Abstract
Irinotecan is widely used in the treatment of metastatic colorectal cancer (mCRC) despite its severe toxicities. Toxicity is often associated with the UGT1A1*28/*28 genotype. An explanation for idiopathic toxicity beyond the UGT1A1 biomarker, however, remains a major concern for clinicians. One of the main irinotecan transporters is P-glycoprotein (P-gp), which is a hepatic efflux pump encoded by ABCB1. P-gp is involved in the biliary excretion of irinotecan and its active metabolite SN-38. We aimed to assess whether functional variants in ABCB1 also contribute to identifying patients at risk of toxicity. A cohort of 308 mCRC patients treated with irinotecan-based regimens were genotyped for polymorphisms in ABCB1 (rs1128503, rs2032582, and rs1045642). The effect of these variants and their haplotypes on irinotecan-induced severe toxicity (diarrhea, neutropenia, asthenia, nausea, and mucositis) was assessed. After adjusting for the relevant clinical and pathological parameters in the multivariate analysis, we found rs1128503 was significantly associated with severe diarrhea and mucositis (P=0.014 and P=0.002, respectively). Additionally, rs2032582 was associated with severe mucositis (P<0.001). Our results show that rs1128503 genotyping could help to predict severe gastrointestinal toxicity induced by irinotecan.
Keywords: ABCB1, P-glycoprotein, irinotecan (CPT-11), gastrointestinal toxicity, diarrhea, mucositis, biomarker, colorectal cancer
Introduction
Irinotecan (CPT-11) is an antitumor agent that is broadly used in metastatic colorectal cancer (mCRC) patients and normally co-administered with infusional 5-fluorouracil/leucovorin (FOLFIRI regimen). Its use is hampered by toxicities such as severe diarrhea and neutropenia in more than one-third of the patients (Fuchs et al., 2003).
In the liver, CPT-11 is converted to 7-ethyl-10-hydroxy-camptothecin (SN-38), which is responsible for both the efficacy and the toxicity of the drug. SN-38 is mainly eliminated through glucuronidation by the uridine 5′-diphospho-(UDP)-glucuronosyltransferase (UGT) isoform 1A1, which is encoded by the UGT1A1 gene. The presence of seven TA repeats (UGT1A1*28 allele) in the promoter region of this gene reduces enzymatic activity (Bosma et al., 1995) and patients homozygous for this variant have a higher risk of irinotecan-induced toxicity (Innocenti et al., 2004; Marcuello et al., 2004; Rouits et al., 2004; Riera et al., 2018). However, UGT1A1 genetic status does not always explain severe toxicity, suggesting that other mechanisms contribute to the appearance of side effects.
An ATP-powered pump encoded by the ABCB1 gene, P-glycoprotein (P-gp), participates in the biliary excretion of CPT-11 and SN-38. The enhancement of P-gp expression increases biliary secretion of SN-38 and reduces its plasma concentrations, conferring a higher risk of intestinal toxicity but a lower risk of neutropenia (Iyer et al., 2002; Mathijssen et al., 2003; Han et al., 2009; Filipski et al., 2014; Li et al., 2018). The most well-known ABCB1 variants, rs1128503 (1236 C>T), rs2032582 (2677 G>T/A), and rs1045642 (3435 C>T), modulate P-gp expression (Hoffmeyer et al., 2000; Haenisch et al., 2007), CPT-11 and SN-38 plasma concentrations, and renal clearance (Mathijssen et al., 2003; Sai et al., 2003). However, the influence of these variants on irinotecan-induced severe toxicity remains inconclusive (Han et al., 2007; Innocenti et al., 2009; Glimelius et al., 2011; Teft et al., 2015; Li et al., 2018; Salvador-Martín et al., 2018).
Based on the relevance of P-gp on irinotecan pharmacokinetics, we conducted a study in a cohort of mCRC patients treated with an irinotecan-containing therapy in whom the UGT1A1 gene had previously been analyzed. The aim was to elucidate whether the aforementioned ABCB1 variants could also help to identify patients who develop severe irinotecan-induced toxicity.
