Abstract
The apelin and Elabela proteins constitute a spatiotemporal double-ligand system that controls apelin receptor (APJ) signal transduction. Phosphorylation of multiple sites within the C-terminus of APJ is essential for the recruitment of β-arrestins. We sought to determine the precise mechanisms by which apelin and Elabela promote APJ phosphorylation, and to elucidate the influence of β-arrestin phosphorylation on G-protein-coupled receptor (GPCR)/β-arrestin-dependent signaling. We used techniques including mass spectrometry (MS), mutation analysis, and bioluminescence resonance energy transfer (BRET) to evaluate the role of phosphorylation sites in APJ-mediated G-protein-dependent and β-dependent signaling. Phosphorylation of APJ occurred at five serine residues in the C-terminal region (Ser335, Ser339, Ser345, Ser348 and Ser369). We also identified two phosphorylation sites in β-arrestin1 and three in β-arrestin2, including three previously identified residues (Ser412, Ser361, and Thr383) and two new sites, Tyr47 in β-arrestin1 and Tyr48 in β-arrestin2. APJ mutations did not affect the phosphorylation of β-arrestins, but it affects the β-arrestin signaling pathway, specifically Ser335 and Ser339. Mutation of Ser335 decreased the ability of the receptor to interact with β-arrestin1/2 and AP2, indicating that APJ affects the β-arrestin signaling pathway by stimulating Elabela. Mutation of Ser339 abolished the capability of the receptor to interact with GRK2 and β-arrestin1/2 upon stimulation with apelin-36, and disrupted receptor internalization and β-arrestin-dependent ERK1/2 activation. Five peptides act on distinct phosphorylation sites at the APJ C-terminus, differentially regulating APJ signal transduction and causing different biological effects. These findings may facilitate screening for drugs to treat cardiovascular and metabolic diseases.
Keywords: Apelin receptor (APJ), Phosphorylation, Mass spectrometry, Signal transduction, Bioluminescence resonance energy transfer (BRET), Internalization
1. Introduction
The apelin receptor (APJ) is a member of the A family of G-protein-coupled receptors (GPCRs) that is widely expressed in the central nervous system and peripheral tissues [1]. APJ functions predominantly in the maintenance of fluid homeostasis [2], and plays a pivotal role in the cardiovascular system [[3], [4], [5]] glucose metabolism [6], and food intake [7]. These functions are mediated by binding of APJ to its endogenous ligand, apelin [8]. The apelin propeptide contains several basic amino acid doublets, which represent endopeptidase cleavage sites that give rise to several bioactive C-terminal fragments, including apelin-36, apelin-17 and apelin-13.
The Elabela/Toddler (ELA) proteins belong to a group of endogenous active peptides recently discovered in zebrafish [9,10]; these proteins are functionally similar to apelin in vivo and thus constitute the Elabela/APJ system. In addition to playing important roles in embryonic development, feeding, and fluid balance, ELA/APJ signaling also lowers blood pressure, promotes angiogenesis, regulates the heart rate, and protects the kidneys. Human ELA is synthesized as a peptide of 54 amino acids, consisting of a signal peptide and a 32-amino-acid mature peptide (ELA-32). In addition to the 32-amino acid isoform (ELA-32), ELA also has two shorter isoforms of 21 or 11 amino acids, corresponding to the C-terminus of ELA-32; both are functional [11]. The cellular signaling profile of APJ is complex and remains under active investigation. Apelin activation of ERK1/2 is mediated by protein kinase C, indicative of coupling to either Gαi or Gαq/11 [12]. ELA-32 binds APJ, resulting in activation of the Gαi1 and β-arrestin signaling pathways and leading to receptor internalization [13]. However, little is known about the interactions of APJ with other intracellular proteins such as the β-arrestins, which are adaptor proteins that promote internalization of GPCRs and transduce signals to multiple effector pathways.
In the traditional model, agonist-occupied GPCRs initiate conformational changes that stimulate G-protein binding, followed by phosphorylation of the receptor C-terminus by GPCR kinases (GRKs). β-arrestin is then recruited and binds with high affinity [13,14]. Arrestins inhibit G-protein activation and mediate GPCR internalization, and may also stimulate β-arrestin signaling [15,16]. Phosphorylation of multiple sites within the C-terminus or intracellular loops of GPCRs is essential for the recruitment of β-arrestins [17]. Other studies have suggested that different phosphorylation patterns on the intracellular C-terminal tail (the “phosphorylation barcode”) of GPCRs can induce conformational distinct active states of arrestins that result in a variety of cellular outcomes [18].
These events lead to the dissociation of G-protein from the receptor and facilitate association of the receptor with clathrin, resulting in GPCR internalization [19]. In addition, upon binding to GPCRs, β-arrestins also serve as adaptors and scaffolding proteins to initiate alternative β-arrestin-dependent pathways that orchestrate the GPCR signaling network [20]. Numerous studies implicated the presence or absence of serine and threonine residues in the receptor C-terminus as a key determinant of the affinity of β-arrestin recruitment and the pattern of intracellular GPCR trafficking [21]. Because APJ is a GPCR, its C-terminal residues are required for receptor phosphorylation and internalization [22].
Apelin and Elabela constitute a spatiotemporal double-ligand system that controls APJ signaling transduction. Our previous research showed that mutation of serine 348 at the C-terminus led to elimination of apelin-13-induced β-arrestin recruitment to APJ. Moreover, APJ internalization and β-arrestin-dependent activation of ERK1/2 were also abolished by a point mutation at serine 348 [23]. However, the precise mechanisms by which apelin and Elabela promote APJ phosphorylation, as well as the influence of β-arrestin phosphorylation on GPCR/β-arrestin-dependent signaling, remain unclear.
In this study, we analyzed the interactions between APJ, APJ mutants, and β-arrestin1/2, as well as β2-adaptin (AP2) and apelin and Elabela stimulation. Furthermore, we characterized the functional impact of key C-terminal residues of APJ on receptor internalization and intracellular signaling events. In addition, using mass spectrometry, we also investigated the impact of APJ stimulation on β-arrestin1 and 2 phosphorylation. Our data provide evidence for the existence of a mechanism by which APJ phosphorylation could modulate the interaction between β-arrestin and receptor complexes, thereby impacting APJ signaling.
2. Experimental procedures
2.1. Materials
Human apelin-36, apelin-17, apelin-13, ELA-32, and ELA-21 were purchased from Phoenix Pharmaceuticals (Belmont, MA, USA). Lipofectamine 2000 was obtained from Invitrogen (Grand Island, NY, USA). FSK and 3-isobutyl-1-methylxanthine (IBMX) were obtained from Sigma-Aldrich Shanghai Trading (Shanghai, China). Anti-phospho-ERK1/2 antibody, anti-ERK1/2 antibody, anti-HA antibody, anti-β-arrestin1 antibody, anti-β-arrestin2 antibody, and anti-β-actin antibody were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.2. Cell culture and transfection
HEK293 cells were cultured at 37 °C and 5% CO2 in Dulbecco's modified Eagle's medium (DMEM; Gibco) containing 10% fetal calf serum (FCS). Twenty-four hours after seeding of cells, transient transfections were carried out using Lipofectamine 2000 (Thermo Fisher Scientific).
2.3. Protein preparation and mass spectrometry
For mass spectrometry experiments, HEK293 cells expressing wild-type (WT) mutant APJ were treated with 100 nM apelin-36, apelin-17, apelin-13, ELA-32, or ELA-21 at 37 °C for 30 min. The cells were washed and lysed for 30 min on ice in RIPA buffer containing protease and phosphatase inhibitors, and protein preparations and phosphopeptides were purified as described previously [24]. Peptide composition was determined by LC-MS/MS mass spectrometry.
LC-MS/MS measurements were carried out on a Shimadzu HPLC system (Columbia, MD, USA) coupled to an SCIEX Triple TOF 5600 mass spectrometer (Applied Biosystems, Foster City, CA, USA). All other details were as described previously [23]. The MS/MS spectra were processed with the Mascot search engine (Matrix Science, London, UK) for database correlation analysis. Search parameters through Mascot were set for phosphorylation emphasis and to search for biological modifications. Each filtered MS/MS spectrum exhibiting possible phosphorylated peptides was manually checked and validated.
2.4. Identification of APJ and β-arrestin interactions that induce β-arrestin phosphorylation sites by mass spectrometry
Cells were transfected with APJ or APJ mutants and β-arrestin1 or β-arrestin2, and then 48 h later were treated with a different ligand (100 nM) for 20 min at 37 °C. Extracted proteins were immunoprecipitated with anti-β-arrestin1 or anti-β-arrestin2 antibodies, as appropriate, and then centrifuged at 12000 g. For immunoprecipitation (IP), 5 μL antibody and 50 μL protein A/G-beads (Santa Cruz Biotechnology, Dallas, TX, USA) were added to the sample and incubated overnight at 4 °C. Immunoprecipitated complexes were separated by SDS-PAGE, and a gel slice corresponding to the position of β-arrestin1/2 was cut out. Phosphorylated peptides were desalted on a MonoTip C18 (Shimadzu), concentrated and loaded, and calibrated using the Shimadzu Laser BioLab MALDI Calibration kit. The peptide parent ion was analyzed by MS/MS [25], and the resultant product ion signal was uploaded to the Swiss-Prot database for identification of the phosphorylation site.
2.5. Generation of phosphorylation site-specific APJ antisera
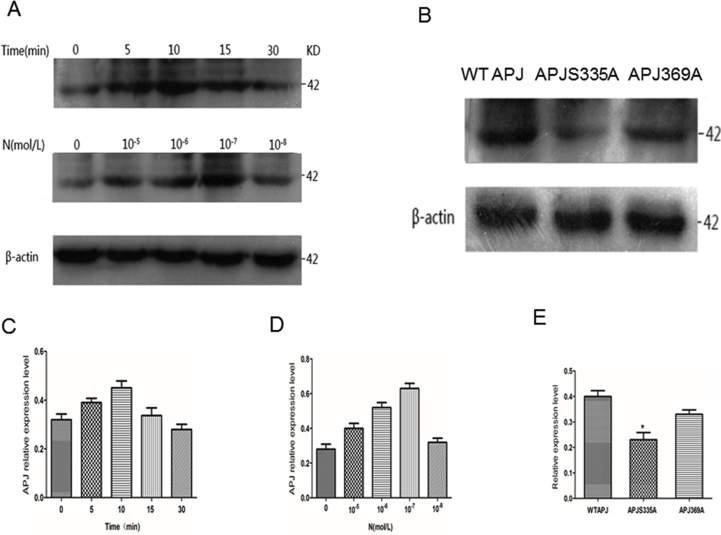
Phosphorylation-specific antisera were raised against the peptide Cys + RCAGTS-pHSS, which corresponds to amino acids 330–338 of APJ in which Ser335 is phosphorylated. Immunizations were performed by Atagenix (Wuhan Institute of Biotechnology, Wuhan, China). The resultant antiserum was reactive to the immunizing peptide.
2.6. Plasmid constructs and mutagenesis
Plasmids pcDNA3.1(+)-APJ and pcDNA3.1(+)-HA-tagged-APJ plasmids were purchased from Missouri S&T cDNA Resource Center (Rolla, MO, USA). A series of APJ mutants were generated by overlap extension PCR using high-fidelity Pfu polymerase and mutagenic primers. The mutated APJ cDNA was cut sequentially with EcoRI and HindIII, and then ligated back into the original pcDNA3.1(+). All mutated cDNAs were confirmed by sequence analysis of both strands. All constructs were verified by sequencing. Gαi2-Rluc and Gαq-Rluc fusion proteins were constructed as described previously [26]. Plasmid encoding GRK2 was kindly provided by Prof. Christian E. Elling, 7TM Pharma A/S, 2970 Hørsholm, Denmark. Plasmid encoding β2 subunit of the clathrin adaptor AP2-YFP (AP2-YFP) was kindly provided by Professor Michel Bouvier (Department of Biochemistry and Molecular Medicine, Faculty, Université de Montréal, Canada).
