Abstract
Modelling of human organs has long been a task for scientists in order to lower the costs of therapeutic development and understand the pathological onset of human disease. For decades, despite marked differences in genetics and etiology, animal models remained the norm for drug discovery and disease modelling. Innovative biofabrication techniques have facilitated the development of organ-on-a-chip technology that has great potential to complement conventional animal models. However, human organ as a whole, more specifically the human heart, is difficult to regenerate in vitro, in terms of its chamber specific orientation and its electrical functional complexity. Recent progress with the development of induced pluripotent stem cell differentiation protocols, made recapitulating the complexity of the human heart possible through the generation of cells representative of atrial & ventricular tissue, the sinoatrial node, atrioventricular node and Purkinje fibers. Current heart-on-a-chip approaches incorporate biological, electrical, mechanical, and topographical cues to facilitate tissue maturation, therefore improving the predictive power for the chamber-specific therapeutic effects targeting adult human. In this review, we will give a summary of current advances in heart-on-a-chip technology and provide a comprehensive outlook on the challenges involved in the development of human physiologically relevant heart-on-a-chip.
Keywords: drug testing, cardiomyocytes, platform, tissue engineering, chamber-specific, disease modeling, toxicity, atrial, ventricular, purkinje, SA node, cardiotoxicity, screening, heart, maturation, heart-on-a-chip, organ-on-a-chip
Graphical abstract:
On average, the human heart beats 3 billion times during a lifetime, starting as early as 3 weeks of gestation age and stopping right before death.[3]. It is reported that human cardiac muscle has a limited ability to regenerate, with approximately 50% of adult human cardiomyocytes (CM), being throughout a lifetime of 80 years. [4–6] Because of the scarce availability of human adult CMs, cardiac researches have to rely on animal models for studying human cardiac diseases, despite the inter-species differences. However, the emergence of induced pluripotent stem cells (iPSC) and the recent advancement made in differentiation techniques [7] have greatly transformed this conventional research paradigm. Directed differentiation of human pluripotent stem cells (PSCs) can now generate large numbers of cardiomyocytes readily available for research.[8] Thus, cardiac tissue engineering becomes a pivotal conduit to explore the applications of these cells for drug testing and patient-specific disease modeling purposes.
Despite the advances made in tissue engineering to revolutionize the field of therapeutic discovery, the effort to re-create a physiological-relevant heart remains a foreseeable challenge. The human heart is a muscular organ that is characterized by its distinctive chambers orientation and the built-in electrical conducting system that stimulate the muscle contraction. However, conventional bench-top studies lack the capability to mimic this complex 3D orientation. Heart-on-a-chip, one of the more recent tissue engineering technologies, provides an interesting alternative. It adopts cardiac cell developmental biology and allows precise physical control of microenvironment to create miniaturized 3D cardiac tissue model that offer more reliable clinically relevant biological functions and disease mechanisms. [9]
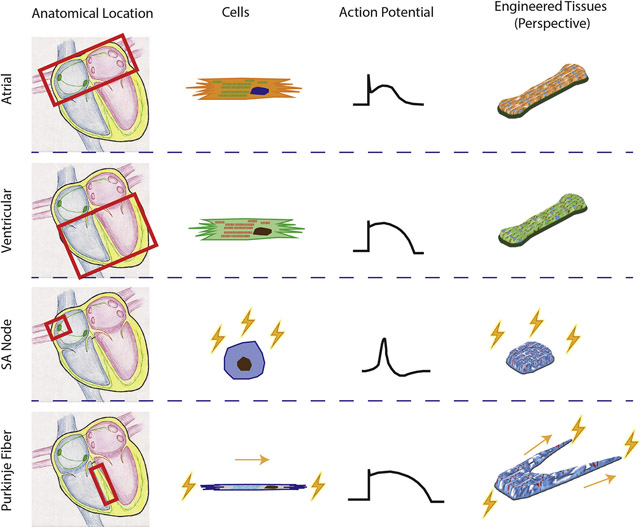
In reality, current heart-on-a-chip platforms should be viewed as ventricle-on-a-chip platforms since the majority of the current studies use ventricular CMs and only study drug responses and diseases modeling related to ventricle chambers. This is because ventricular dysfunction is the primary cause of heart failure[10] and ventricular CMs is the first chamber-specific CM population that can be differentiated with high purity[11]. Nevertheless, other compartments of the heart, such as the atria, sinoatrial node, and Purkinje fibers, are just as important as ventricles during the investigation of drug-related toxicity and cardiac disease mechanisms (Figure 1). For example, conditions of the atrium, such as atrial fibrillation (AF), can elicit sudden death or severe complications during the medical intervention of ventricular chambers.[12] Failure of sinoatrial node function due to congenital disease or aging results in slowing of the heart rate, inefficient blood circulation and arrhythmia.[13] Therefore, it is imperative to obtain these compartment-specific tissue models and to condition them to reach adult maturity for relevant evaluation of their responses to drug dosage and therapeutic intervention.
Figure 1. Heart anatomy and chamber-specific physiology.
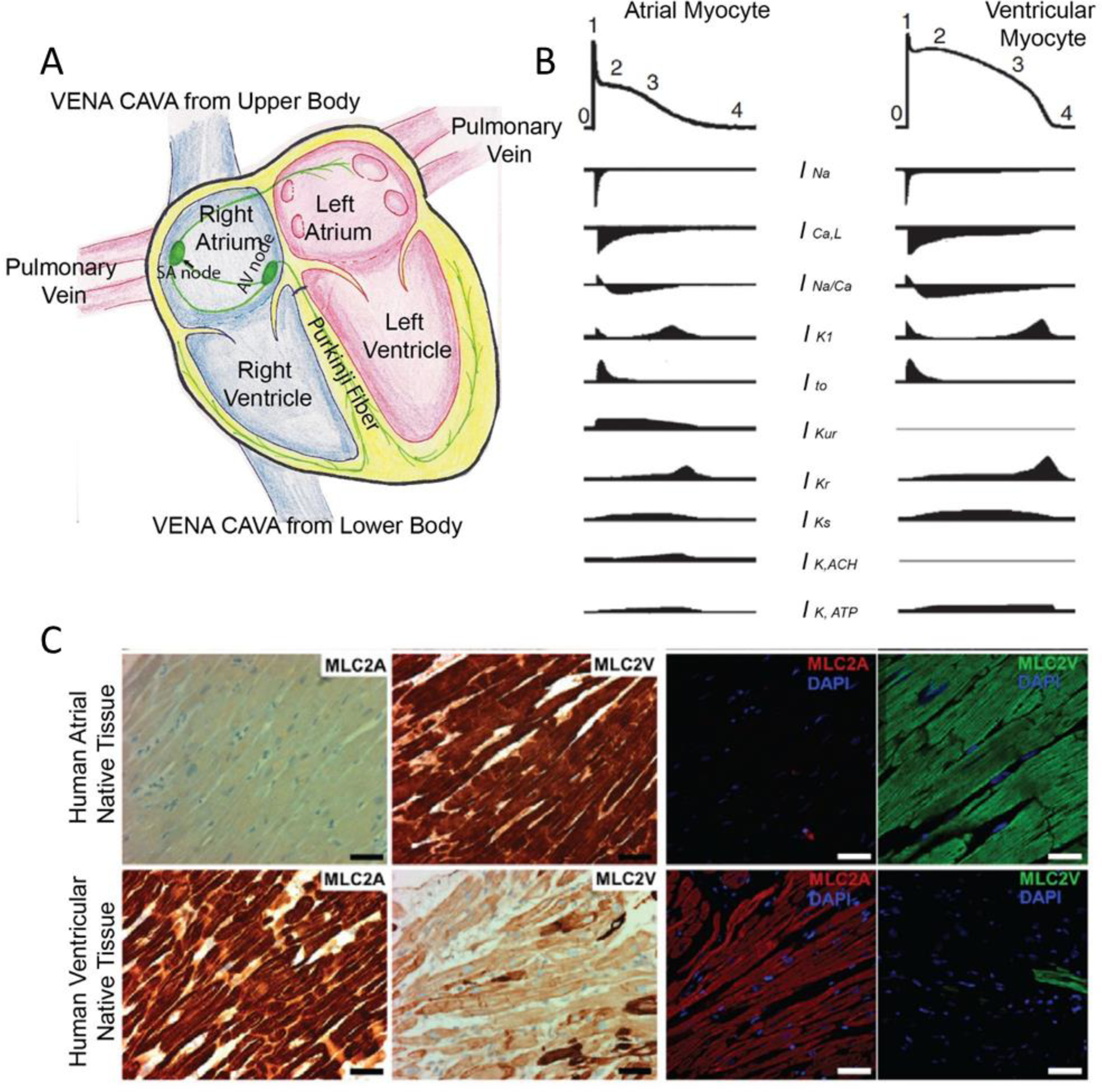
A) Anatomy of the heart. B) Chamber-specific action potential profiles and ion channels. [1] C) Atrial-specific protein, MLC2a, and ventricular-specific protein, MLC2v, in native human tissues. [2] Figures are adapted with permission from their original references.
This review will highlight the current progress of cardiac tissue engineering of specific heart chambers and discuss the challenges to generate a physiologically-relevant heart-on-a-chip model for drug testing applications and disease modeling.
Brief History of Cardiac Tissue Engineering and Heart-on-a-Chip
The main paradigm tissue engineering is to use cells and other biological materials to build functional tissues. Organ-on-a-chip technology, as the convergence of tissue engineering and microfluidics, aims to build functional miniaturized tissues in vitro, simulating the functional characteristics of in vivo tissues or organs for the purposes of drug screening and disease modeling.[14, 15]
Long before the invention of the terms “tissue engineering” or “organ-on-a-chip”, Moscona et al were the first to aggregate chicken embryonic myocytes into a spheroid shaped construct by a continous spinning of the fresh cells in flasks, back in 1950s[16]. This culture method is considered the first in vitro platform in the cardiac field, that enables 3D tissue culture. It has been adapted and improved for culturing various neonatal animal cardiac cells by many groups. In 1999, Akins et al defined a better and more detailed protocol for culturing sphere-shaped 3D structures, resulting in tissues that were structurally-organized presenting with spontaneous and rhythmic beating patterns[17]. Despite the success of simulating cardiac organogenesis in vitro, the morphology and organization of the resulting organoids was not comparable to the native heart muscle.
Heart is a complex organ, which requires sophisticated organization and synchronized behavior of cardiomyocytes. Many efforts were made in the 1990s towards building a functional in vitro platforms. Terracio et al performed chronic cyclic stretch on 2D cell sheets.[18] Carrier et al. used a polyglycolic acid (PGA) scaffold together with laminar flow to form tissue-like structures (50–70 μm thick) in which myocytes were organized in multiple layers in a 3D configuration. [19, 20] Bursac et al seeded cardiomyocytes into patch-shaped PGA scaffolds culturing them in the spinning flask based bioreactor, which enabled improved oxygen supply, thus promoting a better cardiac function. The platform also enabled the electrophysiological assessments on the patch. [19, 20] Similarly, the groups of Li and Leor seeded rat neonatal myocytes into gelatin or alginate scaffolds respectively, which were later engrafted into rat hearts.[21, 22] Tissues seeded on patterned surfaces obtained improved cellular alignment and enhanced tissue function. [23, 24]
Instead of reconstructing the whole heart in vitro, an easier solution is to recapitulate the function and morphology of the basic unit of cardiac muscle. With this in mind, in 1997, Eschenhagen et al were the first to invent the biaxial culture platform with two Velcro-covered glass tubes, which created cardiac tissues recapitulating cardiac muscle bundles. The system can provide anchor points for tissue attachment and static stretching forces during cell compaction and tissue formation for cellular alignment.[25] The arrangement allows the measurement of the isometric contraction force generated by the engineered cardiac tissues. [26] They were also the first group to use collagen I gel aiding cardiac tissue formation. [25] In the later work from this group, the rigid tissue anchor was replaced by posts made of flexible and biocompatible materials, PDMS, significantly increasing the throughput. The mechanical properties of PDMS and the bending motion resulting from tissue contraction could be monitored non-invasively for miniaturized tissue culture systems with a higher throughput.[27] The system has been adapted and modified by multiple research groups and became the earliest version of heart-on-a-chip in the field.[28–35]
In parallel to developing improved tissue culture systems, significant enhancements in cell sources were also achieved over the past two decades. The first engineered cardiac tissue was generated by murine neonatal myoyctes. However, through generation of human embryonic stem cells (hESC), human stem cell derived cardiomyocytes were gradually introduced into engineered platforms. Caspi et al were among the pioneers who introduced human stem cell derived cardiomyoyctes into 3D structure [36]. Shortly after the discovery of human induced pluripotent stem cells (hiPSC)[37], hiPSC derived cardiomyocytes quickly became available to replace the conventional animal cell sources and hESC derived cardiomyocytes [7, 38] in tissue engineering and organ-on-a-chip applications.[30, 35, 39] Chamber-specific cardiomyocytes became available shortly after to create chamber-specific tissues.[2, 28]
Ventricular Tissue Engineering
Ventricles have thick muscle walls and are the primary driving forces for blood flow through the entire body. The diminished ventricular function negatively impacts the quality of life of the individuals and has become one of the primary research foci on therapeutic strategies. Ventricular differentiation method has been well established since the discovery of iPSCs more than a decade ago [40] and produce consistently reproducible results among species.[41] The emerging challenge is to fully exploit these ventricular CMs for more clinically relevant drug testing. Engineered cardiac tissue targeting drug testing applications has been envisioned as a miniaturized, functional 3D tissue that not only recapitulates human adult myocardium, but also can be easily reproduced and tested in a high throughput manner.[42]
3D Ventricular Tissue Models
3D cardiac tissue is commonly preferred since it has more physiologically relevant cell-cell cross talk and cell-matrix interactions compared to 2D monolayer cultures. [43] These biological cues are particularly relevant in cardiac tissue engineering, since the coordinated contraction at the tissue level depends on the intercellular ion exchanges, electrical coupling, and mechanical transduction. [44] Cardiac organoids are created by self-organization into a sophisticated architecture that closely resembles the organogenesis of native cardiac tissues.[15, 45–48] The organoids are typically small in size, i.e. 100 μm, with multiple cardiac lineages and are commonly maintained in a relatively high throughput manner. Unfortunately, there is minimal control over the development of organoids in terms of their structural and functional consistency, due to our limited knowledge of cardiac tissue development. More importantly, their functional output, as well as drug responses, suggest a low level of maturity and little resemblance to the adult myocardium.[49–51]
Organ-on-a-chip technology, more specifically heart-on-a-chip, enables precise control of the physical environment that provides topographical guidance, nutrient supply, flow pattern and shear stress and facilitates functional readouts with built-in sensors. [15, 39, 52–55] Muscle thin film[56] (Figure 2A), as an example, uses 3D printing technique to print force sensors within the polymer scaffold that hold orientated CM layers. The sensors can translate forces into electrical signals and therefore potentially facilitate efficient on-line force analysis. However, muscle films are too thin to be considered as 3D tissues and their physiological relevance is not ideal. [57]
Figure 2. Representative template-guided heart-on-a-chip devices.
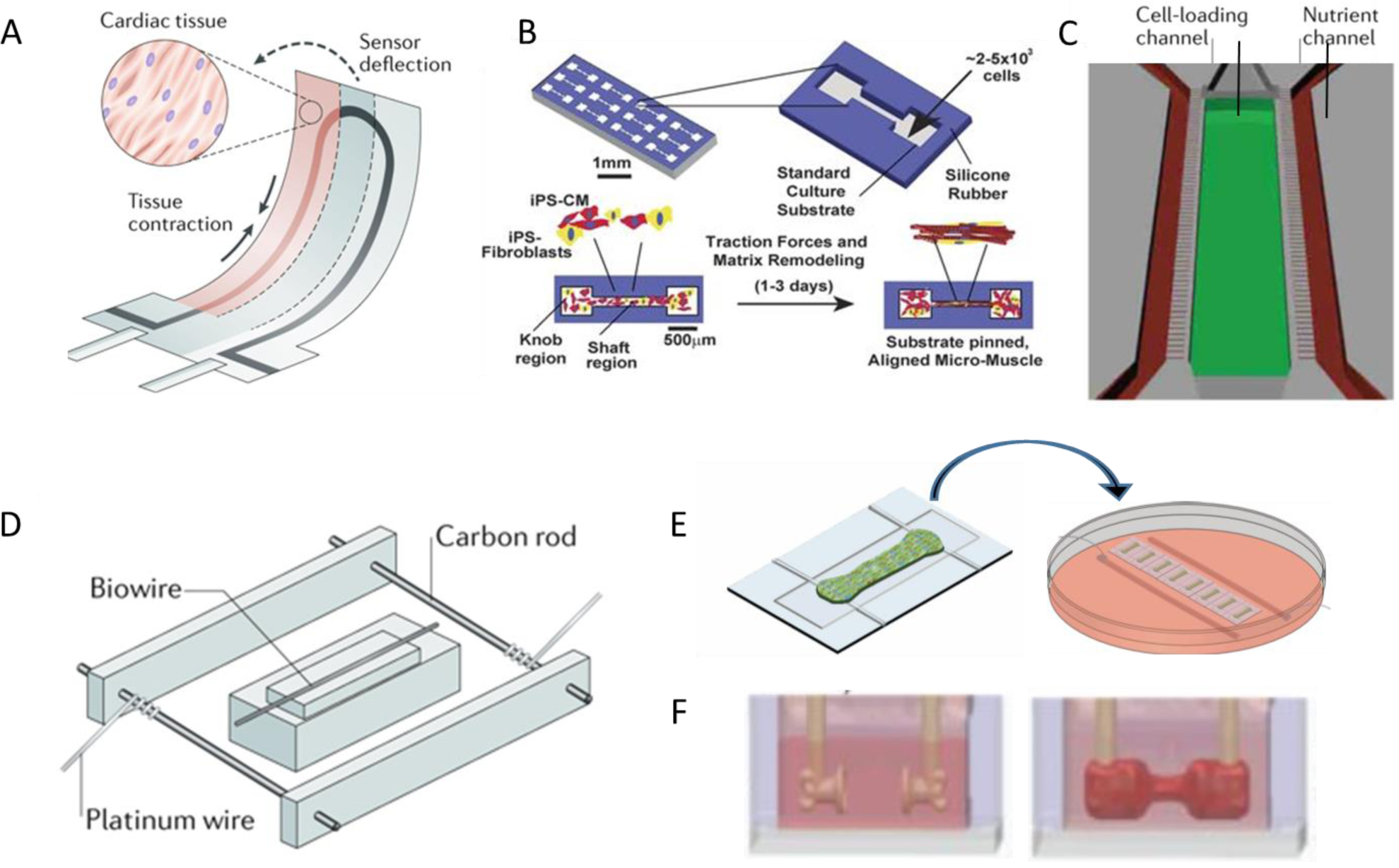
Cardiomyocytes can compact A) adhering as muscle thin films on top of 3D printed sensors[57, 62], B) within microgrooves[59], C) along patterned perfusable channels [62, 63], D) around single suture template [62, 64], E) around double elastic force sensors[28], and F) upside down force-sensing posts[32]. Figures are adapted with permission from their original references.
