattern when the intensity of the BBN was increased. Surprisingly, mean (+/−SEM, n=6) TF latencies with 90, 100, 110 and 120 dB BBN were all much shorter than the latency for the ambient NS condition. There was a significant interaction between the control group and fentanyl group that depended on intensity (F (10, 1 df) =26.71, p<0.001); mean latencies at 90, 100 and 110 dB SPL were significantly different between the sham control group and the fentanyl group (Newman-Keuls post-hoc, p<0.05). There was a significant effect of intensity (F (5, 1 df) = 10.36, p<0.001). Within the fentanyl group, TF latencies at 90, 100, 110 and 120 dB SPL were significantly less than for the NS condition (p<0.05, Newman-Keuls post-hoc) indicative of audio-hyperalgesia.
3.3. Experiment III: Noise-induced hearing loss increases pain sensitivity
Cochlear hearing loss can lead to neuroplastic changes in the central nervous system that can affect multisensory integration. To determine if cochlear hearing loss could alter thermal pain sensitivity, rats were unilaterally exposed to a 126 dB SPL noise for 2-h. Mean ABR thresholds in the noise-exposed ear ranged from 83 dB SPL at 4 kHz to 100 dB SPL at 32 kHz (Fig. 4). Mean ABR thresholds in the noise-exposed ears were significantly higher than those in the plugged ear and also significantly higher than those in the sham control group (F (2, 15 df) = 456, p<0.0001). Thresholds in the exposed ears were significantly higher than those in the unexposed ears at 4, 12, 24 and 32 kHz (Bonferroni post-hoc comparison, p<0.001) and also significantly higher than those in the sham control ears at 4, 12, 24, and 32 kHz (Bonferroni post-hoc comparison, p<0.001). ABR thresholds in the plugged ears of the noise-exposed rats were significantly higher than those in the sham control group at 4 kHz (p<0.01), 12 kHz (p<0.001), 24 kHz (p<0.001) and 32 kHz (p<0.001) (Bonferroni post-hoc comparisons). These results confirm that the unilateral noise exposure induced a significant hearing loss in the exposed ear (48–60 dB) as well as a mild hearing loss (16–20 dB) hearing loss in the plugged ear of the noise exposed rats.
Figure 4:

Mean (+/− SEM, n=6/group). ABR thresholds are elevated 4-weeks after the 126 dB noise exposure. ABR thresholds in the noise-exposed ear are 46–53 dB higher than thresholds in the contralateral plugged ear at 4, 12, 24 and 24 kHz (***, p<0.001). Mean (+/− SEM) ABR thresholds are moderately higher (11–20 dB) in the plugged ear than the sham control ear at 4 (**, p<0.01), 12 (***, p<0.001), 24 (***, p<0.001) and 32 kHz (***, p<0.001) (see text for details).
To determine if noise-induced hearing loss would affect thermal pain sensitivity, TF latencies were measured in ambient NS condition before and 1 to 4-weeks post-exposure. Mean (+/−SEM, n=6) TF latencies in the sham rats remained relatively constant (~6.5 sec) over the pre-exposure and 4-week post-exposure testing period (Figure 5). However, mean TF latencies (+/−SEM, n=6/group) in the noise-exposed rats gradually declined from a pre-treatment value of approximately 6.4 sec to roughly 3 sec 4-weeks post-exposure. A two-way repeated measures ANOVA revealed a significant difference between the sham group and the noise-exposed group (F (1, 10 df) = 11.64, p<0.0067). TF latencies at 3-weeks and 4-weeks post-exposure were significantly smaller in the noise-exposed group than the sham group (p<0.001). These results indicate that noise-induced hearing loss can lead to a heightened state of thermal pain sensitivity several weeks following an intense noise exposure.
Figure 5:

Thermal tail pain sensitivity increases following a severe noise-induced hearing loss. Mean TF latencies (+/− SEM, n=6/group) are measured in ambient background noise, but TF latencies decrease during 3–4 weeks following the 2-h, 126 dB noise exposure in the noise-exposed group, but not in the sham control group. TF latencies 3 and 4 weeks post-exposure are significantly shorter in noise-exposed group than sham control group (#, p<0.001).
4. Discussion
4.1. High intensity sounds enhance thermal pain
Numerous studies have found that moderate intensity sounds up to approximately 90 dB SPL reduce thermal pain sensitivity (Benedek et al., 1985; Cepeda et al., 2006; Helmstetter et al., 1994; Marone, 1968; Shankar et al., 1999; Stevens et al., 1973). Our TF latency results (Fig. 1) are therefore consistent with these earlier reports of audio-analgesia using moderate sound intensities. A major new finding of our study was that TF latencies declined as BBN intensity increased from 90 to 120 dB SPL. Audio-analgesia was largely abolished at 110 dB SPL and at 120 dB SPL TF latencies became shorter than normal at an intensity near the aural threshold of pain (Franks et al., 1996; Gierke et al., 1953). Our results show for the first time that audio-analgesia in rats is limited to intensities below 100 dB SPL, that thermal pain sensitivity begins to increase above 110 dB SPL and that audio-hyperalgesia emerges for intensities near the aural thresholds of pain. These results together with other neuroanatomical and electrophysiological observations suggest that auditory and pain pathways can interact and influence one another (Dobek et al., 2014; Manohar et al., 2016; Norena et al., 2018; Tamari et al., 1974; Wang et al., 2019). While the interpretation of these new results are intriguing, additional studies need to be carried out with other forms of thermal pain, mechanical or chemical pain to confirm the generality of these findings (Kim et al., 2006; Li et al., 2011; Quintao et al., 2010; Szikszay et al., 1985). While our fentanyl studies suggest that pain pathways likely produce some of the observed effects, additional experiments should be conducted to determine the potential contribution of noise-induced stress and anxiety (Helmstetter et al., 1994; Watkins and Mayer, 1982) as well as the contribution of stimulus and response parameters which are known to influence audio-analgesia (Cranney, 1988; Szikszay et al., 1985).
