Abstract
Objective:
To determine the relationship between iron deficiency (or iron-deficient, ID) and neural correlates of recognition memory depending on ID timing (gestation vs. infancy) and infant age at testing (9 vs. 18 months).
Study design:
Event-related potentials (ERP) were used in a visual recognition memory task (mother vs. stranger face) to compare healthy term infants according to iron status at birth and 9 months. Fetal-neonatal ID was defined as cord serum ferritin < 75 μg/l or zinc protoporphrin/heme ratio > 118 μmol/mol, postnatal ID as ≥ 2 abnormal iron measures at 9 months with normal cord-blood iron status, and iron-sufficient as not ID at birth or 9 months. Recognition of mother faces was measured by negative component (Nc) and late slow wave (LSW). These ERP components reflect attention and memory updating processes, respectively.
Results:
All groups showed differences in Nc amplitude elicited by mother and stranger faces at 9 months. At 18 months, only postnatal ID and iron-sufficient groups showed condition differences in Nc amplitude. However, the 2 groups were different in the involved brain regions. For LSW, only the 2 ID groups showed condition differences in amplitude at 9 months. At 18 months, condition differences were not observed in any group.
Conclusions:
This study indicates that the timing of ID in early life (fetal-neonatal vs. postnatal) modulates the impact of ID on recognition memory. Such impact also varies depending on the age of infants at testing (9 vs. 18 months).
Keywords: infancy, fetal-neonatal iron deficiency, postnatal iron deficiency, recognition memory, event-related potentials
INTRODUCTION
Iron deficiency (or iron-deficient, ID) affects an estimated 2 billion people worldwide, 20-30% of whom are pregnant women and young children.(1) Iron deficiency anemia (IDA) in infancy is associated with poorer developmental outcomes, with deficits that persist despite iron therapy.(2) However, most earlier studies focused on ID identified during the period of peak prevalence (6-24 months) and did not consider iron status at birth. Fetal-neonatal ID was largely ignored due to the previous assumption that the fetus is protected from maternal ID. Studies now show that the fetus is only partly buffered.(2) Because ID is common in pregnancy, it is important to assess potential neurodevelopmental effects of fetal-neonatal ID and determine if they differ from those observed with postnatal ID. Identifying such patterns may help better understand the relationship between ID and infant development and point to opportunities to improve prevention and intervention.
Animal studies that model early-life ID typically span late fetal and early postnatal life. They demonstrate ID effects on myelination, dendritic arborization, neurotransmitter function, and neurometabolism in multiple brain regions.(3) Impairments are prominent in both cortical and subcortical brain regions, such as the striatum, amygdala, and hippocampus, which work together to encode, consolidate, and store memory traces.(2, 3) Event-related potentials (ERP) provide a noninvasive way to examine the brain’s electrophysiological responses to cognitive tasks and can be used to study memory even in infants. Two studies used ERP to assess recognition memory in children who had IDA identified at 6-24 months – one during the IDA period (4) and the other at a 10-year follow-up.(5) Both reported poorer memory outcomes, but fetal-neonatal iron status was unknown. Only 2 ERP studies considered effects of fetal-neonatal ID on learning and memory. The first studied newborn infants with ID related to maternal diabetes during pregnancy and found impaired auditory recognition memory.(6) In a Chinese sample without such pregnancy complications, we demonstrated that infants with ID at birth also showed impaired auditory recognition memory at 2 months.(7) However, to our knowledge, no study has investigated whether the learning and memory effects of ID depend on when ID occurred, i.e., predominantly prenatal or postnatal. This is critical to screening and prevention of ID in order to prevent long-term effects, as the development of many neural systems span the fetal-neonatal and infancy periods.(8) The design of the China study allowed us to investigate the relations of fetal-neonatal (at birth) and postnatal (9 months) ID to recognition memory at 9 and 18 months. During the first 2 years of life, memory systems develop rapidly, which might make memory susceptible to ID in this period as reported by previous studies. (4, 5, 7) This study tested if ages at testing (represent different maturational level of memory systems) modulate the associations between ID and memory. Generally, we predicted that ERP responses would differ depending on ID timing and age of infants at testing.
METHODS
Participants
Participants were part of a collaborative Zhejiang University – University of Michigan longitudinal study on developmental and neuro-functional effects of ID in early life. Participants were from Fuyang County, Zhejiang Province, China. Between December 2008 and November 2011, women were randomly screened at routine visits at 36-37 weeks gestation (gestational age determined by ultrasound). Those with uncomplicated singleton pregnancies were invited to participate. Enrolled infants met the following entrance criteria:(7) singleton birth ≥ 37 weeks gestation, birth weight ≥ 2,500 g, no delivery complications or maternal and infant health problems, and normal hearing by screening brainstem auditory evoked potential response. Parents provided signed informed consent. The Institutional Review Boards of the University of Michigan and the Children’s Hospital of Zhejiang University approved the study.
At birth, 436 families agreed to participate in this developmental study. At 9 months, 246 of them underwent ERP assessment of recognition memory. At 18 months, 186 were assessed with ERP: 124 had had ERP testing at 9 months and 62 had not, generally due to parents’ work schedules or other scheduling conflicts. After preprocessing and excluding infants without enough data for analyses (see below), 87 infants (35%) were included for analyses at 9 months (30 fetal-neonatal ID, 20 postnatal ID, 37 iron-sufficient at both time points, Figure 1), and 112 (60%) at 18 months (43 fetal-neonatal ID, 26 postnatal ID, 43 iron-sufficient at both times, Figure 1). Approximately a third of infants with fetal-neonatal ID had ID or IDA at 9 or 18 months (see Table 1 and 2). Infants with IDA received iron therapy per clinical practice and infants with ID were monitored but not treated. Thirty-three infants had sufficient data for analysis at both 9 and 18 months.
Figure 1.
