Abstract
Dendritic cells (DCs) are the most important antigen-presenting cells that determine cancer immune responses by regulating immune activation and tolerance, especially in the initiation stage of specific responses. Manipulation of DCs to enhance specific antitumor immune response is considered to be a powerful tool for tumor eradication. Nanotechnology, which can incorporate multifunction components and show spatiotemporal control properties, is of great interest and is widely investigated for its ability to improve immune response activity against cancer and even for prevention and avoiding recurrence. In this mini-review, we aim to provide a general view of DC-based immunotherapy, including that involving the promising nanotechnology. Particularly we discuss: (1) manipulation or engineering of DCs for adoptive vaccination, (2) employing DCs as a combination to more existing therapeutics in tumor treatment, and (3) direct modulation of DCs in vivo to enhance antigen presentation efficacy and priming T cells subsequently. We comprehensively discuss the updates on the application of nanotechnology in DC-based immunotherapy and provide some insights on the challenges and opportunities of DC-based immunotherapeutics, including the potential of nanotechnology, against cancers.
Keywords: nanotechnology, dendritic cell, cancer, immunotherapy, antigen delivery, cross-presentation
Introduction
Cancer therapies have been evolving with advances in oncology (Couzin-Frankel, 2013). There has been some success with the use of traditional cancer therapies such as surgery (Krook et al., 1991; Wolf, 1991; Macdonald et al., 2001; Coffey et al., 2003), chemotherapy (Liu et al., 2011; Al-Lazikani et al., 2012; Koppelmans et al., 2012; Chen et al., 2017), and radiotherapy (Sauer et al., 2004; Abe et al., 2005). However, low-specificity, drug resistance, and side effects hinder the efficacies of these tumor treatments (Lippert et al., 2008; Guth et al., 2011; Housman et al., 2014; Rothermundt, 2015; Leary et al., 2018). Inducing or boosting up the patients’ own immune responses enable specific recognition and killing of tumor cells. Besides, immune response-mediated tumor eradication reduces the potential risks of toxicity and drug resistance (Beltran et al., 2011; Zhong et al., 2017). Therefore, in recent years, cancer immunotherapy is burgeoning and is considered to be the fourth important therapeutic method to deal with cancers. For example, the first tumor vaccine Sipuleucel-T was approved by the U.S. Food and Drug Administration (FDA) in 2010 for prostate cancer treatment (Higano et al., 2009; Kantoff et al., 2010). In the next year, the first immune-checkpoint inhibitor antibody Ipilimumab (anti-CTLA4 antibody) was approved for the treatment of advanced melanoma (Robert et al., 2011). In the next few years, multiple immune-checkpoint blockage antibodies were approved in various cancers and achieved great success (Brahmer et al., 2015; Le et al., 2015; Reck et al., 2016). Encouragingly, the Nobel prize in physiology and medicine honored James P. Allison and Tasuku Honjo for their discovery of the efficacy of inhibiting negative immune regulation in cancer therapy. Additionally, Tisagenlecleucel, the first chimeric antigen receptor-T cell-based adoptive transfer treatment for pediatric and young adult acute lymphoblastic leukemia and adult diffuse large B-cell lymphoma was approved in 2018 by the FDA (June et al., 2018; Maude et al., 2018).
Although great achievements have been made in cancer immunotherapy, only some percentage of patients can benefit from those promising immunotherapeutics, mainly owing to the immunosuppressive microenvironment of the solid tumor and immune tolerance to mono-therapeutics (Albini et al., 2018; Binnewies et al., 2018; Costa et al., 2018; Feng et al., 2018; Knudson et al., 2018; Zhao et al., 2018). Therefore, maximizing the potential and ability of the immune system to overcome tumors is critical (Zhang and Bevan, 2011; Nicholson, 2016). Dendritic cells (DCs) are the initiators of specific immune responses, and when activated effectively can lead to priming of T cells to elicit immune antitumor responses for tumor destruction (Rescigno et al., 1997; Leifer, 2017). More importantly, results of whole exome sequencing and RNA sequencing present the possibility of developing DC-targeting vaccines and DC-based immunotherapy (Leitner et al., 1999; Mathan et al., 2017). Manipulating DCs by taking advantage of the controllable and modifiable features of nanotechnology shows promising antitumor responses both in vitro and in vivo.
DCs in Immune System
DCs are originated from dedicated hematopoietic precursor cells and transformed into DC in variable stimuli in physiological environment. (Thomas and Lipsky, 1996; Ardavin et al., 2001; Ardavin, 2003; Wang et al., 2019b). Circulating blood cells such as monocytes, white blood cells may differentiate to matured DCs owing to the crosstalk among diverse signals coordination (Rutella et al., 2006; Williams et al., 2013). DCs were demonstrated to play a crucial role in mediating innate and adaptive immune responses in 1970s by Ralph Steinman and Zanvil A. Cohn, who were honored with the Noble Prize for their discovery. Since then, DCs have been documented as the most effective antigen-presenting cells that activate primary and subsequent memory immune responses (Kadowaki, 2009).
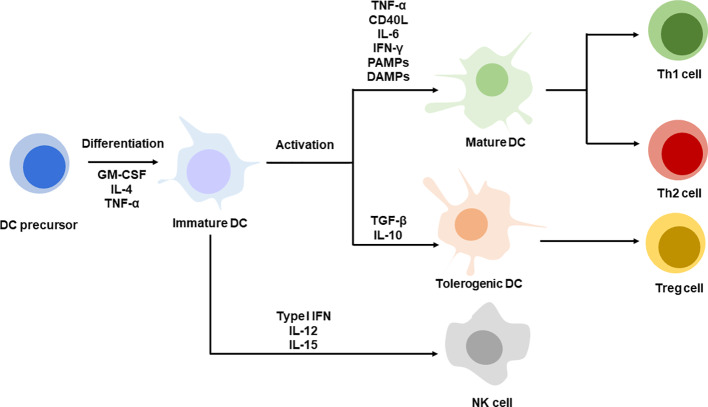
DCs as antigen-presenting cells encounter antigens and present them to T cell for priming in the form of antigen peptide-major histocompatibility complex (MHC) (Cohen et al., 2003; Zehn et al., 2004). Antigens may circulate through peripheral blood and stimulate DCs or captured by tissue resident DCs such as lymph node, spleen. Whatever, DCs’ received antigens are required to migrate to the lymphoid organs which determines the subsequent T cell activation efficacy due to the abundance of T cells in the lymphoid organs (Mempel et al., 2004) (Segura and Villadangos, 2009). The success of DCs’ activation requires two signals, antigens and activation stimuli. The second activation signals may be provided exogenously such as by lipopolysaccharide (Morrison and Kline, 1977), Toll-like receptor (TLR) ligands (Kawai and Akira, 2007), and antibodies targeting activation of receptors like tumor necrosis factor (Schnurr et al., 2000), proinflammatory cytokines IFN-γ, etc (Simmons et al., 2012; Minton, 2014). Endogenous damage-associated molecular patterns such as high mobility group proteins (Raucci et al., 2007), calreticulin (Li et al., 2015), and heat shock proteins with immunogenic features are also capable of maturing DCs (Bethke et al., 2002). Similarly, two signals are required for T cell priming; one is a specific antigen-MHC I/II complex and the other one is a costimulatory signal expressed on activated DCs. mDCs with antigen-MHC complex expression induce T cells to differentiate into Th1 or Th2 cells under the condition of variable cytokines, for example, IL-12, which is essential to activate cytotoxic T cells. In some other cases, under the condition of IL-4, it may switch immunity with antibody secretion. Mostly, these activated CD8+ T cells are fully functional, with cytotoxicity and ability to secrete IFN-γ for highly effective and specific killing of cancer cells. Other than cytotoxic T cell stimulation, matured DCs (mDCs) may also function to neutralize antibody secretion by B cells in the way of IL-10 and IL-33 secretion, driving Th2 immunity and influencing immunoglobulin subtype polarization (Segura et al., 2013). Additionally, DCs with inflammatory features induce Th17 differentiation, which promotes cytotoxic T cell responses and regresses tumor (Murugaiyan and Saha, 2009; Segura et al., 2013; Guéry and Hugues, 2015). Apart from stimulating specific adaptive immunity, mDCs can also be decorated with IL-12, IL-15, and type I IFNs which are positive to NK cell functions in innate immunity. Thus, the DCs act as a bridge between innate and adaptive immunity for host defenses ( Figure 1 ). As discussed above, the immature DCs that are usually found in peripheral lymphoid tissues process antigens without activation stimuli and are capable of presenting antigen-MHC to naïve T cells, resulting in tolerance to T cell responses (Probst et al., 2005).
