Abstract
Autophagy is an intracellular catabolic pathway in which cellular constituents are engulfed by autophagosomes and degraded upon autophagosome fusion with lysosomes. Autophagy serves as a major cytoprotective process by maintaining cellular homeostasis and recycling cytoplasmic contents. However, emerging evidence suggests that autophagy is a primary mechanism of cell death (autophagic cell death, ACD) and implicates ACD in several aspects of mammalian physiology, including tumor suppression and psychological disorders. However, little is known about the physiological roles and molecular mechanisms of ACD. In this review, we document examples of ACD and discuss recent progress in our understanding of its molecular mechanisms.
Subject terms: Macroautophagy, Macroautophagy
Cell death: cell component recycling in health and disease
A natural cellular recycling process could be harnessed or targeted for the treatment of multiple diseases including cancer and psychological disorders. Autophagy is a process that occurs within cells, whereby damaged, toxic, or obsolete cellular components are degraded and recycled to release energy and maintain balance. However, scientists now recognize that autophagy can trigger cell death under certain conditions. Seong-Woon Yu and co-workers at the Daegu Gyeongbuk Institute of Science and Technology, South Korea, reviewed recent research into the mechanisms and role of autophagy in health and disease. Autophagic cell death is implicated in the suppression of tumor development; for example, inducing autophagy led to the death of precancerous cells in mice. Autophagy may also regulate immune cell populations, and play a role in the death of brain cells during chronic stress-related disorders.
Introduction
Autophagy/macroautophagy is a lysosome-dependent catabolic process characterized by increased formation of double-membrane autophagosomes for the sequestration of cytoplasmic components and subsequent degradation after autophagosome fusion with lysosomes1,2. Autophagy occurs during normal development at the basal level, as well as under stress conditions. Autophagy is generally considered as a cell survival/protection mechanism because it removes toxic or obsolete proteins and organelles and recycles the degradation products for use as sources for energy and metabolites in anabolic pathways3. However, autophagy has also been recognized as a cell death pathway, first in Drosophila and recently in mammalian systems4,5. Nevertheless, the definition of autophagic cell death (ACD) has been neither universally understood nor unanimously accepted in the field4. Therefore, the relationship between autophagy and cell death remains unclear and warrants further study to harness autophagy for the treatment of various human diseases.
Autophagy is induced by adverse environmental conditions, such as starvation, growth factor deprivation, and pathogen infection6. Extracellular cues, including those of hormones and cytokines, can also regulate autophagy. For example, Th1 cytokines, including interferon-γ, tumor necrosis factor-α, interleukin (IL)-2, IL-6, and transforming growth factor-β, stimulate autophagy, whereas Th2 cytokines, including IL-4, IL-10, and IL-13, inhibit autophagy and thus regulate inflammatory mediators7. Insulin and insulin-like growth factor 1 are known to inhibit autophagy. In a fasting state, increased glucagon and epinephrine and norepinephrine secretion induce autophagy, and glucocorticoids have also been shown to induce autophagy by stimulating the transcription of autophagy genes such as ATG5, LC3, and Beclin-1 in various tissues8. Including those of autophagy-inducing signals, the molecular details of autophagy and the techniques to assess autophagy flux have been well documented in other reviews1,9. The beneficial roles of autophagy in diverse aspects of human physiology and diseases, including development, metabolism, neurodegeneration, and aging, are also well covered elsewhere10–13. In addition, cell death subroutines have been recently classified on the basis of mechanical and molecular aspects of cell death processes5. Therefore, in this review, we avoid a lengthy repetition of the description of autophagy and cell death processes and focus on the death-promoting roles of autophagy and the intertwined connection between autophagy and apoptosis. We also present recent findings on the molecular mechanisms underlying ACD.
Programmed cell death
Programmed cell death (PCD), as described by Lockshin and Williams14, is defined as controlled cell death evoked by intracellular systems. PCD has fundamental functions in tissue development and homeostasis, as PCD is activated to sculpt or remove structures, regulate cell numbers, and eliminate unnecessary or dysfunctional cells. Therefore, the abnormal regulation of PCD is associated with numerous human diseases, including cancers and neurodegenerative diseases. The Nomenclature Committee on Cell Death has recently classified 12 major cell death modes5. However, the classification of PCD into apoptosis (type I), ACD (type II), and necroptosis (type III)10,15 adequately serve for our discussion.
Apoptosis
Apoptosis is the most well-known mode of PCD and is characterized by specific morphological and biochemical changes in dying cells, including cell shrinkage, chromatin condensation, nuclear fragmentation, membrane blebbing, and chromosomal DNA cleavage16,17. Apoptosis can be categorized into extrinsic and intrinsic pathways17. The extrinsic pathway, also known as the death receptor pathway, is stimulated by the binding of death ligands to cognate death receptors, including the tumor necrosis factor receptor and Fas receptor18. After ligand binding, a death-inducing signaling complex is formed, and procaspase 8 is activated, followed by the activation of downstream executioner caspases, such as caspases 3 and 719. The intrinsic or mitochondrial pathway is initiated by nonreceptor-mediated cellular stressors such as radiation, hypoxia, DNA damage, and oxidative stress17. Cellular stress increases mitochondrial membrane permeability, leading to the release of cytochrome c from the mitochondrial intermembrane space into the cytosol. Then, cytochrome c binds to apoptotic protease-activating factor-1 (APAF-1) and procaspase 9, forming the apoptosome complex, which activates caspase-9 and then executioner caspases, leading to cell death20,21. Extrinsic apoptosis is often interconnected with intrinsic apoptosis through proapoptotic Bcl-2 family members17.
Necroptosis
Previously, necrosis was regarded as an accidental and uncontrolled form of cell death, but it is now recognized that necrosis can be executed in a controlled manner. Therefore, the term “necroptosis” was coined to reflect its regulated nature22. Necroptotic cells show morphological characteristics such as cell swelling and rupture of the plasma membrane, and the presence of necroptotic cells is usually associated with inflammation23. Receptor-interacting protein kinases 1 and 3 (RIP1 and RIP3) act as key molecules in necroptosis, and the development of inhibitors specific to these kinases has contributed to the current understanding of the regulated nature of necroptosis23–25.
