Abstract
Adult neurogenesis, i.e., the generation of neurons from neural stem cells (NSCs) in the adult brain, contributes to brain plasticity in all vertebrates. It varies, however, greatly in extent, location and physiological characteristics between species. During the last decade, the teleost zebrafish (D. rerio) was increasingly used to study the molecular and cellular properties of adult NSCs, in particular as a prominent NSC population was discovered at the ventricular surface of the dorsal telencephalon (pallium), in territories homologous to the adult neurogenic niches of rodents. This model, for its specific features (large NSC population, amenability to intravital imaging, high regenerative capacity) allowed rapid progress in the characterization of basic adult NSC features. We review here these findings, with specific comparisons with the situation in rodents. We specifically discuss the cellular nature of NSCs (astroglial or neuroepithelial cells), their heterogeneities and their neurogenic lineages, and the mechanisms controlling NSC quiescence and fate choices, which all impact the neurogenic output. We further discuss the regulation of NSC activity in response to physiological triggers and non-physiological conditions such as regenerative contexts.
Keywords: neural stem cell, zebrafish, pallium, adult neurogenesis, quiescence, radial glia, cell fate choice decisions
Adult neurogenesis, first identified as such in birds (Goldman and Nottebohm, 1983), has been documented in all vertebrate species studied (Altman and Das, 1965; Eriksson et al., 1998; Byrd and Brunjes, 2001; Suh et al., 2007). The persistence of neuronal production in the adult brain is the product of specialized neural precursor cells, the neural stem cells (NSCs). In rodents, newly-born neurons are physiologically important for the plasticity of specific circuits, notably involved in learning and memory, and impaired adult neurogenesis can correlate with emotional disorders (Anacker and Hen, 2017; Jorgensen, 2018; Toda et al., 2019). NSCs have also been postulated to be at the origin of some brain tumors (Fan et al., 2019; Matarredona and Pastor, 2019). The fundamental importance of NSCs stimulated an explosive research field during the last 20-years, and, more recently, the development of a new study model: the zebrafish adult brain. The large amount of adult NSCs in this system, their widespread distribution and varied properties, and their reactivity toward regeneration, all propelled the zebrafish model to the forefront of adult NSC research, as a complementary and synergistic model to rodents (Anand and Mondal, 2017; Lindsey et al., 2018; Zambusi and Ninkovic, 2020). The time to reach sexual maturity in zebrafish (3 months) and the adult lifespan also approximate those of mouse, allowing to draw direct temporal parallels. With specific focus on NSCs of the dorsal telencephalon, we will review here these different attributes, stressing the contribution of the zebrafish model to understand basic NSC properties such as their lineages, quiescence, fate choices, heterogeneities, population behavior and their physiological and pathological recruitment.
Neural Stem Cells: a Variety of Progenitor Cell Subtypes Drive Neurogenesis in the Adult Zebrafish Central Nervous System
Active Neurogenesis From Multiple Neurogenic Niches
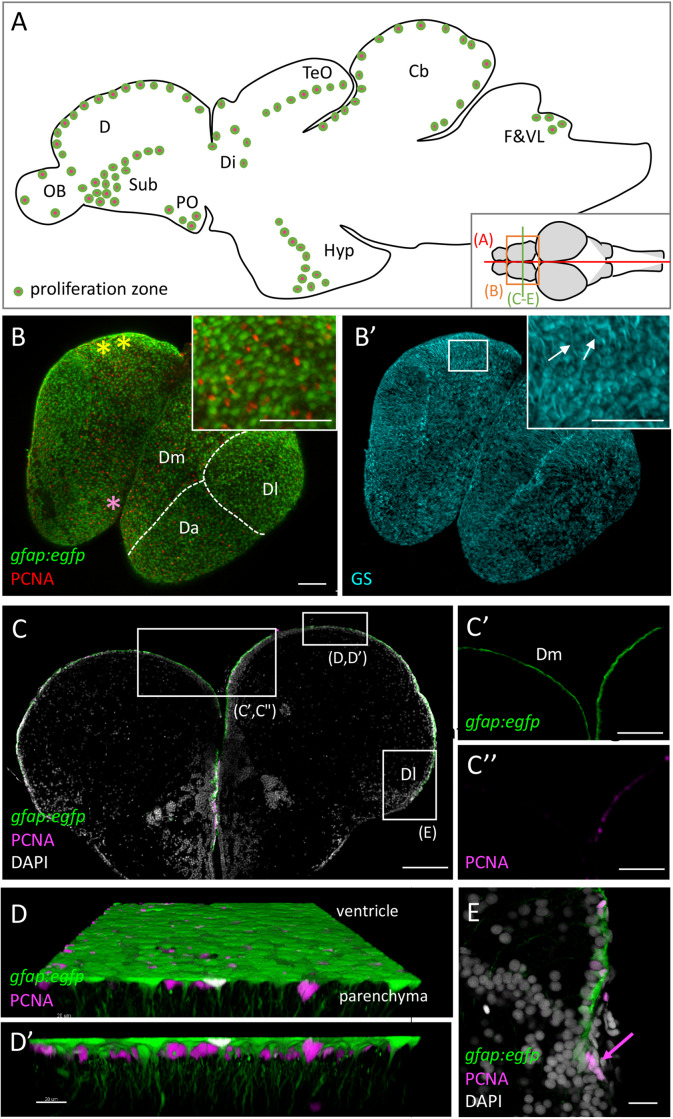
The persistent and widespread neurogenic activity of the zebrafish adult brain was first recognized using classical tracing methods employing thymidine analogs: 16 proliferation domains, present across all brain subdivisions, proved to be at the origin of neurons within a few weeks of chase (Figure 1A; Adolf et al., 2006; Grandel et al., 2006). Using similar approaches, physiologically silent but activatable neural progenitors were also identified in the adult zebrafish spinal cord (Reimer et al., 2009). These constitutive and facultative neurogenic niches raised important interest. Indeed, by their variety, they permit comprehensive comparisons of neurogenic progenitor identities and properties, and of neurogenesis modes, in the adult vertebrate central nervous system.
FIGURE 1.
Progenitor cells in the zebrafish adult brain at 3 months-post-fertilization (mpf). (A) Scheme of a mid-sagittal section (anterior left) showing the localization of proliferation zones (colored dots) (Adolf et al., 2006; Grandel et al., 2006). (B,B’) Dorsal view of a whole-mount telencephalon from a gfap:egfp transgenic animal, processed in triple immunohistochemistry for GFP, PCNA (B), and GS (B’). Anterior is bottom left. Note the continuous layer of progenitor cells visible from the dorsal surface. Pallial territories are indicated by the dotted lines (see Dray et al., 2015). Yellow stars indicate the location of the territory homologous to the hippocampus (Ganz et al., 2015, and see Rodríguez et al., 2002 in goldfish), and the pink star the territory homologous to the amygdala (von Trotha et al., 2014). Anti-GS immunohistochemistry (B’) permits to see basal RG processes (arrows). (C–E) Cross-section of a telencephalon from a gfap:egfp transgenic animal, processed in double immunohistochemistry for GFP and PCNA and counter-stained with DAPI (C) and high magnifications of the domains boxed (C’,C”,E). In addition, a high magnification view of the ventricular zone of Dm is shown (D,D’) in 3D (Imaris software) to appreciate radial glial cell morphology. (E) Focus on NE progenitors at the pallial edge (arrow). Scale bars: (B,B’,C) 100 mm; (C’,C”) 30 mm; (D,D’) 20 mm; (E) 50 mm. Cb, cerebellum; D, dorsal part of the telencephalon (pallium) (Da: anterior part of D, Dm: medial part of D; Dl, lateral part of D); Di, diencephalon; F&VL, facial and vagal lobes; Hyp, hypothalamus; OB, olfactory bulb; PO, preoptic area; TeO, tectum opticum.
We will focus in this review on adult neurogenesis in the zebrafish telencephalon, which hosts the territories homologous to two main neurogenic niches of adult rodents: the sub-ependymal zone of the lateral ventricle (SEZ) and the sub-granular zone (SGZ) of the dentate gyrus of the hippocampus [for completeness on other territories, the reader is referred to other recent reviews or articles (Than-Trong and Bally-Cuif, 2015; Anand and Mondal, 2017; Lindsey et al., 2018)]. Following a process of eversion, likely involving both morphogenetic cell shape changes and anisotropic growth, the ventricle of the zebrafish dorsal telencephalon (pallium) becomes exposed dorsally, covered by an enlarged choroid plexus, with its dorsal midline flipped to lateral positions (Folgueira et al., 2012). This results in a medio-lateral inversion of homologous pallial territories between zebrafish and mammals. A tentative correspondence, based on ontogenetic and functional grounds, has been proposed (von Trotha et al., 2014; Dray et al., 2015; Ganz et al., 2015).
Neural Stem Cells and Neural Progenitors in the Adult Zebrafish Telencephalon
A variety of genetic and non-genetic tracing methods (Table 1), coupled with precise immunohistochemical or molecular characterizations, identified several neural progenitor subtypes in the adult zebrafish telencephalon. Some of them, notably radial glia (RG) of the pallium, are considered NSCs (discussion of the “NSC” versus “neural progenitor” nomenclature in Box 1, and see below).
TABLE 1.