Figure 1.
DC differentiation and maturation. DC precursors are differentiating into immature DC. Immature DCs are activated with specific stimuli, TNF-α, CD40L, IL-6, IFN-γ, PAMPs, DAMPs promote DC maturation, corresponding to Th1 or Th2 cells priming. DCs stimulate with TGF-β, IL-10 present tolerogenic phenotype leading to Treg differentiation. Immature DC decorated with type I IFN, IL-12 and IL-15 favor NK cell function.
Manipulation of DCs for Vaccination
DCs that initiate adaptive immune responses, including antitumorigenesis, are of great interest worldwide, owing to their ability to be able to present tumor-associated antigens and prime subsequent antitumor responses. Because of their critical role with professional presentation expertise in the immune system, exploiting DC for vaccination is considered to provide a powerful tool to prevent and cure infections and cancers (Lizotte et al., 2016; Roden and Stern, 2018; Wang et al., 2019a; Wen et al., 2019). As initiators of adaptive immunity, DCs are ideal targets for ex vivo education and adoptive vaccination, which can induce specific antitumor immune responses in patients in vivo (Lövgren et al., 2018). Conventional techniques to develop DC-based vaccines involved isolation or culture precursor cells from patients’ peripheral blood, loading them with antigens in vitro and applying certain maturation stimuli to promote DC maturation. Precursor cells such as CD34+ hematopoietic cells, monocytes cultured with certain stimuli enabled differentiating into immature DCs. In order to differentiate and expand DCs in vitro, immature DCs are loaded with tumor antigens such as tumor lysates, peptides, proteins, nucleotides, or fused with tumor cells, under the condition of stimulatory molecules. Challenges remained although more techniques have been developed. Peptides can be loaded directly on MHC molecules; however, it requires clear and definite information not only on the epitopes such as sequence and conserved motif, but also on an individual’s HLA configuration that determines the type of immune responses and adaptive immunity. Clinical study is ongoing to confirm the immunogenicity of peptide-loaded DC vaccination (NCT02334735). Instead of peptides, loading of proteins or lysates has also been used for numerous cancer treatments (NCT00045968). Although these require further intracellular processing, the major advantage of using whole protein processing is its potential to induce both CD4+ and CD8+ T cell responses (Constantino et al., 2016; Wang et al., 2017; Sharma et al., 2018). To realize the DC function of initiating specific antitumor immunity, DCs loaded with tumor-associated antigens require stimuli (example, CD40L or TLR agonist) for maturation. The mDCs are then transferred back to the patients to mediate specific immune response. The whole process is complicated and requires skilled operation and high cost. Most clinical studies on DC vaccines are in combination with chemotherapy (NCT03688178, NCT03657966, NCT03047525) (Kongsted et al., 2017), radiotherapy (NCT03226236,), targeted agents (Ogasawara et al., 2018; Palma et al., 2018), or immunotherapeutic regimens (NCT03546426, NCT03735290, NCT03450044, NCT03735589). Clinical studies implied that technologies that combine antigen loading and various combination agents are required and of great potential.
Nanotechnology refers to the technology utilizing nanosized materials that range from 10 to 100 nm in size; these are emerging as an ideal tool for cancer therapy. Nanomaterials with divergent compositions enable passive or active targeting, which enhances the efficacy and reduces the toxicity of treatment (Liu et al., 2018; Wang and Mooney, 2018; Feng et al., 2019). The ability of DCs to capture, process, and present antigen to induce T cell priming is essential in adaptive antitumor immunity. Therefore, nanomaterials targeting DCs are considered to be a promising tool to boost an efficient and specific anticancer immune response. Nanomaterials also incorporate multifunctional molecules together to modulate part or whole of the antigen presentation (Parvanian et al., 2017; Sangtani et al., 2017). Functional nanomaterials with lysosome escaping ability have been widely reported; these nanomaterials are favorable for antigen delivery to the cytoplasm and their intracellular digestion, which further contributes to antigen processing and presentation (Jiang et al., 2018; Zhong et al., 2019). Lipid-based nanoparticles and polymeric nanoparticles (Reichmuth et al., 2016; Zhou et al., 2016; Gulla et al., 2019; Wen et al., 2019; Lin et al., 2020), with better biocompatibility, have been reported in the development of DC targeted vaccines for anticancer and antiviral treatments. Liposome-based nanoparticles containing molecules such as trivalent influenza antigen, OVA, and heat shock proteins, have been investigated for their ability to activate the immune system in disease treatment. Delivery of protein, peptide, and nucleic acid antigens, which can induce corresponding immune responses, to DCs via nanomaterials might probably increase their circulation and reduce their degradation in vivo, which are necessary for future approaches that aim to directly activate DCs in vivo (Rogel et al., 1985; Paglia et al., 1996; Ghadersohi and Sood, 2001; Goodwin and Huang, 2017; Wongso et al., 2017; Dellacherie et al., 2018). Moreover, nanomaterials (e.g., gold, aluminum nanoparticles) probably enhance internalization at the single cell level (Wang et al., 2015; Li et al., 2016; Wang et al., 2016). Aluminum, which was documented as an adjuvant for Th2 stimulation, failed to generate CD8+ T cell responses (Ho et al., 2018). However, using nanosized aluminum particles (100 nm) stabilized by PEG-containing polymer showed higher internalization in antigen-presenting cells and activated CD8+ T cells for curing cancer. Thus, nanotechnology not only improves the bio- or physiochemical features of antigen formulation through antigen protection, quantity improvement, function combination, and antigen presentation pathway, but also redefines the efficacy of traditional agents or materials with potential novel functions in antitumor immunity modulation (Zupančič et al., 2017; Smith et al., 2018; Zhong et al., 2019), which are all beneficial for the subsequent adaptive immunity against cancers. Importantly, a few cases that used nanotechnology for DC vaccination have been reported. For instance, liposome based Tecemotide, AS15, DepoVax, decorating with tumor-associated antigens are in clinical trials (Berinstein et al., 2012; Butts et al., 2014; Vansteenkiste et al., 2014). Virus-like nanoparticles CYT004-MelQbG10 delivering melanoma-associated antigen peptide are in Phase II clinical trials (Speiser et al., 2010). These pre/clinical trials evidenced the potential targeting DC for antitumor immunotherapy.