An alternative approach to recreate a papillary-like tissue is to use template guided cellular assembly. Soft lithography with polydimethylsiloxane (PDMS) molding is a commonly used technique to fabricate the platform with complex template structures.[28, 39, 58] These templates can be a single wire[39], a single perfusable tube[54], double wires[28, 58], or opposing cantilevers[30, 31, 34, 59], residing in microwells (Figure 2B–F), where they simultaneously serve as tissue anchors and force sensors based on their mechanical properties. When CMs with or without hydrogel are placed into the microwells, these templates allow cell self-organization around the structures and generation of static tension within the tissue. These topographical features promote the elongation and alignment of cells.[60, 61] The resulting tissues resemble the papillary muscle bundles at structural and functional levels.
Ventricular Tissue Maturation towards Adult Phenotype
Although human PSC-CMs in 3D tissues and fetal CMs have similar morphological structures and function, they are drastically different from adult CMs (Table 1) [65] Similar to fetal CMs, PSC-CMs are commonly spherical [66] with small sizes[67] and have a low percentage of binucleated cells [68, 69]. Gap junction proteins, such as connexin-43, (Cx43) in PSC-CMs spread out on the cell edge and do not have anisotropic distribution [70–72], confined to intercalated discs as in the adult CMs, which explains the low conduction velocity (1–15cm/s[68, 69]) in the tissues. Although large variation exists among studies with different cell lines and experimental conditions, action potential profiles of PSC-CMs are mostly underdeveloped [73–78]. The randomly distributed sarcomeres in PSC-CMs are on average 0.5–0.6 μm shorter than in adult CMs [77, 78] and only have z disc, I- and A-bands[79, 80]. All PSC-CMs exhibit spontaneous beating[81–83] with the low force of contraction (0.15–0.3 mN/mm2) [84, 85]. Positive force-frequency-relationship (FFR) from 1–3 Hz [86] and pronounced post-rest potentiation (PRP)[87] are two key unique features of adult myocardium, which are not present in either fetal myocardium[88] or PSC-CM derived tissues[89, 90]. Moreover, PSC-CMs and fetal CMs depend on glycolysis for their metabolisms with a minimal level of transition to β-oxidation of fatty acids [91], which is the primary energy source in the adult heart [92]. Mitochondria in PSC-CMs are also less mature, low in quantity and randomly distributed within the cytoplasm. The status of mitochondria confirms the low level of energy production efficiency in PSC-CMs with minimal β-oxidation. [93] Due to these large differences, PSC-CM derived tissues are considered poor approximations of adult myocardium. Further improvements are vital to produce clinically relevant results through the use of these tissues for drug testing applications.
Table 1.
Maturation Benchmarks Set by Native CMs and Current Progress of PSC-CM
| Maturation Parameters | Human Adult CMs | Human Neonatal CMs | Human Fetal CMs | PSC-CM | PSC-CM after Maturation |
|---|---|---|---|---|---|
| Cell Volume (μm3) (Area μm2) | ⩾80000 [94] (Area 1000–2500 [95]) | 5,854 ± 818 (0–1yr) [94] | Similar to PSC-CM[77] | (Area 500–600 [39, 67, 96]) | (Area 917–1700 [39, 67, 96]) |
| Cell Shape | Rod-like[66] | Rod-like[66] | Rod-like[97] | Spherical[66] | Rod-like [39] |
| Cell Surface Area (μm2) | 10,212–14,418 [98] | 4395 ±436 [98] | 1171–1261 [77] | 1445 ± 74.39 [77] | 2169 ± 110.2 [77] |
| Binucleation | 25%–30%[4, 99] | 25% [4] | None[65, 100] | ~0–15% [68, 69] | ~0–20% [32, 68, 101] |
| Sarcomere Structure | Z-, I-, A-, H-, and M-bands[102] | Z-, I-, A-, H-, and M-bands[81, 103] | Missing M-band [81, 103] | Z-, I- and A-bands[80, 104] | Z-, I-, A-, H-, and M-bands o |
| Sarcomere Length (μm) | 2.15 [105] | 2.2 [88] | 1.8 [77] | 1.6 – 1.7 [78] | 2.2 [32] |
| T-tubule | Fully Developed | Fully developed by 2–3 weeks [106] | Minimal [107] | None [106, 108] | Present [28, 32] |
| Gap Junction | Anisotropic [70–72] | Gradually more anisotropic [71, 106, 109] | Cell edges, punctate [106] | Cell edges [70–72] | Cell edges [28]; Anisotropic ends [32] |
| Conduction Velocity (cm/s) | 30–100 [29] | 30 [110] | 40–70 [111] | 1–15 [73, 74] | 31.8 ± 7.9 [28] |
| Action potential Duration (ms) | 228–259 [112] | 324–416 [76] | 200–500 [73–77] | 100–150 [39] | |
| Action potential Amplitude (mV) | 102–110 [112] | 20–27 [76] | 77–116 [75–77] | ~70 [39] or ~100 [28] | |
| Vmax (V/s) | 254–303 [112] | 150 [113] | 5–13 [76] | 6–40 [75–77] | 176–201 [78] or 125 [39] Or 108.8 ± 19.6 [28] |
| Resting Membrane Potentials (mV) | −90 [114] | −37–40 [76] | 37–71 [76–78] | −66–70 [78] or −100 [39] | |
| Automaticity | No[81] | No[82] | Yes[82] | Yes[81–83] | No[32] or minimal [39] |
| Contractile Forces (mN/mm2) | 51±8[105] | 0.8–1.7 (<2week neonatal)[88, 108] | 0.4 (second trimester) [77] | 0.15–0.30 [84, 85] | 0.05–23.2 [28, 31–33] |
| FFR (1–3 Hz) | yes[86] | Improving from flat to positive [88] | Flat[88] | Negative[89, 90, 115] | Positive [28, 32, 33, 90, 116] |
| PRP | yes[87] | Improving from minimal | Minimal [32] | yes [28, 32, 90] | |
| Metabolic activity | β-oxidation of fatty acids [92] | β-oxidation of fatty acids after 7 days [117, 118] | Glycolysis [93] | Glycolysis[91] | Glycolysis and β-oxidation of fatty acids [28, 32] |
| Presence of Mitochondria | Highly organized and occupy 20–40% intracellular space[67] | More developed, ovular shape[117, 118] | Immature, round [93] | Immature and randomly distributed | More developed, packed along the sarcomeres. [32] |
estimated from graphs in the original reference
Native cardiomyocytes reach full maturity after 6–10 years of development [70]. Tissue models for drug testing, however, should achieve ideal maturity within a much shorter time frame, e.g. weeks, after differentiation for an appropriate throughput. To facilitate better tissue maturation within a shorter time frame, many maturation strategies have been investigated over the years. To date, advances of cardiomyocyte maturation have been made through the prolonged culture[78, 119], non-myocyte co-cultures[60, 101, 120], mechanical[31, 35, 101], electrical[32, 39, 121], chemical[33, 122] conditioning and the combinations of these treatments, which all attempt to emulate native physiological microenvironment (Table 2).
Table 2.
Maturation Strategies and Tissue Improvement
| External Stimulus | Parameter approaching the adult level | Other improved Parameters |
|---|---|---|
| Static or Dynamic Stretch | N/A | Better cellular alignment, cell area and higher expression of MYH7, TNNT2, NPPA, NPPB, CACNA1C, RYR2 and ATP2A2[129] More Cx43 and TnT proteins [132] |
| Mechanical and Electrical Stimulation (Fixed pacing frequency at 1–2 Hz) | N/A | Improved cell areas and Frank-Starling mechanism and FFR, improved expression of SR-related proteins [131] Higher contractile forces and better structural proteins[130] |
| β-adrenergic | N/A | Improved contractile forces, structural proteins, gene expression of ANF and MYH7/MYH6 [129] Cell Hypertrophy[104] |
| Triiodothyronine | APD90 | Contractile stress and cell surface area are higher than human fetal control[77]; Improved AP parameters and sarcomere length[77] |
| Dynamic Culture (nutrient availability and shear stress) | Near adult level contractile force 23.2 ± 1.6 mN/mm2 and conduction velocity 25.8±1.2 cm/s [31] | Cellular hypertrophy [31] |
| Electrical Stimulation (Step-up frequency from 2 to 6 Hz) | Positive FFR, t-tubule formation, M-line, Oxidative metabolism, Mitochondria density 30% [32] Conduction velocity 31.8±7.9 cm/s [28] |
Pronounced Post-rest potentiation, better action potential profiles with Ito notch [28] |
| Prolonged culture | Rod-like shape (90 day EB)[133] M-lines (360 days EB)[119] | Improved AP profiles, cell morphology, % multinucleation, and sarcomere structure[29, 119, 133] |
| Co-culture with cardiac fibroblasts | Higher conduction velocity 25.1 ± 7.7 cm/s[60] | Better tissue formation and improved gene expression of MYL7, MYL2, ANP, BNP, and MYH7/MYH6 [60] Improved APD[134] |
estimated from graphs in the original reference
Bioreactor systems have also been demonstrated to improve functional cardiomyocyte phenotypes and tissue maturation. Godier-Furnemont et al demonstrated that electro-mechanical stimulation on the rat engineered heart muscle at a physiological frequency is a necessity to obtain a positive FFR.[123] The combination of mechanical and electrical stimulation was used to mimic the complexities of the native tissue environment.[124] The timing between mechanical and electrical stimulation is important for cardiomyocyte and myocardial tissue maturation in a dual electromechanical bioreactor system.[125] Bioreactors can also provide chemical and mechanical cues, such as hypoxic or dynamic flow conditions, for certain specialized tissue culture models. For example, the rocking/rotating shaker promotes dynamic flow in the bioreactor and enhanced delivery of nutrients, compared to static systems.[126] Hypoxic conditions in the bioreactors have been used to simulate cardiac ischemic conditions in vitro.[127]
Studies have shown that long-term (up to 6 months) monolayer culture can gradually promote human PSC-CMs maturation by improving morphology, myofibril density, alignment, Z-disc registration and action potential (AP) profiles[78]. Jackman and his colleagues have demonstrated that increased nutrient availability by dynamic cultures (rocking the plate) enhanced the force of contraction (23.2 mN/mm2) to the half of the force in adult heart (51 mN/mm2). [31] Tiburcy et al. have shown that fully defined medium can improve the FFR from negative to positive and increase sarcomere length in comparison to fetal CMs.[33] Adding triiodothyronine also improves the force of contraction and AP parameters.[77, 128] β-adrenergic stimulation was shown to improve cellular hypertrophy and the force of contraction through increased expressions of structural proteins.[129] Co-culture with cardiac fibroblasts significantly improves tissue morphology and conduction velocity.[60] While static stretching has moderate effects on increasing contractility, tissue stiffness, cell alignment and size [101, 129], a combination of stretching and low-frequency electrical pacing synergistically enhance cardiac tissue functional development.[130, 131] Nunes et al have shown that gradual increase of the electrical pacing frequency up to 6 Hz in a week can greatly improve calcium handling, AP profiles and cell morphology. Ronaldson-Bouchard et al.[32] slightly decreased the frequency step-up from 0.83 Hz per day to 0.33 Hz per day. The modified electrical stimulation regime was shown to facilitate the formation of cardiac tissues with many hallmarks of adult myocardium, such as positive FFR, T-tubule formation, partially anisotropic gap junction protein distribution and myofibril and mitochondria organization, reporting the M-line presence for the first time and full sarcomere length achievement in engineered cardiac tissues. Another study with a slower frequency ramping up protocol obtained tissues with over 200% PRP, faster conduction velocity, and AP profiles with Ito notch and better refractory phase.[28]
However, many of these studies only assess a subset of these maturation parameters and can hardly perceive the actual maturity of their tissues in the natural cardiac developmental process. The level of maturation in PSC-CMs requires multifaceted evaluations, which should include intracellular structure development, tissue function assessment such as contractile forces, conduction velocity, calcium handling, and electrophysiology; metabolic activities and cellular morphology. The benchmarks of native CMs would allow us to better evaluate the level of CM maturation and determine whether they are improved towards the adult myocardium and therefore will be more suitable for drug testing applications.
By comparing with the benchmarks of native CMs, several maturation strategies can successfully improve a subset of parameters to the level of neonatal CMs or adult CMs (Table 1 and 2). Thus, ventricular engineered tissues after maturation regimes are ideally comparable to post-natal stages with several aspects approaching adult myocardium. [28, 31, 32, 119, 135]Although the tissue model would be more suitable as a surrogate for drugs targeting infants[88], it would be interesting to compare the drug testing results from engineered tissue model and adult myocardium to understand the differences.
We have compiled a list of commonly used drugs and the comparison of drug responses in PSC-CMs, matured PSC-CMs, and adult myocardium (Table 3). Isoproterenol is a potent β-adrenergic agonist that induce positive chronotropic and inotropic responses. The inotropic EC50 in matured engineered cardiac tissues and increased maximum force are comparable to these in adult myocardium. However, inotropic EC50 is much higher [34] with minimal force increase[30, 119] in non-matured cardiac tissue. Dofetilide, an hERG channel blocker, has a high risk of causing QT interval prolongation and arrhythmias. In adult CMs, prolongation of the action potential duration (APD) by 20% is observed at 220 nM [136]. Matured PSC-CMs have approximately 20–40% longer APD at 100–1000nM[28] which is comparable with the adult CMs responses. On the other hand, non-matured PSC-CMs are highly sensitive to dofetilide and have approximately 1000% APD increase at 1000 nM [137]. Oversensitivity of non-matures PSC-CMs is a critical issue in drug development, as it may potentially lead to inappropriate elimination of potentially useful compounds. Lidocaine, a sodium channel blocker, has a negative chronotropic effect on matured PSC-CMs with EC50 at 10–20μM range[28], which is at the same magnitude as EC50 of sodium channel activity in adult CMs [138]. Diltiazem and verapamil, calcium channel blockers, also have similar EC50s in matured CMs[28] and adult CMs [139, 140]. This evidence suggests that the engineered cardiac tissue with maturity approaching adult level can be used to capture some drug responses in adult cardiac muscle strips. However, there are also several exceptions. Both non-matured and matured CMs have different sensitivity of Ca2+ compared to adult CMs. It is also unexpected to see matured CMs becomes much less sensitive to nifedipine compared to adult CMs. To sum up, PSC-CMs with higher maturity have indeed improved the reliability of the drug response evaluation, nevertheless, more sophisticated strategies should be developed to further improve the cell maturity, which eventually closes the gap between matured engineered tissues and adult myocardium.
Table 3.
Drug response comparison among PSC-CMs, matured PSC-CMs and Adult CMs.
| Drug | Matured PSC-CMs in 3D Tissues | Human Adult CMs | PSC-CMs in 2D or 3D Tissues |
|---|---|---|---|
| Isoproterenol | Both chronotropic and inotropic EC50~100nM [32] Inotropic EC50 =10±1 nM while paced [33] (>100% force increase) |
EC50 = 11–80nM [141–144] EC50 = 20–120nM [139] (>100% force increase) |
Minimal inotropic effect Chronotropic effect at 1–10μM [30, 119] Chronotropic EC50=12.9nM[145] Inotropic EC50=750nM [34] |
| Dofetilide | EC50 between 10nM and 100nM (APD) 20–40% elongation of APD50 and APD90 at 100 and 1000 nM[28] |
20% increase in repolarization at 220nM[136] | Increased APD60 by 99 ± 77% at 30 nM [146] Increased APD90 by 1000% at 1000 nM [137] |
| Lidocaine | Reduced frequency by half at 20μM[28] | Na+ EC50=38 μM [138] | Cessation of beating at 50 μM - 1mM [147] |
| Verapamil | APD30 and APD50 EC50 ~ 0.1-lnM[28] | Ca2+ EC50=4.2–24.2μM[148, 149] Inotropic EC50= 0.14–0.79μM[139] |
Inotropic EC50=0.61μM [34] |
| Diltiazem HCL | Negative inotropic effect EC50 between 10–20 μM[28] | EC50= 0.18–0.69 μM [139] Inotropic EC50 is around 10μM [140] |
|
| Ca2+ | EC50~0.4–1.2 nM [32] EC50~0.5mN [33] |
EC50=2.47±0.1μM (pCa=5.61±0.02)[105] pCa=5.67±0.02[150] |
EC50 = 0.8–1.0mM [35, 151] or 1.8mM[34] or 0.4mM [152] |
| Nifedipine | EC50=4.5±1.4μM for force and 3.1±1.9μM for Ca2+ transients[28] | Ca2+ EC50=16nM [148] EC50 = 50–200nM (inotropic; ventricular muscle)[139, 153] |
Ca2+ EC50=39 nM [146] EC50 <100nM (inotropic)[151] |
estimated from graphs in the original reference
Atrial Tissue Engineering
CMs from atrial and ventricular myocardium have profound physiological differences, such as genetic profiles[154], calcium handling [155], electrophysiology [156–160], and structural and functional protein expressions [154] (Figure 1 B, C). Compared to ventricular cells, atrial cells are smaller in size and surface area. They have thinner and fewer traverse tubules with calcium handling machinery different from ventricular cells. In atria, calcium propagation is delayed and shorter than in ventricles, shows lower expression of Ryanodine receptor (RYR), higher expression of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) and sarcolipin (SERCA inhibitor). [155]
Atria and ventricles show a distinct pattern of gene expression. For example, atria express MLC2a, sarcolipin or connexin 40 whereas ventricles express MLC2v and phospholamban [154]. Atrial cells have differential channel activities and expressions which result in a triangle-like AP shape[156]. (Figure 1B) More specifically, potassium channel KCNA5 and its current Ikur are responsible for fast repolarization in atrial AP. Low activity of IK1, IKs, IKr, and INa in atrial cells, also contributes to atrial specific AP shape. IKACh, T type Ca2+ channel and Ca2+ activated K+ channels are also atrial specific channels [157–160]. Thus, several compounds are atrial-selective due to these atrial-specific ion channels. Carbachol (Figure 3A–B), an M2 muscarinic receptors agonist which acts on IKACh, reduces action potential duration[161]; serotonin, acting through the atrial-specific 5HT4 receptor, increases calcium current and induces an inotropic response in atrial cells[162]. These divergent drug responses in different heart chambers, increases the complexity of the drug screening process. The ideal therapeutic strategy for the ventricular disease should have minimal impact in atria, and vice versa. Off-target effects should be actively screened and identified for drugs like adenosine, which targets supraventricular tachycardias with off-target effect on IKACh potentially inducing atrial arrhythmia (off-target effect of known compounds on atria is reviewed elsewhere [163]).