Material and Methods
Study Population
We retrospectively included patients from Hospital de la Santa Creu i Sant Pau (HSCSP, Barcelona, Spain) who were diagnosed between 2001 and 2016 with mCRC and treated with an irinotecan-containing regimen. All patients had an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 and age ≥ 18. Irinotecan-containing regimens were biweekly FOLFIRI (irinotecan 180 mg/m2), irinotecan in monotherapy every 3 weeks (350 mg/m2, except for one patient with the UGT1A1*28/*37 genotype who received a reduced dose of 150 mg/m2), and other regimens containing irinotecan (irinotecan 125–350 mg/m2). We excluded those patients without available germline DNA or without all the toxicities properly collected. The toxicities assessed were diarrhea, neutropenia, asthenia, nausea, and mucositis. For each side-effect, we recorded the highest grade of toxicity experienced by each patient. Toxicities were graded (1 to 4) on the basis of the Common Terminology Criteria for Adverse Events v5.0 (CTCAE) of the National Cancer Institute. Grade 3 to 4 toxicities were considered severe toxicities. The study was approved by the Institutional Ethics Committee at HSCSP and written informed consent was obtained from all participants.
Genotyping
Germline DNA was extracted from blood cells by the salting-out procedure (Miller et al., 1988). Three functional ABCB1 gene polymorphisms, rs1128503 (1236 C>T), rs2032582 (2677 G>T/A), and rs1045642 (3435 C>T), were genotyped using TaqMan PCR assays (Applied Biosystems, Foster City, CA, USA). Due to the rs2032582 triallelic condition, two probes (C_11711720C_30 and C_11711720D_40) were needed to genotype this variant. The other probes used were C_7586662_10 for rs11128503, and C_7586657_20 for rs1045642. Genotyping was performed on a 7500 Real Time PCR System (Applied Biosystems, Foster City, CA, USA).
Statistics
Allele frequencies for each single nucleotide polymorphism (SNP) were checked by direct counting and compared to published data (1000 Genomes Project Consortium et al., 2015). Genotype data were tested for Hardy-Weinberg equilibrium using chi-squared tests. The triallelic condition of the rs2032582 variant results in six possible genotypes (GG, GT, GA, TT, TA, and AA). To simplify the statistical analyses, we combined patients with GT or GA genotypes, and also patients with TT, TA, or AA genotypes. For haplotype analysis, GA, TA, and AA patients were excluded, as PLINK removes triallelic SNP genotype calls. We performed phased haplotype analyses (using the E-M algorithm) including the three ABCB1 variants or using the window option for diplotype analyses. Associations between toxicities and genetic variants/haplotypes were measured using chi-squared tests. Multivariate analyses were carried out, including sex, age, chemotherapy treatment, line of chemotherapy, and UGT1A1 and ABCB1 genotypes. P-values <0.05 were considered statistically significant. Bonferroni correction for multiple comparisons was applied (P-values <2.4·10−4). All statistical analyses were conducted using SPSS (v25.0, IBM) and PLINK (v1.07, Shaun Purcell).
Results
Patient Population
We studied a total of 308 patients. Most patients (n=283) were treated with irinotecan in combination with 5-fluorouracil and leucovorin (FOLFIRI). A total of 176 patients had been enrolled in clinical trials (Marcuello et al., 2011; Páez et al., 2019). The presence of the UGT1A1*28, UGT1A1*37, and UGT1A1*36 alleles was determined in a previous study (Riera et al., 2018). Table 1 shows patients’ clinical characteristics along with UGT1A1 genotype status. Supplementary Table 1 shows toxicity grades according to CTCAE v5.0.
Table 1.
Baseline patient characteristics (n=308).
| n | % | |||
|---|---|---|---|---|
| Sex Male Female Age Mean Range |
63 24–83 |
194 114 |
63.0% 37.0% |
|
| Primary tumor site Right colon Left colon |
76 232 |
24.7% 75.3% |
||
| Chemotherapy treatment FOLFIRI Irinotecan monotherapy Other irinotecan combinations |
283 13 12 |
91.9% 4.2% 3.9% |
||
| Line of chemotherapy First line ≥ Second line UGT1A1 genotype UGT1A1 *1/*1 UGT1A1 *1/*28 UGT1A1 *28/*28 UGT1A1 *28/*37 |
244 64 136 143 28 1 |
79.2% 20.8% 44.2% 46.4% 9.1% 0.3% |
||
FOLFIRI, combination of irinotecan, 5-fluorouracil, and leucovorin, UGT1A1, uridine 5′-diphospho-(UDP)-glucuronosyltransferase (UGT) isoform 1A1
Genetic Studies
Allelic frequencies for all polymorphisms were within the probability limits of Hardy–Weinberg equilibrium (P>0.05), and their minor allele frequencies were in accordance with European population frequencies reported in the 1000 Genomes Project (1000 Genomes Project Consortium et al., 2015). Genotyping was successful in 307 patients (99.7%).
