Abstract
The allophycocyanin (APC) protein purified from Phormidium sp. A09DM was investigated for its in vivo antioxidant and anti-aging potential in Caenorhabditis elegans. An increased mean lifespan of APC-treated (100 μg/ml) worms (wild type) were observed from 16 ± 0.2 days (control) to 20 ± 0.1 days (treated). APC-treated worms also showed improved physiological marker of aging such as the rate of pharyngeal pumping and higher rate of survival against oxidative and thermal stress. Furthermore, APC was found to moderate the expression of human amyloid beta (Aβ1–42) as well as associated Aβ-induced paralysis in the transgenic C. elegans CL4176 upon increase in temperature. Furthermore, RNA interference (RNAi)-mediated studies revealed the dependence of downstream regulator daf-16, independent of stress-induced resistance gene skn-1 in the APC treated C. elegans. In the present study, we tried to demonstrate the anti-aging activity, longevity and protective effects of APC against cellular stress in C. elegans, which can lead to the use of this biomolecule in drug development for age-related disorders.
Electronic supplementary material
The online version of this article (10.1007/s13205-020-02314-1) contains supplementary material, which is available to authorized users.
Keywords: Cyanobacteria, Allophycocyanin, Caenorhabditis elegans, Alzheimer disease, Oxidative stress
Introduction
Phycobilliproteins (PBPs) and linker proteins are reciprocally organized to shape multi-molecular complexes known as phycobilisomes (PBS, 4–8 MDa) associated with light harvesting function in red algae (Rhodophyta), cyanobacteria (blue-green algae) and cryptomonad (Chryophyta) (Grossman et al. 1995). The three major phycobilliproteins based on the light absorbing properties are phycoerythrin (PE; λmax: 540–570 nm), phycocyanin (PC; λmax: 610–620 nm) and allophycocyanin (APC; λmax: 650–655 nm) (Singh et al. 2015). The PBPs form the core and rod structures, with the core located on the periphery of the thylakoid membrane and rods diverging out from the core. The core contains invariable APC, whereas rods contain variable PC and PE (Sonani et al. 2016). Structurally, APC is made up of two subunits, i.e., α (~ 15.5 kDa) and β (~ 17.0 kDa) subunits (Sonani et al. 2015b). These two subunits join together to form a heterodimer; the spatial arrangement of three heterodimers forms the trimeric disc (Grossman et al. 1995). It has been reported that the core APC comprises antioxidant potential, which might be valuable as a potent anti-aging therapeutic biomolecule (Sonani et al. 2014b). The various applications of APC have been reported, such as a dye in color industries, food colorant in nutraceutical industries, and fluorescence tagging in immunofluorescence and other biomedical applications (Sonani et al. 2016). Other than these applications, PBPs are also reported as good therapeutic agents (Sonani et al. 2015a).
The process of aging is reported to involve the progression of reactive oxygen species (ROS) accumulations and downstream regulator gene daf-16 and stress resistance gene skn-1 (Wan et al. 2020, 2019). Out of various hypotheses stated for aging, the free radical theory of aging is widely accepted, which suggests the accumulation of free radicals as well as generation of metabolic imbalance, plays a crucial role in the progression of aging (Blagosklonny 2008). The free radicals can be reduced or declined by increasing the endogenous antioxidant generation or supplementing exogenous antioxidants (Yu et al. 2017). There are various lines of evidences, which exhibit the anti-aging efficacy of various antioxidant compounds such as α-tocopherol, Trolox and α-lipoic acid (Andriollo-Sanchez et al. 2005; Patel et al. 2018). Different genes (daf-16 and skn-1) are also reported to be involved in stress tolerance and other adverse effects, resulting in the extension of lifespan (Andriollo-Sanchez et al. 2005). C. elegans showed a highly conserved gene for diseased and phenotypic characteristic to human disease such as declined body movement, reduced rate of reproduction and elevated accumulation of toxic metabolites (Harrington and Harley 1988; Iwasa et al. 2010). Due to the existence of aging-associated phenotypes, C. elegans is appraised as a good model for analyzing the effect of therapeutic compounds, which may restrict aging (Ishii et al. 2004; Singh et al. 2016). APC can be used as a functional food augmented for its anti-oxidative, anti-inflammatory, immunomodulatory and anti-hyperanalgesic possessions has been reported (Sonani et al. 2015a; Uno and Nishida 2016).
Herein, we examined the role of APC on lifespan using wild-type (N2 Bristol) and different mutants of C. elegans strain. We investigated the antioxidant potential of APC by exposing worms to ROS inducer (paraquat), and RNAi knockout strategy was used to study the involvement of DAF-16 and SKN-1 stress response factor in the longevity of worms. In C. elegans, various transcription factors such as oxidative stress (skn-1) activate the expression of chains of important proteins and molecular chaperons required to balance proteostasis under the respective stress conditions (Morton and Lamitina 2013). The RNAi-mediated gene knockout method was used to investigate the effect of skn-1 gene with APC-treated and -untreated worms. In this study, we explored the effect of APC on longevity, stress resistance and protective effect against age-associated disease. Thus with the antioxidant property of APC, we further explored the possible involvement of DAF-16 and SKN-1 which are important regulators in anti-aging.
Materials and methods
APC purification and characterization
Phormidium sp. A09DM culture was grown in ASN III medium with 12:12 h light: dark cycles at 27 ± 2 °C. The 28 days old culture was harvested and lysed by simple repetitive freeze (− 80 °C)–thaw (4 °C) cycles to extract out all the intracellular proteins (Sonani et al. 2015b). These crude proteins were further subjected to three-step ammonium sulfate precipitation of 20%, 40% and 70% ammonium sulfate saturation. The 0.1% Triton X-100 was added after the 40% ammonium sulfate purification step. The pellet obtained after 70% ammonium sulfate precipitation was dialyzed and the molecular grade of APC protein was obtained by ion exchange chromatographic technique (DEAE Sepharose FF, AKTA Pure system, GE Healthcare). The column was pre-equilibrated with 20 mM Tris–HCl buffer, pH 8.0, dialyzed with 70% ammonium sulfate pellet (5 ml) loaded to the column and the flow rate was kept at 5 ml/min. Protein was eluted by applying 0–500 mM gradient of NaCl in 20 mM Tris–HCl buffer, pH 8.0. The amount of APC was calculated at each step according to Bennet and Bogorad method (1973) [APC] = (OD652 − 0.208OD615)/5.09 (Bennett and Bogorad 1973). The purity of APC at each step of purification was recorded as (purity ratio) calculated by the formula A653/A280. The purified APC protein was characterized by SDS-PAGE and native PAGE analysis to check the purity and homogeneity. The UV–visible absorbance spectrum of APC was recorded by UV–visible spectrophotometer (Specord 210, AnalytikJena AG, Germany) and purity of APC was accessed by taking the purity ratio of absorbance 653–280 nm (Sonani et al. 2014b).