4.2. Audio-hyperalgesia and aural pain threshold
Normal hearing subjects report that pure tones around 100 dB SPL are uncomfortably loud (Sherlock and Formby, 2005) whereas sound between 120 and 140 dB SPL evoke aural pain (Franks et al., 1996). Patients with hyperacusis, however, report that intensities well below 100 dB are uncomfortably loud. One report indicates that the average loudness discomfort level in hyperacusis patients was approximately 85 dB HL although loudness discomfort levels were much lower than this in some cases (Sheldrake et al., 2015). Some hyperacusis patients also experience pain sensations in and around the ear at intensities much lower than 120 dB SPL (Pollard, 2018). It has been suggested that the aural threshold for pain and pain hyperacusis might involve central mechanisms in which the gain of the central auditory system has been increased excessively to compensate for the cochlear hearing loss (Aazh et al., 2014; Diehl and Schaette, 2015; Sun et al., 2009; Tyler et al., 2014).
The neural mechanisms that give rise to aural pain and pain hyperacusis are poorly understood, although a number of studies have provided intriguing clues. Abnormally low aural pain thresholds and pain hyperacusis have been reported in patients with multiple sclerosis, a demyelinating disease that slows neural transmission in the auditory brainstem (Weber et al., 2002). Others have reported that hyperacusis is associated with pain, numbness and burning around the ear in patients with tonic tensor tympani syndrome, a condition associated with reduced tensor tympani reflex thresholds, middle ear muscle spasms and middle ear inflammation. These disturbances are believed to be caused by aberrant neural activity in the trigeminal nerve complex which innervates the tensor tympani and integrates sensory information from the head and neck (Norena et al., 2018; Westcott et al., 2013). Cochlear damage leads to increased expression of pain and inflammatory genes and proteins in the cochlear nucleus (Baizer et al., 2015; Manohar et al., 2016), one of several auditory regions that receives inputs from the trigeminal system (Jain and Shore, 2006; Li and Mizuno, 1997).
4.3. Fentanyl-induced delayed-onset sound-evoked thermal pain hyperalgesia
Systemic administration of naltrexone, a μ-opioid receptor antagonist, suppressed audio-analgesia shortly after administration suggesting that opioid signaling plays an important role in auditory and pain processing (Apfel et al., 1995; Bellgowan et al., 1998; Flor et al., 2002; Goldstein, 2013; Helmstetter et al., 1994). Targeted drug delivery of a μ-opioid receptor antagonist into the periaqueductal gray blocked audio analgesia, whereas local delivery of a kappa opioid receptor antagonist did not, results suggesting that hypoalgesia is strongly regulated by μ-opioid receptors in the periaqueductal gray (Bellgowan et al., 1998).
Fentanyl is a potent μ-opioid receptor agonist. When we systemically administered fentanyl to rats, it temporarily suppressed pain sensitivity as evidenced by increased TF latencies (Fig. 2). However, by 3-days post-exposure, its analgesic properties had completely disappeared. In contrast to earlier studies in mice and rats using different methods of pain assessment, we did not observe an immediate post-treatment period of enhanced thermal pain sensitivity (Celerier et al., 2006; Celerier et al., 2000; Chang et al., 2018; Rivat et al., 2002) for reasons that are unclear. One factor that could account for these differences is the dose of fentanyl administered to the subjects. In one study that reported evidence of fentanyl-induced hyperalgesia 1–2-days post-treatment (Chang et al., 2018), the dose of fentanyl that induced hyperalgesia was significantly different than the one used in our study. Moreover, in this earlier study, hyperalgesia was assessed with mechanical pressure to the tail whereas we assessed hyperalgesia with thermal tail pain. Resolution of these discrepancies will need further studies with different drug doses and assessment techniques.
Interestingly, when TF latencies were evaluated with sound stimulation 10-days post-treatment, we discovered for the first time that audio-analgesia (90–100 dB SPL) was completely abolished and unexpectedly replaced by delayed-onset sound-evoked hyperalgesia (90–110 dB SPL) (Fig. 3). One mechanism through which μ-opioids are believed to induce persistent hyperalgesia is by inducing a sustained increase in glutamate synaptic transmission mediated through N-methyl-D-aspartate (NMDA) receptors (Celerier et al., 2000). The NMDA-pronociceptive process can lead to long-lasting hyperalgesia (Celerier et al., 2001; Laulin et al., 1998). This long-lasting opiate-induced hyperalgesia could be blocked by NMDA-antagonists (Laulin et al., 1998; Rivat et al., 2002). This sustained increase in NMDA-mediated glutamatergic synaptic activity could enhance signaling within the aural pain pathway and promote audio-hyperalgesia in brain regions where auditory and pain information converge. Further research is needed to determine if the delayed-onset sound-evoked hyperalgesia is in fact mediated through the NMDA signaling pathway (Wala and Holtman, 2011). One area where opiate-mediated pain and auditory information converge is the periaqueductal gray (Bellgowan et al., 1998; McNally et al., 2004).
Figure 3:.