2.7. Cell-surface expression assay
HEK293 cells were transiently transfected with equal amounts of pcDNA3.1(+) expressing HA-tagged WT APJ or HA-tagged APJ-S339A/APJ-S345A. Twenty-four hours after transfection, cells were fixed, washed, and incubated in blocking solution (3% BSA) for 1 h. Subsequently, the cells were incubated with rabbit polyclonal anti-HA antibody overnight at 4 °C. After washing with PBS, the cells were incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature. After extensive washing, immunoreactivity was detected by the addition of TMB Plus substrate (Santa Cruz Biotechnology); the enzymatic reaction was stopped with 0.2 M H2SO4. Absorbance at 450 nm was measured on a microplate reader (Bio-Rad, USA). Expression levels of mutant receptors were calculated as percentages of WT APJ expression. Receptor internalization was measured by treating cells with 100 nM apelin-36 for 60 min at 37 °C and then performing the cell-surface ELISA procedure. The expression levels of mutant receptors and the percentage of mutant receptors internalized were defined as described previously [27].
2.8. cAMP assay
Intracellular cAMP was detected using an Epac cAMP bioluminescence resonance energy transfer (BRET) biosensor, which consists of an N-terminal truncated variant of EPAC tagged with Rluc and YFP at the N- and C-termini, respectively. Briefly, YFP-Epac-RLuc plasmids and WT or APJ mutants (APJ-S335A, APJ-S339A, APJ-S345A, APJ-S348 APJ-S369) were co-transfected into HEK293 cells. After 24 h, cells were collected, seeded in 96-well white opaque microplates, and cultured for another 24 h in HEPES-buffered phenol-red-free medium. Cells were then washed with PBS and resuspended in D-PBS. BRET between Rluc and YFP was measured at 20 °C in the presence of coelenterazine H (5 μM) and the nonspecific phosphodiesterase inhibitor IBMX (40 μM) for 20 min, and the cells were stimulated for 10 min with FSK (10 μM). Then, after incubation for 5 min with various concentrations of apelin-36, ELA-32 or ELA-21, BRET readings were collected on a Mithras LB940 plate reader (Berthold Technologies, Bad Wildbad, Germany).
2.9. Intracellular calcium assay
HEK293 cells expressing WT or APJ mutants were plated in 96-well poly-d-lysine-coated black plates (Corning) at 5 × 104 cells/well. Un-transfected HEK293 cells were used as a negative control. Fluo-4 NW calcium assay kits (Invitrogen, Eugene, OR, USA) were used to detect calcium fluorescence. Cells were incubated at 37 °C for 30 min in Fluo-4 NW working solution, and then equilibrated at room temperature for an additional 30 min. Subsequently, cells were stimulated with apelin-36 (0.01–1000 nM) for 180 s, and calcium fluorescence was immediately detected at an excitation wavelength of 485 nm and an emission wavelength of 515 nm on a Mithras LB940 plate reader. Data were imported into GraphPad Prism 5 for statistical analysis and graphing.
2.10. Concentration-response and real-time kinetic BRET assays
HEK293 cells were transiently co-transfected with the indicated Rluc- and EGFP (or Venus)-tagged constructs. Twenty-four hours after transfection, cells were harvested in HEPES-buffered phenol red-free medium containing 5% FCS and seeded in poly-d-lysine-coated 96-well white microplates (Corning 3600). Coelenterazine H substrate was added to a final concentration of 5 μM. BRET measurements were performed immediately on a Mithras LB940 plate reader. BRET saturation curves were obtained by transfecting increasing amounts of the EGFP (or Venus)-tagged construct and a constant amount of the Rluc-tagged donor construct. The total amount of plasmid DNA was kept constant by addition of empty plasmid. Kinetic BRET assays were performed as described previously [28]. The BRET ratio observed between interacting proteins was normalized by subtracting the background BRET ratio; the vehicle-treated sample represents the background ratio. This signal is defined as the ligand-induced BRET ratio.
2.11. Immunostaining and confocal microscopy
HEK293 cells were plated on poly-d-lysine-coated glass coverslips in 6-well plates, grown to 60% confluence, and transiently transfected with various plasmids as indicated in the figures. Twenty-four hours after transfection, the cells were incubated with 100 nM apelin-36 for the indicated periods of time. The cells were then fixed, washed, incubated with 3% BSA for 1 h at room temperature, and incubated overnight at 4 °C with anti-HA antibody. After the cells were washed with PBS, they were incubated with TRITC-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature. Following a wash step, the cells were examined under a Leica DMRE laser scanning confocal microscope (Leica, Milton Keynes, UK).
2.12. Co-immunoprecipitation
Immunoprecipitations were performed as described previously (28). HEK293 cells were transiently transfected with HA-APJ (or HA-APJ339) and Myc-GRK2. 36 h after transfection, cells were serum-starved overnight and stimulated with 100 nM apelin-36 for 15 min. For co-immunoprecipitations, cells were lysed and the supernatant fractions were collected and then incubated with anti-HA-agarose beads overnight at 4 °C with end-over-end rotation. The beads were washed three times with TBST and precipitates were eluted with SDS sample buffer containing β-mercaptoethanol. The supernatants were then analyzed with SDS-PAGE and immunoblotting for anti-Myc antibody immunoreactivity.
2.13. Western blotting
HEK293 cells expressing WT APJ or APJ-S339A in six-well plates were serum-starved for 4 h and stimulated with apelin-36. The cells were washed, harvested, and lysed in RIPA lysis buffer. Ten micrograms of cell extract were subjected to SDS-PAGE, and proteins were transferred to polyvinylidene fluoride membranes. Phosphorylation status of ERK1/2 was detected by immunoblotting with antibody against phospho-ERK1/2. As a control for loading, the same membranes were stripped of antibody and re-probed with anti-total ERK1/2 antibody. ECL reagents (Amersham Biosciences, Little Chalfont, UK) were applied to the membrane, films were scanned on a CanoScan LiDE 700F scanner, and bands were analyzed by densitometry using Scion Image.
For β-arrestin1/2 shRNA experiments, HEK-293 cells stably expressing APJ or APJ-S339A were transiently transfected with β-arrestin1 shRNA (Sigma-Aldrich, Clone ID: NM_004041.3-828s21c1), β-arrestin2 shRNA (Sigma-Aldrich, Clone ID: NM_004313.3-309s21c1), or shRNA Negative Control Med GC (Sigma-Aldrich, pLKO.1-puro) using the Lipofectamine 2000 reagent. After 48 h, cells were stimulated with apelin-36 and lysed for ERK1/2 assays as described above.
2.14. Data analysis
All data are shown as means ± S.E. Data were analyzed in Prism 5.0 (GraphPad, San Diego, CA, USA). Sigmoidal curves were fitted to the concentration-response data by non-linear regression. Statistical analyses were performed by one-way analysis of variance (ANOVA) followed by Tukey's multiple comparisons post-test. Direct comparison of two groups was performed with Student's t-test. P-values less than 0.05 were considered statistically significant.
3. Results
3.1. Ser residues in the C-terminal domain of APJ were phosphorylated in response to stimulation by five different peptides
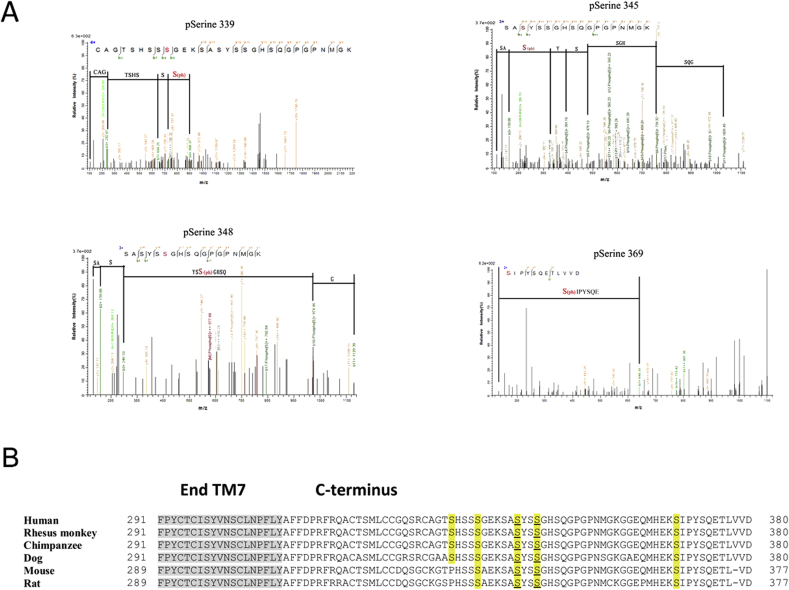
To determine whether the C-terminus of APJ is phosphorylated in an agonist-dependent manner, we performed mass spectrometry. Specifically, we stimulated HEK293 cells stably transfected with APJ with five different peptides: apelin-36, apelin-17, apelin-13, ELA-32, and ELA-21. The five peptides induced phosphorylation of APJ at four sites: Ser-339, Ser-345, Ser-348, and Ser-369 (Table 1). We conducted a mass-spectrometric analysis of the phosphoacceptor sites on APJ; four serine phospho-acceptor sites in the C-terminal tail of APJ are shown in Fig. 1A. Putative APJ phosphorylation sites were identified using the NetPhos 2.0 prediction software. Sequence analysis revealed that serines 339, 345, 348, and 369 were highly conserved C-terminal phosphorylation sites among humans, mice, rats, and other organisms (Fig. 1B).
Table 1.
APJ C-terminal phosphorylation sites by mass spectrometry.
| Apelin-13 | S345 | S348 | |
| Apelin-36 | S339 | S345 | S369 |
| Apelin-17 | S348 | ||
| Elabela-32 | S348 | ||
| Elabela-21 | S345 | S348 |
Fig. 1.
Identification of phosphorylation sites on APJ by mass spectrometry.
A, HEK293 cells expressing WT APJ were treated with 100 nM five different peptides include apelin-36, apelin-17, apelin-13, ELA-32 and ELA-21 for 10 min. Whole cell protein was extracted and subjected to tryptic digestion, and the peptides were analyzed by mass spectrometry. Shown are typical LC-MS/MS traces that identify serine 339, serine 345, serine 348 and serine 369 as phosphorylation sites. The phosphorylated residue is highlighted in red. B, C-terminal amino acid sequence alignment of APJ residues from human, rhesus monkey, chimpanzee, dog, mouse, and rat. Agonist-induced APJ phosphorylation sites identified with LC-MS/MS are highlighted in yellow. These sites were mutated from serine to alanine. Gray shading represents transmembrane helix 7. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To find more phosphorylation sites at the C-terminus of APJ, we used phosphorylation site-specific (amino acids 330–338 of APJ in which Ser335 is phosphorylated) antibodies to identify the phosphorylation site of APJ at the C-terminal residue Ser335. To characterize the phosphosite-specific antibody, we generated various APJ mutants. Immunoblot analysis revealed that ELA-32 stimulated phosphorylation at APJ WT, S335A, and S369A. APJ constructs with an S335A point mutation exhibited weak binding of the anti-pSer335 antibody after stimulation with ELA-32 (Supplementary Fig. S1), but a strong anti-pSer 335 antibody signal was detectable in APJ WT and S369A mutant. Together, these results suggested that ELA-32 induced phosphorylation of Ser335 in the C-terminal tail of APJ, but not S369. Although we used five APJ ligands to identify the phosphorylation sites of mass spectrometry, we still cannot rule out the phosphorylation of other sites, and will further study.