Tracing neural progenitors and/or their progeny in the adult zebrafish telencephalon.
| Method | Principle | Output (and limitations) | Princeps publications |
| Thymidine analogs | These compounds (BrdU, CldU, EdU) incorporate into the DNA of cycling cells during the S phase. They are revealed by immuno-histochemistry or click-chemistry. | Labeling of dividing cells only (thus low efficiency to label dormant cells). Identification of the progeny cells becoming post-mitotic or dividing infrequently post-labeling (dilution of the label at each division round, so rapidly dividing progeny cells are lost). Detection of cells dividing infrequently, when detected together with a proliferation marker (PCNA, MCM proteins) after a chase (“label retention assay”). | Adolf et al., 2006; Grandel et al., 2006; Pellegrini et al., 2007 |
| Retroviruses | Cells with ventricular contact are infected upon intra-ventricular injection of the viral suspension. Following infection, the genetic material carried by the virus is reverse transcribed and integrates into the host cell genome. | Integration into dividing cells for simple retroviruses, and into non-dividing cells as well for lentiviruses, the genetic material of which can cross nuclear pores. Permanent labeling of the progenitor and its progeny. Cell specificity of expression can be achieved using specific promoters. | Rothenaigner et al., 2011 |
| DNA electroporation or lipofection | Cells with ventricular contact are targeted upon intra-ventricular injection of the virus suspension and electroporation. DNA remains episomal. | Cell specificity of expression can be achieved using specific promoters. Labeling is transmitted to progeny cells but is a priori not permanent. Bias toward targeting cells with a large apical surface. | Chapouton et al., 2010; Alunni et al., 2013 |
| Conditional Cre-lox-mediated genetic tracing | Double transgenic animals (driver-reporter) are used. Expression of Cre-ER is driven from the driver transgene by neural progenitor-specific promoters, and nuclear translocation is temporally controlled by tamoxifen treatment. It recombines the reporter transgene at LoxP sites to express a reporter, usually driven by a ubiquitous promoter. | Cell specificity of the recombination is achieved using specific promoters (so far: her4.1; gfap; nestin); these promoters may not recapitulate the endogenous pattern in all lines, and need to drive strong expression for recombination to be efficient. Labeling is permanent in the progenitor and all its progeny cells. Various extents of recombination can be used (from clonal to full). | Kroehne et al., 2011 |
| Tet-rtTA-mediated genetic tracing | Double transgenic animals (driver-reporter) are used. Expression of Tet is driven from the driver transgene by neural progenitor-specific promoters, and its activity is temporally controlled by doxycylin treatment. It then activates the reporter transgene. | Cell specificity of induction is achieved using specific promoters (so far: her4.1). Labeling is transient in the progenitor following arrest of the doxycycline treatment. If the reporter protein is fused with a histone (e.g., H2B), it will be diluted in the progenitor cell upon division, but stably maintained in post-mitotic cells generated soon after induction, hence also serving as a birth dating method; like with thymidine analogs, rapidly dividing progeny cells will be lost by label dilution. Various extents of induction can be used (from clonal to full); full inductions can also be used to track non-dividing progenitor cells that retain the label (although with caution, as expression levels at induction may be variable). | Furlan et al., 2017 |
| Intravital imaging | 2P: Semi-transparent adult animals (casper or nacre) are used, anesthetized and imaged using 2P microscopy. Progenitor cells are tracked using specific transgenic reporter backgrounds or following reporter electroporation. 3P: transgenic casper adults are used, anesthetized and imaged using 3P microscopy. | Individual progenitor cells can be tracked over some weeks. Tracking of progeny cells is transient as they leave the progenitor niche to reach deep parenchymal areas. Only applicable so far to the dorsal-most pallial areas (Da, Dm). Individual progenitors can be imaged, as well as cells located much deeper in the parenchyma (at least 200 mm below the NSC layer), e.g., neurons. Howerver the method has not been used yet for repetitive imaging. | Barbosa et al., 2015a; Dray et al., 2015; Guesmi et al., 2018 |
Box 1 |.
Neural Stem Cells and neural progenitors.
By definition, stem cells are individual cells endowed with long-term self-renewal and at least bi-potency. This initial definition is in line with a classical scheme where a stem cell upon division generates another stem cell and a differentiated progeny. However, clonal tracing in a number of adult stem cell systems rather supports a model where stem cells are self-renewing and bi-potent at the population level, choosing stochastically between balanced numbers of amplifying, asymmetric or differentiative divisions. This is no exception in the adult brain where several studies, both in mouse and zebrafish, are compatible, at least in part, with such “population asymmetry” ensuring both neural stem cell maintenance and neuronal production. These converging observations suggest to revise the strict definition of a neural stem cell toward that of neural stem cell population(s), characterized by their capacity, as a whole, to maintain themselves and generate neurons and/or post-mitotic glial cells. In the zebrafish adult pallium, these properties are met by radial glial cells (although to varying degrees). One can further distinguish constitutive and facultative neural stem cells (or population), the former being active physiologically and the latter being normally silent but becoming active, e.g., upon lesion (as is for example the case in the zebrafish spinal cord). The term “neural progenitor” is generally used more broadly, (i) to mention progenitors that are further committed along the neurogenesis lineage than neural stem cells (for example, the “activated neural progenitors” -NPs- of the zebrafish adult pallium or the equivalent “transit amplifying progenitors” -TAPs- of adult mouse neurogenic niches), (ii) to refer to neurogenic cells whose self-renewal potential has not been clearly assessed, (iii) or to jointly refer to all cells with neurogenic capacity (for example, NSCs + NPs).
Pallial Radial Glial Cells Are Molecularly and Cellularly Similar to Rodent Adult NSCs
Pallial RG are organized as a tight monolayer with their cell bodies lining the pallial ventricle. They exhibit overt apico-basal polarity, exposing a small apical membrane domain to the cerebrospinal fluid and extending a long and highly branched basolateral process across the pallial parenchyma (Figures 1C–D’). Pallial RG express astroglial markers (Glial Fibrilary Acidic Protein - gfap-, Brain Lipid-Binding Protein - blbp-, Nestin, Glutamine Synthetase -GS-) as well as S100β, which highlights NSCs and ependymal cells in rodents, and Aromatase B (Adolf et al., 2006; Grandel et al., 2006; Pellegrini et al., 2007; März et al., 2010a). Parenchymal astrocytes are absent from the adult zebrafish pallium before aging (Ogino et al., 2016); this observation and the expression of factors encoding astrocytic function in RG (GS, and the glutamate transporters Glast and Glt1) suggest that pallial RG serve the function of parenchymal astrocytes, and extend this function into the parenchyma via their basal process. Overall, the morphology and astroglial markers of pallial RG resemble those of adult NSCs in the mouse SEZ and SGZ (Than-Trong and Bally-Cuif, 2015). They also morphologically resemble radial glial cells of the developing mouse cortex, but differ from these in several other aspects such as their proliferation potential and activity, and their transcriptome (Götz et al., 2016) (and see below). In detail, distinct morphologies were described among pallial RG depending on their location (März et al., 2010a). To date, these differences have not been related to functional (whether astrocytic or stem) properties.
Like adult mouse NSCs, pallial RG co-express progenitor markers, such as the transcription factors Sox2, Hey1, and Her4 (mouse Hes5) (Kroehne et al., 2011; Than-Trong et al., 2018; Than-Trong et al., 2020). Of these, only the function of Hey1 was tested to date in zebrafish, and shown to be necessary for the maintenance of progenitor potential in vivo (Than-Trong et al., 2018). Hes5 (together with the related Hes factor Hes1) as well as Sox2 are, however, necessary for NSC maintenance in adult mouse (Ehm et al., 2010; Boareto et al., 2017; Sueda et al., 2019), and are likely to play a similar role in zebrafish.
At any given time, approximately 5% of pallial RG are found within the cell cycle (i.e., express the proliferation parkers PCNA or MCM2/5; these cells are referred to as “activated”). The remaining 95% are out of the cell cycle and interpreted as quiescent (see below) (Chapouton et al., 2010; März et al., 2010a). This interpretation as well as the self-renewal and neurogenic potential of the pallial RG population are supported by a number of converging arguments, including: (i) tracing assays demonstrating that individual RGs can oscillate between the activated and quiescent states, (ii) pharmacological assays or experimental injuries demonstrating that all, or most, RG can be brought into the activated state, (iii) genetic tracing identifying RG progeny at the individual and populational levels (Chapouton et al., 2010; März et al., 2010a; Kroehne et al., 2011; Alunni et al., 2013; Than-Trong et al., 2020). The latter experiments are particularly important as they demonstrate that her4-positive RG generate both pallial neurons and persisting pallial RG that are themselves neurogenic. Thus, at least at the population level, her4-positive RG act as NSCs. For comparison, RG of the developing mouse cortex do not exhibit quiescence phase, and their neurogenic activity is limited to the embryonic period (Götz et al., 2016).
Non-glial Pallial Progenitors
In addition, non-glial neural progenitors (NPs) (negative for astroglial markers and for her4) are present interspersed among RGs along the pallial ventricle (Ganz et al., 2010; März et al., 2010a). NPs are identified as progenitors according to their expression of Sox2, the fact that around 50% of them co-express proliferation markers at any time, and their neurogenic fate (assessed by retroviral tracing at short term) (Rothenaigner et al., 2011; Than-Trong et al., 2020). Cre-lox tracing with short chase times indicates these cells originate from pallial RG (Than-Trong et al., 2020). This population, however, lacks specific markers and to date was only relatively superficially analyzed. It is possibly heterogeneous, and in particular the properties of Sox2+;PCNA- cells have not been directly defined. NPs are classically considered equivalent to the Transit Amplifying Progenitors (TAPs) described in mouse (März et al., 2010a).
Neuroepithelial Progenitor Cells Are Maintained at the Adult Pallial Edge
Neuroepithelial (NE) cells are also present laterally and posteriorly at the junction of the pallium with the choroid plexus, a location corresponding to the dorsal midline (Figure 1E). These cells are ventricular and apico-basally polarized, express neither astroglial markers nor her4, and are proliferating. Their lack of astroglial markers and her4 expression, and their cuboidal as opposed to radial shape, distinguish them from RG. “Negative” tracing of NE cells in the adult pallium, using as landmark a neighboring her4.1:ERT2CreERT2 traced domain, suggests that these cells generate neurogenic RG in the postero-lateral pallial domain, as well as maintain themselves, acting as a local growth zone akin to the ones described in the adult optic tectum and retina (Dirian et al., 2014). Their exact lifespan and fate, however, remain to be studied in detail.
Highly Neurogenic Radial Glia Line the Subpallial Ventricle
RG cells also border the subpallial ventricle. They differ from pallial RG for their high levels of BLBP expression, their thick morphology, their higher proliferative potential, the interkinetic migration of their nuclei, and their generation of neurons that populate both the (subpallial) parenchyma and the olfactory bulb (Ganz et al., 2010; März et al., 2010a). Progenitors fated to the OB follow a longitudinal anterior-ward migration, akin to the rostral migratory stream of rodents, although glial corridors have not been observed (Kishimoto et al., 2011).
