Abstract
Background:
Risk for childhood psychopathology is complex and multifactorial, implicating direct and interacting effects of familial and environmental factors. The role of environmental neurotoxicants in psychiatric risk is of growing concern, including polycyclic aromatic hydrocarbons (PAH), common in air pollution. Prenatal PAH exposure is linked to adverse physical, behavioral, and cognitive outcomes as well as increasing psychiatric risk. It is unclear whether environmental exposures, like PAH, magnify the effects of exposure to early life stress (ELS), a critical risk factor for psychopathology. The current work aimed to test potential interactions between prenatal PAH exposure and psychosocial/socioeconomic stress on psychiatric symptoms in school-age children.
Methods:
Data were from the Columbia Center for Children’s Environmental Health Mothers and Newborns longitudinal birth cohort study. Prenatal PAH exposure was ascertained though air monitoring during pregnancy and maternal PAH-DNA adducts at delivery. Mothers reported on ELS (child age 5) and on child psychiatric symptoms across childhood (child age 5, 7, 9 and 11) using the Child Behavior Checklist (CBCL).
Results:
Significant prenatal airborne PAH × ELS interactions (FDR-corrected) predicted CBCL Attention (β=0.22, t(307)=3.47, p<.001,pfdr=.003) and Thought Problems T-scores (β=0.21, t(307)=3.29, p=.001,pfdr=.004) at age 11 (n=319). Relative to those with lower exposure, children with higher prenatal PAH exposure exhibited stronger positive associations between ELS and CBCL Attention and Thought Problem T-scores. This interaction was also significant examining convergent ADHD measures (Conners, DuPaul) and examining maternal PAH-DNA adducts (β=0.29,t(261)=2.48,p=.01;n=273). A 3-way interaction with assessment wave indicated that the PAH × ELS interaction on Attention Problems was stronger later in development (β=0.03, t(1601)=2.19,p=.03;n=477).
Conclusions:
Prenatal exposure to PAH, a common neurotoxicant in air pollution, may magnify or sustain the effects of early life psychosocial/socioeconomic stress on psychiatric outcomes later in child development. This work highlights the critical role of air pollution exposure on child mental health.
Keywords: ADHD, adversity, child behavior checklist, child development, toxicants
Introduction
Risk for childhood psychopathology is complex and multifactorial, implicating direct and interacting effects of numerous familial and environmental risk factors. The effects of environmental neurotoxicants on psychiatry risk are of growing concern. For example, prenatal exposure to air pollution has been linked to increased risk for a range of psychiatric outcomes, including autism spectrum disorder in the first 5 years of life (Volk et al., 2013, von Ehrenstein et al., 2014, Becerra et al., 2013, Volk et al., 2011) and attention deficit hyperactivity disorder (ADHD) assessed through age 9 (Perera et al., 2014, Peterson et al., 2015, Perera et al., 2012). Exposure to air pollution, modelled at age 17, has also been linked to increases in psychotic experiences at age 18 (Newbury et al., 2019). Air pollution contains numerous potentially harmful compounds including, polycyclic aromatic hydrocarbons (PAH), which are common neurotoxicants released into the environment during incomplete combustion of fossil fuel, tobacco, and other organic material (Bostrom et al., 2002). Animal models have shown that prenatal exposure to PAH results in a number of downstream alterations, including decrements in neural mRNA expression, long-term potentiation, and learning and memory performance (Wormley et al., 2004, Grova et al., 2007, Schellenberger et al., 2013, Brown et al., 2007). These effects on early brain development may underlie long-term risk for the negative outcomes associated with prenatal exposure to PAH. Human epidemiologic studies have demonstrated associations between prenatal exposure to PAH specifically–either measured using personal air monitors or using PAH-DNA adducts, which are PAH-specific biomarkers–and multiple adverse behavioral and cognitive outcomes, including developmental delay at 2–3 years of age (Perera et al., 2006b, Tang et al., 2008), reduced cognitive ability/IQ at 5–7 years of age (Perera et al., 2009, Edwards et al., 2010, Jedrychowski et al., 2015, Lovasi et al., 2014), symptoms of anxious/depressed and attention problems (Perera et al., 2012); and reductions in the development of self-regulatory capacity across childhood that mediated social problems at age 11 (Margolis et al., 2016).
Exposure to stress and adversity, particularly during early life, is a critical risk factor for a number of forms of psychopathology across the lifespan (Kessler et al., 2010). A wide variety of stressors have been examined as risk factors for psychiatric illness, for example abuse and neglect (Kendler et al., 2004, Molnar et al., 2001), exposure to violence (Kessler and Magee, 1994, Golding, 1999), economic hardship (Spence et al., 2002, Gilman et al., 2003, Gilman et al., 2002), parental psychopathology. (Williamson et al., 2004, Lieb et al., 2002), or isolation/lack of social support (Kendler et al., 2005, Teo et al., 2013). These types of exposures often cluster and co-occur in the general population and exposure during early childhood is a potent risk factor for psychiatric illness, likely acting through a number of different mediating mechanisms. These may include epigenetic changes (Wankerl et al., 2014, Swartz et al., 2016, Oberlander et al., 2008), prenatal hormonal factors (e.g. exposure to elevated cortisol in utero (Davis et al., 2007)), postnatal psychosocial factors (e.g. parenting (Kiernan and Huerta, 2008, Dougherty et al., 2013)), psychological factors (e.g. impaired emotion regulation in the child (Silk et al., 2006, Abravanel and Sinha, 2015, Pagliaccio et al., 2015), biological factors (e.g. exaggerated cortisol or inflammatory reactivity in the child (Pace et al., 2006, Pagliaccio and Barch, 2016, Pagliaccio et al., 2014), and neural factors (e.g. altered brain structure and function in the child (Pagliaccio and Barch, 2016, Pagliaccio et al., 2014).
Growing awareness of the complexities of interactions between different exposures on health and development highlights the need to study exposures to understand how prenatal and early postnatal exposures affect developmental trajectories. Some exposures are more likely than others to co-occur. The location of outdoor pollution sources typically places individuals from low-income, urban, and minority communities at increased risk of exposure to PAH (Heritage, 1992, Pirkle et al., 1996, Olden and Poje, 1995, Nieves and Wernette, 1992, Metzger et al., 1995, Wagenknecht et al., 1993). Similarly, individuals from these low-income, urban, and minority communities are at increased risk of experiencing early life stress (ELS) (Nurius et al., 2016, Hatch and Dohrenwend, 2007, Cronholm et al., 2015, Mickelson and Kubzansky, 2003). Prior findings point to a potential interaction between exposure to prenatal PAH and material hardship, one potential aspect of ELS, (e.g. (Perera et al., 2017, Vishnevetsky et al., 2015). Understanding the interaction between exposure to prenatal PAH and ELS may elucidate critical windows of exposure and create opportunities to buffer effects of exposure. Importantly this type of information may help shape successful public health messaging and personalized prevention programs.
Herein, we investigated the potential interactions between exposure to prenatal PAH and postnatal ELS on child psychiatric symptoms using a more comprehensive measure of ELS that includes multiple domains of stress exposure. Given prior findings that exposure to both prenatal PAH and postnatal ELS are associated with increased symptoms of a broad range of child psychopathologies, and that they may act through similar biological mechanisms (e.g. neurotransmitter alteration, oxidative stress, inflammation (Pace et al., 2006, Saunders et al., 2006b, Block and Calderon-Garciduenas, 2009), we hypothesized that these exposures would interact, such that exposure to both prenatal PAH and postnatal ELS would result in higher levels of child psychiatric symptoms in late childhood than would either exposure alone. As prior studies have implicated these risk factors in various internalizing and externalizing behaviors, we probed a broad range of psychiatric symptom domains. We also investigated whether the strength of the prenatal PAH by postnatal ELS interaction might change over the course of childhood.
Methods
Participants: The Columbia Center for Children’s Environmental Health (CCCEH)
Details on CCCEH Mothers and Newborns birth cohort and study design have been published previously (Perera et al., 2006b). Briefly, African-American and Dominican women residing in Washington Heights, Harlem, or the South Bronx in New York City (NYC) were recruited between 1998 and 2006 through local prenatal care clinics. Women were included if they were between 18 and 35 years who were non-users of tobacco products or illicit drugs, free of diabetes, hypertension, or known HIV, and if they had initiated prenatal care by the 20th week of pregnancy. The full cohort included data from 727 women/children. The Institutional Review Board of Columbia University approved the study. Mothers provided informed consent for themselves and their children; beginning at age 7 children provided assent.
Prenatal PAH assessment
Prenatal exposure to PAH was measured using two methods: personal air monitoring collected an external measure of exposure to PAH components for all participants; maternal PAH-DNA adducts were also collected from a smaller subset (N=581) of the sample as biomarker of the biologically effective dose of PAH. Air monitoring of PAH was the primary predictor of interest in the current analyses as this directly measures external PAH concentrations and was measured in the entire cohort. Complementary analyses evaluated maternal PAH-DNA adducts as a biological dosimeter of PAH exposure reflecting both the mother’s exposure to PAH and her toxicokinetic and toxicodynamic capacity (e.g. differences in absorption, metabolic activation, and DNA repair). Therefore, PAH-DNA adducts provide an indicator of the biologically effective dose of PAH, thus capturing inter-individual variability in potential risk.