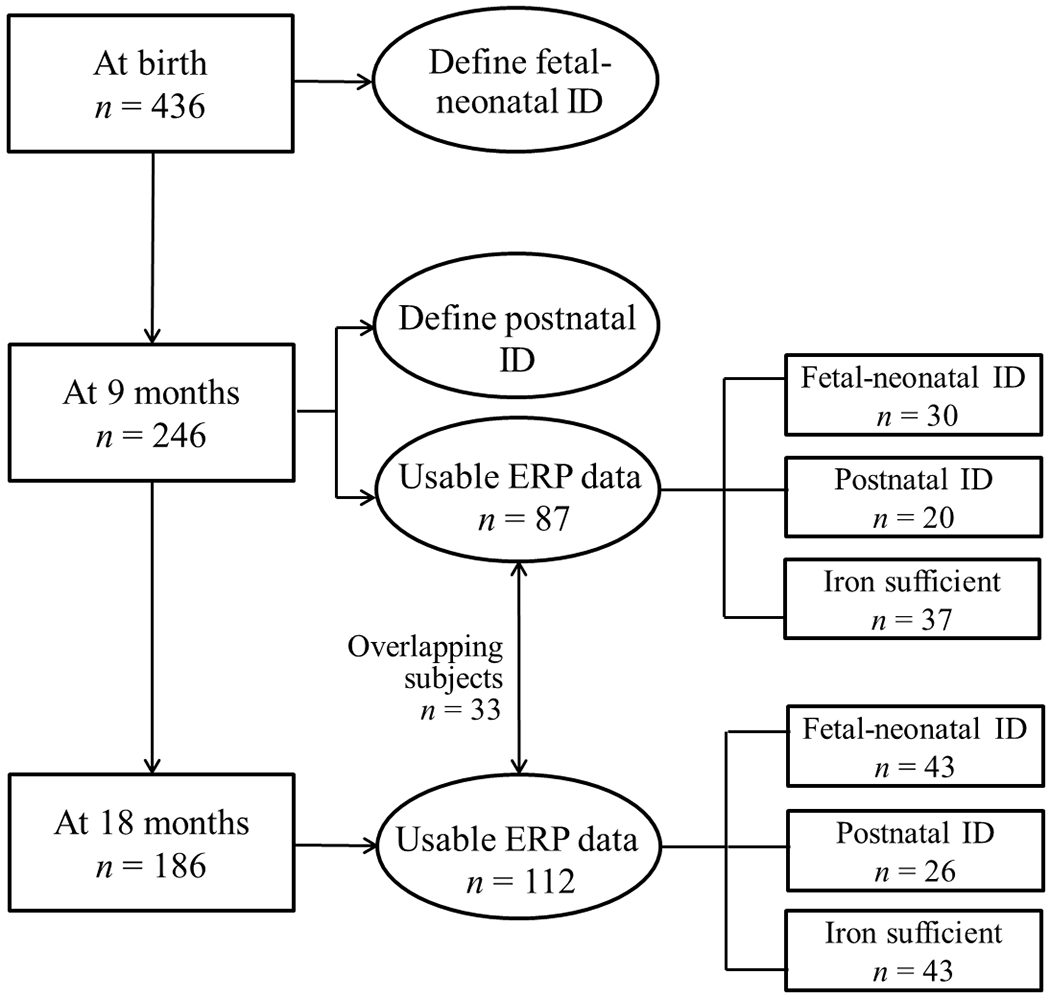
Scheme of this study
Table 1.
9- month-old sample characteristics and hematology variables by iron status group*
| Iron group | Fetal-neonatal ID | Postnatal ID | Control |
|---|---|---|---|
| n | 30 | 20 | 37 |
| Infant characteristics | |||
| Sex, % male (n) | 73.3 (22) | 45.0 (9) | 43.2 (16) |
| Delivery type, % vaginal (n) | 13.3 (4) | 20 (4) | 35.1 (13) |
| Birth weight, kg | 3.4 (0.5) | 3.3 (0.4) | 3.5 (0.4) |
| Age at ERP testing, d | 277 (10.1) | 286 (10.9) | 281 (11.7) |
| Weight-for-age z-score at ERP testing | 0.9 (1.0) | 0.6 (0.9) | 0.9 (1.3) |
| Parental education, % ≥ high school (n) | 60.0 (18) | 50.0 (9) | 41.7 (15) |
| Low-income family, % yes (n)¶ | 20.0 (6) | 50.0 (11) | 55.6 (21) |
| Fetal-neonatal iron status (cord blood) | |||
| Hemoglobin, g/L | 150.4 (17.8) | 152.8 (29.6) | 145.3 (16.8) |
| Zinc protoporphrin/heme (ZPP/H), μmol/mol § | 129.8 (41.1) | 95.9 (13.5) | 85.1 (18.8) |
| Soluble transferrin receptor (sTfR), mg/L § | 4.7 (3.2) | 5.0 (2.4) | 4.7 (3.1) |
| Ferritin, μg/L | 80.6 (70.1) | 191.1 (84.1) | 169.1 (59.5) |
| 9-month iron status | |||
| Hemoglobin, g/L | 110.6 (10.3) | 106.4 (7.7) | 116.5 (8.5) |
| Zinc protoporphrin/heme (ZPP/H), μmol/mol § | 111.6 (35.3) | 123.6 (31.4) | 90.7 (26.4) |
| Soluble transferrin receptor (sTfR), mg/L § | 4.8 (3.1) | 4.8 (2.3) | 4.0 (1.9) |
| Mean corpuscular volume (MCV), fl | 75.9 (5.7) | 73.4 (4.8) | 79.0 (3.1) |
| Red cell distribution width (RDW), % | 13.9 (2.0) | 15.1 (1.2) | 12.8 (0.7) |
| Ferritin, μg/L | 34.6 (23.7) | 29.2 (29.5) | 44.2 (21.8) |
| ID without anemia at 9 months, % (n) | 10.0 (3) | 20.0 (4) | 0.0 (0) |
| IDA at 9 months, % (n) | 23.3 (7) | 80.0 (16) | 0.0 (0) |
Values are means (standard deviation) for continuous variables and % (n) for categorical ones. Variables in bold indicate significant group differences. Ns vary slightly due to missing data.
Low income (<50,000 yuan per year) was based on Fuyang County Housing. Assistance Policy for Low Income Families, 2012.
Values are geometric means for these variables, which were log-transformed for analysis.
Table 2.