Embryonic Origin of Adult Pallial Radial Glia: Heterogeneity, Functional Impact, and Comparison With NSC-Generating Lineages in Rodents
Cre-lox lineage tracing indicates that pallial RG of the dorsal, medial and anterior pallial territories originate from embryonic RG that border the telencephalic ventricle at 1 day-post-fertilization (dpf) (Dirian et al., 2014). These embryonic RG, like their adult counterparts, express the Her transcription factor Her4. In contrast, as discussed above, adult RG of the postero-lateral pallium originate from the NE progenitor pool maintained at the pallial edge, itself deriving from dorsal NE progenitors located at the tel-diencephalic junction at 1 dpf (Dirian et al., 2014). NE progenitors express typical Her factors found at neural tube boundaries, such as Her6 and Her9, and her9 expression is maintained into adulthood (Figure 2A). The two pallial RG populations remain separated by a cryptic boundary, and differ in a number of ways: posterior RG have a higher proliferation rate, higher expression of blbp and lower expression of GS (these three features possibly being related with their relatively “younger” age). Further, this population can be replenished from the NE pool if RG are depleted at larval stages.
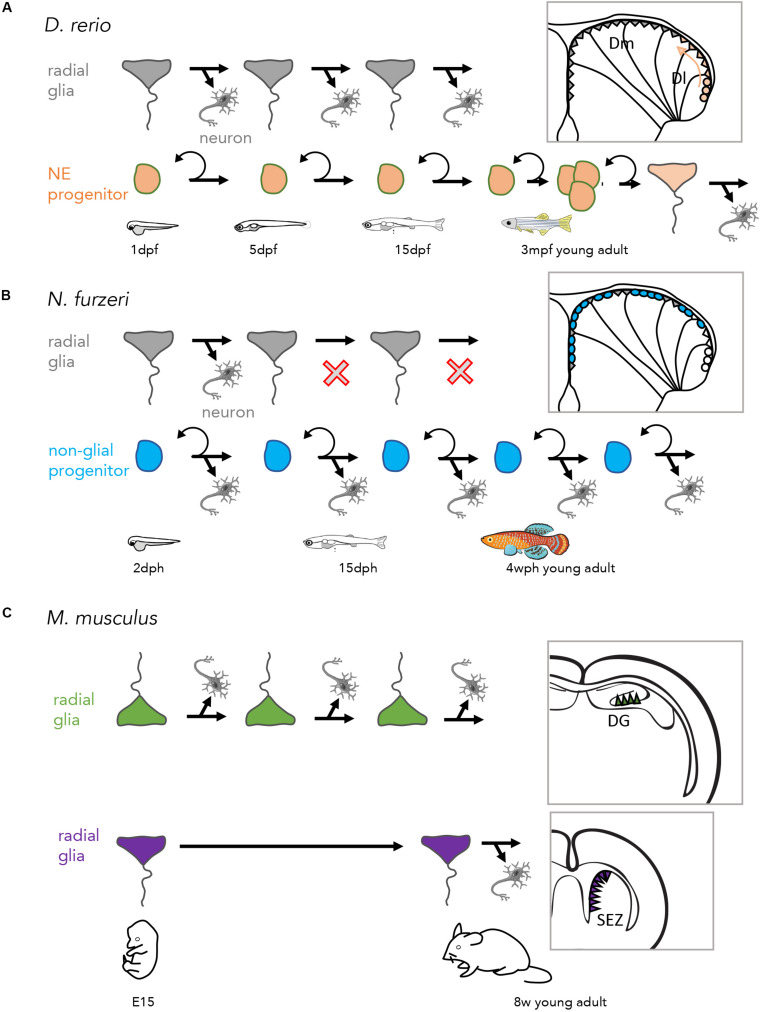
FIGURE 2.
Lineages at the origin of adult neurogenic progenitors in the vertebrate pallium. (A) Lineages in zebrafish, generating adult RG from embryonic RG (top) and NE progenitors (bottom). (B) Lineages in the killifish, where neurogenesis in adults is ensured by a long-lasting non-glial embryonic lineage (blue) dph: days post-hatching, wph: weeks post-hatching. (C) Lineages in mouse, where distinct modes of NSC production are described in the DG (top) and SEZ (bottom) (Dirian et al., 2014; Furutachi et al., 2015; Song et al., 2018; Berg et al., 2019; Coolen et al., 2020).
Teleost fish encompass over 26,000 species across a large variety of habitats, and display a number of adaptations including in the morphology, growth rates or sizes of their pallium. As a response to its ephemeral habitat, N. furzeri follows an explosive development to its adult size (Blažek et al., 2013), including accelerated pallial growth and neurogenesis. Recent work demonstrates that this is not due to the enhanced efficiency of existing lineages, but rather to the long-term persistence until adulthood of a highly neurogenic embryonic lineage (Figure 2B; Coolen et al., 2020). This study, which points to the variety of neurogenic adaptations in the adult vertebrate brain, illustrates the power of fish models to uncover the different natural strategies that can be used to amplify neurogenesis.
Finally, in addition to their embryonic origin, a potential determinant of NSC properties is the duration of their neurogenic activity. Genetic tracing and birth dating experiments indicate that most, likely all, RG of the dorso-medial and anterior pallial domain originate from a constitutively neurogenic lineage, i.e., generating neurons without interruption from embryo to adult (Figure 2A; Dirian et al., 2014; Furlan et al., 2017). This was later shown to be also the case for NSCs of the adult mouse DG (Song et al., 2018; Berg et al., 2019). In apparent contrast, NSCs of the adult mouse SEZ were shown to derive from cells entering quiescence at mid-embryonic stages, hence pausing prior to being remobilized in adults (Figure 2C; Furutachi et al., 2015). It is possible, however, that quiescence instatement in the SEZ is more gradual and that an asynchrony exists in the control of quiescence entry and neurogenic activity among SEZ NSCs, reconciling the different models. Finally, it remains to be formally demonstrated whether the NE progenitors located at the pallial edge, and the young RG that they progressively generate de novo in the adult pallium, have an equivalent in rodents.
Adult Neurogenic Lineages in the Zebrafish Pallium Are Devoid of Amplification and Drive Neuronal Addition
Different Amplification Strategies in Teleosts and Rodents
Downstream of NSCs, adult neurogenesis in mouse involves TAPs, i.e., non-stem neuronal progenitors of limited self-renewal. The amplification potential of TAPs greatly varies between the SEZ and DG: in average, a TAP would divide three to four times in the SEZ (Ponti et al., 2013), but once or twice in the DG (Seri et al., 2004; Encinas et al., 2011; Lugert et al., 2012; Figure 3D). TAP-like progenitors are also present in the developing mouse cortex, notably as basal progenitors expressing the transcription factor Tbr2. These basal progenitors originate from RG and generate cortical neurons following 1 or 2 divisions (Hevner, 2019). Tbr2 expression is also found in the adult SEZ in amplifying progenitors generated from the TAPs (Lugert et al., 2012; Nelson et al., 2020). tbr2 (eomesa) expression in the adult zebrafish pallium is largely regional and has not been directly associated with NPs (Ganz et al., 2015). The lineage amplification by pallial NPs is minimal, with at most one or two divisions, akin to TAPs of the DG (Figure 3C; Rothenaigner et al., 2011; Furlan et al., 2017). Hence, in the zebrafish pallium, extensive neuronal production is ensured by the continuous neurogenic activity of RG (notably in the cortical area, where neurogenesis is shut-down after birth in mammals) and the de novo addition of neurogenic RG into the system. The latter occurs through the activity of NE progenitors at the pallial edge, and currently unidentified “source cells” disseminated at the pallial ventricle (see below) (Than-Trong et al., 2020).
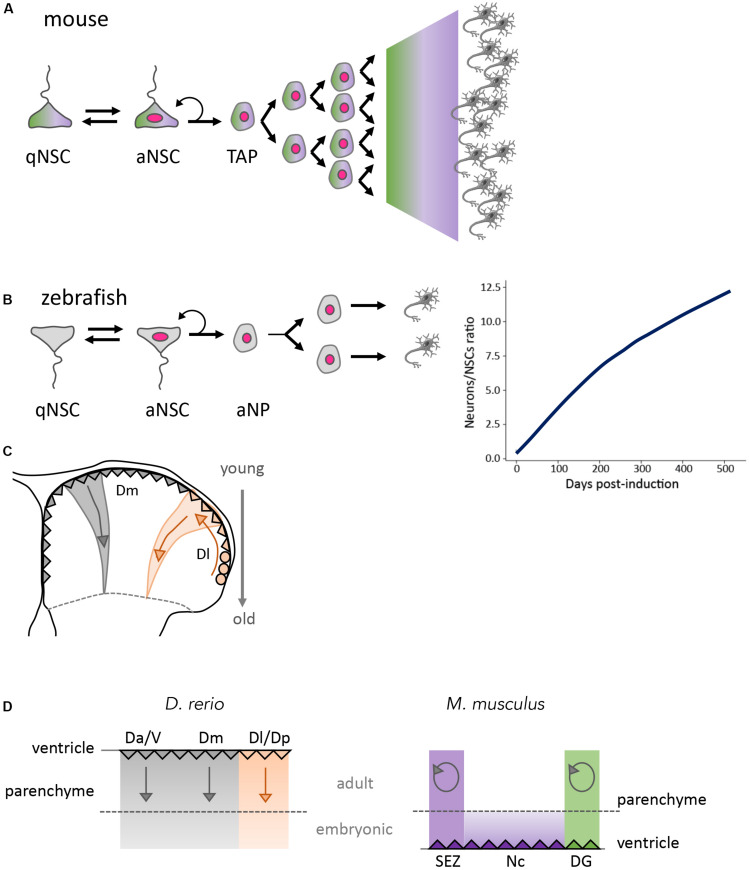
FIGURE 3.
