Abstract
Objectives:
Patients with Crohn Disease often produce antibodies against flagellated intestinal bacteria. There are mixed data as to whether such antibodies are present in patients with spondyloarthritis. Our objectives were to evaluate for the presence of antibodies against intestinal organisms in children with enthesitis related arthritis (ERA).
Methods:
Children with ERA and healthy controls were recruited at three sites. Sera were plated on a nitrocellulose array and incubated with labelled antibodies to human IgA and IgG.
Results:
At UAB, patients and controls had similar antibody levels against the majority of the bacteria selected, with the exception of increased IgA antibodies among ERA patients against Prevotella oralis (1231 [IQR 750, 2566] versus 706 [IQR 428, 1106], p = 0.007.) These findings were partially validated at a second but not at a third site.
Conclusions:
ERA patients may produce increased IgA antibodies against P. oralis. The possible significance of this finding bears further exploration.
Keywords: Antibodies, Enthesitis-Related Arthritis, Immunoglobulin A, Prevotella, Spondyloarthritis
1. Introduction
The human intestine is colonized with an estimated 100 trillion bacteria, a process that begins shortly after birth [1]. Long ignored, it has become increasingly clear that these bacteria play important roles in immune function as well as in a variety of autoimmune and inflammatory disorders [2, 3]. Particular interest has been afforded the role of the microbiota in the related conditions of inflammatory bowel disease (IBD) and spondyloarthritis (SpA), with studies in both diseases demonstrating differences in the contents of the fecal microbiota, including increased or decreased relative abundance of organisms such as Faecalibacterium prausnitzii [4, 5], Ruminococcus gnavus [6, 7], and the Bacteroides genus, including B. fragilis [8, 9]. Patients with IBD also have dysregulated response to enteric antigens. Specifically, through use of a phage display library, our group previously identified bacterial flagellins as important antigenic targets in patients with IBD [10]. Although the work was conducted in mice, anti-flagellin antibodies are also present in about 50% of patients with Crohn Disease [10], where they serve as markers of poor prognosis [11]. There are mixed data as to whether these antibodies are present in patients with SpA, with some [12, 13] but not all [14] studies showing elevated levels of IBD-associated antibodies in this population. There are no pediatric data, however.
Our group developed an antigen array system which we have used to screen patients for IgG and IgA antibodies against enteric antigens [15]. To evaluate for the presence of antibodies against enteric antigens, we applied this array with serum from children with enthesitis related arthritis (ERA). To our surprise, we found that while flagellin reactivity among these subjects was not elevated, they did demonstrate increased IgA reactivity against Prevotella oralis.
2. Methods
2.1. Patients
This study was conducted at three sites: Children’s of Alabama / University of Alabama at Birmingham (UAB), Cincinnati Children’s Hospital Medical Center (CCHMC), and Boston Children’s Hospital (BCH). The derivation of the CCHMC cohort was described previously and included treatment-naïve subjects recruited at CCHMC (n = 9), the Children’s Hospital of Philadelphia (n = 10), St. Vincent’s Hospital in Indianapolis (n = 3), and one each from the North Shore / Long Island Jewish Medical Center and Toledo Children’s Hospital [16], Patients meeting the International League of Associations for Rheumatology criteria for ERA [17] were enrolled, modified to allow inclusion of children with a family (but not personal) history of psoriasis. Children with IBD were excluded from the initial arrays. Healthy controls at UAB and BCH consisted of children referred to our clinics to evaluate for arthritis, but found to have a non-inflammatory cause of joint pain; additional controls at UAB were recruited from the general population through advertisements or through relatives affiliated with the hospital. Healthy control recruitment at CCHMC took place through the Cincinnati Children’s Genomic Control Cohort, which is a population representative sample of 1020 children age 3 – 18 years from the 7 counties that comprise the Greater Cincinnati Region. Controls for the current study were selected so as to ensure matching based upon age at serum collection, sex, and race. Informed consent was obtained from all guardians, and assent / consent were obtained from pediatric patients as per institutional guidelines. Institutional Review Board approval at the UAB as well as at the satellite sites was obtained (UAB IRB approval # IRB-130507003.)