In Vivo Activation of DC for Enhancing Antigen Presentation
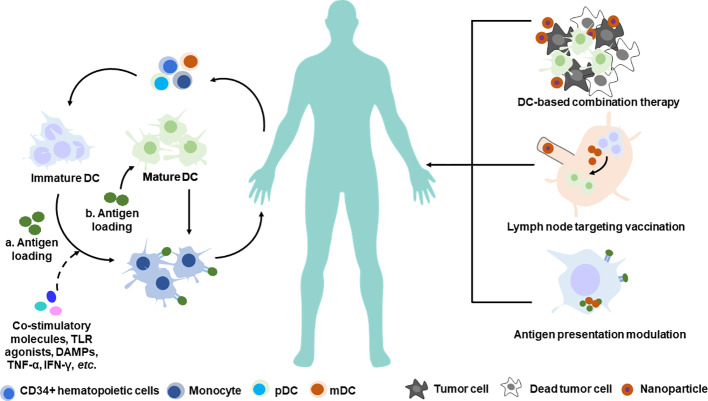
The traditional approach of activating DCs in vitro and transferring them back into patients is expensive, labor-dependent, and difficult to evaluate antitumor immunity generation parallelly in patients. Targeting DCs for in vivo activation with antitumor immunity enhancement is another promising DC-based therapeutic strategy to induce specific antitumor immune responses; this approach directly activates DCs in vivo and can potentially generate a large number of antitumor responses. Nanotechnology-enabled spatiotemporal delivery of formulations aimed to directly activate DCs has been demonstrated in numerous cases ( Figure 2 ). These formulations can be classified into three categories: (1) those that target and activate local DC response in the tumor microenvironment in addition to chemotherapy/radiotherapy (Sau et al., 2018); (2) those that target and activate lymph node-resident DCs, and in addition, target activation receptors on DCs, thereby boosting specific T cell response (De Koker et al., 2016; Jiang et al., 2017); and (3) those that modulate intracellular antigen presentation process for higher cross-presentation efficacy (Sil et al., 2019; Wang et al., 2019a).
Figure 2.
Nanotechnology-based manipulation of DCs therapy. Conventional strategy of DC-based adoptive transfer therapy (left) and novel strategy employing nanotechnology for future DC-based immunotherapy (right). a. Antigen loading on immature DC in the scenario of DC maturation stimuli. b. Antigen loading on mature DC directly. pDC, plasmacytoid dendritic cell; mDC, myeloid dendritic cell.
DC-Based Combination Therapy
Because of the heterogeneous features of variable tumors and their immune microenvironment, “cold” tumors with low immunogenicity show enrichment of immunosuppressive cells such as macrophages and myeloid-derived suppressor cells around the microenvironment to escape from the immune surveillance (Smyth et al., 2006; Dadi et al., 2016; Lussier and Schreiber, 2016; Haanen, 2017). Therefore, combination therapies aimed to change the tumor immunogenic phenotype provide a means to reduce the mortality associated with cancers (Da Silva et al., 2016; Nam et al., 2019). Chemo-immunotherapy approach that incorporates doxorubicin (DOX) and indoximod (IND) with phospholipid as a prodrug shows improved pharmacokinetics and accumulation in 4T1 breast tumor model. In such an approach, the chemo drugs induce tumor cell death, eliciting immunogenic reactions along with the debris being taken up by DCs, leading to further antigen presentation and naïve T cell priming. The activated T cells present cytotoxic effects on tumor cells through perforin and release of IFN-γ for robust killing of both primary and metastatic tumors. Not only does the dual-functional liposomes exert synergistic antitumor effects via DOX and IND components, but also the immune-checkpoint blockage combination treatment which further boosts immune responses allowing metastatic tumor eradication (Johnson et al., 2017; Lu et al., 2018). Radiotherapy is also known to promote CD8+ T cells around the tumor, however, radiation-induced immunosuppression may lead to treatment failure. Bismuth sulfide nanoparticles that conjugate immunoactive polysaccharide can increase radiotherapy sensitivity and activate DCs. Meanwhile, the nanoparticle can further enhance DC maturation and their distribution in tumor. Several studies have indicated that photodynamic therapy, which induces tumor cell lysates, is usually accompanied with damage-associated molecular pattern release that promotes DC activation, leading to specific antitumor immunity. Formulating photosensitizer is a major challenge in photodynamic therapy and further acceptance in clinic. The application of nanotechnology is a stride forward in solving this problem to some extent (Lucky et al., 2015), which favors photodynamic and DC combination therapy (Chen et al., 2016; Yu et al., 2019).
Lymph Node Targeting Vaccination
Peripheral DCs that encounter antigens are required to be trafficked to the lymph node where abundant lymphocytes are located and enabled highly efficient T cell priming. Moreover, diverse cytokines in the lymph node further promote DC maturation and exert function (Manfredi et al., 2008; Martín-Fontecha et al., 2009; Granot et al., 2017). Modifying antigens to target the lymph node and enhance lymph node-resident DC uptake was demonstrated to be a promising strategy in vaccine designing to initiate effective immune responses (Van Herck et al., 2018). Developing delivery systems with variable size can greatly influence the lymph node trafficking and DC uptake of antigens (Bachmann and Jennings, 2010; Kuai et al., 2017; Zhang et al., 2018). Proteins or subunit viral antigens within 10 nm are mostly incorporated with adjuvants to form larger-sized particles or aggregates. Supramolecular antigen formulations such as virus-like particles range from 20 to 200 nm. Virosomes with liposomes and antigens are approximately 100–200 nm (Jennings and Bachmann, 2007; Bachmann and Jennings, 2010). These antigen formulations are presented in the form of nano- or microparticles. Zhang et al. (2018) systemically investigated the influence of size and charge on the micelle-like formulations and their contributions to modulating immune responses, including lymph node accumulation, and DC internalization and immunogenicity, which provide an insight into the micelles and the generated immune responses. Lymph node accumulation and interaction properties of antigen-presenting cells are the two most important factors that influence the immunogenicity of nanoparticulated/nanoformulated antigens. Lymph node draining and DC uptake capability are both size-dependent processes, with a diameter of 10–200 nm considered to be optimal to stimulate immune response. Additionally, the authors claimed that the optimized size range was relatively independent of the materials, as the efficacy of lymph node draining and DC internalization is critically attributed to the particle size. However, variable materials with surface charges might influence the internalization capability of the antigen-presenting cells. Micelles with positive charged surface are beneficial for the enhancement of host antibody production (Reddy et al., 2007; Irvine et al., 2013). The previously published reviews provide an overview of materials on physiochemistry properties of antigen formulation in exerting effective immune responses.
Zhang et al. (2019) designed and synthesized a hybrid nanoparticle including the TLR 7/8 agonist imiquimod, TLR4 agonist monophosphoryl lipid A, polycaprolactone–polyethylene–polycaprolactone copolymer, dioleoyl-3-trimethylammonium propane, and 1,2-Distearoyl-sn-Glycero-3-Phosphoethanolamine-mannos. The hybrid nanoparticles enabled trafficking to secondary lymphoid tissues, spatiotemporal delivery and stimulation of both extracellular and intracellular TLRs, which was critical for efficient DC activation. It was demonstrated that the hybrid nanoparticles favorably enhanced the DC uptake of tumor antigen and cytokine secretion by mDCs. Additionally, homogenous tumor cells re-inoculated into C57BL/6 mice were inhibited upon treatment with the hybrid nanoparticles, highlighting the promising specific antitumor immune responses mediated by the nanoparticles.
Modulating Antigen Presentation for DC Vaccination
Antigen processing is an essential process for the final antigen presentation efficacy followed by lymph node draining accumulation and uptake by antigen-presenting cells. Thus, regulating intracellular antigen processing is important for antigen presentation and subsequent T cell priming. DCs are considered with well capacity for lysosomal proteolysis. It was documented that macrophages containing abundant lysosomal proteases enabled internalized protein degradation rapidly. In contrast, DCs with limited lysosomal capacity due to poor proteolysis degraded antigens slowly in vivo thus preserved antigen for an extended period. The limited proteolysis capacity benefits antigen presentation (Delamarre et al., 2005). Moderate lysosomal capacity of DCs favored antigen presentation implied us the opportunity of other pathway modulation in enhancing antigen degradation and presentation. It has been reported that inducing autophagy, an intracellular degradation and clearance process of unnecessary or dysfunctional components, is beneficial for antigen processing by DCs and leads to highly effective T cell stimulation. Wang et. al. combined a model antigen peptide OVA257–264, autophagy inducing peptide Beclin1, and a pH sensitive polymer together by covalent conjugation and fabricated an autophagy-inducing nanoactivator, which could induce autophagy in DCs, and evaluated the antigen-specific immune responses both in vitro and in vivo (Wang et al., 2015; Wang et al., 2019a). They demonstrated that induction of autophagy improved the antigen-presenting efficacy and T cell activation. Notably, the MHC I-OVA257–264 complex expression was significantly decreased on combining the nanoactivator with an autophagy inhibitor. Furthermore, cross-presentation DCs and antigen-specific T cells increased, along with the appearance of specific antitumor immune responses, tumor infiltrated cytotoxic T cell accumulation, and tumor growth inhibition, indicating that the nanoactivators were capable of initiating antigen-specific immune responses in vivo for the eradication of established solid tumors.