Cultivation and maintenance of C. elegans
Different strains of C. elegans used in this study were N2 Bristol (wild type), CL4176 (dvIs27[pAF29(myo-3/Ab 1–42/let UTR) + pRF4(rol-6(su1006)]) and TJ356 (zls356 [daf-16p::daf-16a/b::GFP + rol-6(su1006)]. The wild-type strain of C. elegans was cultured at 16 °C and maintained in a nematode growth medium plate (NGM), which was preseeded with E. coli OP50 as a nutrient source for the worms. The E. coli OP50, E. coli HT115 (DE3) and RNAi constructs were cultured at 37 °C. To study the effect of APC, the NGM agar plate was prepared with different concentrations of APC (10, 50, and 100 µg/ml). All the worm strains and food source of worms were obtained from the Caenorhabditis Genetics Center (CGC), University of Minnesota, USA.
Lifespan and health span study
Three different concentrations (10, 50 and 100 µg/ml) of APC were used to carry out the lifespan and health span study at 20 °C (Sonani et al. 2014a; Singh et al. 2016). For the lifespan study, the worms were synchronized and L4 staged worms were transferred to control and experimental plates containing APC of different concentrations. The total number of live and dead worms was calculated on every alternate day till all the worms died. The reaction in response to mechanical stimulus and pharyngeal pumping was considered as a surrogate indicator to scrutinize dead animals. The health of the worms was checked by manually counting pharyngeal pumping rate at the 5th and 10th-day post-transferring of the L4 staged worms. All assays are performed in triplicate and worm details are given in supplementary Table 1.
Table 1.
APC protein purification, yield and purity
| Total protein concentration (mg) | APC concentration (mg) | Purity Ratio (A653/A280) | APC content out of total protein (%) | Yield (%) | |
|---|---|---|---|---|---|
| Crude | 80 | 4.7 | 0.47 | 5.87 | 100 |
| Ammonium sulfate fraction 70% | 10 | 3.7 | 1.05 | 37 | 78.72 |
| Ion exchange chromatography | 4.2 | 2.8 | 3.86 | 66.66 | 59.57 |
Stress tolerance assay
Stress tolerance assay was performed by exposing N2 (wild type) worms to thermal and oxidative stress. For oxidative stress assay, worms were synchronized up to post-adulthood and exposed to oxidative stress using 10 mM H2O2 for 2 h in liquid medium supplemented with E. coli OP50. The worms were then transferred to experimental (100 µg/ml APC) and control plates and percentage recovery from stress was calculated after 16 h of incubation at 20 °C (Cai et al. 2011).
The thermal stress was provided by increasing the temperature from 20 to 35 °C and the numbers of live worms were counted in experimental (100 µg/ml APC) and control plates at an interval of 2 h till the death of all worms.
DCHF-DA staining
In vivo antioxidant potential of APC was checked by quantifying intracellular ROS precisely inside the C. elegans body via ROS-specific dye DCFHA-DA. This dye can penetrate through the cell membrane and fluoresce when it comes in contact with free radicals (Rastogi et al. 2010). Paraquat (herbicide) was used to induce oxidative stress by means of generating a high amount of ROS. The L4 stage-synchronized worms were exposed to 10 mM paraquat on experimental (100 µg/ml APC) and control plate to induce ROS (reactive oxygen species) production and incubated for 24 h at 20 °C. Thereafter, formation of ROS within the treated as well as untreated worms was checked by staining the worms with 5 µM DCFH-DA dye (Patel et al. 2018).
RNAi experiment
Synchronized worms were grown on NGM plates with and without APC at 20 °C. L4 stage worms were fed with skn-1 and daf-16 RNAi (HT115 E. Coli) culture as reported previously (Timmons and Fire 1998; Kamath and Ahringer 2003) for three consecutive generations to complete knockout of skn-1 and daf-16. Further, worms were permitted to lay eggs for 1 day. The offspring obtained from the eggs were used as RNAi mutant/knockout for a particular gene (Sonani et al. 2014a) and the lifespan was studied in experimental (100 µg/ml APC) and control plates.
DAF-16::GFP nuclear localization
The L1 stage worms of TJ356 were grown with and without APC (100 μg/ml) containing NGM plates. One set of treated and untreated L4 staged worms were shifted to 36 °C temperature to induce heat shock in the worms and then imaged under fluorescence microscopy, whereas another set of treated and untreated worms were directly imaged (at 10 ×) using a Nikkon DS-Ri2 fluorescence microscope (Eclipse Ni-E, Nikon). The GFP transgenic lines were observed using excitation and emission filter of 400/30 and 508/20, respectively. Translocation of DAF-16 was observed by evaluating the existence of GFP agglomeration in the nuclei.
Paralysis assay
The CL4176 transgenic animal containing human amyloid (Aβ1-42) in muscle cells were grown, with and without APC containing NGM plates for 48 h at 16 °C. The (Aβ1-42) production in the muscle cell was induced by increasing the temperature to 25 °C for 20 h and the worms were scored for paralysis at an interval of 2 h till all the worms were paralyzed. Then, the paralyzed worms were differentiated by means of a“halo” formed as a result of the movement of the head during feeding, as well as no response to the mechanical stimulus was also considered as indication of a paralyzed worm.
In vivo amyloid β staining by Thioflavin-T on C. elegans (CL4176)
The wild-type N2 bristol worms and Alzheimer’s disease model CL4176 worms were employed for this analysis. The staining of APC-treated and -untreated worms was performed by washing worms with S-basal medium for more than three times to get the maximum number of worms for the assay as described in the protocol (Link et al. 2003). Thioflavin-T (0.125%) was used to stain the worms, which werethen incubated for 30 min in dark at room temperature. Subsequently, destaining was done with 50% ethanol till the absolute stain disappeared from the solution. The fluorescence intensity of Aβ aggregate on the body wall of APC-treated and -untreated worms was measured by fluorescence microscopy (Eclipse Ni-E, Nikon) and ImageJ software.