3.2. Site-directed mutagenesis and expression of WT and mutated APJs
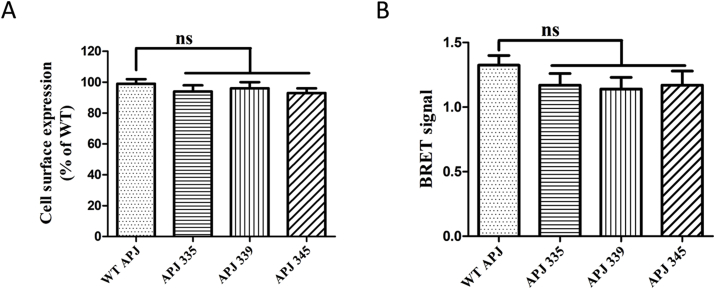
To determine which of the identified phosphorylation sites is most important for APJ function, we generated serine-to-alanine mutants via site-directed mutagenesis. After constructing a series of mutants, we investigated the cell-surface expression levels of WT APJ, APJ-S335A, APJ-S339A, and APJ-S345A in HEK293 cells. BRET data indicating proximity between the cell surface marker Venus-Kras and Rluc-tagged WT or mutant receptors (S335A, S339A, S345A). ELISA and BRET assays indicated that the three point mutants had very little effect on cell-surface expression of the receptor, in comparison with WT APJ (Fig. 2A and B). These data indicate that the APJ mutants were correctly synthesized and expressed on the plasma membrane.
Fig. 2.
Mutations at serine phosphorylation site do not alter APJ receptor expression or localization to the cell membrane.
A, Cell surface expression of WT and APJ mutants (S335A, S339A, S345A), quantified by ELISA. The results are expressed as the percentage of WT APJ cell surface expression, after correction for nonspecific expression in cells transfected with an empty vector (n = 5; one-way analysis of variance; ns, no significant difference, P > 0.05). B, BRET data indicating proximity between the cell surface marker Venus-Kras and Rluc-tagged WT or mutant receptors (S335A, S339A, S345A) (n = 5; one-way analysis of variance; ns, no significant difference, P > 0.05).
3.3. Apelin-36-induced APJ mutants do not affect cAMP and calcium levels and their G-protein interactions
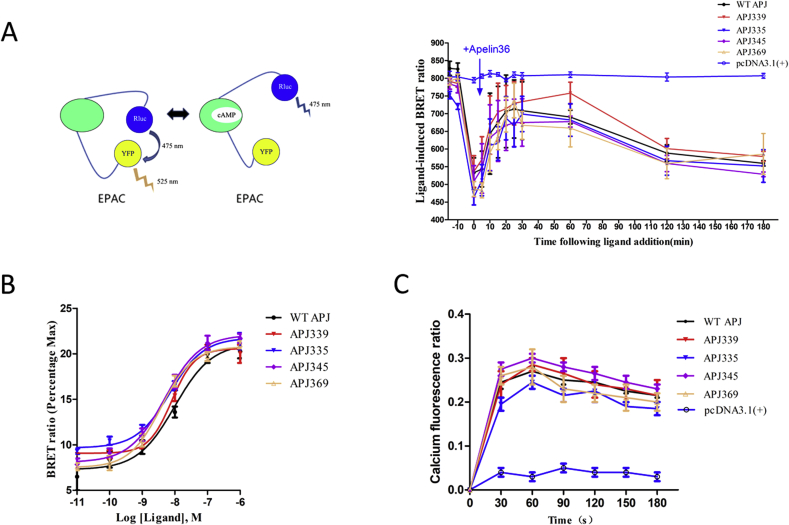
We measured the apelin-36-induced cAMP response using a cAMP BRET biosensor, as described previously [29]. Upon binding of cAMP, the biosensor signal decreases due to a conformational change that increases the distance between the donor and acceptor (Fig. 3A, left). The reduction in the BRET signal upon treatment with forskolin (FSK) in HEK293 cells expressing WT or mutant APJ was significantly increased by apelin-36 (Fig. 3A, right). The cAMP production by apelin-36 stimulation did not differ significantly between WT and APJ mutants. Our data suggest that the APJ C-terminal residues Ser335A, S339A, S345A and S369A have no effect on inhibition of the adenylate cycle. The results also showed that cAMP level generated by APJ through Gαi.
Fig. 3.
Apelin-36 induced cAMP and intracellular calcium accumulations are not affected by serine mutations.
A, Apelin-36 induced a cAMP response for WT and APJ mutants (S335A, S339A, S345A, S369A, only pcDNA3.1(+)) measured with an EPAC-based BRET biosensor. Schematic diagram of the presumptive molecular rearrangement of the EPAC protein with and without cAMP (left). Measurement of BRET signals in HEK293 cells co-expressing the cAMP biosensor and WT or APJ mutants and stimulated with apelin-36 for 180 min (right). The data correspond to the means ± S.E. from five independent experiments. B, Concentration–response curves of intracellular calcium fluorescence with various concentrations of apelin-36 (0.01–1000 nM) in HEK293 cells expressing WT or APJ mutants. C, Variations in intracellular calcium fluorescence over 180 s with 100 nM apelin-36, assessed with Fluo-4 NW Calcium Assay Kits. The data correspond to the means ± S.E. from five independent experiments.
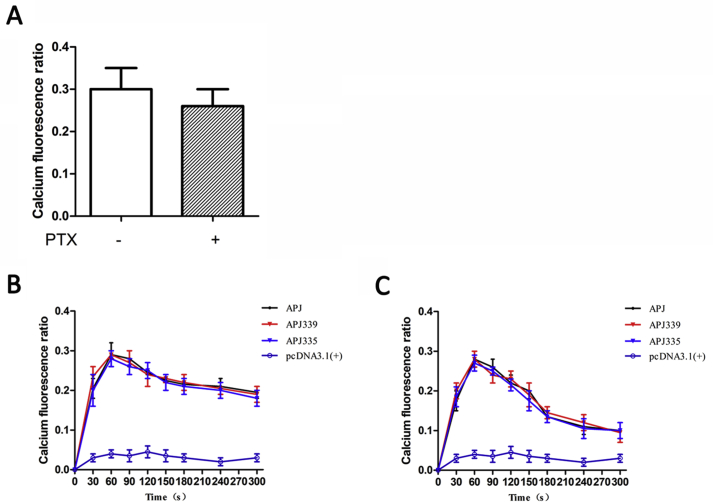
We then investigated whether APJ mutation would affect the elevation of intracellular calcium induced by apelin-36. Intracellular calcium increased in a concentration- and time-dependent manner in both WT and APJ mutants (S335A, S339A, S345A and S369A) (Fig. 3B and C), indicating that the APJ mutants were functional and that their roles in activating G-protein-dependent signaling pathways remained unchanged.
In order to distinguish the downstream calcium signal induced by Gαi protein or Gαq protein, we blocked the Gαi protein signal pathway with the Gαi protein blocker (PTX), and observed the effect of Gαq protein (Supplementary Fig. S2A). The results showed that the blocking of Gαi protein had little effect on the expression of calcium fluorescence, so we speculated that calcium levels are mainly released by receptor coupling to Gαq protein.
To identify the relative importance of the external Ca2+ influx and/or intracellular Ca2+ release induced by apelin-36, we compared experiments performed in the presence or absence of external Ca2+ as illustrated in Supplemental Figs. S2B and 2C. In the presence of external Ca2+, apelin-36 induced a rapid increase of calcium fluorescence and remained at a relatively high level (Supplementary Fig. S2B). In the absence of external Ca2+, the peak phase remained unchanged, but calcium fluorescence drops rapidly (Supplementary Fig. S2C). The persistence of the peak of calcium signal response to apelin-36 in the absence of extracellular Ca2+ shows that the initial increase of calcium signal originated from the release of the intracellular pool. The extracellular Ca2+ entry plays an important role in maintaining the long-term signal of calcium.
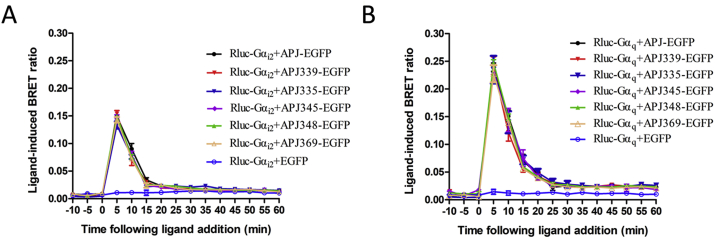
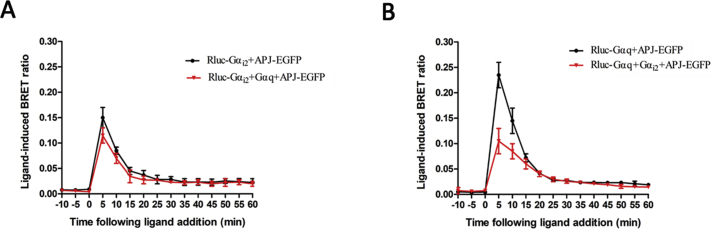
Next, we performed a real-time BRET analysis to detect the effect of apelin-36 on the interaction of G protein with WT or mutant APJ. Administration of apelin-36 to cells co-expressing EGFP-tagged WT or mutant APJ, or APJ and Rluc-tagged Gαi2 resulted in rapid and significant increases in the ligand-induced BRET signal, indicating that Gαi2 was recruited to the activated receptors. The BRET signal peaked at 5 min after treatment and decreased rapidly thereafter (Fig. 4A). Similar treatment of cells co-expressing WT or mutant APJ and Gαq also induced an increase in the BRET signal with a similar kinetic profile, indicating that Gαq was recruited to activated receptors (Fig. 4B). Overall, the BRET data clearly show that the five APJ mutants (S335A, S339A, S345A, S348A and S369A) interact with Gαi2 and Gαq in the same manner as WT APJ.
Fig. 4.
APJ mutants does not alter the interaction between the receptor and G proteins.
Real-time analysis of the interaction between APJ, APJ-S339A, APJ-S335A, APJ-S345A, APJ-S348A, APJ-S369A and Gαi2 (A) or Gαq (B) using BRET in living cells. HEK293 cells were transiently transfected with EGFP-tagged receptors and Rluc-Gαi2 (or Rluc-Gαq), then coelenterazine H was added and detected as above. The results are means ± S.E. from at least five independent experiments.
Furthermore, we transfected a constant amount of unfused Gαq protein to observe the BRET signal between Gαi2 and APJ. The BRET signal did not decrease significantly (Supplementary Fig. S3A). However, unfused Gαi2 protein was transfected, the BRET signal significantly reduced (Supplementary Fig. S3B). All the results suggest that the binding of APJ to Gαi2 protein has priority in a variety of G proteins coupled with APJ receptor.