Conclusion
Considering that DCs play a vital role in priming naïve T cell and inducing adaptive immunity, tremendous efforts have been made in manipulating DC for cancer immunotherapy. It is documented that adoptive DC transfer can elicit specific immune responses in approximately 70% patients (Draube et al., 2011). Controlled parallel studies and comparative analysis are limited but essential for outcome evaluation and further clinical translation of adoptive DC vaccination. Moreover, immunosuppressive patients and intrinsic tumor-associated factors such as inhibitory ligands and Tregs do not favorably respond to DC-based immunotherapy. Hence, there is a need to improve therapeutic efficacy by manipulating DCs independently in the primary step. Developing combination strategies are expected to be promising in the exploitation of DC vaccines (Kalinski et al., 2013). Taking the advantages of nanotechnology, manipulation, and engineering of DCs in vitro can be simplified and its activation efficiency can be improved, which could increase the therapeutic efficacy of adoptive DC transfer treatment. In addition, nano-engineering can allow in vivo DC activation, mimicking natural antigen presentation followed by priming processes. Directly activating DCs in vivo fully employed the moderate lysosomal proteolysis feature that degraded antigens slowly which enabled sustaining antigen presentation. Making use of nanotechnology can also allow the conjugation of DCs to extraordinary functional molecules, which can further enhance antigen presentation, mediating sophisticated crosstalk inside the antigen-presenting cells and or work together with other cells, thereby contributing to the efficacy of DC-based immunotherapy. Another challenge to conventional DC-based therapeutics is that activated DCs are required to migrate to lymph nodes and prime T cells for specific antitumor responses; however, the majority of the DCs are unable to reach the lymph nodes, leading to limited specific immune responses. In contrast, nanoparticles enable higher lymph node accumulation, contributing to highly effective lymph node draining. Emerging nano- and bio-engineering techniques that fabricate lymphoid organs are promising to provide an artificial environment for DCs and T cell priming. Thus, nanotechnology is a promising approach for DC-based therapeutics and can promote multifield development with great impact on clinical cancer treatment.
Author Contributions
CQ, L-JY, and HC wrote the manuscript. HC provided advice both on the review writing and discussion.
Funding
This work was supported by Beijing Municipal Science & Technology Commission (Z171100001717020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Abe O., Abe R., Enomoto K., Kikuchi K., Koyama H., Masuda H., et al. (2005). Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 366, 2087–2106. 10.1016/S0140-6736(05)67887-7 [DOI] [PubMed] [Google Scholar]
- Albini A., Bruno A., Noonan D. M., Mortara L. (2018). Contribution to Tumor Angiogenesis From Innate Immune Cells Within the Tumor Microenvironment: Implications for Immunotherapy. Front. Immunol. 9, 1–9. 10.3389/fimmu.2018.00527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Lazikani B., Banerji U., Workman P. (2012). Combinatorial drug therapy for cancer in the post-genomic era. Nat. Biotechnol. 30, 679–691. 10.1038/nbt.2284 [DOI] [PubMed] [Google Scholar]
- Ardavin C., Del Hoyo G. M., Martin P., Anjuere F., Arias C. F., Marin A. R., et al. (2001). Origin and differentiation of dendritic cells. Trends Immunol. 22, 691–700. 10.1016/S1471-4906(01)02059-2 [DOI] [PubMed] [Google Scholar]
- Ardavin C. (2003). Origin, precursors and differentiation of mouse dendritic cells. Nat. Rev. Immunol. 3, 582–590. 10.1038/nri1127 [DOI] [PubMed] [Google Scholar]
- Bachmann M. F., Jennings G. T. (2010). Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 10, 787–796. 10.1038/nri2868 [DOI] [PubMed] [Google Scholar]
- Beltran H., Beer T. M., Carducci M. A., De Bono J., Gleave M., Hussain M., et al. (2011). New Therapies for Castration-Resistant Prostate Cancer: Efficacy and Safety. Eur. Urol. 60, 279–290. 10.1016/j.eururo.2011.04.038 [DOI] [PubMed] [Google Scholar]
- Berinstein N. L., Karkada M., Morse M. A., Nemunaitis J. J., Chatta G., Kaufman H., et al. (2012). First-in-man application of a novel therapeutic cancer vaccine formulation with the capacity to induce multi-functional T cell responses in ovarian, breast and prostate cancer patients. J. Trans. Med. 10, 156. 10.1186/1479-5876-10-156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bethke K., Staib F., Distler M., Schmitt U., Jonuleit H., Enk A. H., et al. (2002). Different efficiency of heat shock proteins (HSP) to activate human monocytes and dendritic cells: superiority of HSP60. J. Immunol. 169, 6141–6148. 10.4049/jimmunol.169.11.6141 [DOI] [PubMed] [Google Scholar]
- Binnewies M., Roberts E. W., Kersten K., Chan V., Fearon D. F., Merad M., et al. (2018). Understanding the tumor immune microenvironment (TIME) for effective therapy. Nat. Med. 24, 541–550. 10.1038/s41591-018-0014-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahmer J., Reckamp K. L., Baas P., Crino L., Eberhardt W. E. E., Poddubskaya E., et al. (2015). Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. New Engl. J. Med. 373, 123–135. 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts C., Socinski M. A., Mitchell P. L., Thatcher N., Havel L., Krzakowski M., et al. (2014). Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non-small-cell lung cancer (START): a randomised, double-blind, phase 3 trial. Lancet Oncol. 15, 59–68. 10.1016/S1470-2045(13)70510-2 [DOI] [PubMed] [Google Scholar]
- Chen Q., Xu L., Liang C., Wang C., Peng R., Liu Z. (2016). Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 7, 13193. 10.1038/ncomms13193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W. S., Ouyang J., Liu H., Chen M., Zeng K., Sheng J. P., et al. (2017). Black Phosphorus Nanosheet-Based Drug Delivery System for Synergistic Photodynamic/Photothermal/Chemotherapy of Cancer. Adv Mater 29, 1–7. 10.1002/adma.201603864 [DOI] [PubMed] [Google Scholar]
- Coffey J. C., Wang J. H., Smith M. J. F., Bouchier-Hayes D., Cotter T. G., Redmond H. P. (2003). Excisional surgery for cancer cure: therapy at a cost. Lancet Oncol. 4, 760–768. 10.1016/S1470-2045(03)01282-8 [DOI] [PubMed] [Google Scholar]
- Cohen C. J., Denkberg G., Lev A., Epel M., Reiter Y. (2003). Recombinant antibodies with MHC-restricted, peptide-specific, T-cell receptor-like specificity: new tools to study antigen presentation and TCR–peptide–MHC interactions. J. Mol. Recog 16, 324–332. 10.1002/jmr.640 [DOI] [PubMed] [Google Scholar]
