Introduction:
As a public health problem, chronic liver disease (CLD) is a major cause of morbidity and mortality in the United States. It affects approximately 1.8% of the population and leads to 13 deaths per 100,000 persons[1]. A variety of etiologies can cause CLDs, including nonalcoholic fatty liver disease (NAFLD), chronic hepatitis virus infection, alcohol abuse, primary sclerosing cholangitis, primary biliary cirrhosis, and autoimmune hepatitis[2]. Cellular death and inflammation caused by CLD are central elements in hepatic fibrogenesis, which will progress to cirrhosis associated with the development of life-threatening complications of portal hypertension (PHTN), hepatic decompensation, and hepatocellular carcinoma [3]. There is compelling evidence indicate that with the removal of the underlying etiologies, liver fibrosis may regress or stabilize[4]. If treated early, the hepatic parenchyma may even return to almost normal[5]. Therefore, early detection of fibrosis and accurate diagnosis of etiology are essential for monitoring treatment efficacy, disease progression, and for establishing prognosis in patients with CLDs.
Liver biopsy is the gold standard for assessing hepatic inflammation, cellular injury, and fibrosis. However, it is an invasive procedure associated with complications of pain and bleeding, which results in frequent refusal by patients for serial measurements. Other major disadvantages include sampling errors and substantial inter- and intra-observer variation that limits the suitability of biopsy for providing dynamic information for assessing treatment efficacy and disease progression[6 7]. Serum laboratory tests, such as platelet count, aspartate aminotransferase-to-platelet radio index, or APRI[8], FIB-4[9], are useful and easy to perform but have limited accuracy in diagnosing early stage of inflammation and fibrosis[10]. Ultrasound-based elastography technologies (e.g., transient elastography, shear wave elastography, etc.) are inexpensive non-invasive methods for assessing liver stiffness. However, they evaluate only a small portion of the liver with a single parameter, which may yield substantial sampling error and incomplete information for disease assessment. Another limitation of ultrasound examinations is that they may fail in patients with obesity, ascites, or narrow intercostal space [11].
MR elastography (MRE) uses a modified phase-contrast imaging sequence to detect propagating shear waves within the liver. It enables the evaluation of a large portion of the liver and provides multiple mechanical properties that are associated with different pathophysiologic states. With significant advances made in MR technology, MRE has been demonstrated to be a highly accurate non-invasive diagnostic tool in detecting and monitoring various CLDs[12–14].
This comprehensive review summarizes current knowledge of the technical advances and innovations of hepatic MRE development (Table 1), and the clinical applications in various hepatic diseases.
Table.1.
Summary of advances in MRE of liver and their utility
| Advances/Innovations | Utility |
|---|---|
| Spin-echo MRE (SE-MRE) |
|
| 3D-MRE |
|
| Multifrequency MRE | Provides multiple mechanical parameters that can evaluate different pathologic processes |
| Flexible driver | Improve patient comfort |
| Free-breathing MRE | Improve tolerance, particularly pediatric subjects and sedated patients |
| Automated liver stiffness estimation | Reduce inter-observer variation in liver stiffness measurements |
Technology development
Image quality enhancement
Liver MRE is a well-accepted substitute for biopsy in screening and follow-up. To further improve its technical reliability and discover new imaging biomarkers, investigators have made numerous improvements in imaging sequence, active/passive actuator, and elastographic inversion algorithms [15–18].