Global outputs of adult neurogenesis in zebrafish and mouse. (A) Scheme of a typical neurogenesis lineage in adult mouse. Upon quiescence exit, NSCs generate neurons via TAPs. TAPs have variable amplification capacity, high in the SEZ, lower in the DG. Green and purple shades are meant to represent shared cells and attributes between the SEZ and DG (color code in Figure 2C, with proliferating cells indicated with a pink nucleus). (B) Scheme of a typical neurogenic lineage in the adult zebrafish pallium (left) and neuronal output (right). Neurons are generated via an intermediate progenitor (NP: neural progenitor) of limited amplification potential. Because adult-generated neurons persist, however, the number of neurons generated per NSC increases over time in genetically traced lineages from individual NSCs. (C) Spatio-temporal distribution of the neurogenesis output in the zebrafish pallium, from embryonic stages until adult life. Radial glia (triangles) generate neurons that stack in age-related order within the telencephalic parenchyma. Old neurons, at the pallial-subpallial boundary, were generated in the embryo and early larva. In the lateral pallium (orange), the same process operates but radial glia are generated during juvenile and adult stages from NE progenitors (circles). Arrows indicate the spatial organization of neurogenesis over time. (D) Compared output of neurogenesis in the pallium of zebrafish and mouse from embryo to adult, represented on schematic cross-sections where the dotted line separates neurons generated at embryonic versus post-embryonic stages. Neurogenesis is continuous and additive (straight arrows) in zebrafish in all pallial subdivisions (left panel). Neurogenesis stops at birth in the mouse neocortex, spatially isolating the two persisting neurogenic niches SEZ and SGZ. Neurogenesis in these niches is mostly used for neuron replacement (circular arrows) (right panel). Color code as in Figure 1 (Seri et al., 2004; Encinas et al., 2011; Rothenaigner et al., 2011; Lugert et al., 2012; Ponti et al., 2013; Furlan et al., 2017; Than-Trong et al., 2020). D, dorsal part of the telencephalon (pallium); Da, anterior part of D; Dm, medial part of D; Dl, lateral part of D; aNSC, activated neural stem cell; qNSC, quiescent neural stem cell; NP, neural progenitor; TAP, transit amplifying progenitor; V, ventral telencephalon (sub-pallium).
Adult Neurogenesis in Zebrafish Is Additive
Adult neurogenesis in mouse is globally understood to drive neuronal replacement, following the selective maintenance of a subset of adult-born neurons in the functional circuitry -while most adult-generated neurons would be eliminated (Figure 3B). Some publications, however, report neuronal addition, both in the DG (Bayer, 1985; Dranovsky et al., 2011) and OB (Platel et al., 2019). The output of pallial neurogenesis in zebrafish primarily drives neuronal addition. No cell death was observed, and the pallial parenchyma (as well as the OB) increases its neuronal population during adult life and grows (Than-Trong et al., 2020). Genetic birth dating and lineage tracing experiments showed that newborn neurons delaminate from the ventricular zone and stack into the parenchyma in age-related layers until adulthood (Furlan et al., 2017; Figure 3C). Because there is no extensive neuronal migration, and little or no death, this process results in an adult pallium where superficial structures are composed of young (late-born) neurons and central structures of old (early-born) neurons, still including neurons born at embryonic and early juvenile stages. This also applies to the lateral pallium, with in addition a lateral to medial gradient in RG age (Figures 3A,B; Furlan et al., 2017).
To date, the identity of adult-born pallial neurons, as well as their projection pattern and function, remain largely unknown in zebrafish. Like in the mouse, some adult-born neurons in the zebrafish OB are TH-positive (Adolf et al., 2006). In the pallial parenchyma proper, only candidate markers have been tested to date to characterize RG-derived neurons, including some transcription factors and neurotransmitters (identifying for example GABA-ergic and glutamatergic neurons) (von Trotha et al., 2014; Furlan et al., 2017). A neuron atlas was recently generated from the zebrafish telencephalon at 21 dpf using scRNAseq (Raj et al., 2018), and such a description is long awaited in adult, to permit both functional studies -still conducted currently through laborious screening to associate molecularly defined subpopulation with a given function (Lal et al., 2018)- and information on how NSCs generate different neuronal types. In the developing mammalian cerebral cortex and the Drosophila optic lobe, columnar organization is generated through sequential expression of specific transcription factors (Mattar et al., 2015; Doe, 2017). The zebrafish pallium is also built through a sequential stacking process, but in contrast to the mouse, the “migration-free death-free” neurogenesis process of the adult zebrafish pallium makes it possible to readily identify neurons born at adulthood by their (superficial) position (Furlan et al., 2017). This will then make it straightforward to attribute them with molecular signatures. Determining whether neurons at different depths have different identities and when they are generated would therefore represent an important step to know whether there is a temporal heterogeneity in NSCs and how it might be encoded. Moreover, since the same NSCs remain active in an adult brain which keeps on growing, one important question would then be whether NSCs maintain a similar level of plasticity throughout life, either physiologically or in a regenerative context. Finally, identifying neural subpopulations in the pallium could also reveal depth-independent areal heterogeneities, perhaps to be correlated with areal differences in NSC potential.
Pallial Neurogenesis in Zebrafish Is the Output of a Proliferative Hierarchy Involving Functionally Specialized NSC Sub-Pools
The zebrafish adult pallium is particularly amenable to NSC fate studies for several reasons: (i) its superficial location permits intravital imaging hence the direct tracing, during several weeks, of NSC fate in the absence of biased genetic tools and under non-invasive conditions (Barbosa et al., 2015b; Dray et al., 2015), (ii) its small size permits analyzing clones in whole-mount preparations, avoiding the risk of losing cells that occurs when studying brain sections, and (iii) the absence of cell death and migrations makes it easier to quantify clones in their entirety (Than-Trong et al., 2020). We made use of these attributes, and of broad promoters such as her4 and gfap that encompass the largest progenitor population, to determine the dynamics of NSC fates in the adult pallium between 3 and 18 mpf (Than-Trong et al., 2020). The combination of intravital imaging, long-term clonal genetic tracing (Figures 4A–C) and biophysical modeling revealed that NSC population dynamics is compatible with an organization in 3 hierarchically-organized sub-populations, each endowed with a specific function: NSC population growth (“source pool”), self-renewal (“reservoir pool”), and neurogenic activity (“operational pool”) (Figure 4D). The “source” population accounting for growth remains poorly defined. In contrast, division modes and transition rates could be inferred for the reservoir and operation sub-populations, highlighting the heterogeneities of NSC properties and, within the operational pool, their stochastic fate choices.
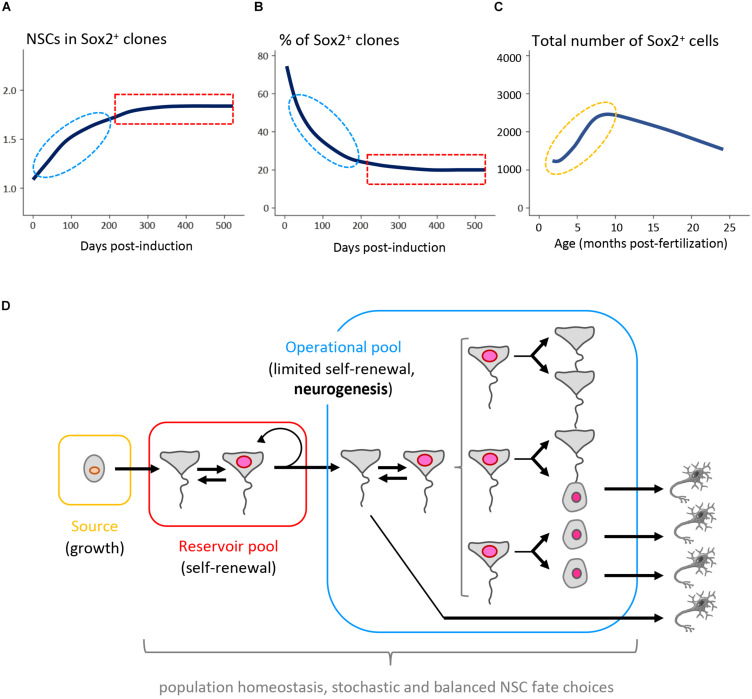
FIGURE 4.
Long-term NSC and neurogenesis dynamic in the adult zebrafish pallium. (A,B) Genetic clonal analysis driven by the her4:ERT2CreERT2 transgene with chase time over 500 days. The number of NSCs per clone containing at least one Sox2+ cell (A) and the proportion of clones containing at least one Sox2+ cell (B) display a bi-phasic dynamics at long term. At early time points after induction, neutral drift is observed -red-. At later time points, a behavior characteristic of single cell-based self-renewal appears -blue-. These two dynamics reflect the behavior of two embedded populations (operational and reservoir, respectively). (C) Total number of Sox2+ cells in the adult Dm between 3 and 25 months post-fertilization (mpf). The Sox2+ population increases in size in the young adult (3–8 mpf), reflecting the NSC-generating activity of a “source” population (orange). (D) Schematic of the proliferative hierarchy of NSC sub-populations sustaining overall NSC maintenance in Dm. Color code as in (A–C) (Than-Trong et al., 2020).
In contrast to this unifying conclusion, the results of a large number of careful clonal studies in mouse diverge, documenting NSC loss, maintenance or even gain, in the SEZ and/or DG (Lugert et al., 2010; Bonaguidi et al., 2011; Dranovsky et al., 2011; Encinas et al., 2011; Fuentealba et al., 2012; Calzolari et al., 2015; Urbán et al., 2016; Basak et al., 2018; Bast et al., 2018; Obernier et al., 2018; Pilz et al., 2018; Berg et al., 2019). The zebrafish data suggest that these discrepant results could be interpreted by the targeting of distinct NSC sub-populations, although a unifying model in mouse remains to be established.
Neural Stem Cell Quiescence and Its Impact on Neurogenesis
Quiescence Is an Actively Maintained State Shared Between Zebrafish and Mouse Adult Neural Stem Cells
Quiescence is a prominent cell state in adult NSCs, as illustrated in both zebrafish and mice. It is therefore important to consider how it may affect NSC biology and neurogenesis output, likely in a similar way in these species. The quiescence phase of adult NSCs generally corresponds to the G0 state of the cell cycle. In Drosophila, NSCs can also undergo a G2 quiescence phase at late embryonic stages (Otsuki and Brand, 2018), and the existence of a long G2 phase has been suggested in NE progenitors of the medaka optic tectum at post-embryonic stages, based on the expression of G2-M arrest genes (Dambroise et al., 2017). G2 quiescence, however, remains to be demonstrated in vertebrate adult brains.