18- month-old sample characteristics and hematology variables by iron status group*
| Iron group | Fetal-neonatal ID | Postnatal ID | Control |
|---|---|---|---|
| n | 43 | 26 | 43 |
| Infant characteristics | |||
| Sex, % male (n) | 51.2 (22) | 46.2 (12) | 53.5 (23) |
| Delivery type, % vaginal (n) | 16.3 (7) | 23.1 (6) | 25.6 (11) |
| Birth weight, kg | 3.5 (0.4) | 3.2 (0.4) | 3.5 (0.4) |
| Age at ERP testing, d | 554 (10.6) | 555 (16.6) | 555 (10.9) |
| Weight-for-age z-score at ERP testing | 0.5 (0.9) | 0.6 (0.8) | 0.5 (0.9) |
| Parental education, % ≥ high school (n) | 64.1 (25) | 80.0 (20) | 69.0 (29) |
| Low-income family, % yes (n)¶ | 42.1 (21) | 60.0 (16) | 52.4 (23) |
| Fetal-neonatal iron status (cord blood) | |||
| Hemoglobin, g/L | 147.2 (18.4) | 147.7 (25.3) | 143.2 (16.1) |
| Zinc protoporphrin/heme (ZPP/H), μmol/mol § | 117.7 (27.8) | 95.7 (14.4) | 87.1 (14.0) |
| Soluble transferrin receptor (sTfR), mg/L § | 4.9 (3.3) | 4.2 (2.9) | 3.9 (2.4) |
| Ferritin, μg/L | 84.1 (74.3) | 187.7 (77.0) | 176.5 (48.1) |
| 9-month iron status | |||
| Hemoglobin, g/L | 110.6 (7.4) | 106.6 (8.2) | 115.7 (8.8) |
| Zinc protoporphrin/heme (ZPP/H), μmol/mol § | 110.7 (35.8) | 124.9 (54.9) | 94.7 (28.1) |
| Soluble transferrin receptor (sTfR), mg/L § | 4.7 (2.6) | 5.5 (3.4) | 4.4 (2.5) |
| Mean corpuscular volume (MCV), fl | 76.6 (5.2) | 74.0 (4.8) | 79.5 (2.7) |
| Red cell distribution width (RDW), % | 13.7 (1.4) | 14.7 (1.3) | 12.8 (0.9) |
| Ferritin, μg/L | 46.0 (34.9) | 37.3 (43.4) | 54.2 (39.4) |
| ID without anemia at 9 months, % (n) | 11.6 (5) | 30.8 (8) | 0 (0) |
| IDA at 9 months, % (n) | 20.9 (9) | 69.2 (18) | 0 (0) |
Values are means (standard deviation) for continuous variables and % (n) for categorical ones. Variables in bold indicate significant group differences. Ns vary slightly due to missing data.
Low income (<50,000 yuan per year) was based on Fuyang County Housing. Assistance Policy for Low Income Families, 2012.
Values are geometric means for these variables, which were log-transformed for analysis.
We compared iron status, background characteristics, and temperament between infants excluded or included at 9 months as well as between those followed up or not followed up between 9 and 18 months. We found no differences between groups with respect to iron status, background characteristics, or temperament (e.g., activity level, fearfulness, distress to limitation, and soothability as measured by the Infant Behavior Questionnaire) (ps > .10).(9)
Procedure
Iron status: we measured iron status at birth from cord blood and at 9 months using venous blood. Measures of iron status included hemoglobin (Hb), serum ferritin (SF), serum transferrin receptor (sTfR), and zinc protoporphyrin/heme ratio (ZPP/H) and, at 9 months, mean corpuscular volume (MCV) and red cell distribution width (RDW). The assay methods were documented in a previous publication.(10) Fetal-neonatal ID was defined as cord SF < 75 μg/l or ZPP/H > 118 μmol/mol.(11–13) Postnatal ID was defined as normal cord iron status at birth and ≥ 2 abnormal iron measures at 9 months using the following cutoffs for 4 iron measures: MCV < 74 fl,(14) RDW > 14.5%,(15) SF < 12.0 μg/l,(16) and ZPP/H > 69 μmol/mol.(17) sTfR was not used in classification, because methods differ and there was no standard cutoff in infancy. IDA was defined as ID plus anemia (Hb < 110 g/l).(1)
ERP paradigm, recording and preprocessing: The ERP paradigm used a visual recognition memory task.(4) Mother’s and stranger’s faces were randomly presented to infants one by one on a screen 65 centimeters away. Faces, varying for each infant, were presented for 500 ms, each face repeated 60 times. No face was presented consecutively more than twice. New trials started when the experimenter judged that the infant was looking at the screen, with inter-trial intervals randomized between 1500 and 2000 ms. The procedure lasted 4-5 minutes.
ERP data were recorded with a 128-channel Hydrocel Geodesic Sensor net, NetAmps hardware, and Net Station data acquisition software. ERP recordings were performed for quiet and alert infants who were seated on the mother’s lap in an electronically shielded room. Four Electrooculography (EOG) channels (channels 125, 126, 127, and 128) were excluded. The vertex electrode was the reference channel. The signal was filtered online with 0.1-100 Hz bandpass and sampled at 500 Hz/channel. Impedance for all channels was kept lower than 50 kΩ during recording. Experimenters were blind to infant iron status.
Data preprocessing used Net Station 4.4 (Electrical Geodesics Inc., Eugene, OR), including filtering, artifact rejection, interpolation, and re-reference. The ERP data were filtered offline with a 30-Hz low-pass and segmented into 100 ms before and 1700 ms after stimuli onset. Ocular artifacts were inspected using the remaining 7 eye channels (1, 8, 14, 17, 21, 25, and 32). Other channels were checked for artifacts (i.e., movement artifact, drift). Data for an individual channel in each segment were rejected if any artifact was detected or the difference between the maximum and minimum amplitude was higher than 200 μV. Segments with > 18 rejected channels or ocular artifacts or other significant noise were rejected. Spherical spline interpolation was applied to construct rejected channels. The averaged data of all segments for each channel were re-referenced to the average potential of all channels. Infants with at least 10 artifact-free segments for each condition were included in final analyses.