Nowadays, the most widely available commercial MRE technique is gradient-recalled echo MRE (GRE-MRE)[19]. It has been well-validated in several large cohorts of clinical studies[20–23]. However, the conventional GRE-MRE technique can have technical failures due to susceptibility artifacts (e.g., iron overloaded liver) and insufficient shear wave penetration/encoding in the deeper structure of the liver (e.g., obesity)[24 25]. Iron-overloaded liver and obesity are not unusual in patients with CLDs[26]. Thus, researchers have developed a dedicated spin-echo based echo-planar (SE-EPI) MRE sequence. It has been demonstrated to be intrinsically insensitive to T2* susceptibility[27 28] and allow rapid acquisition with mitigated motion artifacts[29] (Figure 1). SE-EPI MRE is considered to have a significantly higher technical success rate than GRE-MRE [24 25 30–32].
Figure 1.
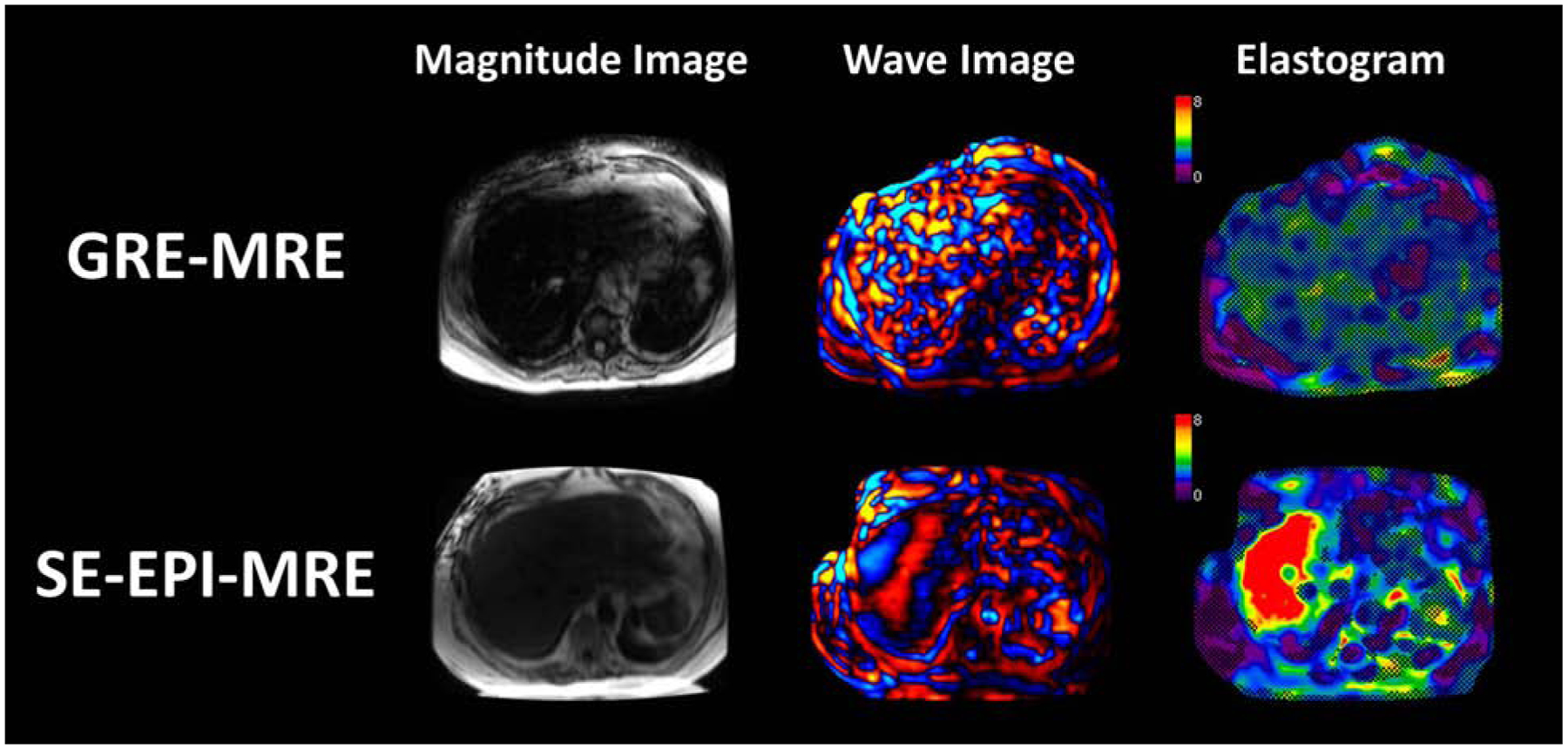
Representative images of MRE in a patient with increased iron deposition in the liver. Magnitude, wave, and elastograms with overlayed confidence map (>0.95 confidence level in the checkboard) of GRE-MRE (top row) and SE-EPI-MRE (bottom row) are shown from left to right. The confidence level is calculated based on goodness of fit, as well as signal/phase to noise ratios in both magnitude and wave images.
SE-EPI MRE allows rapid data acquisition of the x, y, and z components of the vector tissue motion over a large volume of the liver in a reasonable time. It allows a 3D vector-based inversion algorithm for data processing (Figure 2). The tissue stiffness estimation based on this 3D MRE method is more robust and accurate than 2D scalar MRE because it requires fewer assumptions about the polarization and propagation direction of the waves and thus can handle more complex shear wave motion in organs with complicated shapes such as the liver, spleen, and pancreas [33–36]. It has been demonstrated that the 3D MRE has higher diagnostic accuracy than 2D MRE in diagnosing NAFLD advanced fibrosis [37].
Figure 2.

Scan coverage of 4-slice 2D scalar GRE-MRE and 32-slice 3D vector EPI-MRE in the liver.
MRE research is motivated by clinical implementations to provide high sensitivity of elasticity, viscosity, and poroelastic properties to structural variations of biological tissues at multiple scales(15). The frequency dispersion of parameters measured by MRE has been explored to characterize the dynamic responses of structure elements in biological tissues[38–40]. Some investigations demonstrated the feasibility of low-frequency MRE, which is potentially more sensitive to the fluid phase of the tissue[41 42].
Multiple parameters calculation
Compared to 2D-MRE, 3D-MRE allows a more comprehensive analysis of the steady-state dynamic shear wave propagation in the entire liver. Thus, it enables calculation of multiple MR parameters that are sensitive to viscoelastic and compressible alterations of liver tissue in the progression of CLDs.
It has been demonstrated that the MRE-assessed liver stiffness has a static component that is mainly determined by extracellular matrix composites and liver structure (e.g., hepatic fibrosis, necrosis, loss of hepatocytes, regeneration, etc.), and a dynamic component that is affected by intrahepatic hemodynamic changes (e.g., perfusion, congestion, and inflammation)[43 44].
Advanced elastography methods explore multiple mechanical quantities include the model-free properties and model-based viscoelastic parameters [45–54] (Figure3). Among them, liver viscosity was found to be correlated with fibrosis, but not to steatosis or disease activity (inflammation) [55]. The dispersions of shear wave velocity and attenuation were found to be associated with the degree of steatosis [56]. The damping ratio and the loss modulus were found to increase significantly at the early onset of liver injury or necroinflammation [57]. The volumetric strain was found to be a promising biomarker in predicting portal hypertension in a preclinical study[58]. Another potential application is slip interface imaging [59], which can be used to characterize boundary conditions of the focal lesions to predict interface adhesiveness, which may provide promises in determining the invasiveness and malignancy of the tumor.
Figure 3.
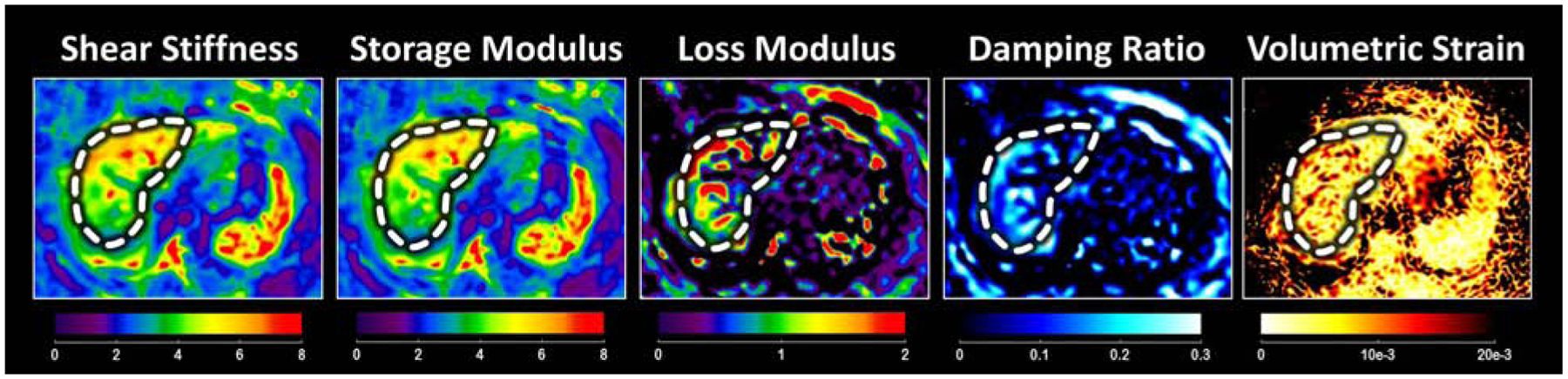
Multiple mechanical properties derived from 3D vector MRE.