Statistical analysis
All data obtained were statistically represented as standard error means (SEM). We used two-tailed Student’s t test for comparing two sets of data. For lifespan, the log rank tests obtained was considered statistically significant at p < 0.05 and a graph was plotted using Prism 4 software. P < 0.001 was considered statistically significant (Supplementary Table 1).
Results
Purification and characterization of APC
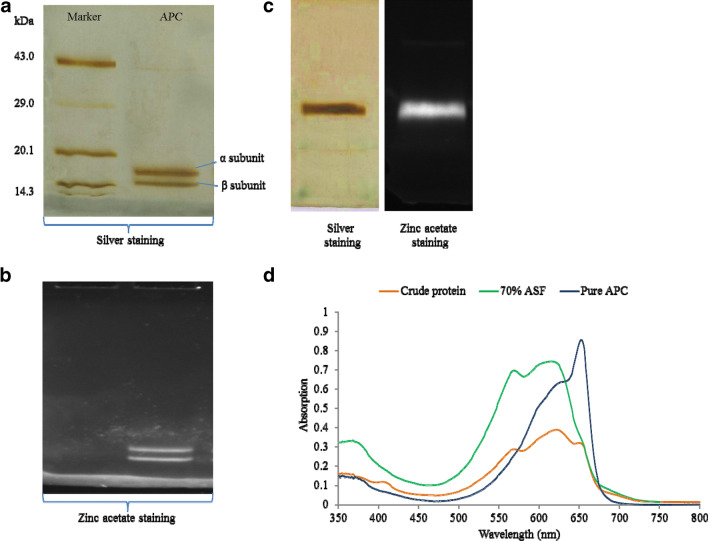
The repetitive cycles of freeze–thaw yields the extraction of a majority of intracellular proteins. The cellular impurities in the greenish pellet were precipitated after 20% ammonium sulfate fractionation and phycoerythrin was separated by 40% ammonium sulfate treatment. The addition of 0.1% Triton-X 100 helped in the separation of PBPs. Other phycobiliproteins were precipitated at 70% ammonium sulfate, and the pellet of 70% ammonium sulfate fractionation was desalted by dialysis against 20 mM Tris–HCl buffer, pH 8.0. Further, DEAE Sepharose ion exchange chromatography was employed to obtain a high purity of protein. Sodium chloride salt gradient (0–500 mM) was used for elution of proteins. Pure APC was obtained at 220 mM NaCl concentration containing 20 mM Tris–HCl buffer, pH 8.0 (Sonani et al. 2015b). The purified protein fractions were analyzed for their purity by UV–visible spectroscopy, SDS-PAGE and native PAGE analysis Fig. 1. The absorbance spectrum recorded ranges from 200 to 800 nm. APC concentration as well as the purity ratio obtained after each purification step is shown in Table 1. APC obtained after ion exchange chromatography shows absorption maxima at 653 nm, having purity ratio 3.86 as shown in Fig. 1d. The SDS-PAGE analysis shows two distinct bands of alpha and beta subunits of APC having molecular weight 15.5 kDa and 17.0 kDa, respectively (Fig. 1a, b). Two orange fluorescent bands on SDS-PAGE after UV illumination of zinc acetate staining further confirm the presence of APC associated with chromospheres (Fig. 1b). Similar fluorescence outcome was observed in the Native PAGE with single intact prominent band after zinc acetate staining. Native PAGE analysis shows one distinct band of APC after silver as well as zinc acetate staining (Fig. 1c).
Fig. 1.
APC purification and characterization by PAGE and UV–visible spectrum analysis. a Purified APC isolated from Phormidium sp. A09DM was analyzed on 15% SDS-PAGE. Protein standard molecular weight markers are shown in the lane denoted as “Marker” and two distinct bands of α (15.5 kDa) and β (17.7 kDa) subunits were observed on the SDS-PAGE stained with silver nitrate. b Zinc acetate staining of 15% SDS-PAGE confirms the presence of two distinct fluorescence subunits α (15.5 kDa) and β (17.7 kDa). c Native PAGE showed the intact purified APC stained with silver nitrate and zinc acetate. d Overlay UV–visible spectrum of crude, 70% ASF and pure APC. Λmax at 653 nm confirmed the presence of APC. Increase in the peak at 653 nm was observed after concurrent purification steps
APC enhances lifespan and health span of C. elegans
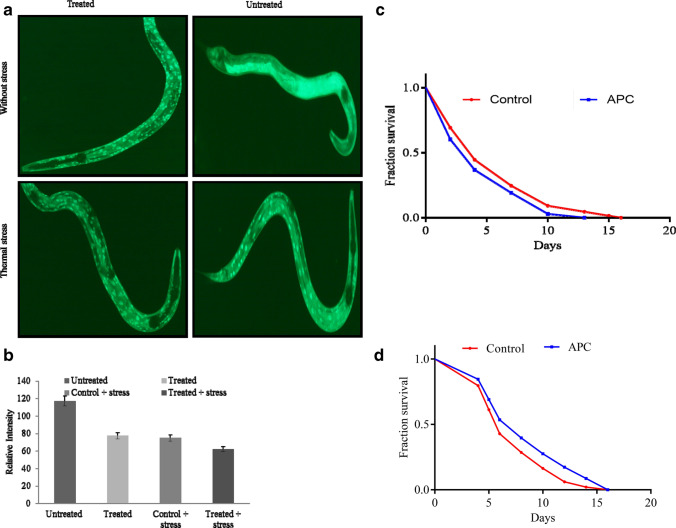
The lifespan of C. elegans was determined by treating worms with APC under different concentration (control, 10, 50, and 100 µg/ml) using the L4 animals at 20 °C (Sonani et al. 2014a; Singh et al. 2016). The number of dead worms was verified on every alternate day. The mean lifespan of control worms was observed to be 16 ± 0.2 days (log-rank test, p < 0.05). APC-treated worms at 10 and 50 µg/ml showed no significant difference as compared to the control, but remarkable increase in lifespan at 100 µg/ml of APC-treated worms was observed (20 ± 0.1 days; log-rank test, p < 0.05) as shown in Fig. 2a. An increased lifespan of APC-treated worms was observed as compared to the control in a dose-dependent manner. We measured the rate of pharyngeal pumping in APC-treated (100 µg/ml) and untreated worms on the 5th day and 10th day of post-adulthood. The rate of pharyngeal pumping for control animals was observed to be 112 ± 5.161 min−1 and 80 ± 5.5 min−1 on the 5th and 10th days, respectively, whereas the treated (100 µg/ml) worms exhibited 150 ± 4.874 and 130 ± 6.841 min−1 on the 5th and 10th days, respectively (Fig. 2b).