Associations Between ABCB1 Variants and Severe Side Effects
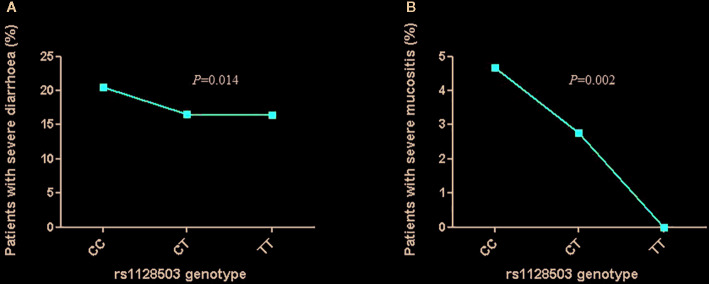
In the univariate analysis, we found no significant associations between the tested ABCB1 variants and the presence of irinotecan-induced side effects. In the multivariate analysis, rs1128503 was significantly associated with severe diarrhea and mucositis (P=0.014 and P=0.002, respectively). Patients harboring the CC genotype for rs1128503 presented a higher prevalence of severe diarrhea and mucositis than those with CT and TT genotypes ( Figure 1 ). In addition, rs2032582 was associated with severe mucositis (P=0.0001). This association retained its significance after Bonferroni correction. Table 2 shows the genotyping distributions and associations between the three polymorphisms and the toxicities assessed. When analyzing gene interactions, we found no significant interactions between the UGT1A1 and the ABCB1 variants tested.
Figure 1.
Association between rs1128503 genotype and irinotecan-induced gastrointestinal severe toxicities [(A) diarrhea; (B) mucositis)].
Table 2.
Univariate and multivariate associations between ABCB1 genotypes and grade 3–4 toxicities.
| Genotype | n (%) | Diarrhea, n (%) | Neutropenia, n (%) | Asthenia, n (%) | Nausea, n (%) | Mucositis, n (%) |
|---|---|---|---|---|---|---|
| rs1128503 (1236 C>T) | ||||||
| CC | 107 (34.9%) | 22 (20.6%) | 29 (27.1%) | 24 (22.4%) | 10 (9.4%) | 5 (4.7%) |
| CT | 145 (47.2%) | 24 (16.6%) | 27 (18.6%) | 29 (20.0%) | 11 (7.6%) | 4 (2.8%) |
| TT | 55 (17.9%) | 9 (16.4 %) | 11 (20.0%) | 11 (20.0%) | 6 (10.9%) | 0 (0%) |
| P-value (univariate analysis) | P = 0.676 | P = 0.256 | P = 0.883 | P = 0.737 | P = 0.245 | |
| P-value (multivariate analysis) | P = 0.014 | P = 0.990 | P = 0.236 | P = 0.615 | P = 0.002 | |
| rs2032582 (2677 G>T/A) | ||||||
| GG | 102 (33.2%) | 18 (17.7%) | 28 (27.5%) | 20 (19.6%) | 8 (7.8%) | 2 (2.0%) |
| GT | 144 (46.9%) | |||||
| GA | 7 (2.3%) | |||||
| GT+GA | 26 (17.2%) | 28 (18.5%) | 33 (21.9%) | 14 (9.3%) | 5 (3.3%) | |
| TT | 51 (16.6%) | |||||
| TA | 3 (1.0%) | |||||
| TT+TA | 11 (20.4%) | 11 (20.4%) | 11 (20.4%) | 5 (9.3%) | 2 (3.7%) | |
| P-value (univariate analysis) | P = 0.871 | P = 0.233 | P = 0.907 | P = 0.917 | P = 0.768 | |
| P-value (multivariate analysis) | P = 0.095 | P = 0.213 | P = 0.586 | P = 0.713 | P = 0.0001 a | |
| rs1045642 (3435 C>T) | ||||||
| CC | 90 (29.3%) | 15 (16.7%) | 24 (26.7%) | 16 (17.8%) | 6 (6.7%) | 3 (3.3%) |
| CT | 149 (48.5%) | 25 (16.8%) | 27 (18.1%) | 34 (22.8%) | 15 (10.1%) | 6 (4.0%) |
| TT | 68 (22.2%) | 15 (22.1%) | 16 (23.5%) | 14 (20.6%) | 6 (8.8%) | 0 (0%) |
| P-value (univariate analysis) | P = 0.600 | P = 0.279 | P = 0.648 | P = 0.667 | P = 0.255 | |
| P-value (multivariate analysis) | P = 0.197 | P = 0.207 | P = 0.240 | P = 0.659 | P = 0.352 |
The bold values indicate the statistically significant P-values (P < 0.05).
aSignificant after Bonferroni correction (P < 2.4·10−4).