Air monitoring.
Personal air monitoring was carried out during the third trimester of pregnancy. As previously describes, participants wore a backpack designed to measure air quality for 48 hours and slept with it next to their bed (Perera et al., 2003). Vapors and particles of ≤2.5μm in diameter were collected on a precleaned quartz microfiber filter and a precleaned polyurethane foam cartridge backup (University Research Glassware, Chapel Hill, NC). Samples were analyzed at Southwest Research Institute for benzo[a]pyrene (B[a]P), benz[a]anthracene, chrysene, benzo[b]fluroanthene, benzo[k]fluroanthene, indeno[1,2,3-cd]pyrene, dibenz[a,h]anthra-cene, and benzo[g,h,i]perylene. Additionally, each personal air monitoring result was assessed for completeness of documentation and accuracy of flow rate and time. Exposure levels were totaled across the components examined and total scores were log transformed, as in prior work, and examined as a continuous predictor.
Maternal PAH-DNA adducts.
At the time of delivery, research staff collected maternal blood. Biospecimens were transported to the CCCEH Molecular Epidemiology Laboratory within several hours of collection. The buffy coat, packed red blood cells, and plasma were separated and stored at –70 °C. DNA adducts of the representative PAH, B[a]P, were analyzed in extracted white blood cell DNA using a high-performance liquid chromatography (HPLC)/fluorescence method which detects B[a]P tetrols (Alexandrov et al., 1992, Perera et al., 2004). As in prior work, adduct levels were dichotomized based on the limit of detection (0.25 adducts per 108 adducts) to group mothers who had detectable (“high adducts”) vs. non-detectable (“low adducts”) maternal blood DNA samples levels. Adducts reflect multiple possible biological pathways for the pathogenic effects of PAH, representing DNA damage (genotoxicity), detoxification, and DNA repair (Godschalk et al., 2003, Veglia et al., 2008). They may also play a role in epigenetic alterations (DNA methylation) (Herbstman et al., 2012). The assay specifically measures the adducts formed by benzo[a] pyrene as a proxy for PAH-DNA because it is considered a representative PAH and is highly correlated with other PAH class members (Perera et al., 2006a).
Early Life Stress (ELS)
Data were aggregated from a structured interview with the parent at child age 5 and used to create a composite measure assessing multiple aspects of ELS exposure. The interview includes items from several published scales (see Appendix S1) measuring six domains of early life stress: 8 items regarding material hardship in the past year (Mayer and Jencks, 1989), 14 items regarding mother’s perceived stress in the past month (Cohen et al., 1994), 12 items regarding experience of intimate partner violence in the child’s lifetime (Straus et al., 1996), 8 items regarding lack of current social support (Cohen et al., 2000, Cohen et al., 1985), 32 items about current neighborhood quality (Sampson and Raudenbush, 1999, Sampson et al., 1997, Kim et al., 2008), and 27 items regarding nonspecific maternal distress or demoralization in the past year (Dohrenwend et al., 1980). These scales generally show good reliability and psychometric properties (Straus, 2017, Cohen et al., 2000, Cohen, 1988, Cohen et al., 1983, Vega and O’Leary, 2007). All item responses were rescaled to 0–1 for the current analysis with higher scores indicating more stress exposure. Responses were averaged within each of the 6 domains, and then averaged across domains to create a composite score (range=0–1). The composite score was used as the independent variable; scores from each stress domain were utilized in complementary analyses.
Maternal report of stress in two of these domains (material hardship and demoralization) was also collected during the third trimester of pregnancy and was used as a measure of prenatal exposure to stress (other stress domains were not measured prenatally). Primary analyses examined postnatal stress; prenatal stress effects were examined in complementary analyses.
Child psychiatric outcomes
CBCL.
Parent-report on the Child Behavior Checklist (CBCL) (Achenbach and Rescorla, 2001) was acquired at child age 3–5, 7, 9, and 11-years old. The first assessment included the preschool (1 ½ −5 years) version and the subsequent visits included the school-age (6–18 years) version. Age- and sex-normed T-scores were examined for the eight main subscales: Anxious/Depressed, Withdrawn/Depressed, Somatic Complaints, Social Problems, Thought Problems, Attention Problems, Rule Breaking Behavior, and Aggressive Behavior.
Conners.
ADHD symptom severity was assessed with the Conners Parent Report Scale (CPRS)–Revised: 80-item Version (Conners, 1997) at child age 9-years old under the guidance of trained research staff. The CPRS provides inattentive and hyperactive-impulsive subscales as well as a total score, derived from the DSM-IV (American Psychiatric Association, 2000) to screen for ADHD-behavior problems. Normed T-scores were examined for these indices.
DuPaul.
Mothers completed the ADHD Rating Scale-IV consisting of 18-items (0–3 Likert scale) regarding children’s ADHD symptom severity (DuPaul et al., 1998) at child age 9–14 years old. Mothers rated the frequency of their child’s ADHD behaviors both over the prior six months and during the period of the child’s worst symptoms ever. This scale was administered as part of an additional study examining other exposures in this cohort (Rauh et al., 2014).
Statistical analyses
All statistical analyses were performed using R v3.5.1 (Team, 2015). All statistical models controlled for potential confounding variables and covariates commonly associated with ELS or PAH exposure and/or psychiatric and behavioral outcomes: sex, gestational age (weeks), ethnicity (binary: African American vs. Dominican/Hispanic), presence of a smoker in the home (binary), maternal IQ (Test of Non-Verbal Intelligence-Third Edition [TONI] (Brown et al., 1997), maternal years of education, quality of the proximal caretaking environment at age 3 (Caldwell and Bradley’s Home Observation for Measurement of the Environment [HOME] (Bradley, 1994), and change of residence by age 5 (i.e., subsequent to PAH air monitoring). Distributions of independent and dependent variables were examined for outliers. Given the large sample size included in the main analysis (N=319), linear regression is generally robust to violations of the assumption of normality of residuals. Standardized regression coefficients (β) are presented in the results. Only complete cases were examined; i.e., list-wise deletion for missing data. All tests were two-tailed; significance thresholds were set at p<.05 and the main analysis was corrected for multiple comparisons using false discovery rate (FDR; R p.adjust function).
Primary analyses.
Linear regression analyses were run to examine whether prenatal airborne PAH and postnatal ELS measured at child age 5 were associated with child psychiatric symptoms at child age 11. Specifically, centered ELS, log transformed total PAH scores, and the interaction term were entered into a linear regression predicting CBCL T-scores at child age 11. T-tests and chi-squared tests evaluated whether those with complete data in the main analyses (n = 319) differed from those excluded for missing data (n=408). The main effects of ELS, PAH, and their interaction predicting each of the eight CBCL subscales at child age 11 were FDR-corrected for multiple comparison (24 p-values). To determine whether any non-significant results indicated the absence of an effect or inconclusive data, Bayesian regression analyses were conducted using the R BayesFactor package (Morey et al., 2015) to evaluate the strength of evidence for the prenatal PAH × postnatal ELS interaction. Specifically, the Bayes factor (BF) was extracted, comparing the above model with and without the prenatal PAH × postnatal ELS interaction, to determine whether adding this interaction improved the model’s description of the data. Bayes factors <1 indicate no evidence to support the alternative hypothesis while scores >20 indicate strong evidence for the alternative hypothesis (recomputed with 100,000 iterations). Complementary analyses further exploring and confirming results from the primary analyses are detailed in the Appendix S1.
Patterns of development over time.
To investigate the effect of early life stress and PAH exposure on patterns of development, significant associations between the prenatal PAH × postnatal ELS interaction term and child psychiatric symptoms at age 11 were examined across all available assessment waves using a repeated measures model. Specifically, we examined the 3-way prenatal PAH × postnatal ELS × time (of assessment: age 3–5, 7, 9, and 11 years) interaction in a linear mixed-effects model, using all available data at each timepoint and allowing for missing data, with a random effect for participant and the same covariates used in the main regression analyses (sample size with complete data by visit: n=469 at 3–5-year, n=438 at 7-year, n=321 at 9-year, 319 at 11-year assessment).
Results
Participants
Of the 727 children in the sample, 527 had available prenatal PAH and 5-year ELS data. Of these, 200 children were missing predictors of interest (176 were missing postnatal ELS scores and 40 were missing useable prenatal PAH scores). Intercorrelations between prenatal PAH, postnatal ELS and its component measures, and CBCL T-scores at age 11 are presented in Table S1. There was no significant association between prenatal exposure to PAH and postnatal ELS (r=−.01, t(525)=−0.32, p=.74). Table 1 presents a summary of all predictors and covariates for the subsamples of children with complete data for all covariates and the primary outcomes of interest (CBCL T-scores at age 11: n=319; see Table S2, for secondary analysis subsets). The 319 children included in the main CBCL 11-year analyses did not show significant differences in demographic covariates or ELS but did exhibited slightly higher prenatal PAH air monitoring values on average, relative to the 408 children with any missing data (Table S3).