Fig. 2.
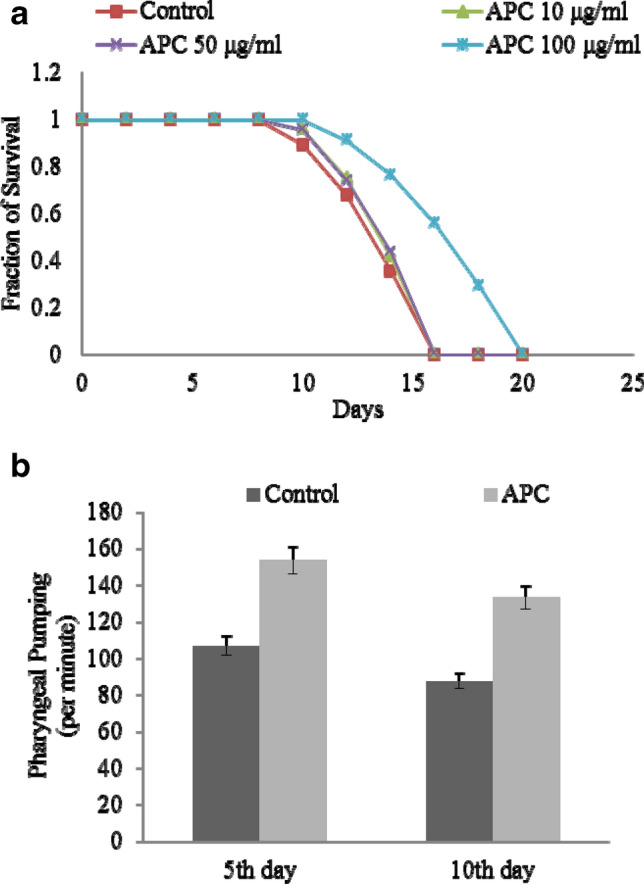
APC treatment enhances lifespan and improves health span in C.elegans: a Result of APC treatment increases survival of on N2 Bristol (adult worm) in a dose-dependent manner. Mean survival duration—control, 16 ± 0.2 days (mean ± SEM; log-rank test, p < 0.05), APC (100 μg/ml) 20 ± 0.1 days (mean ± SEM; log-rank test, p < 0.05). Data are represented as mean ± SEM. APC-treated worms showed increased lifespan as compared to the control and 10 μg/ml and 50 μg/ml of APC-treated worms. b APC treatment improves pharyngeal pumping rate with aging. Effect of APC on the pharyngeal pumping of C. elegans on the 5th day and 10th day—control, 112 ± 5.161 min−1 and 80 ± 5.5 min−1 (t test, p < 0.001), respectively, and on the 10th day 150 ± 4.874 min−1 and 130 ± 6.841 min−1 (t test, p < 0.001). Data are represented as mean ± SEM. APC-treated worms showed improved health span as compared to the control
APC increases stress tolerance in C. elegans
The thermal and oxidative stress assays were performed to determine the preventive role of APC against the stress by exposing the adult worms with APC (treated; 100 µg/ml) and without APC (untreated). The thermotolerance assay was performed by exposing the pre-exposed worms to 35 °C (treated and untreated) and the numbers of dead worms were counted at every 2 h interval until all the worms were scored dead. APC-treated worms exhibited a significantly higher rate of survival 20 ± 2.96 h (t test, p < 0.001) as compared to untreated worms 18 ± 2.96 h (t test, p < 0.001) as shown in Fig. 3a. The oxidative stress assay was carried out by exposing the worms to 10 mM H2O2 for 2 h to generate oxidative stress and thereafter worms were counted at 16 h post-recovery. APC-treated worms showed significantly higher rate of recovery (85 ± 0.7%; t test, p < 0.001) as compared to the control (54 ± 2.2%; t test, p < 0.001) as shown in Fig. 3b. The above results demonstrate the significant role of APC in the promotion of health span in C. elegans.
Fig. 3.
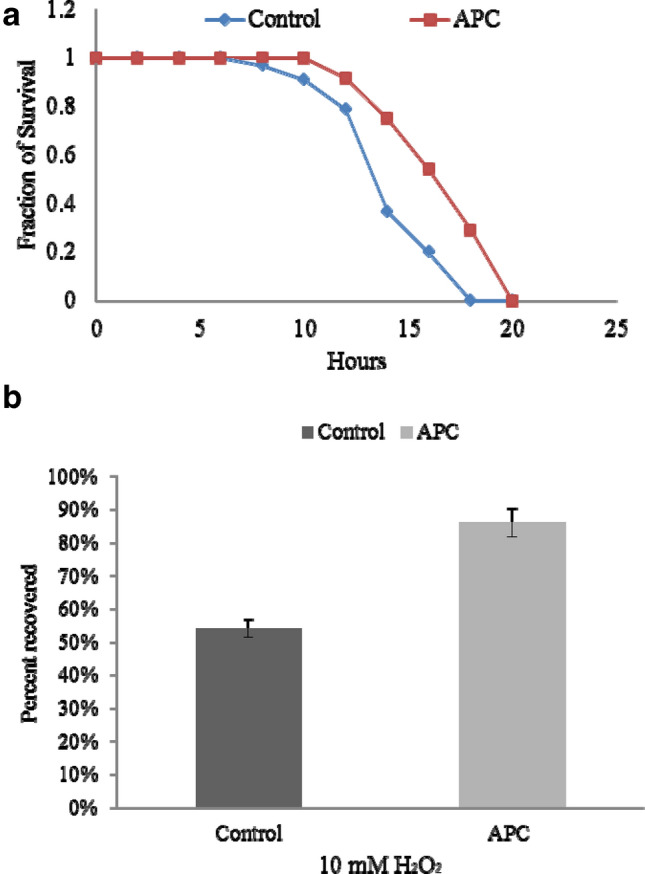
APC treatment enhanced stress tolerance in C. elegans. a Effect of APC treatment on thermal stress tolerance. N2 animals (young adult stage) treated and untreated with APC of 100 μg/ml were 20 ± 2.96 h (t test, p < 0.001) and 18 ± 2.96 h (t test, p < 0.001), respectively, at 35 ˚C. The dead animals were counted at every 2 h until all worms died and represented as survival curves. APC-treated worms showed higher protective resistance against thermal stress as compared to the control. b APC supplement increased oxidative stress tolerance. APC-treated (85% ± 0.7%; test, p < 0.001) and -untreated N2 animals (54 ± 2.2%; t test, p < 0.001) (young animal adults) were subjected to 10 mM hydrogen peroxide for 2 h at 20 ˚C. APC-treated worms showed higher protective resistance against thermal stress as compared to the control
In vivo ROS scavenging potential of APC
We checked the effect of APC under artificial oxidative stress using 10 mM paraquat (PQ). PQ increases the intracellular ROS in C. elegans N2 wild-type body as evidenced by increase in fluorescence intensity (Fig. 4a). In vivo ROS scavenging potential of APC was measured by feeding the protein to C. elegans as the dietary antioxidant supplement. We checked the effect of APC (100 μg/ml) feeding on ROS production induced by PQ in N2 wild- type C. elegans by probing ROS generation using DCFH-DA fluorescence dye. It was noticed that APC feeding reduces the ROS-associated relative fluorescence intensity from 24.409 ± 2.9 (PQ) to 18.99 ± 3.2 (PQ + APC) under normal condition (Fig. 4b). This indicates that APC could protect C. elegans under oxidative stress.