2.2. Antigen array
The array was performed as described [15]. Briefly, proteins, including flagellin antigens [18] as well as bacterial lysates and tetanus toxoid as a control antigen, were diluted in TRIS buffer (pH 8.0) with 0.5% SDS at 0.2 mg/mL and printed onto FAST slides (Global Life Science Solutions, Sanford, ME) using an Array IT robot (Sunnyvale, CA) in quadruplicate. Slides were air-dried overnight, blocked with Super Block (Thermo Scientific, Rockford, IL), and probed with human sera at 1:100 dilution for one hour. After washing, slides were incubated with DyLight 650-labelled Donkey anti-human IgG (Invitrogen, Carlsbad, CA) and DyLight 550-labelled goat anti-human IgA (Immuno Reagents, Raleigh NC) and read on an Axon GenePix 4000B dual laser microarray reader (Molecular Devices, Sunnyvale, CA). The peptides and whole bacterial lysates included on the arrays are shown in Tables 1 and 2, respectively. Preparation of the outer membrane (OM) proteins was performed largely as described [19], except for use of sonication (three 30-second pulses at 70%) instead of French press. Preparation of the extracellular polysaccharide was performed as described [20].
Table 1.
Peptide antigens represented on the microarray
| Name | Protein ID | Function | Primary species |
|---|---|---|---|
| 28_4b | N/A | Unknown | Unspecified Lachnospiraceae |
| A4 Fla2 | Flagellin | Motility | Unspecified Lachnospiraceae |
| CBir1 | Flagellin | Motility | Unspecified Lachnospiraceae |
| OmpC | OMP from E. coli | Porin or transport | Escherichia coli |
| Tetc | Tetanus toxoid | Pathogenicity | Clostridium tetani |
Table 2.
Bacteria represented on the array
| Species | Phylum | Source |
|---|---|---|
| 28–4 | Firmicutes | Murine cecum [18] |
| 28–4 EPS | Firmicutes | Murine cecum [18] |
| A4 | Firmicutes | Murine cecum [18] |
| Alistipes putredinis | Bacteroidetes | ATCC 29800 |
| Fusobacterium nucleatum | Fusobacter | Gifted from Hui Wu |
| Prevotella buccalis | Bacteroidetes | ATCC 35310 |
| Prevotella copri | Bacteroidetes | DSMZ 18205 |
| Porphyromonas gingivalis | Bacteroidetes | ATCC 33277 |
| Prevotella intermedia | Bacteroidetes | ATCC 25611 |
| Prevotella melaninogenica | Bacteroidetes | ATCC 25845 |
| Prevotella oralis | Bacteroidetes | ATCC 33269 |
| P. oralis outer membrane | Bacteroidetes | Prepared from ATCC 33269 |
| Prevotella pallens | Bacteroidetes | ATCC 700821 |
| Rikenella microfusus | Bacteroidetes | ATCC 29728 |
| Streptococcus gordonii | Firmicutes | Gifted from Hui Wu |
| Streptococcus mutans | Firmicutes | Gifted from Hui Wu |
| Streptococcus parasanguinis | Firmicutes | Gifted from Hui Wu |
| Streptococcus pyogenes | Firmicutes | Gifted from Hui Wu |
| Streptococcus sanguinis | Firmicutes | Gifted from Hui Wu |
Abbreviations: ATCC, American Type Culture Collection; DSMZ, Deutsche Sammlung von Mikroorganismen und Zellkulturen; EPS, extracellular polysaccharide.