Table 1:
Characteristics of Main Analysis Sample
| N=319 Subsample | Mean (SD)/N (%) | Range | n |
|---|---|---|---|
| Sex (Female) | 142 (44.5%) | Female/Male | 319 |
| Ethnicity (African American) | 121 (37.9%) | African American/Dominican | 319 |
| Gestational Age (weeks) | 39.27 (1.44) | 30.00–42.00 | 319 |
| Maternal IQ (TONI) | 84.97 (13.05) | 60.00–135.00 | 319 |
| Maternal Education (years) | 11.95 (1.93) | 2–19 | 319 |
| HOME caretaking scores | 39.60 (5.91) | 20.00–52.00 | 319 |
| Change of residence by 5-year visit | 222 (69.6%) | Yes/No | 319 |
| Smoker in the home at baseline visit | 116 (36.4%) | Yes/No | 319 |
| Early Life Stress (ELS) | 0.28 (0.11) | 0.07 – 0.78 | 319 |
| Log PAH | 0.95 (0.69) | −0.72 – 3.60 | 319 |
| Maternal Adducts >.25 | 108 (39.6%) | Present/Absent | 273 |
| CBCL Anxious/Depressed | 53.24 (5.42) | 50–78 | 319 |
| CBCL Withdrawn/Depressed | 55.18 (6.95) | 50–90 | 319 |
| CBCL Somatic Complaints | 55.12 (6.95) | 50–78 | 319 |
| CBCL Social Problems | 53.86 (5.31) | 50–79 | 319 |
| CBCL Thought Problems | 53.01 (5.17) | 50–86 | 319 |
| CBCL Attention problems | 54.25 (6.55) | 50–100 | 319 |
| CBCL Rule breaking Behavior | 53.65 (5.36) | 50–83 | 319 |
| CBCL Aggressive Behavior | 53.20 (5.11) | 50–76 | 319 |
| Conners Total | 49.67 (9.86) | 18–90 | 318 |
| DuPaul Total - Current | 9.73 (10.09) | 0–54 | 331 |
| DuPaul Total - Worst | 10.89 (11.80) | 0–54 | 309 |
Sample characteristics are presented here to summarize the predictor, covariate, and main outcomes for the subsample of 319 participants who had complete data for the key predictors of interest (Early Life Stress [ELS] and log transformed PAH) and main outcome of interest, CBCL T-scores at age 11. Mean, standard deviation (SD) values, and score ranges are presented for all continuous measures. The N and percent are presented for binary variables. The number of participants with available data on each measure are indicated in the n column. Sample characteristics for subsamples in the complementary analyses are similarly described in Table S2. CBCL = Child Behavior Checklist at age 11; HOME = Home Observation for Measurement of the Environment; log PAH = log transformed polycyclic aromatic hydrocarbons
Primary effects on child psychiatric outcomes
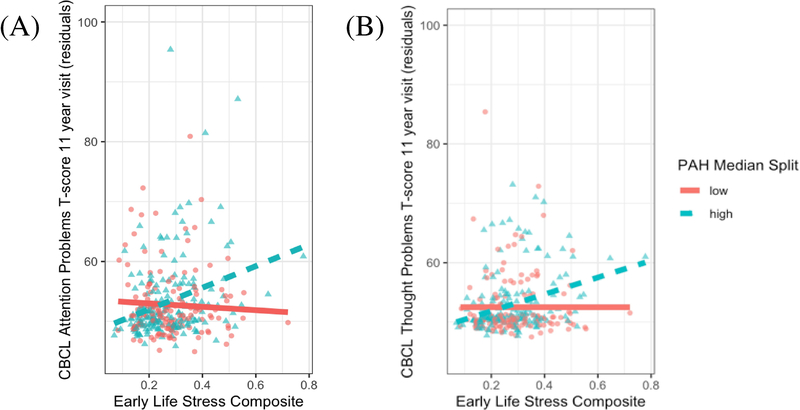
We detected significant prenatal airborne PAH × postnatal ELS interaction effects (passing FDR correction for multiple comparisons; Tables S4 and S5) that predicted CBCL Attention Problems T-scores (β=0.22, t(307)=3.47,p<.001, pfdr=.003, BF=64.67 ± 2.12%) and Thought Problems (β=0.21, t(307)=3.29,p=.001, pfdr=.004, BF=38.72 ± 1.75%) at child age 11. Children with higher prenatal PAH exposure showed stronger positive associations between postnatal ELS and CBCL Attention Problems and Thought Problems T-scores. For display purposes, Figure 1 indicates that higher levels of ELS were associated with higher CBCL Attention and Thought Problems T-scores for children at high, but not low, PAH exposure based on a median split (median=2.28). Examining exposure as continuous variables, region of significance analyses indicated that greater postnatal ELS exposure significantly predicted more severe CBCL problems when log PAH values were elevated (>0.65 for thought or >0.73 for attention problems; Johnson-Neyman plots in Figure S1), while this association was not significant at lower PAH exposure. See Appendix S2 for outlier analyses. A similar PAH × ELS interaction was obtained examining PAH DNA adducts predicting CBCL Attention problems (n=273; β=0.29, t(261)=2.48,p=.01; Figure S2, see Appendix S2). Bayes factor analyses demonstrated no evidence for (i.e. the absence of an effect of) non-significant airborne PAH × ELS interactions predicting the other six CBCL subscales, rather than inconclusive data (all p>.05, Bayes factors < 1.53; Table S4). Regression main effects indicated that higher levels of ELS, but not PAH, were associated with greater symptom severity on all but one CBCL subscale (pfdr >.05; Table S4). Complementary analyses of gold-standard Attention Disorder Hyperactivity Disorder measures (DuPaul and Conner’s) replicated the PAH × ELS interaction (see Appendix S2). The primary PAH × ELS interaction predicting CBCL Thought Problems was driven by effects on specific subscale items (repetitive thoughts, strange behaviors, strange thoughts; see Appendix S2). The primary PAH × ELS interactions were driven by the maternal demoralization, material hardship, perceived stress, and intimate partner violence stress domains (see Appendix S2).
Figure 1: Early life stress × prenatal PAH interactions predicting thought and attention problems at age 11.
Associations between early life stress composite scores and Child Behavioral Checklist (CBCL) thought (A) and attention (B) problems T-scores are presented here grouped by a median split of log total PAH scores (median=2.28). All interaction models were tested with continuous values of PAH and the median split is only used for display purposes here. Participants with low PAH exposure are denoted by red circles and solid fit line while those with high PAH exposure are denoted by blue triangles with dashed fit line. Greater early life stress scores associated with greater CBCL thought and attention problems among children with greater prenatal PAH exposure. Values represent residualized CBCL scores covarying for all covariates in the main analysis models: sex, gestational age, ethnicity, presence of a smoker in the home, maternal IQ, maternal years of education, quality of the proximal caretaking environment at age 3, and change of residence by age 5.
Patterns of development over time
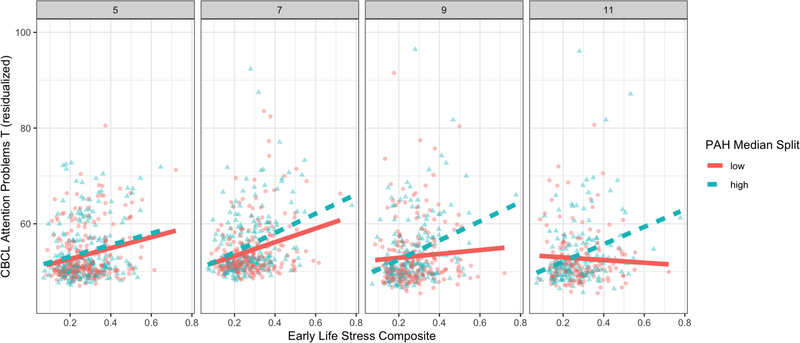
In linear-mixed effects models examining CBCL T-scores as a repeated measure at child age 3–5, 7, 9, and 11-year assessments (n=477 children with at least one wave of CBCL data; n=1617 total CBCL scores), we detected a significant prenatal PAH × postnatal ELS × time interaction on CBCL Attention Problems T-scores (β=0.02, t(1241)=2.68,p=.008; Figure 2). The prenatal PAH × postnatal ELS interaction effect was significantly associated with CBCL Attention Problems T-scores at age 11 (as in main analysis above) and age 9 (n=391; β=0.12, t(379)=2.13,p=.03), but not at child age 3–5 (n=469) or 7 years (n=438). At the two later assessments, ELS associated with increased attention problems among children with higher, but not lower PAH exposure. The main effect indicated that, at mean PAH, postnatal ELS was positively associated with greater CBCL Attention Problems T-scores at all four times (ages 3–5, 7, 9, and 11; all t>4.20, p<.05). Furthermore, in a regression analysis, the prenatal PAH × postnatal ELS interaction effect was associated with worsening attention problems at child age 11 over and above attention problems at child age 5 (when ELS was measured; β=0.19, t(299)=3.03,p=.003).
Figure 2: Early life stress × prenatal PAH × assessment wave interaction predicting CBCL Attention Problems across development.