- Constantino J., Gomes C., Falcão A., Cruz M. T., Neves B. M. (2016). Antitumor dendritic cell–based vaccines: lessons from 20 years of clinical trials and future perspectives. Trans. Res. 168, 74–95. 10.1016/j.trsl.2015.07.008 [DOI] [PubMed] [Google Scholar]
- Costa A., Kieffer Y., Scholer-Dahirel A., Pelon F., Bourachot B., Cardon M., et al. (2018). Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer. Cancer Cell 33, 463–46+. 10.1016/j.ccell.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Couzin-Frankel J. (2013). Cancer immunotherapy. Science 342, 1432–1433. 10.1126/science.342.6165.1432 [DOI] [PubMed] [Google Scholar]
- Da Silva C., Rueda F., Löwik C., Ossendorp F., Cruz L. J. (2016). Combinatorial prospects of nano-targeted chemoimmunotherapy. Biomaterials 83, 308–320. 10.1016/j.biomaterials.2016.01.006 [DOI] [PubMed] [Google Scholar]
- Dadi S., Chhangawala S., Whitlock B. M., Franklin R. A., Luo C. T., Oh S. A., et al. (2016). Cancer immunosurveillance by tissue-resident innate lymphoid cells and innate-like T cells. Cell 164, 365–377. 10.1016/j.cell.2016.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Koker S., Cui J., Vanparijs N., Albertazzi L., Grooten J., Caruso F., et al. (2016). Engineering Polymer Hydrogel Nanoparticles for Lymph Node-Targeted Delivery. Angewandte Chemie Int. Ed 55, 1334–1339. 10.1002/anie.201508626 [DOI] [PubMed] [Google Scholar]
- Delamarre L., Pack M., Chang H., Mellman I., Trombetta E. S. (2005). Differential Lysosomal Proteolysis in Antigen-Presenting Cells Determines Antigen Fate. Science 307, 1630. 10.1126/science.1108003 [DOI] [PubMed] [Google Scholar]
- Dellacherie M. O., Li A. W., Lu B. Y., Mooney D. J. (2018). Covalent conjugation of peptide antigen to mesoporous silica rods to enhance cellular responses. Bioconjug Chem. 29, 733–741. 10.1021/acs.bioconjchem.7b00656 [DOI] [PubMed] [Google Scholar]
- Draube A., Klein-Gonzalez N., Mattheus S., Brillant C., Hellmich M., Engert A., et al. (2011). Dendritic cell based tumor vaccination in prostate and renal cell cancer: a systematic review and meta-analysis. PloS One 6, 1–11. 10.1371/journal.pone.0018801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng B., Zhou F. Y., Hou B., Wang D. G., Wang T. T., Fu Y. L., et al. (2018). Binary Cooperative Prodrug Nanoparticles Improve Immunotherapy by Synergistically Modulating Immune Tumor Microenvironment. Adv Mater 30, 1–10. 10.1002/adma.201803001 [DOI] [PubMed] [Google Scholar]
- Feng X., Xu W., Li Z., Song W., Ding J., Chen X. (2019). Immunomodulatory Nanosystems. Adv Sci. 6, 1900101. 10.1002/advs.201900101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadersohi A., Sood A. K. (2001). Prostate epithelium-derived Ets transcription factor mRNA is overexpressed in human breast tumors and is a candidate breast tumor marker and a breast tumor antigen. Clin. Cancer Res. 7, 2731–2738. [PubMed] [Google Scholar]
- Goodwin T. J., Huang L. (2017). Investigation of phosphorylated adjuvants co-encapsulated with a model cancer peptide antigen for the treatment of colorectal cancer and liver metastasis. Vaccine 35, 2550–2557. 10.1016/j.vaccine.2017.03.067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granot T., Senda T., Carpenter D. J., Matsuoka N., Weiner J., Gordon C. L., et al. (2017). Dendritic Cells Display Subset and Tissue-Specific Maturation Dynamics over Human Life. Immunity 46, 504–515. 10.1016/j.immuni.2017.02.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guéry L., Hugues S. (2015). Th17 Cell Plasticity and Functions in Cancer Immunity. BioMed. Res. Int. 2015, 314620. 10.1155/2015/314620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulla S. K., Rao B. R., Moku G., Jinka S., Nimmu N. V., Khalid S., et al. (2019). In vivo targeting of DNA vaccines to dendritic cells using functionalized gold nanoparticles. Biomater Sci. 7, 773–788. 10.1039/C8BM01272E [DOI] [PubMed] [Google Scholar]
- Guth U., Myrick M. E., Schotzau A., Kilic N., Schmid S. M. (2011). Drug switch because of treatment-related adverse side effects in endocrine adjuvant breast cancer therapy: how often and how often does it work? Breast Cancer Res. Treat 129, 799–807. 10.1007/s10549-011-1668-y [DOI] [PubMed] [Google Scholar]
- Haanen J. B. (2017). Converting cold into hot tumors by combining immunotherapies. Cell 170, 1055–1056. 10.1016/j.cell.2017.08.031 [DOI] [PubMed] [Google Scholar]
- Higano C. S., Schellhammer P. F., Small E. J., Burch P. A., Nemunaitis J., Yuh L., et al. (2009). Integrated Data From 2 Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trials of Active Cellular Immunotherapy With Sipuleucel-T in Advanced Prostate Cancer. Cancer 115, 3670–3679. 10.1002/cncr.24429 [DOI] [PubMed] [Google Scholar]
- Ho N. I., Huis in ‘T Veld L. G. M., Raaijmakers T. K., Adema G. J. (2018). Adjuvants Enhancing Cross-Presentation by Dendritic Cells: The Key to More Effective Vaccines? Front. Immunol. 9, 1–12. 10.3389/fimmu.2018.02874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housman G., Byler S., Heerboth S., Lapinska K., Longacre M., Snyder N., et al. (2014). Drug Resistance in Cancer: An Overview. Cancers 6, 1769–1792. 10.3390/cancers6031769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine D. J., Swartz M. A., Szeto G. L. (2013). Engineering synthetic vaccines using cues from natural immunity. Nat. Mater 12, 978–990. 10.1038/nmat3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jennings G. T., Bachmann M. F. (2007). Designing recombinant vaccines with viral properties: a rational approach to more effective vaccines. Curr. Mol. Med. 7, 143–155. 10.2174/156652407780059140 [DOI] [PubMed] [Google Scholar]
- Jiang H., Wang Q., Sun X. (2017). Lymph node targeting strategies to improve vaccination efficacy. J. Controlled Release 267, 47–56. 10.1016/j.jconrel.2017.08.009 [DOI] [PubMed] [Google Scholar]
- Jiang H., Wang Q., Li L., Zeng Q., Li H., Gong T., et al. (2018). Turning the Old Adjuvant from Gel to Nanoparticles to Amplify CD8+ T Cell Responses. Adv Sci. 5, 1700426. 10.1002/advs.201700426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. S., Mcgaha T., Munn D. H. (2017). “Chemo-immunotherapy: role of indoleamine 2, 3-dioxygenase in defining immunogenic versus tolerogenic cell death in the tumor microenvironment,” in Tumor Immune Microenvironment in Cancer Progression and Cancer Therapy and Cancer Therapy (Springer; ), 1036, 91–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- June C. H., O’connor R. S., Kawalekar O. U., Ghassemi S., Milone M. C. (2018). CAR T cell immunotherapy for human cancer. Science 359, 1361–1365. 10.1126/science.aar6711 [DOI] [PubMed] [Google Scholar]
- Kadowaki N. (2009). The divergence and interplay between pDC and mDC in humans. Front. Biosci 14, 808–817. 10.2741/3279 [DOI] [PubMed] [Google Scholar]
