Abstract
Mixed Plasmodium malaria infections can lead to severe malaria. This systematic review and meta-analysis aimed to explore the prevalence of severe mixed Plasmodium malaria infection and to compare it with the prevalence of severe P. falciparum malaria mono-infection across the included studies. Original English-language research articles from PubMed, Scopus, and ISI Web of Science were identified and screened. Articles reporting the number of mixed infections and the number of severe mixed infections were used to determine the main outcome of this study, while the number of P. falciparum infections and the number of severe P. falciparum infections were used to determine the secondary outcome of this study. For the main outcome, the pooled prevalence and 95% confidence interval (CI) of severe mixed infections was analysed using STATA software version 15.0 (Stata Corp, College Station, TX, USA). For the secondary outcome, the rate of severe mixed infections compared to severe P. falciparum infections was analysed using the meta-analysis approach, and summary odds ratios (ORs) and 95% CIs were calculated. Random-effects models were used to produce the summary ORs. The Mantel–Haenszel method and calculated I2 were also reported to test whether there was heterogeneity among the included studies. Publication bias was also assessed using funnel plots. The meta-analysis of secondary outcomes was conducted using Review Manager 5.3 software (Cochrane Community). A total of 894,561 malaria patients were reported in all 16 included studies. Overall, a pooled analysis showed that 9% (2,006/35,768, 95% CI 7.0–12.0%) of patients with mixed Plasmodium infection had severe mixed infection. A meta-analysis of 14 studies demonstrated that patients with mixed Plasmodium infection (1,999/35,755) and patients with P. falciparum malaria (9,249/294,397) had an equal risk of developing severe malaria (OR 0.93, 95% CI 0.59–1.44). Both mixed infection and P. falciparum mono-infection showed a similar trend of complications in which severe anaemia, pulmonary failure, and renal impairment were the three most common complications found. However, patients with mixed infection had a higher proportion of severe anaemia and pulmonary complications than those with P. falciparum infection. Moreover, patients with mixed infection had a higher proportion of multiple organ failure than those with P. falciparum mono-infection. Mixed Plasmodium spp. infections were common but often unrecognized or underestimated, leading to severe complications among these malaria patients. Therefore, in routine clinical laboratories, using an accurate combination of diagnostic procedures to identify suspected patients with mixed infections is crucial for therapeutic decisions, prompt treatment, and effective patient management.
Subject terms: Diseases, Medical research, Signs and symptoms
Introduction
Human malaria is caused by five species of Plasmodium spp. that include P. falciparum, P. vivax, P. malariae, P. ovale, and P. knowlesi1. Molecular methods have demonstrated the existence of two distinct species of P. ovale: P. ovale curtisi and P. ovale wallikeri2. P. knowlesi naturally occurs in macaques inhabiting forested areas of Southeast Asia and is the fifth species of Plasmodium causing human malaria1,3. In some areas where more than one species of Plasmodium is endemic, mixed Plasmodium spp. infections can frequently occur4. Mixed Plasmodium spp. infections are often unrecognized or underestimated, as a low proportion (2%) is detected by microscopy5,6. This might be due to observer error, technical difficulties, and low parasite densities4. If the mixed infection is misdiagnosed as a P. vivax mono-infection, treatment of P. vivax will increase the risk of P. falciparum parasitaemia, leading to anti-malarial drug resistance and, eventually, development of severe P. falciparum malaria7. Therefore, in routine clinical laboratories, the use of the most accurate diagnostic procedures to identify Plasmodium species in cases of suspected mixed malaria infection is crucial for therapeutic decisions and management among those patients7,8. A research study indicated that mixed P. falciparum and P. vivax infection led to an increase in the disease severity among children9,10. Another study demonstrated that mixed infection with P. falciparum and P. vivax led to suppression of the severity of P. falciparum infection11. Although mixed P. falciparum and P. vivax malaria is common, systematic review and meta-analysis of severe mixed infection has been limited. No recent study has demonstrated the prevalence and differences between mixed Plasmodium infection and P. falciparum malaria infection. This is very important for physicians to plan therapeutic options and determine the prognostic signs of severity during drug treatment. Therefore, this systematic review and meta-analysis aimed to explore the prevalence of severe Plasmodium mixed infection and to compare it with that of severe P. falciparum malaria infection across the included studies.
Methods
Search strategy
The protocol for this systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (PRISMA Checklist S1). The search strategy started by searching the key terms “(Severe OR complicated OR Complication) AND (Plasmodium OR Malaria) AND (“Mixed infection” OR “Mix infection”)” indexed in PubMed, Scopus, and the ISI Web of Science. The articles published through 25 Jan 2020 were retrieved and reviewed by two independent reviewers. Any discrepancy was judged by the third reviewer (FRM).
Definition of severe malaria
The major complications of severe mixed malaria were considered to be the same as those defined for P. falciparum by the World Health Organization (WHO) and included respiratory distress or acidosis (a base deficit of > 8 meq/L, a plasma bicarbonate of < 15 mM or venous plasma lactate > 5 mM), pulmonary oedema (radiologically confirmed, or oxygen saturation < 92% on room air with a respiratory rate > 30/min), impaired consciousness (a Glasgow Coma Score < 11 in adults or a Blantyre coma score < 3 in children), convulsions (more than two episodes within 24 h), prostration (generalized weakness so that the person is unable to sit, stand or walk without assistance), hypotension/shock (systolic blood pressure < 70 mmHg in children or < 80 mmHg in adults), jaundice [plasma bilirubin > 50 µM/L (3 mg/dL)], severe anaemia (A haemoglobin concentration < 5 g/dL), bleeding/Disseminated Intravascular Coagulation (DIC) (recurrent or prolonged bleeding from the nose, gums, or venepuncture sites; haematemesis or melaena), hyperparasitemia (P. falciparum parasitaemia > 10%), and hypoglycaemia [blood or plasma glucose < 2.2 mM (< 40 mg/dL)]12. Cerebral malaria, one criterion of severe P. falciparum malaria in the former version of the WHO definition, was assigned to the group “impaired consciousness” and described as “impaired consciousness/cerebral malaria” for further analysis and demonstration in the results section.