Associations between early life stress composite scores and Child Behavioral Checklist (CBCL) Attention Problems T-scores are presented here grouped by a median split of log total PAH scores (median=2.28) at the 3–5, 7, 9, and 11 year visits (facet panels from left to right). Participants with low PAH exposure are denoted by red circles and solid fit line while those with high PAH exposure are denoted by blue triangles with dashed fit line. Greater early life stress scores associated with greater CBCL Attention Problems T-scores across all waves of assessment and this positive association was maintained over time strongest among children with greater prenatal PAH exposure. All interaction models were tested with continuous values of PAH and the median split is only used for display purposes here. Values represent residualized CBCL scores covarying for all nuisance variables in the main analysis models: sex, gestational age, ethnicity, presence of a smoker in the home, maternal IQ, maternal years of education, quality of the proximal caretaking environment at age 3, and change of residence by age 5. Note: some mothers completed the CBCL 18–24 months prior to the 5-year ELS interview.
The prenatal PAH × postnatal ELS × time interaction on CBCL Thought Problems T-scores was not significant (β=0.01, t(1269)=0.79,p=.43). Significant prenatal PAH × postnatal ELS interactions were observed at all ages (3–5, 9, and 11) and this did not vary significantly over time. The main effect indicated that, at mean PAH, was positively associated with greater CBCL Thought Problems T-scores at all four timepoints (all t>3.56, p<.05). In addition, the prenatal PAH × postnatal ELS interaction effect was associated with increasing CBCL Thought Problems T-scores at child age 11 over and above CBCL Thought Problems T-scores at child age 5 (when ELS was measured; β=0.18, t(299)=2.71,p=.007).
Discussion
The current study examined the association between exposure to prenatal PAH and early life stress on psychiatric symptoms across childhood from age 5 to 11 years old. Higher levels of ELS at child age 5, encompassing both socioeconomic and psychological stress, predicted significantly higher symptoms on all but one CBCL subscale at age 11. Critically, we identified a significant interaction between exposure to prenatal PAH and postnatal ELS predicting elevated T-scores on the CBCL Attention Problems and Thought Problems subscales at age 11. Bayes factors document that the lack of interaction effects predicting other CBCL subscales indicates the absence of an effect and not inconclusive data. Further, prenatal PAH exposure moderated the association between postnatal ELS and attention problems more strongly at older versus younger ages. These age-specific findings suggest that effects of earlier exposure emerge over development, either as symptoms worsen or psychiatric disorders emerge, or as an effect of changing context e.g. ADHD symptoms are more salient at older ages when school or other context requires more attention and behavioral control, even though the problems were actually present at a younger age. Last, the prenatal PAH × postnatal ELS exposure interaction was reproduced when examining the presence of maternal PAH-DNA adducts during the third trimester of pregnancy as a biological dosimeter of PAH exposure. These findings highlight the multifactorial etiology of psychiatric risk in children and the negative sequalae of early exposure to air pollution.
Attention and thought problems
Prenatal exposure to PAH moderated the association between ELS and CBCL Attention and Thought Problems at age 11, such that ELS more strongly predicted symptom severity among children with higher relative to lower prenatal PAH exposure. The prenatal PAH × postnatal ELS interaction effect was also confirmed with two measures specifically probing DSM symptom severity of ADHD, underscoring the robustness of these findings. These convergent results also indicated that the PAH × ELS interaction predicted higher levels of inattentive and hyperactive symptoms of ADHD. Further, the strength of the observed prenatal PAH × postnatal ELS interaction on attention problems varied over the course of development. At older ages, higher levels of ELS were associated with more attention problems among children with higher but not lower prenatal PAH exposure. At younger ages, greater ELS predicted more attention problems regardless of the level of PAH exposure. Thus, risk for attention problems in late childhood appears to be highest among those with exposure to both high prenatal PAH and postnatal ELS. Further, this interaction was associated with attention problems at age 11, over and above symptoms at age 5, pointing to the worsening of symptoms over time. Our findings are consistent with prior work documenting that prenatal PAH exposure and material hardship are risk factors for attention/ADHD problems in early childhood (Perera et al., 2012, Perera et al., 2011, Perera et al., 2014, Perera et al., 2018). Moreover, studies have shown that prenatal PAH exposure as measured by maternal PAH-DNA adducts is associated with an altered developmental trajectory of self-regulatory capacity (Margolis, 2016), and that the presence of prenatal PAH-DNA adducts interacts with prenatal and postnatal material hardship to predict children’s working memory performance at age 7 (Vishnevetsky et al., 2015). Such factors may contribute to the manifestation of ADHD symptoms. Herein, we extend prior work later into childhood. We document the long-lasting effects of prenatal PAH exposure and the complexities of environmental determinants on neurodevelopment. We highlight the importance of a holistic assessment of psychosocial, environmental, and socioeconomic determinants of developmental outcomes.
Higher levels of prenatal PAH exposure also moderated the association between ELS and CBCL Thought Problems T-scores at child age 11, again over and above symptoms at age 5. CBCL Thought Problems T-scores have been linked to a number of different outcomes including psychotic symptoms and risk in youth (Salcedo et al., 2018, Simeonova et al., 2014) and autism spectrum disorder (Mazefsky et al., 2011, Duarte et al., 2003). The thought problems subscale of the CBCL characterizes a heterogenous set of symptoms with a generally low base rate of endorsement, e.g. hallucinations, OCD-symptoms, strange thoughts and behaviors, and self-harm. Although this subscale shows relatively lower internal consistency and long-term stability than other subscales (Achenbach and Rescorla, 2001), its unidimensional nature and measurement invariance have been confirmed in large samples (Abdellaoui et al., 2012). Nonetheless, we detected a significant prenatal PAH × postnatal ELS interaction on three items that drove the overall CBCL Thought Problems effect: can’t get mind off certain things, do things that others find strange, or have thoughts that others find strange. Further, these specific items have been included in a proposed autistic CBCL subscale (Rescorla, 1988) and a proposed obsessive compulsive scale (Hudziak et al., 2006). Thus, the specificity to risk for a specific disorder is hard to determine from this current data and should explored more deeply in future research. Though these results do build on prior findings and highlight links between air pollution and symptoms associated with risk for autism spectrum disorder (Volk et al., 2013, von Ehrenstein et al., 2014, Becerra et al., 2013, Volk et al., 2011) and psychotic experiences (Newbury et al., 2019).
Domains of stress
Increased levels of ELS at child age 5, encompassing both socioeconomic and psychological stress, were associated with increased psychiatric symptoms in all but one CBCL subscale at all of the ages that we studied. Such findings are consistent with the extant literature documenting the deleterious effects of ELS on psychiatric outcomes (Green et al., 2010, Kessler et al., 1997, Carr et al., 2013). Of the different domains of stress that we examined, material hardship, maternal perceived stress, experience of intimate partner violence, and maternal distress/demoralization each interacted with levels of prenatal PAH to predict psychiatric symptoms. Neighborhood quality and social support did not interact with levels of prenatal PAH in predicting psychiatric symptoms. These stress-specific factors point to important targets for personalized prevention which will have greatest impact on child health outcomes. Last, a significant interaction between prenatal PAH and prenatal maternal demoralization on attention problems was mediated by the interaction between prenatal PAH and postnatal ELS. Although the continuity of stress exposure over time makes it difficult to pinpoint a specific vulnerable window of development, these results highlight that exposure to prenatal and/or early postnatal psychosocial and socioeconomic stress represents a key component of psychiatric risk that is moderate the impact of prenatal PAH exposure.
Shared biological pathways for ELS and PAH exposure
A number of different mechanisms have been theorized to underlie the effects of both ELS and PAH on neurodevelopmental and psychiatric outcomes. Herein, we studied exposure to both PAH and ELS, which independently alter multiple downstream pathways but also may serve as a ‘double hit’ on shared mechanistic pathways. Stress exposure likely leads to wide ranging changes in, for example, epigenetic expression, cortisol, inflammation, and brain structure and function (e.g. (Wankerl et al., 2014, Swartz et al., 2016, Oberlander et al., 2008, Davis et al., 2007, Pace et al., 2006, Pagliaccio and Barch, 2016, Pagliaccio et al., 2014). The mechanism underlying the effects of PAH are still being interrogated, however, alterations in brain structure/function and inflammation represent possible shared mechanistic pathways. For example, PAH has been linked to widespread alterations in cortical structure and downstream associations with cognitive functioning and ADHD symptoms (Peterson et al., 2015). The maturation of cortical architecture has also been suggested to underlie the link between perinatal stress and ADHD outcomes (Bock and Braun, 2011). Further, inflammation and oxidative stress are linked to ELS exposure (e.g. (Pace et al., 2006, Bierhaus et al., 2003, Bierhaus et al., 2006)), PAH exposure (Saunders et al., 2006a), and ADHD (Joseph et al., 2015). Our findings are also consistent with mouse models showing an interaction between prenatal exposure to air pollution (diesel exhaust particles) and nest material restriction (stress) on offspring cognitive outcomes that operates through an inflammatory mechanism (Bolton et al., 2013). Other ‘double hit’ interaction effects have been found in human work, for example, showing stronger adverse associations between organophosphate pesticide exposure and IQ in children experiencing greater adversity (Stein et al., 2016). Low socio-economic status/ high deprivation environments, more generally, have also been suggested to magnify the link between air pollution and autism spectrum disorder in children (McGuinn et al., 2019, O’Lenick et al., 2017). Understanding the importance of stress and environmental exposure and the relevant timing of critical windows of vulnerability and critical windows for manifesting symptomatology can highlight targets for personalized prevention and intervention programs.