3.4. Mutation of Ser339 APJ impairs the receptor's interaction with GRK2 and β-arrestin1/2
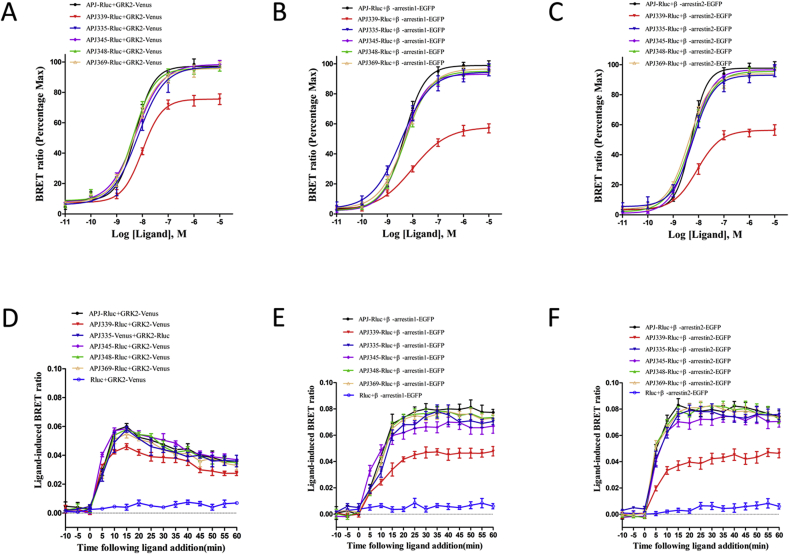
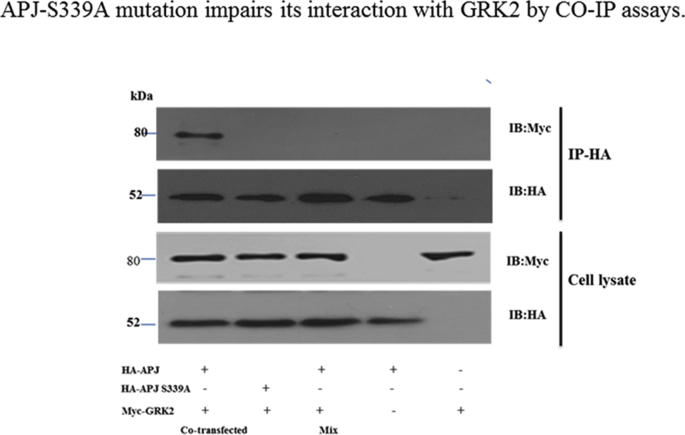
Next, we used the BRET assay to measure the apelin-36-induced concentration-response curves of GRK2 recruitment to APJ at 10 min post-stimulation. Apelin-36 caused a robust concentration-dependent increase in the BRET signal for WT and the five APJ mutants, indicating that GRK2 was recruited to activated receptors (Fig. 5A). The EC50 values for GRK2 were as Table 2. GRK2 was recruited to APJ-S335A, APJ-S345A, APJ-S348A, and APJ-S369A similarly to WT APJ. By contrast, GRK2 was recruited to APJ-S339A much less efficiently and affinity, with an EC50 value of 9.91 ± 1.54 nM. The interaction between APJ and GRK2 has also been confirmed by a cellular co-immunoprecipitation assay. The results indicated that co-immunoprecipitations could be detected in cells transfected with Myc GRK2 (Supplementary Fig. S4) and HA-APJ plasmids with apelin-36 treatment, but not in cells transfected with either vector alone. The co-immunoprecipitations could not be detected in the cells expressing Myc-GRK2 and HA-APJ339.
Fig. 5.
APJ-S339A mutation impairs the receptor's interactions with GRK2 and β-arrestins via apelin-36 stimulation.
A-C, Concentration–response curves of the recruitment of GRK2 or β-arrestin1/2 to WT and APJ mutates, measured by BRET assay. HEK293 cells were transiently transfected with Rluc-tagged receptors and Venus-tagged GRK2 (A), Rluc-tagged receptors and EGFP-tagged β-arrestin1 (B), or Rluc-tagged receptors and EGFP-tagged β-arrestin2 (C) and then treated with increasing concentration of apelin-36. The BRET signals between receptor and GRK2 were recorded at 10 min after agonist stimulation, the BRET signals between receptor and β-arrestin1/2 were recorded at 15 min after agonist stimulation. Data are expressed as means ± S.E. from five independent experiments.
D-F, Kinetic-response curves of the recruitment of GRK2 or β-arrestin1/2 to WT and APJ mutants following apelin-36 stimulation, measured by BRET in living cells. Real-time analysis of the interaction between WT or mutant APJ and GRK2 (D), WT or mutant APJ and β-arrestin1 (E), and WT or mutant APJ and β-arrestin2 (F). Coelenterazine H was added to transiently co-transfected HEK293 cells and then the BRET signal was recorded after apelin-36 stimulation. Data are expressed as means ± S.E. from five independent experiments.
Table 2.
APJ-S339A mutation impairs the receptor's interactions with GRK2 and β-arrestins via apelin-36 stimulation.
| EC50 (nM) |
|||
|---|---|---|---|
| GRK2 | β-Arrestin1 | β-Arrestin2 | |
| APJ WT | 5.33 ± 0.63 | 4.54 ± 0.52 | 5.18 ± 0.65 |
| APJ S-335A | 5.89 ± 0.80 | 4.5 ± 0.40 | 5.01 ± 0.45 |
| APJ S-339A | 9.91 ± 1.54 | 9.72 ± 0.85 | 8.74 ± 1.02 |
| APJ S-345A | 5.62 ± 0.86 | 4.36 ± 0.48 | 5.24 ± 0.43 |
| APJ S-348A | 4.82 ± 0.47 | 4.95 ± 0.36 | 4.98 ± 0.63 |
| APJ S-369A | 5.04 ± 0.76 | 4.99 ± 0.45 | 4.89 ± 0.46 |
We then used the BRET assay to measure the apelin-36-induced concentration-response curves of β-arrestin1/2 recruitment to WT APJ 15 min post-stimulation. Apelin-36 caused a robust concentration-dependent increase in the BRET signal for WT APJ, APJ-S335A, APJ-S345A, APJ-S348A, and APJ-S369A, indicating that β-arrestin1 and 2 were recruited to activated receptors (Fig. 5B and C). EC50 values were as Table 2. By contrast, the efficacy of β-arrestin1/2 recruitment to APJ-S339A was significantly reduced (EC50 values: 9.72 ± 0.85 nM for β-arrestin1; 8.74 ± 1.02 nM for β-arrestin2). This is consistent with recruitment of GRK2 to the activated receptor in a ligand-dependent manner.
Next, we used real-time BRET analysis to investigate the effect of C-terminal serine mutations on the interaction between APJ and GRK2, or β-arrestin1/2 upon apelin-36 stimulation. In cells expressing APJ-Rluc and GRK2-Venus, we observed a rapid increase in the BRET signal that peaked 15 min after apelin-36 addition (Fig. 5D); a similar increase was observed with the APJ-S335A, APJ-S345A, APJ-S348A, and APJ-S369A mutants. However, the BRET signal of the APJ-S339A mutant was significantly lower, indicating that the mutation of Ser339 to alanine affected the interaction between APJ and GRK2. Similarly, we observed a significant reduction in the BRET signal after apelin-36 stimulation of HEK293 cells expressing β-arrestin1/2-EGFP and APJ-S339A-Rluc, indicating that serine 339 is also crucial for binding β-arrestins to APJ (Fig. 5E and F).
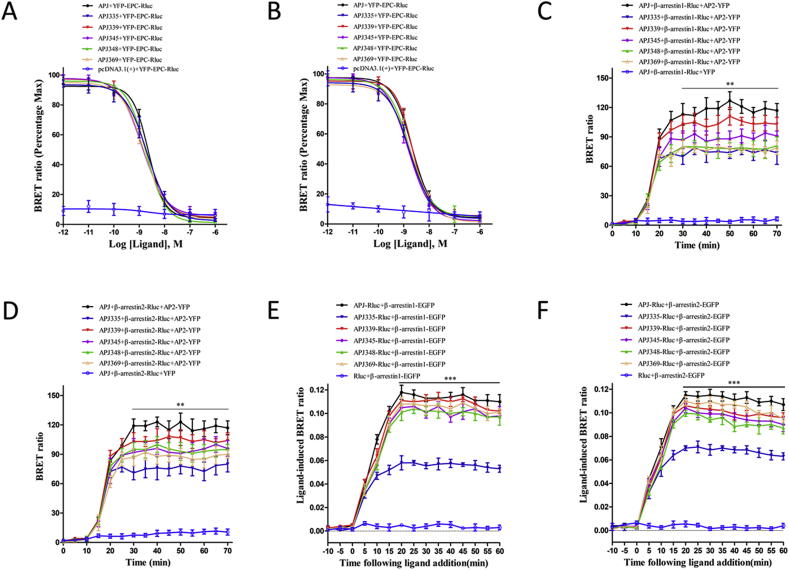
3.5. Mutation of APJ Ser335 mutation impairs the receptor's interaction with β-arrestin1/2 and AP2 following Elabela stimulation, but does not alter cAMP levels
We used the cAMP BRET biosensor to determine whether APJ-S335A affected the accumulation of cAMP upon ELA-32 or ELA-21 stimulation. For this purpose, cells expressing APJ or APJ-S335A were treated with ELA-32 (0.01–1000 nM, Fig. 6A) or ELA-21 (0.01–1000 nM, Fig. 6B), resulting in a concentration-dependent increase in cAMP generation. Both ELA-32 and ELA-21 were equipotent in stimulating cAMP production, with EC50 values of 10.35 ± 0.85 nM and 10.67 ± 1.00 nM, respectively. We observed no significant difference between APJ and APJ mutants in the stimulation of cAMP production by ELA-32 or ELA-21.
Fig. 6.
ELA-32-induced cAMP levels are not affected by APJ-S335 mutations, but this mutation can impair the interaction of receptors with AP2 and β-arrestin.
A, B ELA-32 and ELA-21 induced a cAMP response for WT and APJ mutants (S335A, S339A, S345A, S348A, S369A, only pcDNA3.1(+)) measured with an EPAC-based BRET biosensor. Concentration–response curves of intracellular cAMP levels with various concentrations of ELA-32 (Fig. 6, A) or ELA-21 (Fig. 6, B) (0.001–1000 nM) in HEK293 cells expressing WT or APJ mutants. Data are expressed as means ± S.E. from five independent experiments. C, D Kinetic-response curves of interactions between β-arrestin1 and AP2 or β-arrestin 2 and AP2 induced by APJ and APJ mutants upon activation by the ELA-32 agonist, obtained from real-time dynamic BRET determination. Data are expressed as means ± S.E. from five independent experiments. **P < 0.01 APJ + β-arrestin1-Rluc + AP2-YFP vs APJ335 + β-arrestin1-Rluc + AP2-YFP, **P < 0.01APJ + β-arrestin2-Rluc + AP2-YFP vs APJ335 + β-arrestin2-Rluc + AP2-YFP. E, F Kinetic-response curves of interactions between β-arrestin1 or β-arrestin 2 induced by APJ and APJ mutants upon activation by the ELA-32 agonist, obtained from real-time dynamic BRET determination. Data are expressed as means ± S.E. from five independent experiments. APJ-Rluc + β-arrestin1-EGFP vs APJ335-Rluc + β-arrestin1-EGFP, ***P < 0.001 APJ-Rluc + β-arrestin2-EGFP vs APJ335-Rluc + β-arrestin2-EGFP.