Figure 3. Atrial tissue engineering and drug testing.
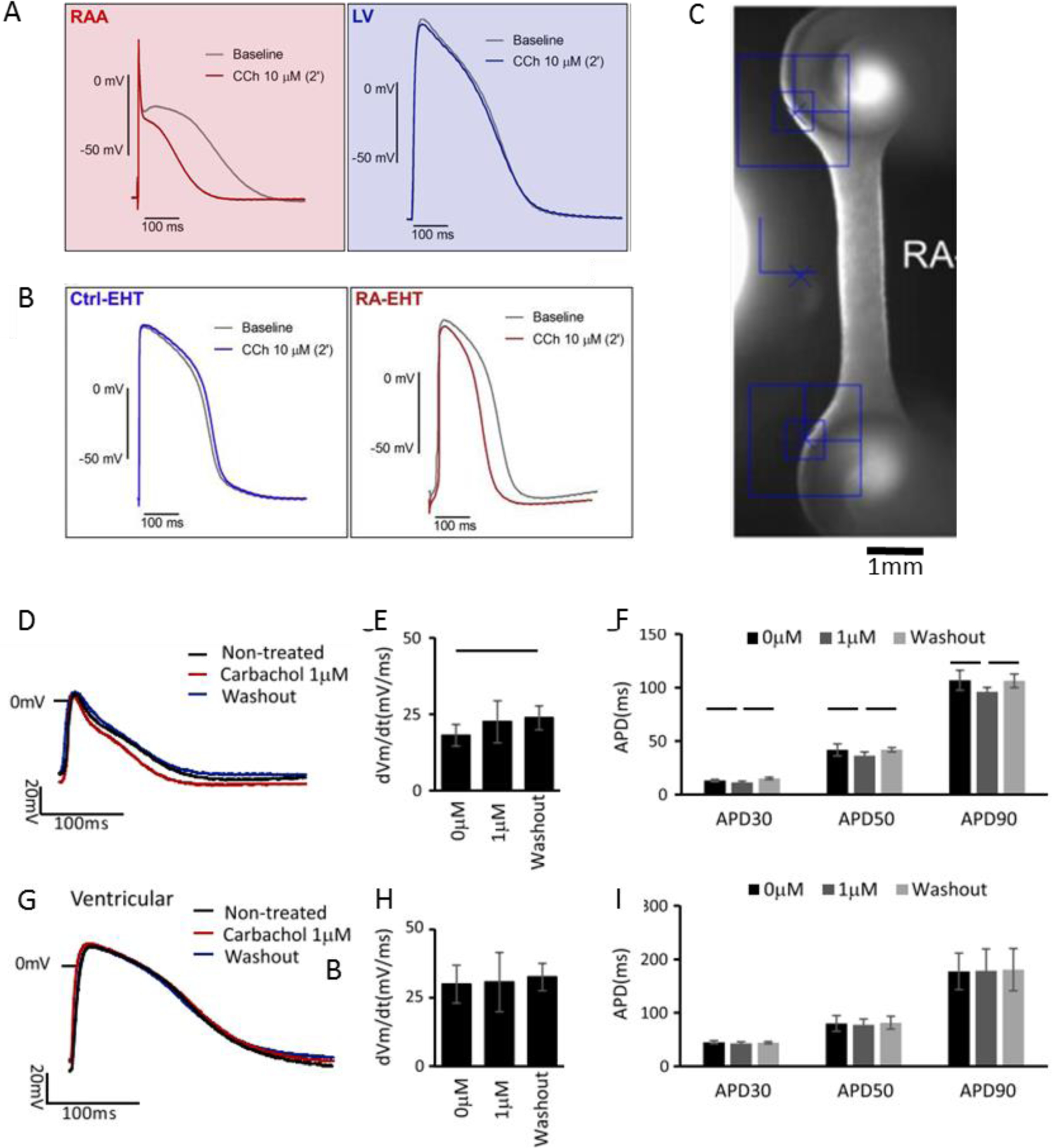
Carbachol-induced atrial-specific responses on action potential parameters in A) native human heart tissues; engineered human heart tissues on the B, C) post-based platform[2], and D-I) Biowire II tissues[28]. Figures are adapted with permission from their original references.
Therefore, chamber-specific engineered tissues are imperative in drug screening to minimize the cardiac off-target effect in human. While significant progress has been made in ventricular tissue engineering, generation of engineered atrial tissues is still at its infancy stage. This lag is partly due to the limited availability of atrial specific differentiation protocols [164]. Recently, the use of retinoic acid (RA) has been studied to direct atrial CMs differentiation from hPSC cells [160, 165–167]. Zhang et al added noggin and retinoic acid in cardiac differentiation protocol, and obtained impure atrial population. A purer population of atrial cells was generated by applying retinoic acid after induction of mesodermal stage[6, 166]. Lee et al reported an efficient atrial differentiation protocol for the generation of atrial linages through the use of developmental signaling gradients that specify atrial mesoderm precursors.[6] These advances provide a strong foundation for the creation of functional engineered atrial tissues.
Several groups have reported engineered atrial tissues. Zhao et al have successfully cultured and improved the maturity of atrial tissues[28] on Biowire II platform with two opposing elastic wires as a template. Cyganek and Lemme have created ring-shaped and rod-shaped atrial cardiac tissues using the two post platform [2, 116] (Figure 3C). These tissues showed distinct AP profiles with much shorter APD30 relative to ventricular tissue, resembling adult atrial APD30, measured both by potentiometric dyes and sharp microelectrode recording. (Table 4) When compared with atrial adult myocardium atrial phenotype of these tissues was confirmed by atrial specific carbachol response which reduces APD and hyperpolarizes membrane potential (Figure 3D–I). Tissues from Lemme et al201 and Zhao et al[28] showed unique responses to low doses of 4AP, IKur blocker by APD extension. These atrial tissues had less force compared to their ventricular counterparts and did not show positive FFR. Instead, they showed flat FFR, a characteristic of atrial tissue [2, 28, 116]. All reported atrial tissues had around 100 times less force than human adult atria. [168] Atrial tissue from Zhao’s study had approximately the same level of post rest potentiation compared to that of atrial adult myocardium. Atrial myocytes are known to have faster calcium handling. They show smaller post rest twitch and in a shorter time frame139,140. The AP parameters, including APD30 and APD30/90, are also slightly improved towards adult level when atrial PSC-CMs are incorporated in engineered tissues. Moreover, atrial PSC-CMs and atrial engineered tissues both have higher than 1Hz spontaneous beat which shows both immaturity and contamination with a small population of pacemaker cells. The engineered tissues are also showing AP profile of immature myocytes, with lower Upstroke velocity, a sign of lower adult INa activity, hyperpolarized membrane potential which indicates lower IK1 activity. The APD is also shorter than adult atrial CMs. To date, the ability to generate pacemaker free atrial population and development of a maturation protocol that would generate quiescent cells remain as challenges in atrial tissue generation.
Table 4.
Parameter comparison among atrial PSC CMs, atrial PSC-CM in 3D tissues and CMs in atrial adult tissue
| Parameters | Adult Atrial CMs or Myocardium | Atrial PSC-CMs in Engineered Tissues | Atrial PSC-CMs |
|---|---|---|---|
| Force (mN/mm2) | 8–10[168] | 0.12 [2] or 0.25 [116] or 1μN/ tissue at 1Hz[28] | NA |
| FFR (1–3 Hz) | Positive from 0.5–1.5 Hz and negative between 1.5Hz to 3Hz[169] | Flat [2, 28] | NA |
| PRP | Max at 10s after rest Increased over 50%[168] | 100% [2, 28] | NA |
| APD90 (ms) | 150–500 [157] | 90–120 [28] | 150 [166] or ~200 [6] |
| APD30 (ms) | ~10–20 [170] | ~13–19[28] | ~15[6] |
| APD50 (ms) | ~100 [170] | ~40–66[28] | ~50 [6] |
| APD30/90 | 0.1[171–173] | ~0.14[28] | <0.3[6] |
| Action potential Vmax | 150–300[157] | 30[28] or 97[2] (measured by dye) | 45[166] |
| MDP | −65 to −80[157] | −65 and −70 [2, 28] | −65[166] |
| Amplitude | Around 100 [174] | 85–90 [2, 28] | 80[166] |
estimated from graphs in the original reference
Tissue engineering of the conduction system
Development of pacemaker has great therapeutic importance for patient suffering from brachychardia, in this regard, several groups applied different approaches to purify and characterize cells from the cardiac conduction system; sinoatrial node, atrioventricular node or Purkinje fibers (Figure 1A). The primary attempts in the generation of nodal cells were the introduction of genes with an active role in conduction systems in stem cells differentiation, expression of mink-Lacz under chicken GATA6 enhancer in mouse ES, expression of HCN2 /4 in NKX2.5 myocytes and differentiation HCN4 expressing cells.[175, 176] Addition of certain chemicals, like B12 or SKCa activator 1-ethyl-2-benzimidazolinone to cardiac differentiation, increased the population of nodal cells.[177, 178]. A mixed population of pacemaker cells was generated by induction of C-MYC regulated NKX2–5 and TGF-beta inhibition[165] or by controlling the expression of BMP and Wnt at specific time points from the mesoderm stage. Portze et al developed the first SA nodal specific differentiation protocol by treating mesoderm differentiated PSCs by bFGF and low amount of activin A, BMP4 then inhibition bFGF, TGF-β, and Wnt signaling in the presence of BMP4 and RA.[13]
ESC reporter line derived from Contactin2:EGFP BAC gene was first used to separate small population of cells in cardiac differentiation that show Purkinje fiber like gene expression and AP profile, using small molecule screening strategies in ventricular differentiation. It was reported that the addition of sodium nitroprusside between day 6 to 11 increases Purkinje cells population from less than 1% to more than 33%.[179, 180]
The first engineered tissue of the conduction system was made using neonatal rat ventricular myocytes co-cultured with HCN1 transfected human mesenchymal cells. The tissue showed hyperpolarized membrane potential and higher spontaneous beating rate [181]. Another 3D structure was generated from cells with sinoatrial or atrioventricular AP and gene expression profile. These tissues were generated by selection of cells based on SHOX2 promoter and Cx30.2 enhancer and constructing spheroid shaped aggregates [182]. Co-culture of endothelin treated embryonic rat cardiac cells with endothelial progenitor cells in Matrigel generated high spontaneous beating vascularized tissue like pacemaker [183]. Further efforts need to be done on the direct differentiation protocol for Purkinje fibers and AV nodal cells to enable a complete setup of cardiac conductive tissue model.
Conductive biomaterials aiding functional maturation
Other than the biological niches used in differentiation protocols, specialized biomaterials, especially conductive biocompatible materials, can potentially be incorporated as scaffolding materials to promote the electrical function of the engineered conduction systems.
Cardiac muscle fibers throughout the entire heart rely on the adequate electrical signal propagation to generate chamber-specific synchronized contraction. In case of large injuries caused by ischemic myocardial infarction, conductive materials can be incorporated into the scaffolds of large implantable cardiac tissue patches to quickly re-establish the signal propagation through the infarct sites. The use of conductive materials can potentially accelerate the electromechanical integration of the grafts with the host tissues, compensating for the functional loss of cardiomyocytes in the infarct site and minimizing the possibility of arrhythmia.[184, 185] For in vitro applications, miniaturized engineered cardiac tissues can benefit from the incorporation of conductive materials at the early phase of the tissue formation and functional maturation. Shortly after cell seeding, cell-cell junctions and gap junction proteins are not well-developed to provide sufficient conduction for adequate intercellular communication. Some cardiomyocytes begin to spontaneously contract, but they are unable to propagate the electrical impulses to the adjacent cells for synchronized contraction. Thus, introducing conductive materials at the early stage can be beneficial to ameliorate the conductive properties of the cardiac tissues and help with the initiation of tissue level synchronized contraction. [186]
The biocompatible materials and conductive materials are commonly chemically or covalently incorporated together as composite materials for cardiac tissue engineering. Carbon nanotubes blended with collagen hydrogel[187], GelMA[188] or polyesters[189], and reduced graphene oxide dispersed in GelMA[190] or silk materials[191, 192], have been previously reported and shown to promote functional improvements in the engineered cardiac tissues. Conductive and electroactive polymers work in the similar fashion. Polypyrrole has been incorporated with polycaprolactone[193], chitosan[194] or silk[195] as scaffold materials to improve electrophysiology of cardiomyocytes, synchronization of tissue contraction and myocardial electrical impulse propagation across the tissue constructs. PEDOT:PSS[196] as well as polyaniline[197] were both tested in a similar fashion for in vitro cardiac tissue engineering.
Disease models
Disease models are essential to allow a better understanding of disease mechanisms and accelerate drug development. Conventional in vitro cardiac disease studies rely mostly on the use of heterologous cell culture models[9]. However, these cell sources are often accompanied by marked interspecies differences and confounding genetic information, thus cannot fully elucidate human pathology[198]. Human-derived disease models have great potential in precisely dissecting disease mechanisms and developing compounds for treating patient-specific disorders, and identifying individualized therapies[199].
Genetic diseases present as major causes of cardiac conditions, and have been the focus of personalized disease modeling[200]. The advent of iPSC technology presents exciting opportunities for generating personalized diseased cardiac tissues[9]. As human iPSC-derived cardiac cells inherit physiological and pathological traits of the differentiation origin[201], they often possess disease-specific genotypes that can significantly affect disease onset and progression[201–203]. Besides using patient-specific cells, cardiac myocytes carrying disorders can also be generated from healthy individuals through genetic editing on specific loci. The emergence of genome editing techniques such as the clustered regularly interspaced short palindromic repeat (CRISPR) system has provided another useful approach to create disease alleles into healthy cells[204].
Various genetic cardiac models have been generated using iPSC lines from patients with defined genetic disorders[28, 201]. Among these models, monogenic diseases have been intensively studied[205]. Monogenetic diseases normally present high penetrance and clear phenotypes[205]. Hereditary arrhythmia-related cardiomyopathies are a family of well-characterized monogenic conditions [199]. Gene mutations in ion channels associated with the repolarization process can cause long QT syndrome (LQT), which is characterized by prolonged QT intervals on the electrocardiograms and severe ventricular arrhythmias[206]. LQT syndrome type 1 (LQT1) is caused by a mutation in the KCNQ1 gene, leading to abnormalities in encoding the channels that regulate potassium current[206]. Moretti et al generated iPSCs-cardiomyocytes derived from patients with LQT1 with mutations in KCNQ1, which demonstrated prolonged APD, increased the occurrence of catecholamine-induced tachyarrhythmia, and negative trafficking defect associated with IKs reduction[207]. Many other patient-specific PSCs based disease models have been used to study different types of model LQT syndrome and demonstrated the applicability of patient-derived stems in modeling cardiac disease[206]. As in the case of other cardiac diseases, Lan at al reported the generation of patient-specific PSCs from HCM patients carrying a mutation in the MYH7 gene. Abnormal Ca2+ transients, arrhythmias and morphological remodeling were observed[208]. More diseases, such as Timothy syndrome[209] with mutation in the L-type calcium channel CaV1.2; LEOPARD syndromes[210] with a mutation in the PTPN11 gene; and arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) [211] with desmosome gene mutations, have been investigated to demonstrate the development of pathological phenotypes in terms of cellular morphology, intercellular structural protein organization, calcium transients, electrophysiology and contractile behaviours.
More importantly, iPSCs offer the possibility to recapitulate and investigate the pathogenesis of cardiac disease with 3D cardiac microtissues to model cardiomyopathy in vitro. Two recent studies investigated the diseased phenotypes human iPSC derived CMs in the engineered tissue environment. In recent work, human engineered cardiac tissues from a post-based platform were used for evaluating the pathogenicity of sarcomeric protein titin gene variants in four patients[212] (Figure 4A). The effect of titin truncations can be successfully demonstrated with affected sarcomerogenesis-related remodeling, impaired intrinsic contractility, reduced responses to stress and altered gene and protein expressions, which all contributes to the progression of dilated cardiomyopathies[212]. In another study, two patient-specific PSCs were differentiated into cardiomyocytes and were constructed using thin muscle film tissue model to recapitulate the pathophysiology underlying Barth syndrome (BTHS) cardiomyopathy [213] (Figure 4B). The abnormalities associated with mitochondrial protein taffazin mutation were captured at metabolic, structural and functional levels. The platform enabled the study to capture the changes of twitch forces (Figure 4B) due to the impairment of sarcomeres organization and metabolic activity resulted from mitochondrial dysfunction. [213]
Figure 4. Disease modeling with heart-on-a-chip.