PAH air monitoring versus adducts
Findings from our primary analyses examining air-monitored prenatal PAH measures were largely consistent with findings when we instead examined the presence of maternal DNA adducts as a measure of PAH exposure. Specifically, PAH measured either by air monitoring during the third trimester, an external marker of exposure, or by the presence of maternal DNA adducts, an internal biomarker of the downstream effects of PAH on the mother, interacted with postnatal ELS to predict CBCL Attention Problems T-scores and approached significance for Thought Problems T-scores. It is noteworthy that the results reported here were consistent for both air monitoring measures of PAH and PAH-DNA adducts in maternal blood. While these metrics are related, they are not associated significantly in these data and reflect different underlying constructs. PAH in air is a summed combination of eight individual PAH components whereas PAH-DNA adducts using HPLC reflect only benzo(a)pyrene, one of the eight PAH included in the air measure. Although the single 48-hour PAH air monitoring measure has been shown to correlate highly with three sequential 2-week integrated indoor air samples (r = 0.57–0.76; (Rundle et al., 2012), PAH-DNA adducts have a half-life of several months (Mooney et al., 1995) and thus may reflect a longer exposure period. Finally, PAH in air indicates the exposure that is available to be inhaled whereby PAH-DNA adducts represent an integrated index of exposure from all routes (e.g., inhalation, ingestion, absorption) as well as individual metabolic differences in detoxification. The observation that models using either metric of PAH exposure support similar inferences support the robustness of these findings.
Limitations and conclusions
A number of limitations should be noted here. First, although the Mothers and Newborns Cohort study importantly focused on understanding the effects of environmental exposure among African-American and Dominican women in NYC, this may limit the representativeness of the results to other populations and areas. Yet, it is important to note that low-income, urban, and minority communities are at disproportionate risk for PAH exposure (Heritage, 1992, Pirkle et al., 1996, Olden and Poje, 1995, Nieves and Wernette, 1992, Metzger et al., 1995, Wagenknecht et al., 1993), and thus, this targeted research is critical to understanding threats to development in children in these communities. Second, although the community sampling of the study was a strength in acquiring an unbiased, random sample of participants and given the typical prevalence of ADHD, most children in the sample were within normative ranges of symptom severity. Future work over-sampling for psychopathology may further extend these findings to larger samples of children meeting diagnostic criteria for ADHD. Third, our assessment of stress exposure and psychiatric symptom outcomes relied on maternal report. Although we cannot fully rule out reporter bias, i.e. that mothers experiencing more stress may report greater symptoms in their children, the observed prenatal PAH × postnatal ELS subscales interactions remained significant when we controlled for maternal distress/demoralization. Furthermore, maternal report can be supplemented by child self-report in later waves of the study. It is also important to note that the prospective longitudinal nature of the study was a key strength, which circumvents the challenges of retrospective report of stress exposure where recall bias is a concern, e.g. neuroticism has been linked to greater retrospective recall of childhood adversity than reported during prospective assessment (Reuben et al., 2016). Fourth, it is important to note that the PAH and ELS assessments capture exposure at specific timepoints. Measuring PAH during the prenatal period captures potential effects of exposure on fetal brain development that may affect developmental trajectories. Measuring ELS at child age 5 may capture a cumulative measure of stress experienced during early childhood, but ELS items generally assessed stress in the prior month or year. Thus, the level of stress experienced could vary over childhood as a function of a family’s changing socioeconomic status. Fifth, it is possible that a gene-environment correlation exists between ELS exposure and psychiatric risk, which should be examined more in future research. Sixth, although we highlight interactions between prenatal PAH and broadband assessment of psychosocial and socioeconomic stress, data were not available regarding the occurrence of specific stressful life events. Seventh, the prenatal assessment only examined two domains of stress, which limited our ability to fully parse the temporal specificity of the stress effects to the prenatal vs. postnatal period. Finally, fewer mothers reported severe lack of social support, relative to the other stress domains examined, which may limit our power to identify specific associations with this factor.
The current study highlights the moderating effects of prenatal exposure to PAH, a neurotoxicant compound common in air pollution, on associations between exposure to stress in early childhood and attention and thought problems in later childhood. While alleviating stress can be difficult, particularly in minority and low-income communities, it is critical that we continue to identify modifiable risk factors for mental health problems, like environmental exposure (Kioumourtzoglou, 2019). Critically, work has shown that the economic cost of PAH exposure is due to not only physical health sequalae but, for example, preschool special education costs due to associated developmental delays (Weiland et al., 2011). As prenatal exposure to PAH can likely magnify the deleterious effects of early life stress on children’s mental health, taking steps to reduce the population’s exposure to neurotoxic air pollutants is central to healthy development.
Supplementary Material
Appendix S1. Supplementary methods.
Appendix S2. Supplementary results.
Table S1. Intercorrelation among variables of interest.
Table S2. Sub-sample characteristics restricted by different outcomes.
Table S3. Characteristics of included vs. excluded subsamples
Table S4. ELS and ELS × PAH effects for all eight CBCL subscale scores.
Table S5. Primary regression analyses predicting CBCL thought and attention problems.
Figure S1. Johnson-Neyman plots of early life stress × PAH air monitoring interaction.
Figure S2. Early life stress × maternal DNA adducts interaction.
Key points.
Prenatal exposure to polycyclic aromatic hydrocarbons (PAH) is linked to adverse physical, behavioral, and cognitive outcomes
Early life stress is a risk factor for childhood psychiatric disorders, but it is unknown whether stress and environmental exposures, including PAH, show independent or interacting effects on psychiatric outcomes
Data from the Columbia Center for Children’s Environmental Health Mothers and Newborns longitudinal birth cohort study showed that prenatal exposure to PAH magnifies the association between stress and attention and thought problems across childhood
Exposure to neurotoxicants in air pollution have long lasting effects on child mental health
Acknowledgements
Funding was provided by the National Institute for Environmental Health Sciences (NIEHS) and the U.S. Environmental Protection Agency (US EPA): NIEHS/EPA P01ES09600/RD82702701, NIEHS/EPA P01ES09600/RD832141, NIEHS/EPA P01ES09600/RD834509, NIEHS/EPA P50ES09600/RD83615401, NIEHS K23ES026239. NIEHS R01ES014393, NIEHS RC2ES018784, NIEHS R01ES13163, and NIEHS R01ES08977, NIEHS 5P50ES009600, the New York Community Trust, Trustees of the Blanchette Hooker Rockefeller Fund, and the John and Wendy Neu Foundation. The authors have declared that they have no competing or potential conflicts of interest.
Footnotes
Supporting information
Additional supporting information may be found online in the Supporting Information section at the end of the article:
Conflict of interest statement: No conflicts declared.