Previous studies indicate that mother’s and stranger’s faces elicit 2 ERP components related to memory (4, 7), specifically, negative component (Nc) and late slow wave (LSW). Nc, defined as a negative peak in frontal regions occurring between 300 and 600 ms after stimuli onset, is thought to measure the attention process modulated by memory. LSW, defined as a positive-going slow wave in anterior temporal electrodes and a negative-going slow wave in posterior temporal electrodes occurring about 1100 ms after stimuli onset, is thought to reflect memory updating or encoding. Based on previous studies (4, 7) and the characteristics of our data, regions of interest (ROI) were defined (Figure 2): 3 for Nc, left frontal, middle frontal, and right frontal and 4 for LSW, left anterior temporal, right anterior temporal, left posterior temporal, and right posterior temporal. The average of all channels in each region across timing windows was computed to quantify the amplitude of Nc and LSW. Differences in Nc and/or LSW amplitude elicited by mother vs. stranger faces suggest that infants differentiate the 2 faces (i.e., infants recognize their mothers’ faces). Nc latency findings were not reported due to no significant differences between iron groups.
Figure 2.
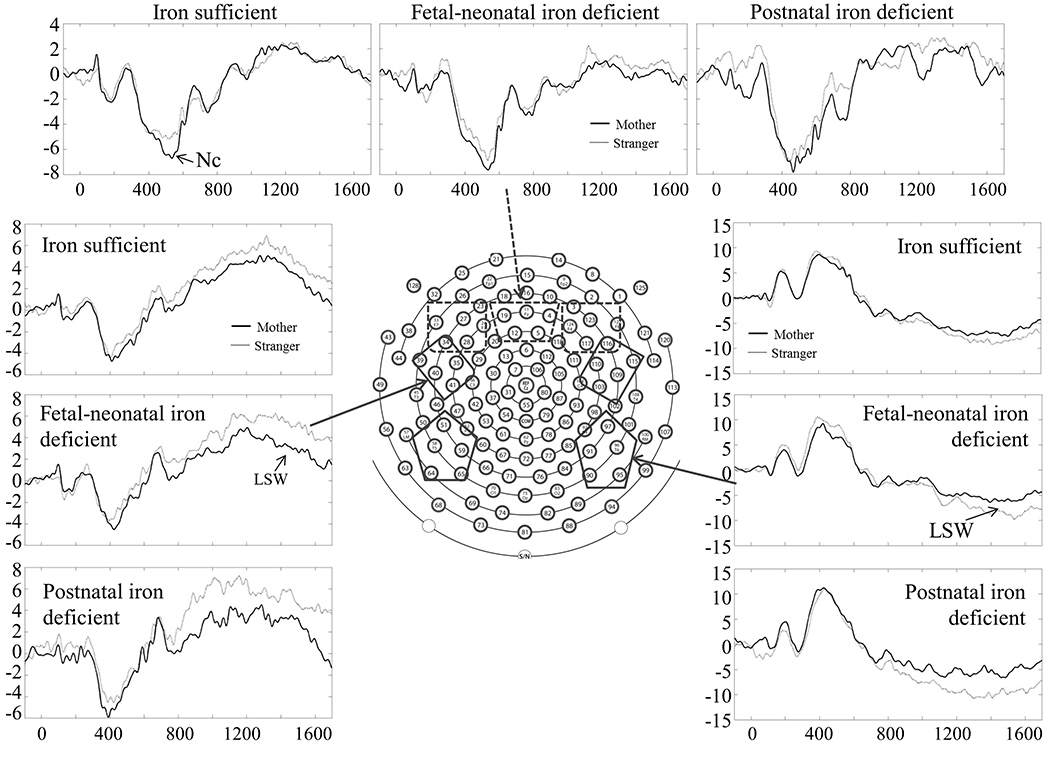
Regions of interest and waveforms of Nc and LSW (9-month data as an example). Dashed and solid boxes mark regions of interest for Nc and LSW, respectively.
Statistical analysis
IBM SPSS Statistics 20 (IBM Corp., Chicago, IL) was used to carry out statistical analyses. ANOVA or chi-square tests were run to test age or iron group differences in background characteristics. Differences showing p-values ≤ .10 were considered as potential confounding variables.
Nc and LSW amplitude from 9- and 18-month ERP recordings were separately entered as dependent measures in repeated measure ANCOVA models. For Nc, Condition (mother and stranger) and Hemisphere (left, middle, and right) were entered as repeated measures. For LSW, Condition (mother and stranger), Anterior-Posterior, and Hemisphere (left, middle, and right) were repeated measures. For both Nc and LSW, Age and Iron Group were between-subject factors. Background variables showing differences between Age or/and Iron Groups were entered as continuous covariates. For statistically significant findings involving interactions between Condition, Iron group, and/or Age, further analyses were carried out to understand the interactions with background variables included. Post-hoc analyses were not corrected for multiple comparisons due to hypotheses-testing nature of the study and the argument that automatic correcting for multiple comparisons is not in the best interest of good empirical science (18). Additionally, power analyses indicated that we needed 123 subjects to get significant ID effects with power value as 0.80, or 153 subjects with power value as 0.90.
RESULTS
Sample characteristics
Tables 1 and 2 show background characteristics and iron status measures for infants with usable ERP data at 9 and 18 months, respectively. A 9 months, iron status groups differed in age (F (2, 87) = 4.42, p = .02), sex (χ2 (2, N = 87) = 5.34, p = .03), family income (χ2 (2, N = 84) = 9.17, p = .01), and delivery type (suggestive trend, p = .10). At 18 months, groups differed in birth weight (F (2, 102) = 3.85, p = .02). Weight-for-age z-scores (F (1, 196) = 5.02, p = .03) and parental education (χ2 (2, N = 190) = 7.74, p = .005) differed between 9- and 18-month samples. Some fetal-neonatal ID infants were also ID in infancy (9-month sample: 7 IDA and 3 ID without anemia; 18-month sample: 9 IDA and 5 ID without anemia). The postnatal ID group consisted of 16 IDA and 4 ID without anemia in the 9-month sample and 18 IDA, 8 ID without anemia in the 18-month sample. Iron status was good at 18 months (data not shown): only 1 infant had IDA and 3 had ID without anemia.
Statistical details for post-hoc analyses of Nc and LSW within each iron group were presented in Table 3. The observed effect sizes for the results of post-hoc analyses were generally high with η squared (η2) between 0.12 and 0.40.(19)
Table 3.