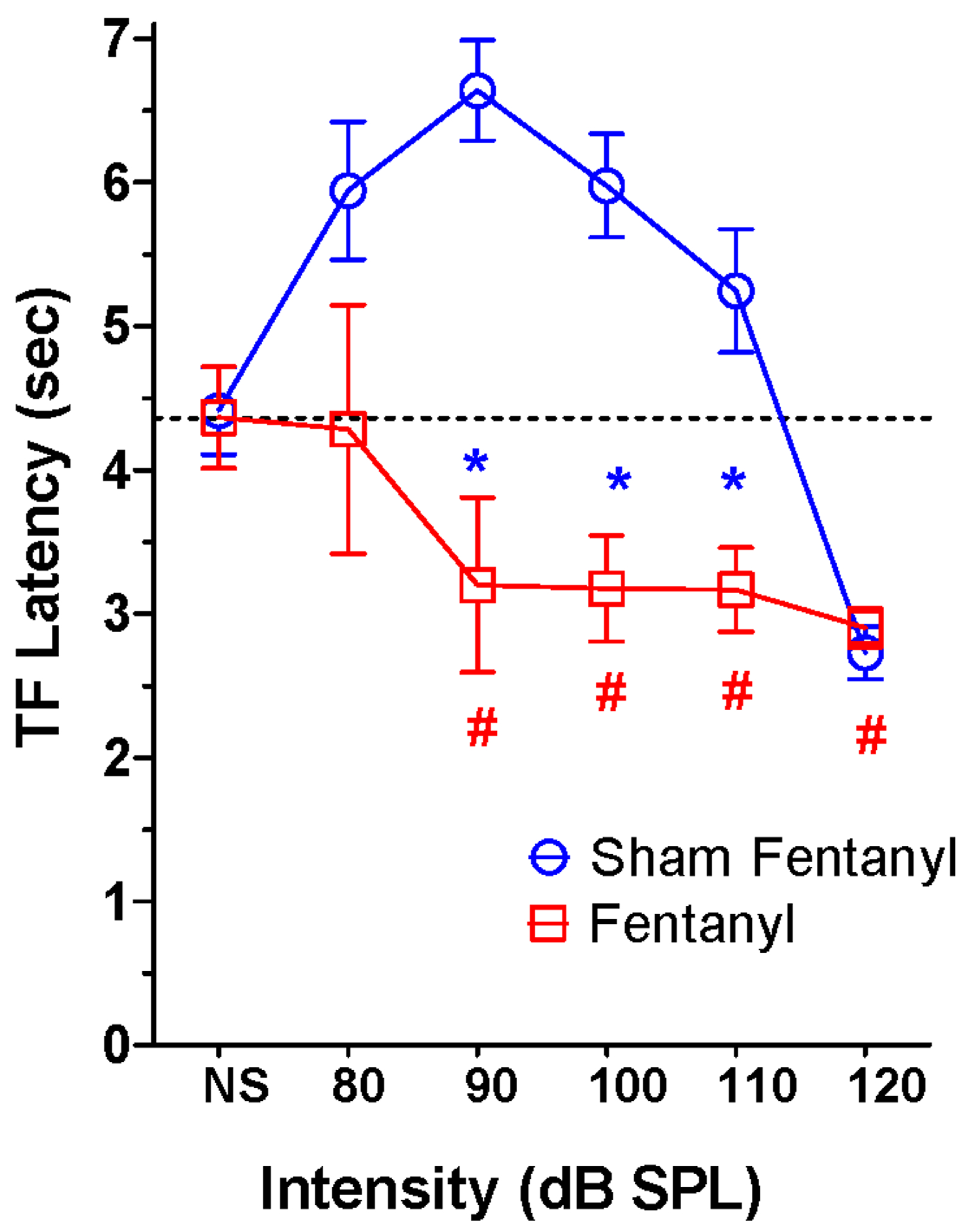
Mean (+/− SEM, n= 6/group). Sound-evoked TF latencies are dramatically altered 10-d post-fentanyl TF latencies are significantly smaller in the fentanyl group than the control group at 90, 100 and 110 dB SPL (* p<0.05, Newman-Keuls post-hoc). In the fentanyl group, TF latencies are significantly less at 90, 100, 110 and 120 dB SPL than in the NS condition (#, p<0.05, Newman-Keuls post-hoc).
Previous studies have shown that systemic administration of naltrexone, a μ-opioid receptor antagonist, suppressed audio analgesia around the time of administration (Apfel et al., 1995; Bellgowan et al., 1998; Flor et al., 2002; Goldstein, 2013; Helmstetter et al., 1994). This early-onset effect was the first to link the μ-opioid receptor to neural centers involved in auditory-pain processing. Our results add to this body of information by showing that prior fentanyl treatment induced a period of delayed-onset thermal pain hyperalgesia. Taken together, these results suggest that the μ-opioid receptor alone or in conjunction with NMDA receptors can significantly alter the way in which the auditory and pain pathways interact to influence thermal pain processing (Celerier et al., 2000).
Imaging studies have revealed numerous brain regions where auditory and pain signaling interact during audio-analgesia (Dobek et al., 2014). One intriguing candidate region is the periaqueductal gray; an area in which μ-opioid receptors have been implicated in hypoalgesia (Bellgowan et al., 1998; Rodriguez-Munoz et al., 2012) and in which imaging studies have revealed robust changes during audio-analgesia (Dobek et al., 2014). The periaqueductal gray receives collaterals from ascending temperature and pain fibers in the spinothalamic tract (Zhang et al., 1990). In addition, it receives inputs from several auditory nuclei and responds to acoustic stimulation (Halladay and Blair, 2012; McNally et al., 2004; Parsons et al., 2014; Radmilovich et al., 1991; Wang et al., 2019).
4.3. Noise-induced hearing loss enhances thermal pain sensitivity
Cochlear hearing loss can give rise to neuroplastic changes in the central nervous system leading to profound changes in neural function in brain regions associated with sensory processing and multisensory integration (Allman et al., 2009; Bizley and King, 2009; Meredith et al., 2012; Meredith et al., 2016; Moshourab et al., 2017; Shore et al., 2008; Zeng et al., 2012). To determine if noise-induced hearing loss might disrupt thermal pain sensitivity, we measured thermal TF latencies before and after inducing a significant unilateral hearing loss (Fig. 5). TF latencies gradually declined with post-exposure time in the noise-exposed group, but not in sham control group. Latencies in the noise group were significantly shorter than normal 3- and 4-weeks post-exposure. To our knowledge, these results show for the first time that noise-induced hearing loss can lead to increased thermal pain sensitivity.
The mechanisms through which hearing loss alters pain sensitivity are unclear, but a number of intriguing possibilities exist. Ascending temperature sensitive fibers from the spinothalamic tract and auditory system enter the periaqueductal gray, an important pain processing center in the midbrain (Zhang et al., 1990). Ascending spinothalamic tract fibers also project to other auditory processing areas such as the inferior colliculus and medial geniculate body (Berkley and Hand, 1978; Ledoux et al., 1987; Winer and Morest, 1983). Following cochlear damage, extensive remodeling occurs in auditory structures that integrate information from the spinal trigeminal nucleus (Basura et al., 2012; Dehmel et al., 2012; Illing et al., 2005; Jain et al., 2006; Reisch et al., 2007). Imaging studies of trigeminal nociception have revealed strong connectivity with many pain centers including the periaqueductal gray, insula, somatosensory cortex and thalamus during trigeminal nociception (Schulte et al., 2016).