References
- ABDELLAOUI A, DE MOOR MH, GEELS LM, VAN BEEK JH, WILLEMSEN G & BOOMSMA DI (2012). Thought problems from adolescence to adulthood: measurement invariance and longitudinal heritability. Behav Genet, 42, 19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ABRAVANEL BT & SINHA R (2015). Emotion dysregulation mediates the relationship between lifetime cumulative adversity and depressive symptomatology. Journal of Psychiatric Research, 61, 89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ACHENBACH & RESCORLA (2001). Manual for the Achenbach system of empirically based assessment school-age forms profiles. Burlington, VT: Aseba. [Google Scholar]
- ALEXANDROV K, ROJAS M, GENESTE O, CASTEGNARO M, CAMUS A-M, PETRUZZELLI S, GIUNTINI C & BARTSCH H (1992). An improved fluorometric assay for dosimetry of benzo (a) pyrene diol-epoxide-DNA adducts in smokers’ lung: comparisons with total bulky adducts and aryl hydrocarbon hydroxylase activity. Cancer Research, 52, 6248–6253. [PubMed] [Google Scholar]
- AMERICAN PSYCHIATRIC ASSOCIATION (2000). Diagnostic and statistical manual of mental disorders DSM-IV-TR fourth edition (text revision). [Google Scholar]
- BECERRA TA, WILHELM M, OLSEN J, COCKBURN M & RITZ B (2013). Ambient air pollution and autism in Los Angeles county, California. Environ Health Perspect, 121, 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIERHAUS A, HUMPERT PM & NAWROTH PP (2006). Linking stress to inflammation. Anesthesiology Clinics of North America, 24, 325–340. [DOI] [PubMed] [Google Scholar]
- BIERHAUS A, WOLF J, ANDRASSY M, ROHLEDER N, HUMPERT PM, PETROV D, FERSTL R, VON EYNATTEN M, WENDT T & RUDOFSKY G (2003). A mechanism converting psychosocial stress into mononuclear cell activation. Proceedings of the National Academy of Sciences, 100, 1920–1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLOCK ML & CALDERON-GARCIDUENAS L (2009). Air pollution: mechanisms of neuroinflammation and CNS disease. Trends Neurosci, 32, 506–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOCK J & BRAUN K (2011). The impact of perinatal stress on the functional maturation of prefronto-cortical synaptic circuits: implications for the pathophysiology of ADHD? Prog Brain Res, 189, 155–169. [DOI] [PubMed] [Google Scholar]
- BOLTON JL, HUFF NC, SMITH SH, MASON SN, FOSTER WM, AUTEN RL & BILBO SD (2013). Maternal stress and effects of prenatal air pollution on offspring mental health outcomes in mice. Environ Health Perspect, 121, 1075–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOSTROM CE, GERDE P, HANBERG A, JERNSTROM B, JOHANSSON C, KYRKLUND T, RANNUG A, TORNQVIST M, VICTORIN K & WESTERHOLM R (2002). Cancer risk assessment, indicators, and guidelines for polycyclic aromatic hydrocarbons in the ambient air. Environ Health Perspect, 110 Suppl 3, 451–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRADLEY RH (1994). The Home Inventory: review and reflections. [DOI] [PubMed] [Google Scholar]
- BROWN L, SHERBENOU RJ & JOHNSEN SK (1997). Test of Nonverbal Intelligence. Third edition IL:Scholastic Testing Service, Inc, Bensenville. [Google Scholar]
- BROWN LA, KHOUSBOUEI H, GOODWIN JS, IRVIN-WILSON CV, RAMESH A, SHENG L, MCCALLISTER MM, JIANG GC, ASCHNER M & HOOD DB (2007). Down-regulation of early ionotrophic glutamate receptor subunit developmental expression as a mechanism for observed plasticity deficits following gestational exposure to benzo(a)pyrene. Neurotoxicology, 28, 965–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARR CP, MARTINS CM, STINGEL AM, LEMGRUBER VB & JURUENA MF (2013). The role of early life stress in adult psychiatric disorders: a systematic review according to childhood trauma subtypes. J Nerv Ment Dis, 201, 1007–1020. [DOI] [PubMed] [Google Scholar]
- COHEN S (1988). Perceived stress in a probability sample of the United States The social psychology of health. (pp. 31–67). Thousand Oaks, CA, US: Sage Publications, Inc. [Google Scholar]
- COHEN S, KAMARCK T & MERMELSTEIN R (1983). A Global Measure of Perceived Stress. Journal of Health and Social Behavior. [PubMed] [Google Scholar]
- COHEN S, KAMARCK T & MERMELSTEIN R (1994). Perceived stress scale. Measuring stress: A guide for health and social scientists, 235–283. [Google Scholar]
- COHEN S, MERMELSTEIN R, KAMARCK T & HOBERMAN HM (1985). Measuring the functional components of social support Social support: Theory, research and applications. (pp. 73–94). Springer. [Google Scholar]
- COHEN S, UNDERWOOD LG & GOTTLIEB BH (2000). Social support measurement and intervention: A guide for health and social scientists: Oxford University Press. [Google Scholar]
- CONNERS CK (1997). Conners’ Rating Scales--revised: User’s Manual: Multi-Health Systems, Incorporated. [Google Scholar]
- CRONHOLM PF, FORKE CM, WADE R, BAIR-MERRITT MH, DAVIS M, HARKINS-SCHWARZ M, PACHTER LM & FEIN JA (2015). Adverse Childhood Experiences: Expanding the Concept of Adversity. Am J Prev Med, 49, 354–361. [DOI] [PubMed] [Google Scholar]
- DAVIS EP, GLYNN LM, SCHETTER CD, HOBEL C, CHICZ-DEMET A & SANDMAN CA (2007). Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry, 46, 737–746. [DOI] [PubMed] [Google Scholar]
- DOHRENWEND BP, SHROUT PE, EGRI G & MENDELSOHN FS (1980). Nonspecific Psychological Distress and Other Dimensions of Psychopathology - Measures for Use in the General-Population. Archives of General Psychiatry, 37, 1229–1236. [DOI] [PubMed] [Google Scholar]
- DOUGHERTY LR, TOLEP MR, SMITH VC & ROSE S (2013). Early exposure to parental depression and parenting: associations with young offspring’s stress physiology and oppositional behavior. J Abnorm Child Psychol, 41, 1299–1310. [DOI] [PubMed] [Google Scholar]
- DUARTE CS, BORDIN IA, DE OLIVEIRA A & BIRD H (2003). The CBCL and the identification of children with autism and related conditions in Brazil: pilot findings. J Autism Dev Disord, 33, 703–707. [DOI] [PubMed] [Google Scholar]
- DUPAUL GJ, POWER TJ, ANASTOPOULOS AD & REID R (1998). ADHD Rating Scale—IV: Checklists, norms, and clinical interpretation: Guilford Press. [Google Scholar]
- EDWARDS SC, JEDRYCHOWSKI W, BUTSCHER M, CAMANN D, KIELTYKA A, MROZ E, FLAK E, LI Z, WANG S, RAUH V & PERERA F (2010). Prenatal exposure to airborne polycyclic aromatic hydrocarbons and children’s intelligence at 5 years of age in a prospective cohort study in Poland. Environ Health Perspect, 118, 1326–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GILMAN SE, KAWACHI I, FITZMAURICE GM & BUKA SL (2002). Socioeconomic status in childhood and the lifetime risk of major depression. International journal of epidemiology, 31, 359–367. [PubMed] [Google Scholar]
- GILMAN SE, KAWACHI I, FITZMAURICE GM & BUKA SL (2003). Socio-economic status, family disruption and residential stability in childhood: relation to onset, recurrence and remission of major depression. Psychological Medicine, 33, 1341–1355. [DOI] [PubMed] [Google Scholar]
- GODSCHALK RW, VAN SCHOOTEN F-J & BARTSCH H (2003). A critical evaluation of DNA adducts as biological markers for human exposure to polycyclic aromatic compounds. Journal of Biochemistry and Molecular Biology, 36, 1–11. [DOI] [PubMed] [Google Scholar]
- GOLDING JM (1999). Intimate partner violence as a risk factor for mental disorders: A meta-analysis. Journal of family violence, 14, 99–132. [Google Scholar]
- GREEN JG, MCLAUGHLIN KA, BERGLUND PA, GRUBER MJ, SAMPSON NA, ZASLAVSKY AM & KESSLER RC (2010). Childhood Adversities and Adult Psychiatric Disorders in the National Comorbidity Survey Replication I Associations With First Onset of DSM-IV Disorders. Archives of General Psychiatry, 67, 113–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GROVA N, VALLEY A, TURNER JD, MOREL A, MULLER CP & SCHROEDER H (2007). Modulation of behavior and NMDA-R1 gene mRNA expression in adult female mice after sub-acute administration of benzo(a)pyrene. Neurotoxicology, 28, 630–636. [DOI] [PubMed] [Google Scholar]
- HATCH SL & DOHRENWEND BP (2007). Distribution of traumatic and other stressful life events by race/ethnicity, gender, SES and age: a review of the research. Am J Community Psychol, 40, 313–332. [DOI] [PubMed] [Google Scholar]
- HERBSTMAN JB, TANG D, ZHU D, QU L, SJÖDIN A, LI Z, CAMANN D & PERERA FP (2012). Prenatal exposure to polycyclic aromatic hydrocarbons, benzo [a] pyrene–DNA adducts, and genomic DNA methylation in cord blood. Environmental Health Perspectives, 120, 733–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERITAGE J (1992). Environmental protection—has it been fair. EPA J, 18, 1–64. [Google Scholar]
- HUDZIAK JJ, ALTHOFF RR, STANGER C, VAN BEIJSTERVELDT CE, NELSON EC, HANNA GL, BOOMSMA DI & TODD RD (2006). The Obsessive Compulsive Scale of the Child Behavior Checklist predicts obsessive-compulsive disorder: a receiver operating characteristic curve analysis. J Child Psychol Psychiatry, 47, 160–166. [DOI] [PubMed] [Google Scholar]