- Kalinski P., Muthuswamy R., Urban J. (2013). Dendritic cells in cancer immunotherapy: vaccines and combination immunotherapies. Expert Rev. Vaccines 12, 285–295. 10.1586/erv.13.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantoff P. W., Higano C. S., Shore N. D., Berger E. R., Small E. J., Penson D. F., et al. (2010). Sipuleucel-T Immunotherapy for Castration-Resistant Prostate Cancer. New Engl. J. Med. 363, 411–422. 10.1056/NEJMoa1001294 [DOI] [PubMed] [Google Scholar]
- Kawai T., Akira S. (2007). TLR signaling Semin Immunon. 19, 24–32. [DOI] [PubMed] [Google Scholar]
- Knudson K. M., Hicks K. C., Luo X. L., Chen J. Q., Schlom J., Gameiro S. R. (2018). M7824, a novel bifunctional anti-PD-L1/TGF beta Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. Oncoimmunology 7, 1–14. 10.1080/2162402X.2018.1426519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongsted P., Borch T. H., Ellebaek E., Iversen T. Z., Andersen R., Met Ö, et al. (2017). Dendritic cell vaccination in combination with docetaxel for patients with metastatic castration-resistant prostate cancer: a randomized phase II study. Cytotherapy 19, 500–513. 10.1016/j.jcyt.2017.01.007 [DOI] [PubMed] [Google Scholar]
- Koppelmans V., Breteler M. M. B., Boogerd W., Seynaeve C., Gundy C., Schagen S. B. (2012). Neuropsychological Performance in Survivors of Breast Cancer More Than 20 Years After Adjuvant Chemotherapy. J. Clin. Oncol. 30, 1080–1086. 10.1200/JCO.2011.37.0189 [DOI] [PubMed] [Google Scholar]
- Krook J. E., Moertel C. G., Gunderson L. L., Wieand H. S., Collins R. T., Beart R. W., et al. (1991). Effective surgical adjuvant therapy for high-risk rectal-carcinoma. New Engl. J. Med. 324, 709–715. 10.1056/NEJM199103143241101 [DOI] [PubMed] [Google Scholar]
- Kuai R., Ochyl L. J., Bahjat K. S., Schwendeman A., Moon J. J. (2017). Designer vaccine nanodiscs for personalized cancer immunotherapy. Nat. Mater 16, 489–496. 10.1038/nmat4822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövgren T., Wolodarski M., Edbäck U., Martell E., Markland K., Nyström M., et al. (2018). Abstract CT032: Adoptive T cell transfer combined with DC vaccination in patients with metastatic melanoma. Cancer Research 78. 10.1158/1538-7445.AM2018-CT032 [DOI] [Google Scholar]
- Le D. T., Uram J. N., Wang H., Bartlett B. R., Kemberling H., Eyring A. D., et al. (2015). PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. New Engl. J. Med. 372, 2509–2520. 10.1056/NEJMoa1500596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leary M., Heerboth S., Lapinska K., Sarkar S. (2018). Sensitization of Drug Resistant Cancer Cells: A Matter of Combination Therapy. Cancers 10, 1–18. 10.3390/cancers10120483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leifer C. A. (2017). Dendritic cells in host response to biologic scaffolds. Semin. Immunol. 29, 41–48. 10.1016/j.smim.2017.01.001 [DOI] [PubMed] [Google Scholar]
- Leitner W. W., Ying H., Restifo N. P. (1999). DNA and RNA-based vaccines: principles, progress and prospects. Vaccine 18, 765–777. 10.1016/S0264-410X(99)00271-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zeng X., He L., Yuan H. (2015). Dendritic cell activation and maturation induced by recombinant calreticulin fragment 39-272. Int. J. Clin. Exp. Med. 8, 7288. [PMC free article] [PubMed] [Google Scholar]
- Li F., Chen W.-L., You B.-G., Liu Y., Yang S.-D., Yuan Z.-Q., et al. (2016). Enhanced cellular internalization and on-demand intracellular release of doxorubicin by stepwise pH-/reduction-responsive nanoparticles. ACS Appl. Mater Interfaces 8, 32146–32158. 10.1021/acsami.6b09604 [DOI] [PubMed] [Google Scholar]
- Lin Y.-X., Wang Y., Blake S., Yu M., Mei L., Wang H., et al. (2020). RNA Nanotechnology-Mediated Cancer Immunotherapy. Theranostics 10, 281. 10.7150/thno.35568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippert T. H., Ruoff H. J., Volm M. (2008). Intrinsic and acquired drug resistance in malignant tumors. Arzneimittelforschung-Drug Res. 58, 261–264. 10.1055/s-0031-1296504 [DOI] [PubMed] [Google Scholar]
- Liu H. Y., Chen D., Li L. L., Liu T. L., Tan L. F., Wu X. L., et al. (2011). Multifunctional Gold Nanoshells on Silica Nanorattles: A Platform for the Combination of Photothermal Therapy and Chemotherapy with Low Systemic Toxicity. Angewandte Chemie-International Ed 50, 891–895. 10.1002/anie.201002820 [DOI] [PubMed] [Google Scholar]
- Liu Z., Jiang W., Nam J., Moon J. J., Kim B. Y. S. (2018). Immunomodulating Nanomedicine for Cancer Therapy. Nano Lett. 18, 6655–6659. 10.1021/acs.nanolett.8b02340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizotte P., Wen A., Sheen M., Fields J., Rojanasopondist P., Steinmetz N., et al. (2016). In situ vaccination with cowpea mosaic virus nanoparticles suppresses metastatic cancer. Nat. Nanotechnol 11, 295. 10.1038/nnano.2015.292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Liu X., Liao Y.-P., Wang X., Ahmed A., Jiang W., et al. (2018). Breast cancer chemo-immunotherapy through liposomal delivery of an immunogenic cell death stimulus plus interference in the IDO-1 pathway. ACS Nano 12, 11041–11061. 10.1021/acsnano.8b05189 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lucky S. S., Soo K. C., Zhang Y. (2015). Nanoparticles in Photodynamic Therapy. Chem. Rev. 115, 1990–2042. 10.1021/cr5004198 [DOI] [PubMed] [Google Scholar]
- Lussier D. M., Schreiber R. (2016). Cancer immunosurveillance: immunoediting. Immunity Patho. Tumors 4, 396–405. 10.1016/B978-0-12-374279-7.17001-8 [DOI] [Google Scholar]
- Macdonald J. S., Smalley S. R., Benedetti J., Hundahl S. A., Estes N. C., Stemmermann G. N., et al. (2001). Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. New Engl. J. Med. 345, 725–730. 10.1056/NEJMoa010187 [DOI] [PubMed] [Google Scholar]
- Manfredi A. A., Capobianco A., Esposito A., De Cobelli F., Canu T., Monno A., et al. (2008). Maturing Dendritic Cells Depend on RAGE for In Vivo Homing to Lymph Nodes. J. Immunol. 180, 2270. 10.4049/jimmunol.180.4.2270 [DOI] [PubMed] [Google Scholar]
- Martín-Fontecha A., Lanzavecchia A., Sallusto F. (2009). “Dendritic Cell Migration to Peripheral Lymph Nodes,” in Dendritic Cells. Eds. Lombardi G., Riffo-Vasquez Y. (Berlin, Heidelberg: Springer Berlin Heidelberg; ), 31–49. [DOI] [PubMed] [Google Scholar]
- Mathan T. S. M., Textor J., Skold A. E., Reinieren-Beeren I., Van Oorschot T., Bruning M., et al. (2017). Harnessing RNA sequencing for global, unbiased evaluation of two new adjuvants for dendritic-cell immunotherapy. Oncotarget 8, 19879–19893. 10.18632/oncotarget.15190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maude S. L., Laetsch T. W., Buechner J., Rives S., Boyer M., Bittencourt H., et al. (2018). Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. New Engl. J. Med. 378, 439–448. 10.1056/NEJMoa1709866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mempel T. R., Henrickson S. E., Von Andrian U. H. (2004). T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature 427, 154–159. 10.1038/nature02238 [DOI] [PubMed] [Google Scholar]