Fig. 4.
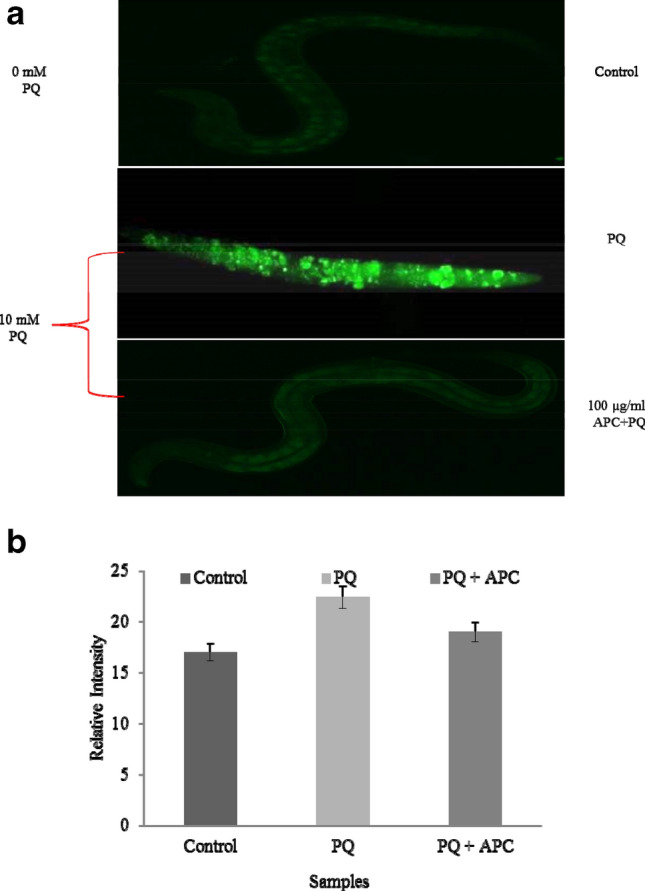
APC reduces intracellular production of reactive oxygen species in N2 worm under oxidative stress. a Indicative fluorescence microscopic images of DCFH-DA stained control and APC (100 μg/ml) treated L4 worms of N2 types were analyzed under normal and Paraquat (20 mM) induced oxidative stress condition. b The relative fluorescence intensity in APC-treated and -untreated worms quantified by Image J software
Extension of lifespan by APC is independent of SKN-1 pathway and partially dependent on DAF-16
To study the consequence of APC on stress response factor, we observed the existence of DAF-16 forkhead transcription factor in the cytoplasm of TJ356, which was translocated to the nucleus upon APC treatment (Fig. 5a). The intensity of cytoplasmic DAF-16::GFP obtained was reduced in the APC-treated worms (Fig. 5b). Thus, confirmatory logical deductions can be made that upon APC treatment, DAF-16::GFP is translocated to the nucleus, which resulted in a significant decrease in the DAF-16 levels in the cytoplasm. The relative fluorescence intensity obtained without stress in TJ356 DAF-16::GFP worms showed reduction in fluorescence from 117.065 ± 1.56 (without stress + untreated) to 77.412 ± 4.91 (without stress + treated), whereas relative fluorescence intensity obtained with stress (temperature) on TJ356 DAF-16::GFP worms supplemented with and without APC showed reduction in fluorescence from 74 0.851 ± 3.4 (stress + untreated) to 62.228 ± 3.89 (stress + treated) as shown in Fig. 5b. DAF-16::GFP transgenics were found to show less GFP fluorescence intensity in cytoplasm upon APC treatment. Thus, it might be dependent on the DAF-16 forkhead transcription factor directly to enhance the longevity of worms. The same was also examined by observing the effect of APC treatment on the lifespan of the DAF-16 mutant. APC treatment showed a decrease in the lifespan of DAF-16 mutant worms with the mean value 12 ± 0.294 (log-rank test, p < 0.05) days compared to untreated control worms having 15 ± 0.322 days (log-rank test, p < 0.05) (Fig. 5a–ii). The above-mentioned result supported the DAF-16-dependent increase in the lifespan of worms.
Fig. 5.
Effect of APC on lifespan is dependent of DAF-16 and independent of SKN-1. a Representative image showing nuclear localization of DAF-16::GFP. APC-treated and -untreated worms were exposed to heat shock at 35 °C for 15 min to induce DAF-16::GFP nuclear localization in TJ356 worms. b Quantification of relative intensity of GFP fluorescence measured by Image J software. c Effect of APC-supplemented RNAi (DAF-16)-fed worms (12 ± 0.294 days; log-rank test, p < 0.05) showed insignificant difference with untreated worms (16 ± 0.029 days; log-rank test, p < 0.05) (RNAi DAF-16), indicating APC effects are DAF-16 dependent. d Effect of APC treatment on lifespan of SKN-1 (RNAi). APC exposure increased mean lifespan of SKN-1 (RNAi) mutant (16 ± 0.015 days; log-rank test, p < 0.05) as compared to untreated (15 ± 0.322 days; log-rank test, p < 0.05) indication APC effects are independent of SKN-1
We also checked the effect of APC on the oxidative stress resistance gene skn-1 expression in RNAi mutant/knockout worm. APC treatment showed equal increase in the lifespan of SKN-1 mutant worms with the mean value of 15 ± 0.322 days compared to untreated control worms having 15 ± 0.015 days (log-rank test, p < 0.05) as shown in Fig. 5b
The results obtained signify that DAF-16 is responsible for APC-mediated lifespan extension in C. elegans irrespective of stress response factor SKN-1.