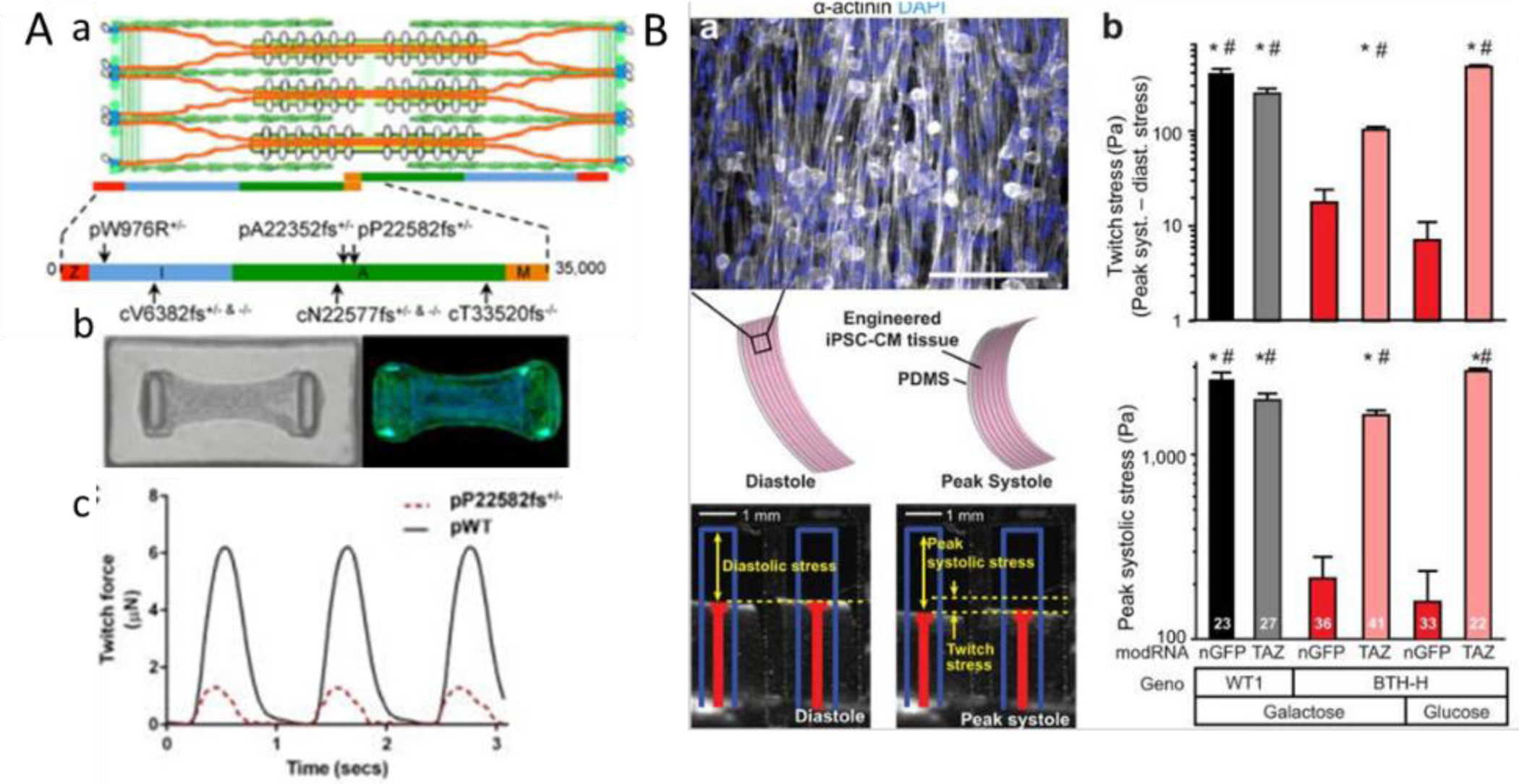
A) Engineered PSC-CM microtissues with titin mutations have impaired intrinsic contractility and responses to stress. [212] a) Schematic of the cardiac sarcomere with titin (orange), b) Images of bright field and Phalloidin labelled tissues suspended between two PDMS pillars, c) twitch forces comparison between wild type and mutant tissues. B) Depressed contractile stress generation [213] by a) diseased myocardial tissue constructs (mitochondrial protein taffazin mutation) demonstrated with muscle thin film (MTF) platform. Cardiomyocyte stress generation reduces the radius of curvature of the construct as it contracts from diastole to peak systole. b) Twitch stress and peak systolic stress generated by MTFs from patient-derived PSC-CMs and control PSC-CMs. Figures are reproduced with permission from their original references.
Polygenetic diseases, one the other hand, originate from a set of genes and involve various unknown loci[205]. Although polygenic diseases are the most common form of genetic disorders, they present complex disease phenotypes and are challenging to model using PSC cells[205]. Polygenic disorders often involve mutations in genes that have not been fully identified thus cannot be easily corrected using genomic editing methods. Some of these challenges can be overcome in order to model certain complex disorders. For example, cardiac hypertrophy generally occurs as a polygenic disease. It is poorly understood how different variants in sarcomeric proteins can lead to changes underlying this condition. To model this polygenic disease, it is critically required to provide a chronically increased workload to the cardiac tissue over a prolonged time period. [28] Tissue maturation methods are proven to facilitate the modeling of polygenic cardiac hypertrophy[214]. Zhao et al found electrical conditioning for up to 8 months enabled modeling of polygenic left ventricular hypertrophy starting from patient cells[214].
The cardiac disease can be potentiated through environmental factors. Polygenic conditions are particularly prone to be affected by external cues. The onset of polygenetic disease often remains unclear, owing to environmental modifications on phenotype development. The interplay between genetic and environmental factors is crucial to disease progression. For example, hypertrophy-promoting factors, such as energy depletion, the increased workload associated with prolonged hypertension can both accelerate cardiac hypertrophy progression and eventually cause left ventricular dysfunction and heart failure[99]. It is important to generate 3D tissue models that enable prolonged biophysical stimulation through tissue cultivation. The environmental factors can be mimicked through modulating electromechanical stimulation, metabolism, matrix stiffness, and oxidative conditions[198]. The applications of PSCs in differentiating cardiac cells combined with external pathological cues have important implications in constructing functional and diseased cardiac tissues[201].
Currently, most of the disease models are generated using ventricular cardiomyocytes derived from patient cells[6]. Given their major role in supplying blood to the human body, ventricular functional inefficiency is a significant cause of heart failure. Great strides have been made in modeling of ventricular arrhythmia[199], left ventricular hypertrophy[9], dilated cardiomyopathy[203] and channelopathy[215]. However, atrial disease modeling has not yet been extensively studied. Atrial originating cardiac diseases such as AF present as rising healthcare challenges. AF, which is a cardiac electrical conduction system disorder which leads to irregular and fast heartbeats, is emerging as an epidemic heart disease of aging population[216]. AF generally increases mortality and hospitalization rate as it is associated with a higher risk of stroke, heart failure, sudden cardiac death, and premature dementia[217, 218]. It is important to develop platforms that enable the creation of chamber-specific tissues for disease modeling. Several atrial disease models have been studied, enabled by the development of atrial specific protocols. Laksman et al generated a circular sheet of confluent Hes3 derived cells71. Arrhythmia was induced in the cell sheet by fast rate pacing, and the behaviour of the formed rotor was studied with two known antiarrhythmic drugs flecainide and dofetilide[219]. In another study, Olsen et al created a KCNA5 mutation model of atrial iPSC derived myocytes on Matrigel coated coverslip[220]. Although these cells had extended APD at baseline, they did not have arrhythmic effects. The addition of cholinergic agonist charbachol was found to shrink APD and eventually induce arrythmia in this model[221]
Cardiac Tissue Engineering for Drug Discovery
A major focus in drug discovery has to be set on avoiding a drug-induced fatal ventricular arrhythmia - torsades de pointes (TdP)[222–224]. Binding to ether-a-go-go-related (hERG) potassium channel has been used to predict the risk of drug inducing TdP[224] through the use of patch-clamp systems or binding assays[225]. However, drug cardiotoxicity can be multifaceted and the screening methods cannot detect cardiotoxicity related to multiple ion channels and other mechanisms. Chamber-specific toxicity should also be a factor to consider in screening potential drug candidates.[28] More comprehensive approaches are being developed to detect drug-induced, chamber-specific, structural and contractile cardiotoxicity[28] to avoid unexpected failure at later stages of drug discovery.
The evaluation of electrophysiological influence of new drug candidates is critical in understanding drug safety and mechanisms for clinical translation. For instance, electrophysiological responses of iPSC-CMs to 28 blinded compounds were used to predict the risk of lethal ventricular tachyarrhythmia by using microelectrode array, computational model[226] and intracellular recording[227]. On the other hand, contractility is another pivotal functional index in drug discovery, since changes in cardiac contractility are representative of the changes in ejection fraction in clinical setting. In patients, changes in contractility can indicate altered ventricular wall movements and post treatment cardiac hemodynamics.[228] Unwanted contractility related toxicity may also result in systemic hemodynamic effects such as drug-induced orthostatic hypotension[229]. Heart-on-a-chip models can serve as an ideal in vitro platform and provide these functional parameters for drug assessment.
To employ heart-on-a-chip in the development of drug discovery, a key focus should be set on developing simplistic, high-throughput, automated cell culture and testing systems to screen large libraries of drug leads. Agarwal et al developed PDMS-based micron-size cantilevers through semi-automated laser-based fabrication technique to engineer 2D anisotropic cardiac tissues, allowing measurement of diastolic and systolic stresses of the tissues[230]. The positive inotropic effect of isoproterenol on cardiac contractility was demonstrated with the platform. The platforms can quantify contractility and tissue structure, and have a throughput of 35 tissues on one chip device. A muscle-on-a-chip (MTF) model developed as a thin muscle film to monitor muscle contractility was fitted in a 24-well plate and can be easily scalable to 96-well plate format[231].
In terms of 3D tissue, the throughput of culture and testing are slightly lower[33] due to the complexity of the platform. However, compared to monolayer cultures and MTF, the 3D engineered tissues have demonstrated advanced maturation in genetic, functional and structural aspects, which can improve the physiological relevance of the results. With non-invasive long-term monitoring, a chronic cardiotoxicity from catecholamine was demonstrated with this platform recapitulating hallmarks of heart failure (e.g., contractile dysfunction, cardiomyocyte death, and release of N-terminal pro B-type natriuretic peptide)[33]. Biowire II platform has been scaled up from 8 microtissues per 10 cm culture dish, to the format of 96-well plate, which facilitates tissue culture and drug testing in a much higher throughput[58]. Schneider et al [232] demonstrated a centrifugally-assisted cell loading approach to robustly construct and culture 8 individual cardiac microtissues at single seeding. Tissues cultured in the centrifugal chip can be maintained for 1 month, with calculation of temporal and spatial beating kinetics observed by an optical method. This study innovated an automated cell loading system, which standardizes the tissue culture procedures, eliminates the inconsistency of the conventional manual cell seeding method, and enables the generation of multiple reproducible cardiac tissues. Nevertheless, more non-invasive functional assessments need to be incorporated into the system. [232]
There are still many remaining challenges to the use of heart-on-a-chip technology for drug discovery. First is to standardize the tissue culture and testing protocol for reliable tissue manufacture with reproducible, high throughput, and high content functional readouts. Culture and testing platform should be amenable to chamber-specific cardiac tissues for targeted drug responses. At the same time, the connections between functional readouts from in vitro platform and clinical assessments should be established. With the proper translation, drug safety and toxicity can be obtained and understood prior to the clinical trials and therefore significantly improve the success rate.
Microscale fabrication technologies and their applications in heart-on-a-chip
Significant advances have been made in current microscale technologies, such as additive manufacturing and micropatterning methods. These powerful fabrication methods can build complex microstructures with various designed chemical and mechanical properties to create customized microphysiological environment for heart-on-a-chip, resulting in the significant advances for cardiac tissue models.
Lind et al developed a cardiac microphysiological device by multimaterial 3D printing with six customized inks.[233] By an innovative combination of these ink materials, a high-conductance carbon black/thermoplastic polyurethane composite was used to fabricate the device. The device has a biocompatible culture surface for cardiomyocytes, and a highly conductive inner material with piezo-resistive shield to sense the contractile function of the seeded cardiac tissues. 3D printing technology can also incorporate cells directly. Lee et al demonstrated 3D bioprinting of cardiac ventricles using cardiomyocytes and collagen hydrogel. The resulting constructs had synchronized contractions and directional action potential propagation.[234] To further increase the cell seeding density to a physiologically relevant level, organoids in hydrogels were printed instead of cellular suspensions using sacrificial writing. [235] The resulting tissue constructs had high cell density of 180 million/mL and customized perfusable vascular channels.
Another commonly used technology is soft-lithography. The method is commonly used to produce micropatterned molds with ultra-high resolutions. For example, Ribeiro et al demonstrated Matrigel micropatterns generated by microcontact printing from PDMS molds. Customized shape generated from Matrigel micropatterns and appropriate stiffness of polyacrylamide substrate induced myofibril alignment and affected the contractility of single iPSC-derived cardiomyocytes by increasing translation of sarcomere shortening.[236] The platform also allows the in situ force measurement through analyzing videos of live-cell imaging.[237] Huebsch et al presented a cardiac microphyciological system with 2500 cardiomyocytes per construct on micropatterned PDMS template. This miniaturized cardiac system promotes cardiomyocyte alignment and uniaxial tissue contraction.[238] “I-wire” platform provided two conductive rigid wires as anchor points. The force probe was placed at the center of the cardiac tissue to measure the force of contraction.[239–241]
Challenges and Future Steps
Technological challenges
To date, ventricular tissue models are the most established and most intensively investigated in vitro models. Because of their similarities in tissue organization and functions, atrial tissue models can be improved by adaptation and modification of the existing ventricular tissue models, including the tissue formation on platforms and maturation strategies. However, proper adjustments are needed based on the atriums physiological characteristics. For example, due to the shorter refractiveness of atrial cells, maturation of atrial tissues should be electrically conditioned at a much faster ramp up protocol.[28] Similarly, cells from SA node have automaticity and will require different maturation strategies and evaluation criteria. Thus, developing standard strategies to mature cardiac cells is therefore utmost important, especially when understanding disease models. This is because most of the cardiac diseases associated with aging and environmental factors are often manifest to adult patients. Nevertheless, native adult CMs from different cardiac compartments should always be used as the benchmarks for tissue maturity.
Taking a step further, once these matured engineered tissues from different cardiac compartments are readily available, building integrated tissue with cells from different chamber origins can improve drug screening efficiency and set foundations for an integrated heart-on-a-chip platform. The recently published paper in Cell is the first to report a heteropolar tissue model made with functional atrial and ventricular zones using Biowire II platform.[28] This model is suitable for duo assessments of drug responses on AP and calcium transients (potentiometric or calcium dye) in single preparation. Serotonin and ranolazine have been used to demonstrate their atrial-selective drug responses on the heteropolar tissue. By monitoring the calcium transients, this heteropolar tissue model is the first to demonstrate the inotropic responses of these drugs. However, the atrial and ventricular chambers in the heart are connected with atrioventricular conduction system (Purkinje fiber). Therefore, a better heteropolar platform that incorporates the conductive system to electrically connect two chamber-specific tissues is needed and will provide a more physiological-relevant drug response.
Furthermore, conventional drug testing protocol introduces pharmaceutical compounds by directly bathing the tissues in different doses of the drugs in culture media. In reality, these drugs are delivered to the tissue through the vascular system. Interactions between drugs and endothelial cells can provide new insights on cardiovascular adverse events. Therefore, a sophisticated functional vasculature network would be beneficial in emulating a more physiological-relevant drug delivery. However, cardiac tissue with vasculature is a more complicated system to recapitulate. Simple co-culture of CMs with endothelial cells will only result in premature lumen structures embedded within the tissue.[101] These micro-vasculatures are not sufficient to support perfusion.[101] On the other hand, designing a defined vasculature within the parenchymal space requires biomaterials with adequate mechanical stability.[53, 55] The rigidity of the materials reduces the flexibility of the cellular organization and the tissue shortening during contraction would be obstructed. Moreover, the setup is difficult to fabricate and standardize. The functional readouts for drug testing with these vascularized cardiac tissues is more difficult to interpret and less likely to enable high throughput assessment. Therefore, more effort should be focused on incorporating stable, native-like vasculatures within cardiac tissue while facilitating its highly compacted, aligned, fully contractible cellular organization.
To sum up, an ideal heart on a chip system should be envisioned as a multi-compartment tissue with pacemaker cells, Purkinje conduction system, and several chamber-specific myocardium integrated with a built-in vasculature for proper drug delivery. By utilizing standardized maturation protocol, tissues from each compartment would achieve adult-like phenotypes and functions to provide drug testing results relevant to human native physiology. During drug testing, tested compound perfused through the vasculature network will emulate the actual drug entry route and interactions between the drug and endothelial cells. At the same time, chamber-specific tissues will be assessed and analyzed for chamber-specific drug responses. In addition, uniquely designed conditioning protocol, such as tuning the cardiac workload, hormone level, blood sugar level, would be customized after the maturation process to trigger disease phenotypes in these tissues by emulating environmental factors of polygenic diseases.
To realize this ultimate goal, a multi-disciplinary collaboration between cell engineers and platform engineers is therefore needed. Cell engineers are tasked with developing more sophisticated strategies to obtain matured, purified cardiac cell population, whereas, platform engineers are assigned to create a versatile platform that can provide physiological relevant microenvironment while maintaining user-friendly interface with an aim for high throughput culturing and testing efficiency.
Commercialization challenges
Heart-on-a-chip technology poses superior advantages for the applications in drug screening and personalized medicine. Many pioneers in the field have recognized the growing opportunity and have initiated the commercialization efforts of the heart-on-a-chip products in the past 5–10 years, through companies such as TARA Biosystems, myriamed, EHT Technologies, NovoHeart etc. Novoheart, EHT technologies and myriamed are using post-based platforms, whereas TARA biosystems exploits the platform using elastic wires for cultivation of strips of human myocardium. These companies can provide both products as well as testing services for academic collaborators and industrial partners. During the early technological development, the start-up companies commonly seek interest and collaborations from pharmaceutical industry and regulatory departments to tailor the technology development for better usability and clinical translation. At this stage, tissue-based drug screening platforms can effectively be used to answer specific questions on the mechanism of action or drug toxicity. However, they cannot be used alone in the process of bringing a drug to the market due to the current regulatory framework and the need to further validate these systems.