ABCB1, ATP binding cassette subfamily B member 1.
Associations Between ABCB1 Haplotypes and Severe Side Effects
In the univariate analysis, we found that the diplotypes rs1045642T-rs1128503C (frequency=10.5%) and rs1128503C-rs2032582T (frequency=1.9%) were associated with a higher risk of diarrhea (P=0.034 and P=0.002, respectively). The rs1128503C-rs2032582T diplotype was also associated with a higher risk of mucositis (P=3.8·10−8), asthenia (P=0.043), and nausea (P=0.034). The association with mucositis remained statistically significant after applying Bonferroni test.
In the multivariate analysis, the associations of both diplotypes with diarrhea remained significant (P=0.035 for rs1045642T-rs1128503C and P=0.005 for rs1128503C-rs2032582T). The associations of the diplotype rs1128503C-rs2032582T with mucositis and nausea also retained their significance after multivariate adjustments (P=0.00018 and P=0.049, respectively), but only the association with mucositis retained its significance after Bonferroni correction. No other associations between ABCB1 diplotypes and the occurrence of severe side effects were found. These results are shown in the Supplementary Table 2 .
The haplotype analyses considering the three ABCB1 gene variants (rs1128503-rs2032582-rs1045642) identified seven of the eight possible haplotypes in our cohort of patients. The most frequent haplotypes were CGC and TTT, with a prevalence of 47.5% (n=282) and 37.4% (n=222), respectively ( Table 3 ). In contrast, the haplotype CTC was rare (0.3%) and the TGT haplotype was not harbored by any patient.
Table 3.
Univariate and multivariate associations between ABCB1 haplotypes and grade 3–4 toxicities.
| Haplotype (rs1128503-rs2032582-rs1045642)* | |||||||
|---|---|---|---|---|---|---|---|
| TTT (n=222) | TTC (n=13) | CTT (n=9) | TGC (n=16) | CGT (n=50) | CGC (n=282) | ||
| Diarrhoea | Unaffected (n=485) Affected (n=107) P-value (univariate analysis) P-value (multivariate analysis) |
185 (38.1%) 37 (34.6%) 0.511 0.459 |
10 (2.1%) 3 (2.8%) 0.703 0.888 |
4 (0.8%) 5 (4.7%) 0.005 0.012 |
15 (3.1%) 1 (0.9%) 0.213 0.193 |
38 (7.8%) 12 (11.2%) 0.256 0.306 |
233 (48.0%) 49 (45.8%) 0.680 0.791 |
| Neutropenia | Unaffected (n=458) Affected (n=134) P-value (univariate analysis) P-value (multivariate analysis) |
179 (39.1%) 43 (32.1%) 0.151 0.122 |
10 (2.2%) 3 (2.2%) 0.963 0.631 |
5 (1.1%) 4 (3.0%) 0.140 0.234 |
13 (2.8%) 3 (2.2%) 0.708 0.815 |
38 (8.3%) 12 (9.0%) 0.812 0.361 |
213 (46.5%) 69 (51.5%) 0.303 0.408 |
| Asthenia | Unaffected (n=468) Affected (n=124) P-value (univariate analysis) P-value (multivariate analysis) |
176 (37.6%) 46 (37.1%) 0.935 0.975 |
12 (2.6%) 1 (0.8%) 0.246 0.203 |
4 (0.9%) 5 (4.0%) 0.015 0.047 |
13 (2.8%) 3 (2.4%) 0.829 0.979 |
40 (8.5%) 10 (8.1%) 0.901 0.765 |
223 (47.6%) 59 (47.6%) 0.984 0.777 |
| Nausea | Unaffected (n=538) Affected (n=54) P-value (univariate analysis) P-value (multivariate analysis) |
201 (37.4%) 21 (38.9%) 0.812 0.799 |
13 (2.4%) 0 (0%) 0.263 0.708 |
6 (1.1%) 3 (5.6%) 0.014 0.029 |
14 (2.6%) 2 (3.7%) 0.633 0.753 |
47 (8.7%) 3 (5.6%) 0.447 0.427 |
257 (47.8%) 25 (46.3%) 0.832 0.866 |
| Mucositis | Unaffected (n=579) Affected (n=13) P-value (univariate analysis) P-value (multivariate analysis) |
219 (37.8%) 3 (23.1%) 0.214 0.251 |
13 (2.2%) 0 (0%) 0.906 0.921 |
7 (1.2%) 2 (15.4%) 8.8·10−6a 0.002 |
16 (2.8%) 0 (0%) 0.541 0.998 |
50 (8.6%) 0 (0%) 0.302 0.458 |
274 (47.3%) 8 (61.5%) 0.353 0.733 |
*The haplotype CTC was present in two patients. Due to its low frequency, it was excluded from the statistical analysis. The haplotype TGT was not harbored by any patient.
aSignificant after Bonferroni correction (P < 2.4·10-4).