To identify the early events leading to β-arrestin- and clathrin-dependent internalization of GPCR [24], we measured BRET between β-arrestin1-Rluc or β-arrestin2-Rluc and β2-adaptin-EYFP (the β2 subunit of clathrin adaptor AP-2) in HEK293 cells co-transfected with WT or mutant APJ. Interesting, after treatment with ELA-32, (1) BRET signals in cells expressing β-arrestin1 and AP2 significantly differed (**P < 0.01) between HEK293-APJ and HEK293-APJ-S335A (Fig. 6C), (2) BRET signal of the APJ-S335A mutant was significantly lower (**P < 0.01). Similarly, we also observed a significant reduction in the BRET signal after ELA-32 stimulation in HEK293 cells expressing APJ-S335A along with β-arrestin2-EGFP and AP2, indicating that Ser335 is also crucial for binding AP2 to APJ and subsequent internalization (Fig. 6D). These results indicate that the APJ-S335A mutant is defective in APJ internalization induced by ELA-32.
When HEK293 cells expressing β-arrestin1-EGFP and WT or mutant APJ-Rluc were treated with ELA-32 (100 nM). The BRET signal showed that activation of APJ and APJ mutants (S339A, S348A, S348S, S369A) increased β-arrestin1. After the addition of ELA-32, the BRET signal increased rapidly, reaching a peak in 20 min (Fig. 6E). However, ELA-32 treatment of HEK293 cells co-expressing β-arrestin1-EGFP and APJ-S335A resulted in a decrease in the BRET value, Impair the functioning of APJ-S335A interaction with β-arrestin1. Similarly, ELA-32-dependent recruitment of β-arrestin2 was observed in APJ and APJ mutants (Fig. 6F), but the BRET signal of the APJ-S335A mutant was significantly lower (***P < 0.001). Together, these results showed that APJ-S335A decreases the recruitment and function of β-arrestin1 and 2. We will demonstrate in future studies that when multiple residues are mutated simultaneously, we can see a more significant loss of β-arrestins recruitment.
3.6. Phosphorylation of β-arrestin1 and 2 in APJ and APJ mutants upon stimulation with five peptides, as determined by mass spectrometry
We then examined the effect of WT and mutant APJ stimulation on the phosphorylation status of β-arrestin1/2. Stimulation with apelin-36, apelin-17, or apelin-13 resulted in phosphorylation of the Tyr47 and Ser 412 sites of β-arrestin1, and the Tyr48, Ser 361, and Thr383 sites of β-arrestin2. The same sites were phosphorylated when cells were stimulated with ELA-32 and ELA-21 stimulation, i.e., the phosphorylation sites of β-arrestins were consistent under stimulation with apelin and Elabela. Notably in this regard, when the APJ-S339A and APJ-S335A mutants were stimulated by apelin-36 and ELA-32, respectively, the same residues of the β-arrestins were still phosphorylated. Thus, APJ mutations did not affect the phosphorylation of β-arrestins (Supplementary Fig. S5).
3.7. Mutation of Ser339 prevents apelin-36-induced receptor internalization
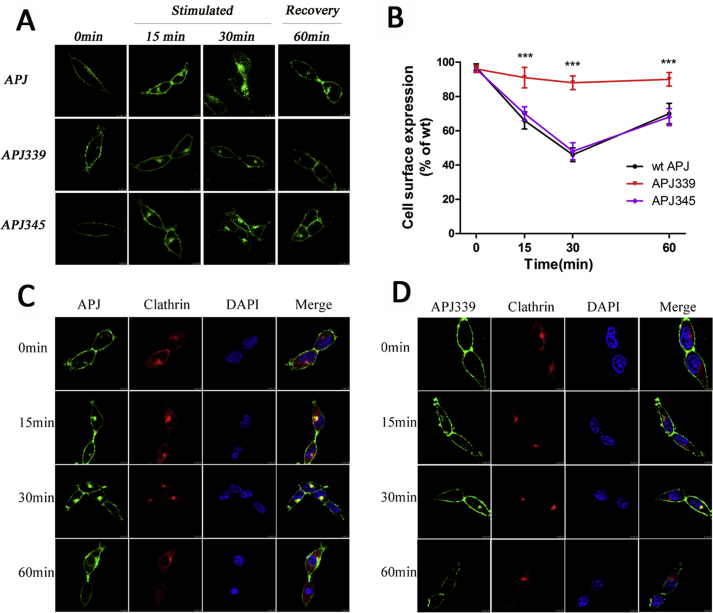
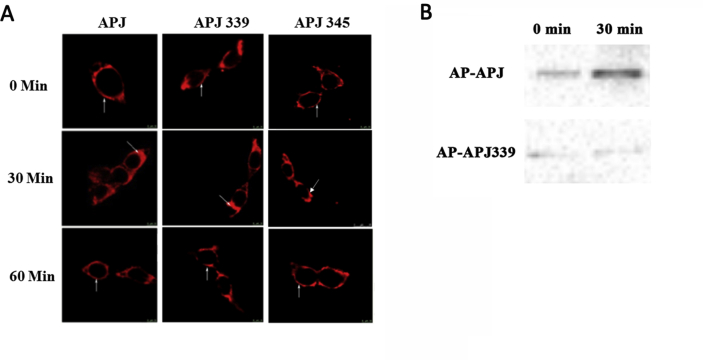
In general, receptor phosphorylation is typically essential for agonist-induced receptor internalization. Hence, we performed imaging to determine the subcellular location of EGFP-tagged receptors (Supplementary Fig. S6A), and conducted ELISA assays (Supplementary Fig. S6B) to define the extent of WT and mutational receptor internalization after exposure to apelin-36. In HEK293 cells expressing WT APJ, apelin-36 (100 nM) caused robust time-dependent receptor internalization peaking 30 min after the addition of the agonist. GPCR internalization primarily occurs via a clathrin-mediated mechanism, usually after receptor phosphorylation by GRKs. To image cell-surface expression and receptor internalization, we transiently co-transfected HEK293 cells with EGFP-tagged WT or mutational APJs and mCherry-tagged clathrin. Figs. S4C and S4D show that prior to agonist stimulation (at 0 min), APJ (shown in green) was clearly localized at the cell surface, whereas fluorescent clathrin protein (shown in red) was evenly distributed throughout the cytosol. In addition, the results showed that ELA-32 does not cause S339 site phosphorylation (Table 1). HEK293 cells were transiently HA-tagged WT or APJ S335 or APJ S345 mutant (Supplementary Fig. S7A), shows that prior to ELA-32 stimulation (at 0 min), the distribution of APJ (shown in red) was clearly localized at the cell surface. Upon stimulation with the appropriate agonist ELA-32 (100 nM) for 30 min, a significant internalization was observed in HEK293 cells expressing WT APJ. APJ was then redistributed back to the cell surface at 60 min. APJ-S335A and APJ-S345A showed a similar cell surface expression and internalization process compared with the WT APJ.
Proximity biotinylation was also used to examine the interaction between clathrin and APJ. Since biotin and streptavidin form a tight interaction with a slow off-rate (of days), biotinylated APJ would remain biotinylated even if the complex dissociated. Here, we detected biotinylation using western blot and found that acceptor peptide (AP)-APJ can be biotinylated, while AP-APJ339 cannot be biotinylated, demonstrating that mutation of Ser339 prevents Apelin-36-induced receptor internalization (Supplementary Fig. S7B). In summary, our results indicate that the application of proximity biotinylation method further confirms that Apelin-36 can prevent the internalization of APJ 339.
3.8. The Ser339 mutation in APJ decreases β-arrestin-dependent ERK1/2 activation
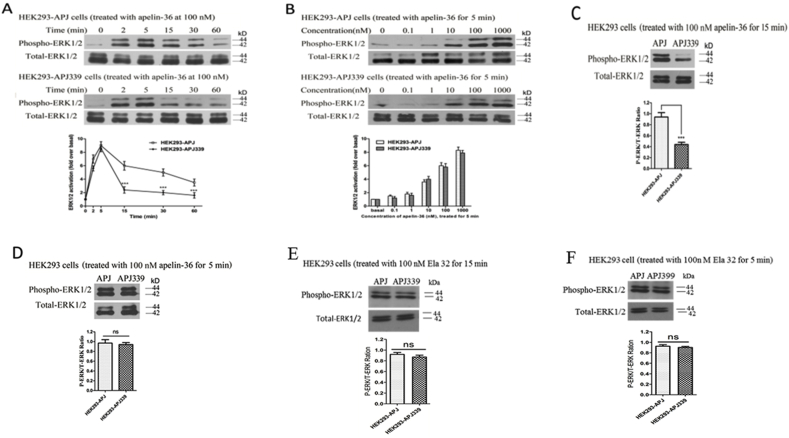
To determine whether the APJ mutants affect fast or slow ERK1/2 activation, we examined the time- and concentration-dependent effects of apelin-36 on ERK1/2. In cells in WT APJ, we observed a significant increase in ERK1/2 phosphorylation after 2 min treatment with apelin-36. The phosphorylation level peaked at 5 min, and phospho-ERK1/2 levels remained high for 30 min (Fig. 7A, upper). In cells expressing the APJ-S339A mutant, ERK1/2 phosphorylation was similar to that observed in WT APJ 5 min after agonist stimulation, but subsequently the phospho-ERK1/2 level decreased to near-basal levels within 15 min. This effect was β-arrestin-dependent (Fig. 7, middle) activation of ERK1/2.
Fig. 7.
The effects of Ser-339 mutation on ERK1/2 phosphorylation.
A, Time-dependent ERK1/2 phosphorylation in HEK293 cells expressing APJ (upper) or APJ-S339A (middle) after 100 nM apelin-36 stimulation for different lengths of time. The time-dependent curves of the two groups were quantified and the results shown in the lower panel. B, Concentration-dependent ERK1/2 phosphorylation in HEK293 cells expressing APJ (upper) or APJ-S339A (middle) after apelin-36 stimulation at different concentrations for 5 min. The concentration-dependent effects in the two groups were quantified and the results are shown in the lower panel. C and D, ERK1/2 phosphorylation in HEK293 cells expressing APJ or APJ-S339A (upper) after 100 nM apelin-36 stimulation for 15 min (C) or 5 min (D). ERK1/2 phosphorylation in HEK293 cells expressing APJ or APJ-S339A after 100 nM ELA-32 treatment for 15 min (E) or 5 min (F). The P-ERK/T-ERK ratio of the two groups was quantified and is shown in the lower panels. The density of the bands corresponding to 44 and 42 kDa was quantified with an imaging densitometer, normalized to total ERK. The data correspond to the means ± S.E. from five independent experiments. APJ-S339A group compared with WT APJ group, ***P < 0.001; ns, no significant difference, P > 0.05.
We then assessed concentration-dependent ERK1/2 phosphorylation in cells expressing mutant APJ-S339A and found that administration of apelin-36 (0.1–1000 nM) for 5 min induced ERK1/2 activation (Fig. 7, middle). These data were consistent with those for WT APJ (Fig. 7B, upper) and exhibit a concentration-dependent trend.
Next, we observed the effect of the duration of apelin-36 application on ERK1/2 phosphorylation in cells expressing WT APJ or APJ-S339A. The results revealed that 15 min apelin-36 treatment induced less ERK1/2 activation in cells expressing APJ-S339A than in those expressing WT APJ (Fig. 7C). However, when the duration of agonist application was reduced, we observed similar ERK1/2 activation, with no statistical difference between cells expressing WT and APJ-S399A (Fig. 7B and D). Further, in the cells expressing mutant APJ-S339A, the ERK1/2 activation induced by ELA-32 (100 nM) treatment for 5 and 15 min was not different from that of WT APJ (Fig. 7E and F).