Statistical details for the analyses of Nc and LSW
| ERP indices | Age group | Fetal-neonatal ID | Postnatal ID | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Main effects or Interactions | F | p | η2 | Main effects or Interactions | F | p | η2 | Main effects or Interactions | F | p | η2 | ||
| Nc | 9 months | ||||||||||||
| 18 months | Condition × Hemisphere | 4.13 | .03 | .16 | Condition effect | 5.10 | .03 | .12 | |||||
| Condition effect in right hemisphere | 5.78 | .03 | .21 | ||||||||||
|
| |||||||||||||
| LSW | 9 months | Condition × Anterior-Posterior × Hemisphere | 13.68 | .001 | .36 | Condition × Anterior-Posterior | 5.65 | .03 | .30 | ||||
| Condition × Hemisphere in anterior area | 9.98 | .004 | .29 | Condition effect in anterior area | 8.5 | .01 | .40 | ||||||
| Condition effect in left anterior temporal area | 12.85 | .001 | .35 | Condition × Hemisphere in posterior area | 7.09 | .02 | .35 | ||||||
| Condition effect in right posterior temporal area | 4.66 | .05 | .26 | ||||||||||
| 18 months | |||||||||||||
Nc
The repeated measures ANCOVA indicated Condition × Hemisphere (F (2, 346) = 3.34, p = .039, η2 = 0.02) and Condition × Hemisphere × Age (suggestive trend, F (2, 346) = 2.82, p = .06, η2 = 0.02) interactions. To understand these interactions, we ran separate repeated measures ANCOVA for 9 months (age, delivery type, and sex as covariates) and 18 months (birth weight as covariate).
9 months
Nc amplitude was more negative in mother vs. stranger condition, implying that infants invested different amounts of attention resources to process mother vs. stranger faces at this age (F (1, 76) = 7.20, p = .01, η2 = 0.09). There were no significant iron group differences.
18 months
There was an interaction between Condition, Hemisphere, and Iron Group. Further analyses revealed a main effect of Condition in the iron-sufficient group, with more negative Nc amplitude in mother vs. stranger condition (Figure 3). We also found an interaction between Condition and Hemisphere in the postnatal ID group. Postnatal ID infants showed more negative Nc amplitude in mother vs. stranger condition in the right hemisphere (Figure 3). These findings suggested that postnatal ID and iron-sufficient groups engaged more brain resources to process mother vs. stranger faces but differed in the involved brain regions. There was no condition difference in Nc amplitude in the fetal-neonatal ID group.
Figure 3.
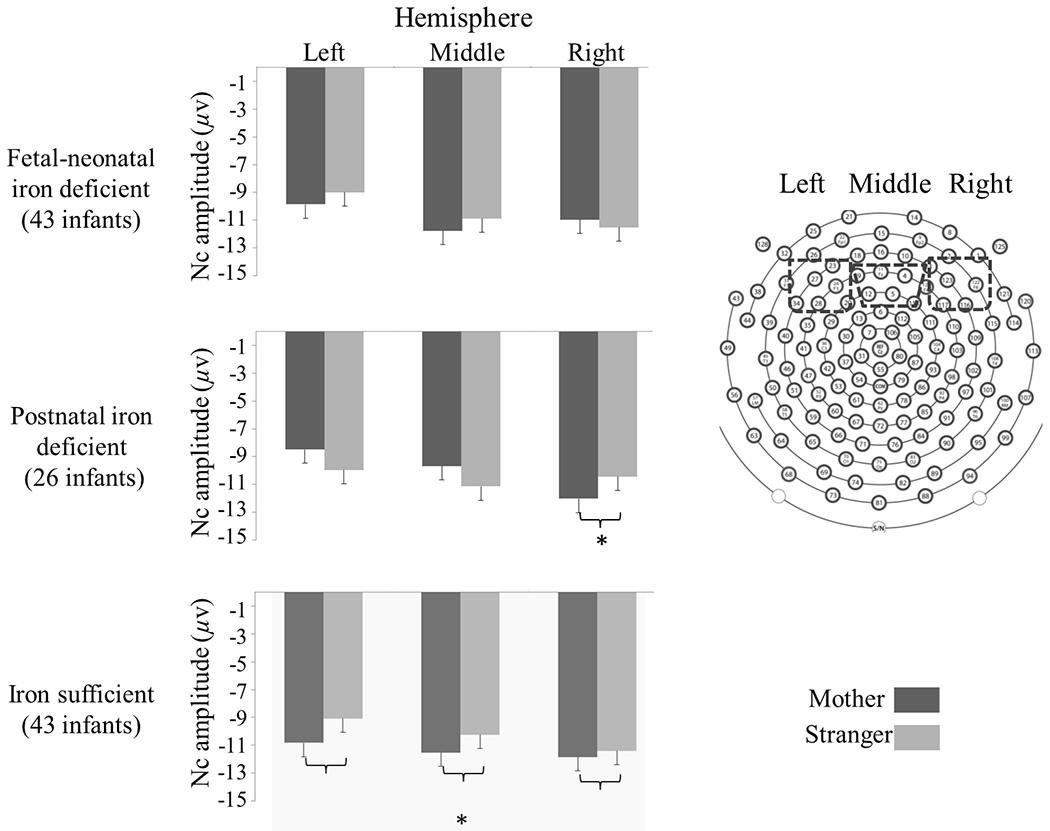
Mean and standard error (SE, error bar) of Nc peak amplitude in each condition and region for 18-month-olds. The postnatal ID group showed a condition difference only in the right hemisphere; the iron-sufficient group showed a main effect of condition without hemisphere difference (*p < .05).
To understand the Condition × Hemisphere × Iron Group interaction better, we tested Condition × Iron Group in each hemisphere. This 2-way interaction was significant only in the left hemisphere (F (1, 101) = 3.09, p = .05, η2 = 0.06). Post-hoc analyses indicated that postnatal ID and iron-sufficient groups showed distinct patterns in the condition difference in Nc amplitude (p = .015, difference value = 3.149, 95% CI: 0.63 to 5.66, Figure 3).
LSW
The analyses indicated Condition × Anterior-Posterior × Hemisphere × Iron Group (F (2, 173) = 7.90, p = .001, η2 = 0.08) and Condition × Anterior-Posterior × Age × Iron Group (suggestive trend, F (2 173) = 2.54, p = .08, η2 = 0.03) interactions. With the same covariates as for Nc, we ran separate repeated measures ANCOVA for 9 and 18 months.