Practically, quiescent NSCs are negatively defined by the absence of proliferation markers. Until now, a positive core signature for quiescent NSCs has not been defined, although RNASeq data in both mouse and zebrafish brought deeper understanding of the molecular players of NSC quiescence: generally, pathways involved in transcription, translation, DNA replication and DNA repair, and cell cycle progression, are downregulated (Codega et al., 2014; Dulken et al., 2017), while cell-cell communication (Shin et al., 2015; Basak et al., 2018), cell adhesion (Codega et al., 2014; Shin et al., 2015), cell signaling and lipid metabolism (Llorens-Bobadilla et al., 2015; Than-Trong et al., 2018) are upregulated (Figure 5).
FIGURE 5.
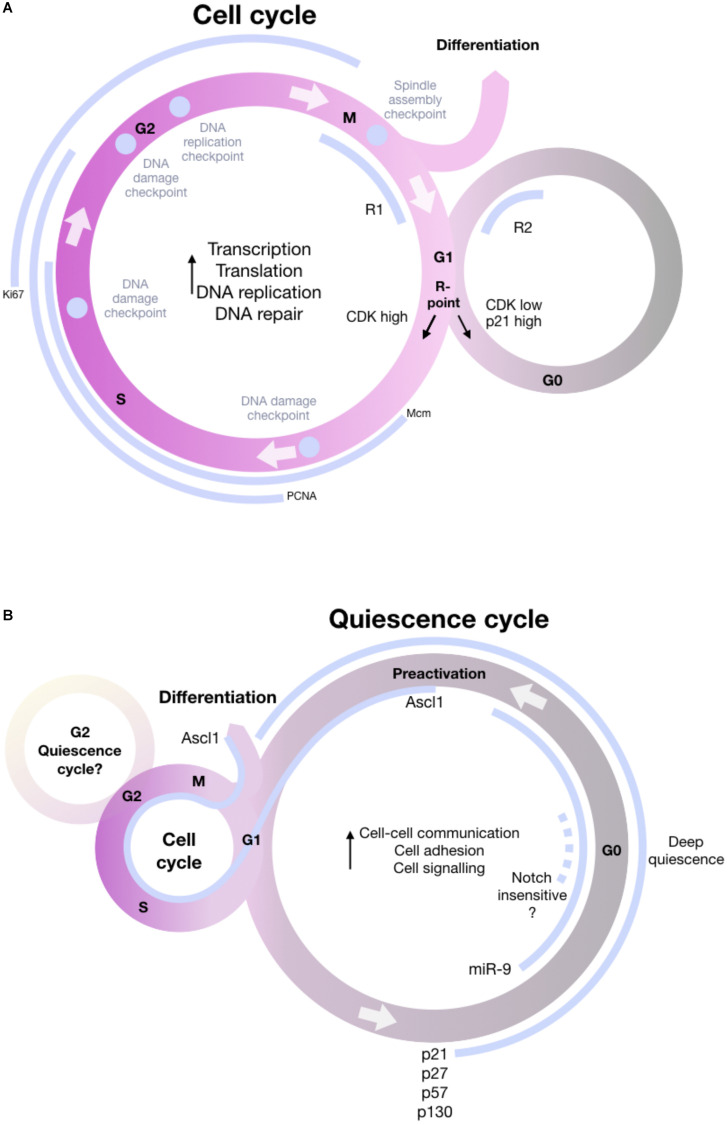
Schematic of the cell cycle including the most important information about the decision to enter quiescence, remain in cycle or differentiate. (A) General cell cycle knowledge, illustrating phases G1, S, G2, and M and the most important checkpoints (purple). During the cell cycle, proteins involved in transcription, translation, DNA replication and DNA repair are upregulated. The schematic includes proliferation markers MCM, PCNA, and Ki67 (gray) that are expressed in different phases of the cell cycle and commonly used to define proliferating NSCs. During the cell cycle, cells can enter into the quiescence state in G1, the decisions for entry happening at a R-point in G1. After passing the R-point, cells are committed to fulfill another cell cycle. Another important check-point is the bifurcation point right after mitosis, a window in which cells are sensitive to mitogen signals that influence CDK2 (R1 and R2 window on the schematic). Cells with a normal level of CDK2 will keep cycling, whereas cells with low levels of CDK2 will enter a transient quiescence and will face a second restriction window at the end of G1, controlled by the CDK inhibitor p21. Only cells that built up enough CDK will be able to bypass quiescence and eventually re-enter quiescence. (B) NSC-specific quiescence cycle. Quiescence can be entered in G1, or G2 (this remains to be shown for vertebrates). During quiescence, genes involved in cell-cell communication, cell adhesion and cell signaling are upregulated, stressing that quiescence is an actively maintained state. Some data (e.g., the dynamics of miR-9 expression) suggest that quiescence can be seen as a cycle, but alternative models exist. Quiescent cells express p21, p27, p57, and p130. Quiescence is heterogeneous, and deeper and shallower sub-states exist. miR-9 is nuclear in deeply quiescent cells. Some NSCs that are insensitive to Notch blockade can also be interpreted as deeply quiescent. A “pro-activated” state precedes activation proper. In this state, NSCs express ascl1, which will also be maintained during activation and differentiation (Pardee, 1974; Alunni et al., 2013; Spencer et al., 2013; Andersen et al., 2014; Katz et al., 2016).
Quiescence is classically linked with the maintenance of stem cell properties (stemness, i.e., self-renewal and differentiation potential, see Box 1). This link is, however, not obligatory, as illustrated in the gut and skin, where adult stem cells proliferate continuously while staying in homeostasis. In the brain, however, quiescence is believed necessary for stemness -hence neurogenesis potential-, avoiding DNA, protein or mitochondria damage that could lead to senescence or malignant transformations. But this has been difficult to demonstrate, both in mouse and zebrafish, in particular because testing for stemness requires functional assays where NSCs will divide, and because many quiescence control factors have pleiotropic effects and in particular are actors of the neurogenesis cascade itself (see below). Nevertheless, several studies to some extent disentangled the two properties. For example, in adult mouse, physical exercise leads to increased SGZ NSC proliferation, but is not followed by exhaustion of the NSC pool (Van Praag et al., 1999; Wu et al., 2008). In the adult zebrafish pallium, bulk RNAseq profiling of quiescent versus activated NSCs or in the presence or absence of Notch3 activity showed that Notch3 promotes quiescence and stemness in part via distinct molecular cascades (Than-Trong et al., 2018). While the transcription factor Hey1 mediates Notch3 activity on stemness, the candidate Notch3 effectors controlling quiescence remain to be experimentally validated. In mouse, the direct effect of Notch on stemness remains to be unraveled, as well as whether Hey1 is a target of Notch and could potentially control stemness. In the mouse SGZ, Notch2 drives expression of the transcription factor-encoding gene Id4. However, unlike the depletion of Notch2, the depletion of Id4 induces NSC activation but does not promote neuronal differentiation (Zhang et al., 2019). Thus, in mouse, NSC quiescence and stemness could also be molecularly uncoupled downstream of Notch2, Id4 controlling only its quiescence-promoting effect.
Quiescence Instatement, Length, and Depth in Adult Neural Stem Cells: Variable Geometry Parameters?
Quiescence Length Remains to Be Measured With Precision
Through genetic lineage tracings and live imaging in zebrafish and mouse, we know now that NSCs can re-enter quiescence after activation (Berg et al., 2010; März et al., 2010a; Bonaguidi et al., 2011; Dray et al., 2015; Pilz et al., 2018; Than-Trong et al., 2020). It remains, however, unclear, and debated, whether NSCs keep the same properties (fate, quiescence length…) upon division (Bonaguidi et al., 2011; Urbán et al., 2016; Than-Trong et al., 2020). Quiescence length, as well the duration of cell cycle phases, also remain to be precisely defined in NSCs, and key studies on these issues are summarized in Table 2. Overall, S-phase can last between 4 and 8 h (Encinas et al., 2011; Ponti et al., 2013), and the complete adult NSC cell cycle will take 10–35 h (Encinas et al., 2011; Ponti et al., 2013; Roccio et al., 2013). The time between 2 divisions can lie between 14 and 36 days, as observed by live imaging in the SGZ, but the upper limits of quiescence were not explored (Pilz et al., 2018). In the zebrafish adult pallium, mathematical models predict average quiescence times reaching 143 days, which is yet to be confirmed experimentally (Than-Trong et al., 2020). It is likely that the zebrafish pallium will be highly instrumental to fill these gaps, as the superficial location of the pallial progenitor zone (contrasting with the deep location of mammalian NSCs) permits long-term intravital imaging.
TABLE 2.