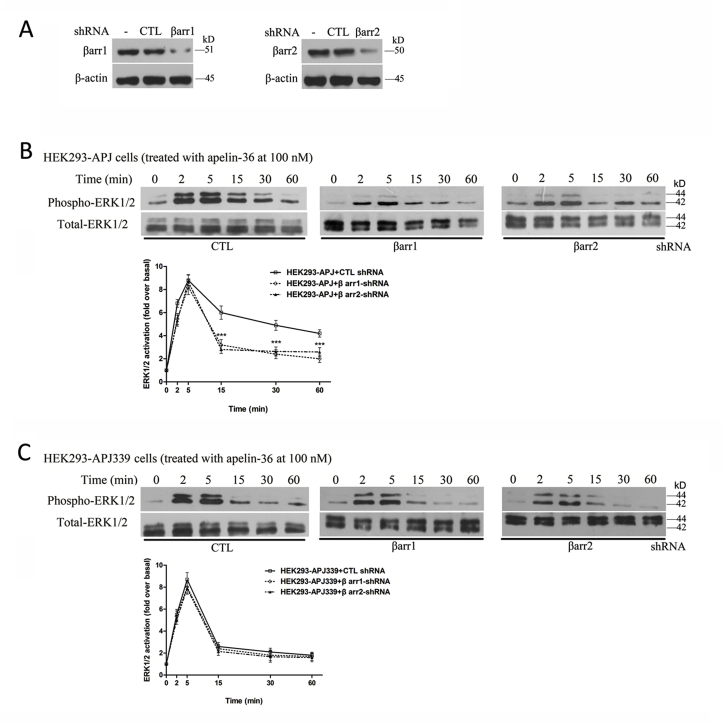
We analyzed the time course of apelin-36-induced ERK1/2 phosphorylation after depleting the cellular levels of β-arrestin1 or β-arrestin2 by transfecting cells with shRNA specific for each isoform. A non-targeted control shRNA had no effect on apelin-36-induced ERK1/2 phosphorylation in cells expressing APJ (compare Fig. 7, Fig. 8B). When serum-starved cells were treated with 100 nM apelin-36, and cell lysates were analyzed for ERK1/2 phosphorylation and β-arrestin (Fig. 8A). However, in WT APJ-expressing cells transfected with β-arrestin1 or β-arrestin2 shRNA, ERK1/2 phosphorylation was significantly reduced from 15 min onward (Fig. 8B). These data show that late ERK1/2 activity is β-arrestin dependent. Transfecting β-arrestin shRNA into cells expressing the mutant APJ-S339A made no difference to the apelin-36-induced ERK1/2 signaling. This signaling was at a low level in all conditions, and no statistically significant differences were observed (Fig. 8C).
Fig. 8.
Effects of β-arrestin shRNA on APJ-stimulated ERK1/2 phosphorylation.
Serum-starved cells were treated with 100 nM apelin-36 and cell lysates were analyzed for ERK1/2 phosphorylation and β-arrestin (A). HEK293 cells stably expressing APJ (B) or APJ-S339A (C) were transfected with the indicated shRNA. Serum-starved cells were treated with 100 nM apelin-36 and cell lysates were analyzed for ERK1/2 phosphorylation and β-arrestin (A). The density of the bands corresponding to 44 and 42 kDa was quantified with an imaging densitometer, normalized to total ERK. The data correspond to the means ± S.E. from five independent experiments. ***P < 0.001 compared with the control treatment group.
4. Discussion
GPCRs, the largest superfamily of cell-surface receptors, regulate nearly all known physiological processes in mammals [30]. Accordingly, they are the targets of up to 40% of current pharmaceuticals [31]. Different patterns of GRK-mediated phosphorylation on receptors can induce distinct arrestin conformations, which are in turn capable of mediating specific functions, such as internalization, desensitization, and downstream signaling [32].
Apelin and Elabela constitute a spatiotemporal double-ligand system for control of APJ signal transduction with distinct receptor binding and intracellular trafficking features. In this study, we used LC-MS/MS analytical methods to demonstrate that five peptides induce phosphorylation at four sites (Ser 339, 345, 348, and 369). Phosphorylation of S335, which was not seen by MS, was detected using a site-specific antibody to show that phosphorylation of APJ occurs at one serine residue in region of the C-terminus (Ser 335), but not Ser 369. Of particular interest, it was found that only ELA-32 induced phosphorylation of S335. In this way, five phosphorylation sites were found, which provided a solid foundation for future research.
Apelin-36 and apelin-13 are endogenous ligands of APJ that have different receptor binding affinities and consequently different abilities to affect the intracellular trafficking of the receptor. For example, apelin-36 has longer-lasting effects on APJ activation than apelin-13 [33]. Other data show that apelin-36 and apelin-13 do not have any differences in affinity for the native human receptor [34], We speculated that the potential difference between apelin-36 and apelin-13 might be the result of downstream signaling. Ma et al. have showed that apelin-36 produces a more durable response that could not be recapitulated after a washout period [35]. Our previous results are consistent with this, showing that mutation of Ser348 eliminated apelin-13-induced recruitment of GRKs and β-arrestin1/2 to APJ [23]. To complicate matters, different ligands can favor specific phosphorylation events, raising the possibility of ligand-specific phosphorylation [36]. We used mass spectrometry to identify apelin-36-induced APJ phosphorylation sites, as distinct from the apelin-13-induced sites. In addition, we used BRET assays to analyze the interactions between APJ, APJ mutants, and GRK2 or β-arrestin1/2 upon apelin-36 stimulation. The interaction between APJ and GRK2 has also been confirmed by a cellular co-immunoprecipitation assay. These data confirmed that mutation of serine 339 to alanine in APJ greatly prevents recruitment with GRK2 under apelin-36 stimulation, in which APJ-S339A may fail to induce the β-arrestin-dependent signaling pathway. APJ S339 phosphorylation was not detected with any of the other apelin/ELA ligands by MS (Table 1), therefore the S339A mutation would not be predicted to affect GRK2 or β-arrestin1/2 recruitment in response to any of these other ligands. APJ S339A was unable to induce the β-arrestin-dependent signaling pathway, but had no effect on G-protein binding or subsequent G-protein-dependent intracellular signaling. The phosphorylation site at Ser 339 was crucial for the interactions with GRK2 and β-arrestin, as well as for the internalization of APJ following treatment with apelin-36. This phosphorylation barcode is translated into specific β-arrestin conformations that dictate selective signaling and intracellular functions.
Elabela and apelin were shown to exhibit the varying structural features and distribution patterns, resulting in different modes of binding and potencies for activation of apelin receptor. Both these two peptides were also reported to bind to APJ, which activates Gαi2 and β-arrestin signaling pathway followed by receptor internalization. Our results show that there is no significant difference in BRET signal between β-arrestin 2 and APJ induced by apelin-36 and ELA-32 compared with β-arrestin 1.
We utilized site-directed mutagenesis to introduce serine-to-alanine mutations, and used BRET assays to demonstrate that the potency of β-arrestin1/2 recruitment to APJ-335A was significantly reduced. Upon ELA-32 stimulation, the APJ-S335A mutant was impaired in the interaction with β-arrestin1/2, but the mutation did not alter cAMP levels, or influence induced G-protein activation or the G-protein-dependent signaling pathway. Conversely, with apelin-36 treatment, in which APJ-S339A may fail to induce the β-arrestin-dependent signaling pathway, but APJ-S335A, as same as like WT APJ, induces the β-arrestin-dependent signaling pathway. However, the results revealed that Ser335 plays an important role in the interactions of APJ with β-arrestin under stimulation with ELA-32. However, the various C-terminal phosphorylation sites of APJ play different roles in signal transduction through different ligands such as ELA-32 and aplin-36 induced the corresponding phosphorylation site (Ser 335 and Ser 339) respectively. In this context, we can speculate that the processing of ELA may be the same as apelin. Different cell types and external environments have differences, resulting in different ligand-mediated biological effects. Therefore, the study found that apelin and ELA seem to constitute a spatiotemporal dual ligand system to control cardiac APJ signals [37].
Another mechanism that might underlie the β-arrestin dependence of receptor-operated signaling is the phosphorylation of β-arrestins themselves [38]. β-arrestins are important signal transducers and that a number of biological functions exerted by GPCRs are mediated by β-arrestin-dependent signaling. Our findings show that mutation in APJ affects binding to β-arrestins and do not affect cAMP and calcium levels and their G-protein interactions, but what about the phosphorylation of β-arrestins themselves? To answer this question, we used mass spectrometry to study the phosphorylation of β-arrestin1 and 2 in APJ and APJ mutants in response to five peptides. Stimulation by apelin-36, apelin-17, apelin-13, ELA-32, and ELA-21 caused the Y47 and S412 sites of β-arrestin1, and the Y48, S361, and T383 sites of β-arrestin2 to be phosphorylated. Of particular interest, when the APJ S335A and S339A mutants were stimulated with ELA-32 and apelin-36, respectively, phosphorylation of β-arrestins was still detected. Thus, APJ mutations do not affect phosphorylation of β-arrestins. We identified two sites phosphorylated on β-arrestin1 and three on β-arrestin2, including three previously identified residues (Ser412, Ser361, and Thr383) and two new sites: Tyr 47 on β-arrestin1 and Tyr48 on β-arrestin2 (Figure supplement 3). The influence of β-arrestin phosphorylation on APJ operated β-arrestin-dependent signaling such as Erk1/2 activation, trafficking these detailed functions of the newly discovered phosphorylation sites on β-arrestins will be examined in future studies.
Different peptides act on different phosphorylation sites in the APJ C-terminus to regulate distinct aspects of APJ signal transduction. In addition to their role in desensitization, β-arrestins also play important roles in directing GPCR internalization via clathrin-mediated endocytic machinery. Compared with WT APJ, APJ-S335A and APJ-S345A showed similar cell surface expression and internalization processes when stimulated by apelin-36. APJ-S339A was significantly less able to bind β-arrestins than WT APJ, the results show that the phosphorylation site Ser339 is specifically involved in the receptor internalization process. Both ELISA and cellular localization assays revealed a large reduction in apelin-36-induced internalization of APJ-S339A. Furthermore, after apelin-36 stimulation, APJ-S339A was co-localized with clathrin to a significantly lesser degree than WT APJ. Thus, mutation of APJ Ser339 to alanine is sufficient to block apelin-36 induced APJ internalization. In addition, another ligand is used, the results show that ELA-32 and apelin-36 play different roles in the internalization of APJ-S339A and do not affect ELA-32 induced APJ internalization. It is also proved that apelin-36 plays a unique role in the phosphorylation of Ser339 during the internalization of APJ. We can conclude that phosphorylation of Ser339 plays an important role in β-arrestin and receptor interaction, and in apelin-36-induced APJ internalization.
We also observed a significant reduction in the BRET signal following ELA-32 stimulation in HEK293 cells expressing APJ-S335A with β-arrestin-EGFP and β2 subunit of the clathrin adaptor AP2 (AP2), indicating that serine 335 is also crucial for binding of clathrin to APJ and subsequent internalization. The results indicate that the APJ-S335A mutation affects ELA-32-induced APJ internalization. The results also showed that clathrin directly interact with APJ and β1/2-arrestin to induce its internalization. The demonstrated that clathrin plays an important role in the APJ internalization by ELA 32.