Inclusion and exclusion criteria
Original research articles published in the English language were included in the current analysis if they met the following criteria: (1) malaria positivity confirmed by any combination of rapid diagnostic tests (RDTs), microscopy, or polymerase chain reaction (PCR); (2) enrolled both uncomplicated and complicated malaria; (3) the numbers of mixed infections and severe mixed infections were reported, and (4) all complications in the patients with severe mixed infections were reported. Case reports, animal studies, experimental studies, clinical trials, book or book chapters, letters to the editor, editorials, reviews or systematic reviews, conference papers, short surveys, and studies of co-infection of Plasmodium with other agents were excluded from the present study.
Data extraction
For all articles included in the analysis, the following information was extracted: name of the authors, year of publication, country of the participants, duration of the study, the total number of malaria patients, number of severe mixed infections, number of mixed infections, number of severe P. falciparum infections, number of P. falciparum infections, complications of severe mixed infections, and complications of P. falciparum infections. The number of mixed infections and the number of severe mixed infections was used to determine the main outcome of this study, while the number of P. falciparum infections and the number of severe P. falciparum infections were used to determine the secondary outcome of this study.
Statistical analysis
For the main outcome, the pooled prevalence and 95% confidence interval (CI) of severe mixed infection was analysed using STATA software version 15.0 (Stata Corp, College Station, TX, USA). For the secondary outcome, the rate of severe mixed infection compared to severe P. falciparum infection was analysed using the meta-analysis approach and summary odds ratios (ORs) and 95% CI were calculated. Random-effects models were used to produce summary ORs as described previously13. The Mantel–Haenszel method and the calculated I2 were also reported to determine whether there was heterogeneity among the included studies. Publication bias was also assessed using funnel plots and Egger's test as described elsewhere14. The meta-analysis of the secondary outcomes was conducted using Review Manager 5.3 software (Cochrane Community).
Results
Characteristics of the included studies
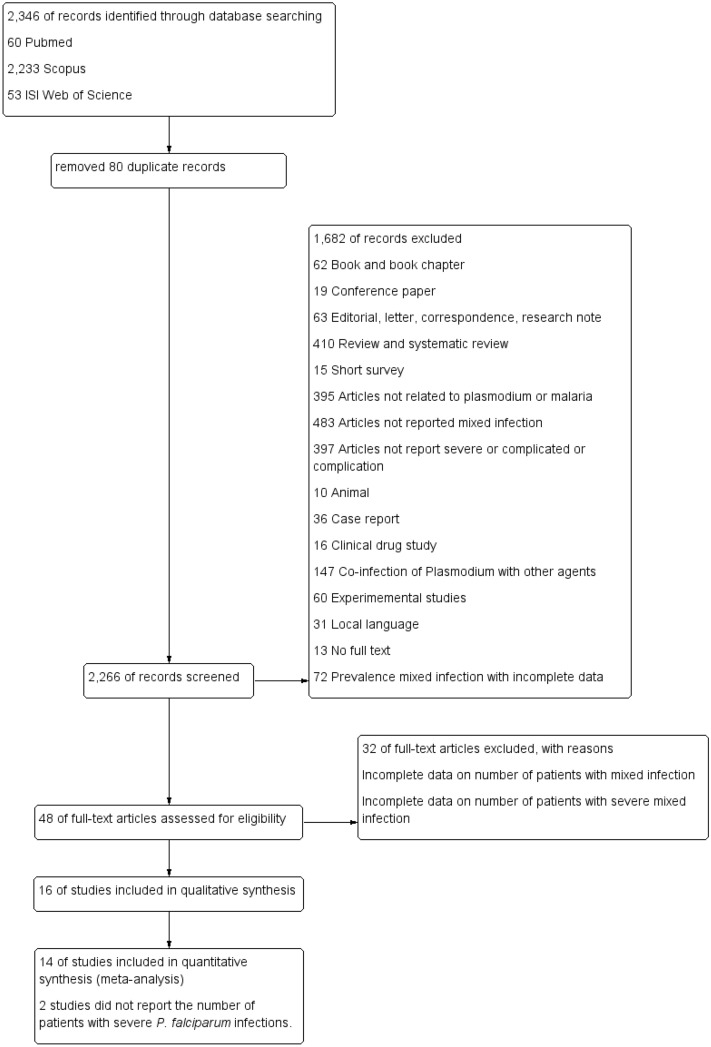
The flow diagram of this study according to the PRISMA guidelines is shown in Fig. 1. All 2,346 articles were retrieved from three research databases, including 60 from PubMed, 2,233 from Scopus, and 53 from ISI Web of Science. After 80 duplicated articles were removed, 2,266 articles were processed through title and abstract screening. After title and abstract screening, 48 full-text articles were extensively reviewed, resulting in 16 studies that passed the inclusion and exclusion criteria review. A total of 894,561 malaria patients were reported in all 16 included studies15–30. Most of the included studies (56.3%, 9/16) were descriptive studies or cross-sectional observational designs15–17,20,23,24,26,27,30. Six studies (37.5%, 6/16) were prospective studies or prospective cohort studies18,19,21,22,28,29. One study was a retrospective observational study25. The majority of patients in all included studies were infected with P. vivax (62.5%, 558,705/894,561), followed by P. falciparum (32.9%, 294,397/894,561). Almost all of the included studies reported that P. falciparum/P. vivax mixed infection was frequently found among those with mixed Plasmodium spp. infection. One study reported patients with P. vivax/P. malariae mixed infection and other types of mixed infection24. The majority of malaria patients were identified from the SIVIGILA study conducted in Colombia (547,542 participants)16. Half of the studies were conducted in India (9/16, 56.3%), followed by Colombia (3/16, 18.8%). Half of the included studies (8/16, 50%) used combined microscopy techniques and other techniques to confirm the parasite species. Four studies used PCR to confirm the Plasmodium parasite species (Table 1). The quality of all included studies was shown in Table 2.
Figure 1.
Flow diagram.
Table 1.