APC ameliorates protein aggregation (Aβ reduction) on CL4176
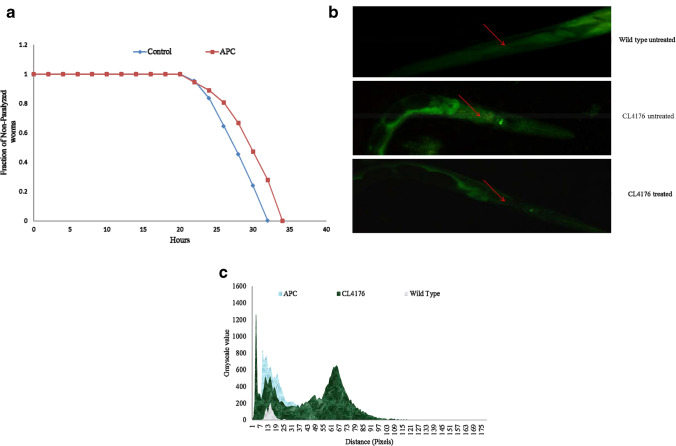
Since the occurrence and progression of Alzheimer’s disease and Aβ toxicity are mediated by oxidative stress (Chaubey et al. 2019), we used APC as an antioxidant. We analyzed the effect of APC on Abeta aggregation using C. elegans. Synchronized eggs were seeded on NGM plate with (100 µg/ml) and without APC and incubated for 36 h at 16 °C, followed by temperature increase of L4 worms to 25 °C to induce Aβ expression (paralysis phenotype). A noteworthy delay in paralysis phenotype was observed in the APC-treated worms (34 h ± 0.90 h) as compared to the untreated control (32 h ± 0.45 h) (Fig. 6a). Significant delay (~ 6%) in paralysis was observed in APC-treated CL4176 as compared to untreated worms. From the above result, we conclude that APC might be effective against Aβ-induced paralysis. Furthermore, Thioflavin-T assay was used to determine whether APC is effective in minimizing the production of Aβ in transgenic CL4176 (Alzheimer model). The APC-untreated transgenic worms show high fluorescence intensity on their body wall as compared to APC-untreated transgenic worms as shown in Fig. 6b, c. Our findings suggest that APC could be effective in the reduction of Aβ-mediated toxicity and can be used as a putative therapeutic agent for Alzheimer’s as well as other neurodegenerative diseases.
Fig. 6.
APC-treated CL4176 worms are effective in preventing protein aggregation and Aβ reduction. a Effect of APC on the reduction of Aβ(1–42)-associated paralysis phenotype in CL4176 animals. APC-treated worms showed delay in paralysis as compared to the control. b Representative image of APC-treated and -untreated CL4176 worms were visualized by using Thioflavin-T staining. APC-treated worms (CL4176) showed reduced intensity compared to the control (untreated CL4176) and higher than that of wild type (N2 Bristol). c Image J software quantification showed the fluorescence intensity in the following order: CL4176 > APC > wild type
Discussion
Exponentially growing cyanobacterial cell mass (28 days old) was taken for the extraction and purification of APC. A freeze–thaw cycle of cyanobacterial cell breached the cell wall and enabled whole intracellular protein extraction (Parmar et al. 2010). Further, crude lysate was subjected to ammonium sulfate precipitation in the presence of Triton X-100 followed by ion exchange chromatography. Pure APC obtained after ion exchange chromatography was further subjected to its characterization (Sonani et al. 2014b). The purity of the protein was affirmed by SDS-PAGE as well as UV–visible spectrum analysis. SDS-PAGE corresponds to two prominent bands, α and β subunits of purified APC. Moreover, UV–Vis absorption maxima of purified APC were found at 653 nm along with a shoulder peak at 620 nm, as also reported previously (Parmar et al. 2010; Su et al. 2010). UV–visible spectrum of APC over the protein specific peak at 280 nm indicated the absence of impurities of other cellular proteins. Even though APC proportion is very low as compared to PE and PC, we successfully purified and characterized dimeric protein from the Phormidium sp. A09DM.
The “free radical theory of aging” stated that the compound having antioxidant activity might have anti-aging effects (Kazuko et al. 1992; Blagosklonny 2008). Accordingly, we checked the effects of APC on the lifespan, health span, stress assay and RNAi using eukaryotic model organism C. elegans. Our results showed the efficacy of APC, which increases lifespan and improves health span, as indicated by the rate of pharyngeal pumping or feeding rate in C. elegans, which are appraised as the characteristics of aging (Huang et al. 2004). The results of APC supplementation on the characteristics of aging exhibit this protein as a potent biomolecule in the current research of drug discovery. Aging in an organism is naturally conveyed by overabundance of multifaceted problems and related pathologies (Pandey et al. 2019). The antioxidant virtue of APC reported in previous studies showed that it can be used as an anti-aging compound (Sonani et al. 2014b). The improved health span and lifespan prolonging action of APC is found to be associated with the antioxidant virtue.
The supplementation of APC counterbalances diverse ROS species and eventually detoxifies the effect of thermal stress by endogenous antioxidant defense system in C. elegans (Kampkötter et al. 2007). The above results indicate that APC reduces free radicals and their aggregation induced by oxidative stress in C. elegans (Sonani et al. 2014a; Singh et al. 2016). Similarly, the exogenous exposure of oxidative stress in the form of PQ increases the intracellular ROS level in C. elegans as probed by DCFH-DA dye (Takanashi et al. 1997; Drechsel and Patel 2009). Thus, APC shows quenching and ameliorates the protective effect against oxidative stress in worms.
It is well established that longevity of worms is routed through stress response factor DAF-16 (ortholog of mammalian FOXO) and SKN-1 (ortholog of mammalian Nrf-2). RNAi mutant/knockout DAF-16 worms clearly indicate the involvement of DAF-16 in longevity, whereas SKN-1 does not show any involvement in the longevity (Robida-Stubbs et al. 2012; Sonani et al. 2014a). Furthermore, we observed significant nuclear localization of DAF-16 in TJ356 worms, which clearly confirmed the DAF-16-dependent APC-mediated longevity. The underlying mechanism beneath activation and nuclear localization of DAF-16 is unknown and further detailed study is needed. We therefore concluded that expansion of lifespan in C. elegans is dependent on DAF-16 and independent of SKN-1.