Microglia activation plays an important role in neuropathic pain following peripheral nerve injury (Bartel and Finger, 2013; Durrenberger et al., 2004; Hulsebosch, 2012; Inoue and Tsuda, 2009; Lewis, 2013). The 126 dB SPL noise exposure used in this study induced strong microglia activation in the cochlear nuclei, a region in the auditory brainstem that integrates acoustic information with somatosensory signals from the face and spinal cord (Shore, 2005; Zeng et al., 2012; Zhou and Shore, 2004). We observed strong microglia activation in the cochlear nuclei 1-month post-exposure (Baizer et al., 2015); microglia activation spatially and temporally overlapped auditory nerve fiber degeneration patterns for months following the exposure. The same noise exposure also significantly upregulated the expression of several genes involved in neuroinflammation and neuropathic pain 2-weeks post-exposure (Manohar et al., 2016). Our results suggest that noise-induced neuroinflammation and microglia activation in the cochlear nuclei do not begin immediately after noise exposure, but is delayed because of the time it takes for auditory nerve fibers to begin degenerating.
The noise-induced changes in nociception observed in this study could conceivably arise from locomotor changes caused by damage to the vestibular system or noise-induced changes in anxiety. The 126 dB SPL noise exposure used in this study caused significant unilateral hearing loss in the exposed ear (Fig. 3), but we did not observe any behavioral evidence of unilateral vestibular function (e.g., circling behavior). We have shown previously that this unilateral noise exposure causes considerable hair cell loss in the noise-exposed ear and considerable auditory nerve loss and microglia activation in the cochlear nuclei on the side of the noise exposure. The nerve fiber degeneration and microglia activation in the cochlear nuclei began several weeks post-exposure and persisted for more than 6 months. However, we found no evidence of fiber degeneration or microglia activation in adjacent brainstem vestibular nuclei (Baizer et al., 2015; Kraus et al., 2011). Thus, the functional changes noted above are unlikely due to locomotor changes to the vestibular damage. We did not carry our studies to test for noise-induced changes in anxiety in this study, but in other ongoing studies using the same 126 dB SPL exposure, we could not find evidence of anxiety-like behavior on the open field test or elevated plus maze. Thus it is unlikely that changes in anxiety could play a role in the nociceptive changes reported above.
We also observed some differences in mean TF latencies between rats in Experiment I and Experiments III. The reasons for these differences are unclear, but could be related to two procedural differences. First, different groups of animals were used in Experiment I and Experiment II. Second, different investigators measured the TF latencies in Experiment I and Experiment II. Despite these differences between experiments, these variables are unlikely to affect the results because the two factors were held constant within each experiment. Therefore, the significant differences between sham and experimental animals seen in Experiment I and Experiment III are likely valid, an interpretation supported by the clear-cut trends that occurred within each individual experiment.
4.4. Limitations
We observed compelling evidence for sound-evoked hypoalgesia (80–90 dB SPL) and hyperalgesia (120 dB SPL) by applying heat to the tail (Fig. 1), but it remains to be seen whether sound stimulation could modulate cold or mechanical sensitivity applied to the tail or other regions of the body or if the same trends are observed at higher or lower water temperatures. Similarly, it is unclear whether fentanyl pre-treatment or hearing loss could modulate cold or mechanical sensitivity in the same way as it did for thermal stimulation. In order to compare our finding with earlier works on audio-analgesia and to avoid fluctuations in analgesia associated with the estrus cycle, we used male rats only (Bellgowan et al., 1998; Helmstetter et al., 1994; Ryan and Maier, 1988; Shankar et al., 1999). Having observed a clear bidirectional effect of intensity on pain sensitivity in males, further research is needed to determine if there are gender differences. Because individuals with pain hyperacusis often experience pain in regions around the ear, regions innervated by the trigeminal nerve complex, a logical extension of this study would be to examine sound-induced hypoalgesia and hypoalgesia using mechanical, cold or heat stimulation delivered around the ear or face. While previous studies have reported a post-fentanyl period of hyperalgesia, we found no evidence of this in our study. This could be due to our testing schedule, strain of rat being tested, the type of noxious stimulus and/or the region of the body where the noxious stimulus was delivered.
5. Conclusion
The present study elucidates for the first time the bidirectional interactions between sound intensity and thermal nociception (Fig. 1) and provides new insights into the complex interplay that can occur between the auditory and pain pathways. The interactions between auditory and pain signaling were dramatically altered by prior fentanyl administration. Our results showed for the first time that fentanyl pre-treatment enhances audio-hyperalgesia (Fig. 2) possibly due to prolonged changes in NMDA and/or μ-opioid signaling pathways in the central nervous system. Further work is needed to elucidate the mechanism(s) underlying this effect. Importantly, we showed for the first time that severe noise-induced hearing loss gradually leads to an increase in thermal pain sensitivity raising the possibility that hearing loss may contribute to chronic pain possibly due to chronic neuroinflammation (Ji et al., 2016; Taves et al., 2013). Evidence for noise-induced chronic neuroinflammation has been observed in regions of the auditory brainstem where somatosensory and auditory information converge (Baizer et al., 2015; Manohar et al., 2016). However, further research is needed to gain a mechanistic understanding of how this occurs. Taken together, these studies provide fertile new ground for exploring the complex interactions between auditory and pain signaling that may improve our understanding of pain hyperacusis and the neuroplastic changes that can occur in the central nervous system as the results of cochlear hearing loss.
Supplementary Material
Highlights.