Improve patient experience
Nowadays, in most published studies, a rigid plastic pneumatic driver was used in hepatic MRE scans, which may cause discomfort for patients. To improve the patient experience during the scan, a flexible and soft pneumatic driver has been developed recently[60]. Compared to the rigid driver, the flexible and soft driver conforms better to the anterior chest wall, and is closer to the liver, which enables the propagation of more uniform shear waves and potential improvement in liver stiffness estimation accuracy[66] (Figure 4). There are studies proved that the repeatability and reproducibility of the flexible driver are as good as the rigid one[16].
Figure 4.
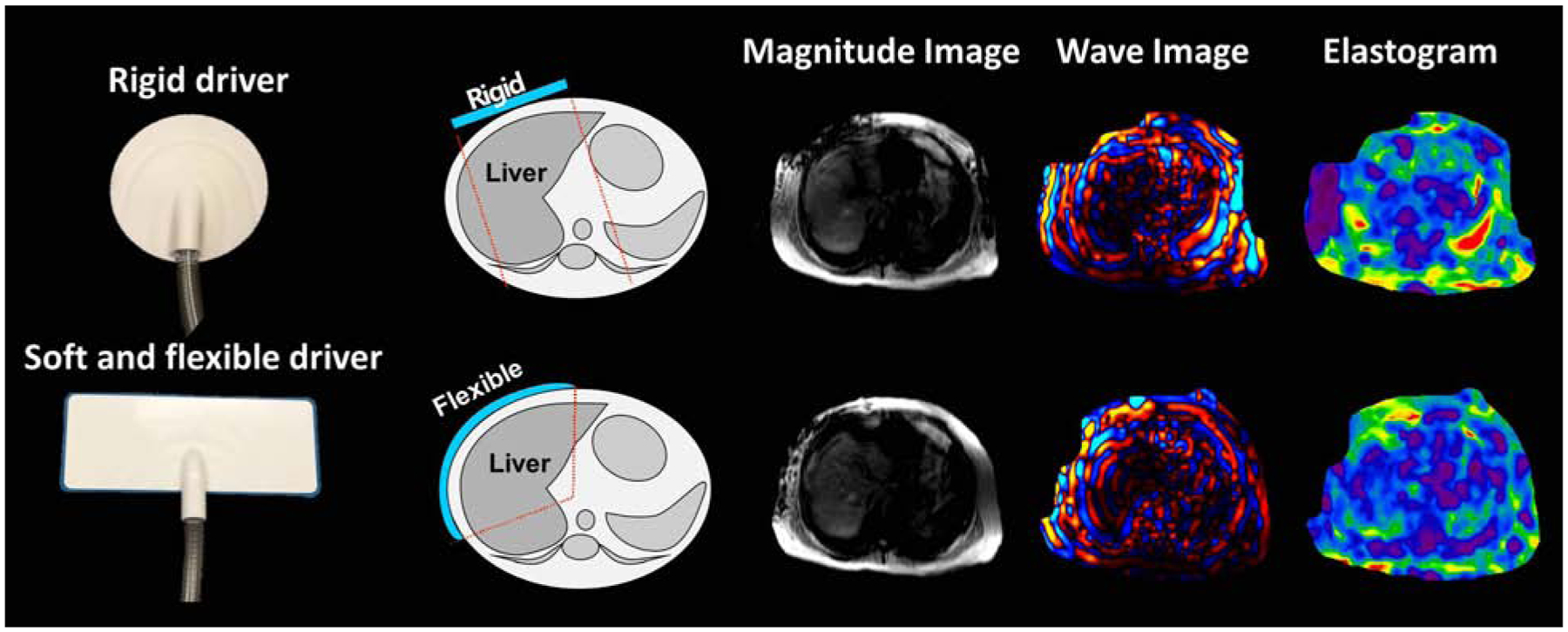
Images showing different driver designs and corresponding MRE images obtained in the same subject.
Courtesy of J. Chen, PhD, Rochester, MN.
Another technical concern related with patient experience is that the conventional hepatic MRE should be performed using expiration breath holds to avoid respiratory motion artifacts in the images [17, 18]. However, some patients have difficulty performing adequate end-expiration breath holds (e.g., pediatric and sedated patients). To eliminate the need for breath holds, investigators developed a non-gated, free-breathing, single-shot, multi-slice 2D EPI-MRE technique with a view-sharing-based reconstruction strategy[61], which can generate elastograms every 0.8 seconds and accomplish 100 time points within 1.5min. This implementation of free-breathing MRE has comparable repeatability and provides accurate averaged liver stiffness measurement compared with conventional breath-held MRE[62]. Additionally, this non-gate free-breathing MRE is capable of using the respiratory cycle to measure liver stiffness and other third-order mechanical parameters that may be helpful in disease diagnosis[63] (Figure 5). Another group demonstrated the feasibility of a respiratory-triggered (RT) SE-EPI MRE, which also yields comparable results of breath-held MRE[15]. Free-breathing MRE technique will be very beneficial for pediatric and sedated patients and will improve the comfort and patient experience for the general population as well.
Figure 5.

A. Images of free-breathing MRE in a healthy volunteer acquired at different time points during the scan. B. Graph plot showing excellent correlation between liver stiffness measured from free-breathing MRE and with breath hold, and illustrating excellent agreement between the two methods
Minimize inter-observer variation
MRE was introduced in 2007 for the clinical application of measuring liver stiffness, and are widely available on many MRI vendors, such as GE, Siemens, and Philips, with standardized hardware (Resoundant, Inc.) and inversion software (MMDI). To further improve inter-observer reproducibility and remove the need for manual analysis in MRE, a fully automated segmentation algorithm has been developed for calculating liver stiffness. This automated method is highly consistent with the measurements manually performed by expert readers in both 2D MRE and 3D MRE[64 65].