A characteristic of Alzheimer’s disease is the aggregation of amyloid-β peptides (Aβ) in the human brains (Chaubey et al. 2019). Over-expression of Aβ peptides have been observed to generate neurotoxic consequences in diverse cell lines and animal models (Smith and Luo 2003; Cohen et al. 2006). APC on age-related diseases such as Alzheimer was studied, using CL4176 strain which shows the temperature-sensitive paralysis. The APC supplementation delayed the paralysis, as well as Thioflavin-T assay showed that APC significantly abolished the Aβ aggregation in CL4176 transgenic worms. Thus, our results demonstrate APC as a potent therapeutic molecule against aging and age-associated disease in humans.
Conclusion
The present results showed the novel role of APC in increasing the lifespan and health span of C. elegans. It was observed that APC not only increases the lifespan, but also improved the physiological aging characteristics in worms such as improved feeding rate. The APC was also found to increase the stress tolerance and plays an important role in delaying the Aβ-mediated paralysis in C. elegans (Alzheimer’s disease model). The involvement of DAF-16 and SKN-1 upon APC treatment was also investigated. We found APC-mediated longevity is DAF-16 dependent and SKN-1 independent. Overall, our results clearly indicate that APC may be used as a potential anti-aging molecule, which may be exploited in conducting the anti-aging research and development of various anti-aging drugs by different pharmaceutical industries.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
NKS acknowledges Science and Engineering Research Board (SERB), Department of Science and Technology (DST), Ministry of Science and Technology, Government of India, for Young Scientist fellowship under the scheme of startup research grant (SB/YS/LS-290/2013). MGC also acknowledges SERB for Senior Research Fellowship under the scheme of startup research grant (SB/YS/LS-290/2013).
Abbreviations
- C. elegans
Caenorhabditis elegans
- PE
Phycoerythrin
- PC
Phycocyanin
- NGM
Nematode growth medium agar plate
- CGC
Caenorhabditis Genetic Center
- ROS
Reactive oxygen species
- Aβ
Amyloid-beta
- PBP
Phycobiliprotein
- SDS
Sodium dodecyl sulfate
- PAGE
Polyacrylamide gel electrophoresis
- L4
Larval stage 4
- H2O2
Hydrogen peroxide
- DCFH-DA
Dichloro-dihydro-fluorescein-diacetate
- PQ
Paraquat
Author contributions
Conceptualization: MGC, SNP; DM, RPR, NKS. Methodology: MGC, SNP; DM, NKS, RPR. Formal analysis and investigation: MGC, SNP; DM, NKS, RPR. Writing—original draft preparation: MGC. Writing—review and editing: DM, NKS, RPR. Resources: Shri. A. N. Patel P. G. Institute of Science and Research, Anand, Post Graduate Department of Bioscience, Sardar Patel University, Anand. Supervision: NKS, RPR.
Funding
The research was supported by the Department of Science and Technology (DST), Ministry of Science and Technology, Government of India under the scheme of startup research grant (SB/YS/LS-290/2013)
Compliance with ethical standards
Conflict of interest
Mukesh Ghanshyam Chaubey, Stuti Nareshkumar Patel, Rajesh Prasad Rastogi, Datta Madamwar and Niraj kumar Singh declare that they have no conflict of interest.
Ethics approval
No human or animal rights are applicable to this study.
Contributor Information
Rajesh Prasad Rastogi, Email: raj_rastogi@rediffmail.com.
Niraj Kumar Singh, Email: nirajbiotech@gmail.com.
References
- Andriollo-Sanchez M, Hininger-Favier I, Meunier N, et al. Age-related oxidative stress and antioxidant parameters in middle-aged and older European subjects: the ZENITH study. Eur J Clin Nutr. 2005;59:S58. doi: 10.1038/sj.ejcn.1602300. [DOI] [PubMed] [Google Scholar]
- Bennett A, Bogorad L. Complementary chromatic adaptation in a filamentous blue-green alga. J Cell Biol. 1973;58:419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagosklonny MV. Aging: ROS or TOR. Cell Cycle. 2008;7:3344–3354. doi: 10.4161/cc.7.21.6965. [DOI] [PubMed] [Google Scholar]
- Cai W-J, Huang J-H, Zhang S-Q, et al. Icariin and its derivative icariside II extend healthspan via insulin/IGF-1 pathway in C. elegans. PLoS ONE. 2011;6:e28835. doi: 10.1371/journal.pone.0028835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaubey MG, Patel SNK, Rastogi RP, et al. Therapeutic potential of cyanobacterial pigment protein phycoerythrin: in silico and in vitro study of BACE1 interaction and in vivo Aβ reduction. Int J Biol Macromol. 2019 doi: 10.1016/j.ijbiomac.2019.05.006. [DOI] [PubMed] [Google Scholar]
- Cohen E, Bieschke J, Perciavalle RM, et al. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- Drechsel DA, Patel M. Paraquat-induced production of reactive oxygen species in brain mitochondria. Methods Enzymol. 2009;456:381–393. doi: 10.1016/S0076-6879(08)04421-2. [DOI] [PubMed] [Google Scholar]
- Grossman AR, Bhaya D, Apt KE, Kehoe DM. Light-harvesting complexes in oxygenic photosynthesis: diversity, control, and evolution. Annu Rev Genet. 1995;29:231–288. doi: 10.1146/annurev.ge.29.120195.001311. [DOI] [PubMed] [Google Scholar]
- Harrington LA, Harley CB. Effect of vitamin E on lifespan and reproduction in Caenorhabditis elegans. Mech Ageing Dev. 1988;43:71–78. doi: 10.1016/0047-6374(88)90098-X. [DOI] [PubMed] [Google Scholar]
- Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci USA. 2004;101:8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Senoo-Matsuda N, Miyake K, et al. Coenzyme Q10 can prolong C. elegans lifespan by lowering oxidative stress. Mech Ageing Dev. 2004;125:41–46. doi: 10.1016/j.mad.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Iwasa H, Yu S, Xue J, Driscoll M. Novel EGF pathway regulators modulate C. elegans healthspan and lifespan via EGF receptor, PLC-γ, and IP3R activation. Aging Cell. 2010;9:490–505. doi: 10.1111/j.1474-9726.2010.00575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Ahringer J. Genome-wide RNAi screening in Caenorhabditis elegans. Methods. 2003;30:313–321. doi: 10.1016/S1046-2023(03)00050-1. [DOI] [PubMed] [Google Scholar]