2.3. Statistical analysis
Comparisons between dichotomous variables were performed with the Mann-Whitney test (two groups) or Kruskal-Wallis test (three groups), while comparison of continuous variables was performed with the Spearman correlation coefficient.. Correction for multiple comparisons was performed with the Benjamini-Hochberg test. Analyses were performed with R, version 3.6.3.
3. Results
3.1. Patients
Clinical and demographic features of the subjects are shown in Table 3. By design, patients and controls recruited through CCHMC were well-matched by age and sex, while at BCH and UAB, controls were slightly older and slightly younger, respectively, than the ERA patients. All of the subjects at BCH and CCHMC were naïve to immunosuppressive therapy, while the UAB subjects were heterogeneous with respect to prior therapies.
Table 3.
Subjects included in the array.
| Feature | Controls | ERA |
|---|---|---|
| Children’s of Alabama | ||
| N | 20 | 40 |
| Age at diagnosis (years ±SD) | Not applicable | 12 ± 3.2 |
| Age at collection (years ±SD) | 10.5 ± 4.9 | 13.2 ± 2.9 |
| Males | 11 (55%) | 20 (50%) |
| Race | ||
| Caucasian | 16 (80%) | 29 (72%) |
| African-American | 2 (10%) | 11 (28%) |
| Other | 2 (10%) | 0 (0%) |
| HLA-B27+ | Not measured | 12 / 37 (32%) |
| Medicines | ||
| None | 20 (100%) | 17 (42%) |
| Conventional DMARDs alone | 6 (15%) | |
| TNFi ± conventional DMARD | 17 (42%) | |
| Boston Children’s Hospital | ||
| N | 18 | 17 |
| Age at diagnosis (years ±SD) | Notapplicable | 11.2 ± 3.4 |
| Age at collection (years ±SD) | 14.5 ± 3.6 | 12.5 ± 3.7 |
| Males | 6 (33%) | 11 (65%) |
| Race | ||
| Caucasian | 13 (72%) | 14 (82%) |
| African-American | 2 (11%) | 1 (5.9%) |
| Other | 3 (17%) | 2 (12%) |
| HLA-B27+ | 1/1 (100%) | 3/6 (50%) |
| Medicines | None | None |
| Cincinnati Children’s Hospital Medical Center | ||
| N | 24 | 24 |
| Age at diagnosis (years ± SD) | Not applicable | 12.1 ± 2.7 |
| Age at collection (years ± SD) | 13.8 ± 3.0 | 14.0 ± 3.2 |
| Males | 18 (75%) | 18 (75%) |
| Race | ||
| Caucasian | 23 (96%) | 22 (92%) |
| African-American | 1 (4.2%) | 0 |
| Other | 2 (8.4%) | |
| HLA-B27+ | Not available | 10 / 15 (67%) |
| Medicines | None | None |
Abbreviations: DMARD, disease-modifying anti-rheumatic drugs; TNFi, tumor necrosis factor inhibitor
3.2. Antigen array studies
Antigens present on the array are shown in Tables 1 and 2. As shown, UAB patients and controls had similar levels of IgA (Figure 1A) and IgG (Figure 2A) antibody production against the majority of the bacteria selected, the only exception being Prevotella oralis. Clear separation of the groups is observed, with ERA patients demonstrating increasing net fluorescent intensity of 1231 (IQR 750, 2566) to 706 (IQR 428, 1106) (p = 0.007), albeit the finding was non-significant at 0.161 when corrected with the Benjamini-Hochberg test. No other comparisons achieved even an uncorrected statistical significance. Among the children with ERA, there were no associations between P. oralis reactivity and age of serum collection, sex, HLA-B27 status, use of immunosuppressive therapy, ESR, or CRP. For example, male patients demonstrated reactivity of 1243 (IQR 803, 2566) as compared to 1167 (IQR 750, 2282) for female patients (p = 0.914), and if anything, patients previously exposed to immunosuppressive therapy may have had increased reactivity (1702, IQR 915, 3075) compared to 952 (IQR 659, 1804) for treatment-naïve patients (p = 0.091). With respect to IgG reactivity, ERA patients demonstrated a non-significant increase in reactivity against P. oralis of 1582 (IQR 736, 2485) compared to 776 (IQR 538, 1656), p = 0.067; with nominally significant decreases in reactivity against P. copri (418 [IQR 255, 624] versus 622 [434, 858], p = 0.005) and P. intermedia (7104 [IQR 5086, 10748] versus 12016 [IQR 7956, 15172], p = 0.007), although none of these differences withstood correction for multiple comparisons.