- Minton K. (2014). IFN-dependent DC maturation. Nat. Rev. Immunol. 14, 67–67. 10.1038/nri3618 [DOI] [Google Scholar]
- Morrison D. C., Kline L. F. (1977). Activation of the classical and properdin pathways of complement by bacterial lipopolysaccharides (LPS). J. Immunol. 118, 362–368. [PubMed] [Google Scholar]
- Murugaiyan G., Saha B. (2009). Protumor vs Antitumor Functions of IL-17. J. Immunol. 183, 4169. 10.4049/jimmunol.0901017 [DOI] [PubMed] [Google Scholar]
- Nam J., Son S., Park K. S., Zou W., Shea L. D., Moon J. J. (2019). Cancer nanomedicine for combination cancer immunotherapy. Nat. Rev. Mater 4, 398–414. 10.1038/s41578-019-0108-1 [DOI] [Google Scholar]
- Nicholson L. B. (2016). The immune system. Essays Biochem. 60, 275–301. 10.1042/EBC20160017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara M., Miyashita M., Ota S. (2018). Vaccination of Urological Cancer Patients With WT1 Peptide-Pulsed Dendritic Cells in Combination With Molecular Targeted Therapy or Conventional Chemotherapy Induces Immunological and Clinical Responses. Ther. Apheresis Dialysis 22, 266–277. 10.1111/1744-9987.12694 [DOI] [PubMed] [Google Scholar]
- Paglia P., Chiodoni C., Rodolfo M., Colombo M. P. (1996). Murine dendritic cells loaded in vitro with soluble protein prime cytotoxic T lymphocytes against tumor antigen in vivo. J. Exp. Med. 183, 317–322. 10.1084/jem.183.1.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palma M., Hansson L., Mulder T. A., Adamson L., Näsman-Glaser B., Eriksson I., et al. (2018). Lenalidomide as immune adjuvant to a dendritic cell vaccine in chronic lymphocytic leukemia patients. Eur. J. Haematol 101, 68–77. 10.1111/ejh.13065 [DOI] [PubMed] [Google Scholar]
- Parvanian S., Mostafavi S. M., Aghashiri M. (2017). Multifunctional nanoparticle developments in cancer diagnosis and treatment. Sens. Bio-sensing Res. 13, 81–87. 10.1016/j.sbsr.2016.08.002 [DOI] [Google Scholar]
- Probst H. C., Mccoy K., Okazaki T., Honjo T., Van Den Broek M. (2005). Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat. Immunol. 6, 280–286. 10.1038/ni1165 [DOI] [PubMed] [Google Scholar]
- Raucci A., Palumbo R., Bianchi M. E. (2007). HMGB1: a signal of necrosis. Autoimmunity 40, 285–289. 10.1080/08916930701356978 [DOI] [PubMed] [Google Scholar]
- Reck M., Rodriguez-Abreu D., Robinson A. G., Hui R. N., Csoszi T., Fulop A., et al. (2016). Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. New Engl. J. Med. 375, 1823–1833. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- Reddy S. T., Van Der Vlies A. J., Simeoni E., Angeli V., Randolph G. J., O’neil C. P., et al. (2007). Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 25, 1159–1164. 10.1038/nbt1332 [DOI] [PubMed] [Google Scholar]
- Reichmuth A. M., Oberli M. A., Jaklenec A., Langer R., Blankschtein D. (2016). mRNA vaccine delivery using lipid nanoparticles. Ther. Deliv 7, 319–334. 10.4155/tde-2016-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescigno M., Winzler C., Delia D., Mutini C., Lutz M., Ricciardi-Castagnoli P. (1997). Dendritic cell maturation is required for initiation of the immune response. J. Leukoc Biol. 61, 415–421. 10.1002/jlb.61.4.415 [DOI] [PubMed] [Google Scholar]
- Robert C., Thomas L., Bondarenko I., O’day S., Weber J., Garbe C., et al. (2011). Ipilimumab plus Dacarbazine for Previously Untreated Metastatic Melanoma. New Engl. J. Med. 364, 2517–2526. 10.1056/NEJMoa1104621 [DOI] [PubMed] [Google Scholar]
- Roden R. B., Stern P. L. (2018). Opportunities and challenges for human papillomavirus vaccination in cancer. Nat. Rev. Cancer 18, 240. 10.1038/nrc.2018.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogel A., Popliker M., Webb C. G., Oren M. (1985). p53 cellular tumor antigen: analysis of mRNA levels in normal adult tissues, embryos, and tumors. Mol. Cell. Biol. 5, 2851–2855. 10.1128/MCB.5.10.2851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothermundt C. (2015). Management of side effects and adherence to oral cancer therapies in metastatic renal cell cancer. Oncol. Res. Treat 38, 17–17. [Google Scholar]
- Rutella S., Danese S., Leone G. (2006). Tolerogenic dendritic cells: cytokine modulation comes of age. Blood 108, 1435–1440. 10.1182/blood-2006-03-006403 [DOI] [PubMed] [Google Scholar]
- Sangtani A., Nag O. K., Field L. D., Breger J. C., Delehanty J. B. (2017). Multifunctional nanoparticle composites: progress in the use of soft and hard nanoparticles for drug delivery and imaging. Wiley Interdiscip. Rev: Nanomed Nanobiotechnol 9, e1466. 10.1002/wnan.1466 [DOI] [PubMed] [Google Scholar]
- Sau S., Alsaab H. O., Bhise K., Alzhrani R., Nabil G., Iyer A. K. (2018). Multifunctional nanoparticles for cancer immunotherapy: a groundbreaking approach for reprogramming malfunctioned tumor environment. J. Controlled Release 274, 24–34. 10.1016/j.jconrel.2018.01.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R., Becker H., Hohenberger W., Rodel C., Wittekind C., Fietkau R., et al. (2004). Preoperative versus postoperative chemoradiotherapy for rectal cancer. New Engl. J. Med. 351, 1731–1740. 10.1056/NEJMoa040694 [DOI] [PubMed] [Google Scholar]
- Schnurr M., Then F., Galambos P., Scholz C., Siegmund B., Endres S., et al. (2000). Extracellular ATP and TNF-α synergize in the activation and maturation of human dendritic cells. J. Immunol. 165, 4704–4709. 10.4049/jimmunol.165.8.4704 [DOI] [PubMed] [Google Scholar]
- Segura E., Villadangos J. A. (2009). Antigen presentation by dendritic cells in vivo. Curr. Opin. Immunol. 21, 105–110. 10.1016/j.coi.2009.03.011 [DOI] [PubMed] [Google Scholar]
- Segura E., Touzot M., Bohineust A., Cappuccio A., Chiocchia G., Hosmalin A., et al. (2013). Human Inflammatory Dendritic Cells Induce Th17 Cell Differentiation. Immunity 38, 336–348. 10.1016/j.immuni.2012.10.018 [DOI] [PubMed] [Google Scholar]
- Sharma M. D., Rodriguez P. C., Koehn B. H., Baban B., Cui Y., Guo G., et al. (2018). Activation of p53 in immature myeloid precursor cells controls differentiation into Ly6c+ CD103+ monocytic antigen-presenting cells in tumors. Immunity 48, 91–106. e106. 10.1016/j.immuni.2017.12.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil P., Zhao F., Muse G. W., Wong S.-W., Kolb J. P., Degraff L. M., et al. (2019). Non-canonical autophagy in dendritic cells restricts cross-presentation and anti-tumor immunity. bioRxiv 789867. 10.1101/789867 [DOI] [Google Scholar]