Auditory stimulation induces both audio-analgesia and audio-hyperalgesia
Fentanyl disrupts multi-sensory interactions between auditory and pain pathways
Fentanyl pre-treatment enhances audio-analgesia
Noise-induced hearing loss enhances pain sensitivity
Noise-induced hearing loss may promote pain hyperacusis
Acknowledgments
Research supported in part by a grant from NIH National Institute on Deafness and Other Communicative Disorders (R01DC014452, RS) and Hearing Health Foundation (72364, SM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aazh H, McFerran D, Salvi R, Prasher D, Jastreboff M, Jastreboff P 2014. Insights from the first international conference on hyperacusis: Causes, evaluation, diagnosis and treatment. Noise Health 16, 123–6. [DOI] [PubMed] [Google Scholar]
- Allman BL, Keniston LP, Meredith MA 2009. Adult deafness induces somatosensory conversion of ferret auditory cortex. Proc Natl Acad Sci U S A 106, 5925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apfel SC, Newel M, Dormia C, Kessler JA 1995. Kappa opioid receptors participate in nerve growth factor-induced hyperalgesia. Neuroscience 68, 1199–206. [DOI] [PubMed] [Google Scholar]
- Baghdadi ZD 2000. Evaluation of audio analgesia for restorative care in children treated using electronic dental anesthesia. Journal of Clinical Pediatric Dentistry 25, 9–12. [PubMed] [Google Scholar]
- Baizer JS, Wong KM, Manohar S, Hayes SH, Ding D, Dingman R, Salvi RJ 2015. Effects of acoustic trauma on the auditory system of the rat: The role of microglia. Neuroscience 303, 299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban CD 2011. Migraine, vertigo and migrainous vertigo: Links between vestibular and pain mechanisms. J Vestib Res 21, 315–21. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Finger TE 2013. Reactive microglia after taste nerve injury: comparison to nerve injury models of chronic pain. F1000Res 2, 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basura GJ, Koehler SD, Shore SE 2012. Multi-sensory integration in brainstem and auditory cortex. Brain Res 1485, 95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellgowan PS, Helmstetter FJ 1996. Neural systems for the expression of hypoalgesia during nonassociative fear. Behav Neurosci 110, 727–36. [DOI] [PubMed] [Google Scholar]
- Bellgowan PS, Helmstetter FJ 1998. The role of mu and kappa opioid receptors within the periaqueductal gray in the expression of conditional hypoalgesia. Brain Res 791, 83–9. [DOI] [PubMed] [Google Scholar]
- Benedek G, Szikszay M 1985. Sensitization or tolerance to morphine effects after repeated stresses. Prog Neuropsychopharmacol Biol Psychiatry 9, 369–80. [DOI] [PubMed] [Google Scholar]
- Benison AM, Chumachenko S, Harrison JA, Maier SF, Falci SP, Watkins LR, Barth DS 2011. Caudal granular insular cortex is sufficient and necessary for the long-term maintenance of allodynic behavior in the rat attributable to mononeuropathy. J Neurosci 31, 6317–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkley KJ, Hand PJ 1978. Efferent projections of the gracile nucleus in the cat. Brain Res 153, 263–83. [DOI] [PubMed] [Google Scholar]
- Bizley JK, King AJ 2009. Visual influences on ferret auditory cortex. Hear Res 258, 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blass BC 1975. Sound analgesia. Journal of the American Podiatry Association 65, 963–71. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Xu F, Gainetdinov RR, Caron MG 2000. Potentiated opioid analgesia in norepinephrine transporter knock-out mice. J Neurosci 20, 9040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G 2001. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci 21, 4074–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Gonzalez JR, Maldonado R, Cabanero D, Puig MM 2006. Opioid-induced hyperalgesia in a murine model of postoperative pain: role of nitric oxide generated from the inducible nitric oxide synthase. Anesthesiology 104, 546–55. [DOI] [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G 2000. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology 92, 465–72. [DOI] [PubMed] [Google Scholar]
- Cepeda MS, Carr DB, Lau J, Alvarez H 2006. Music for pain relief. Cochrane Database of Systematic Reviews, CD004843. [DOI] [PubMed] [Google Scholar]
- Chang L, Ye F, Luo Q, Tao Y, Shu H 2018. Increased Hyperalgesia and Proinflammatory Cytokines in the Spinal Cord and Dorsal Root Ganglion After Surgery and/or Fentanyl Administration in Rats. Anesth Analg 126, 289–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranney J 1988. Analgesia Following Startle-Eliciting Stimuli. Psychobiology 16, 67–69. [Google Scholar]
- de Klaver MJ, van Rijn MA, Marinus J, Soede W, de Laat JA, van Hilten JJ 2007. Hyperacusis in patients with complex regional pain syndrome related dystonia. J Neurol Neurosurg Psychiatry 78, 1310–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehmel S, Pradhan S, Koehler S, Bledsoe S, Shore S 2012. Noise overexposure alters long-term somatosensory-auditory processing in the dorsal cochlear nucleus--possible basis for tinnitus-related hyperactivity? J Neurosci 32, 1660–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl PU, Schaette R 2015. Abnormal Auditory Gain in Hyperacusis: Investigation with a Computational Model. Front Neurol 6, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobek CE, Beynon ME, Bosma RL, Stroman PW 2014. Music modulation of pain perception and pain-related activity in the brain, brain stem, and spinal cord: a functional magnetic resonance imaging study. J Pain 15, 1057–68. [DOI] [PubMed] [Google Scholar]
- Durrenberger PF, Facer P, Gray RA, Chessell IP, Naylor A, Bountra C, Banati RB, Birch R, Anand P 2004. Cyclooxygenase-2 (Cox-2) in injured human nerve and a rat model of nerve injury. Journal of the Peripheral Nervous System 9, 15–25. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ 2017. Acquired hearing loss and brain plasticity. Hear Res 343, 176–190. [DOI] [PubMed] [Google Scholar]