One persisting question from the pharmaceutical industry is focused on proving that the organ-on-a-chip platforms are better than the current approaches that rely on cell lines in 2D culture and animal models. Moreover, as commercial products, reproducibility among batches of hearts-on-a-chip is essential. In some cases, this may be difficult to achieve due to the large biological variability of various iPSC lines and iPSC derived cell products. Although cell suppliers, such as Cellular dynamics and Ncardia, have done an impressive quality control, biological variability still contributes significantly to the variability of the tissue-based platforms despite the incorporation of GMP. Patient-specific tissue models have become the primary focus of several companies, however, many disease phenotypes only manifest after the adult-like maturation or aging, which may be difficult to achieve over a short time in culture (e.g. several weeks) in these tissue-based platform. These are the challenges for the field going forward.
Conclusions
In summary, this review highlighted and discussed the current progress in cardiac tissue engineering of different compartments of the heart, ventricular, atrial and SA nodal and their uses in drug testing applications. As the most established cardiac tissue model, ventricular tissues have shown similar maturity and drug responses that are comparable to bench mark of native adult CMs. Recent progress in stem cell differentiation has demonstrated atrial CMs can be generated in high purity and atrial tissues generated from these cells displayed atrial-like features and response to atrial specific drugs. However, better maturation strategies and removal of pacemaker cells side populations are still critical to produce high fidelity atrial tissue. More work remains before the generation of physiological-relevant Purkinje fiber-like tissues and SA nodal tissue. Finally, proof-of-concept study demonstrated the possibility to model polygenic diseases in vitro, which further substantiate the necessity of matured CMs for disease modeling and personalized medicine.
Acknowledgements:
Our work is funded by the Canadian Institutes of Health Research (CIHR) Operating Grant No. MOP-137107, the University of Toronto McLean Award, the CIHR Operating Grant (MOP-126027), the Heart and Stroke Foundation Grant No. G-16-00012711, National Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant (RGPIN-2015-05952) and National Institute of Health Grant (2R01HL076485-12).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest:
Conflict of interest disclosure: Y.Z. and M.R. are co-founders of TARA Biosystems Inc, and hold equity in this company. All other authors have no conflicts of interest.
References:
- [1].Li GR, Dong MQ, Pharmacology of cardiac potassium channels, Advances in pharmacology, 59 (2010) 93–134. [DOI] [PubMed] [Google Scholar]
- [2].Lemme M, Ulmer BM, Lemoine MD, Zech ATL, Flenner F, Ravens U, Reichenspurner H, Rol-Garcia M, Smith G, Hansen A, Christ T, Eschenhagen T, Atrial-like Engineered Heart Tissue: An In Vitro Model of the Human Atrium, Stem cell reports, 11 (2018) 1378–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Levine HJ, Rest heart rate and life expectancy, Journal of the American College of Cardiology, 30 (1997) 1104–1106. [DOI] [PubMed] [Google Scholar]
- [4].Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J, Evidence for cardiomyocyte renewal in humans, Science, 324 (2009) 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kattman SJ, Witty AD, Gagliardi M, Dubois NC, Niapour M, Hotta A, Ellis J, Keller G, Stage-specific optimization of activin/nodal and BMP signaling promotes cardiac differentiation of mouse and human pluripotent stem cell lines, Cell stem cell, 8 (2011) 228–240. [DOI] [PubMed] [Google Scholar]
- [6].Lee JH, Protze SI, Laksman Z, Backx PH, Keller GM, Human Pluripotent Stem Cell-Derived Atrial and Ventricular Cardiomyocytes Develop from Distinct Mesoderm Populations, Cell stem cell, 21 (2017) 179–194 e174. [DOI] [PubMed] [Google Scholar]
- [7].Lian X, Zhang J, Azarin SM, Zhu K, Hazeltine LB, Bao X, Hsiao C, Kamp TJ, Palecek SP, Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/beta-catenin signaling under fully defined conditions, Nature protocols, 8 (2013) 162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Dunn KK, Palecek SP, Engineering Scalable Manufacturing of High-Quality Stem Cell-Derived Cardiomyocytes for Cardiac Tissue Repair, Frontiers in medicine, 5 (2018) 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Fischer B, Meier A, Dehne A, Salhotra A, Tran TA, Neumann S, Schmidt K, Meiser I, Neubauer JC, Zimmermann H, Gentile L, A complete workflow for the differentiation and the dissociation of hiPSC-derived cardiospheres, Stem Cell Res, 32 (2018) 65–72. [DOI] [PubMed] [Google Scholar]
- [10].Mosterd A, Hoes AW, Clinical epidemiology of heart failure, Heart, 93 (2007) 1137–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bhattacharya S, Burridge PW, Kropp EM, Chuppa SL, Kwok WM, Wu JC, Boheler KR, Gundry RL, High efficiency differentiation of human pluripotent stem cells to cardiomyocytes and characterization by flow cytometry, Journal of visualized experiments : JoVE, (2014) 52010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Eisen A, Ruff CT, Braunwald E, Nordio F, Corbalan R, Dalby A, Dorobantu M, Mercuri M, Lanz H, Rutman H, Wiviott SD, Antman EM, Giugliano RP, Sudden Cardiac Death in Patients With Atrial Fibrillation: Insights From the ENGAGE AF-TIMI 48 Trial, Journal of the American Heart Association, 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Protze SI, Liu J, Nussinovitch U, Ohana L, Backx PH, Gepstein L, Keller GM, Sinoatrial node cardiomyocytes derived from human pluripotent cells function as a biological pacemaker, Nature biotechnology, 35 (2017) 56–68. [DOI] [PubMed] [Google Scholar]
- [14].Ronaldson-Bouchard K, Vunjak-Novakovic G, Organs-on-a-Chip: A Fast Track for Engineered Human Tissues in Drug Development, Cell stem cell, 22 (2018) 310–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Takebe T, Zhang B, Radisic M, Synergistic Engineering: Organoids Meet Organs-on-a-Chip, Cell stem cell, 21 (2017) 297–300. [DOI] [PubMed] [Google Scholar]
- [16].Moscona A, Moscona H, The dissociation and aggregation of cells from organ rudiments of the early chick embryo, Journal of anatomy, 86 (1952) 287–301. [PMC free article] [PubMed] [Google Scholar]
- [17].Akins RE, Boyce RA, Madonna ML, Schroedl NA, Gonda SR, McLaughlin TA, Hartzell CR, Cardiac organogenesis in vitro: reestablishment of three-dimensional tissue architecture by dissociated neonatal rat ventricular cells, Tissue engineering, 5 (1999) 103–118. [DOI] [PubMed] [Google Scholar]
- [18].Terracio L, Miller B, Borg TK, Effects of cyclic mechanical stimulation of the cellular components of the heart: in vitro, In vitro cellular & developmental biology : journal of the Tissue Culture Association, 24 (1988) 53–58. [DOI] [PubMed] [Google Scholar]
- [19].Carrier RL, Papadaki M, Rupnick M, Schoen FJ, Bursac N, Langer R, Freed LE, Vunjak-Novakovic G, Cardiac tissue engineering: cell seeding, cultivation parameters, and tissue construct characterization, Biotechnol Bioeng, 64 (1999) 580–589. [DOI] [PubMed] [Google Scholar]
- [20].Bursac N, Papadaki M, Cohen RJ, Schoen FJ, Eisenberg SR, Carrier R, Vunjak-Novakovic G, Freed LE, Cardiac muscle tissue engineering: toward an in vitro model for electrophysiological studies, The American journal of physiology, 277 (1999) H433–444. [DOI] [PubMed] [Google Scholar]
- [21].Li RK, Jia ZQ, Weisel RD, Mickle DA, Choi A, Yau TM, Survival and function of bioengineered cardiac grafts, Circulation, 100 (1999) II63–69. [DOI] [PubMed] [Google Scholar]
- [22].Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, Granot Y, Cohen S, Bioengineered cardiac grafts: A new approach to repair the infarcted myocardium?, Circulation, 102 (2000) III56–61. [DOI] [PubMed] [Google Scholar]
- [23].Khademhosseini A, Eng G, Yeh J, Kucharczyk PA, Langer R, Vunjak-Novakovic G, Radisic M, Microfluidic patterning for fabrication of contractile cardiac organoids, Biomedical microdevices, 9 (2007) 149–157. [DOI] [PubMed] [Google Scholar]
- [24].Karp JM, Yeo Y, Geng W, Cannizarro C, Yan K, Kohane DS, Vunjak-Novakovic G, Langer RS, Radisic M, A photolithographic method to create cellular micropatterns, Biomaterials, 27 (2006) 4755–4764. [DOI] [PubMed] [Google Scholar]
- [25].Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schafer H, Bishopric N, Wakatsuki T, Elson EL, Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 11 (1997) 683–694. [DOI] [PubMed] [Google Scholar]
- [26].Zimmermann WH, Fink C, Kralisch D, Remmers U, Weil J, Eschenhagen T, Three-dimensional engineered heart tissue from neonatal rat cardiac myocytes, Biotechnology and bioengineering, 68 (2000) 106–114. [PubMed] [Google Scholar]
- [27].Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T, Tissue engineering of a differentiated cardiac muscle construct, Circulation research, 90 (2002) 223–230. [DOI] [PubMed] [Google Scholar]
- [28].Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K, Pahnke A, Protze S, Lee JH, Davenport Huyer L, Jekic D, Wickeler A, Naguib HE, Keller GM, Vunjak-Novakovic G, Broeckel U, Backx PH, Radisic M, A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling, Cell, 176 (2019) 913–927 e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Yang X, Pabon L, Murry CE, Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes, Circulation research, 114 (2014) 511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Boudou T, Legant WR, Mu A, Borochin MA, Thavandiran N, Radisic M, Zandstra PW, Epstein JA, Margulies KB, Chen CS, A microfabricated platform to measure and manipulate the mechanics of engineered cardiac microtissues, Tissue engineering. Part A, 18 (2012) 910–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Jackman CP, Carlson AL, Bursac N, Dynamic culture yields engineered myocardium with near-adult functional output, Biomaterials, 111 (2016) 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ronaldson-Bouchard K, Ma SP, Yeager K, Chen T, Song L, Sirabella D, Morikawa K, Teles D, Yazawa M, Vunjak-Novakovic G, Advanced maturation of human cardiac tissue grown from pluripotent stem cells, Nature, 556 (2018) 239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tiburcy M, Hudson JE, Balfanz P, Schlick S, Meyer T, Chang Liao ML, Levent E, Raad F, Zeidler S, Wingender E, Riegler J, Wang M, Gold JD, Kehat I, Wettwer E, Ravens U, Dierickx P, van Laake LW, Goumans MJ, Khadjeh S, Toischer K, Hasenfuss G, Couture LA, Unger A, Linke WA, Araki T, Neel B, Keller G, Gepstein L, Wu JC, Zimmermann WH, Defined Engineered Human Myocardium With Advanced Maturation for Applications in Heart Failure Modeling and Repair, Circulation, 135 (2017) 1832–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Turnbull IC, Karakikes I, Serrao GW, Backeris P, Lee JJ, Xie C, Senyei G, Gordon RE, Li RA, Akar FG, Hajjar RJ, Hulot JS, Costa KD, Advancing functional engineered cardiac tissues toward a preclinical model of human myocardium, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 28 (2014) 644–654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Schaaf S, Shibamiya A, Mewe M, Eder A, Stohr A, Hirt MN, Rau T, Zimmermann WH, Conradi L, Eschenhagen T, Hansen A, Human engineered heart tissue as a versatile tool in basic research and preclinical toxicology, PloS one, 6 (2011) e26397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S, Tissue engineering of vascularized cardiac muscle from human embryonic stem cells, Circulation research, 100 (2007) 263–272. [DOI] [PubMed] [Google Scholar]
- [37].Takahashi K, Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors, Cell, 126 (2006) 663–676. [DOI] [PubMed] [Google Scholar]
- [38].Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP, Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling, Proceedings of the National Academy of Sciences of the United States of America, 109 (2012) E1848–1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Masse S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G, Radisic M, Biowire: a platform for maturation of human pluripotent stem cell-derived cardiomyocytes, Nature methods, 10 (2013) 781–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE, Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts, Nature biotechnology, 25 (2007) 1015–1024. [DOI] [PubMed] [Google Scholar]
- [41].Fonoudi H, Ansari H, Abbasalizadeh S, Blue GM, Aghdami N, Winlaw DS, Harvey RP, Bosman A, Baharvand H, Large-Scale Production of Cardiomyocytes from Human Pluripotent Stem Cells Using a Highly Reproducible Small Molecule-Based Differentiation Protocol, Journal of visualized experiments : JoVE, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhao Y, Korolj A, Feric N, Radisic M, Human pluripotent stem cell-derived cardiomyocyte based models for cardiotoxicity and drug discovery, Expert opinion on drug safety, 15 (2016) 1455–1458. [DOI] [PubMed] [Google Scholar]
- [43].Antoni D, Burckel H, Josset E, Noel G, Three-dimensional cell culture: a breakthrough in vivo, International journal of molecular sciences, 16 (2015) 5517–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Parker KK, Ingber DE, Extracellular matrix, mechanotransduction and structural hierarchies in heart tissue engineering, Philosophical transactions of the Royal Society of London. Series B, Biological sciences, 362 (2007) 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, Knoblich JA, Publisher Correction: Guided self-organization and cortical plate formation in human brain organoids, Nature biotechnology, 36 (2018) 1016. [DOI] [PubMed] [Google Scholar]
- [46].Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, Knoblich JA, Guided self-organization and cortical plate formation in human brain organoids, Nature biotechnology, 35 (2017) 659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Takebe T, Zhang RR, Koike H, Kimura M, Yoshizawa E, Enomura M, Koike N, Sekine K, Taniguchi H, Generation of a vascularized and functional human liver from an iPSC-derived organ bud transplant, Nature protocols, 9 (2014) 396–409. [DOI] [PubMed] [Google Scholar]
- [48].Takebe T, Sekine K, Enomura M, Koike H, Kimura M, Ogaeri T, Zhang RR, Ueno Y, Zheng YW, Koike N, Aoyama S, Adachi Y, Taniguchi H, Vascularized and functional human liver from an iPSC-derived organ bud transplant, Nature, 499 (2013) 481–484. [DOI] [PubMed] [Google Scholar]
- [49].Mills RJ, Titmarsh DM, Koenig X, Parker BL, Ryall JG, Quaife-Ryan GA, Voges HK, Hodson MP, Ferguson C, Drowley L, Plowright AT, Needham EJ, Wang QD, Gregorevic P, Xin M, Thomas WG, Parton RG, Nielsen LK, Launikonis BS, James DE, Elliott DA, Porrello ER, Hudson JE, Functional screening in human cardiac organoids reveals a metabolic mechanism for cardiomyocyte cell cycle arrest, Proceedings of the National Academy of Sciences of the United States of America, 114 (2017) E8372–E8381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Cyprotex, Spontaneously beating cardiac microtissues: 3D structural cardiovascular toxicity assay, in, 2016.