- JEDRYCHOWSKI WA, PERERA FP, CAMANN D, SPENGLER J, BUTSCHER M, MROZ E, MAJEWSKA R, FLAK E, JACEK R & SOWA A (2015). Prenatal exposure to polycyclic aromatic hydrocarbons and cognitive dysfunction in children. Environ Sci Pollut Res Int, 22, 3631–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOSEPH N, ZHANG-JAMES Y, PERL A & FARAONE SV (2015). Oxidative Stress and ADHD: A Meta-Analysis. J Atten Disord, 19, 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENDLER KS, KUHN JW & PRESCOTT CA (2004). Childhood sexual abuse, stressful life events and risk for major depression in women. Psychological Medicine, 34, 1475–1482. [DOI] [PubMed] [Google Scholar]
- KENDLER KS, MYERS J & PRESCOTT CA (2005). Sex differences in the relationship between social support and risk for major depression: A longitudinal study of opposite-sex twin pairs. American Journal of Psychiatry, 162, 250–256. [DOI] [PubMed] [Google Scholar]
- KESSLER RC, DAVIS CG & KENDLER KS (1997). Childhood adversity and adult psychiatric disorder in the US National Comorbidity Survey. Psychological Medicine, 27, 1101–1119. [DOI] [PubMed] [Google Scholar]
- KESSLER RC & MAGEE WJ (1994). Childhood family violence and adult recurrent depression. Journal of Health and Social Behavior, 13–27. [PubMed] [Google Scholar]
- KESSLER RC, MCLAUGHLIN KA, GREEN JG, GRUBER MJ, SAMPSON NA, ZASLAVSKY AM, AGUILAR-GAXIOLA S, ALHAMZAWI AO, ALONSO J, ANGERMEYER M, BENJET C, BROMET E, CHATTERJI S, DE GIROLAMO G, DEMYTTENAERE K, FAYYAD J, FLORESCU S, GAL G, GUREJE O, HARO JM, HU CY, KARAM EG, KAWAKAMI N, LEE S, LEPINE JP, ORMEL J, POSADA-VILLA J, SAGAR R, TSANG A, USTUN TB, VASSILEV S, VIANA MC & WILLIAMS DR (2010). Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br J Psychiatry, 197, 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIERNAN KE & HUERTA MC (2008). Economic deprivation, maternal depression, parenting and children’s cognitive and emotional development in early childhood. Br J Sociol, 59, 783–806. [DOI] [PubMed] [Google Scholar]
- KIM SY, NAIR R, KNIGHT GP, ROOSA MW & UPDEGRAFF KA (2008). Measurement Equivalence of Neighborhood Quality Measures for European American and Mexican American Families. J Community Psychol, 37, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIOUMOURTZOGLOU MA (2019). Identifying Modifiable Risk Factors of Mental Health Disorders-The Importance of Urban Environmental Exposures. JAMA Psychiatry. [DOI] [PubMed] [Google Scholar]
- LIEB R, ISENSEE B, HOFLER M, PFISTER H & WITTCHEN HU (2002). Parental major depression and the risk of depression and other mental disorders in offspring: a prospective-longitudinal community study. Arch Gen Psychiatry, 59, 365–374. [DOI] [PubMed] [Google Scholar]
- LOVASI GS, ELDRED-SKEMP N, QUINN JW, CHANG HW, RAUH VA, RUNDLE A, ORJUELA MA & PERERA FP (2014). Neighborhood Social Context and Individual Polycyclic Aromatic Hydrocarbon Exposures Associated with Child Cognitive Test Scores. J Child Fam Stud, 23, 785–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARGOLIS AE, HERBSTMAN JB, DAVIS KS, THOMAS VK, TANG D, WANG Y, WANG S, PERERA FP, PETERSON BS & RAUH VA (2016). Longitudinal effects of prenatal exposure to air pollutants on self-regulatory capacities and social competence. J Child Psychol Psychiatry, 57, 851–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAYER SE & JENCKS C (1989). Poverty and the distribution of material hardship. Journal of Human resources, 88–114. [Google Scholar]
- MAZEFSKY CA, ANDERSON R, CONNER CM & MINSHEW N (2011). Child Behavior Checklist Scores for School-Aged Children with Autism: Preliminary Evidence of Patterns Suggesting the Need for Referral. Journal of Psychopathology and Behavioral Assessment, 33, 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MCGUINN LA, WINDHAM GC, MESSER LC, DI Q, SCHWARTZ J, CROEN LA, MOODY EJ, RAPPOLD AG, RICHARDSON DB, NEAS LM, GAMMON MD, SCHIEVE LA & DANIELS JL (2019). Air pollution, neighborhood deprivation, and autism spectrum disorder in the Study to Explore Early Development. Environmental Epidemiology, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- METZGER R, DELGADO JL & HERRELL R (1995). Environmental health and Hispanic children. Environmental Health Perspectives, 103, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MICKELSON KD & KUBZANSKY LD (2003). Social distribution of social support: the mediating role of life events. Am J Community Psychol, 32, 265–281. [DOI] [PubMed] [Google Scholar]
- MOLNAR BE, BUKA SL & KESSLER RC (2001). Child sexual abuse and subsequent psychopathology: results from the National Comorbidity Survey. American journal of public health, 91, 753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOONEY L, SANTELLA RM, COVEY L, JEFFREY AM, BIGBEE W, RANDALL MC, COOPER TB, OTTMAN R, TSAI W-Y & WAZNEH L (1995). Decline of DNA damage and other biomarkers in peripheral blood following smoking cessation. Cancer Epidemiology and Prevention Biomarkers, 4, 627–634. [PubMed] [Google Scholar]
- MOREY RD, ROUDER JN, JAMIL T & MOREY MRD (2015). Package ‘BayesFactor’. URLh http://cran/r-projectorg/web/packages/BayesFactor/BayesFactorpdfi (accessed 1006 15). [Google Scholar]
- NEWBURY JB, ARSENEAULT L, BEEVERS S, KITWIROON N, ROBERTS S, PARIANTE CM, KELLY FJ & FISHER HL (2019). Association of Air Pollution Exposure With Psychotic Experiences During Adolescence. JAMA Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIEVES L & WERNETTE D (1992). Breathing Polluted Air. EPA Journal, 18, 16–17. [Google Scholar]
- NURIUS PS, GREEN S, LOGAN-GREENE P, LONGHI D & SONG C (2016). Stress pathways to health inequalities: embedding ACEs within social and behavioral contexts. International public health journal, 8, 241. [PMC free article] [PubMed] [Google Scholar]
- O’LENICK CR, WINQUIST A, MULHOLLAND JA, FRIBERG MD, CHANG HH, KRAMER MR, DARROW LA & SARNAT SE (2017). Assessment of neighbourhood-level socioeconomic status as a modifier of air pollution-asthma associations among children in Atlanta. J Epidemiol Community Health, 71, 129–136. [DOI] [PubMed] [Google Scholar]
- OBERLANDER TF, WEINBERG J, PAPSDORF M, GRUNAU R, MISRI S & DEVLIN AM (2008). Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics, 3, 97–106. [DOI] [PubMed] [Google Scholar]
- OLDEN K & POJE J (1995). Environmental justice and environmental health. Bull Soc Occup Environ Health, 4, 3–4. [Google Scholar]
- PACE TW, MLETZKO TC, ALAGBE O, MUSSELMAN DL, NEMEROFF CB, MILLER AH & HEIM CM (2006). Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry, 163, 1630–1633. [DOI] [PubMed] [Google Scholar]
- PAGLIACCIO D & BARCH D (2016). Early Life Adversity and Risk for Depression: Alterations in Cortisol and Brain Structure and Function as Mediating Mechanisms. Systems Neuroscience in Depression. [Google Scholar]
- PAGLIACCIO D, LUBY JL, BOGDAN R, AGRAWAL A, GAFFREY MS, BELDEN AC, BOTTERON KN, HARMS MP & BARCH DM (2014). Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology, 39, 1245–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAGLIACCIO D, LUBY JL, BOGDAN R, AGRAWAL A, GAFFREY MS, BELDEN AC, BOTTERON KN, HARMS MP & BARCH DM (2015). Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. J Abnorm Psychol, 124, 817–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERERA F, RAUH V, WHYATT RM, TSAI W-Y, TANG D, DIAZ D, HOEPNER L, BARR D, TU Y-H, CAMANN D & KINNEY P (2006a). Effect of Prenatal Exposure to Airborne Polycyclic Aromatic Hydrocarbons on Neurodevelopment in the First 3 Years of Life among Inner-City Children. Environmental Health Perspectives, 114, 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERERA F, TANG D, WANG S, VISHNEVETSKY J, ZHANG B, DIAZ D, CAMANN D & RAUH V (2012). Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ Health Perspect, 120, 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERERA FP, CHANG HW, TANG D, ROEN EL, HERBSTMAN J, MARGOLIS A, HUANG TJ, MILLER RL, WANG S & RAUH V (2014). Early-life exposure to polycyclic aromatic hydrocarbons and ADHD behavior problems. PLoS One, 9, e111670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERERA FP, LI Z, WHYATT R, HOEPNER L, WANG S, CAMANN D & RAUH V (2009). Prenatal airborne polycyclic aromatic hydrocarbon exposure and child IQ at age 5 years. Pediatrics, 124, e195–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERERA FP, RAUH V, TSAI WY, KINNEY P, CAMANN D, BARR D, BERNERT T, GARFINKEL R, TU YH, DIAZ D, DIETRICH J & WHYATT RM (2003). Effects of transplacental exposure to environmental pollutants on birth outcomes in a multiethnic population. Environ Health Perspect, 111, 201–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERERA FP, RAUH V, WHYATT RM, TSAI WY, TANG D, DIAZ D, HOEPNER L, BARR D, TU YH, CAMANN D & KINNEY P (2006b). Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environ Health Perspect, 114, 1287–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERERA FP, TANG D, TU Y-H, CRUZ LA, BORJAS M, BERNERT T & WHYATT RM (2004). Biomarkers in maternal and newborn blood indicate heightened fetal susceptibility to procarcinogenic DNA damage. Environmental Health Perspectives, 112, 1133–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERERA FP, WANG S, VISHNEVETSKY J, ZHANG B, COLE KJ, TANG D, RAUH V & PHILLIPS DH (2011). Polycyclic aromatic hydrocarbons-aromatic DNA adducts in cord blood and behavior scores in New York city children. Environ Health Perspect, 119, 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERERA FP, WHEELOCK K, WANG Y, TANG D, MARGOLIS AE, BADIA G, COWELL W, MILLER RL, RAUH V, WANG S & HERBSTMAN JB (2017). Combined effects of prenatal exposure to polycyclic aromatic hydrocarbons and material hardship on child ADHD behavior problems. Environ Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERERA FP, WHEELOCK K, WANG Y, TANG D, MARGOLIS AE, BADIA G, COWELL W, MILLER RL, RAUH V, WANG S & HERBSTMAN JB (2018). Combined effects of prenatal exposure to polycyclic aromatic hydrocarbons and material hardship on child ADHD behavior problems. Environ Res, 160, 506–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETERSON BS, RAUH VA, BANSAL R, HAO X, TOTH Z, NATI G, WALSH K, MILLER RL, ARIAS F, SEMANEK D & PERERA F (2015). Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry, 72, 531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PIRKLE JL, FLEGAL KM, BERNERT JT, BRODY DJ, ETZEL RA & MAURER KR (1996). Exposure of the US population to environmental tobacco smoke: the Third National Health and Nutrition Examination Survey, 1988 to 1991. JAMA, 275, 1233–1240. [PubMed] [Google Scholar]