Characteristics of the included studies.
| No. | Author | Study area (years of the survey) | Study design | Method for malaria detection | Plasmodium sp. | Severe Pf infection (%) | Total malaria | Mixed infection of Plasmodium spp. | Number of mixed infection (%) | Severe mixed infection (%) | Complications of mixed infections |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Chaparro et al.15 |
Colombia Data from SIVIGILA 2010 |
Descriptive study |
Microscopy RDT |
P. falciparum 32,777 P. vivax 82,856 P.malariae 47 |
282 (0.86) | 117,108 | Pf/Pv | 1,428 (1.22) | 32 (2.24) |
Cerebral malaria = 6 Renal impairment = 10 Jaundice = 14 Pulmonary = 1 Unreported = 1 |
| 2 | Chaparro‑Narváez et al.16 |
Colombia (2007–2013) Data from SIVIGILA |
Descriptive study |
Microscopy RDT |
P. falciparum 150,026 P. vivax 390,944 |
1,274 (0.85) | 547,542 | Pf/Pv | 6,570 (1.2) | 153 (2.32) |
Jaundice = 11 Convulsions = 15 Cerebral malaria = 24 Severe anemia = 20 Bleeding/DIC = 10 Shock = 6 Pulmonary = 15 |
| 3 | Dayanand et al.17 | India (2013–2016) | Descriptive study | Microscopy |
P. falciparum 2,456 P. vivax 15,334 |
10 (0.41) | 18,936 | Pf/Pv | 1,146 (6.05) | 7 (0.61) |
Impaired consciousness = 6 Renal impairment = 5 Pulmonary = 6 Hemoglobinuria = 2 Shock = 2 Multi-organ dysfunction = 6 |
| 4 | Devineni et al.18 | India (2014–2015) | Prospective study |
Microscopy RDT |
P. falciparum 62 P. vivax 114 |
NA | 180 | Pf/Pv | 4 (2.22) | 4 (100) |
Renal impairment = 4 Pulmonary = 4 Bleeding/DIC = 2 Impaired consciousness = 4 Hyperparasitemia = 4 Hypoglycemia = 2 Death = 4 |
| 5 | Genton et al.19 |
Papua New Guinea (1997–2004) |
Prospective cohort study | Microscopy |
P. falciparum 6,886 P. vivax 1,946 P.malariae 328 P.ovale 27 |
261 (3.79) | 9,537 | Pf/Pv | 350 (3.67) | 24 (6.86) |
Pulmonary = 14 Impaired consciousness = 2 Severe anemia = 7 |
| 6 | Hermansyah et al.20 | Indonesia (2011–2013) | Descriptive study |
Microscopy RDT PCR |
P. falciparum 8 P. vivax 12 (severe only) |
NA | 29 | Pf/Pv | 9 (NA) | 3 (33.3) |
Cerebral malaria = 2 Convulsion = 1 |
| 7 | Kochar et al.21 | India (2007–2008) | Prospective study |
Microscopy RDT PCR |
P. falciparum 555 P. vivax 485 |
274 (44.5) | 1,123 | Pf/Pv | 83 (7.4) | 44 (53) |
Cerebral malaria = 5 Severe anemia = 17 Jaundice = 25 Renal impairment = 6 |
| 8 | Kochar et al.22 | India (2007–2008) | Prospective study |
Microscopy RDT PCR |
P. falciparum 185 P. vivax 103 |
79 (42.7) | 303 | Pf/Pv | 15 (4.95) | 2 (13.3) |
Severe anemia = 1 Multiorgan Dysfunction = 1 |
| 9 | Laman et al.23 |
Papua New Guinea |
Descriptive observational study | Microscopy |
P. falciparum 78 P. vivax 3 |
58 (74.3) | 87 | Pf/Pv | 6 (6.9) | 4 (66.7) |
Cerebral malaria = 1 Convulsion = 1 Severe anemia = 2 |
| 10 | Langford et al.24 | Indonesia (2004–2013) | Descriptive study | Microscopy |
P. falciparum 100,078 P. vivax 65,306 P. ovale 120 P. malariae 5,097 |
6,361 (6.36) | 196,380 |
Pf/Pm 148 Pv/Pm 93 (No data on other mixed species) |
25,779 (13.1) | 1,666 (6.46) |
Renal impairment = 84 Pulmonary = 343 Severe anemia = 1,239 |
| 11 | Limaye et al.25 | India (2009) | Retrospective observational study |
Microscopy RDT |
P. falciparum 206 P. vivax 338 |
64 (31) | 680 | Pf/Pv | 136 (20) | 14 (10.3) |
Cerebral malaria = 22 Severe anemia = 16 Renal impairment = 14 Pulmonary = 12 Jaundice = 54 Shock = 1 Death = 14 |
| 12 | Medina-Morales et al.26 | Colombia (2013) | Descriptive cross-sectional study | Microscopy |
P. falciparum 17 P. vivax 313 |
3 (17.6) | 349 | Pf/Pv | 19 (5.4) | 3 (15.8) |
Pulmonary = 2 Severe anemia = 1 |
| 13 | Mittal et al.27 | India (2011) | Descriptive study |
Microscopy RDT |
P. falciparum 66 P. vivax 128 |
52 (78.8) | 198 | Pf/Pv | 4 (2) | 4 (100) |
Cerebral malaria = 1 Severe anemia = 1 More than 1 complications = 2 |
| 14 | Mohapatra et al.28 | India (2007–2009) | Prospective study |
Microscopy RDT |
P. falciparum 770 | 440 (57.1) | 888 | Pf/Pv | 118 (13.3) | 21 (17.8) |
Cerebral malaria = 4 Jaundice = 2 Severe anemia = 8 More than 1 complications = 7 |
| 15 | Nayak et al.29 | India (2010–2011) | Prospective study |
Microscopy RDT PCR |
P. falciparum 147 P. vivax 459 |
68 (46.3) | 642 | Pf/Pv | 36 (5.6) | 12 (33.3) |
Severe anemia = 3 Pulmonary = 6 Cerebral malaria = 1 Hypoglycemia = 1 Renal impairment = 1 Bleeding = 5 More than 1 complications = 3 |
| 16 | Punnath et al.30 | India (2013–2015) | Descriptive cross-sectional study | Microscopy |
P. falciparum 150 P. vivax 364 |
23 (15.3) | 579 | Pf/Pv | 65 (11.2) | 13 (20) |
Shock = 3 Pulmonary = 2 Renal impairment = 1 Jaundice = 4 Severe anemia = 5 Cerebral malaria = 1 More than 1 complications = 9 |
| Total |
India = 9/16 (56.3%) Colombia = 3/16 (18.8%) Papua New Guinea = 2/16 (12.5%) Indonesia = 2/16 (12.5%) |
Descriptive study = 9/16 (56.3%) Prospective study = 6/16 (37.5%) Retrospective observational study = 1/16 (6.3%) |
Microscopy alone = 6/16 (37.5%) Microscopy with other technique = 8/16 (50%) |
P. falciparum 294,397 (32.9%) P. vivax 558,705 (62.5%) P. malariae 5,472 (0.6%) P. ovale 147 (0.02%) |
9,222 (3.13) | 894,561 | 35,768 (4) | 2,006 (6.7) |
Cerebral malaria/impaired consciousness = 79 (3.94%) Renal impairment = 125 (6.23%) Jaundice = 110 (5.48%) Pulmonary = 420 (20.9%) Convulsions = 17 (0.85%) Severe anemia = 1,320 (65.8%) Bleeding/DIC = 17 (0.85%) Shock = 12 (0.6%) Hyperparasitemia = 4 (0.2%) Hypoglycemia = 3 (0.15%) Death = 18 (0.9%) More than 1 complications = 27 (13.1%) |
Table 2.