- [51].Snodgrass R, Xian H-Q, Bruening-Wright A, Obejero-Paz C, Pym L, Tung K, Keller G, VALIDATION OF HUMAN STEM CELL-DERIVED “MICRO-HEART” CARDIOTOXICITY ASSAY, in, VistaGen Therapeutics, Inc, ChanTest Corp., Capsant Neurotechnologies, McEwen Centre for Regenerative Medicine; 2011. [Google Scholar]
- [52].Ahadian S, Civitarese R, Bannerman D, Mohammadi MH, Lu R, Wang E, Davenport-Huyer L, Lai B, Zhang B, Zhao Y, Mandla S, Korolj A, Radisic M, Organ-On-A-Chip Platforms: A Convergence of Advanced Materials, Cells, and Microscale Technologies, Advanced healthcare materials, 7 (2018). [DOI] [PubMed] [Google Scholar]
- [53].Lai BFL, Davenport Huyer D, Lu RXZ, Drecun S, Radisic M, Zhang B, InVADE: Integrated Vasculature for Assessing Dynamic Events, Advanced functional materials, 27 (2017). [Google Scholar]
- [54].Xiao Y, Zhang B, Liu H, Miklas JW, Gagliardi M, Pahnke A, Thavandiran N, Sun Y, Simmons C, Keller G, Radisic M, Microfabricated perfusable cardiac biowire: a platform that mimics native cardiac bundle, Lab on a chip, 14 (2014) 869–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zhang B, Montgomery M, Chamberlain MD, Ogawa S, Korolj A, Pahnke A, Wells LA, Masse S, Kim J, Reis L, Momen A, Nunes SS, Wheeler AR, Nanthakumar K, Keller G, Sefton MV, Radisic M, Biodegradable scaffold with built-in vasculature for organ-on-a-chip engineering and direct surgical anastomosis, Nature materials, 15 (2016) 669–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Feinberg AW, Feigel A, Shevkoplyas SS, Sheehy S, Whitesides GM, Parker KK, Muscular thin films for building actuators and powering devices, Science, 317 (2007) 1366–1370. [DOI] [PubMed] [Google Scholar]
- [57].Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park SJ, Kotikian A, Nesmith AP, Campbell PH, Vlassak JJ, Lewis JA, Parker KK, Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing, Nature materials, 16 (2017) 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhao Y, Wang EY, Davenport LH, Liao Y, Yeager K, Vunjak-Novakovic G, Radisic M, Zhang B, A Multimaterial Microphysiological Platform Enabled by Rapid Casting of Elastic Microwires, Advanced healthcare materials, (2019) e1801187. [DOI] [PubMed] [Google Scholar]
- [59].Huebsch N, Loskill P, Deveshwar N, Spencer CI, Judge LM, Mandegar MA, Fox CB, Mohamed TM, Ma Z, Mathur A, Sheehan AM, Truong A, Saxton M, Yoo J, Srivastava D, Desai TA, So PL, Healy KE, Conklin BR, Miniaturized iPS-Cell-Derived Cardiac Muscles for Physiologically Relevant Drug Response Analyses, Scientific reports, 6 (2016) 24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Thavandiran N, Dubois N, Mikryukov A, Masse S, Beca B, Simmons CA, Deshpande VS, McGarry JP, Chen CS, Nanthakumar K, Keller GM, Radisic M, Zandstra PW, Design and formulation of functional pluripotent stem cell-derived cardiac microtissues, Proceedings of the National Academy of Sciences of the United States of America, 110 (2013) E4698–4707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Salameh A, Dhein S, Effects of mechanical forces and stretch on intercellular gap junction coupling, Biochimica et biophysica acta, 1828 (2013) 147–156. [DOI] [PubMed] [Google Scholar]
- [62].Zhang B, Korolj A, Lai BFL, Radisic M, Advances in organ-on-a-chip engineering, Nature Reviews Materials, 3 (2018) 21. [Google Scholar]
- [63].Mathur A, Loskill P, Shao K, Huebsch N, Hong S, Marcus SG, Marks N, Mandegar M, Conklin BR, Lee LP, Healy KE, Human iPSC-based cardiac microphysiological system for drug screening applications, Scientific reports, 5 (2015) 8883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Sun X, Nunes SS, Biowire platform for maturation of human pluripotent stem cell-derived cardiomyocytes, Methods, 101 (2016) 21–26. [DOI] [PubMed] [Google Scholar]
- [65].Feric NT, Radisic M, Towards adult-like human engineered cardiac tissue, Advanced drug delivery reviews, 96 (2016) 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Gerdes AM, Kellerman SE, Moore JA, Muffly KE, Clark LC, Reaves PY, Malec KB, McKeown PP, Schocken DD, Structural remodeling of cardiac myocytes in patients with ischemic cardiomyopathy, Circulation, 86 (1992) 426–430. [DOI] [PubMed] [Google Scholar]
- [67].Denning C, Borgdorff V, Crutchley J, Firth KS, George V, Kalra S, Kondrashov A, Hoang MD, Mosqueira D, Patel A, Prodanov L, Rajamohan D, Skarnes WC, Smith JG, Young LE, Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform, Biochimica et biophysica acta, 1863 (2016) 1728–1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Dai DF, Danoviz ME, Wiczer B, Laflamme MA, Tian R, Mitochondrial Maturation in Human Pluripotent Stem Cell Derived Cardiomyocytes, Stem cells international, 2017 (2017) 5153625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Veerman CC, Kosmidis G, Mummery CL, Casini S, Verkerk AO, Bellin M, Immaturity of human stem-cell-derived cardiomyocytes in culture: fatal flaw or soluble problem?, Stem cells and development, 24 (2015) 1035–1052. [DOI] [PubMed] [Google Scholar]
- [70].Peters NS, Severs NJ, Rothery SM, Lincoln C, Yacoub MH, Green CR, Spatiotemporal relation between gap junctions and fascia adherens junctions during postnatal development of human ventricular myocardium, Circulation, 90 (1994) 713–725. [DOI] [PubMed] [Google Scholar]
- [71].Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG, Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium, Circulation research, 80 (1997) 88–94. [DOI] [PubMed] [Google Scholar]
- [72].Jansen JA, van Veen TA, de Bakker JM, van Rijen HV, Cardiac connexins and impulse propagation, Journal of molecular and cellular cardiology, 48 (2010) 76–82. [DOI] [PubMed] [Google Scholar]
- [73].Burridge PW, Thompson S, Millrod MA, Weinberg S, Yuan X, Peters A, Mahairaki V, Koliatsos VE, Tung L, Zambidis ET, A universal system for highly efficient cardiac differentiation of human induced pluripotent stem cells that eliminates interline variability, PloS one, 6 (2011) e18293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Thompson SA, Burridge PW, Lipke EA, Shamblott M, Zambidis ET, Tung L, Engraftment of human embryonic stem cell derived cardiomyocytes improves conduction in an arrhythmogenic in vitro model, Journal of molecular and cellular cardiology, 53 (2012) 15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Doss MX, Di Diego JM, Goodrow RJ, Wu Y, Cordeiro JM, Nesterenko VV, Barajas-Martinez H, Hu D, Urrutia J, Desai M, Treat JA, Sachinidis A, Antzelevitch C, Maximum diastolic potential of human induced pluripotent stem cell-derived cardiomyocytes depends critically on I(Kr), PloS one, 7 (2012) e40288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Mummery C, Ward-van Oostwaard D, Doevendans P, Spijker R, van den Brink S, Hassink R, van der Heyden M, Opthof T, Pera M, de la Riviere AB, Passier R, Tertoolen L, Differentiation of human embryonic stem cells to cardiomyocytes: role of coculture with visceral endoderm-like cells, Circulation, 107 (2003) 2733–2740. [DOI] [PubMed] [Google Scholar]
- [77].Ribeiro MC, Tertoolen LG, Guadix JA, Bellin M, Kosmidis G, D’Aniello C, Monshouwer-Kloots J, Goumans MJ, Wang YL, Feinberg AW, Mummery CL, Passier R, Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro--correlation between contraction force and electrophysiology, Biomaterials, 51 (2015) 138–150. [DOI] [PubMed] [Google Scholar]
- [78].Lundy SD, Zhu WZ, Regnier M, Laflamme MA, Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells, Stem cells and development, 22 (2013) 1991–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Nunes SS, Miklas JW, Liu J, Aschar-Sobbi R, Xiao Y, Zhang B, Jiang J, Massé S, Gagliardi M, Hsieh A, Thavandiran N, Laflamme MA, Nanthakumar K, Gross GJ, Backx PH, Keller G, Radisic M, Biowire: a platform for maturation of human pluripotent stem cell–derived cardiomyocytes, Nature Methods, 10 (2013) 781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Snir M, Kehat I, Gepstein A, Coleman R, Itskovitz-Eldor J, Livne E, Gepstein L, Assessment of the ultrastructural and proliferative properties of human embryonic stem cell-derived cardiomyocytes, American journal of physiology. Heart and circulatory physiology, 285 (2003) H2355–2363. [DOI] [PubMed] [Google Scholar]
- [81].Scuderi GJ, Butcher J, Naturally Engineered Maturation of Cardiomyocytes, Frontiers in cell and developmental biology, 5 (2017) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Keung W, Boheler KR, Li RA, Developmental cues for the maturation of metabolic, electrophysiological and calcium handling properties of human pluripotent stem cell-derived cardiomyocytes, Stem cell research & therapy, 5 (2014) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Satin J, Kehat I, Caspi O, Huber I, Arbel G, Itzhaki I, Magyar J, Schroder EA, Perlman I, Gepstein L, Mechanism of spontaneous excitability in human embryonic stem cell derived cardiomyocytes, The Journal of physiology, 559 (2004) 479–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kita-Matsuo H, Barcova M, Prigozhina N, Salomonis N, Wei K, Jacot JG, Nelson B, Spiering S, Haverslag R, Kim C, Talantova M, Bajpai R, Calzolari D, Terskikh A, McCulloch AD, Price JH, Conklin BR, Chen HS, Mercola M, Lentiviral vectors and protocols for creation of stable hESC lines for fluorescent tracking and drug resistance selection of cardiomyocytes, PloS one, 4 (2009) e5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Hazeltine LB, Simmons CS, Salick MR, Lian X, Badur MG, Han W, Delgado SM, Wakatsuki T, Crone WC, Pruitt BL, Palecek SP, Effects of substrate mechanics on contractility of cardiomyocytes generated from human pluripotent stem cells, International journal of cell biology, 2012 (2012) 508294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Mulieri LA, Hasenfuss G, Leavitt B, Allen PD, Alpert NR, Altered myocardial force-frequency relation in human heart failure, Circulation, 85 (1992) 1743–1750. [DOI] [PubMed] [Google Scholar]
- [87].Pieske B, Sutterlin M, Schmidt-Schweda S, Minami K, Meyer M, Olschewski M, Holubarsch C, Just H, Hasenfuss G, Diminished post-rest potentiation of contractile force in human dilated cardiomyopathy. Functional evidence for alterations in intracellular Ca2+ handling, The Journal of clinical investigation, 98 (1996) 764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Wiegerinck RF, Cojoc A, Zeidenweber CM, Ding G, Shen M, Joyner RW, Fernandez JD, Kanter KR, Kirshbom PM, Kogon BE, Wagner MB, Force frequency relationship of the human ventricle increases during early postnatal development, Pediatric research, 65 (2009) 414–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Mannhardt I, Breckwoldt K, Letuffe-Breniere D, Schaaf S, Schulz H, Neuber C, Benzin A, Werner T, Eder A, Schulze T, Klampe B, Christ T, Hirt MN, Huebner N, Moretti A, Eschenhagen T, Hansen A, Human Engineered Heart Tissue: Analysis of Contractile Force, Stem cell reports, 7 (2016) 29–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Godier-Furnemont AF, Tiburcy M, Wagner E, Dewenter M, Lammle S, El-Armouche A, Lehnart SE, Vunjak-Novakovic G, Zimmermann WH, Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation, Biomaterials, 60 (2015) 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Ulmer BM, Stoehr A, Schulze ML, Patel S, Gucek M, Mannhardt I, Funcke S, Murphy E, Eschenhagen T, Hansen A, Contractile Work Contributes to Maturation of Energy Metabolism in hiPSC-Derived Cardiomyocytes, Stem cell reports, 10 (2018) 834–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].N. V., Heart muscle: ultrastructural studies, in, Cambridge University Press, New York, 1987. [Google Scholar]
- [93].Porter GA Jr., Hom J, Hoffman D, Quintanilla R, de Mesy Bentley K, Sheu SS, Bioenergetics, mitochondria, and cardiac myocyte differentiation, Progress in pediatric cardiology, 31 (2011) 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Mollova M, Bersell K, Walsh S, Savla J, Das LT, Park SY, Silberstein LE, Dos Remedios CG, Graham D, Colan S, Kuhn B, Cardiomyocyte proliferation contributes to heart growth in young humans, Proceedings of the National Academy of Sciences of the United States of America, 110 (2013) 1446–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Goktepe S, Abilez OJ, Parker KK, Kuhl E, A multiscale model for eccentric and concentric cardiac growth through sarcomerogenesis, Journal of theoretical biology, 265 (2010) 433–442. [DOI] [PubMed] [Google Scholar]
- [96].Besser RR, Ishahak M, Mayo V, Carbonero D, Claure I, Agarwal A, Engineered Microenvironments for Maturation of Stem Cell Derived Cardiac Myocytes, Theranostics, 8 (2018) 124–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ribeiro MC, Tertoolen LG, Guadix JA, Bellin M, Kosmidis G, D’Aniello C, Monshouwer-Kloots J, Goumans MJ, Wang YL, Feinberg AW, Mummery CL, Passier R, Functional maturation of human pluripotent stem cell derived cardiomyocytes in vitro - Correlation between contraction force and electrophysiology, Biomaterials, 51 (2015) 138–150. [DOI] [PubMed] [Google Scholar]
- [98].Li RK, Mickle DA, Weisel RD, Carson S, Omar SA, Tumiati LC, Wilson GJ, Williams WG, Human pediatric and adult ventricular cardiomyocytes in culture: assessment of phenotypic changes with passaging, Cardiovascular research, 32 (1996) 362–373. [DOI] [PubMed] [Google Scholar]
- [99].Olivetti G, Cigola E, Maestri R, Corradi D, Lagrasta C, Gambert SR, Anversa P, Aging, cardiac hypertrophy and ischemic cardiomyopathy do not affect the proportion of mononucleated and multinucleated myocytes in the human heart, J Mol Cell Cardiol, 28 (1996) 1463–1477. [DOI] [PubMed] [Google Scholar]
- [100].Kim HD, Kim DJ, Lee IJ, Rah BJ, Sawa Y, Schaper J, Human fetal heart development after mid-term: morphometry and ultrastructural study, Journal of molecular and cellular cardiology, 24 (1992) 949–965. [DOI] [PubMed] [Google Scholar]
- [101].Tulloch NL, Muskheli V, Razumova MV, Korte FS, Regnier M, Hauch KD, Pabon L, Reinecke H, Murry CE, Growth of engineered human myocardium with mechanical loading and vascular coculture, Circulation research, 109 (2011) 47–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Squire JM, Architecture and function in the muscle sarcomere, Current opinion in structural biology, 7 (1997) 247–257. [DOI] [PubMed] [Google Scholar]
- [103].Chacko KJ, Observations on the ultrastructure of developing myocardium of rat embryos, Journal of morphology, 150 (1976) 681–709. [DOI] [PubMed] [Google Scholar]
- [104].Nunes SS, Feric N, Pahnke A, Miklas JW, Li M, Coles J, Gagliardi M, Keller G, Radisic M, Human Stem Cell-Derived Cardiac Model of Chronic Drug Exposure, Acs Biomater Sci Eng, 3 (2017) 1911–1921. [DOI] [PubMed] [Google Scholar]
- [105].van der Velden J, Klein LJ, van der Bijl M, Huybregts MA, Stooker W, Witkop J, Eijsman L, Visser CA, Visser FC, Stienen GJ, Force production in mechanically isolated cardiac myocytes from human ventricular muscle tissue, Cardiovascular research, 38 (1998) 414–423. [DOI] [PubMed] [Google Scholar]
- [106].Liau B, Zhang D, Bursac N, Functional cardiac tissue engineering, Regenerative medicine, 7 (2012) 187–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Ziman AP, Gomez-Viquez NL, Bloch RJ, Lederer WJ, Excitation-contraction coupling changes during postnatal cardiac development, Journal of molecular and cellular cardiology, 48 (2010) 379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lieu DK, Liu J, Siu CW, McNerney GP, Tse HF, Abu-Khalil A, Huser T, Li RA, Absence of transverse tubules contributes to non-uniform Ca(2+) wavefronts in mouse and human embryonic stem cell-derived cardiomyocytes, Stem cells and development, 18 (2009) 1493–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Vreeker A, van Stuijvenberg L, Hund TJ, Mohler PJ, Nikkels PG, van Veen TA, Assembly of the cardiac intercalated disk during pre- and postnatal development of the human heart, PloS one, 9 (2014) e94722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Zhu H, Scharnhorst KS, Stieg AZ, Gimzewski JK, Minami I, Nakatsuji N, Nakano H, Nakano A, Two dimensional electrophysiological characterization of human pluripotent stem cell-derived cardiomyocyte system, Scientific reports, 7 (2017) 43210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Gennser G, Nilsson E, Excitation and impulse conduction in the human fetal heart, Acta physiologica Scandinavica, 79 (1970) 305–320. [DOI] [PubMed] [Google Scholar]
- [112].Koncz I, Szel T, Bitay M, Cerbai E, Jaeger K, Fulop F, Jost N, Virag L, Orvos P, Talosi L, Kristof A, Baczko I, Papp JG, Varro A, Electrophysiological effects of ivabradine in dog and human cardiac preparations: potential antiarrhythmic actions, European journal of pharmacology, 668 (2011) 419–426. [DOI] [PubMed] [Google Scholar]
- [113].Schram G, Pourrier M, Melnyk P, Nattel S, Differential Distribution of Cardiac Ion Channel Expression as a Basis for Regional Specialization in Electrical Function, Circulation Research, 90 (2002) 939–950. [DOI] [PubMed] [Google Scholar]
- [114].Drouin E, Charpentier F, Gauthier C, Laurent K, Le Marec H, Electrophysiologic characteristics of cells spanning the left ventricular wall of human heart: evidence for presence of M cells, Journal of the American College of Cardiology, 26 (1995) 185–192. [DOI] [PubMed] [Google Scholar]
- [115].Sasaki D, Matsuura K, Seta H, Haraguchi Y, Okano T, Shimizu T, Contractile force measurement of human induced pluripotent stem cell-derived cardiac cell sheet-tissue, PloS one, 13 (2018) e0198026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cyganek L, Tiburcy M, Sekeres K, Gerstenberg K, Bohnenberger H, Lenz C, Henze S, Stauske M, Salinas G, Zimmermann WH, Hasenfuss G, Guan K, Deep phenotyping of human induced pluripotent stem cell-derived atrial and ventricular cardiomyocytes, JCI insight, 3 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Yatscoff MA, Jaswal JS, Grant MR, Greenwood R, Lukat T, Beker DL, Rebeyka IM, Lopaschuk GD, Myocardial hypertrophy and the maturation of fatty acid oxidation in the newborn human heart, Pediatric research, 64 (2008) 643–647. [DOI] [PubMed] [Google Scholar]