- Flor H, Birbaumer N, Schulz R, Grusser SM, Mucha RF 2002. Pavlovian conditioning of opioid and nonopioid pain inhibitory mechanisms in humans. Eur J Pain 6, 395–402. [DOI] [PubMed] [Google Scholar]
- Foo H, Helmstetter FJ 1999. Hypoalgesia elicited by a conditioned stimulus is blocked by a mu, but not a delta or a kappa, opioid antagonist injected into the rostral ventromedial medulla. Pain 83, 427–31. [DOI] [PubMed] [Google Scholar]
- Fournier P, Schonwiesner M, Hebert S 2014. Loudness modulation after transient and permanent hearing loss: implications for tinnitus and hyperacusis. Neuroscience 283, 64–77. [DOI] [PubMed] [Google Scholar]
- Franks JR, Stephenson MMD, Merry CJ 1996. Preventing occupational hearing loss : a practical guide U.S. Dept. of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, Division of Biomedical and Behavioral Science, Physical Agents Effects Branch, Bethesda, Md.? [Google Scholar]
- Gardner WJ, Licklider JC 1959. Auditory analgesia in dental operations. Journal of the American Dental Association 59, 1144–9. [DOI] [PubMed] [Google Scholar]
- Gierke HEV, Davis H, Eldredge DH, Hardy JD 1953. Aural pain produced by sound In Benox Report, An Exploratory Study of the Biological Effects of noise. Project NR 144079. Office of Naval Research. University of Chicago Press. [Google Scholar]
- Goldstein A 2013. Thrills in response to music and other stimuli. Physiological Psychology 8, 126–129. [Google Scholar]
- Halladay LR, Blair HT 2012. The role of mu-opioid receptor signaling in the dorsolateral periaqueductal gray on conditional and unconditional responding to threatening and aversive stimuli. Neuroscience 216, 82–93. [DOI] [PubMed] [Google Scholar]
- Handley LJ 1970. A “Sound” approach to analgesia. Journal of the Rocky Mountain Analgesia Society 2, 39–40. [PubMed] [Google Scholar]
- Hauser W, Bock F, Engeser P, Tolle T, Willweber-Strumpfe A, Petzke F 2014. Long-term opioid use in non-cancer pain. Dtsch Arztebl Int 111, 732–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstetter FJ, Bellgowan PS 1994. Hypoalgesia in response to sensitization during acute noise stress. Behavioral Neuroscience 108, 177–85. [DOI] [PubMed] [Google Scholar]
- Horowitz LG 1992. Audiotaped relaxation, implosion, and rehearsal for the treatment of patients with dental phobia. General Dentistry 40, 242–7. [PubMed] [Google Scholar]
- Hulsebosch CE 2012. Special issue on microglia and chronic pain. Exp Neurol 234, 253–4. [DOI] [PubMed] [Google Scholar]
- Illing RB, Kraus KS, Meidinger MA 2005. Reconnecting neuronal networks in the auditory brainstem following unilateral deafening. Hear Res 206, 185–99. [DOI] [PubMed] [Google Scholar]
- Inoue K, Tsuda M 2009. Microglia and neuropathic pain. Glia 57, 1469–79. [DOI] [PubMed] [Google Scholar]
- Irimia P, Cittadini E, Paemeleire K, Cohen AS, Goadsby PJ 2008. Unilateral photophobia or phonophobia in migraine compared with trigeminal autonomic cephalalgias. Cephalalgia 28, 626–30. [DOI] [PubMed] [Google Scholar]
- Ison JR, Allen PD, O’Neill WE 2007. Age-related hearing loss in C57BL/6J mice has both frequency-specific and non-frequency-specific components that produce a hyperacusis-like exaggeration of the acoustic startle reflex. J Assoc Res Otolaryngol 8, 539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain R, Shore S 2006. External inferior colliculus integrates trigeminal and acoustic information: unit responses to trigeminal nucleus and acoustic stimulation in the guinea pig. Neuroscience Letters 395, 71–5. [DOI] [PubMed] [Google Scholar]
- Janssen PA, Niemegeers CJ, Dony JG 1963. The inhibitory effect of fentanyl and other morphine-like analgesics on the warm water induced tail withdrawl reflex in rats. Arzneimittel-Forschung 13, 502–7. [PubMed] [Google Scholar]
- Ji RR, Chamessian A, Zhang YQ 2016. Pain regulation by non-neuronal cells and inflammation. Science 354, 572–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Mittal DP, Iadarola MJ, Dionne RA 2006. Genetic predictors for acute experimental cold and heat pain sensitivity in humans. J Med Genet 43, e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipper M, Van Dijk P, Nunes I, Ruttiger L, Zimmermann U 2013. Advances in the neurobiology of hearing disorders: recent developments regarding the basis of tinnitus and hyperacusis. Prog Neurobiol 111, 17–33. [DOI] [PubMed] [Google Scholar]
- Kovelowski CJ, Bian D, Hruby VJ, Lai J, Ossipov MH, Porreca F 1999. Selective opioid delta agonists elicit antinociceptive supraspinal/spinal synergy in the rat. Brain Res 843, 12–7. [DOI] [PubMed] [Google Scholar]
- Kraus KS, Ding D, Jiang H, Lobarinas E, Sun W, Salvi RJ 2011. Relationship between noise-induced hearing-loss, persistent tinnitus and growth-associated protein-43 expression in the rat cochlear nucleus: does synaptic plasticity in ventral cochlear nucleus suppress tinnitus? Neuroscience 194, 309–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ 2010. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience 167, 1216–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laulin JP, Larcher A, Celerier E, Le Moal M, Simonnet G 1998. Long-lasting increased pain sensitivity in rat following exposure to heroin for the first time. Eur J Neurosci 10, 782–5. [DOI] [PubMed] [Google Scholar]
- Ledoux JE, Ruggiero DA, Forest R, Stornetta R, Reis DJ 1987. Topographic organization of convergent projections to the thalamus from the inferior colliculus and spinal cord in the rat. J Comp Neurol 264, 123–46. [DOI] [PubMed] [Google Scholar]
- Lewis S 2013. Pain: microglia take control in chronic pain. Nat Rev Neurosci 14, 154. [DOI] [PubMed] [Google Scholar]
- Li H, Mizuno N 1997. Single neurons in the spinal trigeminal and dorsal column nuclei project to both the cochlear nucleus and the inferior colliculus by way of axon collaterals: a fluorescent retrograde double-labeling study in the rat. Neurosci Res 29, 135–42. [DOI] [PubMed] [Google Scholar]