ACD
In many cases, dying cells develop autophagosomes, leading to the idea of “autophagic” cell death (ACD). Initially, “ACD” was simply a morphological term to describe dying cells showing features of autophagy without implying a causative role for autophagy in cell death5. Autophagy may be activated to overcome cell death; on the other hand, apoptosis may impair autophagy to complete cell death. When autophagic flux is impaired, autophagosome maturation is suspended, and autophagosomes may accumulate9. Therefore, the use of “ACD” as a descriptive term without mechanistic implications for the role of autophagy in cell death led to confusion. To make matters more complicated, autophagy may precede and trigger apoptosis or necroptosis, leading to the term “autophagy-mediated cell death”26. In autophagy-mediated cell death, autophagy accompanies and is required for the activation of other cell death modes. In these cases, inhibition of autophagy can prevent cell death, even though cell death is not executed through autophagy. Thus, the term “ACD” should be applied only when the following criteria are met: (1) cell death occurs without the involvement of other types of PCD, (2) autophagic flux is elevated, and (3) pharmacological or genetic inhibition of autophagy blocks cell death27. In the following section, we minimize the introduction of cases of autophagy-mediated cell death and cell death in which autophagy and apoptosis overlap to focus mainly on authentic examples of ACD.
ACD in model systems
ACD in Drosophila
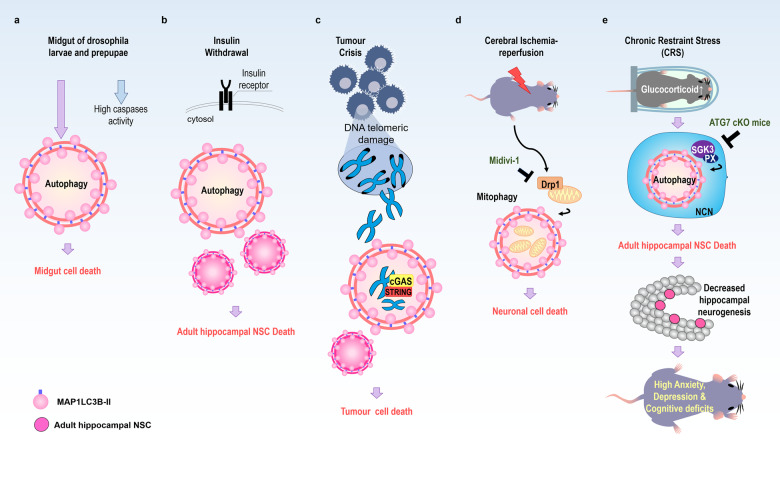
A plausible example of ACD was first presented during developmental cell death in Drosophila. In dying larval salivary glands, autophagy-related (Atg) genes, as well as apoptotic genes such as those for caspases, are induced, and both autophagy and apoptosis are required for the complete loss of salivary glands28,29. Therefore, salivary gland cell death does not meet the criteria of ACD. However, an authentic case of ACD in Drosophila was discovered later, in midgut cell death30. Decay, a Drosophila caspase, is active in the dying midgut and contributes to the activation of other caspases, dronc, and drice1. However, the inhibition of decay or all three caspases cannot block midgut cell death, and the canonical apoptotic pathway is not required for midgut regression. Interestingly, midgut cell death is accompanied by an increase in the number of autophagosomes. Midgut cell death is delayed in Atg1- and Atg18-mutant flies. Similarly, knocking down Atg2 and Atg18 also significantly suppresses cell death in the midgut. Therefore, autophagy, not apoptosis, is crucial for Drosophila midgut cell death (Fig. 1a). Midgut removal is a developmental process controlled by the Drosophila steroid hormone ecdysone. Decapentaplegic, Drosophila bone morphogenic protein/transforming growth factor-β ligand, blocks autophagy-dependent midgut degeneration by inhibiting ecdysone production and thereby impairs the correct timing of development31.
Fig. 1. Representative cases of autophagic cell death (ACD).
a The midgut of Drosophila larvae and pupae shows high caspase activity and autophagy flux, but midgut cell death depends only on autophagy. b Adult hippocampal neural stem cells (NSCs) undergo ACD following insulin withdrawal without the involvement of apoptosis or necroptosis. c During replicative crisis, DNA with telomeric damage is released into the cytosol and is recognized by cGAS and STRING, which induces ACD. d Cerebral ischemia–reperfusion induces mitophagy in a DRP1-dependent manner with subsequent neuronal cell death. e Chronic restraint stress (CRS) or corticosterone treatment induces ACD in adult hippocampal NSCs via SGK3 in vivo and in vitro. CRS decreases adult hippocampal neurogenesis, which is accompanied by anxiety, depression, and cognitive deficits.
ACD in Dictyostelium
In Dictyostelium discoideum, triggering the differentiation of vegetative cells under starvation conditions induces the programmed death of stalk cells, which is characterized by early massive vacuolization and late membrane lesions but intact nuclei and the absence of apoptosis markers such as DNA fragmentation32. This developmental cell death requires two successive but separable exogenous signals: (1) starvation/cAMP for the induction of autophagy and (2) differentiation factor DIF-1 for the induction of cell death. Autophagy induced by the first signal does not lead to cell death, and DIF-1 does not induce cell death when added to nonstarved cells. An Atg1 mutation prevents both starvation-induced autophagy and DIF-1 exposure-induced cell death, showing that autophagy is required for ACD, with Atg1 being a critical inducer33. ACD induction by DIF-1 is prevented by mutations in iplA (IP3R), TalB (talinB), DcsA (cellulose synthase), GbfA, ugpB, glcS (glycogen synthase), and atg134. As mammalian homologs of some of these molecules, such as glycogen synthase kinase 3-beta (GSK3B), also play important roles in ACD (as discussed in the following section), comparative studies between Dictyostelium and mammalian cells may provide novel mechanical insights into the molecular and genetic regulation of ACD.
ACD in mammalian systems
ACD in cancer cells
As apoptosis is defective in most cancer cells, due consideration has been given to ACD as an attractive cancer treatment modality to induce PCD by using anticancer agents. ACD has been reported in various cancer cells. In A549 lung cancer cells, resveratrol treatment induced cell death with increased autophagy flux35. Cell death occurred in the absence of apoptosis markers, including the cleaved forms of caspases 9, 8, and 3, and Atg7-, Atg12-, Beclin-1-, or Ulk1-knockdown increased cell viability and reduced autophagy activation. Signalome-wide screening with shRNAs led to the identification of glucocerebrosidase as a mediator of ACD in resveratrol-treated A549 cells. In pancreatic cancer cells, LZ1, a peptide derived from snake venom cathelicidin, suppressed cell growth both in vitro and in vivo by inducing ACD by binding to and degrading cell surface-expressed nucleolin, subsequently activating AMP-activated protein kinase (AMPK)36. As2O3 specifically triggered ACD in human malignant glioma cells (U373-MG, U251, U87-MG, A172, T98G, and GB1)37. An increased number of autophagosomes was apparent after As2O3 treatment, but no apoptotic features were observed by electron microscopy, and Z-VAD, an apoptosis inhibitor with broad-spectrum caspase inhibition activity, did not prevent As2O3-induced cell death.