The repeatability coefficient of 2D MRE has been claimed as 19% by Quantitative Imaging Biomarkers Alliance (QIBA), which means a measured change in hepatic stiffness of 19% or larger indicates that a true change in stiffness has occurred with 95% confidence[66]. In a pilot repeatability study of 9 healthy volunteers and 6 patients, free-breathing MRE has a comparable RC value of 21% compared with that of breath-held MRE of 20%. In the same study cohort, 3D MRE provides a superior RC value of 10%[62].
Clinical applications
NAFLD
With the rising prevalence of obesity, NAFLD has become the leading cause of CLDs in the Western world[67]. Approximately 20–25% of NAFLD patients develop nonalcoholic steatohepatitis (NASH), leading to faster fibrosis progression to end-stage liver disease and hepatocellular carcinoma, which are established risk factors of liver-related death[68]. In preclinical studies, the liver stiffness derived from MRE has been proved to be sensitive to anti-fibrotic treatment, which supports the use of MRE as a non-invasive method to evaluate treatment efficacy longitudinally[69]. The multi-parametric 3D MRE combined with MRI-assessed proton density fat fraction shows high accuracy in predicting the NAFLD activity score (NAS) and NASH diagnosis in both preclinical models and clinical patients with NAFLD [43 44].
Even though MRE has been demonstrated to be highly accurate in diagnosing advanced fibrosis in patients with NAFLD[37 70 71], there are still diagnostic challenges in the detection of NASH[72]. In recent studies, the damping ratio and loss modulus have been shown to differentiate early onset of inflammation from fibrosis, even before the development of histologically detectable inflammatory cellular invasion[73]. A streamlined imaging protocol for NASH clinic has been recently established for virtual NAS prediction. This abbreviated imaging protocol takes as little as 5 minutes magnet time with the combination of multi-frequency 3D MRE and multi-echo Dixon imaging. It offers the advantage to predict not only NAS (the most commonly used surrogate end-point in NASH trials) but also separate estimations of the three components of NAS (steatosis, inflammation/ballooning, and fibrosis), which are individually targeted in certain experimental monotherapies[43] (Figure 6). This virtual NAS also reflects the histologic changes of NASH resolution in patients after bariatric surgery[74]. It is conceivable that this streamlined liver imaging protocol can be appropriately counseled about the risk of NAFLD disease progression and advised to implement therapeutic interventions.
Figure 6.

Examples of imaging analyses and predicted probabilities of NASH and NAS with 68% confidence intervals from (A, B) two clinical patients. The prediction model is composed of 3 imaging parameters, including shear stiffness, damping ratio, and fat fraction.
Chronic viral hepatitis
In patients with viral hepatitis, MRE has been demonstrated to be more accurate than aspartate aminotransferase-to-platelet ratio index (APRI) and many other non-invasive biomarkers in detecting significant fibrosis[75]. Additionally, MRE provides a cutoff value of 2.8kPa as a threshold for initiating antiviral therapy in patients with hepatitis C virus (HCV) infection [76].
Portal hypertension
The MRE-assessed liver stiffness has previously performed well in the detection of clinically significant PHTN[77 78], and in the estimation of the presence of esophageal varices[79]. One group found that the MRE-assessed liver stiffness was significantly higher in patients with cirrhotic PHTN, when compared with non-cirrhotic PHTN[80]. Recent work showed that the ratio between the spleen and liver stiffness can distinguish cirrhotic PHTN and non-cirrhotic PHTN[81]. Moreover, there was a preclinical study showing that a prediction model with multiple parameters derived from MRE has the potential to monitor PHTN progression[58].
Hepatocellular carcinoma
As the most common primary hepatic malignancy, the prognosis of patients with HCC is related to the aggressiveness and recurrence of the tumor[82]. One group found that each 1-kPa increase in tumor stiffness was associated with a 16.3% increase in the risk for tumor recurrence[83], which demonstrated that MRE has high diagnostic accuracy for predicting HCC development and stratifying the risk of HCC development.
The MRE-derived slip interface imaging may be related to the microvascular invasion of HCC, which can be used to stage malignancy and predict prognosis[84] (Figure 7).
Figure 7.

A 57-year-old female with a complete slip interface and no microvascular invasion at pathology. There is a stiffer HCC in left lobe compared with background liver tissue. The HCC-liver interface is clearly delineated in the octahedral shear strain (OSS) map, which indicates this HCC was well-incapsulated. The risk of microvascular invasion may be lower compared with those patients without clear slip interface shown in between HCC and liver.
Courtesy of J. Wang, MD, Guangzhou, Guangdong, People’s Republic of China.
Summary
Hepatic MRE has been demonstrated to be the most accurate non-invasive technique in diagnosing fibrosis and cirrhosis with liver stiffness measurement. With recent technology developments, multiparametric MRE can provide more promising parameters for evaluating pathogenic changes during disease progression of CLD, with substantially improved patient experience via more rapid, comfortable, and reliable imaging.
Synopsis.
Magnetic Resonance Elastography (MRE) has been well-accepted as the most accurate non-invasive technique in diagnosing fibrosis and cirrhosis in patients with chronic liver disease (CLD). To further improve the technical reliability and discover new MRE biomarkers, investigators made innovative progress in imaging sequence design, active/passive actuator design, and elastographic inversion algorithms. The accuracy of hepatic MRE in distinguishing the severity of disease has been validated in different studies of patients with CLDs, including nonalcoholic fatty liver disease, chronic hepatitis virus infection, portal hypertension, and hepatocellular carcinoma. The advanced hepatic MRE has been established as a reliable, comfortable, and inexpensive alternative to liver biopsy for disease diagnosing, progression monitoring, and clinical decision making in patients with CLD.
This comprehensive review will summarize current knowledge of the technical advances and innovations of hepatic MRE development, and the clinical applications in various hepatic diseases.
Key Points.
MR elastography (MRE) has been well-accepted as the most accurate non-invasive assessment of hepatic fibrosis and cirrhosis in patients with chronic liver disease (CLD).
Investigators have made numerous improvements in imaging sequence, active/passive actuator, and elastographic inversion algorithms to enhance the technical reliability and discover new MRE biomarkers.