Figure 1.
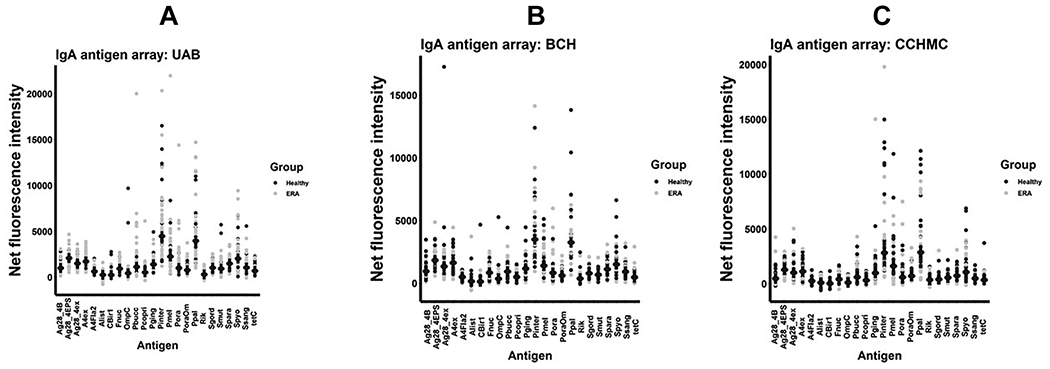
IgA antigen arrays. Net fluorescent intensities (mean, SD) are shown for the IgA antigen arrays performed on serum obtained from subjects at the University of Alabama at Birmingham (A), Boston Children’s Hospital (B), and the Cincinnati Children’s Hospital Medical Center (C).
Figure 2.
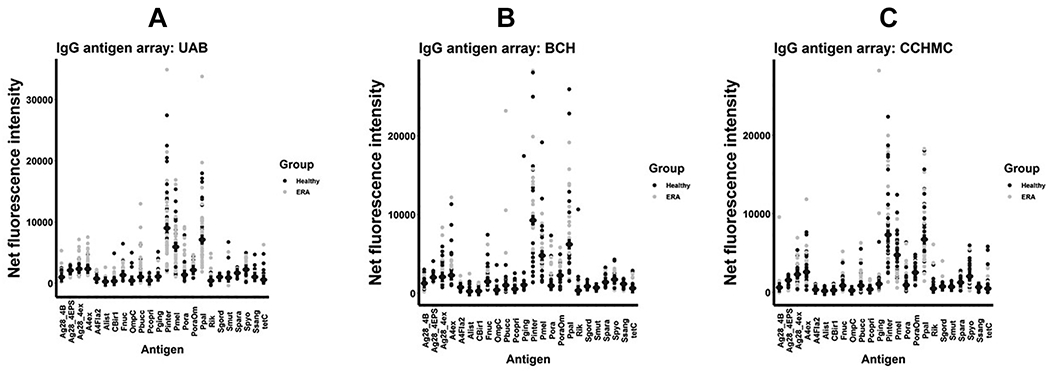
IgG antigen arrays. Net fluorescent intensities (mean, SD) are shown for the IgG antigen arrays performed on serum obtained from subjects at the University of Alabama at Birmingham (A), Boston Children’s Hospital (B), and the Cincinnati Children’s Hospital Medical Center (C).