The bold values indicate the statistically significant P-values (P < 0.05).
ABCB1, ATP binding cassette subfamily B member 1.
In the univariate analysis, the CTT haplotype (frequency= 1.5%) was significantly associated with a higher risk of severe diarrhea, mucositis, asthenia, and nausea (P=0.005, P=8.8·10−6, P=0.015, and P=0.014, respectively). These associations retained their significance after multivariate adjustments (P=0.012, P=0.002, P=0.047, and P=0.029, respectively) (see Table 3 ). Only the association with mucositis in the univariate analysis remained statistically significant after applying the Bonferroni test.
Discussion
This study shows the ABCB1 rs1128503 variant is a promising predictor of irinotecan-induced severe gastrointestinal toxicity, in particular diarrhea and mucositis. This result is reinforced by the significant associations found between the diplotypes and haplotypes containing the rs1128503-C allele and the referred toxicities. The rs2032582 variant also appears to be associated with severe mucositis.
Irinotecan-induced toxicities, the most common being neutropenia and diarrhea, usually lead to dose reduction or treatment withdrawal (Douillard et al., 2000). The Food and Drug Administration (FDA) drug label for irinotecan includes therapeutic recommendations for UGT1A1*28 homozygous patients to reduce the risk of developing neutropenia [Irinotecan (CAMPTOSAR) drug label. Food and Drug Administration. (Accession date: 24/02/2020)]. However, no recommendations are given to reduce the likelihood of irinotecan-induced severe gastrointestinal toxicity. The variability observed in the development of toxicity in clinical practice suggests that other polymorphisms or haplotypes could be involved. A growing body of evidence shows that variants in CPT-11 transporter genes could be helpful to identify patients at risk of severe side effects (Innocenti et al., 2009; Chen et al., 2015; Teft et al., 2015). ABCB1 gene is one of the most promising candidates, considering the relevance of the P-gp on the biliary secretion of irinotecan and its metabolites from the liver (Iyer et al., 2002). Great attention has been paid to rs1128503 (1236 C>T), rs2032582 (2677 G>T/A), and rs1045642 (3435 C>T), variants found to be in strong linkage disequilibrium and associated with drug exposure (Mathijssen et al., 2003; Han et al., 2007; Li et al., 2018).
Rhodes et al. analyzed 11 polymorphisms in 54 patients with metastatic colorectal cancer, and reported significant associations with grade 3–4 toxicity and grade 3–4 neutropenia when combining ABCB1, C1236T, and SLCO1B1 T521C polymorphisms (Rhodes et al., 2007). More recently, Salvador-Martín et al. analyzed the association between variants in ABC efflux transporter genes and irinotecan adverse effects, and found that the ABCB1 variants were significantly associated with overall toxicity (P<0.01) and with higher hematological toxicity including, but not limited to, neutropenia (P<0.01) (Salvador-Martín et al., 2018). However, other studies conducted in mCRC patients treated with irinotecan in whom ABCB1 variants were analyzed did not show significant associations with severe neutropenia (De Mattia et al., 2013; Chen et al., 2015; Teft et al., 2015; Yan et al., 2016). Our findings support these negative results as we found no associations with severe neutropenia in the 307 patients analyzed. These data, together with the significant association obtained with the UGT1A1*28 marker (P = 0.037) in a previous study by our group (Riera et al., 2018), lead us to infer that UGT1A1 is a more powerful predictor of severe neutropenia than ABCB1.