9 months
There was a 4-way interaction: Condition × Anterior-Posterior × Hemisphere × Iron Group (F (2, 76) = 5.20, p = .008, η2 = 0.12). Further analysis indicated that the iron-sufficient group did not show condition differences in any region, whereas there was an interaction between Condition, Anterior-Posterior, and Hemisphere in infants with fetal-neonatal ID. The Condition × Hemisphere interaction was observed for the anterior regions. The fetal-neonatal ID group showed lower LSW amplitude for mother vs. stranger condition in the left-anterior hemisphere (Figure 4), indicating that infants with fetal-neonatal ID used more brain resources to update their memories of the stranger face. There was no effect for posterior regions.
Figure 4.
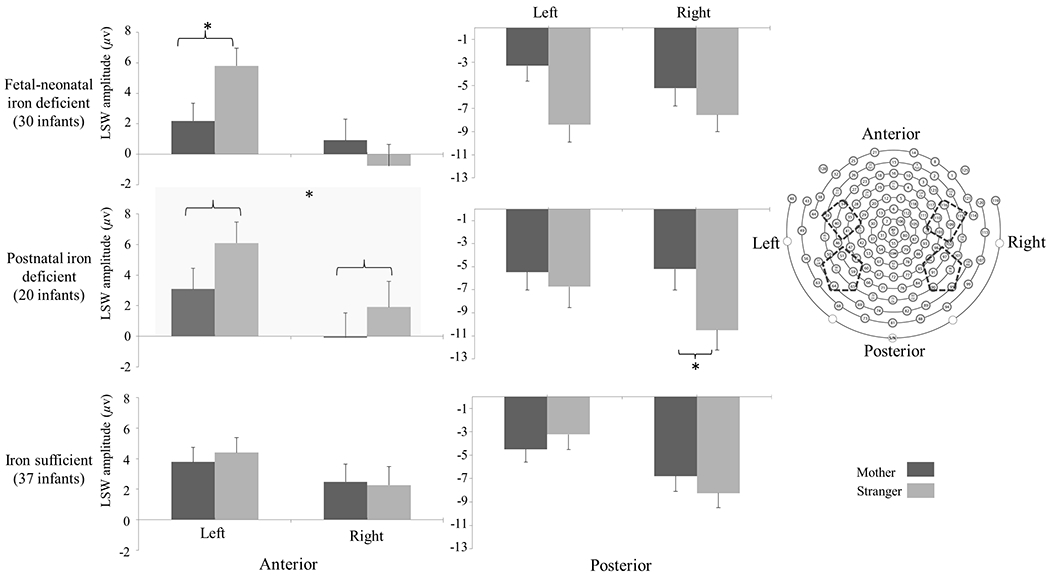
Mean and standard error (SE, error bar) of LSW amplitude in each condition and region for 9-month-olds. The fetal-neonatal ID group showed more positive LSW amplitude in stranger vs. mother condition in the left-anterior region. The postnatal ID group showed the main effect of condition in the anterior and right-posterior regions (*p < .05).
For postnatal ID, there was also an interaction between Condition and Anterior-Posterior. The postnatal ID group showed lower LSW in mother vs. stranger condition in the anterior leads (Figure 4). For the posterior leads, there was an interaction between Condition and Hemisphere. Further analysis indicated that LSW amplitude was more negative in stranger vs. mother condition in right-posterior hemisphere (Figure 4). As with fetal-neonatal ID, these findings implied that the postnatal ID group invested a different amount of brain resources to update their memories of mother and stranger faces, although the engaged brain regions appeared to differ between the ID groups. The iron-sufficient group did not show condition differences in any region.
To understand the Condition × Anterior-Posterior × Hemisphere × Iron Group interaction better, we tested the 3-way Condition × Hemisphere × Iron Group interaction for anterior and posterior regions separately. The 3-way interaction was significant only in the posterior leads (F (2, 76) = 3.21, p = .046, η2 = 0.08). Further tests indicated that fetal-neonatal ID and iron-sufficient groups showed distinct patterns in the condition difference in LSW amplitude only in the left-posterior hemisphere (p = .005, Figure 4).
18 months
There were no condition or group differences in LSW.
Exploratory analyses of subgroups of ID infants
Effects of fetal-neonatal ID might be driven by infants who were also ID at 9 months. A related question is whether the findings were due to IDA or ID without anemia at 9 months. Sample size limited our ability to test these questions with confidence, but we addressed them in exploratory analyses. There were no statistically significant differences in 9- or 18-month ERP responses between infants with fetal-neonatal ID who did or did not have ID or IDA at 9 months. There were also no significant differences in ERP responses between infants with IDA or ID without anemia at 9 months, regardless of fetal-neonatal iron status.
DISCUSSION
The current study contributes to the field by considering how ID timing and infant age at testing modulate the association between ID and memory. The results indicate that both ID timing and infant age at testing have effects. The effects differed between Nc and LSW, the 2 ERP components used for measuring recognition memory.
For Nc, 9-month-old infants invested more attention resources to process mother vs. stranger faces, regardless of iron status. In contrast, at 18 months, iron-sufficient infants continued showing this pattern, but fetal-neonatal ID infants did not show the difference in any area, and postnatal ID infants showed it only in the right hemisphere. Therefore, there was an ID effect at 18 months but not at 9 months. Although children start transitioning from allocating more attention to the primary caregiver’s faces to other faces during the latter part of the first year of life,(20) stranger faces did not elicit greater Nc amplitude than mother faces for either iron-sufficient or ID infants. Instead, we found that only for iron-sufficient infants, mother faces attracted more attention than stranger faces at both 9 and 18 months. The explanation for these findings is unclear. It is possible that the stranger faces, which were shown repetitively in this study, did not have enough novelty to attract infants’ attention. The findings might also relate to the association between ID and socio-emotional development.(21) Our previous study indicated that socioemotional deficits associated with ID can partly explain the relation between ID and cognitive abilities.(22) For instance, young children with IDA showed less social looking toward their mothers compared to the controls,(23) which might be the reason that only iron sufficient infants consistently showed more negative Nc amplitude for mother vs. stranger faces at both 9 and 18 months. Fetal-neonatal and postnatal ID were different in their influences on processing mother and stranger faces at 18 months, which implied that ID timing modulated the influence of ID on socioemotional development.