To validate our findings, serum samples were obtained from two additional sites: CCHMC and BCH. Given the geographic distance between all three sites, it is highly unlikely that any single subject was enrolled more than once; indeed, a thorough review of the medical records of the UAB patients did not identify any who had been treated at BCH or any of the sites that provided samples to CCHMC. Among the BCH patients, there appeared to be a subset demonstrating increased reactivity (1126 [IQR 453, 2657] for the patients versus 690 [IQR 505, 976] among the controls), albeit with an outlier among the controls (Figure 1C), the differences were statistically insignificant at p = 0.113. No IgA comparisons at BCH achieved nominal significance, while the only IgG comparison at BCH that did so was decreased reactivity against Streptococcus pyogenes among patients (1371 [IQR 921, 2352) as compared to controls (2008 [IQR 1720, 2594], p = 0.035. There were no differences in IgG reactivity among the subjects at BCH. At CCHMC, however, reactivity against P. oralis was virtually identical between the two groups. Decreased IgA reactivity against Porphyromonas gingivalis (806 [IQR 537, 1220) versus 1148 [833, 1612], p = 0.050) and Rikenella microfusus (250 [IQR 62, 404] versus 480 [IQR 320, 682], p = 0.014) were observed; as with BCH, decreased reactivity was observed against S. pyogenes (1750 [IQR 1262, 2199] versus 2901 [IQR 1825, 3140], p = 0.006. Controls at all three sites had similar IgA titers against P. oralis (UAB 706 [IQR 428, 1106], BCH 690 [ICR 505, 976], CCHMC 740 [IQR 306, 1220), p = 0.976; while among the patients, the levels at CCHMC were lower than those at the other two sites (UAB 1231 [IQR 750, 2566], BCH 1126 [ICR 453, 2657], CCHMC 551 [IQR 307, 1289), p = 0.020. When data from the three sites were pooled, the only significant difference in IgA reactivity was against P. oralis (1031 [IQR 512, 2333] versus 693 [IQR 409, 1157), p = 0.010. Likewise, when data from the three IgG arrays were pooled, increased reactivity to P. oralis (1497 [IQR 684, 3014] versus 869 [IQR 634, 1691], p = 0.040), along with decreased reactivity to Streptococcus pyogenes (1728 [IQR 921, 2621] versus 2525 [IQR 1716, 2988], p = 0.002) and possibly P. gingivalis (1029 [IQR 751, 1302] versus 1186 [IQR 878, 1618], p = 0.047) emerged. As with UAB, there were no associations between sex and P. oralis titers among the subjects at BCH and CCHMC, nor among HLA-B27 status and IgA or IgG P. oralis titers at CCHMC; the low numbers of subjects tested for HLA-B27 among the BCH cohort precluded analysis. As BCH and CCHMC both consisted of inception cohorts, it was not possible to include treatment status as a variable. Small associations between age at serum collection and both IgA (r = 0.275, p = 0.013) and IgG (r = 0.217, p = 0.052) P. oralis titers among the cohort as a whole were observed.
Flagellin antibodies associated with Crohn Disease [10, 11] were included on the array. Among the ERA patients at UAB, there were no differences in IgA or IgG reactivity against any of these antigens, nor among children in the CCHMC or BCH cohorts (Figures 1, 2.) We ran a repeat array that included 8 pediatric patients with Crohn Disease and 30 healthy controls recruited at UAB. Here, as expected, there was substantially increased IgG reactivity to Fla2 among the Crohn Disease patients (192 [IQR 147, 344) versus the controls (30 [IQR 5.9, 64]), p < 0.001), with otherwise similar IgG reactivity to the remainder of the antigens (not shown).
4. Discussion
We screened three cohorts of children with ERA for IgA and IgG antibodies against a panel of oral and intestinal bacteria. Our results suggested that at UAB and possibly at BCH, children with ERA demonstrated increased IgA antibodies against a single organism, P. oralis, as compared to healthy control subjects. In addition, pooled together, the subjects had increased IgA as well as IgG antibodies against P. oralis, although the differences in IgG reactivity were not significant at any of the sites. These differences were largely specific to P. oralis.