Few studies have analyzed ABCB1 variants as determinants of gastrointestinal toxicity in mCRC patients treated with irinotecan, and most of them produced negative results (De Mattia et al., 2013; Chen et al., 2015; Teft et al., 2015; Yan et al., 2016; Salvador-Martín et al., 2018). Nevertheless, in a cohort of 56 mCRC patients receiving an irinotecan-based treatment, Cortejoso et al. found that patients with the CC genotype for the rs1045642 variant had a higher probability of diarrhea than patients with CT and TT genotypes (P=0.039) (Cortejoso, et al., 2013). In line with this study, and after adjusting for clinicopathological covariates and the UGT1A1*28 genotype in a larger sample, we found that patients with the CC genotype for the ABCB1 rs1128503 variant were at highest risk of diarrhea (P=0.014) and mucositis (P=0.002). Furthermore, diplotypes and haplotypes including the rs1128503-C allele were significantly associated with severe gastrointestinal toxicity, suggesting this allele contributes to the risk of toxicity. Of note, Mathijssen et al. reported that patients homozygous for the rs1128503-T allele showed greater exposure to both irinotecan (P=0.038) and SN-38 (P=0.031) than the other patients in the trial (Mathijssen et al., 2003). They also postulated that this synonymous variant could alter RNA stability and affect the expression of ABCB1. Thus, in keeping with our findings, we speculate that patients carrying the TT genotype may present less biliary excretion and a lower risk of gastrointestinal toxicity.
In an attempt to improve the pharmacogenetic prediction of irinotecan-induced severe toxicity, we focused our study on the three most informative ABCB1 variants. Although this approach allowed us to achieve a higher statistical power, it has the limitation of losing the genetic effect of other variants located in the ABCB1 gene or in other hepatic efflux transporters, such as ABCC2 or ABCG2. A second limitation of our study could be the similar side-effect profile of 5-fluorouracil and irinotecan. However, the ABCB1 transporter has not been found to be involved in the pharmacokinetics of 5-fluorouracil, suggesting that the associations observed are likely due to irinotecan effects. A third limitation is that we did not record whether toxicity appeared in the first or later cycles or whether diarrhea was early-onset or delayed. Neither did we record the prophylactic anti-diarrheal agents received by each patient. Taken together, these data would have been useful to interpret the results. Finally, the associations found in this study should be considered exploratory because only the results with a P<0.001 survived Bonferroni correction for multiple comparisons. We note, however, that the Bonferroni correction was too conservative as the ABCB1 variants were in linkage disequilibrium and the procedure ignored the dependencies between these variants. This limitation could be overcome by validating our findings in a similar and independent cohort of patients, and also by performing functional studies.
In conclusion, our findings in a large and homogeneous cohort of mCRC patients show that rs1128503 and the diplotypes/haplotypes containing the C allele of this variant could be useful predictors of irinotecan-induced severe gastrointestinal toxicity. Further studies are needed to increase evidence regarding the utility of the rs1128503 variant in clinical practice.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Ethics Committee at Hospital de la Santa Creu i Sant Pau. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
PR, AA-B, JS, and DP contributed to the conception and design of the study. PR, AA-B, and JS performed genotyping and collected data. AS and AV collected data. PR, AA-B, JS, MA, and DP analyzed the data and performed statistical analyses. PR, AA-B, JS, and DP wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
AS reports receiving travel grants from Merck, Amgen, Sanofi, and Roche. AV has honoraria from Speakers Bureau of Amgen, Sanofi, and Kyowa Kirin, declares a scientific advisory role for Roche and Amgen, and reports receiving travel grants from Merck, Roche, Amgen, Sanofi, MSD, and Servier. DP has honoraria from Speakers Bureau of Merck Serono and F. Hoffmann-La Roche Ltd, and declares a scientific advisory role for Amgen and Sanofi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This work was supported by the Instituto de Salud Carlos III (CM18/00207 for PR).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2020.00973/full#supplementary-material