Z-VAD killed mouse L929 fibrosarcoma cells, with the appearance of numerous autophagic vacuoles and morphology that was distinct from that of apoptotic cells38. Z-VAD-induced cell death associated with autophagy was also observed in U937 human leukemia cells, a mouse RAW264.7 macrophage cell line, and primary mouse peritoneal macrophages38. In this study, pharmacological inhibition and knockdown of Atg7 and Beclin-1 inhibited Z-VAD-induced cell death with a parallel decrease in autophagic vacuole formation. These data suggest that autophagy is required for PCD in these various types of cells following Z-VAD treatment. RIP and Jun amino-terminal kinase, but neither p38 nor extracellular signal-regulated kinase, were found to be components of a signaling pathway that led to the activation of autophagy38. Of interest, caspase 8 was identified as a target of Z-VAD, as caspase 8 inhibition led to an increase in cell death associated with autophagy features38. Further study showed that cell death induced by caspase inhibition is mediated by catalase degradation and subsequent reactive oxygen species (ROS) accumulation, processes that are blocked by autophagy inhibition39.
Apoptosis inhibition-induced ACD was also reported in multiple myeloma. In several multiple myeloma cell lines, caspase 10 catalytic activity and cFLIPL expression, driven by IRF4, are required for cell viability irrespective of genetic abnormalities40. Caspase 10 inhibition by a broad-spectrum caspase inhibitor, Q-VD-OPH or a more selective caspase 10 inhibitor kills myeloma cells without the hallmarks of apoptosis but through the induction of ACD. Posttranslational cleavage of BCL-2-associated transcription factor 1 (BCLAF1) has been identified in a mechanism of ACD suppression induced by caspase 10. Therefore, overexpression and knockdown of BCLAF1 induced and mitigated ACD, respectively, in multiple myeloma cells with inhibited caspase 1040. Apoptosis inhibition-induced ACD may have some clinical relevance when apoptosis is considered as a means of tumor suppression.
In cancer cells, ACD induction by ROS-generating agents has also been observed. In HEK293, U87, and HeLa cells, hydrogen peroxide (H2O2) and 2-methoxyestradiol (2-ME)-induced ACD, whereas cell death was inhibited by 3-methyladenine (3-MA) or the deletion of Beclin-1, Atg6, or Atg7 but not by Z-VAD41. Inhibition of the mitochondrial electron transport chain also induced ACD in transformed and cancer cell lines through the generation of ROS42. Blocking autophagy failed to reduce ROS generation, positioning ROS upstream of autophagy, which differs from the finding in Z-VAD-induced death of L929 cells41,42. Interestingly, neither H2O2 nor 2-ME could induce autophagy or cell death in mouse primary astrocytes, suggesting a difference in signaling mechanisms between transformed or cancer cells and nontransformed cells41,42.
In the HCT116 human colon cancer cell line, oxidative stress leads to acetylation of FoxO1, a forkhead O family protein, by inducing its dissociation from sirtuin-2. Then, acetylated FoxO1 binds to ATG7 in the cytosol, leading to ACD and tumor suppression activity. Regressed tumor growth was observed in a xenograft nude mouse model after transplantation of FoxO1-expressing cancer cells but not after transplantation of FoxO1-expressing cancer cells upon a stable knockdown of Atg7, demonstrating that FoxO1 exerts tumor-suppressor activity by inducing ACD43.
Neferine, a natural alkaloid isolated from Nelumbo nucifera, induces ACD via calcium release after the activation of ryanodine receptor (RYR) and ULK1–PERK and AMPK–mTOR signaling cascades, especially in apoptosis-resistant cancer cell lines, including HeLa, H1299, HepG2, and Lo2 cells44. Plasma-activated medium (PAM), which was developed for ovarian cancer suppression, induced ACD in some types of endometrial cancer cells45. PAM treatment increased ACD by inactivating the mTOR pathway, providing a potential novel treatment for endometrial cancer.
ACD in other mammalian cells
ACD has also been documented in various noncancerous mammalian cell types. Mouse embryonic fibroblasts deficient in both BAX and BAK are resistant to apoptosis and instead undergo nonapoptotic death induced by various apoptotic stimuli46,47. This nonapoptotic death is associated with a significant increase in the number of autophagosomes/autolysosomes, can be blocked by the autophagy inhibitor 3-MA or by knockdown of Atg5 or Beclin-146,47, and requires lysosomal membrane permeability48.
ACD is the mechanism of PCD in senescent keratinocytes. Keratinocyte senescence is caused by the accumulation of oxidative damage to the nucleus and mitochondria, which can be replicated by applying a subtoxic level of H2O249. Senescent cells ultimately undergo cell death, not by apoptosis but by ACD49.
The number of immune T cells is under tight control through two cell death pathways: (1) activation-induced cell death upon prolonged T-cell receptor activation and (2) activated T-cell-autonomous death. The latter is induced when survival signals, such as those from growth factors, are limited. In activated mouse CD4+ T cells, growth factor abrogation-induced cell death can be prevented by blocking autophagy with 3-MA or by knocking down Beclin-1 or Atg750. In addition to the reported involvement of apoptosis and necrosis in the death of activated T cells, this report suggests that autophagy is also important for the regulation of CD4+ T-cell homeostasis.