Important caveats here are the cross-sectional nature of the study and its small sample size, which impacted statistical power and underscored the exploratory nature of these findings. Furthermore, the demographics of the patients and controls at BCH and UAB were dissimilar with respect to age of the patients versus controls. It appears unlikely that this would account for our findings; the ERA patients were older than the controls at UAB and younger than the controls at BCH. The CCHMC cohort was not matched geographically, as the ERA subjects were derived from several sites, while the controls were all local; however, limiting the analysis to the subjects recruited at CCHMC did not alter the results (data now shown). It is puzzling that the differences in IgA and IgG reactivity were observed against whole P. oralis, but not against its outer membrane. We suspect this reflects limited statistical power and the presence of outliers among the controls, as visual inspection of the graphs (Figures 1 and 2) demonstrates some clustering with both the UAB and BCH cohorts for both IgA and IgG.
It bears mention that the presence of IgA antigenic targets does not necessarily indicate that either these targets or the antibodies directed against them are pathogenic; indeed, SpA has developed in patients lacking B cells completely [21]. However, such antibodies are a marker of class-switching and a mature response, thus potentially indicating T cell involvement. Although CD8 T cells are not required for disease in the HLA-B27 transgenic rat model [22], CD4 T cells are essential [23]. If this putative SpA-associated antigen is indeed targeted by CD4 T cells, then the basis for the association of HLA-B27 with SpA remains elusive, leaving our findings compatible with theories involving HLA-B27 protein misfolding as a basis for the unfolded protein response [24, 25]. It thus bears emphasis that among patients with ERA there was no association at UAB or CCHMC or among the group as a whole between IgA or IgG reactivity against P. oralis and HLA-B27 status.
Given the possibility of an antigenic trigger and the well-recognized association of sub-clinical ileitis in adult [26] and pediatric [27, 28] SpA, it is not implausible that such as trigger may be found within the gastrointestinal microbiota. The intestinal microbiota is the largest mass of adjuvant to which our immune system is exposed, and has profound lifelong effects on the maturation of our immune system [29]. Furthermore, the microbiota is clearly a trigger in IBD, in which several flagellin antigens are present in a large percentage of Crohn Disease patients, serving as markers of poor prognosis [10, 11, 18]. Additional supporting evidence for the role of the microbiota in SpA comes from animal studies showing abrogation of the disease in the germ-free state [30], as well as multiple studies in humans showing altered microbiota [8, 31–34].
Although our studies cannot exclude the possibility that antibodies to P. oralis represent a consequence of the disease process, the organism is an oral commensal that is scarcely present in the stool of children with ERA, some of whom were included in our previous studies on the fecal microbiota [4, 8]. Thus, elevated IgA antibodies against this organism in some ERA patients do not appear to represent a response to organisms present in an inflamed intestinal milieu. However, our data do not permit speculation as to the mechanism underlying this potential finding, nor the significance of the more robust association with IgA versus IgG antibodies. This latter finding is not unusual, as several immunologic conditions involving the gut are associated with stronger IgA responses to specific antigens, such as the tissue transglutaminase antibody in celiac disease [35] and the anti-Saccharomyces cerevisiae and outer membrane protein C antibodies associated with Crohn Disease [36].
In conclusion, our data suggest that oral and intestinal organisms such as P. oralis may be targeted in children with ERA. The possible significance of this finding bears further exploration.
5. Acknowledgements
The authors would like to acknowledge Dr. Hui Wu for kindly providing us with several of the whole bacteria present on the arrays. MHC was supported by National Institute of Health / National Institute of Allergy and Infectious Diseases (NIAID) T32AI007512 and a Scientist Development Award from the Rheumatology Research Foundation. PAN was supported by the National Institute of Health / National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) R01-AR065538 and P30-AR070253 grants and by the Arbuckle Family Fund for Arthritis Research, and RAC was supported by the NIAMS Intramural Research Program (Z01 AR041184).