- [118].Ellen Kreipke R, Wang Y, Miklas JW, Mathieu J, Ruohola-Baker H, Metabolic remodeling in early development and cardiomyocyte maturation, Seminars in cell & developmental biology, 52 (2016) 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Kamakura T, Makiyama T, Sasaki K, Yoshida Y, Wuriyanghai Y, Chen J, Hattori T, Ohno S, Kita T, Horie M, Yamanaka S, Kimura T, Ultrastructural maturation of human-induced pluripotent stem cell-derived cardiomyocytes in a long-term culture, Circulation journal : official journal of the Japanese Circulation Society, 77 (2013) 1307–1314. [DOI] [PubMed] [Google Scholar]
- [120].Correction for Thavandiran et al. , Design and formulation of functional pluripotent stem cell-derived cardiac microtissues, Proceedings of the National Academy of Sciences of the United States of America, 112 (2015) E3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Hirt MN, Boeddinghaus J, Mitchell A, Schaaf S, Bornchen C, Muller C, Schulz H, Hubner N, Stenzig J, Stoehr A, Neuber C, Eder A, Luther PK, Hansen A, Eschenhagen T, Functional improvement and maturation of rat and human engineered heart tissue by chronic electrical stimulation, Journal of molecular and cellular cardiology, 74 (2014) 151–161. [DOI] [PubMed] [Google Scholar]
- [122].Pillekamp F, Haustein M, Khalil M, Emmelheinz M, Nazzal R, Adelmann R, Nguemo F, Rubenchyk O, Pfannkuche K, Matzkies M, Reppel M, Bloch W, Brockmeier K, Hescheler J, Contractile properties of early human embryonic stem cell-derived cardiomyocytes: beta-adrenergic stimulation induces positive chronotropy and lusitropy but not inotropy, Stem cells and development, 21 (2012) 2111–2121. [DOI] [PubMed] [Google Scholar]
- [123].Godier-Furnémont AF, Tiburcy M, Wagner E, Dewenter M, Lämmle S, El-Armouche A, Lehnart SE, Vunjak-Novakovic G, Zimmermann W-H, Physiologic force-frequency response in engineered heart muscle by electromechanical stimulation, Biomaterials, 60 (2015) 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Stoppel WL, Kaplan DL, Black LD III, Electrical and mechanical stimulation of cardiac cells and tissue constructs, Advanced drug delivery reviews, 96 (2016) 135–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Morgan KY, Black LD III, It’s all in the timing: Modeling isovolumic contraction through development and disease with a dynamic dual electromechanical bioreactor system, Organogenesis, 10 (2014) 317–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Rangarajan S, Madden L, Bursac N, Use of flow, electrical, and mechanical stimulation to promote engineering of striated muscles, Annals of biomedical engineering, 42 (2014) 1391–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Katare RG, Ando M, Kakinuma Y, Sato T, Engineered heart tissue: a novel tool to study the ischemic changes of the heart in vitro, PloS one, 5 (2010) e9275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Jackman C, Li H, Bursac N, Long-term contractile activity and thyroid hormone supplementation produce engineered rat myocardium with adult-like structure and function, Acta biomaterialia, 78 (2018) 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Kensah G, Gruh I, Viering J, Schumann H, Dahlmann J, Meyer H, Skvorc D, Bar A, Akhyari P, Heisterkamp A, Haverich A, Martin U, A novel miniaturized multimodal bioreactor for continuous in situ assessment of bioartificial cardiac tissue during stimulation and maturation, Tissue engineering. Part C, Methods, 17 (2011) 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Miklas JW, Nunes SS, Sofla A, Reis LA, Pahnke A, Xiao Y, Laschinger C, Radisic M, Bioreactor for modulation of cardiac microtissue phenotype by combined static stretch and electrical stimulation, Biofabrication, 6 (2014) 024113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Ruan JL, Tulloch NL, Razumova MV, Saiget M, Muskheli V, Pabon L, Reinecke H, Regnier M, Murry CE, Mechanical Stress Conditioning and Electrical Stimulation Promote Contractility and Force Maturation of Induced Pluripotent Stem Cell-Derived Human Cardiac Tissue, Circulation, 134 (2016) 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Mihic A, Li J, Miyagi Y, Gagliardi M, Li SH, Zu J, Weisel RD, Keller G, Li RK, The effect of cyclic stretch on maturation and 3D tissue formation of human embryonic stem cell-derived cardiomyocytes, Biomaterials, 35 (2014) 2798–2808. [DOI] [PubMed] [Google Scholar]
- [133].Shinozawa T, Imahashi K, Sawada H, Furukawa H, Takami K, Determination of appropriate stage of human-induced pluripotent stem cell-derived cardiomyocytes for drug screening and pharmacological evaluation in vitro, Journal of biomolecular screening, 17 (2012) 1192–1203. [DOI] [PubMed] [Google Scholar]
- [134].Trieschmann J, Bettin D, Haustein M, Koster A, Molcanyi M, Halbach M, Hanna M, Fouad M, Brockmeier K, Hescheler J, Pfannkuche K, Hannes T, The Interaction between Adult Cardiac Fibroblasts and Embryonic Stem Cell-Derived Cardiomyocytes Leads to Proarrhythmic Changes in In Vitro Cocultures, Stem cells international, 2016 (2016) 2936126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Protic O, Islam MS, Greco S, Giannubilo SR, Lamanna P, Petraglia F, Ciavattini A, Castellucci M, Hinz B, Ciarmela P, Activin A in Inflammation, Tissue Repair, and Fibrosis: Possible Role as Inflammatory and Fibrotic Mediator of Uterine Fibroid Development and Growth, Seminars in reproductive medicine, 35 (2017) 499–509. [DOI] [PubMed] [Google Scholar]
- [136].Nguyen N, Nguyen W, Nguyenton B, Ratchada P, Page G, Miller PE, Ghetti A, Abi-Gerges N, Adult Human Primary Cardiomyocyte-Based Model for the Simultaneous Prediction of Drug-Induced Inotropic and Pro-arrhythmia Risk, Frontiers in physiology, 8 (2017) 1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Saxena P, Hortigon-Vinagre MP, Beyl S, Baburin I, Andranovits S, Iqbal SM, Costa A, AP IJ, Kugler P, Timin E, Smith GL, Hering S, Correlation between human ether-a-go-go-related gene channel inhibition and action potential prolongation, British journal of pharmacology, 174 (2017) 3081–3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Bennett PB, Valenzuela C, Chen LQ, Kallen RG, On the molecular nature of the lidocaine receptor of cardiac Na+ channels. Modification of block by alterations in the alpha-subunit III-IV interdomain, Circulation research, 77 (1995) 584–592. [DOI] [PubMed] [Google Scholar]
- [139].Schwinger RH, Bohm M, Erdmann E, Different negative inotropic activity of Ca2(+)-antagonists in human myocardial tissue, Klinische Wochenschrift, 68 (1990) 797–805. [DOI] [PubMed] [Google Scholar]
- [140].Sutton MS, Morad M, Mechanisms of action of diltiazem in isolated human atrial and ventricular myocardium, Journal of molecular and cellular cardiology, 19 (1987) 497–508. [DOI] [PubMed] [Google Scholar]
- [141].White M, Wiechmann RJ, Roden RL, Hagan MB, Wollmering MM, Port JD, Hammond E, Abraham WT, Wolfel EE, Lindenfeld J, et al. , Cardiac beta-adrenergic neuroeffector systems in acute myocardial dysfunction related to brain injury. Evidence for catecholamine-mediated myocardial damage, Circulation, 92 (1995) 2183–2189. [DOI] [PubMed] [Google Scholar]
- [142].Flesch M, Schwinger RH, Schiffer F, Frank K, Sudkamp M, Kuhn-Regnier F, Arnold G, Bohm M, Evidence for functional relevance of an enhanced expression of the Na(+)-Ca2+ exchanger in failing human myocardium, Circulation, 94 (1996) 992–1002. [DOI] [PubMed] [Google Scholar]
- [143].Holubarsch C, Schneider R, Pieske B, Ruf T, Hasenfuss G, Fraedrich G, Posival H, Just H, Positive and negative inotropic effects of DL-sotalol and D-sotalol in failing and nonfailing human myocardium under physiological experimental conditions, Circulation, 92 (1995) 2904–2910. [DOI] [PubMed] [Google Scholar]
- [144].Bohm M, Morano I, Pieske B, Ruegg JC, Wankerl M, Zimmermann R, Erdmann E, Contribution of cAMP-phosphodiesterase inhibition and sensitization of the contractile proteins for calcium to the inotropic effect of pimobendan in the failing human myocardium, Circulation research, 68 (1991) 689–701. [DOI] [PubMed] [Google Scholar]
- [145].Brito-Martins M, Harding SE, Ali NN, beta(1)- and beta(2)-adrenoceptor responses in cardiomyocytes derived from human embryonic stem cells: comparison with failing and non-failing adult human heart, British journal of pharmacology, 153 (2008) 751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Gibson JK, Yue Y, Bronson J, Palmer C, Numann R, Human stem cell-derived cardiomyocytes detect drug-mediated changes in action potentials and ion currents, Journal of pharmacological and toxicological methods, 70 (2014) 255–267. [DOI] [PubMed] [Google Scholar]
- [147].Sheng X, Reppel M, Nguemo F, Mohammad FI, Kuzmenkin A, Hescheler J, Pfannkuche K, Human pluripotent stem cell-derived cardiomyocytes: response to TTX and lidocain reveals strong cell to cell variability, PloS one, 7 (2012) e45963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Kuryshev YA, Brown AM, Duzic E, Kirsch GE, Evaluating state dependence and subtype selectivity of calcium channel modulators in automated electrophysiology assays, Assay and drug development technologies, 12 (2014) 110–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Cao X, Lee YT, Holmqvist M, Lin Y, Ni Y, Mikhailov D, Zhang H, Hogan C, Zhou L, Lu Q, Digan ME, Urban L, Erdemli G, Cardiac ion channel safety profiling on the IonWorks Quattro automated patch clamp system, Assay and drug development technologies, 8 (2010) 766–780. [DOI] [PubMed] [Google Scholar]
- [150].Narolska NA, van Loon RB, Boontje NM, Zaremba R, Penas SE, Russell J, Spiegelenberg SR, Huybregts MA, Visser FC, de Jong JW, van der Velden J, Stienen GJ, Myocardial contraction is 5-fold more economical in ventricular than in atrial human tissue, Cardiovascular research, 65 (2005) 221–229. [DOI] [PubMed] [Google Scholar]
- [151].Mannhardt I, Eder A, Dumotier B, Prondzynski M, Kramer E, Traebert M, Sohren KD, Flenner F, Stathopoulou K, Lemoine MD, Carrier L, Christ T, Eschenhagen T, Hansen A, Blinded Contractility Analysis in hiPSC-Cardiomyocytes in Engineered Heart Tissue Format: Comparison With Human Atrial Trabeculae, Toxicological sciences : an official journal of the Society of Toxicology, 158 (2017) 164–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Streckfuss-Bomeke K, Wolf F, Azizian A, Stauske M, Tiburcy M, Wagner S, Hubscher D, Dressel R, Chen S, Jende J, Wulf G, Lorenz V, Schon MP, Maier LS, Zimmermann WH, Hasenfuss G, Guan K, Comparative study of human-induced pluripotent stem cells derived from bone marrow cells, hair keratinocytes, and skin fibroblasts, European heart journal, 34 (2013) 2618–2629. [DOI] [PubMed] [Google Scholar]
- [153].Godfraind T, Egleme C, Finet M, Jaumin P, The actions of nifedipine and nisoldipine on the contractile activity of human coronary arteries and human cardiac tissue in vitro, Pharmacology & toxicology, 61 (1987) 79–84. [DOI] [PubMed] [Google Scholar]
- [154].Asp J, Synnergren J, Jonsson M, Dellgren G, Jeppsson A, Comparison of human cardiac gene expression profiles in paired samples of right atrium and left ventricle collected in vivo, Physiological genomics, 44 (2012) 89–98. [DOI] [PubMed] [Google Scholar]
- [155].Dobrev D, Wehrens XH, Calmodulin kinase II, sarcoplasmic reticulum Ca2+ leak, and atrial fibrillation, Trends in cardiovascular medicine, 20 (2010) 30–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Gaborit N, Le Bouter S, Szuts V, Varro A, Escande D, Nattel S, Demolombe S, Regional and tissue specific transcript signatures of ion channel genes in the non-diseased human heart, The Journal of physiology, 582 (2007) 675–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].P. SV, Cardiac electrophysiology : from cell to beside, Elsevier Sauders, 2014. [Google Scholar]
- [158].Ehrlich JR, Biliczki P, Hohnloser SH, Nattel S, Atrial-selective approaches for the treatment of atrial fibrillation, Journal of the American College of Cardiology, 51 (2008) 787–792. [DOI] [PubMed] [Google Scholar]
- [159].Nattel S, Calcium-activated potassium current: a novel ion channel candidate in atrial fibrillation, The Journal of physiology, 587 (2009) 1385–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Zhang H, Garratt CJ, Zhu J, Holden AV, Role of up-regulation of IK1 in action potential shortening associated with atrial fibrillation in humans, Cardiovascular research, 66 (2005) 493–502. [DOI] [PubMed] [Google Scholar]
- [161].Heidbuchel H, Vereecke J, Carmeliet E, Three different potassium channels in human atrium. Contribution to the basal potassium conductance, Circulation research, 66 (1990) 1277–1286. [DOI] [PubMed] [Google Scholar]
- [162].Brattelid T, Qvigstad E, Moltzau LR, Bekkevold SV, Sandnes DL, Birkeland JA, Skomedal T, Osnes JB, Sjaastad I, Levy FO, The cardiac ventricular 5-HT4 receptor is functional in late foetal development and is reactivated in heart failure, PloS one, 7 (2012) e45489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Kaakeh Y, Overholser BR, Lopshire JC, Tisdale JE, Drug-Induced Atrial Fibrillation, Drugs, 72 (2012) 1617–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Gintant G, Sager PT, Stockbridge N, Evolution of strategies to improve preclinical cardiac safety testing, Nature reviews. Drug discovery, 15 (2016) 457–471. [DOI] [PubMed] [Google Scholar]
- [165].Birket MJ, Ribeiro MC, Verkerk AO, Ward D, Leitoguinho AR, den Hartogh SC, Orlova VV, Devalla HD, Schwach V, Bellin M, Passier R, Mummery CL, Expansion and patterning of cardiovascular progenitors derived from human pluripotent stem cells, Nature biotechnology, 33 (2015) 970–979. [DOI] [PubMed] [Google Scholar]
- [166].Devalla HD, Schwach V, Ford JW, Milnes JT, El-Haou S, Jackson C, Gkatzis K, Elliott DA, Chuva de Sousa Lopes SM, Mummery CL, Verkerk AO, Passier R, Atrial-like cardiomyocytes from human pluripotent stem cells are a robust preclinical model for assessing atrial-selective pharmacology, EMBO molecular medicine, 7 (2015) 394–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Ryckebusch L, Wang Z, Bertrand N, Lin SC, Chi X, Schwartz R, Zaffran S, Niederreither K, Retinoic acid deficiency alters second heart field formation, Proceedings of the National Academy of Sciences of the United States of America, 105 (2008) 2913–2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Schotten U, Greiser M, Benke D, Buerkel K, Ehrenteidt B, Stellbrink C, Vazquez-Jimenez JF, Schoendube F, Hanrath P, Allessie M, Atrial fibrillation-induced atrial contractile dysfunction: a tachycardiomyopathy of a different sort, Cardiovascular research, 53 (2002) 192–201. [DOI] [PubMed] [Google Scholar]
- [169].Schwinger RH, Bohm M, Koch A, Uhlmann R, Uberfuhr P, Kreuzer E, Reichart B, Erdmann E, Force-frequency-relation in human atrial and ventricular myocardium, Molecular and cellular biochemistry, 119 (1993) 73–78. [DOI] [PubMed] [Google Scholar]
- [170].Van Wagoner DR, Pond AL, Lamorgese M, Rossie SS, McCarthy PM, Nerbonne JM, Atrial L-type Ca2+ currents and human atrial fibrillation, Circulation research, 85 (1999) 428–436. [DOI] [PubMed] [Google Scholar]
- [171].O’Hara T, Virag L, Varro A, Rudy Y, Simulation of the undiseased human cardiac ventricular action potential: model formulation and experimental validation, PLoS computational biology, 7 (2011) e1002061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Koivumaki JT, Korhonen T, Tavi P, Impact of sarcoplasmic reticulum calcium release on calcium dynamics and action potential morphology in human atrial myocytes: a computational study, PLoS computational biology, 7 (2011) e1001067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Dawodu AA, Monti F, Iwashiro K, Schiariti M, Chiavarelli R, Puddu PE, The shape of human atrial action potential accounts for different frequency-related changes in vitro, International journal of cardiology, 54 (1996) 237–249. [DOI] [PubMed] [Google Scholar]
- [174].Wang ZG, Pelletier LC, Talajic M, Nattel S, Effects of flecainide and quinidine on human atrial action potentials. Role of rate-dependence and comparison with guinea pig, rabbit, and dog tissues, Circulation, 82 (1990) 274–283. [DOI] [PubMed] [Google Scholar]
- [175].White SM, Claycomb WC, Embryonic stem cells form an organized, functional cardiac conduction system in vitro, American journal of physiology. Heart and circulatory physiology, 288 (2005) H670–679. [DOI] [PubMed] [Google Scholar]
- [176].Yano S, Miake J, Mizuta E, Manabe K, Bahrudin U, Morikawa K, Arakawa K, Sasaki N, Igawa O, Shigemasa C, Yamamoto Y, Morisaki T, Hidaka K, Kurata Y, Yoshida A, Shiota G, Higaki K, Ninomiya H, Lee JK, Shirayoshi Y, Hisatome I, Changes of HCN gene expression and I(f) currents in Nkx2.5-positive cardiomyocytes derived from murine embryonic stem cells during differentiation, Biomedical research, 29 (2008) 195–203. [DOI] [PubMed] [Google Scholar]
- [177].Kleger A, Seufferlein T, Malan D, Tischendorf M, Storch A, Wolheim A, Latz S, Protze S, Porzner M, Proepper C, Brunner C, Katz SF, Varma Pusapati G, Bullinger L, Franz WM, Koehntop R, Giehl K, Spyrantis A, Wittekindt O, Lin Q, Zenke M, Fleischmann BK, Wartenberg M, Wobus AM, Boeckers TM, Liebau S, Modulation of calcium-activated potassium channels induces cardiogenesis of pluripotent stem cells and enrichment of pacemaker-like cells, Circulation, 122 (2010) 1823–1836. [DOI] [PubMed] [Google Scholar]