Quality of the included studies.
| No. | References | Selection | Compatibility | Exposure | |||||
|---|---|---|---|---|---|---|---|---|---|
| Is the case definition adequate? | Representativeness of the cases | Selection of controls | Definition of controls | Ascertainment of exposure | Same method of ascertainment for cases and controls | Non-response rate | |||
| 1 | Chaparro et al.15 | * | * | * | * | ** | * | * | * |
| 2 | Chaparro‑Narváez et al.16 | * | * | * | * | ** | * | * | * |
| 3 | Dayanand et al.17 | * | * | * | * | ** | * | * | * |
| 4 | Devineni et al.18 | * | * | ** | * | * | * | ||
| 5 | Genton et al.19 | * | * | * | * | ** | * | * | * |
| 6 | Hermansyah et al.20 | * | * | ** | * | * | * | ||
| 7 | Kochar et al.21 | * | * | * | * | ** | * | * | * |
| 8 | Kochar et al.22 | * | * | * | * | ** | * | * | * |
| 9 | Laman et al.23 | * | * | * | * | ** | * | * | * |
| 10 | Langford et al.24 | * | * | * | * | ** | * | * | * |
| 11 | Limaye et al.25 | * | * | * | * | ** | * | * | * |
| 12 | Medina-Morales et al.26 | * | * | * | * | ** | * | * | * |
| 13 | Mittal et al.27 | * | * | * | * | ** | * | * | * |
| 14 | Mohapatra et al.28 | * | * | * | * | ** | * | * | * |
| 15 | Nayak et al.29 | * | * | * | * | ** | * | * | * |
| 16 | Punnath et al.30 | * | * | * | * | ** | * | * | * |
The prevalence of mixed Plasmodium spp. infection
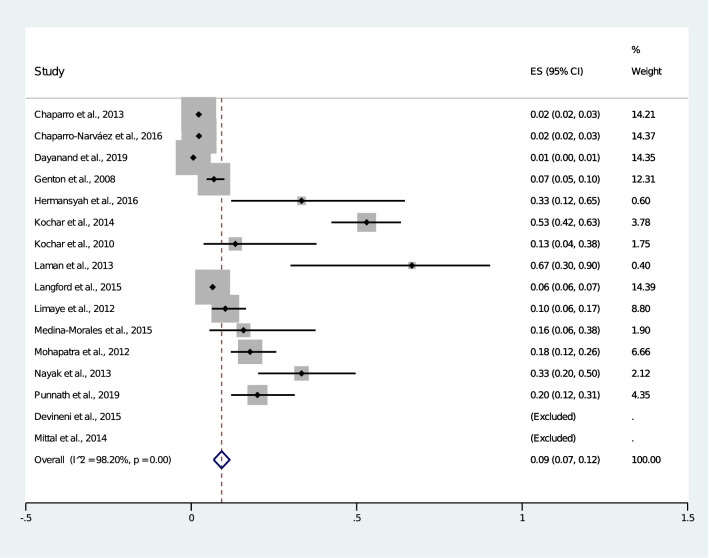
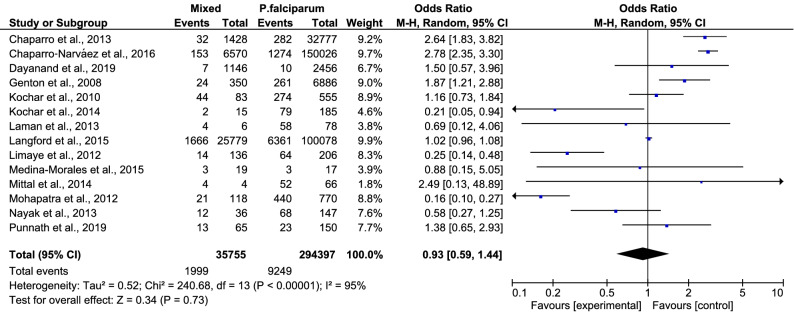
Overall, the pooled analysis showed that 9% (2,006/35,768, 95% CI 7.0–12.0%) of patients with mixed Plasmodium infection had a severe mixed infection (Fig. 2). There was statistical heterogeneity (I2: 98.2%) among the included studies, suggesting a high level of heterogeneity between studies, so random-effects models were used to produce the summary ORs in the present meta-analysis. Among the 16 included studies, only 14 studies were used to perform the meta-analysis, as two studies by Devineni et al., 2015 and Hermansyah et al., 2016 did not report the number of patients who had severe P. falciparum infections. The meta-analysis of these 14 studies demonstrated that patients with mixed Plasmodium infection (1,999/35,755) and those with P. falciparum mono-infection (9,249/294,397) had an equal risk of developing severe malaria (OR 0.93, 95% CI 0.59–1.44) (Fig. 3)15–17,19,21–30. Three studies demonstrated that patients with mixed infection had a significantly lower risk of developing severe malaria than patients with P. falciparum mono-infection15,16,19. Three studies demonstrated that patients with a mixed infection had a significantly higher risk of developing severe malaria than patients with a P. falciparum mono-infection21,25,28.