Current updates about applications of hepatic MRE in disease diagnosing, progression monitoring, and clinical decision making for patients with CLD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
Meng Yin and the Mayo Clinic have intellectual property and a financial interest related to MRE technology
Contributor Information
Jiahui Li, Department of Radiology, Mayo Clinic, Rochester, Minnesota.
Sudhakar Kundapur Venkatesh, Department of Radiology, Mayo Clinic, Rochester, Minnesota.
Meng Yin, Department of Radiology, Mayo Clinic, Rochester, Minnesota.
Reference
- 1.Berzigotti A, Seijo S, Reverter E, Bosch J. Assessing portal hypertension in liver diseases. Expert Rev Gastroenterol Hepatol 2013;7(2):141–55 doi: 10.1586/egh.12.83|. [DOI] [PubMed] [Google Scholar]
- 2.Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet (London, England) 2014;383(9930):1749–61 doi: 10.1016/s0140-6736(14)60121-5|. [DOI] [PubMed] [Google Scholar]
- 3.Alegre F, Pelegrin P, Feldstein AE. Inflammasomes in Liver Fibrosis. Seminars in liver disease 2017;37(2):119–27 doi: 10.1055/s-0037-1601350|. [DOI] [PubMed] [Google Scholar]
- 4.Campana L, Iredale JP. Regression of Liver Fibrosis. Seminars in liver disease 2017;37(1):1–10 doi: 10.1055/s-0036-1597816|. [DOI] [PubMed] [Google Scholar]
- 5.Tsuchida T, Friedman SL. Mechanisms of hepatic stellate cell activation. Nature reviews. Gastroenterology & hepatology 2017;14(7):397–411 doi: 10.1038/nrgastro.2017.38|. [DOI] [PubMed] [Google Scholar]
- 6.Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD. Liver biopsy. Hepatology 2009;49(3):1017–44 doi: 10.1002/hep.22742|. [DOI] [PubMed] [Google Scholar]
- 7.Regev A, Berho M, Jeffers LJ, et al. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. The American journal of gastroenterology 2002;97(10):2614–8 doi: 10.1111/j.1572-0241.2002.06038.x|. [DOI] [PubMed] [Google Scholar]
- 8.Wai CT, Greenson JK, Fontana RJ, et al. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology 2003;38(2):518–26 doi: 10.1053/jhep.2003.50346|. [DOI] [PubMed] [Google Scholar]
- 9.Vallet-Pichard A, Mallet V, Nalpas B, et al. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007;46(1):32–6 doi: 10.1002/hep.21669|. [DOI] [PubMed] [Google Scholar]
- 10.Parkes J, Guha IN, Roderick P, Rosenberg W. Performance of serum marker panels for liver fibrosis in chronic hepatitis C. Journal of hepatology 2006;44(3):462–74 doi: 10.1016/j.jhep.2005.10.019|. [DOI] [PubMed] [Google Scholar]
- 11.Zhou JH, Cai JJ, She ZG, Li HL. Noninvasive evaluation of nonalcoholic fatty liver disease: Current evidence and practice. World journal of gastroenterology 2019;25(11):1307–26 doi: 10.3748/wjg.v25.i11.1307|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh S, Venkatesh SK, Loomba R, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol 2016;26(5):1431–40 doi: 10.1007/s00330-015-3949-z|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh S, Venkatesh SK, Wang Z, et al. Diagnostic performance of magnetic resonance elastography in staging liver fibrosis: a systematic review and meta-analysis of individual participant data. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2015;13(3):440–51 e6 doi: 10.1016/j.cgh.2014.09.046|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guo Y, Parthasarathy S, Goyal P, McCarthy RJ, Larson AC, Miller FH. Magnetic resonance elastography and acoustic radiation force impulse for staging hepatic fibrosis: a meta-analysis. Abdominal imaging 2015;40(4):818–34 doi: 10.1007/s00261-014-0137-6|. [DOI] [PubMed] [Google Scholar]
- 15.Wang H, Tkach JA, Trout AT, Dumoulin CL, Dillman JR. Respiratory-triggered spin-echo echo-planar imaging-based mr elastography for evaluating liver stiffness. Journal of magnetic resonance imaging : JMRI 2019;50(2):391–96 doi: 10.1002/jmri.26610|. [DOI] [PubMed] [Google Scholar]
- 16.Wang K, Manning P, Szeverenyi N, et al. Repeatability and reproducibility of 2D and 3D hepatic MR elastography with rigid and flexible drivers at end-expiration and end-inspiration in healthy volunteers. Abdominal radiology (New York) 2017;42(12):2843–54 doi: 10.1007/s00261-017-1206-4|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin M, Talwalkar JA, Glaser KJ, et al. Assessment of hepatic fibrosis with magnetic resonance elastography. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2007;5(10):1207–13.e2 doi: 10.1016/j.cgh.2007.06.012|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin M, Talwalkar JA, Glaser KJ, et al. Dynamic postprandial hepatic stiffness augmentation assessed with MR elastography in patients with chronic liver disease. AJR. American journal of roentgenology 2011;197(1):64–70 doi: 10.2214/ajr.10.5989|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cunha GM, Glaser KJ, Bergman A, Luz RP, de Figueiredo EH, Lobo Lopes FPP. Feasibility and agreement of stiffness measurements using gradient-echo and spin-echo MR elastography sequences in unselected patients undergoing liver MRI. The British journal of radiology 2018;91(1087):20180126 doi: 10.1259/bjr.20180126|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Loomba R, Wolfson T, Ang B, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology 2014;60(6):1920–8 doi: 10.1002/hep.27362|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi Y, Guo Q, Xia F, et al. MR elastography for the assessment of hepatic fibrosis in patients with chronic hepatitis B infection: does histologic necroinflammation influence the measurement of hepatic stiffness? Radiology 2014;273(1):88–98 doi: 10.1148/radiol.14132592|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ichikawa S, Motosugi U, Ichikawa T, et al. Magnetic resonance elastography for staging liver fibrosis in chronic hepatitis C. Magnetic resonance in medical sciences : MRMS : an official journal of Japan Society of Magnetic Resonance in Medicine 2012;11(4):291–7 doi: 10.2463/mrms.11.291|. [DOI] [PubMed] [Google Scholar]