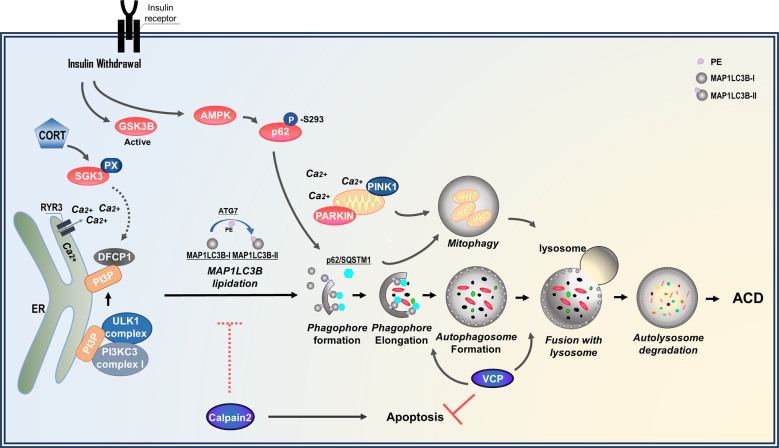
We have also reported ACD in murine adult hippocampal neural stem cells (NSCs) following insulin withdrawal (Fig. 1b)51 with no hallmarks of apoptosis (caspase activation, chromosomal DNA fragmentation, or cell death prevention by Z-VAD)52. In contrast, insulin-deficient hippocampal NSCs exhibited increased autophagy flux, as determined by assessing morphological and biochemical markers of autophagy. Furthermore, knocking down Atg7 or promoting autophagy using rapamycin decreased or increased cell death, respectively, fulfilling the criteria for ACD and indicating that this experimental system can be regarded as a genuine model of ACD27,53. According to this finding, the molecular machinery of ACD following insulin withdrawal in adult hippocampal NSCs has been gradually established (Fig. 2). GSK3B was identified as one of the upstream kinases involved in the initiation of ACD52. Genetic and pharmacological inhibition of GSK3B attenuated ACD, whereas its activation accelerated ACD following insulin withdrawal52. This study demonstrated that GSK3B is a positive regulator of ACD following insulin withdrawal in adult hippocampal NSCs. Mitophagy was also observed following insulin withdrawal. Insulin withdrawal activated AMPK, and AMPK phosphorylated p62 at a novel site, Ser-293/Ser-294 (rat/human sequence, respectively)54. Phosphorylated p62 was then translocated to mitochondria where it induced mitophagy and ACD54. Another important pathway in mitophagy in adult hippocampal NSCs following insulin withdrawal is established by the recruitment of PINK1/PARKIN to depolarized mitochondria55. Insulin withdrawal increased the ratio of depolarized mitochondria and their colocalization with autophagosomes. PARKIN was also upregulated in insulin-deprived adult hippocampal NSCs, and it mediated mitophagy and cell death. One interesting role of PARKIN in mitophagy is its mediation of Ca2+ transfer from the endoplasmic reticulum (ER) to mitochondria and the induction of mitochondrial depolarization during the early steps of mitophagy. These novel functions of PARKIN, in addition to its well-known role in the recognition and loss of depolarized mitochondria, contributes to mitophagy and cell death in adult hippocampal NSCs55. RYR3 is critical for ER calcium release56. These studies have firmly established the central role of autophagy in the death of adult hippocampal NSCs upon insulin withdrawal because the effects of GSK3B, RYR3, and PARKIN were all significantly blunted upon Atg7 knockdown52–55.
Fig. 2. Molecular effectors in ACD of adult hippocampal NSCs following insulin withdrawal or psychological stress.
Insulin withdrawal activates GSK3B and AMPK, followed by AMPK-mediated phosphorylation of p62. RYR3 mediates the efflux of Ca2+ from the ER. PARKIN levels are upregulated in a GSK3B-dependent manner, and PARKIN is involved in the transfer of ER Ca2+ to mitochondria and depolarization of mitochondrial membrane potential. Both p62 and PARKIN promote mitophagy, leading to ACD. Cell death triggered by insulin withdrawal is switched from ACD to apoptosis by calpain 2 and VCP. High levels of corticosterone (CORT) induced by CRS cause ACD via SGK3, which has a PX domain for binding to PI3P and the initiation of autophagy. The dashed lines indicate that the process is not yet experimentally confirmed.
ACD in mammalian pathophysiology
Tumor suppression
Recently, it was reported that autophagy activation is required for cell death and, thereby, the elimination of precancerous cells during replicative crisis caused by telomere dysfunction and that loss of ACD initiates tumorigenesis in fibroblasts and epithelial cells57. During replicative crises, apoptosis markers were not detected, whereas extensive cytoplasmic double-membrane autophagosomes and single-membrane autolysosomes were observed with a reduced level of p62, an autophagy cargo receptor. Moreover, the accumulation of microtubule-associated protein 1 light chain 3-beta-II (MAP1LC3B)-II and p62 after treatment with the autophagy blocker bafilomycin A1 suggested increased autophagy flux during replicative crisis. On the other hand, shRNA against ATG3, ATG5, or ATG7 promoted a bypass of the crisis, continued cell proliferation, and increased genome instability. It was also found that telomeric DNA damage activated ACD via the cGAS-STING pathway57 (Fig. 1c). These findings highlight autophagy as an essential component in tumor-suppressive mechanisms.
Melanoma cells, which are resistant to apoptosis-inducing drugs, can undergo ACD upon treatment with compounds targeting orphan nuclear receptor TR358. Upon treatment, TR3 is translocated to mitochondria via its interaction with the mitochondrial outer membrane protein Nix and dissipates mitochondrial membrane potential to induce massive mitochondrial clearance and ACD. TR3 translocation-triggered autophagy requires TR3 to cross into the mitochondrial inner membrane; therefore, this nuclear receptor becomes integrated into a mitochondrial signaling pathway to induce ACD. However, further details are not yet clear, particularly those that might indicate whether selective removal of mitochondria through mitophagy is required for ACD or whether mitochondrial clearance is simply part of bulk autophagic degradation. Despite the lack of detailed knowledge of the mode of action of TR3-targeting compounds, engagement of TR3 by compounds targeted to it demonstrated antimelanoma activity in the liver and lung in several mouse models58.
The role of autophagy in tumorigenesis is complex, with autophagy having different consequences for cancer development and treatment depending on the types of tumors and their stages59. Autophagy may serve as a tumor-suppressor pathway through several mechanisms: by maintaining genomic stability; by eliminating defective subcellular organelles, including depolarized mitochondria, and thus by removing the cellular sources of oxidative stress; and by regulating inflammation. All of these mechanisms may contribute to the prevention of cancer development. However, the survival- and death-promoting functions of autophagy make its association with cancer treatment very complicated. In contrast to the antitumor roles of autophagy, whereby cancer cells are eradicated by ACD, autophagy can maintain cancer cells viability by providing metabolic substrates under nutrient-limited conditions, delaying the onset of apoptosis of cells challenged by chemotherapeutic drugs or irradiation, and enhancing cancer cell survival under stressful microenvironments, including hypoxia60.
Excitotoxicity
Kainate-induced excitotoxicity combined with hypoxia was used to mimic hypoxia–ischemia in vitro and induced cell death in primary rat cortical neurons. Cell death was blocked by pharmacological autophagy inhibitors and genetic inhibition of autophagy by knocking down Beclin-1 or Atg7, whereas overexpression of Beclin-1 or ATG7 enhanced hypoxic excitotoxicity61. In vivo knockdown via intrastriatal injection of lentivirus-expressing shBeclin-1 reduced striatal damage in a rat model of neonatal hypoxia–ischemia61. No apoptosis activation was observed, and Bcl-2 overexpression or caspase inhibition prevented neuronal cell death.