Figure 2.
Pooled prevalence of severe mixed infection.
Figure 3.
Mixed infection versus P. falciparum infection.
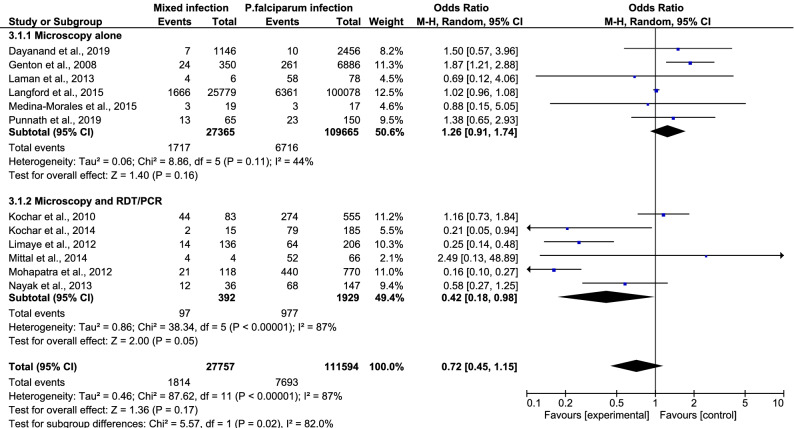
In a subgroup analysis comparing the results from India and non-India areas in 13 studies, the pooled analysis showed that patients with mixed Plasmodium spp. infection and patients with P. falciparum mono-infection had an equal risk of developing severe malaria (OR 0.91, 95% CI 0.58–1.42) (Fig. 4). There was a subgroup difference (P value = 0.02, I2 = 80.4%) in this subgroup analysis, indicating that the study area (India and non-India) was one source of heterogeneity in the present study. Further stratification by diagnostic technique (microscopy alone and microscopy with other techniques) also showed that patients with mixed infection had an equal risk of developing severe malaria compared to those with P. falciparum mono-infection (OR 0.72, 95% CI 0.45–1.15) (Fig. 5). Once again, there was a subgroup difference (P value = 0.02, I2 = 82%) in this subgroup analysis, indicating that diagnostic technique (microscopy alone and microscopy with other techniques) was also a source of heterogeneity in the present study.
Figure 4.
Subgroup analysis of India.
Figure 5.
Subgroup analysis of diagnostic technique.
Complications of severe mixed infection
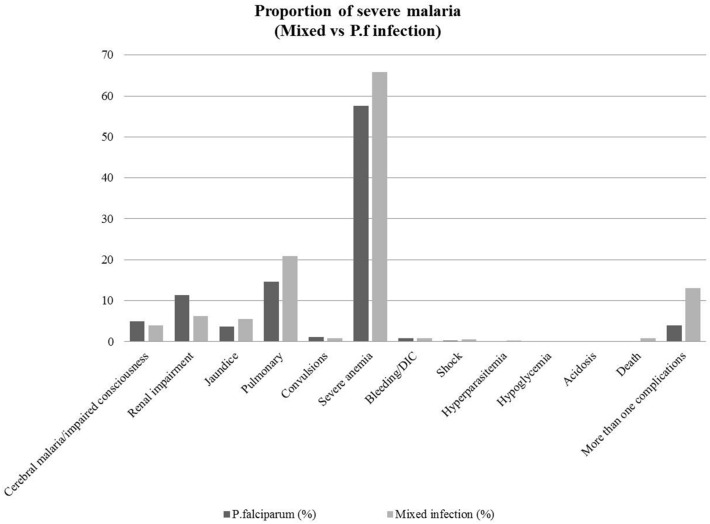
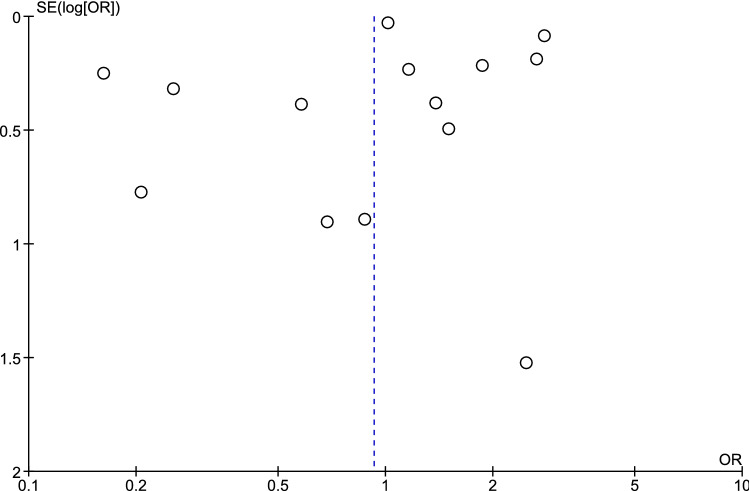
Common severe complications found in patients with mixed malaria infection were severe anaemia (65.8%, 1,320/2006), pulmonary failure (20.9%, 420), renal impairment (6.23%, 125), jaundice (5.48%, 110), cerebral malaria/impaired consciousness (3.94%, 79), convulsions (0.85%, 17), bleeding/DIC (0.85%, 17), shock (0.6%, 12), hyperparasitemia (0.2%, 4), hypoglycaemia (0.15%, 3), and more than one complication (13.1%, 27/2006). The mortality rate of severe mixed infection was 0.9% (18/2006). The most common severe complications of P. falciparum mono-infection were severe anaemia (57.6%, 5,312/9,222), pulmonary complications (14.6), and renal impairment (11.4%). For all complications, the proportions of severe mixed malaria infection and severe P. falciparum infection are shown in Fig. 6. Both mixed infection and P. falciparum mono-infection showed similar trends of severe complications by severe anaemia, pulmonary failure, and renal impairment, which were the three most common complications found in this study. Patients with mixed infection had a higher proportion of severe anaemia (65.8% vs 57.6%) and pulmonary complications (20.9% vs 14.6%) than those with P. falciparum mono-infection. Patients with mixed infection (13.1%) had a higher proportion of multiple organ failure than those with P. falciparum mono-infection (3.95%). The publication bias among studies was assessed by funnel plots (Fig. 7) and Egger's test for small-study effects. The result of Egger's test indicated that no publication bias was found in the present study (P value = 0.857, t = 0.18, 95% CI = − 2.57–3.04).