References
- 1000 Genomes Project Consortium R. A., Auton A., Brooks L. D., Durbin R. M., Garrison E. P., Kang H. M., et al. (2015). A global reference for human genetic variation. Nature 526 (7571), 68–74. 10.1038/nature15393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma P. J., Chowdhury J. R., Bakker C., Gantla S., de Boer A., Oostra B. A., et al. (1995). The genetic basis of the reduced expression of bilirubin UDP-glucuronosyltransferase 1 in Gilbert’s syndrome. New Engl. J. Med. 333 (18), 1171–1175. 10.1056/NEJM199511023331802 [DOI] [PubMed] [Google Scholar]
- Chen S., Villeneuve L., Jonker D., Couture F., Laverdière I., Cecchin E., et al. (2015). ABCC5 and ABCG1 polymorphisms predict irinotecan-induced severe toxicity in metastatic colorectal cancer patients. Pharmacogenet. Genomics 25 (12), 573–583. 10.1097/FPC.0000000000000168 [DOI] [PubMed] [Google Scholar]
- Cortejoso L., García M. I., García-Alfonso P., González-Haba E., Escolar F., Sanjurjo M., et al. (2013). Differential toxicity biomarkers for irinotecan- and oxaliplatin-containing chemotherapy in colorectal cancer. Cancer Chemother. Pharmacol. 71 (6), 1463–1472. 10.1007/s00280-013-2145-6 [DOI] [PubMed] [Google Scholar]
- De Mattia E., Toffoli G., Polesel J., D’Andrea M., Corona G., Zagonel V., et al. (2013). Pharmacogenetics of ABC and SLC transporters in metastatic colorectal cancer patients receiving first-line FOLFIRI treatment. Pharmacogenet. Genomics 23 (10), 549–557. 10.1097/FPC.0b013e328364b6cf [DOI] [PubMed] [Google Scholar]
- Douillard J. Y., Cunningham D., Roth A. D., Navarro M., James R. D., Karasek P., et al. (2000). Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet 355 (9209), 1041–1047. 10.1016/s0140-6736(00)02034-1 [DOI] [PubMed] [Google Scholar]
- Filipski E., Berland E., Ozturk N., Guettier C., van der Horst G. T. J., Lévi F., et al. (2014). Optimization of irinotecan chronotherapy with P-glycoprotein inhibition. Toxicol. Appl. Pharmacol. 274 (3), 471–479. 10.1016/j.taap.2013.12.018 [DOI] [PubMed] [Google Scholar]
- Fuchs C. S., Moore M. R., Harker G., Villa L., Rinaldi D., Hecht J. R. (2003). Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J. Clin. Oncol. 21 (5), 807–814. 10.1200/JCO.2003.08.058 [DOI] [PubMed] [Google Scholar]
- Glimelius B., Garmo H., Berglund A., Fredriksson L. A., Berglund M., Kohnke H., et al. (2011). Prediction of irinotecan and 5-fluorouracil toxicity and response in patients with advanced colorectal cancer. Pharmacogenom. J. 11 (1), 61–71. 10.1038/tpj.2010.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haenisch S., Zimmermann U., Dazert E., Wruck C. J., Dazert P., Siegmund S., et al. (2007). Influence of polymorphisms of ABCB1 and ABCC2 on mRNA and protein expression in normal and cancerous kidney cortex. Pharmacogenom. J. 7 (1), 56–65. 10.1038/sj.tpj.6500403 [DOI] [PubMed] [Google Scholar]
- Han J.-Y., Lim H.-S., Yoo Y.-K., Shin E. S., Park Y. H., Lee S. Y., et al. (2007). Associations of ABCB1, ABCC2, and ABCG2 polymorphisms with irinotecan-pharmacokinetics and clinical outcome in patients with advanced non-small cell lung cancer. Cancer 110 (1), 138–147. 10.1002/cncr.22760 [DOI] [PubMed] [Google Scholar]
- Han J.-Y., Lim H.-S., Park Y. H., Lee S. Y., Lee J. S. (2009). Integrated pharmacogenetic prediction of irinotecan pharmacokinetics and toxicity in patients with advanced non-small cell lung cancer. Lung Cancer 63 (1), 115–120. 10.1016/j.lungcan.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Hoffmeyer S., Burk O., von Richter O., Arnold H. P., Brockmöller J., Johne A., et al. (2000). Functional polymorphisms of the human multidrug-resistance gene: Multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl. Acad. Sci. 97 (7), 3473–3478. 10.1073/pnas.050585397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innocenti F., Undevia S. D., Iyer L., Chen P. X., Das S., Kocherginsky M., et al. (2004). Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J. Clin. Oncol. 22 (8), 1382–1388. 10.1200/JCO.2004.07.173 [DOI] [PubMed] [Google Scholar]
- Innocenti F., Kroetz D. L., Schuetz E., Dolan M. E., Ramírez J., Relling M., et al. (2009). Comprehensive Pharmacogenetic Analysis of Irinotecan Neutropenia and Pharmacokinetics. J. Clin. Oncol. 27 (16), 2604–2614. 10.1200/JCO.2008.20.6300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irinotecan (CAMPTOSAR) drug label. Food and Drug Administration . [Accession date: 02/24/2020].