Peroxynitrite (ONOO–), a representative reactive nitrogen species, activates mitophagy via the PINK1/PARKIN pathway to mediate cerebral ischemia–reperfusion injury (Fig. 1d)62. Increased nitrotyrosine levels were observed in the plasma of ischemic stroke patients and in ischemia–reperfusion injured rat brains; the recruitment of DRP1 to dysfunctional mitochondria with decreased membrane potential activated the PINK1/PARKIN pathway to initiate mitophagic flux and thus remove the damaged mitochondria, which reduced infarct volume and cell death in the ischemia–reperfusion injured brains. FeTMPyP, a peroxynitrite decomposition catalyst, and Mdivi-1, a blocker of mitophagy activation, prevented mitophagy-induced cell death in the ischemia–reperfusion injured brain.
In hippocampal neuronal cell death caused by neonatal hypoxia–ischemia, both caspase-3-dependent and caspase-3-independent cell death pathways are activated with the concomitant induction of autophagy63,64. Nestin-Cre-driven conditional knockout (cKO) of Atg7 in the nervous system prevented both caspase-dependent and caspase-independent neuronal death and reduced hippocampal damage. Interestingly, neuronal death was both caspase-dependent and caspase-independent at the neonatal stage but caspase-independent with more-pronounced autophagy levels at the adult stage. However, because mice deficient in Atg7 undergo neurodegeneration during development, whether neuronal cell death elicited by hypoxia–ischemia is truly attributable to ACD needs further study using an inducible KO adult mouse model.
Psychological stress
Chronic stress or prolonged glucocorticoid administration leads to loss of hippocampal neurons and a reduction in hippocampus size65,66. Glucocorticoid receptors are enriched in the hippocampus67, and adult hippocampal neurogenesis (continuous generation of new neurons in the adult hippocampus over a lifetime) is highly susceptible to psychological stress and is greatly reduced in various models of stress68,69. However, most studies have failed to detect signs of apoptosis; therefore, PCD of hippocampal neurons or adult hippocampal NSCs has not been considered as a mechanism of stress-induced decline in adult hippocampal neurogenesis or hippocampal damage70,71. However, our recent genetic study using adult NSC-specific Atg7-cKO mice demonstrated that chronic restraint stress (CRS) induced ACD in adult hippocampal NSCs in vivo and in vitro (Fig. 1e)72. As autophagy is essential for development and tissue homeostasis, deletion of key autophagy genes in the brain from an early developmental stage causes neurodegenerative symptoms and it is difficult to explore ACD in the adult mouse brain73–75. To overcome this obstacle and study the role of autophagy in the effects of psychological stress on adult hippocampal NSCs, a Nestin-Cre-ERT2 mouse line was crossed with Atg7 flox mice, and Atg7 deletion was induced in the offspring at 7 weeks of age; these NSC-specific cKO mice (Atg7-NSC cKO mice) were subjected to CRS. Histological and electron microscopic examination revealed an increase in autophagy flux but not in apoptosis, in hippocampal NSCs. Loss of NSCs and decreases in adult neurogenesis were blocked by Atg7 deletion72. Furthermore, stress-triggered anxiety and depression, as well as cognitive deficits, were effectively prevented in the Atg7-NSC cKO mice. These findings indicated that ACD is undoubtedly physiologically important in mammals and that autophagy in the adult hippocampus may provide a new therapeutic avenue for the treatment of stress-induced psychological disorders.
In adult hippocampal NSCs, serum/glucocorticoid regulated kinase (SGK) family proteins are suspected to be the signaling mechanism mediating stress-induced ACD, as SGK1 was previously reported to mediate glucocorticoid effects on hippocampal neurogenesis76,77. The SGK family consists of three members: SGK1, SGK2, and SGK378. The SGK family has a three-dimensional structure and sequence similar to those of the protein kinase B (PKB)/AKT family78,79. Importantly, a series of experiments using the CRISPR/CAS9 genome editing technique to knock out SGK1, 2, or 3 revealed that SGK3, but not SGK1 or 2, is a critical mediator of ACD (Fig. 2)72. SGK3 contains a complete Phox homology (PX) domain78,80, which contains a phosphoinositide-binding site. Phosphatidylinositol 3-phosphate (PtdIns3P) is the most common lipid that binds to the PX domain, and it is enriched in endosomes and vacuoles; SGK3 binds PtdIns3P and is located mostly in endosomes79. PtdIns3P is a product of PI3K and regulates the initiation of autophagy81. A point mutation in which Arg-90 is changed to Ala-90 in SGK3 prevented ACD72. Therefore, SGK3 is a critical regulator of stress-induced ACD and has this role by interacting with PtdIns3P in adult hippocampal NSCs. However, additional studies are required to elucidate the details of how SGK3 regulates ACD and to explore SGK3 as a potential therapeutic target for stress-induced psychological disorders.
Molecular intersection of ACD and apoptosis
Why is ACD activated in normal cells equipped with intact apoptosis capability? This question can be answered by examining the molecular pathways that link ACD and apoptosis. Detailed studies on insulin-deficient adult hippocampal NSCs have offered a few glimpses into the complicated intersection of ACD and apoptosis (Fig. 2). Calpain 2 is a major calpain in adult hippocampal NSCs and was identified as a key rheostat of apoptosis with respect to ACD, as suppression of calpain activity promoted ACD, whereas higher calpain activity switched the cell death program from ACD to apoptosis in insulin-deprived adult hippocampal NSCs82. Another interesting effector in the interplay between apoptosis and ACD in adult hippocampal NSCs is valosin-containing protein (VCP), which positively regulates autophagosome maturation at the basal state. However, under conditions of high autophagy flux following insulin withdrawal, VCP regulates the autophagy initiation step83. Of interest, pharmacological, and genetic inactivation of VCP led to apoptosis with a concomitant increase in calpain 2 levels in insulin-deprived adult hippocampal NSCs83. However, the switch from ACD to apoptosis and upregulation of calpain activity by inhibition or knockdown of VCP under insulin-deprived conditions were prevented by Atg7 knockdown, indicating that ACD is a prerequisite for the switch to apoptosis.
ATG5 and Beclin-1 were reported as substrates of calpain. Calpain cleaves ATG5 in HeLa, Jurkat, and MDA-MA-231 cells in response to several apoptotic stimuli, including etoposide, doxorubicin, and staurosporine84. Cleaved ATG5 then translocates from the cytosol to mitochondria, where it associates with Bcl-X1, and triggers cytochrome c release and caspase activation. Calpain-mediated cleavage of Beclin-1 following renal ischemia results in autophagy inhibition and extensive neuronal death85. However, we could not detect cleavage of ATG5 or Beclin-1 in insulin-deprived adult hippocampal NSCs. Therefore, understanding the molecular mechanism by which calpain regulates the switch from ACD to apoptosis in adult NSCs awaits further study.