Estimated lengths of cell cycle phases and quiescence in adult NSCs of the zebrafish and mouse telencephalon.
| Cell cycle phase | Length | Model | Method | References |
| G1 | 0–35 h, most of the time: 6–15 h | Mouse NSC cell culture | Hes5:FUCCI line | Roccio et al., 2013 |
| S/G2/M | 4–12 h, most of the time: between 4 and 9 h | Mouse NSC cell culture | Hes5:FUCCI line | Roccio et al., 2013 |
| Complete cell cycle | 17 h | Mouse NSCs in the SEZ | Thymidine analog | Ponti et al., 2013 |
| S-phase | 4.5 | Mouse NSCs in the SEZ | Thymidine analog | Ponti et al., 2013 |
| S-phase | ANP (amplifying neural progenitors, higher level of proliferation): 12.2 ± 1.1 QNP (quiescent neural progenitors, low level of proliferation): 7.8 ± 0.7 | Mouse NSCs in the SGZ | Thymidine analogs | Encinas et al., 2011 |
| Cell cycle | 28–35 h | Mouse NSCs in the SGZ | Thymidine analogs | Encinas et al., 2011 |
| G0 | 20 ± 4 days | Mouse NSCs in SEZ | Genetic tracing based on Wnt-target Troy:GFP. In the model, qNSCs become activated at constant low rate, and aNSC go to quiescence at constant rate. | Basak et al., 2018 |
| G1 G0 transition | 5 ± 2 days | Mouse NSCs in SEZ | Genetic tracing based on Wnt-target Troy:GFP. In the model, qNSCs become activated at constant low rate, and aNSC go to quiescence at constant rate. | Basak et al., 2018 |
| Division rate | 5 ± 2 h | Mouse NSCs in SEZ | Genetic tracing based on Wnt-target Troy:GFP. In the model, qNSCs become activated at constant low rate, and aNSC go to quiescence at constant rate. | Basak et al., 2018 |
| Non-proliferating | Mouse NSC in the SGZ | Live imaging with inducible Ascl1:tdTomato line | Pilz et al., 2018 | |
| G0 | 24.4 and 143 days | Zebrafish pallium | Genetic tracing based line Tg(her4:RFP). In the mathematical model, qNSCs become activated at 2 different rates. | Than-Trong et al., 2020 |
Quiescence Instatement Is Progressive With a Schedule That May Differ Between Niches and Species
Progenitors in the SGZ produce granule neurons during embryonic and postnatal stages and enter quiescence postnatally. Then, they acquire their radial morphology and organize in the SGZ (Li et al., 2013; Berg et al., 2019). In contrast, in the SEZ, stem cells with quiescence characteristics were identified at embryonic stages by H2B-mediated lineage tracing (Furutachi et al., 2015). These cells would slow down their cell cycle at E13.5, then remain quiescent to re-activate at adult stages (Fuentealba et al., 2012). As mentioned earlier, these differences between the SEZ and SGZ may be apparent and due to tracing some cells only, or due to using indirect measurements. For example, H2B-tracing is based on differential dilution, and a positive read-out necessitates a minimal quiescence length. In zebrafish, pallial neural progenitors start entering quiescence at 5 dpf (Alunni et al., 2013), and the average duration of quiescence -as inferred from the decreasing proportion of PCNA-positive cells within the population- gradually increases until adulthood (Dirian et al., 2014; Katz et al., 2016). It remains unclear whether the data above can directly be compared, as they use different methods with their inherent limitations. Likewise, measures based on the lack of PCNA protein will not distinguish cells in early G1 phase (PCNA transcription and protein stability being low prior to the G1-S transition) (Chang et al., 1990) from cells in G0. Progressive quiescence instatement, concluded from the increasing duration of a PCNA-negative state, may therefore be concluded for cells that in fact progressively lengthen early G1. Overall, it remains urgent for the field to positively label G0.
NSC Quiescence Is a Heterogeneous State
Several analyses support the idea that G0 quiescence is heterogeneous. Some studies suggest different types of quiescence (mainly short versus long-term) depending on the cell and its history (Urbán et al., 2016). Additionally, quiescence can consist of sub-states, defined as transient phases, arguably harboring specific molecular or cellular signatures and properties, that cells transit through during their quiescence phase. Zebrafish adult pallial NSCs were instrumental to experimentally exemplify potential quiescence sub-states. For example, pharmacological blockade of Notch signaling in zebrafish, which globally leads to NSC quiescence exit (see below), revealed different lag phases to re-enter cycling, and approximately 5% of quiescent NSCs did not respond to the blockade (Alunni et al., 2013). Convincingly, a subset of quiescent NSCs express microRNA-9 (miR-9), and BrdU chase experiments suggest that the miR-9-positive state is a transient phase in a quiescence cycle and may reflect deep quiescence (Katz et al., 2016). Indeed BrdU incorporated during the S phase of dividing NSCs becomes associated with miR-9 staining only after long chase, showing that miR-9 is expressed in now deeply quiescent NSCs but that were previously dividing.
scRNAseq and expression analyses conducted in mouse also suggest the existence of a distinct quiescent sub-state close to activation, as was proposed for muscle satellite cells. The first study reporting such heterogeneity in the mouse SEZ identified three non-dividing NSC clusters (Llorens-Bobadilla et al., 2015): a dormant cluster in the deepest state of quiescence, a second cluster containing cells expressing markers related to activation but that do not divide, and a third cluster that falls in between on the spectrum between quiescence and activation. The same group reported the same subpopulation structure in a new study and using a different technology, suggesting that these cells can be reliably and reproducibly grouped into distinct clusters (Kalamakis et al., 2019). Recently a separate group reported the most extensive scRNAseq conducted on NSCs so far (Mizrak et al., 2019), in which they captured close to 40k SEZ astrocytic cells. They identified several independent clusters that also matched distinct regions along the SEZ which differ in proliferation rate. This can be explained if NSCs along the lateral ventricles rest in different depths of quiescence. An important limitation to these experiments in the SEZ, however, is the difficulty to distinguish between astrocytes and bona fide NSCs (Dulken et al., 2017). In the dentate gyrus, astrocytes and RGL-cells formed distinct clusters (Hochgerner et al., 2018). However so far only low numbers of stem cells were captured in scRNAseq experiments conducted on the hippocampus, which prevents proper analysis of their intrinsic heterogeneity. Two reports were recently published in zebrafish, based on NSCs isolated from her4.1-driven transgenes (Cosacak et al., 2019; Lange et al., 2020). However these studies only captured small numbers of NSCs (609 and 76, respectively). One of them conclusively shows the existence of distinct NSC clusters in the pallium (Cosacak et al., 2019), but more extensive studies will be necessary to get a better idea of the level of heterogeneity as well as whether and how these subpopulations differ in quiescence depth.
Control Mechanisms of Quiescence Are Highly Conserved Between Zebrafish and Rodents
Control mechanisms of NSC quiescence in zebrafish and rodents appear similar, yet many mechanisms that were identified in rodents remain to be studied in zebrafish and vice versa. Conditional functional studies in the adult zebrafish remain technically challenging, especially when genetics-based, and this is still slowing down the field. We will focus here only on the control mechanisms that have been studied in the zebrafish pallium and compare them to data in mouse (Figure 6) (but see Table 3). It is to note that, while these studies convincingly implicate various factors in quiescence control, they do not resolve their function in controlling quiescence entry, maintenance, exit, or transition through the different sub-states discussed above.
FIGURE 6.
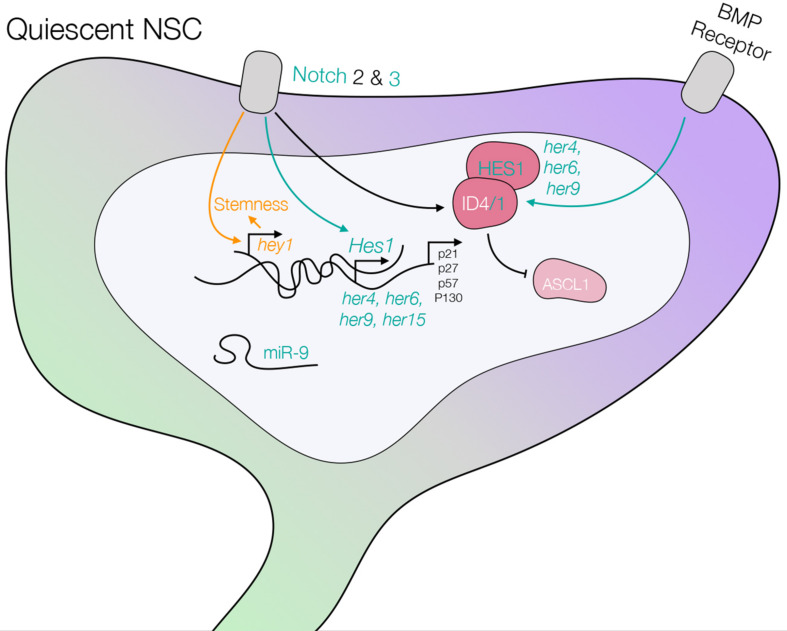
Schematic of a quiescent NSC including the pathways controlling quiescence, which are summarized in this review. The scheme highlights knowledge generated in mouse, and confirmed pathways in zebrafish are illustrated in green. Knowledge generated in zebrafish and later extended to mouse is shown in green as well. Knowledge generated in zebrafish and still to be confirmed in mouse is depicted in yellow. Differences (Notch2 is not expressed in qNSCs in zebrafish), or data that need consolidation in zebrafish (Ascl1 expression and its regulation by ID and Hes1, BMP receptor), are shown in black. See text and Table 3 for references.
TABLE 3.
Quiescence promoting factors.