- 23.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. Journal of magnetic resonance imaging : JMRI 2013;37(3):544–55 doi: 10.1002/jmri.23731|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mariappan YK, Dzyubak B, Glaser KJ, et al. Application of Modified Spin-Echo-based Sequences for Hepatic MR Elastography: Evaluation, Comparison with the Conventional Gradient-Echo Sequence, and Preliminary Clinical Experience. Radiology 2017;282(2):390–98 doi: 10.1148/radiol.2016160153|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Serai SD, Dillman JR, Trout AT. Spin-echo Echo-planar Imaging MR Elastography versus Gradient-echo MR Elastography for Assessment of Liver Stiffness in Children and Young Adults Suspected of Having Liver Disease. Radiology 2017;282(3):761–70 doi: 10.1148/radiol.2016160589|. [DOI] [PubMed] [Google Scholar]
- 26.Kowdley KV. Iron Overload in Patients With Chronic Liver Disease. Gastroenterology & hepatology 2016;12(11):695–98 [PMC free article] [PubMed] [Google Scholar]
- 27.Huwart L, Salameh N, ter Beek L, et al. MR elastography of liver fibrosis: preliminary results comparing spin-echo and echo-planar imaging. European Radiology 2008;18(11):2535–41 doi: 10.1007/s00330-008-1051-5|. [DOI] [PubMed] [Google Scholar]
- 28.Garteiser P, Sahebjavaher RS, Ter Beek LC, et al. Rapid acquisition of multifrequency, multislice and multidirectional MR elastography data with a fractionally encoded gradient echo sequence. NMR in biomedicine 2013;26(10):1326–35 doi: 10.1002/nbm.2958|. [DOI] [PubMed] [Google Scholar]
- 29.DeLaPaz RL. Echo-planar imaging. Radiographics : a review publication of the Radiological Society of North America, Inc 1994;14(5):1045–58 doi: 10.1148/radiographics.14.5.7991813|. [DOI] [PubMed] [Google Scholar]
- 30.Choi SL, Lee ES, Ko A, et al. Technical success rates and reliability of spin-echo echo-planar imaging (SE-EPI) MR elastography in patients with chronic liver disease or liver cirrhosis. Eur Radiol 2019. doi: 10.1007/s00330-019-06496-y|. [DOI] [PubMed] [Google Scholar]
- 31.Zhan C, Kannengiesser S, Chandarana H, Fenchel M, Ream J, Shanbhogue KP. MR elastography of liver at 3 Tesla: comparison of gradient-recalled echo (GRE) and spin-echo (SE) echo-planar imaging (EPI) sequences and agreement across stiffness measurements. Abdominal radiology (New York) 2019;44(5):1825–33 doi: 10.1007/s00261-019-01932-5|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Felker ER, Choi KS, Sung K, et al. Liver MR Elastography at 3 T: Agreement Across Pulse Sequences and Effect of Liver R2* on Image Quality. AJR. American journal of roentgenology 2018;211(3):588–94 doi: 10.2214/ajr.17.19288|. [DOI] [PubMed] [Google Scholar]
- 33.Yin MGR, Manduca A. Rapid EPI-based MR elastography of the liver. ISMRM, 2016. [Google Scholar]
- 34.Hamhaber U, Sack I, Papazoglou S, Rump J, Klatt D, Braun J. Three-dimensional analysis of shear wave propagation observed by in vivo magnetic resonance elastography of the brain. Acta biomaterialia 2007;3(1):127–37 doi: 10.1016/j.actbio.2006.08.007|. [DOI] [PubMed] [Google Scholar]
- 35.Papazoglou S, Hamhaber U, Braun J, Sack I. Algebraic Helmholtz inversion in planar magnetic resonance elastography. Physics in medicine and biology 2008;53(12):3147–58 doi: 10.1088/0031-9155/53/12/005|. [DOI] [PubMed] [Google Scholar]
- 36.Morisaka H, Motosugi U, Glaser KJ, et al. Comparison of diagnostic accuracies of two-and three-dimensional MR elastography of the liver. Journal of magnetic resonance imaging : JMRI 2017;45(4):1163–70 doi: 10.1002/jmri.25425|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loomba R, Cui J, Wolfson T, et al. Novel 3D Magnetic Resonance Elastography for the Noninvasive Diagnosis of Advanced Fibrosis in NAFLD: A Prospective Study. The American journal of gastroenterology 2016;111(7):986–94 doi: 10.1038/ajg.2016.65|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGarry MD, Johnson CL, Sutton BP, et al. Suitability of poroelastic and viscoelastic mechanical models for high and low frequency MR elastography. Medical physics 2015;42(2):947–57 doi: 10.1118/1.4905048|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leiderman R, Barbone PE, Oberai AA, Bamber JC. Coupling between elastic strain and interstitial fluid flow: ramifications for poroelastic imaging. Physics in medicine and biology 2006;51(24):6291–313 doi: 10.1088/0031-9155/51/24/002|. [DOI] [PubMed] [Google Scholar]
- 40.Parker KJ. A microchannel flow model for soft tissue elasticity. Physics in medicine and biology 2014;59(15):4443–57 doi: 10.1088/0031-9155/59/15/4443|. [DOI] [PubMed] [Google Scholar]
- 41.Dittmann F, Hirsch S, Tzschatzsch H, Guo J, Braun J, Sack I. In vivo wideband multifrequency MR elastography of the human brain and liver. Magnetic resonance in medicine 2016;76(4):1116–26 doi: 10.1002/mrm.26006|. [DOI] [PubMed] [Google Scholar]
- 42.Dittmann F, Tzschatzsch H, Hirsch S, et al. Tomoelastography of the abdomen: Tissue mechanical properties of the liver, spleen, kidney, and pancreas from single MR elastography scans at different hydration states. Magnetic resonance in medicine 2017;78(3):976–83 doi: 10.1002/mrm.26484|. [DOI] [PubMed] [Google Scholar]