Figure 6.
The proportion of severe mixed malaria infection and severe P. falciparum mono-infection.
Figure 7.
Funnel plot.
Discussion
The present study showed a high prevalence of severe mixed Plasmodium infection across the included studies (9%), demonstrating for the first time, to our knowledge, that mixed infection can cause a high rate of severe malaria. Although the mixed malaria prevalence was predominantly due to P. falciparum/P. vivax infection, the prevalence of non-P. falciparum/P. vivax mixed infections, such as P. falciparum/P. malariae mixed infections was also reported in our study, but they were not shown in their literature24. This high prevalence of severe mixed malaria partly explains why malaria remains one of the leading causes of morbidity and mortality worldwide despite available interventions, public health control, and management employed. These findings suggested that there is a need for continued detection and monitoring of mixed infection using species-specific RDTs in combination with routine microscopy, or even using PCR as soon as possible, to move towards malaria elimination and to protect against severe malaria resulting in death. A previous study indicated that the severity of mixed P. falciparum/P. vivax infection occurred when P. vivax superinfection occurred over an existing P. falciparum infection. However, P. falciparum superinfection over an existing P. vivax infection results in a lower risk of severe malaria28. The present study demonstrated the equal prevalence of severe mixed malaria infection compared to severe P. falciparum mono-infections across the included studies. However, when considering individual studies, it was demonstrated that three of our included studies reported that patients with mixed malaria infection had a significantly lower risk of developing severe malaria than patients with P. falciparum mono-infection15,16,19. These results were consistent with results from a previous study conducted in Thailand, which observed that severe malaria was less common among patients with mixed infections compared to those with P. falciparum mono-infection31. However, three other included studies demonstrated that patients with mixed infections had a significantly higher risk of developing severe malaria than patients with P. falciparum mono-infections21,25,28. This could be because mixed infections are often unrecognized or underestimated by microscopists32,33, leading to treatment failure, anti-malarial drug resistance, and the development of severe P. falciparum malaria7. Previous studies also demonstrated that age was associated with mixed infection34,35. They found that children under two years of age had a lower frequency of mixed Plasmodium malaria compared to those at an older age. They suggested that maternal antibodies could be the source of mixed infection malaria protection34.
The major complications of severe malaria defined by the World Health Organization (WHO) included respiratory distress, acidosis, pulmonary oedema, death, impaired consciousness, convulsions, prostration, hypotension/shock, jaundice, severe anaemia, bleeding/DIC, hyperparasitemia, and hypoglycaemia12. The present study demonstrated that 9% of severe malaria was caused by mixed infection, whereas a previous study showed that severe malaria accounts for approximately 5% of total malaria-infected patients36. The mortality rate of severe mixed malaria in the present study was 0.9%, which was consistent with the case fatality rate in previously reported P. falciparum malaria mono-infection (0.6–3.8%)4. The present study also indicated that both mixed infection and P. falciparum mono-infection showed similar trends of complications in which severe anaemia, pulmonary failure, and renal impairment were the three most common complications. However, patients with mixed infection had a higher proportion of severe anaemia and pulmonary complications than those with P. falciparum mono-infection. Moreover, patients with mixed infection had a higher proportion of multiple organ failure than those with P. falciparum mono-infection. A study in Thailand indicated that mixed P. falciparum/P. vivax infection could reduce the risk of severe anaemia among patients with falciparum malaria by cross-species immunity37. In Southeast Asia, other possible reasons behind the reduction of the risk for severe anaemia among patients with malaria infections were haemoglobinopathies and enzymatic deficiencies38. Haemoglobinopathies related to the reduced risk of malaria infections or reducing the risk of severe malaria included sickle cell traits39, haemoglobin C40, haemoglobin E41, and thalassemia40. Enzymatic deficiencies related to the reduced risk of malaria infections include glucose-6-phosphate dehydrogenase (G6PD) deficiency42 and pyruvate kinase deficiency43. In addition, individuals with blood type O were less susceptible to severe malaria than individuals who were not blood type O44. The expression of the host RBC surface protein called Duffy antigen receptor for chemokines (DARC) has been shown to protect against malaria infections38. Moreover, altered RBC morphologies such as Southeast Asian ovalocytosis (SAO) could reduce the risk of malaria infection or severe malaria45,46.
The included studies conducted in Papua New Guinea (1997–2004) demonstrated that mixed infection caused more severe anaemia than did the Plasmodium mono-infection alone19. The results of our study were also consistent with the results of studies in India9 and Indonesia47 that reported a high prevalence of severe anaemia among patients with mixed infections. The higher proportion of severe mixed infection than that of P. falciparum and P. vivax mono-infection was due to mixed infection having higher parasite densities19.
The present study had limitations. First, there was a high level of heterogeneity across the included studies. Second, except for the area of the study (India and non-India) and diagnostic method, the source(s) of heterogeneity could not be explored due to the incomplete data among the included studies. Third, a limited number of studies met the criteria for inclusion because many publications included patients with severe complications and infections with etiologic agents other than malaria. Fourth, most of the included studies used microscopy for malaria detection, which might have led to missed detection of Plasmodium mixed infections. The analysis of mixed-species infections compared with P. falciparum mono-infections needs to be carefully interpreted as it is highly likely to be confounded by a proportion of undiagnosed mixed infections in the P. falciparum mono-infection groups. Fifth, a large number of additional factors related to transmission intensity, host immunity, and vectors that likely influenced the large variance seen in the mixed-Plasmodium species infections could not be taken into account because of the inherent data limitations from each study. Lastly, the present Review submits analysis of data which is relevant for the asexual blood stages of Plasmodium spp. infections resulting to severe manifestation and does not take into account hypnozoites and/or submicroscopic co-infections.