- Simmons D. P., Wearsch P. A., Canaday D. H., Meyerson H. J., Liu Y. C., Wang Y., et al. (2012). Type I IFN Drives a Distinctive Dendritic Cell Maturation Phenotype That Allows Continued Class II MHC Synthesis and Antigen Processing. J. Immunol. 188, 3116. 10.4049/jimmunol.1101313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G., Liu Y., Tian J.-H., Massare M. J., Boddapati S., Shane E., et al. (2018). Vaccine compositions having improved stability and immunogenicity. WO/2017/041100
- Smyth M. J., Dunn G. P., Schreiber R. D. (2006). Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 90, 1–50. 10.1016/S0065-2776(06)90001-7 [DOI] [PubMed] [Google Scholar]
- Speiser D. E., Schwarz K., Baumgaertner P., Manolova V., Devevre E., Sterry W., et al. (2010). Memory and Effector CD8 T-cell Responses After Nanoparticle Vaccination of Melanoma Patients. J. Immunother 33, 848–858. 10.1097/CJI.0b013e3181f1d614 [DOI] [PubMed] [Google Scholar]
- Thomas R., Lipsky P. E. (1996). Dendritic cells: origin and differentiation. Stem Cells 14, 196–206. 10.1002/stem.140196 [DOI] [PubMed] [Google Scholar]
- Van Herck S., Deswarte K., Nuhn L., Zhong Z., Portela Catani J. P., Li Y., et al. (2018). Lymph-node-targeted immune activation by engineered block copolymer amphiphiles–TLR7/8 agonist conjugates. J. Am. Chem. Soc. 140, 14300–14307. 10.1021/jacs.8b08595 [DOI] [PubMed] [Google Scholar]
- Vansteenkiste J. F., Cho B., Vanakesa T., De Pas T., Zielinski M., Kim M. S., et al. (2014). Magrit, a Double-Blind, Randomized, Placebo-Controlled Phase III Study to Assess the Efficacy of the Recmage-A3 + As15 Cancer Immunotherapeutic As Adjuvant Therapy in Patients with Resected Mage-A3-Positive Non-Small Cell Lung Cancer (Nsclc). Ann. Oncol. 25, iv409. 10.1093/annonc/mdu347.1 [DOI] [PubMed] [Google Scholar]
- Wang H., Mooney D. J. (2018). Biomaterial-assisted targeted modulation of immune cells in cancer treatment. Nat. Mater 17, 761–772. 10.1038/s41563-018-0147-9 [DOI] [PubMed] [Google Scholar]
- Wang Y., Lin Y. X., Qiao Z. Y., An H. W., Qiao S. L., Wang L., et al. (2015). Self-Assembled Autophagy-Inducing Polymeric Nanoparticles for Breast Cancer Interference In-Vivo. Adv Mater 27, 2627–2634. 10.1002/adma.201405926 [DOI] [PubMed] [Google Scholar]
- Wang S., Huang P., Chen X. (2016). Hierarchical targeting strategy for enhanced tumor tissue accumulation/retention and cellular internalization. Adv Mater 28, 7340–7364. 10.1002/adma.201601498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lin Y.-X., Qiao S.-L., An H.-W., Ma Y., Qiao Z.-Y., et al. (2017). Polymeric nanoparticles enable reversing macrophage in tumor microenvironment for immunotherapy. Biomaterials 112, 3e163. [DOI] [PubMed] [Google Scholar]
- Wang Y., Lin Y.-X., Wang J., Qiao S.-L., Liu Y.-Y., Dong W.-Q., et al. (2019. a). In Situ Manipulation of Dendritic Cells by an Autophagy-Regulative Nanoactivator Enables Effective Cancer Immunotherapy. ACS Nano 13, 7568–7577. 10.1021/acsnano.9b00143 [DOI] [PubMed] [Google Scholar]
- Wang Y., Lin Y. X., Qiao S. L., Wang J., Wang H. (2019. b). Progress in Tumor-Associated Macrophages: From Bench to Bedside. Adv Biosyst. 3, 1800232. 10.1002/adbi.201800232 [DOI] [PubMed] [Google Scholar]
- Wen R., Umeano A. C., Kou Y., Xu J., Farooqi A. A. (2019). Nanoparticle systems for cancer vaccine. Nanomedicine 14, 627–648. 10.2217/nnm-2018-0147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. W., Tjota M. Y., Clay B. S., Vander Lugt B., Bandukwala H. S., Hrusch C. L., et al. (2013). Transcription factor IRF4 drives dendritic cells to promote Th2 differentiation. Nat. Commun. 4, 1–12. 10.1038/ncomms3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf G. T. (1991). Induction chemotherapy plus radiation compared with surgery plus radiation in patients with advanced laryngeal-cancer. New Engl. J. Med. 324, 1685–1690. 10.1056/NEJM199106133242402 [DOI] [PubMed] [Google Scholar]
- Wongso D., Dong J., Ueda H., Kitaguche T. (2017). Flashbody: A Next Generation Fluobody with Fluorescence Intensity Enhanced by Antigen Binding. Anal Chem. 89, 6719–6725. 10.1021/acs.analchem.7b00959 [DOI] [PubMed] [Google Scholar]
- Yu H., Yang Y., Jiang T., Zhang X., Zhao Y., Pang G., et al. (2019). Effective Radiotherapy in Tumor Assisted by Ganoderma lucidum Polysaccharide-Conjugated Bismuth Sulfide Nanoparticles through Radiosensitization and Dendritic Cell Activation. ACS Appl. Mater Interfaces 11, 27536–27547. 10.1021/acsami.9b07804 [DOI] [PubMed] [Google Scholar]
- Zehn D., Cohen C. J., Reiter Y., Walden P. (2004). Extended presentation of specific MHC-peptide complexes by mature dendritic cells compared to other types of antigen-presenting cells. Eur. J. Immunol. 34, 1551–1560. 10.1002/eji.200324355 [DOI] [PubMed] [Google Scholar]
- Zhang N., Bevan M. J. (2011). CD8+ T cells: foot soldiers of the immune system. Immunity 35, 161–168. 10.1016/j.immuni.2011.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R., Smith J. D., Allen B. N., Kramer J. S., Schauflinger M., Ulery B. D. (2018). Peptide Amphiphile Micelle Vaccine Size and Charge Influence the Host Antibody Response. ACS Biomater Sci. Eng. 4, 2463–2472. 10.1021/acsbiomaterials.8b00511 [DOI] [PubMed] [Google Scholar]
- Zhang L., Wu S., Qin Y., Fan F., Zhang Z., Huang C., et al. (2019). Targeted Codelivery of an Antigen and Dual Agonists by Hybrid Nanoparticles for Enhanced Cancer Immunotherapy. Nano Lett. 19, 4237–4249. 10.1021/acs.nanolett.9b00030 [DOI] [PubMed] [Google Scholar]
- Zhao P. F., Wang Y. H., Kang X. J., Wu A. H., Yin W. M., Tang Y. S., et al. (2018). Dual-targeting biomimetic delivery for anti-glioma activity via remodeling the tumor microenvironment and directing macrophagemediated immunotherapy. Chem. Sci. 9, 2674–2689. 10.1039/C7SC04853J [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L. L., Zhao Y. L., Zhang K., Li X., Cui R. J., Yang W. (2017). Recent advances of immune checkpoint in breast cancer. Biomed. Research-India 28, 7268–7273. 10.1186/s12916-019-1326-5 [DOI] [Google Scholar]
- Zhong X., Zhang Y., Tan L., Zheng T., Hou Y., Hong X., et al. (2019). An aluminum adjuvant-integrated nano-MOF as antigen delivery system to induce strong humoral and cellular immune responses. J. Controlled Release 300, 81–92. 10.1016/j.jconrel.2019.02.035 [DOI] [PubMed] [Google Scholar]
- Zhou Q., Zhang Y., Du J., Li Y., Zhou Y., Fu Q., et al. (2016). Different-sized gold nanoparticle activator/antigen increases dendritic cells accumulation in liver-draining lymph nodes and CD8+ T cell responses. ACS Nano 10, 2678–2692. 10.1021/acsnano.5b07716 [DOI] [PubMed] [Google Scholar]
- Zupančič E., Curato C., Paisana M., Rodrigues C., Porat Z., Viana A. S., et al. (2017). Rational design of nanoparticles towards targeting antigen-presenting cells and improved T cell priming. J. Controlled Release 258, 182–195. 10.1016/j.jconrel.2017.05.014 [DOI] [PubMed] [Google Scholar]