Overall, these results are consistent with previous studies showing that the recruitment of β-arrestin to phosphorylated receptors is an essential process for the internalization and trafficking of GPCRs. β-arrestins function as scaffolding proteins for the recruitment of downstream effectors such as ERK [39,40]. Some GPCRs can initiate ERK1/2 signaling through both G-protein-dependent and -independent (i.e., β-arrestin-dependent) pathways, albeit with different activation kinetics and molecular consequences: the G-protein-dependent signals exhibit an early and transient response, whereas β-arrestin-dependent signals are late and sustained [41,42]. We found that the ability of APJ-S339A to recruit β-arrestins in an apelin-36-dependent manner was clearly reduced. To determine whether the APJ-S339A mutant affects the activation of ERK1/2 through either the G-protein or β-arrestin pathway, we monitored the kinetic patterns that characterize ERK1/2 activation via the two pathways. The results revealed that ERK1/2 activation peaked 5 min after apelin-36 stimulation in both the WT and APJ-S339A groups. These data confirmed that apelin-36 activates ERK1/2 via a G-protein-dependent pathway. Furthermore, the phospho-ERK1/2 level after 15 min was significantly lower in the APJ-S339A group than in the WT group. Apelin-36 induced ERK1/2 phosphorylation in a concentration-dependent manner in HEK293 cells expressing WT APJ. By contrast, the APJ-S339A mutant exhibited a similar pattern for fast ERK1/2 activation (5 min) but reduced ERK1/2 activation (15 min) at all concentrations. In addition, After Stimulation with ELA-32, the WT APJ was compared with APJ-S339A and no difference was found between them for the activation of ERK1/2. The results demonstrated that β-arrestin1/2-dependent ERK activation by ELA-32 is not affected by the S339A mutation.
Further studies utilizing shRNA directed against various β-arrestins confirmed that slow ERK1/2 activity is β-arrestin dependent. These data demonstrated that β-arrestin-regulated β-arrestin-dependent activation of ERK1/2 activation was affected by point mutation of Ser339, although activation of ERK1/2 via the G-protein-dependent pathway could still be triggered in the mutant. In an analogous manner, elimination of the phosphorylation sites in the tails of PAR-2 and NK1R abolished β-arrestin-dependent activation of ERK1/2 [43]. These data suggest that apelin-36 activates ERK1/2 via a G-protein-dependent pathway and is not affected by mutation at Ser339. By contrast, ERK1/2 activation via the β-arrestin dependent pathway was clearly decreased by the mutation at serine 339.
However, few examples of natural GPCR mutations with biased agonistic effects have been reported to date [44,45]. Some biased agonists of APJ were identified through high-throughput screening. In our study, mutation of the APJ phosphorylation sites at Ser335 and Ser339 may have also induced biased signaling. The complex properties of biased signaling enrich the function of GPCRs, potentially leading to improved therapeutic properties and reduced side effects or toxicity [46]. Thus, GPCRs present both challenges and opportunities for preclinical drug screening and development [47].
Taken together, our results demonstrate that phosphorylation of APJ occurs at five serine residues of the C-terminus: Ser335, 339, 345, 348, and Ser369. We also identified two phosphorylation sites on β-arrestin1 and three on β-arrestin2, including three previously identified residues (Ser412, Ser361, and Thr383) and two new sites: Tyr 47 for β-arrestin1 and Tyr 48 for β-arrestin2. Mutations decreased binding to β-arrestins but did not affect the role of β-arrestins in phosphorylation. Our results also demonstrate that APJ S335A and APJ S339A have the characteristics of a biased receptor, resulting in a lack of activity in the β-arrestin-dependent pathway. We showed that APJ-S335A mutant damage affects Elabela-induced APJ internalization. Phosphorylation of Ser339 plays an important role in β-arrestin receptor interaction and apelin-36-induced APJ internalization, and disrupts β-arrestin-dependent ERK1/2 activation. Our also results demonstrate that mutations in the apelin receptor decreased binding to β-arrestins but did not affect β-arrestins phosphorylation.
Recent research showed that APJ plays a dual role in cardiac hypertrophy [48]. Apelin-activated APJ evokes a protective response in cardiac hypertrophy via the Gαi pathway, whereas β-arrestin-dependent signaling induces cardiac hypertrophy. Insights gained from this study are likely to provide additional understanding of the functional properties of APJ that allow G-protein-dependent APJ signals to override β-arrestin-dependent signals. This may lead to potential therapeutic interventions for cardiovascular and metabolic diseases.
Author's contributions
J.C conceived and designed the experiments; S. Li, HL. M, YU. J, RM. Z and X. C conducted the experiments; MC. Y, CM.W and XY.C performed the data analysis; J.C and CM.W supervised the project. J.C and XY. C wrote the manuscript.
Declaration of competing interest
The authors declare that they have no conflict of interests.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31600949 and 31271243), the Nature Science Foundation of Shandong Province (ZR2018MC005 and ZR2018PC011), Academic Promotion Plan of Shandong Province (2019QL013), and the Science and Technology Project of Shandong Province (J16LE01).
Abbreviations
The abbreviations used are
- GPCRs
G-protein-coupled receptors
- GRKs
G-protein-coupled receptor kinases
- APJ
apelin receptor
- ELA
Elabela
- BRET
bioluminescence resonance energy transfer
- EGFP
enhanced green fluorescence protein
- Rluc
Renilla luciferase
- ERK1/2
extracellular-signal regulated kinase-1/2
- HEK
Human embryonic kidney
- WT
wild-type
- MS
mass spectrometry
- Ser
Serine
- Tyr
tyrosine
- ANOVA
analysis of variance
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2020.101629.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Identification of ELA-32 regulation of APJ phosphorylation status using phosphorylation site–specific antibody.
HEK 293 cells were induced to express APJ WT were either time or dose-dependent activation of phosphorylation and lysates were then immunoblotted with the antibodies to S335 (A). Blots were stripped and re-probed with antibodies to the β-actin. Blots are representative of n = 5 independent experiments. (B). Characterization of phosphosite-specific (S335) antibody using APJ mutants. HEK293 cells were transfected with APJ WT, or APJ S335A, or APJ S369A by 100 nM ELA-32 for 10 min. Lysates of cells were resolved by SDS-PAGE and phosphorylated receptors detected by immunoblotting with the phospho-Ser335 antibody. Blots were stripped and re-probed with antibodies to the β-actin. Blots are representative of n = 5 independent experiments. APJ WT were either time (C) or dose-dependent (D) activation of phosphorylation. HEK293 cells were transfected with APJ WT, or APJ S335A, or APJ S369A by 100 nM ELA-32 for 10 min (E), APJ-S335A group compared with WT APJ group, *P < 0.05. The densities of the bands corresponding to APJ or APJ S335A or APJ S369S phosphorylation and total β-actin were quantified with an imaging densitometer (Scion Image).
Fig. S2.
Characterization of the Ca2+response to apelin-36.
Effect of PTX blocking Gαi protein signal on calcium fluorescence (A). Variations in calcium fluorescence over 300 s with 100 nM apelin-36 in the presence (B) or absence of external Ca2+ (C). The data correspond to the means ± S.E. from five independent experiments.
Fig. S3.
Selection of different G protein blinding to APJ.
HEK293 cells were transiently transfected with EGFP-tagged APJ, Gαq and Rluc-Gαi2 (A) or EGFP-tagged APJ, Gαi2 and Rluc-Gαq (B), then coelenterazine H was added and detected as above. The results are means ± S.E. from at least five independent experiments.
Fig. S4.
APJ-S348A mutation impairs its interaction with GRK2 by CO-IP assays.
A, HEK293 cells were transfected with expression vectors of both HA-APJ and Myc fusion proteins (co-transfected) or with either vector alone. Samples containing either HA-APJ or Myc-GRK2 were mixed (Mix). Confirmation of the expression of appropriate constructs was obtained by immunoblotting cell lysates with either anti-Myc or anti-HA antibodies (lower panels). The cell lysates were subsequently immunoprecipitated (IP) with anti-HA-agarose beads and followed by immunoblotted (IB) with anti-Myc or anti-HA antibodies (upper panels). The results shown are representative images from at least four independent experiments.
Fig. S5.
Mass spectrometry was used to identify APJ or APJ mutants and β-arrestin interactions to induce β-arrestin phosphorylation sites.
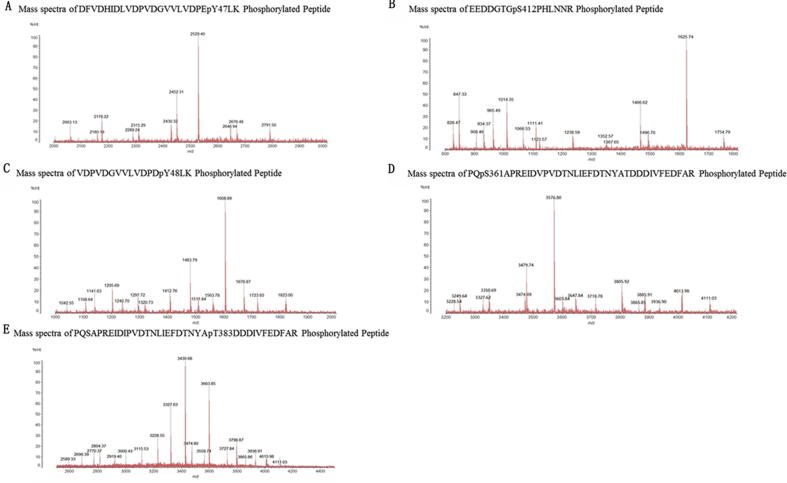
Mass spectrometry identified two phosphorylation sites in β-arrestin1 (Tyr 47 and Ser 412). Mass spectra of DFVDHIDLVDPVDGVVLVDPEpY47LK and EEDDGTGpS412PHLNNR phosphorylated peptides identified from β-arrestin1 were transiently co-expressed with APJ or APJ mutants in HEK-293 cells and immunoprecipitated using β-arrestin1 antibody (Fig. S5 A, B).
Mass spectrometry identified three phosphorylation sites in β-arrestin 2 (Tyr 48, Ser 361 and Thr383). Mass spectra of VDPVDGVVLVDPDpY48LK, PQpS361APREIDVPVDTNLIEFDTNYATDDDIVFEDFAR and PQSAPREIDIPVDTNLIEFDTNYApT383DDDIVFEDFAR phosphorylated peptides identified from β-arrestin2 transiently co-expressed with APJ or APJ mutants in HEK-293 cells and immunoprecipitated using the β-arrestin 2 antibody (Fig. S5 C, D, E).
Fig. S6.
APJ-S339A mutation impairs agonist-induced receptor internalization in HEK293 cells.
A, Internalization of WT and APJ mutants after 100 nM apelin-36 stimulation in HEK293 cells, measured by confocal microscopy. HEK293 cells expressing EGFP-tagged-APJ, APJ-S339A, or APJ-S345A were treated with apelin-36 (100 nM) for 0, 15, 30, and 60 min. The redistribution of WT and APJ mutants (green) after agonist stimulation is shown. The results shown are representative images from at least five independent experiments. Scale bars, 10 μm.
B, Cell surface ELISAs were performed with an anti-HA antibody to detect cell surface receptors in non-permeabilized cells treated with apelin-36 (100 nM) for 0, 15, 30, and 60 min. The results shown are representative images from at least three independent experiments (n = 5; one-way analysis of variance; ***P < 0.001 versus WT APJ group). C-D, HEK293 cells were co-transfected with a clathrin-cherry construct and either APJ-EGFP (C) or APJ-S339A-EGFP (D) at a 1:1 ratio then treated with apelin-36 (100 nM) for 0, 15, 30, and 60 min. The redistribution and co-localization of APJ (green) and clathrin (red) after agonist stimulation is shown. Nuclei were stained with DAPI. Yellow indicates the co-localization of receptors and clathrin. The results shown are representative images from at least five independent experiments. Scale bars, 10 μm.