- Iyer L., Ramírez J., Shepard D. R., Bingham C. M., Hossfeld D.-K., Ratain M. J., et al. (2002). Biliary transport of irinotecan and metabolites in normal and P-glycoprotein-deficient mice. Cancer Chemother Pharmacol. 49 (4), 336–341. 10.1007/s00280-001-0420-4 [DOI] [PubMed] [Google Scholar]
- Li M., Seiser E. L., Baldwin R. M., Ramirez J., Ratain M. J., Innocenti F., et al. (2018). ABC transporter polymorphisms are associated with irinotecan pharmacokinetics and neutropenia. Pharmacogenom. J. 18 (1), 35–42. 10.1038/tpj.2016.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcuello E., Altés A., Menoyo A., Del Rio E., Gómez-Pardo M., Baiget M. (2004). UGT1A1 gene variations and irinotecan treatment in patients with metastatic colorectal cancer. Br. J. Cancer 91 (4), 678–682. 10.1038/sj.bjc.6602042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcuello E., Páez D., Paré L., Salazar J., Sebio A., del Rio E., et al. (2011). A genotype-directed phase I-IV dose-finding study of irinotecan in combination with fluorouracil/leucovorin as first-line treatment in advanced colorectal cancer. Br. J. Cancer 105 (1), 53–57. 10.1038/bjc.2011.206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathijssen R. H. J., Marsh S., Karlsson M. O., Xie R., Baker S. D., Verweij J., et al. (2003). Irinotecan pathway genotype analysis to predict pharmacokinetics. Clin. Cancer Res. 9 (9), 3246–3253. [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. (1988). A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 16 (3), 1215. 10.1093/nar/16.3.1215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Páez D., Tobeña M., Fernández-Plana J., Sebio A., Virgili A. C., Cirera L., et al. (2019). Pharmacogenetic clinical randomised phase II trial to evaluate the efficacy and safety of FOLFIRI with high-dose irinotecan (HD-FOLFIRI) in metastatic colorectal cancer patients according to their UGT1A 1 genotype. Br. J. Cancer 120 (2), 190–195. 10.1038/s41416-018-0348-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes K. E., Zhang W., Yang D., Press O. A., Gordon M., Vallböhmer D., et al. (2007). ABCB1, SLCO1B1 and UGT1A1 gene polymorphisms are associated with toxicity in metastatic colorectal cancer patients treated with first-line irinotecan. Drug Metab. Lett. 1 (1), 23–30. 10.2174/187231207779814328 [DOI] [PubMed] [Google Scholar]
- Riera P., Salazar J., Virgili A. C., Tobeña M., Sebio A., Gallano P., et al. (2018). Relevance of CYP3A4*20 , UGT1A1*37 and UGT1A1*28 variants in irinotecan-induced severe toxicity. Br. J. Clin. Pharmacol. 84 (6), 1389–1392. 10.1111/bcp.13574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouits E., Boisdron-Celle M., Dumont A., Guérin O., Morel A., Gamelin E. (2004). Relevance of different UGT1A1 polymorphisms in irinotecan-induced toxicity: a molecular and clinical study of 75 patients. Clin. Cancer Res. 10 (15), 5151–5159. 10.1158/1078-0432.CCR-03-0548 [DOI] [PubMed] [Google Scholar]
- Sai K., Kaniwa N., Itoda M., Saito Y., Hasegawa R., Komamura K., et al. (2003). Haplotype analysis of ABCB1/MDR1 blocks in a Japanese population reveals genotype-dependent renal clearance of irinotecan. Pharmacogenetics 13 (12), 741–757. 10.1097/00008571-200312000-00005 [DOI] [PubMed] [Google Scholar]
- Salvador-Martín S., García-González X., García M., II, Blanco C., García-Alfonso P., Robles L., et al. (2018). Clinical utility of ABCB1 genotyping for preventing toxicity in treatment with irinotecan. Pharmacol. Res. 136, 133–139. 10.1016/j.phrs.2018.08.026 [DOI] [PubMed] [Google Scholar]
- Teft W. A., Welch S., Lenehan J., Parfitt J., Choi Y.-H., Winquist E., et al. (2015). OATP1B1 and tumour OATP1B3 modulate exposure, toxicity, and survival after irinotecan-based chemotherapy. Br. J. Cancer 112 (5), 857–865. 10.1038/bjc.2015.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L., Wang X., Wei L., Nie Y., Liu J., Zhang L. (2016). Effects of UGT1A1*6, UGT1A1*28, and ABCB1-3435C<T polymorphisms on irinotecan-induced toxicity in Chinese cancer patients. Int. J. Clin. Pharmacol. Ther. 54 (03), 193–199. 10.5414/CP202442 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.