- 43.Allen AM, Shah VH, Therneau TM, et al. The Role of Three-Dimensional Magnetic Resonance Elastography in the Diagnosis of Nonalcoholic Steatohepatitis in Obese Patients Undergoing Bariatric Surgery. Hepatology 2018. doi: 10.1002/hep.30483|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yin Z, Murphy MC, Li J, et al. Prediction of nonalcoholic fatty liver disease (NAFLD) activity score (NAS) with multiparametric hepatic magnetic resonance imaging and elastography. Eur Radiol 2019. doi: 10.1007/s00330-019-06076-0|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Asbach P, Klatt D, Hamhaber U, et al. Assessment of liver viscoelasticity using multifrequency MR elastography. Magnetic resonance in medicine 2008;60(2):373–9 [DOI] [PubMed] [Google Scholar]
- 46.Catheline S, Gennisson JL, Delon G, et al. Measuring of viscoelastic properties of homogeneous soft solid using transient elastography: an inverse problem approach. The Journal of the Acoustical Society of America 2004;116(6):3734–41 [DOI] [PubMed] [Google Scholar]
- 47.Guo J, Posnansky O, Hirsch S, et al. Fractal network dimension and viscoelastic powerlaw behavior: II. An experimental study of structure-mimicking phantoms by magnetic resonance elastography. Physics in medicine and biology 2012;57(12):4041–53 doi: 10.1088/0031-9155/57/12/4041|. [DOI] [PubMed] [Google Scholar]
- 48.Klatt D, Friedrich C, Korth Y, Vogt R, Braun J, Sack I. Viscoelastic properties of liver measured by oscillatory rheometry and multifrequency magnetic resonance elastography. Biorheology 2010;47(2):133–41 [DOI] [PubMed] [Google Scholar]
- 49.Vappou J, Maleke C, Konofagou EE. Quantitative viscoelastic parameters measured by harmonic motion imaging. Physics in medicine and biology 2009;54(11):3579–94 [DOI] [PubMed] [Google Scholar]
- 50.Doyley MM. Model-based elastography: a survey of approaches to the inverse elasticity problem. Physics in medicine and biology 2012;57(3):R35–73 doi: 10.1088/0031-9155/57/3/R35|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sack I, Beierbach B, Wuerfel J, et al. The impact of aging and gender on brain viscoelasticity. Neuroimage 2009;46(3):652–7 doi: 10.1016/j.neuroimage.2009.02.040|. [DOI] [PubMed] [Google Scholar]
- 52.Suki B, Barabasi AL, Lutchen KR. Lung tissue viscoelasticity: a mathematical framework and its molecular basis. J Appl Physiol (1985) 1994;76(6):2749–59 [DOI] [PubMed] [Google Scholar]
- 53.Robert B, Sinkus R, Larrat B, Tanter M, Fink M. A New Rheological Model Based on Fractional Derivatives for Biological Tissues. IEEE Ultrasonics Symposium, 2006:1033–36. [Google Scholar]
- 54.Klatt D, Hamhaber U, Asbach P, Braun J, Sack I. Noninvasive assessment of the rheological behavior of human organs using multifrequency MR elastography: a study of brain and liver viscoelasticity. Physics in medicine and biology 2007;52(24):7281–94 doi: 10.1088/0031-9155/52/24/006|. [DOI] [PubMed] [Google Scholar]
- 55.Deffieux T, Gennisson JL, Bousquet L, et al. Investigating liver stiffness and viscosity for fibrosis, steatosis and activity staging using shear wave elastography. Journal of hepatology 2015;62(2):317–24 doi: 10.1016/j.jhep.2014.09.020|. [DOI] [PubMed] [Google Scholar]
- 56.Barry CT, Mills B, Hah Z, et al. Shear wave dispersion measures liver steatosis. Ultrasound in medicine & biology 2012;38(2):175–82 doi: 10.1016/j.ultrasmedbio.2011.10.019|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yin M, Glaser KJ, Manduca A, et al. Distinguishing between Hepatic Inflammation and Fibrosis with MR Elastography. Radiology 2017:160622 doi: 10.1148/radiol.2017160622|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiahui Li MBH, Glaser Kevin J., Simonetto Douglas A., Shah Vijay, Ehman Richard L., Yin Meng. Assessment of Portal Hypertension with Multi-parametric Hepatic MR Elastography in Mouse Models. ISMRM. Paris, France, 2018. [Google Scholar]
- 59.Yin Z, Glaser KJ, Manduca A, et al. Slip Interface Imaging Predicts Tumor-Brain Adhesion in Vestibular Schwannomas. Radiology 2015;277(2):507–17 doi: 10.1148/radiol.2015151075|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen JSD, Glaser K, Yin M. Ergonomic Flexible Drivers for Hepatic MR Elastography ISMRM. Stockholm, Sweden, 2010. [Google Scholar]
- 61.Glaser KCJ, Ehman R. . Fast 2D hepatic MR elastography for free-breathing and short breath hold applications. ISMRM. Toronto, Ontario, Canada, 2015. [Google Scholar]
- 62.BD Jiahui Li, Glaser Kevin J., Yin Ziying, Chen Jun, Allen Alina, Venkatesh Sudhakar K., Manduca Armando, Shah Vijay, Ehman Richard L., Yin Meng. Repeatability and Clinical Performance of Non-gated, Free-breathing, MR Elastography (MRE) of the Liver. ISMRM; Montreal, Canada, 2019. [Google Scholar]
- 63.Ziying Yin BD, Li Jiahui, Glaser Kevin J., Venkatesh Sudhakar K., Manduca Armando, Ehman Richard L., Yin Meng. . A Feasibility Study of Nonlinear Mechanical Response Assessment of the Liver with MR Elastography (MRE). . ISMRM; Paris, France: 2018. [Google Scholar]
- 64.Dzyubak B, Venkatesh SK, Manduca A, Glaser KJ, Ehman RL. Automated liver elasticity calculation for MR elastography. Journal of magnetic resonance imaging : JMRI 2015. doi: 10.1002/jmri.25072|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dzyubak B, Glaser KJ, Manduca A, Ehman RL. Automated Liver Elasticity Calculation for 3D MRE. Proceedings of SPIE--the International Society for Optical Engineering 2017;10134 doi: 10.1117/12.2254476|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Serai SD, Obuchowski NA, Venkatesh SK, et al. Repeatability of MR Elastography of Liver: A Meta-Analysis. Radiology 2017;285(1):92–100 doi: 10.1148/radiol.2017161398|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang TD, Behary J, Zekry A. Non-alcoholic fatty liver disease (NAFLD): a review of epidemiology, risk factors, diagnosis and management. Internal medicine journal 2019. doi: 10.1111/imj.14709|. [DOI] [PubMed] [Google Scholar]