The complex relationship between autophagy and apoptosis depends on the biological context and is not yet fully understood. Intriguingly, the two pathways share common components, such as Bcl-2 family proteins. Bcl-2 can directly bind to Beclin-1. As Beclin-1 is a core component of the VPS34 complex, which is required for phagophore formation and initiation of autophagy through the generation of PtdIns3P, the binding ability of Bcl-2 to Beclin-1 confers, in addition to its well-known antiapoptotic function, another critical cellular function to Bcl-2: an antiautophagic role. Interestingly, the interaction of Bcl-2 with Beclin-1 does not interfere with the antiapoptotic potential of Bcl-286. However, this interaction can be disrupted by posttranslational modification of Bcl-2 or Beclin-1, including phosphorylation, ubiquitination, or caspase-mediated cleavage87,88. Nevertheless, whether the interaction of ACD with apoptosis is controlled by Bcl-2 family proteins is not yet clear. This indication will be worth more attention in the near future.
Our recent finding that caspase-9 is activated in an APAF-1-independent manner in insulin-deprived adult hippocampal NSCs provides another intriguing illustration of the interaction of autophagy and apoptosis89. Caspase-9 promotes ACD but not apoptosis following insulin withdrawal in adult NSCs89. Elucidation of the molecular mechanism by which autophagy directs caspase-9 into ACD rather than apoptosis will greatly advance our understanding of the interconnection between apoptosis and ACD.
Conclusion and unresolved questions
Studies on the cell death mechanism in adult hippocampal NSCs following insulin withdrawal or psychological stress have greatly contributed to the elucidation of ACD at the molecular level. Adult hippocampal NSCs have intact machinery for apoptosis and necroptosis subroutines, as indicated by staurosporine or H2O2 treatment inducing apoptosis or necroptosis in these cells, and this machinery can be inhibited by appropriate pharmacological inhibitors72. Therefore, the immediately forthcoming question is how ACD rather than apoptosis/necroptosis is predominantly triggered to promote cell death. Another conundrum is the nature of the signaling mechanisms that dictate the contradictory roles of autophagy in cell death and cell survival. As a compromise, it has been assumed that basal, low-level autophagy is cytoprotective, whereas the sustained excessive level of autophagy flux causes cell death. However, this assumption has not yet been tested experimentally. As most techniques to measure autophagy flux are qualitative, quantitative comparisons of autophagy flux between different conditions, even in the same cell type, as well as between different cell types, is technically very challenging. Therefore, the molecular mechanisms of ACD are far from being understood. Nevertheless, we have recently witnessed an increasing recognition of the critical roles of ACD in mammalian pathophysiology, including tumor suppression and mental disorders associated with psychological stress. Elucidation of this uniquely programmed mechanism of cell death holds great potential for applications of autophagy in human health and the treatment of diseases.
Acknowledgements
This work was supported by National Research Foundation of Korea (NRF) grants (2018 8M3C7A1056275 and 2020R1A2C3005215) from the Ministry of Science and ICT of Korea.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yang Z, Klionsky DJ. Eaten alive: a history of macroautophagy. Nat. Cell Biol. 2010;12:814–822. doi: 10.1038/ncb0910-814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Duve C, Wattiaux R. Functions of lysosomes. Annu Rev. Physiol. 1966;28:435–492. doi: 10.1146/annurev.ph.28.030166.002251. [DOI] [PubMed] [Google Scholar]
- 3.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–1075. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kroemer G, Levine B. Autophagic cell death: the story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008;9:1004–1010. doi: 10.1038/nrm2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galluzzi L, et al. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev. Genet. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian M, Fang X, Wang X. Autophagy and inflammation. Clin. Transl. Med. 2017;6:24. doi: 10.1186/s40169-017-0154-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sinha RA, Singh BK, Yen PM. Reciprocal crosstalk between autophagic and endocrine signaling in metabolic homeostasis. Endocr. Rev. 2017;38:69–102. doi: 10.1210/er.2016-1103. [DOI] [PubMed] [Google Scholar]
- 9.Klionsky DJ, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition) Autophagy. 2016;12:1–222. doi: 10.1080/15548627.2015.1100356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chung KM, Yu SW. Interplay between autophagy and programmed cell death in mammalian neural stem cells. BMB Rep. 2013;46:383–390. doi: 10.5483/BMBRep.2013.46.8.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napoletano F, Baron O, Vandenabeele P, Mollereau B, Fanto M. Intersections between regulated cell death and autophagy. Trends Cell Biol. 2019;29:323–338. doi: 10.1016/j.tcb.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Saha S, Panigrahi DP, Patil S, Bhutia SK. Autophagy in health and disease: a comprehensive review. Biomed. Pharmacother. 2018;104:485–495. doi: 10.1016/j.biopha.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Jiang P, Mizushima N. Autophagy and human diseases. Cell Res. 2014;24:69–79. doi: 10.1038/cr.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lockshin RA, Williams CM. Programmed cell death-I. Cytology of degeneration in the intersegmental muscles of the pernyi silkmoth. J. Insect Physiol. 1965;11:123–133. doi: 10.1016/0022-1910(65)90099-5. [DOI] [PubMed] [Google Scholar]
- 15.Clarke PG. Developmental cell death: morphological diversity and multiple mechanisms. Anat. Embryol. (Berl.) 1990;181:195–213. doi: 10.1007/BF00174615. [DOI] [PubMed] [Google Scholar]
