As yet, no direct-acting antiviral drugs have demonstrated efficacy in the disease. In contrast, increasing evidence indicates an immune-mediated pathophysiology that is paving the way for the evaluation of immunomodulation strategies [2]. In support of this view, we would like to highlight the striking similarities between COVID-19 and a rare autoimmune disease: the anti-MDA5-syndrome.
Short abstract
Similarities between COVID-19 and anti-MDA5 syndrome support further evaluation of employing an immunomodulatory strategy in COVID-19 https://bit.ly/3dN6lJ8
To the Editor:
The coronavirus disease 2019 (COVID-19) pandemic has struck worldwide, leading to more than 7 million cases by June 2020, with a ∼5.5% mortality rate, mainly due to acute respiratory distress syndrome (ARDS) [1].
Current conventional treatment is mainly based on support therapy and there is an urgent need for effective, specific treatments.
As yet, no direct-acting antiviral drugs have demonstrated efficacy in the disease. In contrast, increasing evidence indicates an immune-mediated pathophysiology that is paving the way for the evaluation of immunomodulation strategies [2]. In support of this view, we would like to highlight the striking similarities between COVID-19 and a rare autoimmune disease: the anti-MDA5-syndrome.
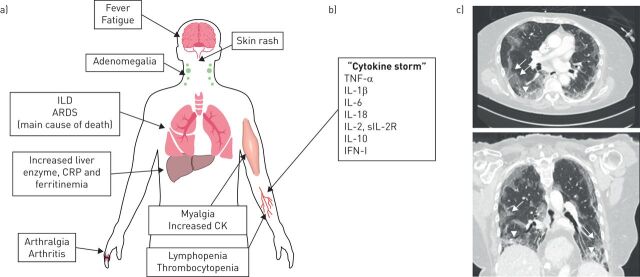
The hallmark of this disease is the presence of auto-antibodies targeting MDA5, an intracellular sensor of viral RNA (including coronavirus) that triggers the innate immune response [3]. The syndrome is characterised by systemic signs resembling COVID-19, and ARDS is the main cause of death (figure 1a) [4, 5]. In addition, chest computed tomography findings [6], as well as blood cytokine profiles [7, 8] are very similar in the two conditions (figure 1b and c), further supporting common pathophysiological mechanisms. So far, there is no evidence that patients with COVID-19 have anti-MDA5 autoantibodies but, while other diseases causing ARDS feature “cytokine storm”, few show such similarities with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection.
FIGURE 1.
a) Clinical and biological features of anti-MDA5 syndrome. b) Cytokines whose levels are increased in anti-MDA5 syndrome patients' serum. c) High-resolution computed tomography of an anti-MDA5 syndrome patient, showing bilateral peripheral subpleural ground-glass opacities prevailing in the lower lobes (arrows), with limited consolidation (arrowheads). ILD: interstitial lung disease; ARDS: acute respiratory distress syndrome; CRP: C-reactive protein; CK: creatine kinase; TNF-α: tumour necrosis factor-α; IL: interleukin; s-IL-2R: soluble IL-2 receptor; IFN-I: interferon type I.
Beyond these similarities, anti-MDA5 syndrome responds to glucocorticoids and immunomodulatory drugs, among which tofacitinib (a JAK inhibitor) [9, 10] and a combination of tacrolimus and cyclophosphamide [11] have recently been shown to improve survival versus conventional strategies. Likewise, even though an immunological reaction is necessary to eliminate SARS-CoV-2 infection and corticosteroids are not currently recommended by the World Health Organization [12], in severe COVID-19 patients, dexamethasone has been reported to improve survival [13] and ruxolitinib (another JAK inhibitor) results in a greater chest tomography improvement and faster clinical improvement with no death compared with standard of care [14]. Moreover, tacrolimus has been shown to inhibit SARS-CoV replication [15].
These data support further evaluation of employing such an immunomodulatory strategy in COVID-19.
Shareable PDF
Acknowledgements
We thank Pragnell Babette for her English support.
Footnotes
Author contributions: M. Giannini: literature search, figures, study design, data collection, data analysis, data interpretation, writing. M. Ohana: figures, data collection, data interpretation. B. Nespola: data interpretation. G. Zanframundo: data interpretation. B. Geny: data interpretation, writing. A. Meyer: literature search, figures, study design, data collection, data analysis, data interpretation, writing.