Fig. S7.
APJ-S339A mutation does not impair receptor internalization and trafficking under ELA-32 stimulation.
A, HEK293 cells were transfected APJ (left), APJ-S339A (middle), APJ-S345A (right). Shown are representative confocal micrographs of APJ or APJ-S339A or APJ-S345A (red) after ELA-32 (100 nM) stimulation for 0, 30, and 60 min. The results shown are representative images from at least five independent experiments. Vertical arrows show the plasma membrane, slanted arrows show endosomes. Scale bars, 10 μm. B, we detected biotinylation using western blot and found that acceptor peptide (AP)-APJ can be biotinylated, while AP-APJ339 cannot be biotinylated.
References
- 1.Medhurst A.D. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J. Neurochem. 2003;84(5):1162–1172. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]
- 2.O'Carroll A.M. The apelin receptor APJ: journey from an orphan to a multifaceted regulator of homeostasis. J. Endocrinol. 2013;219(1):R13–R35. doi: 10.1530/JOE-13-0227. [DOI] [PubMed] [Google Scholar]
- 3.Chandrasekaran B., Dar O., McDonagh T. The role of apelin in cardiovascular function and heart failure. Eur. J. Heart Fail. 2008;10(8):725–732. doi: 10.1016/j.ejheart.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 4.Brash L. Short-term hemodynamic effects of apelin in patients with pulmonary arterial hypertension. JACC Basic Transl. Sci. 2018;3(2):176–186. doi: 10.1016/j.jacbts.2018.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Japp A.G. Acute cardiovascular effects of apelin in humans: potential role in patients with chronic heart failure. Circulation. 2010;121(16):1818–1827. doi: 10.1161/CIRCULATIONAHA.109.911339. [DOI] [PubMed] [Google Scholar]
- 6.Dray C. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metabol. 2008;8(5):437–445. doi: 10.1016/j.cmet.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Taheri S. The effects of centrally administered apelin-13 on food intake, water intake and pituitary hormone release in rats. Biochem. Biophys. Res. Commun. 2002;291(5):1208–1212. doi: 10.1006/bbrc.2002.6575. [DOI] [PubMed] [Google Scholar]
- 8.Tatemoto K. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998;251(2):471–476. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 9.Chng S.C. ELABELA: a hormone essential for heart development signals via the apelin receptor. Dev. Cell. 2013;27(6):672–680. doi: 10.1016/j.devcel.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Read C. International union of basic and clinical pharmacology. CVII. Structure and pharmacology of the apelin receptor with a recommendation that Elabela/Toddler is a second endogenous peptide ligand. Pharmacol. Rev. 2019;71(4):467–502. doi: 10.1124/pr.119.017533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pauli A. Toddler: an embryonic signal that promotes cell movement via Apelin receptors. Science. 2014;343(6172):1248636. doi: 10.1126/science.1248636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masri B. Apelin (65-77) activates extracellular signal-regulated kinases via a PTX-sensitive G protein. Biochem. Biophys. Res. Commun. 2002;290(1):539–545. doi: 10.1006/bbrc.2001.6230. [DOI] [PubMed] [Google Scholar]
- 13.Murza A. Discovery and structure-activity relationship of a bioactive fragment of ELABELA that modulates vascular and cardiac functions. J. Med. Chem. 2016;59(7):2962–2972. doi: 10.1021/acs.jmedchem.5b01549. [DOI] [PubMed] [Google Scholar]
- 14.Lohse M.J., Hoffmann C. Arrestin interactions with G protein-coupled receptors. Handb. Exp. Pharmacol. 2014;219:15–56. doi: 10.1007/978-3-642-41199-1_2. [DOI] [PubMed] [Google Scholar]
- 15.O'Hayre M. Genetic evidence that beta-arrestins are dispensable for the initiation of beta2-adrenergic receptor signaling to ERK. Sci. Signal. 2017;10(484) doi: 10.1126/scisignal.aal3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luttrell L.M. Manifold roles of beta-arrestins in GPCR signaling elucidated with siRNA and CRISPR/Cas9. Sci. Signal. 2018;11(549) doi: 10.1126/scisignal.aat7650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vishnivetskiy S.A. Regulation of arrestin binding by rhodopsin phosphorylation level. J. Biol. Chem. 2007;282(44):32075–32083. doi: 10.1074/jbc.M706057200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer D. Distinct G protein-coupled receptor phosphorylation motifs modulate arrestin affinity and activation and global conformation. Nat. Commun. 2019;10(1):1261. doi: 10.1038/s41467-019-09204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlos N.J., Friedman P.A. GPCR signaling and trafficking: the long and short of it. Trends Endocrinol. Metabol. 2017;28(3):213–226. doi: 10.1016/j.tem.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gurevich V.V., Gurevich E.V. Structural determinants of arrestin functions. Prog. Mol. Biol. Transl. Sci. 2013;118:57–92. doi: 10.1016/B978-0-12-394440-5.00003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oakley R.H. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J. Biol. Chem. 2001;276(22):19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- 22.Masri B. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J. Biol. Chem. 2006;281(27):18317–18326. doi: 10.1074/jbc.M600606200. [DOI] [PubMed] [Google Scholar]
- 23.Chen X. Identification of serine 348 on the apelin receptor as a novel regulatory phosphorylation site in apelin-13-induced G protein-independent biased signaling. J. Biol. Chem. 2014;289(45):31173–31187. doi: 10.1074/jbc.M114.574020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi B.K. Single-step perfusion chromatography with a throughput potential for enhanced peptide detection by matrix-assisted laser desorption/ionization-mass spectrometry. Proteomics. 2003;3(10):1955–1961. doi: 10.1002/pmic.200300558. [DOI] [PubMed] [Google Scholar]
- 25.Cai X. Apelin receptor homodimer-oligomers revealed by single-molecule imaging and novel G protein-dependent signaling. Sci. Rep. 2017;7:40335. doi: 10.1038/srep40335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bai B. Dual-agonist occupancy of orexin receptor 1 and cholecystokinin A receptor heterodimers decreases G-protein-dependent signaling and migration in the human colon cancer cell line HT-29. Biochim. Biophys. Acta Mol. Cell Res. 2017;1864(7):1153–1164. doi: 10.1016/j.bbamcr.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Bai B. Heterodimerization of human apelin and bradykinin 1 receptors: novel signal transduction characteristics. Cell. Signal. 2014;26(7):1549–1559. doi: 10.1016/j.cellsig.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 28.Wang C. Heterodimerization of mouse orexin type 2 receptor variants and the effects on signal transduction. Biochim. Biophys. Acta. 2014;1843(3):652–663. doi: 10.1016/j.bbamcr.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 29.Ji B. Novel signaling of dynorphin at kappa-opioid receptor/bradykinin B2 receptor heterodimers. Cell. Signal. 2017;31:66–78. doi: 10.1016/j.cellsig.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 30.Hamdan F.F. Unraveling G protein-coupled receptor endocytosis pathways using real-time monitoring of agonist-promoted interaction between beta-arrestins and AP-2. J. Biol. Chem. 2007;282(40):29089–29100. doi: 10.1074/jbc.M700577200. [DOI] [PubMed] [Google Scholar]
- 31.Pierce K.L., Premont R.T., Lefkowitz R.J. Seven-transmembrane receptors. Nat. Rev. Mol. Cell Biol. 2002;3(9):639–650. doi: 10.1038/nrm908. [DOI] [PubMed] [Google Scholar]
- 32.Ma P., Zemmel R. Value of novelty? Nat. Rev. Drug Discov. 2002;1(8):571–572. doi: 10.1038/nrd884. [DOI] [PubMed] [Google Scholar]
- 33.Goidescu C.M., Vida-Simiti L.A. The apelin-APJ system in the evolution of heart failure. Clujul Med. 2015;88(1):3–8. doi: 10.15386/cjmed-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maguire J.J. [Pyr1]apelin-13 identified as the predominant apelin isoform in the human heart: vasoactive mechanisms and inotropic action in disease. Hypertension. 2009;54(3):598–604. doi: 10.1161/HYPERTENSIONAHA.109.134619. [DOI] [PubMed] [Google Scholar]
- 35.Ma Y. Structural basis for apelin control of the human apelin receptor. Structure. 2017;25(6):858–866 e4. doi: 10.1016/j.str.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 36.Butcher A.J. Differential G-protein-coupled receptor phosphorylation provides evidence for a signaling bar code. J. Biol. Chem. 2011;286(13):11506–11518. doi: 10.1074/jbc.M110.154526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuba K. Apelin and Elabela/Toddler; double ligands for APJ/Apelin receptor in heart development, physiology, and pathology. Peptides. 2019;111:62–70. doi: 10.1016/j.peptides.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Cassier E. Phosphorylation of beta-arrestin2 at Thr(383) by MEK underlies beta-arrestin-dependent activation of Erk1/2 by GPCRs. Elife. 2017;6 doi: 10.7554/eLife.23777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moore C.A., Milano S.K., Benovic J.L. Regulation of receptor trafficking by GRKs and arrestins. Annu. Rev. Physiol. 2007;69:451–482. doi: 10.1146/annurev.physiol.69.022405.154712. [DOI] [PubMed] [Google Scholar]
- 40.Lefkowitz R.J., Rajagopal K., Whalen E.J. New roles for beta-arrestins in cell signaling: not just for seven-transmembrane receptors. Mol. Cell. 2006;24(5):643–652. doi: 10.1016/j.molcel.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Ahn S. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J. Biol. Chem. 2004;279(34):35518–35525. doi: 10.1074/jbc.M405878200. [DOI] [PubMed] [Google Scholar]
- 42.DeWire S.M. Beta-arrestins and cell signaling. Annu. Rev. Physiol. 2007;69:483–510. doi: 10.1146/annurev.physiol.69.022405.154749. [DOI] [PubMed] [Google Scholar]
- 43.DeFea K.A. The proliferative and antiapoptotic effects of substance P are facilitated by formation of a beta -arrestin-dependent scaffolding complex. Proc. Natl. Acad. Sci. U. S. A. 2000;97(20):11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Grasberger H. A familial thyrotropin (TSH) receptor mutation provides in vivo evidence that the inositol phosphates/Ca2+ cascade mediates TSH action on thyroid hormone synthesis. J. Clin. Endocrinol. Metab. 2007;92(7):2816–2820. doi: 10.1210/jc.2007-0366. [DOI] [PubMed] [Google Scholar]
- 45.Leach K. Identification of molecular phenotypes and biased signaling induced by naturally occurring mutations of the human calcium-sensing receptor. Endocrinology. 2012;153(9):4304–4316. doi: 10.1210/en.2012-1449. [DOI] [PubMed] [Google Scholar]
- 46.DeWire S.M., Violin J.D. Biased ligands for better cardiovascular drugs: dissecting G-protein-coupled receptor pharmacology. Circ. Res. 2011;109(2):205–216. doi: 10.1161/CIRCRESAHA.110.231308. [DOI] [PubMed] [Google Scholar]
- 47.Wootten D., Christopoulos A., Sexton P.M. Emerging paradigms in GPCR allostery: implications for drug discovery. Nat. Rev. Drug Discov. 2013;12(8):630–644. doi: 10.1038/nrd4052. [DOI] [PubMed] [Google Scholar]
- 48.Scimia M.C. APJ acts as a dual receptor in cardiac hypertrophy. Nature. 2012;488(7411):394–398. doi: 10.1038/nature11263. [DOI] [PMC free article] [PubMed] [Google Scholar]