- 68.Angulo P, Kleiner DE, Dam-Larsen S, et al. Liver Fibrosis, but No Other Histologic Features, Is Associated With Long-term Outcomes of Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology 2015;149(2):389–97.e10 doi: 10.1053/j.gastro.2015.04.043|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiahui Li HT, Boehm Stephanie, Mauer Amy, Bradstreet Thomas, Chow Patrick, Malone Harold, Zinker Bradley, Charles Edgar, Hayes Wendy, Liu Huimin, Malhi Harmeet, Ehman Richard, Du Shuyan, Yin Meng. A PEGylated Fibroblast Growth Factor 21 Variant Improves Hepatic Fibrosis in a Mouse Model of Non-Alcoholic Steatohepatitis, as Determined by Magnetic Resonance Elastography AASLD. Boston, MA, US, 2019. [Google Scholar]
- 70.Furlan A, Tublin ME, Yu L, Chopra KB, Lippello A, Behari J. Comparison of 2D Shear Wave Elastography, Transient Elastography, and MR Elastography for the Diagnosis of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. AJR. American journal of roentgenology 2019:1–7 doi: 10.2214/ajr.19.21267|. [DOI] [PubMed] [Google Scholar]
- 71.Ajmera VH, Liu A, Singh S, et al. Clinical utility of an increase in magnetic resonance elastography in predicting fibrosis progression in NAFLD. Hepatology 2019. doi: 10.1002/hep.30974|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Besutti G, Valenti L, Ligabue G, et al. Accuracy of imaging methods for steatohepatitis diagnosis in non-alcoholic fatty liver disease patients: A systematic review. Liver international : official journal of the International Association for the Study of the Liver 2019;39(8):1521–34 doi: 10.1111/liv.14118|. [DOI] [PubMed] [Google Scholar]
- 73.Yin M, Glaser KJ, Manduca A, et al. Distinguishing between Hepatic Inflammation and Fibrosis with MR Elastography. Radiology 2017;284(3):694–705 doi: 10.1148/radiol.2017160622|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alina M VHS Allen, Therneau Terry M., Venkatesh Sudhakar K., Mounajjed Taofic, Larson Joseph J., Mara Kristin C., Kellogg Todd A., Kendrick Michael L., McKenzie Travis J., Greiner Suzanne M., Li Jiahui, Glaser Kevin J., Wells Michael L., Gunneson Timothy J., Ehman Richard L., Yin Meng. Multiparametric Magnetic Resonance Elastography Improves Noninvasive Detection of NASH Regression post Bariatric Surgery. Hepatology communications [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu XY, Wang WS, Zhang QM, et al. Performance of common imaging techniques vs serum biomarkers in assessing fibrosis in patients with chronic hepatitis B: A systematic review and meta-analysis. World journal of clinical cases 2019;7(15):2022–37 doi: 10.12998/wjcc.v7.i15.2022| [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wu WP, Chou CT, Chen RC, Lee CW, Lee KW, Wu HK. Non-Invasive Evaluation of Hepatic Fibrosis: The Diagnostic Performance of Magnetic Resonance Elastography in Patients with Viral Hepatitis B or C. PloS one 2015;10(10):e0140068 doi: 10.1371/journal.pone.0140068|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Attia D, Schoenemeier B, Rodt T, et al. Evaluation of Liver and Spleen Stiffness with Acoustic Radiation Force Impulse Quantification Elastography for Diagnosing Clinically Significant Portal Hypertension. Ultraschall in der Medizin (Stuttgart, Germany : 1980) 2015;36(6):603–10 doi: 10.1055/s-0041-107971|. [DOI] [PubMed] [Google Scholar]
- 78.Jeon SK, Lee JM, Joo I, Yoon JH, Lee DH, Han JK. Two-dimensional Shear Wave Elastography with Propagation Maps for the Assessment of Liver Fibrosis and Clinically Significant Portal Hypertension in Patients with Chronic Liver Disease: A Prospective Study. Academic radiology 2019. doi: 10.1016/j.acra.2019.08.006|. [DOI] [PubMed] [Google Scholar]
- 79.Abe H, Midorikawa Y, Matsumoto N, et al. Prediction of esophageal varices by liver and spleen MR elastography. Eur Radiol 2019;29(12):6611–19 doi: 10.1007/s00330-019-06230-8|. [DOI] [PubMed] [Google Scholar]
- 80.Navin PJ, Gidener T, Allen AM, et al. The Role of Magnetic Resonance Elastography in the Diagnosis of Noncirrhotic Portal Hypertension. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association 2019. doi: 10.1016/j.cgh.2019.10.018|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tolga Gidener PJN, Alina M. Allen, Yin Meng, Torbenson Michael S., Kamath Patrick S., Ehman Richard L., Venkatesh Sudhakar K.. The utility of MR elastography for differentiating non-cirrhotic portal hypertension from cirrhotic portal hypertension. RSNA; Chicago, IL, U: S, 2019. [Google Scholar]
- 82.Colecchia A, Schiumerini R, Cucchetti A, et al. Prognostic factors for hepatocellular carcinoma recurrence. World journal of gastroenterology 2014;20(20):5935–50 doi: 10.3748/wjg.v20.i20.5935|. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tamaki N, Higuchi M, Kurosaki M, et al. Risk assessment of hepatocellular carcinoma development by magnetic resonance elastography in chronic hepatitis C patients who achieved sustained virological responses by direct-acting antivirals. Journal of viral hepatitis 2019;26(7):893–99 doi: 10.1111/jvh.13103|. [DOI] [PubMed] [Google Scholar]
- 84.Bing Hu ZY, Glaser Kevin J., Venkatesh Sudhakar K., Deng Ying, Kuang Sichi, Quan Li Chen Jun, Arani Arvin, Yin Meng, Ehman Richard L. , Wang Jin. Slip-interface imaging preoperatively predicts hepatocellular carcinoma microvascular invasion. ISMRM; Montreal, QC, Canada, 2019. [Google Scholar]