Conclusion
Mixed Plasmodium spp. infections are common but often unrecognized or underestimated, leading to severe complications among malaria patients. Therefore, in routine clinical laboratories, using an accurate combination of diagnostic procedures or repeat blood film examinations by microscopists to identify mixed infection in suspected patients is crucial for therapeutic decisions, prompt treatment, and effective management among those patients.
Supplementary information
Acknowledgements
The authors would like to thank the authors of all the published research that contributed to the data used in this study. This research was partially supported by the new strategic research (P2P) project, Walailak University, Thailand. The funders had a role in the collection, analysis, and interpretation of the data.
Abbreviations
- RDT
Rapid diagnostic test
- PCR
Polymerase chain reaction
- CI
Confidence interval
- ORs
Odds ratios
Author contributions
M.K., K.U.K., G.D.M., and F.R.M. participated in the study design, data analysis, and writing of the paper. All authors read and approved the final paper.
Data availability
The datasets used during the current study are available from the corresponding author based on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-68082-3.
References
- 1.Singh B, Daneshvar C. Human infections and detection of Plasmodium knowlesi. Clin. Microbiol. Rev. 2013;26:165–184. doi: 10.1128/CMR.00079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutherland CJ, et al. Two nonrecombining sympatric forms of the human malaria parasite Plasmodium ovale occur globally. J. Infect. Dis. 2010;201:1544–1550. doi: 10.1086/652240. [DOI] [PubMed] [Google Scholar]
- 3.Singh B, et al. A large focus of naturally acquired Plasmodium knowlesi infections in human beings. Lancet. 2004;363:1017–1024. doi: 10.1016/S0140-6736(04)15836-4. [DOI] [PubMed] [Google Scholar]
- 4.Mayxay M, Pukrittayakamee S, Newton PN, White NJ. Mixed-species malaria infections in humans. Trends Parasitol. 2004;20:233–240. doi: 10.1016/j.pt.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Looareesuwan S, White NJ, Chittamas S, Bunnag D, Harinasuta T. High rate of Plasmodium vivax relapse following treatment of falciparum malaria in Thailand. Lancet. 1987;2:1052–1055. doi: 10.1016/s0140-6736(87)91479-6. [DOI] [PubMed] [Google Scholar]
- 6.McKenzie FE, Bossert WH. Multispecies Plasmodium infections of humans. J. Parasitol. 1999;85:12–18. doi: 10.2307/3285692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee GC, et al. Development and evaluation of a rapid diagnostic test for Plasmodium falciparum, P. vivax, and mixed-species malaria antigens. Am. J. Trop. Med. Hyg. 2011;85:989–993. doi: 10.4269/ajtmh.2011.11-0265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obare P, et al. Misclassification of Plasmodium infections by conventional microscopy and the impact of remedial training on the proficiency of laboratory technicians in species identification. Malar. J. 2013;12:113. doi: 10.1186/1475-2875-12-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gopinathan VP, Subramanian AR. Vivax and falciparum malaria seen at an Indian service hospital. J. Trop. Med. Hyg. 1986;89:51–55. [PubMed] [Google Scholar]
- 10.Bruce MC, et al. Effect of transmission setting and mixed species infections on clinical measures of malaria in Malawi. PLoS ONE. 2008;3:e2775. doi: 10.1371/journal.pone.0002775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mason DP, McKenzie FE. Blood-stage dynamics and clinical implications of mixed Plasmodium vivax–Plasmodium falciparum infections. Am. J. Trop. Med. Hyg. 1999;61:367–374. doi: 10.4269/ajtmh.1999.61.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.WHO. Guidelines for the treatment of malaria. https://www.who.int/malaria/publications/atoz/9789241549127/en/ (2014).
- 13.Tamhane UU, et al. Safety and efficacy of thrombectomy in patients undergoing primary percutaneous coronary intervention for acute ST elevation MI: a meta-analysis of randomized controlled trials. BMC Cardiovasc. Disord. 2010;10:10. doi: 10.1186/1471-2261-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boonchan T, Wilasrusmee C, McEvoy M, Attia J, Thakkinstian A. Network meta-analysis of antibiotic prophylaxis for prevention of surgical-site infection after groin hernia surgery. Br. J. Surg. 2017;104:e106–e117. doi: 10.1002/bjs.10441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chaparro P, Padilla J, Vallejo AF, Herrera S. Characterization of a malaria outbreak in Colombia in 2010. Malar. J. 2013 doi: 10.1186/1475-2875-12-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chaparro-Narvaez PE, et al. Clinical and epidemiological aspects of complicated malaria in Colombia, 2007–2013. Malar. J. 2016;15:11. doi: 10.1186/s12936-016-1323-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dayanand KK, et al. Malaria severity in Mangaluru city in the southwestern coastal region of India. Am. J. Trop. Med. Hyg. 2019;100:275–279. doi: 10.4269/ajtmh.18-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devineni SB, Suneetha O, Harshavardhan N. Study of platelet count in malaria patients and the correlation between the presence and severity of platelet count with type of malaria. J. Evol. Med. Dent. Sci. 2015;4:11734–11746. doi: 10.14260/jemds/2015/1691. [DOI] [Google Scholar]
- 19.Genton B, et al. Plasmodium vivax and mixed infections are associated with severe malaria in children: a prospective cohort study from Papua New Guinea. PLoS Med. 2008;5:0881–0889. doi: 10.1371/journal.pmed.0050127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermansyah B, et al. Clinical features of severe malaria: protective effect of mixed plasmodial malaria. Asian Pac. Trop. Biomed. 2017;7:4–9. doi: 10.1016/j.apjtb.2016.11.001. [DOI] [Google Scholar]
- 21.Kochar DK, et al. A prospective study on adult patients of severe malaria caused by Plasmodium falciparum, Plasmodium vivax and mixed infection from Bikaner, northwest India. J. Vector Borne Dis. 2014;51:200–210. [PubMed] [Google Scholar]