- 16.Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer. 1972;26:239–257. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 19.Kischkel FC, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chinnaiyan AM. The apoptosome: heart and soul of the cell death machine. Neoplasia. 1999;1:5–15. doi: 10.1038/sj.neo.7900003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hill MM, Adrain C, Duriez PJ, Creagh EM, Martin SJ. Analysis of the composition, assembly kinetics and activity of native Apaf-1 apoptosomes. EMBO J. 2004;23:2134–2145. doi: 10.1038/sj.emboj.7600210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galluzzi L, Kroemer G. Necroptosis: a specialized pathway of programmed necrosis. Cell. 2008;135:1161–1163. doi: 10.1016/j.cell.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Kawahara A, Ohsawa Y, Matsumura H, Uchiyama Y, Nagata S. Caspase-independent cell killing by Fas-associated protein with death domain. J. Cell Biol. 1998;143:1353–1360. doi: 10.1083/jcb.143.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, et al. Necroptosis inhibitor necrostatin-1 promotes cell protection and physiological function in traumatic spinal cord injury. Neuroscience. 2014;266:91–101. doi: 10.1016/j.neuroscience.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 25.Cui H, et al. Necrostatin-1 treatment inhibits osteocyte necroptosis and trabecular deterioration in ovariectomized rats. Sci. Rep. 2016;6:33803. doi: 10.1038/srep33803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bialik, S., Dasari, S. K. & Kimchi, A. Autophagy-dependent cell death—where, how and why a cell eats itself to death. J. Cell Sci.131, jcs215152 (2018). [DOI] [PubMed]
- 27.Shen HM, Codogno P. Autophagic cell death: Loch Ness monster or endangered species? Autophagy. 2011;7:457–465. doi: 10.4161/auto.7.5.14226. [DOI] [PubMed] [Google Scholar]
- 28.Berry DL, Baehrecke EH. Growth arrest and autophagy are required for salivary gland cell degradation in Drosophila. Cell. 2007;131:1137–1148. doi: 10.1016/j.cell.2007.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baehrecke EH. Autophagic programmed cell death in Drosophila. Cell Death Differ. 2003;10:940–945. doi: 10.1038/sj.cdd.4401280. [DOI] [PubMed] [Google Scholar]
- 30.Denton D, et al. Autophagy, not apoptosis, is essential for midgut cell death in Drosophila. Curr. Biol. 2009;19:1741–1746. doi: 10.1016/j.cub.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Denton D, Xu T, Dayan S, Nicolson S, Kumar S. Dpp regulates autophagy-dependent midgut removal and signals to block ecdysone production. Cell Death Differ. 2019;26:763–778. doi: 10.1038/s41418-018-0154-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cornillon S, et al. Programmed cell death in Dictyostelium. J. Cell Sci. 1994;107:2691–2704. doi: 10.1242/jcs.107.10.2691. [DOI] [PubMed] [Google Scholar]
- 33.Luciani MF, et al. Atg1 allows second-signaled autophagic cell death in Dictyostelium. Autophagy. 2011;7:501–508. doi: 10.4161/auto.7.5.14957. [DOI] [PubMed] [Google Scholar]
- 34.Giusti C, Tresse E, Luciani MF, Golstein P. Autophagic cell death: analysis in Dictyostelium. Biochim. Biophys. Acta. 2009;1793:1422–1431. doi: 10.1016/j.bbamcr.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 35.Dasari SK, et al. Signalome-wide RNAi screen identifies GBA1 as a positive mediator of autophagic cell death. Cell Death Differ. 2017;24:1288–1302. doi: 10.1038/cdd.2017.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu C, et al. Targeting surface nucleolin induces autophagy-dependent cell death in pancreatic cancer via AMPK activation. Oncogene. 2019;38:1832–1844. doi: 10.1038/s41388-018-0556-x. [DOI] [PubMed] [Google Scholar]
- 37.Kanzawa T, Kondo Y, Ito H, Kondo S, Germano I. Induction of autophagic cell death in malignant glioma cells by arsenic trioxide. Cancer Res. 2003;63:2103–2108. [PubMed] [Google Scholar]
- 38.Yu L, et al. Regulation of an ATG7-beclin 1 program of autophagic cell death by caspase-8. Science. 2004;304:1500–1502. doi: 10.1126/science.1096645. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, et al. Autophagic programmed cell death by selective catalase degradation. Proc. Natl. Acad. Sci. USA. 2006;103:4952–4957. doi: 10.1073/pnas.0511288103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lamy L, et al. Control of autophagic cell death by caspase-10 in multiple myeloma. Cancer Cell. 2013;23:435–449. doi: 10.1016/j.ccr.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Oxidative stress induces autophagic cell death independent of apoptosis in transformed and cancer cells. Cell Death Differ. 2008;15:171–182. doi: 10.1038/sj.cdd.4402233. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, McMillan-Ward E, Kong J, Israels SJ, Gibson SB. Mitochondrial electron-transport-chain inhibitors of complexes I and II induce autophagic cell death mediated by reactive oxygen species. J. Cell Sci. 2007;120:4155–4166. doi: 10.1242/jcs.011163. [DOI] [PubMed] [Google Scholar]
- 43.Zhao Y, et al. Cytosolic FoxO1 is essential for the induction of autophagy and tumour suppressor activity. Nat. Cell Biol. 2010;12:665–675. doi: 10.1038/ncb2069. [DOI] [PubMed] [Google Scholar]
- 44.Law BYK, et al. Neferine induces autophagy-dependent cell death in apoptosis-resistant cancers via ryanodine receptor and Ca(2+)-dependent mechanism. Sci. Rep. 2019;9:20034. doi: 10.1038/s41598-019-56675-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshikawa N, et al. Plasma-activated medium promotes autophagic cell death along with alteration of the mTOR pathway. Sci. Rep. 2020;10:1614. doi: 10.1038/s41598-020-58667-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shimizu S, et al. Role of Bcl-2 family proteins in a non-apoptotic programmed cell death dependent on autophagy genes. Nat. Cell Biol. 2004;6:1221–1228. doi: 10.1038/ncb1192. [DOI] [PubMed] [Google Scholar]
- 47.Arakawa S, et al. Role of Atg5-dependent cell death in the embryonic development of Bax/Bak double-knockout mice. Cell Death Differ. 2017;24:1598–1608. doi: 10.1038/cdd.2017.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karch, J. et al. Autophagic cell death is dependent on lysosomal membrane permeability through Bax and Bak. Elife6, e30543 (2017). [DOI] [PMC free article] [PubMed]
- 49.Deruy E, et al. MnSOD upregulation induces autophagic programmed cell death in senescent keratinocytes. PLoS ONE. 2010;5:e12712. doi: 10.1371/journal.pone.0012712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li C, et al. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J. Immunol. 2006;177:5163–5168. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- 51.Yu SW, et al. Autophagic death of adult hippocampal neural stem cells following insulin withdrawal. Stem Cells. 2008;26:2602–2610. doi: 10.1634/stemcells.2008-0153. [DOI] [PubMed] [Google Scholar]
- 52.Ha S, et al. Regulation of autophagic cell death by glycogen synthase kinase-3beta in adult hippocampal neural stem cells following insulin withdrawal. Mol. Brain. 2015;8:30. doi: 10.1186/s13041-015-0119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clarke PG, Puyal J. Autophagic cell death exists. Autophagy. 2012;8:867–869. doi: 10.4161/auto.20380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ha S, et al. Phosphorylation of p62 by AMP-activated protein kinase mediates autophagic cell death in adult hippocampal neural stem cells. J. Biol. Chem. 2017;292:13795–13808. doi: 10.1074/jbc.M117.780874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park H, et al. Parkin promotes mitophagic cell death in adult hippocampal neural stem cells following insulin withdrawal. Front. Mol. Neurosci. 2019;12:46. doi: 10.3389/fnmol.2019.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chung KM, Jeong EJ, Park H, An HK, Yu SW. Mediation of autophagic cell death by type 3 ryanodine receptor (RyR3) in adult hippocampal neural stem Cells. Front. Cell Neurosci. 2016;10:116. doi: 10.3389/fncel.2016.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nassour J, et al. Autophagic cell death restricts chromosomal instability during replicative crisis. Nature. 2019;565:659–663. doi: 10.1038/s41586-019-0885-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang WJ, et al. Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway. Nat. Chem. Biol. 2014;10:133–140. doi: 10.1038/nchembio.1406. [DOI] [PubMed] [Google Scholar]