| Quiescence promoting factor | Model | Method | References |
| Notch (in general) | Mouse SGZ | Conditional knockout of RBPJk in Glast-expressing NSCs enhances neurogenesis (quiescence exit) and leads to NSC depletion 2 months after induction | Ehm et al., 2010 |
| Mouse SEZ | Conditional knockout of RBPJk in Nes-expressing NSCs enhances neurogenesis (quiescence exit) and leads to NSC depletion 3 months later | Imayoshi et al., 2010 | |
| Notch3 | Adult zebrafish pallium | Pharmacological blockade of gamma-secretase (Notch signaling pathway), notch3 mutant and notch3 MO show that Notch3 maintains quiescence | Alunni et al., 2013 |
| Mouse SEZ | Notch3-null mice and knockdown in adult with lentiviral expressing shRNA targeting Notch3 drive quiescence exit of NSCs, especially in the lateral and ventral wall | Kawai et al., 2017 | |
| Notch2 | Mouse SEZ | Conditional knockout of Notch2 in Hes5-expressing NSCs and short-term lineage tracing of Notch2-expressing cells shows that Notch2 maintains NSCs in quiescence, as loss of function leads to quiescence exit, increased neurogenesis and accelerated NSC exhaustion | Engler et al., 2018 |
| Mouse SGZ | Conditional knockout of Notch2 in Hes5-expressing NSCs leads to proliferation of NSCs and increased neurogenesis | Zhang et al., 2019 | |
| Dll1 | Mouse SEZ | Conditional knockout of Dll1 in Nes-expressing NSCs leads to quiescence exit (feedback to activate Notch in quiescent cells is not ensured anymore) | Kawaguchi et al., 2013 |
| Jagged1 | Mouse SGZ | Conditional knockout of Jagged1 in Nes-expressing NSCs leads to quiescence exit, implying that the interaction between Jagged1 and Notch is important for NSC quiescence and maintenance | Lavado and Oliver, 2014 |
| Fezf2 | Adult zebrafish pallium | Vivo-morpholino against fezf2 (short-term knock-down) leads to quiescence exit and increased proliferation | Berberoglu et al., 2014 |
| Bone morphogenic proteins | Mouse SEZ | BMP7 overexpression (virus-mediated) and Noggin expression (through protein purification or virus-mediated) show that expression of BMP maintains quiescence in type B cells/NSCs and therefore inhibits neurogenesis. It promotes the survival of type A progenitors | Lim et al., 2000 |
| Mouse SGZ | Blocking BMP through Noggin leads to reactivation and expansion of the NSC pool, suggesting that BMP is involved in quiescence control | Bonaguidi et al., 2008 | |
| Mouse SGZ | Intracerebral infusion of Noggin, lentivirus-mediated ablation of BMPR-1A and conditional knockout of Smad4 in Glast-expressing NSCs lead to quiescence exit, increased proliferation and exhaustion | Mira et al., 2010 | |
| NSCs derived from ESCs | NSC culture can be pushed to quiescence by adding BMP4 in 24 h | Martynoga et al., 2013 | |
| Inhibitors of DNA binding | Mouse SGZ | Conditional knockout of Id4 in Glast-expressing NSCs leads to increased ASCL1 expression and reactivation of previously quiescent NSCs | Blomfield et al., 2019 |
| Mouse SGZ | Conditional knockout of Id4 in Gfap-expressing cells using adeno-gfap::Cre viruses leads to NSC activation and cell-cycle entry without inducing neurogenesis | Zhang et al., 2019 | |
| NFIX | NSC culture derived from Mouse ESCs | Knockdown in vitro leads to impaired quiescence | Martynoga et al., 2013 |
| Mouse | NFIX-/- knockout: lethal at 3 weeks. Proportion of cycling NSCs is increased in the mutant. | Martynoga et al., 2013 | |
| Forkhead box O3 | Mouse SEZ and SGZ | Conditional knockout of FOXO1,3,4 in Gfap-expressing cells: decline of NSC pool and neurogenesis. | Paik et al., 2009 |
| Neurospheres from NSC culture | Neurospheres from FOXO3-/- versus FOXO3+/+, genome-wide microarray analysis: FoxO3 induces a program to preserve quiescence | Renault et al., 2009 | |
| Mouse SEZ and SGZ | FOXO3-/-: reduced number of NSCs in vivo (NSCs got activated and lost) | Renault et al., 2009 | |
| Adult mouse primary NSC culture | ChIP: FOXO3 binds proneural genes that are also targeted by Ascl1 possibly as a competitor to repress their expression and maintain NSC identity | Webb et al., 2013 | |
| Mouse SGZ | Conditional knockout of FoxO1,3,4 in Glast-expressing NSCs: leads to quiescence exit, increased proliferation followed by loss of NSC number | Schäffner et al., 2018 | |
| Mouse SEZ | FoxO3-/- knockout results in quiescence exit and increased neurogenesis | Webb et al., 2013 | |
| miR-9 | Zebrafish pallium | Vivo-MO targeting mature miR-9 leads to reactivation of previously quiescent mir-9+ NSCs. | Katz et al., 2016 |
| Gaba | Mouse postnatal SEZ, acute slices | GABAA-R-antagonist bicuculline leads to increase of proliferation in GFAP+ cells | Liu et al., 2005 |
| SGZ adult | Clonal analysis after cKO of gamma2-subunit-containing GABAA receptor in Nes-expressing NSCs – > quiescence exit and symmetrical self-renewal | Song et al., 2012 | |
| SGZ adult | Pharmacological inhibition – > increase of NSC proliferation Genetic deletion of GABAB1 (homozygous mutant) – > increase of NSC proliferation (Sox2+ cells) and differentiation to neuroblasts. Later loss of progenitors and increased neurogenesis | Giachino and Taylor, 2014 |
Notch Signaling Is a Key Quiescence-Promoting Pathway
One of the most prominent NSC quiescence-promoting pathway is Notch signaling, as first demonstrated in the adult zebrafish pallium (Chapouton et al., 2010), and later confirmed to be conserved by numerous studies in mice. In zebrafish and mice, Notch is highly expressed in NSCs. Whereas notch3 is strongly upregulated in quiescent cells, notch1 (notch1b in zebrafish) is strongly expressed in activated NSCs (Aguirre et al., 2010; Chapouton et al., 2010; Basak et al., 2012; Alunni et al., 2013; de Oliveira-Carlos et al., 2013; Kawai et al., 2017; Zhang et al., 2019). notch2 expression was not detected in the zebrafish brain, but is expressed in quiescent NSCs in mice (Basak et al., 2012; Kawai et al., 2017; Zhang et al., 2019). Blocking Notch signaling with a gamma-secretase inhibitor dissolved in fish water leads to activation of NSCs and expansion of the NSC pool by symmetric divisions (Chapouton et al., 2010; Alunni et al., 2013), and this was recapitulated by the selective blockade of Notch3 using morpholinos (Alunni et al., 2013). In mice, Notch inhibition, either at the level of the ligands or the effector RBPjK leads to exit of quiescence and exhaustion of the NSC pool, a phenotype also often understood to mean that NSC quiescence is crucial for stemness maintenance (Ehm et al., 2010; Imayoshi et al., 2010; Kawaguchi et al., 2013; Lavado and Oliver, 2014). In zebrafish, NSC exhaustion was not observed, but long-term Notch blockade at adult stage was not conducted beyond 7 days (Alunni et al., 2013). One transcription factor functionally interacting with Notch signaling is Fezf2 (Fez family Zinc Finger 2), which is expressed at high levels in quiescent NSCs in the zebrafish adult pallium and mouse SGZ (Berberoglu et al., 2014). In zebrafish, fezf2 expression correlates with the nuclear localization of NICD and with high expression level of the Notch target her4, and is necessary for quiescence (Berberoglu et al., 2014).
Other Quiescence Promoting Pathways Are Highly Conserved Between Rodents and Zebrafish
Another important pathway for NSC quiescence is BMP (bone morphogenic protein) signaling. NSCs express components of the BMP pathway like Smads, BMPR I and II (Lim et al., 2000; Bonaguidi et al., 2008; Mira et al., 2010). Overexpressing BMP ligands leads to a decrease in NSC proliferation and differentiation, while overexpression of the BMP inhibitor Noggin leads to increased proliferation and neurogenesis in the SGZ, the SEZ and in vitro (Lim et al., 2000; Bonaguidi et al., 2008; Martynoga et al., 2013). Targets of the BMP pathway include ID transcription factors (“Inhibitor of DNA binding/differentiation”), which are also targeted by the Notch pathway. IDs are strongly expressed in zebrafish adult pallial NSCs, and recent work shows that BMP positively controls id1 expression through conserved enhancers in the adult zebrafish brain (Zhang et al., 2020). In zebrafish, id1 expression is specific of quiescent NSCs, and is necessary and sufficient for quiescence (Diotel et al., 2015; Rodriguez-Viales et al., 2015). In response to injury, id1 is upregulated. It may play a role in maintaining the NSC pool through stabilizing its interactor proteins such as the Her factors Her4 or Her6, also expressed in adult pallial NSCs (Rodriguez-Viales et al., 2015). In mouse NSCs, Id interacts with and stabilizes Hes1, the mammalian ortholog of zebrafish Her6. Hes1 represses the transcription factor Ascl1 (Bai et al., 2007), which itself normally promotes NSC activation (Andersen et al., 2014; Sueda et al., 2019). Id4 does not affect Ascl1 transcription, but binds the normal Ascl1 stabilizing partner E47, leading to Ascl1 clearing (Blomfield et al., 2019).
Finally, the miR-9 quiescence-promoting factor initially identified in adult zebrafish pallial NSCs (see above) (Katz et al., 2016) is also conserved in mouse, as well as its striking sub-cellular localization: in both species, miR-9 is nuclear in NSCs transiting through a deep quiescence sub-state. Further, primary NSCs in culture derived from the SGZ and pushed toward quiescence through BMP relocalize miR-9 to the cell nucleus (Katz et al., 2016). The targets of miR-9 in quiescence control remain unknown.
Overall, a tentative quiescence cycle is presented in Figure 5B, indicating the transient-sub-states (miR-9-positive, Ascl1-positive, Notch-insensitive) that NSCs transit through.
Activating Factors Are Also Shared Between Rodents and Zebrafish
A key promoter of NSC activation, mentioned above, is Ascl1 (achete and scute homolog 1), which directly upregulates the expression of cell cycle genes (Castro et al., 2011; Andersen et al., 2014). Ascl1 is expressed in all activated NSCs and some neural progenitors in the mouse SEZ and SGZ. Conditional loss of function experiments showed that Ascl1-negative NSCs neither proliferate nor differentiate (Andersen et al., 2014). Ascl1 is transcribed in some quiescent NSCs (Blomfield et al., 2019; Zhang et al., 2019), but its expression and activity are repressed during quiescence by Id4 and the Notch target Hes1, which is expressed at high level with moderate oscillation amplitude (Sueda et al., 2019). How high Ascl1 expression levels become induced to drive NSC activation remains to be uncovered. Lower and oscillating levels of Hes1 expression preceding NSC activation can lead to Ascl1 oscillations, themselves driving NSC activation (Sueda et al., 2019). Then, following NSC division, the ubiquitin ligase HUWE1 degrades Ascl1 thus enabling the cell to re-enter quiescence (Urbán et al., 2016). In zebrafish, ascl1a is expressed in activated NSCs (Than-Trong et al., 2018), but its function remains to be studied. Further to this transcription factor, growth factors are also activating factors in NSCs. In the mouse brain, intracerebroventricular infusions of the fibroblast growth factor FGF2 lead to increased proliferation and neurogenesis (Rai et al., 2007). Accordingly, conditional knock-out of FgfR1 in Nestin-expressing NSCs in the SGZ impairs proliferation and neurogenesis (Zhao et al., 2007). In the zebrafish brain, fgfr1-4 are expressed in the dorsal telencephalon. Whereas heat shock-induced expression of dominant negative forms of FGFR1 does not alter NSC activation, the overexpression of FGF8a results in strong proliferation (Topp et al., 2008; Ganz et al., 2010). fgf8a expression is restricted to the ventral telencephalon, but fgf8b, strongly expressed in the pallium (Topp et al., 2008), may play the same role.