For LSW, there were also effects of infant age and ID timing. At 9 months, iron-sufficient infants did not use more brain resources to update or encode their memories of stranger faces than mother faces. Fetal-neonatal ID infants showed difference at left-frontal leads. Postnatal ID infants showed differences at frontal and right-posterior leads. At 18 months, there were no significant LSW amplitude differences between conditions regardless of iron status group. The reason that ID effects in LSW were only detected at 9 months may relate to developmental changes in encoding speed during the first 2 years of life.(24) At 9 months, ID infants might not encode stranger faces as efficiently as iron sufficient infants, which might be the reason that there was ID effect in LSW amplitude at this testing age. At 18 months, neural and cognitive maturation might allow infants, regardless of prior ID, to fully encode stranger faces easily and quickly, resulting in a lack of condition difference in LSW amplitude in all groups.
The ID timing effect in LSW at 9 months might relate to the developing hippocampus. As the neural substrate for memory encoding, the hippocampus develops quickly in later pregnancy and early infancy and thus is vulnerable to ID. Exposure to ID during either gestation or infancy might differentially impair hippocampal maturation, contributing to the ID timing effects on the neurodevelopmental phenotype observed in LSW at 9 months.
The few previous studies of early-life ID without confounding gestational conditions (e.g., maternal diabetes mellitus) found differences in LSW amplitude for familiar vs. unfamiliar stimuli only in iron-sufficient infants.(4, 6, 7) In contrast, we found a condition difference in LSW amplitude only in the ID groups at 9 months. Interpreting such disparate results is challenging, because normal developmental patterns of recognition memory are unclear. Prior studies assessing LSW in typically developing infants have yielded inconsistent findings. Some found a LSW amplitude difference for mother vs. stranger faces in normal 9-month-old infants;(4) others found a differential LSW amplitude between the 2 types of faces at 4-6 months but not later.(25) The lack of agreement may arise from differences in task designs, research techniques, and/or populations.(25) The inconsistencies make it difficult to know when LSW amplitude differences are “normal” and when they indicate maturational delay. Even so, preclinical models demonstrate that early-life ID causes substantial maturational delay in hippocampal development, specifically of the gamma-aminobutyric acid interneuron inhibitory system.(26) Electrophysiological studies in human infants also indicate that ID or IDA are risk factors for delayed CNS maturation (4, 5, 27).
Strengths of the current study include its consideration of age and ID timing effects on 2 cognitive processes involved in recognition memory and its use of ERP methodology to study the neural correlates and circuit function underlying such effects. The current study also included several limitations. This study was limited by lack of behavioral data collected during ERP testing. Our observational study, like others, cannot support causal inferences about the effects of ID or age on ERP indicators of recognition memory. Unusable data is another concern. Although the proportion of infants with unusable data that we observed is within the range reported in previous studies (25-75%)(28) and not biased toward any iron status group, such losses limit sample size, reduce power to get significant findings, and/or introduce other sources of potential bias. Additionally, we used 10 artifact-free ERP trials as threshold for selecting subjects as recommended by previous studies. However, more trials could be better in terms of signal/noise ratio. Finally, due to data loss, there were relatively few infants with analyzable data at both 9 and 18 months. Therefore, the differences observed between these 2 age groups are likely to be affected by unknown confounding variables.
To conclude, this study improves the understanding of ID by indicating that the timing of ID in early life (fetal-neonatal vs. infancy) modulates the impact of ID on the neural correlates of recognition memory. Such impact also varies depending on the age of infants at testing (9 vs. 18 months). Coinciding with periods of rapid brain development, ID during late gestation and/or early infancy may affect the development of related brain areas and their interactions underlying memory development. The influence may differ based on the timing of ID and the time course of brain and behavioral development. Therefore, our results point to the importance of identifying and treating ID early. Especially, there are no current recommendations for screening neonates for ID and no standard definition of ID at birth. Even for older infants, screening often relies only on hemoglobin or red cell indices, and ID without anemia may not be detected. Our findings indicate the value of detecting ID in neonates and infants and considering ID timing and infant age in designing interventions to improve memory and attention in infants with ID in early life.
ACKNOWLEDGEMENTS
We thank the participants and their families and Yaping Shi, Liqin Chen, Zheng Shen, Zhengyan Zhao, Mingyan Li, Chai Ji, Zhiwei Zhu, and other team members for assistance with subject enrollment, data collection, and laboratory analyses.
Statement of financial support: This study was supported by grants from the US National Institutes of Health (NIH) (P01HD039386, to BL) and the National Natural Science Foundation of China (NNSFC) (no.81273085, to JS). ERP analyses were conducted during FG’s post-doctoral fellowship at the University of Michigan.
Data availability statement: The data that support the findings of this study are available from the corresponding author, FG, upon reasonable request.
Footnotes
DISCLOSURE
The authors declare no conflicts of interest. Datasets would be available upon request.