Grant support
This work was supported by the NIH (P60 AR064172; Elson, PI), P30 AR070549 (Thompson, PI), P01 AR048929 (Thompson, PI), Z01 AR041184 (Colbert), and the American College of Rheumatology / Rheumatology Research Foundation (Stoll, PI). The research was also supported in part by the Cincinnati Children’s Research Foundation and its Cincinnati Genomic Control Cohort. The funding sources had no involvement in the design, conduct, or reporting of the study.
Abbreviations
- BCH
Boston Children’s Hospital
- CCHMC
Cincinnati Children’s Hospital Medical Center
- ERA
enthesitis related arthritis
- IBD
inflammatory bowel diseases
- OM
outer membrane
- SpA
spondyloarthritis
- UAB
University of Alabama at Birmingham
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
None
References
- 1.Johnson CL and Versalovic J, The human microbiome and its potential importance to pediatrics. Pediatrics, 2012. 129(5): p. 950–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stoll ML and Cron RQ, The microbiota in pediatric rheumatic disease: epiphenomenon or therapeutic target? Curr Opin Rheumatol, 2016. 28(5): p. 537–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvonen M, et al. , Gut microbiota-host interactions and juvenile idiopathic arthritis. Pediatr Rheumatol Online J, 2016. 14(1): p. 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoll ML, et al. , Altered microbiota associated with abnormal humoral immune responses to commensal organisms in enthesitis-related arthritis. Arthritis Res Ther, 2014. 16(6): p. 486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y, Shen J, and Ran ZH, Association between Faecalibacterium prausnitzii Reduction and Inflammatory Bowel Disease: A Meta-Analysis and Systematic Review of the Literature. Gastroenterol Res Pract, 2014. 2014: p. 872725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Willing BP, et al. , A pyrosequencing study in twins shows that gastrointestinal microbial profiles vary with inflammatory bowel disease phenotypes. Gastroenterology, 2010. 139(6): p. 1844–1854 e1. [DOI] [PubMed] [Google Scholar]
- 7.Breban M, et al. , Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis, 2017. 76(9): p. 1614–1622. [DOI] [PubMed] [Google Scholar]
- 8.Stoll ML, et al. , Age and fecal microbial strain-specific differences in patients with spondyloarthritis. Arthritis Res Ther, 2018. 20(1): p. 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gevers D, et al. , The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe, 2014. 15(3): p. 382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodes MJ, et al. , Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest, 2004. 113(9): p. 1296–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Targan SR, et al. , Antibodies to CBir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology, 2005. 128(7): p. 2020–8. [DOI] [PubMed] [Google Scholar]
- 12.Hoffman IE, et al. , Anti-saccharomyces cerevisiae IgA antibodies are raised in ankylosing spondylitis and undifferentiated spondyloarthropathy. Ann Rheum Dis, 2003. 62(5): p. 455–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallis D, et al. , Elevated serum anti-flagellin antibodies implicate subclinical bowel inflammation in ankylosing spondylitis: an observational study. Arthritis Res Ther, 2013. 15(5): p. R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riente L, et al. , Antibodies to tissue transglutaminase and Saccharomyces cerevisiae in ankylosing spondylitis and psoriatic arthritis. J Rheumatol, 2004. 31(5): p. 920–4. [PubMed] [Google Scholar]