- 59.Marinkovic M, Sprung M, Buljubasic M, Novak I. Autophagy modulation in cancer: current knowledge on action and therapy. Oxid. Med Cell Longev. 2018;2018:8023821. doi: 10.1155/2018/8023821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Levine B. Cell biology: autophagy and cancer. Nature. 2007;446:745–747. doi: 10.1038/446745a. [DOI] [PubMed] [Google Scholar]
- 61.Ginet V, et al. Involvement of autophagy in hypoxic-excitotoxic neuronal death. Autophagy. 2014;10:846–860. doi: 10.4161/auto.28264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng J, et al. Inhibition of peroxynitrite-induced mitophagy activation attenuates cerebral ischemia-reperfusion injury. Mol. Neurobiol. 2018;55:6369–6386. doi: 10.1007/s12035-017-0859-x. [DOI] [PubMed] [Google Scholar]
- 63.Koike M, et al. Inhibition of autophagy prevents hippocampal pyramidal neuron death after hypoxic-ischemic injury. Am. J. Pathol. 2008;172:454–469. doi: 10.2353/ajpath.2008.070876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xie C, et al. Neuroprotection by selective neuronal deletion of Atg7 in neonatal brain injury. Autophagy. 2016;12:410–423. doi: 10.1080/15548627.2015.1132134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee T, Jarome T, Li SJ, Kim JJ, Helmstetter FJ. Chronic stress selectively reduces hippocampal volume in rats: a longitudinal magnetic resonance imaging study. Neuroreport. 2009;20:1554–1558. doi: 10.1097/WNR.0b013e328332bb09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J. Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reul JMHM, Kloet ERD. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2511. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 68.Gould E, Cameron H, Daniels D, Woolley C, McEwen B. Adrenal hormones suppress cell division in the adult rat dentate gyrus. J. Neurosci. 1992;12:3642–3650. doi: 10.1523/JNEUROSCI.12-09-03642.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cameron HA, Gould E. Adult neurogenesis is regulated by adrenal steroids in the dentate gyrus. Neuroscience. 1994;61:203–209. doi: 10.1016/0306-4522(94)90224-0. [DOI] [PubMed] [Google Scholar]
- 70.Lagace DC, et al. Adult hippocampal neurogenesis is functionally important for stress-induced social avoidance. Proc. Natl. Acad. Sci. USA. 2010;107:4436–4441. doi: 10.1073/pnas.0910072107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-kappaB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proc. Natl Acad. Sci. USA. 2010;107:2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jung S, et al. Autophagic death of neural stem cells mediates chronic stress-induced decline of adult hippocampal neurogenesis and cognitive deficits. Autophagy. 2020;16:512–530. doi: 10.1080/15548627.2019.1630222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Komatsu M, et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature. 2006;441:880–884. doi: 10.1038/nature04723. [DOI] [PubMed] [Google Scholar]
- 74.Mo J, Atherton SS, Wang L, Liu S. Autophagy protects against retinal cell death in mouse model of cytomegalovirus retinitis. BMC Ophthalmol. 2019;19:146. doi: 10.1186/s12886-019-1141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hara T, et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature. 2006;441:885–889. doi: 10.1038/nature04724. [DOI] [PubMed] [Google Scholar]
- 76.Sato H, et al. Large-scale analysis of glucocorticoid target genes in rat hypothalamus. J. Neurochem. 2008;106:805–814. doi: 10.1111/j.1471-4159.2008.05489.x. [DOI] [PubMed] [Google Scholar]
- 77.Anacker C, et al. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc. Natl. Sci. USA. 2013;110:8708–8713. doi: 10.1073/pnas.1300886110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bruhn MA, Pearson RB, Hannan RD, Sheppard KE. Second AKT: the rise of SGK in cancer signalling. Growth Factors. 2010;28:394–408. doi: 10.3109/08977194.2010.518616. [DOI] [PubMed] [Google Scholar]
- 79.Basnet R, Gong GQ, Li C, Wang MW. Serum and glucocorticoid inducible protein kinases (SGKs): a potential target for cancer intervention. Acta Pharm. Sin. B. 2018;8:767–771. doi: 10.1016/j.apsb.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem. J. 1999;344:189–197. [PMC free article] [PubMed] [Google Scholar]
- 81.Yu X, Long YC, Shen HM. Differential regulatory functions of three classes of phosphatidylinositol and phosphoinositide 3-kinases in autophagy. Autophagy. 2015;11:1711–1728. doi: 10.1080/15548627.2015.1043076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chung, K. M. et al. Calpain determines the propensity of adult hippocampal neural stem cells to autophagic cell death following insulin withdrawal. Stem Cells33, 3052–3064 (2015). [DOI] [PubMed]
- 83.Yeo BK, et al. Valosin-containing protein is a key mediator between autophagic cell death and apoptosis in adult hippocampal neural stem cells following insulin withdrawal. Mol. Brain. 2016;9:31. doi: 10.1186/s13041-016-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yousefi S, et al. Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis. Nat. Cell Biol. 2006;8:1124–1132. doi: 10.1038/ncb1482. [DOI] [PubMed] [Google Scholar]
- 85.Russo R, et al. Calpain-mediated cleavage of Beclin-1 and autophagy deregulation following retinal ischemic injury in vivo. Cell Death Dis. 2011;2:e144. doi: 10.1038/cddis.2011.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pattingre S, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–939. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 87.Wirawan E, et al. Caspase-mediated cleavage of Beclin-1 inactivates Beclin-1-induced autophagy and enhances apoptosis by promoting the release of proapoptotic factors from mitochondria. Cell Death Dis. 2010;1:e18. doi: 10.1038/cddis.2009.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang R, Zeh HJ, Lotze MT, Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011;18:571–580. doi: 10.1038/cdd.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.An, H. K. et al. CASP9 (caspase 9) is essential for autophagosome maturation through regulation of mitochondrial homeostasis. Autophagy 1–20, 10.1080/15548627.2019.1695398 (2019). [DOI] [PMC free article] [PubMed]