Conflict of interest: M. Giannini has nothing to disclose.
Conflict of interest: M. Ohana has nothing to disclose.
Conflict of interest: B. Nespola has nothing to disclose.
Conflict of interest: G. Zanframundo has nothing to disclose.
Conflict of interest: B. Geny has nothing to disclose.
Conflict of interest: A. Meyer has nothing to disclose.
References
- 1.WHO . Coronavirus disease (COVID-19) situation report – 106. www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports/ Date last updated: 5 May 2020. Date last accessed: 5 May 2020.
- 2.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. The Lancet 2020; 395: 1033–1034. doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dias Junior AG, Sampaio NG, Rehwinkel J. A balancing act: MDA5 in antiviral immunity and autoinflammation. Trends Microbiol 2019; 27: 75–85. doi: 10.1016/j.tim.2018.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allenbach Y, Uzunhan Y, Toquet S, et al. Different phenotypes in dermatomyositis associated with anti-MDA5 antibody: study of 121 cases. Neurology 2020; 95: e70–e78. 10.1212/WNL.0000000000009727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li J, Liu Y, Li Y, et al. Associations between anti-melanoma differentiation-associated gene 5 antibody and demographics, clinical characteristics and laboratory results of patients with dermatomyositis: a systematic meta-analysis. J Dermatol 2018; 45: 46–52. doi: 10.1111/1346-8138.14092 [DOI] [PubMed] [Google Scholar]
- 6.Tanizawa K, Handa T, Nakashima R, et al. HRCT features of interstitial lung disease in dermatomyositis with anti-CADM-140 antibody. Respir Med 2011; 105: 1380–1387. doi: 10.1016/j.rmed.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 7.Gono T, Kaneko H, Kawaguchi Y, et al. Cytokine profiles in polymyositis and dermatomyositis complicated by rapidly progressive or chronic interstitial lung disease. Rheumatology 2014; 53: 2196–2203. doi: 10.1093/rheumatology/keu258 [DOI] [PubMed] [Google Scholar]
- 8.Nishioka A, Tsunoda S, Abe T, et al. Serum neopterin as well as ferritin, soluble interleukin-2 receptor, KL-6 and anti-MDA5 antibody titer provide markers of the response to therapy in patients with interstitial lung disease complicating anti-MDA5 antibody-positive dermatomyositis. Mod Rheumatol 2019; 29: 814–820. doi: 10.1080/14397595.2018.1548918 [DOI] [PubMed] [Google Scholar]
- 9.Chen Z, Wang X, Ye S. Tofacitinib in amyopathic dermatomyositis–associated interstitial lung disease. N Engl J Med 2019; 381: 291–293. doi: 10.1056/NEJMc1900045 [DOI] [PubMed] [Google Scholar]
- 10.Kurasawa K, Arai S, Namiki Y, et al. Tofacitinib for refractory interstitial lung diseases in anti-melanoma differentiation-associated 5 gene antibody-positive dermatomyositis. Rheumatology 2018; 57: 2114–2119. doi: 10.1093/rheumatology/key188 [DOI] [PubMed] [Google Scholar]
- 11.Tsuji H, Nakashima R, Hosono Y, et al. Multicenter prospective study of the efficacy and safety of combined immunosuppressive therapy with high-dose glucocorticoid, tacrolimus, and cyclophosphamide in interstitial lung diseases accompanied by anti–melanoma differentiation–associated gene 5–positive dermatomyositis. Arthritis Rheumatol 2020; 72: 488–498. doi: 10.1002/art.41105 [DOI] [PubMed] [Google Scholar]
- 12.WHO . Clinical management of COVID-19: interim guidance. www.who.int/publications/i/item/clinical-management-of-covid-19 Date last updated: 27 May 2020. Date last accessed: 27 May 2020.
- 13.Ledford H. Coronavirus breakthrough: dexamethasone is first drug shown to save lives. Nature 2020; 582: 469. [DOI] [PubMed] [Google Scholar]
- 14.Cao Y, Wei J, Zou L, et al. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): a multicenter, single-blind, randomized controlled trial. J Allergy Clin Immunol 2020; 146: 137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carbajo-Lozoya J, Müller MA, Kallies S, et al. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res 2012; 165: 112–117. doi: 10.1016/j.virusres.2012.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This one-page PDF can be shared freely online.
Shareable PDF ERJ-01618-2020.Shareable (272.6KB, pdf)