- Li JY, Xie W, Strong JA, Guo QL, Zhang JM 2011. Mechanical hypersensitivity, sympathetic sprouting, and glial activation are attenuated by local injection of corticosteroid near the lumbar ganglion in a rat model of neuropathic pain. Regional Anesthesia and Pain Medicine 36, 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar S, Ramchander PV, Salvi R, Seigel GM 2019. Synaptic Reorganization Response in the Cochlear Nucleus Following Intense Noise Exposure. Neuroscience 399, 184–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar S, Dahar K, Adler HJ, Dalian D, Salvi R 2016. Noise-induced hearing loss: Neuropathic pain via Ntrk1 signaling. Mol Cell Neurosci 75, 101–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manohar S, Spoth J, Radziwon K, Auerbach BD, Salvi R 2017. Noise-induced hearing loss induces loudness intolerance in a rat Active Sound Avoidance Paradigm (ASAP). Hear Res 353, 197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marone JG 1968. Suppression of pain by sound. Psychological Reports 22, 1055–6. [DOI] [PubMed] [Google Scholar]
- McNally GP, Pigg M, Weidemann G 2004. Opioid receptors in the midbrain periaqueductal gray regulate extinction of pavlovian fear conditioning. J Neurosci 24, 6912–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Allman BL 2012. Early hearing-impairment results in crossmodal reorganization of ferret core auditory cortex. Neural Plast 2012, 601591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith MA, Clemo HR, Corley SB, Chabot N, Lomber SG 2016. Cortical and thalamic connectivity of the auditory anterior ectosylvian cortex of early-deaf cats: Implications for neural mechanisms of crossmodal plasticity. Hear Res 333, 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell LA, MacDonald RA, Brodie EE 2006. A comparison of the effects of preferred music, arithmetic and humour on cold pressor pain. Eur J Pain 10, 343–51. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M 1999. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain 80, 67–82. [DOI] [PubMed] [Google Scholar]
- Moller AR, (Ed.) 2007. Neurophysiologic Abnormaliteis in Autism. Nova Science Publishers. [Google Scholar]
- Moshourab R, Begay V, Wetzel C, Walcher J, Middleton S, Gross M, Lewin GR 2017. Congenital deafness is associated with specific somatosensory deficits in adolescents. Sci Rep 7, 4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- N’Gouemo P, Faingold CL 1999. The periaqueductal grey is a critical site in the neuronal network for audiogenic seizures: modulation by GABA(A), NMDA and opioid receptors. Epilepsy Res 35, 39–46. [DOI] [PubMed] [Google Scholar]
- Norena AJ, Fournier P, Londero A, Ponsot D, Charpentier N 2018. An Integrative Model Accounting for the Symptom Cluster Triggered After an Acoustic Shock. Trends Hear 22, 2331216518801725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter JT, Crofford LJ 2013. Chronic opioid use in fibromyalgia syndrome: a clinical review. Journal of Clinical Rheumatology 19, 72–7. [DOI] [PubMed] [Google Scholar]
- Parsons CE, Young KS, Joensson M, Brattico E, Hyam JA, Stein A, Green AL, Aziz TZ, Kringelbach ML 2014. Ready for action: a role for the human midbrain in responding to infant vocalizations. Soc Cogn Affect Neurosci 9, 977–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard B 2018. Unravelling the mystery of hyperacusis with pain. ENT Audiol News 6, 61–62. [Google Scholar]
- Pomper U, Hofle M, Hauck M, Kathmann N, Engel AK, Senkowski D 2013. Crossmodal bias of visual input on pain perception and pain-induced beta activity. Neuroimage 66, 469–78. [DOI] [PubMed] [Google Scholar]
- Quintao NL, da Silva GF, Antonialli CS, Rocha LW, Cechinel Filho V, Ciccio JF 2010. Chemical composition and evaluation of the anti-hypernociceptive effect of the essential oil extracted from the leaves of Ugni myricoides on inflammatory and neuropathic models of pain in mice. Planta Medica 76, 1411–8. [DOI] [PubMed] [Google Scholar]
- Radmilovich M, Bertolotto C, Pena JL, Pedemonte M, Velluti R 1991. A search for a mesencephalic periaqueductal gray-cochlear nucleus connection. Acta Physiol Pharmacol Ther Latinoam 41, 369–75. [PubMed] [Google Scholar]
- Ramar K, Hariharavel VP, Sinnaduri G, Sambath G, Zohni F, Alagu PJ 2016. Effect of Audioanalgesia in 6- to 12-year-old Children during Dental Treatment Procedure. Journal of Contemporary Dental Practice 17, 1013–1015. [PubMed] [Google Scholar]
- Ramos Macias A, Falcon-Gonzalez JC, Manrique Rodriguez M, Morera Perez C, Garcia-Ibanez L, Cenjor Espanol C, Coudert-Koall C, Killian M 2018. One-Year Results for Patients with Unilateral Hearing Loss and Accompanying Severe Tinnitus and Hyperacusis Treated with a Cochlear Implant. Audiol Neurootol 23, 8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisch A, Illing RB, Laszig R 2007. Immediate early gene expression invoked by electrical intracochlear stimulation in some but not all types of neurons in the rat auditory brainstem. Exp Neurol 208, 193–206. [DOI] [PubMed] [Google Scholar]
- Rivat C, Laulin JP, Corcuff JB, Celerier E, Pain L, Simonnet G 2002. Fentanyl enhancement of carrageenan-induced long-lasting hyperalgesia in rats: prevention by the N-methyl-D-aspartate receptor antagonist ketamine. Anesthesiology 96, 381–91. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Munoz M, Sanchez-Blazquez P, Vicente-Sanchez A, Berrocoso E, Garzon J 2012. The mu-opioid receptor and the NMDA receptor associate in PAG neurons: implications in pain control. Neuropsychopharmacology 37, 338–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan SM, Maier SF 1988. The estrous cycle and estrogen modulate stress-induced analgesia. Behav Neurosci 102, 371–80. [DOI] [PubMed] [Google Scholar]