- Kampkötter A, Pielarski T, Rohrig R, et al. The Ginkgo biloba extract EGb761 reduces stress sensitivity, ROS accumulation and expression of catalase and glutathione S-transferase 4 in Caenorhabditis elegans. Pharmacol Res. 2007;55:139–147. doi: 10.1016/j.phrs.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Kazuko S, Kuniko F, Keiko Y, Takashi N. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. J Agric Food Chem. 1992;40:945–948. doi: 10.1021/jf00018a005. [DOI] [Google Scholar]
- Link CD, Taft A, Kapulkin V, et al. Gene expression analysis in a transgenic Caenorhabditis elegans Alzheimer’s disease model. Neurobiol Aging. 2003;24:397–413. doi: 10.1016/S0197-4580(02)00224-5. [DOI] [PubMed] [Google Scholar]
- Morton EA, Lamitina T. Caenorhabditis elegans HSF-1 is an essential nuclear protein that forms stress granule-like structures following heat shock. Aging Cell. 2013;12:112–120. doi: 10.1111/acel.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey T, Sammi SR, Nooreen Z, et al. Anti-ageing and anti-Parkinsonian effects of natural flavonol, tambulin from Zanthoxyllum aramatum promotes longevity in Caenorhabditis elegans. Exp Gerontol. 2019;120:50–61. doi: 10.1016/j.exger.2019.02.016. [DOI] [PubMed] [Google Scholar]
- Parmar A, Singh NK, Madamwar D. Allophycocyanin from a local isolate Geitlerinema sp. A28DM (Cyanobacteria): a simple and efficient purification process 1. J Phycol. 2010;46:285–289. doi: 10.1111/j.1529-8817.2009.00798.x. [DOI] [Google Scholar]
- Patel SN, Sonani RR, Jakharia K, et al. Antioxidant activity and associated structural attributes of Halomicronema phycoerythrin. Int J Biol Macromol. 2018;111:359–369. doi: 10.1016/j.ijbiomac.2017.12.170. [DOI] [PubMed] [Google Scholar]
- Rastogi RP, Singh SP, Häder D-P, Sinha RP. Detection of reactive oxygen species (ROS) by the oxidant-sensing probe 2′, 7′-dichlorodihydrofluorescein diacetate in the cyanobacterium Anabaena variabilis PCC 7937. Biochem Biophys Res Commun. 2010;397:603–607. doi: 10.1016/j.bbrc.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Robida-Stubbs S, Glover-Cutter K, Lamming DW, et al. TOR signaling and rapamycin influence longevity by regulating SKN-1/Nrf and DAF-16/FoxO. Cell Metab. 2012;15:713–724. doi: 10.1016/j.cmet.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NK, Sonani RR, Rastogi RP, Madamwar D. The phycobilisomes: an early requisite for efficient photosynthesis in cyanobacteria. EXCLI J. 2015;14:268–289. doi: 10.17179/excli2014-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NK, Sonani RR, Awasthi A, et al. Phycocyanin moderates aging and proteotoxicity in Caenorhabditis elegans. J Appl Phycol. 2016;28:2407–2417. doi: 10.1007/s10811-015-0772-5. [DOI] [Google Scholar]
- Smith JV, Luo Y. Elevation of oxidative free radicals in Alzheimer’s disease models can be attenuated by Ginkgo biloba extract EGb 761. J Alzheimers Dis. 2003;5:287–300. doi: 10.3233/JAD-2003-5404. [DOI] [PubMed] [Google Scholar]
- Sonani RR, Rastogi RP, Madamwar D. Antioxidant potential of Phycobiliproteins: role in anti-aging research. Biochem Anal Biochem. 2015;4:2161–1009. doi: 10.4172/2161-1009.1000172. [DOI] [Google Scholar]
- Sonani RR, Singh NK, Awasthi A, et al. Phycoerythrin extends life span and health span of Caenorhabditis elegans. Age Dordr Neth. 2014;36:9717. doi: 10.1007/s11357-014-9717-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonani RR, Singh NK, Kumar J, et al. Concurrent purification and antioxidant activity of phycobiliproteins from Lyngbya sp. A09DM: an antioxidant and anti-aging potential of phycoerythrin in Caenorhabditis elegans. Process Biochem. 2014;49:1757–1766. doi: 10.1016/j.procbio.2014.06.022. [DOI] [Google Scholar]
- Sonani RR, Gupta GD, Madamwar D, Kumar V. Crystal structure of Allophycocyanin from marine Cyanobacterium Phormidium sp. A09DM. PLoS ONE. 2015;10:e0124580. doi: 10.1371/journal.pone.0124580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonani RR, Rastogi RP, Patel R, Madamwar D. Recent advances in production, purification and applications of phycobiliproteins. World J Biol Chem. 2016;7:100–109. doi: 10.4331/wjbc.v7.i1.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su H-N, Xie B-B, Chen X-L, et al. Efficient separation and purification of allophycocyanin from Spirulina (Arthrospira) platensis. J Appl Phycol. 2010;22:65–70. doi: 10.1007/s10811-009-9427-8. [DOI] [Google Scholar]
- Takanashi T, Ogura Y, Taguchi H, et al. Fluorophotometric quantitation of oxidative stress in the retina in vivo. Invest Ophthalmol Vis Sci. 1997;38:2721–2728. [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Uno M, Nishida E. Lifespan-regulating genes in C. elegans. NPJ Aging Mech Dis. 2016;2:16010. doi: 10.1038/npjamd.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan Q-L, Fu X, Dai W, et al. Hypotaurine promotes longevity and stress tolerance via the stress response factor DAF-16/FOXO and SKN-1/NRF2 in Caenorhabditis elegans. R Soc Chem. 2019;10:347–357. doi: 10.1039/c9fo02000d. [DOI] [PubMed] [Google Scholar]
- Wan Q-L, Fu X, Dai W, et al. Uric acid induces stress resistance and extends the life span through activating the stress response factor DAF-16/FOXO and SKN-1/NRF2. Aging. 2020;12:2840–2856. doi: 10.18632/aging.102781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu P, Wu Y, Wang G, et al. Purification and bioactivities of phycocyanin. Crit Rev Food Sci Nutr. 2017;57:3840–3849. doi: 10.1080/10408398.2016.1167668. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.