- RAUH V, MARGOLIS A, ARUNAJADAI S & BS P (2014). Atypical Neuropsychological Profiles, Attention Deficit Disorder, Brain Anomalies and Environmental Insecticide Exposure. 69th Annual Meeting of Society for Biological Psychiatry New York, NY. [Google Scholar]
- RESCORLA L (1988). Cluster analytic identification of autistic preschoolers. Journal of Autism and Developmental Disorders, 18, 475–492. [DOI] [PubMed] [Google Scholar]
- REUBEN A, MOFFITT TE, CASPI A, BELSKY DW, HARRINGTON H, SCHROEDER F, HOGAN S, RAMRAKHA S, POULTON R & DANESE A (2016). Lest we forget: comparing retrospective and prospective assessments of adverse childhood experiences in the prediction of adult health. J Child Psychol Psychiatry, 57, 1103–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUNDLE A, HOEPNER L, HASSOUN A, OBERFIELD S, FREYER G, HOLMES D, REYES M, QUINN J, CAMANN D, PERERA F & WHYATT R (2012). Association of childhood obesity with maternal exposure to ambient air polycyclic aromatic hydrocarbons during pregnancy. Am J Epidemiol, 175, 1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALCEDO S, RIZVI SH, FREEMAN LK, YOUNGSTROM JK, FINDLING RL & YOUNGSTROM EA (2018). Diagnostic efficiency of the CBCL thought problems and DSM-oriented psychotic symptoms scales for pediatric psychotic symptoms. Eur Child Adolesc Psychiatry, 27, 1491–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAMPSON RJ & RAUDENBUSH SW (1999). Systematic social observation of public spaces: A new look at disorder in urban neighborhoods. American Journal of Sociology, 105, 603–651. [Google Scholar]
- SAMPSON RJ, RAUDENBUSH SW & EARLS F (1997). Neighborhoods and violent crime: A multilevel study of collective efficacy. Science, 277, 918–924. [DOI] [PubMed] [Google Scholar]
- SAUNDERS CR, DAS SK, RAMESH A, SHOCKLEY DC & MUKHERJEE S (2006a). Benzo (a) pyrene‐induced acute neurotoxicity in the F‐344 rat: role of oxidative stress. Journal of Applied Toxicology: An International Journal, 26, 427–438. [DOI] [PubMed] [Google Scholar]
- SAUNDERS CR, DAS SK, RAMESH A, SHOCKLEY DC & MUKHERJEE S (2006b). Benzo(a)pyrene-induced acute neurotoxicity in the F-344 rat: role of oxidative stress. J Appl Toxicol, 26, 427–438. [DOI] [PubMed] [Google Scholar]
- SCHELLENBERGER MT, GROVA N, FARINELLE S, WILLIEME S, SCHROEDER H & MULLER CP (2013). Modulation of benzo[a]pyrene induced neurotoxicity in female mice actively immunized with a B[a]P-diphtheria toxoid conjugate. Toxicol Appl Pharmacol, 271, 175–183. [DOI] [PubMed] [Google Scholar]
- SILK JS, SHAW DS, SKUBAN EM, OLAND AA & KOVACS M (2006). Emotion regulation strategies in offspring of childhood-onset depressed mothers. J Child Psychol Psychiatry, 47, 69–78. [DOI] [PubMed] [Google Scholar]
- SIMEONOVA DI, NGUYEN T & WALKER EF (2014). Psychosis risk screening in clinical high-risk adolescents: a longitudinal investigation using the Child Behavior Checklist. Schizophr Res, 159, 7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCE SH, NAJMAN JM, BOR W, O’CALLAGHAN MJ & WILLIAMS GM (2002). Maternal anxiety and depression, poverty and marital relationship factors during early childhood as predictors of anxiety and depressive symptoms in adolescence. Journal of Child Psychology and Psychiatry, 43, 457–469. [DOI] [PubMed] [Google Scholar]
- STEIN LJ, GUNIER RB, HARLEY K, KOGUT K, BRADMAN A & ESKENAZI B (2016). Early childhood adversity potentiates the adverse association between prenatal organophosphate pesticide exposure and child IQ: The CHAMACOS cohort. Neurotoxicology, 56, 180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STRAUS MA (2017). The Conflict Tactics Scales and its critics: An evaluation and new data on validity and reliability. Physical violence in American families. (pp. pp. 49–74). [Google Scholar]
- STRAUS MA, HAMBY SL, BONEYMCCOY S & SUGARMAN DB (1996). The revised Conflict Tactics Scales (CTS2) - Development and preliminary psychometric data. Journal of Family Issues, 17, 283–316. [Google Scholar]
- SWARTZ JR, HARIRI AR & WILLIAMSON DE (2016). An epigenetic mechanism links socioeconomic status to changes in depression-related brain function in high-risk adolescents. Molecular Psychiatry, 22, 209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TANG D, LI TY, LIU JJ, ZHOU ZJ, YUAN T, CHEN YH, RAUH VA, XIE J & PERERA F (2008). Effects of prenatal exposure to coal-burning pollutants on children’s development in China. Environ Health Perspect, 116, 674–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEAM RC (2015). R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. [Google Scholar]
- TEO AR, CHOI H & VALENSTEIN M (2013). Social relationships and depression: ten-year follow-up from a nationally representative study. PLoS One, 8, e62396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VEGA EM & O’LEARY KD (2007). Test–Retest Reliability of the Revised Conflict Tactics Scales (CTS2). Journal of family violence, 22, 703–708. [Google Scholar]
- VEGLIA F, LOFT S, MATULLO G, PELUSO M, MUNNIA A, PERERA F, PHILLIPS DH, TANG D, AUTRUP H & RAASCHOU-NIELSEN O (2008). DNA adducts and cancer risk in prospective studies: a pooled analysis and a meta-analysis. Carcinogenesis, 29, 932–936. [DOI] [PubMed] [Google Scholar]
- VISHNEVETSKY J, TANG D, CHANG HW, ROEN EL, WANG Y, RAUH V, WANG S, MILLER RL, HERBSTMAN J & PERERA FP (2015). Combined effects of prenatal polycyclic aromatic hydrocarbons and material hardship on child IQ. Neurotoxicol Teratol, 49, 74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLK HE, HERTZ-PICCIOTTO I, DELWICHE L, LURMANN F & MCCONNELL R (2011). Residential proximity to freeways and autism in the CHARGE study. Environ Health Perspect, 119, 873–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOLK HE, LURMANN F, PENFOLD B, HERTZ-PICCIOTTO I & MCCONNELL R (2013). Traffic-related air pollution, particulate matter, and autism. JAMA Psychiatry, 70, 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VON EHRENSTEIN OS, ARALIS H, COCKBURN M & RITZ B (2014). In utero exposure to toxic air pollutants and risk of childhood autism. Epidemiology, 25, 851–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAGENKNECHT L, MANOLIO T, SIDNEY S, BURKE G & HALEY N (1993). Environmental tobacco smoke exposure as determined by cotinine in black and white young adults: the CARDIA study. Environmental research, 63, 39–46. [DOI] [PubMed] [Google Scholar]
- WANKERL M, MILLER R, KIRSCHBAUM C, HENNIG J, STALDER T & ALEXANDER N (2014). Effects of genetic and early environmental risk factors for depression on serotonin transporter expression and methylation profiles. Transl Psychiatry, 4, e402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEILAND K, NEIDELL M, RAUH V & PERERA F (2011). Cost of developmental delay from prenatal exposure to airborne polycyclic aromatic hydrocarbons. J Health Care Poor Underserved, 22, 320–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMSON DE, BIRMAHER B, AXELSON DA, RYAN ND & DAHL RE (2004). First episode of depression in children at low and high familial risk for depression. Journal of the American Academy of Child & Adolescent Psychiatry, 43, 291–297. [DOI] [PubMed] [Google Scholar]
- WORMLEY DD, RAMESH A & HOOD DB (2004). Environmental contaminant-mixture effects on CNS development, plasticity, and behavior. Toxicol Appl Pharmacol, 197, 49–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary methods.
Appendix S2. Supplementary results.
Table S1. Intercorrelation among variables of interest.
Table S2. Sub-sample characteristics restricted by different outcomes.
Table S3. Characteristics of included vs. excluded subsamples
Table S4. ELS and ELS × PAH effects for all eight CBCL subscale scores.
Table S5. Primary regression analyses predicting CBCL thought and attention problems.
Figure S1. Johnson-Neyman plots of early life stress × PAH air monitoring interaction.
Figure S2. Early life stress × maternal DNA adducts interaction.