REFERENCES
- 1.Stevens GA, Finucane MM, De-Regil LM, Paciorek CJ, Flaxman SR, Branca F, Peña-Rosas JP, Bhutta ZA, Ezzati M. Global, regional, and national trends in haemoglobin concentration and prevalence of total and severe anaemia in children and pregnant and non-pregnant women for 1995-2011: a systematic analysis of population-representative data. Lancet Glob Health. 2013;1:e16–e25. doi: 10.1016/s2214-109x(13)70001-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cusick SE, Georgieff MK, Rao R. Approaches for Reducing the Risk of Early-Life Iron Deficiency-Induced Brain Dysfunction in Children. Nutrients. 2018;10(2):227. Cited in: Pubmed; PMID doi: 10.3390/nu10020227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgieff MK. The role of iron in neurodevelopment: fetal iron deficiency and the developing hippocampus. Biochem Soc Trans. 2008;36:1267–1271. doi: 10.1042/bst0361267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burden MJ, Westerlund AJ, Armony-Sivan R, Nelson CA, Jacobson SW, Lozoff B, Angelilli ML, Jacobson JL. An event-related potential study of attention and recognition memory in infants with iron-deficiency anemia. Pediatrics. 2007;120(2):e336–e345. doi: 10.1542/peds.2006-2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Congdon EL, Westerlund A, Algarin CR, Peirano PD, Gregas M, Lozoff B, Nelson CA. Iron deficiency in infancy is associated with altered neural correlates of recognition memory at 10 years. J Pediatr. 2012;160(6):1027–1033. doi: 10.1016/j.jpeds.2011.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siddappa AM, Georgieff MK, Wewerka S, Worwa C, Nelson CA, Deregnier R-A. Iron deficiency alters auditory recognition memory in newborn infants of diabetic mothers. Pediatr Res. 2004;55(6):1034–1041. [DOI] [PubMed] [Google Scholar]
- 7.Geng F, Mai X, Zhan J, Xu L, Zhao Z, Georgieff M, Shao J, Lozoff B. Impact of fetal-neonatal iron deficiency on recognition memory at 2 months of age. J Pediatr. 2015;167(6):1226–1232. doi: 10.1016/j.jpeds.2015.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nelson CA. Neural development and lifelong plasticity. In: Lerner RM, Jacobs F, Wertlieb D, editors. Promoting positive child, adolescents, and family development: handbook of program and policy interventions. Thousand Oaks: Sage Publications; 2002. p. 31–60. [Google Scholar]
- 9.Gartstein MA, Rothbart MK. Studying infant temperament via the Revised Infant Behavior Questionnaire. Infant Behav Dev. 2003;26(1):64–86. doi: 10.1016/S0163-6383(02)00169-8. [DOI] [Google Scholar]
- 10.Armony-Sivan R, Zhu B, Clark KM, Richards B, Ji C, Kaciroti N, Shao J, Lozoff B. Iron deficiency (ID) at both birth and 9 months predicts right frontal EEG asymmetry in infancy. Dev Psychobiol. 2016;58(4):462–470. doi: 10.1002/dev.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amin SB, Orlando M, Eddins A, MacDonald M, Monczynski C, Wang H. In utero iron status and auditory neural maturation in premature infants as evaluated by auditory brainstem response. J Pediatr. 2010;156(3):377–381. doi: 10.1016/j.jpeds.2009.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tamura T, Goldenberg RL, Hou J, Johnston KE, Cliver SP, Ramey SL, Nelson KG. Cord serum ferritin concentrations and mental and psychomotor development of children at five years of age. J Pediatr. 2002;140(2):165–170. doi: 10.1067/mpd.2002.120688. [DOI] [PubMed] [Google Scholar]
- 13.McLimore HM, Phillips AK, Blohowiak SE, Pham DQD, Coe CL, Fischer BA, Kling PJ. Impact of multiple prenatal risk factors on newborn iron status at delivery. J Pediatr Hematol Oncol. 2013;35(6):473–477. eng. doi: 10.1097/mph.0b013e3182707f2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Center for Disease Control. Healthy people 2000 national health promotion and disease prevention objectives final review. Hyattsville, MD; 2001. Department of Health and Human Services. [Google Scholar]
- 15.Centers for Disease Control and Prevention. Recommendations to prevent and control iron deficiency in the United States. MMWR 1998;47:1–29. [PubMed] [Google Scholar]
- 16.Saarinen UM, Siimes MA. Serum ferritin in assessment of iron nutrition in healthy infants. Acta Pediatr. 1978;67:745–751. doi: 10.1111/j.1651-2227.1978.tb16254.x. [DOI] [PubMed] [Google Scholar]
- 17.Soldin OP, Miller M, Soldin SJ. Pediatric reference ranges for zinc protoporphyrin. Clin Biochem. 2003;36:21–25. doi: 10.1016/S0009-9120(02)00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rothman KJ. No Adjustments Are Needed for Multiple Comparisons. Epidemiology. 1990;1(1):43–46. Full publication date: Jan., 1990. [PubMed] [Google Scholar]
- 19.Murphy KR, Myors B, Wolach A. Statistical power analysis: a simple and general model for traditional and modern hypothesis tests. 4th ed. New York: Routledge; 2014. [Google Scholar]
- 20.Carver LJ, Dawson G, Panagiotides H, Meltzoff AN, McPartland J, Gray J, Munson J. Age-related differences in neural correlates of face recognition during the toddler and preschool years. Dev Psychobiol. 2003;42(2):148–159. doi: 10.1002/dev.10078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.East P, Lozoff B, Blanco E, Delker E, Delva J, Encina P, Gahagan S. Infant iron deficiency, child affect, and maternal unresponsiveness: Testing the long-term effects of functional isolation. Dev Psychol. 2017. 2017/12//;53(12):2233–2244. eng. doi: 10.1037/dev0000385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter RC, Jacobson JL, Burden MJ, Armony-Sivan R, Dodge NC, Angelilli ML, Lozoff B, Jacobson SW. Iron deficiency anemia and cognitive function in infancy. Pediatrics. 2010;126(2):e427–e434. eng. doi: 10.1542/peds.2009-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lozoff B, Corapci F, Burden MJ, Kaciroti N, Angulo-Barroso R, Sazawal S, Black M. Preschool-Aged Children with Iron Deficiency Anemia Show Altered Affect and Behavior. J Nutr. 2007;137(3):683–689. doi: 10.1093/jn/137.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bauer PJ. Event memory. Handbook of Child Psychology. John Wiley & Sons, Inc.; 2007. [Google Scholar]
- 25.Webb SJ, Long JD, Nelson CA. A longitudinal investigation of visual event-related potentials in the first year of life. Dev Sci. 2005;8(6):605–616. doi: 10.1111/j.1467-7687.2005.00452.x. [DOI] [PubMed] [Google Scholar]
- 26.Callahan LSN, Thibert KA, Wobken JD, Georgieff MK. Early-life iron deficiency anemia alters the development and long-term expression of parvalbumin and perineuronal nets in the rat hippocampus. Dev Neurosci. 2013;35(5):427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otero GA, Fernández T, Pliego-Rivero FB, Mendieta GG. Iron therapy substantially restores qEEG maturational lag among iron-deficient anemic infants. Nutr Neurosci. 2019. 2019/05/04;22(5):363–372. doi: 10.1080/1028415X.2017.1391529. [DOI] [PubMed] [Google Scholar]
- 28.DeBoer T, Scott LS, Nelson CA. ERPs in developmental populations. Event-related potentials: a methods handbook. Vol 13. Cambridge, MA: MIT Press; 2005. p. 251–67. [Google Scholar]