- 22.Kochar DK, et al. Clinical features of children hospitalized with malaria—a study from Bikaner, northwest India. Am. J. Trop. Med. Hyg. 2010;83:981–989. doi: 10.4269/ajtmh.2010.09-0633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laman M, Manning L, Siba PM, Davis TME. Short report: Prevalence and implications of cerebrospinal fluid leukocytosis in Papua New Guinean children hospitalized with severe malaria. Am. J. Trop. Med. Hyg. 2013;89:866–868. doi: 10.4269/ajtmh.13-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langford S, et al. Plasmodium malariae infection associated with a high burden of anemia: a hospital-based surveillance study. PLoS. Negl. Trop. Dis. 2015 doi: 10.1371/journal.pntd.0004195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Limaye CS, Londhey VA, Nabar ST. The study of complications of vivax malaria in comparison with falciparum malaria in Mumbai. J. Assoc. Physicians India. 2012;60:15–18. [PubMed] [Google Scholar]
- 26.Medina-Morales DA, Montoya-Franco E, Sanchez-Aristizabal VD, Machado-Alba JE, Rodríguez-Morales AJ. Severe and benign Plasmodium vivax malaria in Emberá (Amerindian) children and adolescents from an endemic municipality in Western Colombia. J. Infect. Public Health. 2016;9:172–180. doi: 10.1016/j.jiph.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 27.Mittal M, Jain R, Talukdar B, Kumar M, Kapoor K. Emerging new trends of malaria in children: a study from a tertiary care centre in northern India. J. Vector Borne Dis. 2014;51:115–118. [PubMed] [Google Scholar]
- 28.Mohapatra MK, Dash LK, Barih PK, Karua PC. Profile of mixed species (Plasmodium vivax and falciparum) malaria in adults. J. Assoc. Physicians India. 2012;60:20–24. [PubMed] [Google Scholar]
- 29.Nayak KC, Meena SL, Gupta BK, Kumar S, Pareek V. Cardiovascular involvement in severe vivax and falciparum malaria. J. Vector Borne Dis. 2013;50:285–291. [PubMed] [Google Scholar]
- 30.Punnath K, et al. Clinical features and haematological parameters among malaria patients in Mangaluru city area in the southwestern coastal region of India. Parasitol. Res. 2019 doi: 10.1007/s00436-019-06540-2. [DOI] [PubMed] [Google Scholar]
- 31.Luxemburger C, et al. The epidemiology of severe malaria in an area of low transmission in Thailand. Trans. R. Soc. Trop. Med Hyg. 1997;91:256–262. doi: 10.1016/s0035-9203(97)90066-3. [DOI] [PubMed] [Google Scholar]
- 32.Mbakilwa H, et al. Quality of malaria microscopy in 12 district hospital laboratories in Tanzania. Pathog. Glob. Health. 2012;106:330–334. doi: 10.1179/2047773212Y.0000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frean J, et al. External quality assessment of national public health laboratories in Africa, 2002–2009. Bull. World Health Organ. 2012;90:191–199A. doi: 10.2471/BLT.11.091876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sitali L, et al. Patterns of mixed Plasmodium species infections among children six years and under in selected malaria hyper-endemic communities of Zambia: population-based survey observations. BMC Infect Dis. 2015;15:204. doi: 10.1186/s12879-015-0935-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerra-Neira A, et al. Plasmodium diversity in non-malaria individuals from the Bioko Island in Equatorial Guinea (West Central-Africa) Int. J. Health Geogr. 2006;5:27. doi: 10.1186/1476-072X-5-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genton B, D'Acremont V. Clinical Features of Malaria in Returning Travelers and Migrants. New York: BC Decker; 2001. pp. 371–392. [Google Scholar]
- 37.Price RN, et al. Factors contributing to anemia after uncomplicated falciparum malaria. Am. J. Trop. Med. Hyg. 2001;65:614–622. doi: 10.4269/ajtmh.2001.65.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goheen MM, Campino S, Cerami C. The role of the red blood cell in host defence against falciparum malaria: an expanding repertoire of evolutionary alterations. Br. J. Haematol. 2017;179:543–556. doi: 10.1111/bjh.14886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Taylor SM, Cerami C, Fairhurst RM. Hemoglobinopathies: slicing the Gordian knot of Plasmodium falciparum malaria pathogenesis. PLoS Pathog. 2013;9:e1003327. doi: 10.1371/journal.ppat.1003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor SM, Parobek CM, Fairhurst RM. Haemoglobinopathies and the clinical epidemiology of malaria: a systematic review and meta-analysis. Lancet Infect. Dis. 2012;12:457–468. doi: 10.1016/S1473-3099(12)70055-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chotivanich K, et al. Hemoglobin E: a balanced polymorphism protective against high parasitemias and thus severe P. falciparum malaria. Blood. 2002;100:1172–1176. doi: 10.1182/blood.V100.4.1172.h81602001172_1172_1176. [DOI] [PubMed] [Google Scholar]
- 42.Uyoga S, et al. Glucose-6-phosphate dehydrogenase deficiency and the risk of malaria and other diseases in children in Kenya: a case–control and a cohort study. Lancet Haematol. 2015;2:e437–444. doi: 10.1016/S2352-3026(15)00152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Bruggen R, et al. Modulation of malaria phenotypes by pyruvate kinase (PKLR) variants in a Thai population. PLoS ONE. 2015;10:e0144555. doi: 10.1371/journal.pone.0144555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Afoakwah R, Aubyn E, Prah J, Nwaefuna EK, Boampong JN. Relative susceptibilities of ABO blood groups to Plasmodium falciparum Malaria in Ghana. Adv. Hematol. 2016;2016:5368793. doi: 10.1155/2016/5368793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Allen SJ, et al. Prevention of cerebral malaria in children in Papua New Guinea by southeast Asian ovalocytosis band 3. Am. J. Trop. Med. Hyg. 1999;60:1056–1060. doi: 10.4269/ajtmh.1999.60.1056. [DOI] [PubMed] [Google Scholar]
- 46.Genton B, et al. Ovalocytosis and cerebral malaria. Nature. 1995;378:564–565. doi: 10.1038/378564a0. [DOI] [PubMed] [Google Scholar]
- 47.Tjitra E, et al. Multidrug-resistant Plasmodium vivax associated with severe and fatal malaria: a prospective study in Papua, Indonesia. PLoS Med. 2008;5:e128. doi: 10.1371/journal.pmed.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used during the current study are available from the corresponding author based on reasonable request.