- [178].Saito M, Sasaki T, Matsuoka H, Vitamin B(12) promotes Cx40 and HCN4 gene expression at an early stage of cardiomyocyte differentiation, Experimental animals, 58 (2009) 57–60. [DOI] [PubMed] [Google Scholar]
- [179].Maass K, Shekhar A, Lu J, Kang G, See F, Kim EE, Delgado C, Shen S, Cohen L, Fishman GI, Isolation and characterization of embryonic stem cell-derived cardiac Purkinje cells, Stem cells, 33 (2015) 1102–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Tsai SY, Maass K, Lu J, Fishman GI, Chen S, Evans T, Efficient Generation of Cardiac Purkinje Cells from ESCs by Activating cAMP Signaling, Stem cell reports, 4 (2015) 1089–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Zhou YF, Yang XJ, Li HX, Han LH, Jiang WP, Genetically-engineered mesenchymal stem cells transfected with human HCN1 gene to create cardiac pacemaker cells, The Journal of international medical research, 41 (2013) 1570–1576. [DOI] [PubMed] [Google Scholar]
- [182].Hashem SI, Claycomb WC, Genetic isolation of stem cell-derived pacemaker-nodal cardiac myocytes, Molecular and cellular biochemistry, 383 (2013) 161–171. [DOI] [PubMed] [Google Scholar]
- [183].Zhang L, Li X, Yu X, Li Y, Sun A, Huang C, Xu F, Guo J, Sun Y, Zhang X, Yang X, Zhang C, Construction of vascularized pacemaker tissues by seeding cardiac progenitor cells and endothelial progenitor cells into Matrigel, Life sciences, 179 (2017) 139–146. [DOI] [PubMed] [Google Scholar]
- [184].Monteiro LM, Vasques-Novoa F, Ferreira L, Pinto-do OP, Nascimento DS, Erratum: Restoring heart function and electrical integrity: Closing the circuit, NPJ Regenerative medicine, 2 (2017) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Monteiro LM, Vasques-Novoa F, Ferreira L, Pinto-do OP, Nascimento DS, Restoring heart function and electrical integrity: closing the circuit, NPJ Regenerative medicine, 2 (2017) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [186].Solazzo M, O’Brien FJ, Nicolosi V, Monaghan MG, The rationale and emergence of electroconductive biomaterial scaffolds in cardiac tissue engineering, APL bioengineering, 3 (2019) 041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Sun HY, Lu SH, Jiang XX, Li X, Li H, Lin QX, Mou YC, Zhao YW, Han Y, Zhou J, Wang CY, Carbon nanotubes enhance intercalated disc assembly in cardiac myocytes via the beta 1-integrin-mediated signaling pathway, Biomaterials, 55 (2015) 84–95. [DOI] [PubMed] [Google Scholar]
- [188].Sun HY, Tang JJ, Mou YC, Zhou J, Qu LL, Duval K, Huang Z, Lin N, Dai RW, Liang CX, Chen Z, Tang LJ, Tian FZ, Carbon nanotube-composite hydrogels promote intercalated disc assembly in engineered cardiac tissues through beta 1-integrin mediated FAK and RhoA pathway, Acta biomaterialia, 48 (2017) 88–99. [DOI] [PubMed] [Google Scholar]
- [189].Ahadian S, Huyer LD, Estili M, Yee B, Smith N, Xu ZS, Sun Y, Radisic M, Moldable elastomeric polyester-carbon nanotube scaffolds for cardiac tissue engineering, Acta biomaterialia, 52 (2017) 81–91. [DOI] [PubMed] [Google Scholar]
- [190].Shin SR, Zihlmann C, Akbari M, Assawes P, Cheung L, Zhang KZ, Manoharan V, Zhang YS, Yuksekkaya M, Wan KT, Nikkhah M, Dokmeci MR, Tang XW, Khademhosseini A, Reduced Graphene Oxide-GelMA Hybrid Hydrogels as Scaffolds for Cardiac Tissue Engineering, Small, 12 (2016) 3677–3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Zhao GX, Qing HB, Huang GY, Genin GM, Lu TJ, Luo ZT, Xu F, Zhang XH, Reduced graphene oxide functionalized nanofibrous silk fibroin matrices for engineering excitable tissues (vol 10, pg 982, 2018), Npg Asia Mater, 11 (2019). [Google Scholar]
- [192].Zhao GX, Qing HB, Huang GY, Genin GM, Lu TJ, Luo ZT, Xu F, Zhang XH, Reduced graphene oxide functionalized nanofibrous silk fibroin matrices for engineering excitable tissues, Npg Asia Mater, 10 (2018) 982–994. [Google Scholar]
- [193].Spearman BS, Hodge AJ, Porter JL, Hardy JG, Davis ZD, Xu T, Zhang X, Schmidt CE, Hamilton MC, Lipke EA, Conductive interpenetrating networks of polypyrrole and polycaprolactone encourage electrophysiological development of cardiac cells, Acta biomaterialia, 28 (2015) 109–120. [DOI] [PubMed] [Google Scholar]
- [194].Cui Z, Ni NC, Wu J, Du GQ, He S, Yau TM, Weisel RD, Sung HW, Li RK, Polypyrrole-chitosan conductive biomaterial synchronizes cardiomyocyte contraction and improves myocardial electrical impulse propagation, Theranostics, 8 (2018) 2752–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Tsui JH, Ostrovsky-Snider NA, Yama DMP, Donohue JD, Choi JS, Chavanachat R, Larson JD, Murphy AR, Kim DH, Conductive Silk-Polypyrrole Composite Scaffolds with Bioinspired Nanotopographic Cues for Cardiac Tissue Engineering, Journal of materials chemistry. B, 6 (2018) 7185–7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Abedi A, Hasanzadeh M, Tayebi L, Conductive nanofibrous Chitosan/PEDOT:PSS tissue engineering scaffolds, Mater Chem Phys, 237 (2019). [Google Scholar]
- [197].Mawad D, Mansfield C, Lauto A, Perbellini F, Nelson GW, Tonkin J, Bello SO, Carrad DJ, Micolich AP, Mahat MM, Furman J, Payne DJ, Lyon AR, Gooding JJ, Harding SE, Terracciano CM, Stevens MM, A conducting polymer with enhanced electronic stability applied in cardiac models, Science advances, 2 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Savoji H, Mohammadi MH, Rafatian N, Toroghi MK, Wang EY, Zhao Y, Korolj A, Ahadian S, Radisic M, Cardiovascular disease models: A game changing paradigm in drug discovery and screening, Biomaterials, 198 (2019) 3–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Brandão KO, Tabel VA, Atsma DE, Mummery CL, Davis RP, Human pluripotent stem cell models of cardiac disease: from mechanisms to therapies, Disease Models & Mechanisms, 10 (2017) 1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Yang C, Al-Aama J, Stojkovic M, Keavney B, Trafford A, Lako M, Armstrong L, Concise Review: Cardiac Disease Modeling Using Induced Pluripotent Stem Cells, STEM CELLS, 33 (2015) 2643–2651. [DOI] [PubMed] [Google Scholar]
- [201].Kothapalli CR, van Veen E, de Valence S, Chung S, Zervantonakis IK, Gertler FB, Kamm RD, A high-throughput microfluidic assay to study neurite response to growth factor gradients, Lab Chip, 11 (2011) 497–507. [DOI] [PubMed] [Google Scholar]
- [202].Garbern JC, Lee RT, Cardiac stem cell therapy and the promise of heart regeneration, Cell stem cell, 12 (2013) 689–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Sun N, Yazawa M, Liu J, Han L, Sanchez-Freire V, Abilez OJ, Navarrete EG, Hu S, Wang L, Lee A, Pavlovic A, Lin S, Chen R, Hajjar RJ, Snyder MP, Dolmetsch RE, Butte MJ, Ashley EA, Longaker MT, Robbins RC, Wu JC, Patient-Specific Induced Pluripotent Stem Cells as a Model for Familial Dilated Cardiomyopathy, Science Translational Medicine, 4 (2012) 130ra147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Chadwick AC, Musunuru K, Genome Editing for the Study of Cardiovascular Diseases, Current cardiology reports, 19 (2017) 22–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [205].Boyle EA, Li YI, Pritchard JK, An Expanded View of Complex Traits: From Polygenic to Omnigenic, Cell, 169 (2017) 1177–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [206].Friedrichs S, Malan D, Sasse P, Modeling long QT syndromes using induced pluripotent stem cells: Current progress and future challenges, Trends in Cardiovascular Medicine, 23 (2013) 91–98. [DOI] [PubMed] [Google Scholar]
- [207].Moretti A, Bellin M, Welling A, Jung CB, Lam JT, Bott-Flügel L, Dorn T, Goedel A, Höhnke C, Hofmann F, Seyfarth M, Sinnecker D, Schömig A, Laugwitz K-L, Patient-Specific Induced Pluripotent Stem-Cell Models for Long-QT Syndrome, New England Journal of Medicine, 363 (2010) 1397–1409. [DOI] [PubMed] [Google Scholar]
- [208].Lan F, Lee Andrew S., Liang P, Sanchez-Freire V, Nguyen Patricia K., Wang L, Han L, Yen M, Wang Y, Sun N, Abilez Oscar J., Hu S, Ebert Antje D., Navarrete Enrique G., Simmons Chelsey S., Wheeler M, Pruitt B, Lewis R, Yamaguchi Y, Ashley Euan A., Bers Donald M., Robbins Robert C., Longaker Michael T., Wu Joseph C., Abnormal Calcium Handling Properties Underlie Familial Hypertrophic Cardiomyopathy Pathology in Patient-Specific Induced Pluripotent Stem Cells, Cell Stem Cell, 12 (2013) 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Yazawa M, Hsueh B, Jia X, Pasca AM, Bernstein JA, Hallmayer J, Dolmetsch RE, Using induced pluripotent stem cells to investigate cardiac phenotypes in Timothy syndrome, Nature, 471 (2011) 230–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Carvajal-Vergara X, Sevilla A, D’Souza SL, Ang YS, Schaniel C, Lee DF, Yang L, Kaplan AD, Adler ED, Rozov R, Ge Y, Cohen N, Edelmann LJ, Chang B, Waghray A, Su J, Pardo S, Lichtenbelt KD, Tartaglia M, Gelb BD, Lemischka IR, Patient-specific induced pluripotent stem-cell-derived models of LEOPARD syndrome, Nature, 465 (2010) 808–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Kim C, Wong J, Wen J, Wang S, Wang C, Spiering S, Kan NG, Forcales S, Puri PL, Leone TC, Marine JE, Calkins H, Kelly DP, Judge DP, Chen HS, Studying arrhythmogenic right ventricular dysplasia with patient-specific iPSCs, Nature, 494 (2013) 105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Hinson JT, Chopra A, Nafissi N, Polacheck WJ, Benson CC, Swist S, Gorham J, Yang L, Schafer S, Sheng CC, Haghighi A, Homsy J, Hubner N, Church G, Cook SA, Linke WA, Chen CS, Seidman JG, Seidman CE, HEART DISEASE. Titin mutations in iPS cells define sarcomere insufficiency as a cause of dilated cardiomyopathy, Science, 349 (2015) 982–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Zweigerdt R, Gruh I, Martin U, Your heart on a chip: iPSC-based modeling of Barth-syndrome-associated cardiomyopathy, Cell stem cell, 15 (2014) 9–11. [DOI] [PubMed] [Google Scholar]
- [214].Zhao Y, Rafatian N, Feric NT, Cox BJ, Aschar-Sobbi R, Wang EY, Aggarwal P, Zhang B, Conant G, Ronaldson-Bouchard K, Pahnke A, Protze S, Lee JH, Davenport Huyer L, Jekic D, Wickeler A, Naguib HE, Keller GM, Vunjak-Novakovic G, Broeckel U, Backx PH, Radisic M, A Platform for Generation of Chamber-Specific Cardiac Tissues and Disease Modeling, Cell, 176 (2019) 913–927.e918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Müller M, Seufferlein T, Illing A, Homann J, Modelling human channelopathies using induced pluripotent stem cells: a comprehensive review, Stem cells international, 2013 (2013) 496501–496501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Chugh Sumeet S, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benjamin Emelia J, Gillum Richard F, Kim Y-H, McAnulty John H, Zheng Z-J, Forouzanfar Mohammad H, Naghavi M, Mensah George A, Ezzati M, Murray Christopher JL, Worldwide Epidemiology of Atrial Fibrillation, Circulation, 129 (2014) 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Eisen A, Ruff Christian T, Braunwald E, Nordio F, Corbalán R, Dalby A, Dorobantu M, Mercuri M, Lanz H, Rutman H, Wiviott Stephen D, Antman Elliott M, Giugliano Robert P, Sudden Cardiac Death in Patients With Atrial Fibrillation: Insights From the ENGAGE AF-TIMI 48 Trial, Journal of the American Heart Association, 5 e003735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Lip GYH, Lane DA, Stroke Prevention in Atrial Fibrillation: A Systematic ReviewStroke Prevention in Atrial FibrillationStroke Prevention in Atrial Fibrillation, JAMA, 313 (2015) 1950–1962. [DOI] [PubMed] [Google Scholar]
- [219].Laksman Z, Wauchop M, Lin E, Protze S, Lee J, Yang W, Izaddoustdar F, Shafaattalab S, Gepstein L, Tibbits GF, Keller G, Backx PH, Modeling Atrial Fibrillation using Human Embryonic Stem Cell-Derived Atrial Tissue, Scientific reports, 7 (2017) 5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Ballew JD, Olson TM, Alekseev AE, Jahangir A, Zingman LV, Bienengraeber M, Sattiraju S, Park S, Liu XK, Terzic A, Kv1.5 channelopathy due to KCNA5 loss-of-function mutation causes human atrial fibrillation, Human Molecular Genetics, 15 (2006) 2185–2191. [DOI] [PubMed] [Google Scholar]
- [221].Marczenke M, Piccini I, Mengarelli I, Fell J, Ropke A, Seebohm G, Verkerk AO, Greber B, Cardiac Subtype-Specific Modeling of Kv1.5 Ion Channel Deficiency Using Human Pluripotent Stem Cells, Frontiers in physiology, 8 (2017) 469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [222].Stockbridge N, Morganroth J, Shah RR, Garnett C, Dealing with global safety issues, Drug safety, 36 (2013) 167–182. [DOI] [PubMed] [Google Scholar]
- [223].Roden D, Cellular basis of drug-induced torsades de pointes, British journal of pharmacology, 154 (2008) 1502–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [224].Gintant G, Sager PT, Stockbridge N, Evolution of strategies to improve preclinical cardiac safety testing, Nature reviews Drug discovery, 15 (2016) 457. [DOI] [PubMed] [Google Scholar]
- [225].Okada J.-i., Yoshinaga T, Kurokawa J, Washio T, Furukawa T, Sawada K, Sugiura S, Hisada T, Screening system for drug-induced arrhythmogenic risk combining a patch clamp and heart simulator, Science advances, 1 (2015) e1400142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Blinova K, Dang Q, Millard D, Smith G, Pierson J, Guo L, Brock M, Lu HR, Kraushaar U, Zeng H, International multisite study of human-induced pluripotent stem cell-derived cardiomyocytes for drug proarrhythmic potential assessment, Cell reports, 24 (2018) 3582–3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Ronaldson K, Feric N, Zhao YM, Zhang BY, Conant G, Panhke A, Aschar-Sobbi R, Vunjak-Novakovic G, Backx P, Radisic M, TARA Biosystems’ Biowire (TM) II: Engineering Mature Human Cardiac Tissues Enables More Predictive Drug Screening, J Pharmacol Tox Met, 88 (2017) 242–242. [Google Scholar]
- [228].Hanton G, Preclinical cardiac safety assessment of drugs, Drugs in R&D, 8 (2007) 213–228. [DOI] [PubMed] [Google Scholar]
- [229].Guth BD, Engwall M, Eldridge S, Foley CM, Guo L, Gintant G, Koerner J, Parish ST, Pierson JB, Ribeiro AJS, Zabka T, Chaudhary KW, Kanda Y, Berridge B, Considerations for an In Vitro, Cell-Based Testing Platform for Detection of Adverse Drug-Induced Inotropic Effects in Early Drug Development. Part 1: General Considerations for Development of Novel Testing Platforms, Frontiers in pharmacology, 10 (2019) 884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [230].Agarwal A, Goss JA, Cho A, McCain ML, Parker KK, Microfluidic heart on a chip for higher throughput pharmacological studies, Lab on a Chip, 13 (2013) 3599–3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [231].Grosberg A, Nesmith AP, Goss JA, Brigham MD, McCain ML, Parker KK, Muscle on a chip: in vitro contractility assays for smooth and striated muscle, Journal of pharmacological and toxicological methods, 65 (2012) 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [232].Schneider O, Zeifang L, Fuchs S, Sailer C, Loskill P, User-Friendly and Parallelized Generation of Human Induced Pluripotent Stem Cell-Derived Microtissues in a Centrifugal Heart-on-a-Chip, Tissue Engineering Part A, 25 (2019) 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Lind JU, Busbee TA, Valentine AD, Pasqualini FS, Yuan H, Yadid M, Park S-J, Kotikian A, Nesmith AP, Campbell PH, Instrumented cardiac microphysiological devices via multimaterial three-dimensional printing, Nature materials, 16 (2017) 303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [234].Lee A, Hudson A, Shiwarski D, Tashman J, Hinton T, Yerneni S, Bliley J, Campbell P, Feinberg A, 3D bioprinting of collagen to rebuild components of the human heart, Science, 365 (2019) 482–487. [DOI] [PubMed] [Google Scholar]
- [235].Skylar-Scott MA, Uzel SG, Nam LL, Ahrens JH, Truby RL, Damaraju S, Lewis JA, Biomanufacturing of organ-specific tissues with high cellular density and embedded vascular channels, Science advances, 5 (2019) eaaw2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Ribeiro AJ, Ang Y-S, Fu J-D, Rivas RN, Mohamed TM, Higgs GC, Srivastava D, Pruitt BL, Contractility of single cardiomyocytes differentiated from pluripotent stem cells depends on physiological shape and substrate stiffness, Proceedings of the National Academy of Sciences, 112 (2015) 12705–12710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [237].Ribeiro AJ, Schwab O, Mandegar MA, Ang Y-S, Conklin BR, Srivastava D, Pruitt BL, Multi-imaging method to assay the contractile mechanical output of micropatterned human iPSC-derived cardiac myocytes, Circulation research, 120 (2017) 1572–1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [238].Huebsch N, Loskill P, Deveshwar N, Spencer CI, Judge LM, Mandegar MA, Fox CB, Mohamed TM, Ma Z, Mathur A, Miniaturized iPS-cell-derived cardiac muscles for physiologically relevant drug response analyses, Scientific reports, 6 (2016) 24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [239].Schroer AK, Shotwell MS, Sidorov VY, Wikswo JP, Merryman WD, I-Wire Heart-on-a-Chip II: Biomechanical analysis of contractile, three-dimensional cardiomyocyte tissue constructs, Acta biomaterialia, 48 (2017) 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [240].Sidorov VY, Samson PC, Sidorova TN, Davidson JM, Lim CC, Wikswo JP, I-Wire Heart-on-a-Chip I: Three-dimensional cardiac tissue constructs for physiology and pharmacology, Acta biomaterialia, 48 (2017) 68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [241].Sonin DL, Wakatsuki T, Routhu KV, Harmann LM, Petersen M, Meyer J, Strande JL, Protease-activated receptor 1 inhibition by SCH79797 attenuates left ventricular remodeling and profibrotic activities of cardiac fibroblasts, Journal of cardiovascular pharmacology and therapeutics, 18 (2013) 460–475. [DOI] [PMC free article] [PubMed] [Google Scholar]