- Schulte LH, Sprenger C, May A 2016. Physiological brainstem mechanisms of trigeminal nociception: An fMRI study at 3T. Neuroimage 124, 518–525. [DOI] [PubMed] [Google Scholar]
- Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I 2015. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci 18, 499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkowski D, Schneider TR, Foxe JJ, Engel AK 2008. Crossmodal binding through neural coherence: implications for multisensory processing. Trends Neurosci 31, 401–9. [DOI] [PubMed] [Google Scholar]
- Shankar N, Awasthy N, Mago H, Tandon OP 1999. Analgesic effect of environmental noise: a possible stress response in rats. Indian Journal of Physiology and Pharmacology 43, 337–46. [PubMed] [Google Scholar]
- Sheldrake J, Diehl PU, Schaette R 2015. Audiometric characteristics of hyperacusis patients. Front Neurol 6, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherlock LP, Formby C 2005. Estimates of loudness, loudness discomfort, and the auditory dynamic range: normative estimates, comparison of procedures, and test-retest reliability. J Am Acad Audiol 16, 85–100. [DOI] [PubMed] [Google Scholar]
- Shore SE 2005. Multisensory integration in the dorsal cochlear nucleus: unit responses to acoustic and trigeminal ganglion stimulation. Eur J Neurosci 21, 3334–48. [DOI] [PubMed] [Google Scholar]
- Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S 2008. Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci 27, 155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkin P, Bolding A 2004. Update on nonpharmacologic approaches to relieve labor pain and prevent suffering. J Midwifery Womens Health 49, 489–504. [DOI] [PubMed] [Google Scholar]
- Stevens MW, Domer FR 1973. Alterations in morphine-induced analgesia in mice exposed to pain, light or sound. Archives Internationales de Pharmocodynamie et de Thérapie 206, 66–75. [PubMed] [Google Scholar]
- Stricker PA, Muhly WT, Jantzen EC, Li Y, Jawad AF, Long AS, Polansky M, Cook-Sather SD 2017. Intramuscular Fentanyl and Ketorolac Associated with Superior Pain Control After Pediatric Bilateral Myringotomy and Tube Placement Surgery: A Retrospective Cohort Study. Anesth Analg 124, 245–253. [DOI] [PubMed] [Google Scholar]
- Suhnan AP, Finch PM, Drummond PD 2017. Hyperacusis in chronic pain: neural interactions between the auditory and nociceptive systems. Int J Audiol 56, 801–809. [DOI] [PubMed] [Google Scholar]
- Sun W, Lu J, Stolzberg D, Gray L, Deng A, Lobarinas E, Salvi RJ 2009. Salicylate increases the gain of the central auditory system. Neuroscience 159, 325–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szikszay M, Benedek G, Hideg J 1985. Non-opiate analgesia following stressful acoustic stimulation. Physiol Behav 35, 135–8. [DOI] [PubMed] [Google Scholar]
- Tamari JW, Naccache A, To’mey GF, Jabbur SJ 1974. Auditory and visual influences on the trigeminal nuclear activity evoked by tooth pulp stimulation in the cat. Exp Neurol 45, 663–6. [DOI] [PubMed] [Google Scholar]
- Taves S, Berta T, Chen G, Ji RR 2013. Microglia and spinal cord synaptic plasticity in persistent pain. Neural Plast 2013, 753656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler RS, Pienkowski M, Roncancio ER, Jun HJ, Brozoski T, Dauman N, Dauman N, Andersson G, Keiner AJ, Cacace AT, Martin N, Moore BC 2014. A review of hyperacusis and future directions: part I. Definitions and manifestations. Am J Audiol 23, 402–19. [DOI] [PubMed] [Google Scholar]
- Van Elstraete AC, Sitbon P, Mazoit JX, Benhamou D 2008. Gabapentin prevents delayed and long-lasting hyperalgesia induced by fentanyl in rats. Anesthesiology 108, 484–94. [DOI] [PubMed] [Google Scholar]
- Wala EP, Holtman JR Jr. 2011. Buprenorphine-induced hyperalgesia in the rat. Eur J Pharmacol 651, 89–95. [DOI] [PubMed] [Google Scholar]
- Wang H, Chen J, Xu X, Sun WJ, Chen X, Zhao F, Luo MH, Liu C, Guo Y, Xie W, Zhong H, Bai T, Tian Y, Mao Y, Ye C, Tao W, Li J, Farzinpour Z, Li J, Zhou JN, Wang K, He J, Chen L, Zhang Z 2019. Direct auditory cortical input to the lateral periaqueductal gray controls sound-driven defensive behavior. PLoS Biol 17, e3000417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Mayer DJ 1982. Organization of endogenous opiate and nonopiate pain control systems. Science 216, 1185–92. [DOI] [PubMed] [Google Scholar]
- Weber H, Pfadenhauer K, Stohr M, Rosler A 2002. Central hyperacusis with phonophobia in multiple sclerosis. Mult Scler 8, 505–9. [DOI] [PubMed] [Google Scholar]
- Westcott M, Sanchez TG, Diges I, Saba C, Dineen R, McNeill C, Chiam A, O’Keefe M, Sharples T 2013. Tonic tensor tympani syndrome in tinnitus and hyperacusis patients: a multi-clinic prevalence study. Noise Health 15, 117–28. [DOI] [PubMed] [Google Scholar]
- Winer JA, Morest DK 1983. The medial division of the medial geniculate body of the cat: implications for thalamic organization. J Neurosci 3, 2629–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BL, Gregoriou GC, Kissiwaa SA, Wells OA, Medagoda DI, Hermes SM, Burford NT, Alt A, Aicher SA, Bagley EE 2017. Endogenous opioids regulate moment-to-moment neuronal communication and excitability. Nat Commun 8, 14611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz P, Diers M, Diener S, Rance M, Wessa M, Flor H 2010. Brain correlates of stress-induced analgesia. Pain 151, 522–9. [DOI] [PubMed] [Google Scholar]
- Zeng C, Yang Z, Shreve L, Bledsoe S, Shore S 2012. Somatosensory projections to cochlear nucleus are upregulated after unilateral deafness. J Neurosci 32, 15791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang DX, Carlton SM, Sorkin LS, Willis WD 1990. Collaterals of primate spinothalamic tract neurons to the periaqueductal gray. J Comp Neurol 296, 277–90. [DOI] [PubMed] [Google Scholar]
- Zhou J, Shore S 2004. Projections from the trigeminal nuclear complex to the cochlear nuclei: a retrograde and anterograde tracing study in the guinea pig. J Neurosci Res 78, 901–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