- 15.Christmann BS, et al. , Human seroreactivity to gut microbiota antigens. J Allergy Clin Immunol, 2015. 136(5): p. 1378–86 e1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barnes MG, et al. , Subtype-specific peripheral blood gene expression profiles in recent-onset juvenile idiopathic arthritis. Arthritis Rheum, 2009. 60(7): p. 2102–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petty RE, et al. , International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol, 2004. 31(2): p. 390–2. [PubMed] [Google Scholar]
- 18.Duck LW, et al. , Isolation of flagellated bacteria implicated in Crohn’s disease. Inflamm Bowel Dis, 2007. 13(10): p. 1191–201. [DOI] [PubMed] [Google Scholar]
- 19.Thein M, et al. , Efficient subfractionation of gram-negative bacteria for proteomics studies. J Proteome Res, 2010. 9(12): p. 6135–47. [DOI] [PubMed] [Google Scholar]
- 20.Gorska S, et al. , Structural and immunochemical studies of neutral exopolysaccharide produced by Lactobacillus johnsonii 142. Carbohydr Res, 2010. 345(1): p. 108–14. [DOI] [PubMed] [Google Scholar]
- 21.Baeten D, et al. , Spondylarthritis in the absence of B lymphocytes. Arthritis Rheum, 2008. 58(3): p. 730–3. [DOI] [PubMed] [Google Scholar]
- 22.Taurog JD, et al. , Spondylarthritis in HLA-B27/human beta2-microglobulin-transgenicrats is not prevented by lack of CD8. Arthritis Rheum, 2009. 60(7): p. 1977–84. [DOI] [PubMed] [Google Scholar]
- 23.Breban M, et al. , T cells, but not thymic exposure to HLA-B27, are required for the inflammatory disease of HLA-B27 transgenic rats. J Immunol, 1996. 156(2): p. 794–803. [PubMed] [Google Scholar]
- 24.Colbert RA, et al. , From HLA-B27 to spondyloarthritis: a journey through the ER. Immunol Rev, 2010. 233(1): p. 181–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Colbert RA, Navid F, and Gill T, The role of HLA-B*27 in spondyloarthritis. Best Pract Res Clin Rheumatol, 2017. 31(6): p. 797–815. [DOI] [PubMed] [Google Scholar]
- 26.Mielants H, et al. , Gut inflammation in the spondyloarthropathies: clinical, radiologic, biologic and genetic features in relation to the type of histology. A prospective study. J Rheumatol, 1991. 18(10): p. 1542–51. [PubMed] [Google Scholar]
- 27.Mielants H, et al. , Gut inflammation in the spondyloarthropathies. Curr Rheumatol Rep, 2005. 7(3): p. 188–94. [DOI] [PubMed] [Google Scholar]
- 28.Stoll ML, Punaro M, and Patel AS, Fecalcal protectin in children with the enthesitis-related arthritis subtype of juvenile idiopathic arthritis. J Rheumatol, 2011. 38(10): p. 2274–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu HJ and Wu E, The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes, 2012. 3(1): p. 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taurog JD, et al. , The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med, 1994. 180(6): p. 2359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aggarwal A, et al. , Gut microbiome in children with enthesitis-related arthritis in a developing country and the effect of probiotic administration. Clin Exp Immunol, 2017. 187(3): p. 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Breban M, et al. , Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann Rheum Dis, 2017. [DOI] [PubMed] [Google Scholar]
- 33.Tito RY, et al. , Brief Report: Dialister as a Microbial Marker of Disease Activity in Spondyloarthritis. Arthritis Rheumatol, 2017. 69(1): p. 114–121. [DOI] [PubMed] [Google Scholar]
- 34.Costello ME, et al. , Intestinal dysbiosis in ankylosing spondylitis. Arthritis Rheumatol, 2015. 67: p. 686–691. [DOI] [PubMed] [Google Scholar]
- 35.Chou R, et al. , Screening for Celiac Disease: Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA, 2017. 317(12): p. 1258–1268. [DOI] [PubMed] [Google Scholar]
- 36.Yao F, et al. , Diagnostic utility of serological biomarkers in patients with Crohn’s disease: A case-control study. Medicine (Baltimore), 2018. 97(32): p. e11772. [DOI] [PMC free article] [PubMed] [Google Scholar]