Stemness-Related Neural Stem Cell Fate Choices
Decisions taken by NSCs along their life include whether to activate (or remain quiescent) but also whether to maintain (or lose) their stemness (Box 1). We will refer to “stemness-related NSC fate choices” the checkpoints when a NSC decides to remain stem or to commit toward expression of the genetic program reflective of another cell type.
NSC Potency: Do NSC Fates Differ Between Zebrafish and Mouse?
In the SEZ, the differentiation potential of individual NSCs is limited to specific neuronal subtypes based on their regional localization (Merkle et al., 2007; Merkle et al., 2014; Chaker et al., 2016; Mizrak et al., 2019). However, fate mapping experiments confirmed that even if most NSCs produce neurons, few NSCs produce oligodendrocytes (Menn et al., 2006) or astrocytes (Sohn et al., 2015). Still, the capacity for a single NSC to produce the 3 lineages in vivo at adult stage remains unclear (but see Levison and Goldman, 1993 for the neonate). In vitro, clonal cultures of primary NSCs are able to generate neurons and oligodendrocytes (Menn et al., 2006) but continuous live-imaging of dividing NSCs revealed their commitment toward oligodendrogenic or neurogenic lineages only (Ortega et al., 2013). Also, ependymal cells were not described to originate from NSCs under physiological conditions (Spassky et al., 2005; Shah et al., 2018). NSCs of the SGZ most probably possess a heterogenous range of self-renewal and fate potential (Bonaguidi et al., 2012). Compared to the SEZ, clearer examples of multipotent NSCs were unraveled by careful analysis of lineage tracing outputs and notably of clones of 3–4 cells, showing that an individual NSC can self-renew and give rise to neurons and astrocytes (Bonaguidi et al., 2011; Encinas et al., 2011). While they do not give rise to oligodendrocytes physiologically, they can do so under conditions of demyelination or following the functional abrogation of inhibitory transcription factors (Nait-Oumesmar et al., 1999; Xing et al., 2014; Harris et al., 2018).
The situation in the adult zebrafish pallium is inherently different, as there are no “specialized” NSCs given that, as mentioned, RG cells also serve the function of parenchymal astrocytes. Thus, stemness maintenance includes the maintenance of astrocytic function (the reverse not being true, as stemness can be lost while astroglial characteristics are maintained; Than-Trong et al., 2018). At present, adult pallial NSCs are viewed as bipotent, able to self-renew and to generate neurons (Adolf et al., 2006; Grandel et al., 2006; Kroehne et al., 2011; Than-Trong et al., 2020). Little is known about neuronal subtypes in the dorsal pallium and it remains unexplored if specific NSC pools give rise to neuronal subtypes (like in the SEZ). The dorsal pallium is deprived of ependymal cells, but hosts an Olig2-positive population of oligodendrocytes. No clear lineage relationship has been made between oligodendrocytes and NSCs. The Olig2-positive population of cells is heterogeneous and located mostly in the parenchyma -although some cells can be found close to the ventricular surface-, comprising mature oligodendrocytes, slow proliferating oligodendrocyte progenitors (OPCs), proliferating OPCs, quiescent OPCs and radial glia-like cells (März et al., 2010b). These observations suggest that oligodendrocytes are produced within the parenchyma from OPCs. A recent publication based on scRNAseq argues that her4.1-positive NSCs express olig2 at very low level, suggesting nascent NSC progeny differentiating toward OPCs (Lange et al., 2020). Likewise, pseudo-lineages inferred from scRNAseq in the mouse SEZ reveal a molecular connection between NSCs and oligodendrocytes (Mizrak et al., 2019). This hypothesis needs to be carefully tested with a lineage tracing approach.
These observations together suggest potential differences between the panel of fates endogenously taken by SEZ, SGZ, and pallial NSCs between mouse and zebrafish. Hence, stemness-related fate choices are complex and not limited to remaining or not stem, but may include the choice of a particular fate. These differences may reflect a different potential, or the presence of different contextual cues.
Stemness-Related Fate Choices in Zebrafish Pallial NSCs Can Be Taken in the Quiescent or Activated States
Direct Neuronal Differentiation Is a Frequent Adult NSC Fate in Zebrafish
Both the quiescent and activated NSC states harbor potential windows where stemness can be maintained or altered. In the zebrafish adult pallium, the generation of neurons directly from quiescent NSCs has been suggested based on intravital imaging methods where NSCs were observed to differentiate after over 6–20 days without division (Barbosa et al., 2015b; Than-Trong et al., 2020). Thus, stemness needs to be actively promoted even during quiescence. Some effectors of NSC stemness maintenance have been identified in mice (Ars2 and Sox2) (Andreu-Agulló et al., 2009; Baser et al., 2019) and in zebrafish (Hey1) (Than-Trong et al., 2018). Interestingly, the depletion of their function in quiescent NSCs leads to a non-stem RG (GS+; Sox2–) fate suggesting that direct neuronal differentiation further requires active neuronal commitment cues.
The Mechanisms Driving NSC Fate Choices at Division Remain Poorly Understood
Adult zebrafish pallial NSCs can take several fates at division and generate two NSCs, one NSC and one aNP, or two aNPs. Models of clonal dynamics are compatible with stochastic decisions under physiological conditions (Than-Trong et al., 2020). Following a mechanical lesion, which leads to enhanced NSC recruitment for neuronal regeneration, a bias toward neurogenic consuming divisions (generating two aNPs) was observed (Barbosa et al., 2015b). In contrast, upon pharmacological Notch blockade, enhanced NSC recruitment is accompanied by a bias toward amplifying divisions (generating two aNSCs). It remains largely unknown how these decisions are taken.
Examples of all three division modes were also directly observed in mouse in vivo by clonal analysis of small clones (Bonaguidi et al., 2011; Encinas et al., 2011; Song et al., 2012; Calzolari et al., 2015; Basak et al., 2018; Obernier et al., 2018) or by live-imaging (Pilz et al., 2018). In both NSC niches, the choice for a given division mode seems to vary in part depending on the driver line used to follow NSCs, and the discrepancies may reflect experimental designs. Still, this observation would argue for the existence of NSC signatures highlighting specific modes of division (Figure 7).
FIGURE 7.
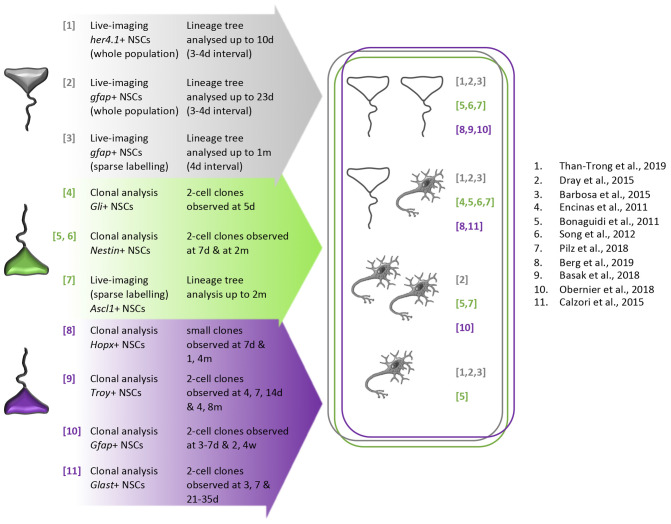
Schematic summary of division modes directly observed in adult mouse telencephalic neurogenic niches and in the zebrafish adult pallium in vivo. To evidence with certainty the existence of each division mode, we listed on the left part of the figure the clonal lineage tracing and live-imaging analyses only. For all the clonal analysis, we also only focused on 2–3-cell clones data at various time of induction/chase. Arrow depict the path leading individual NSC toward a cell fate decision (symmetrical self-renewing division, asymmetrical self-renewing division, symmetrical differentiating division or direct differentiation, illustrated on the right part of the figure). In the zebrafish pallium (gray NSCs), the mouse SEZ (purple NSCs), and the mouse SGZ (green NSCs), the three modes of division were evidenced. Direct neuronal differentiation was observed in the zebrafish adult pallium and mouse SEZ. In the SEZ, Gfap+ and Troy+ NSCs are able to symmetrically self-renew, symmetrically differentiate and asymmetrically divide whereas Glast+ NSCs were only described to asymmetrically divide. In the SGZ, Nestin+ and Ascl1+ NSCs can symmetrically self-renew, symmetrically differentiate and asymmetrically divide although Gli+ NSCs were only observed to asymmetrically divide and Hopx+ NScs to symmetrically self-renew and asymmetrically divide. Numbers refer to publications (see reference list).
Key studies in embryonic neural progenitors, including in zebrafish, pointed to several mechanisms controlling or biasing daughter cell fate at division. These notably include cell cycle dynamics such as the length of G1 or S phases (Calegari and Huttner, 2003; Huttner and Kosodo, 2005; Chen et al., 2015; Turrero García et al., 2016), asymmetrical inheritance of cellular components at division (Knoblich, 2008; Kressmann et al., 2015; Tozer et al., 2017; Lukaszewicz et al., 2019; Taverna and Huttner, 2019), and intra-lineage or niche-mediated bias in Notch signaling (Dong et al., 2012). Corresponding data in adult NSCs are sparse and were generally obtained in vitro, in mouse. For example, in cultured adult SGZ NSCs, G1 lengthening (through CDK4 inhibition) pushes NSCs toward differentiation (Roccio et al., 2013). Speculatively, basal process inheritance could be a fate determinant, as suggested by ex vivo SEZ cultures analyzed with live imaging (Obernier et al., 2018). Cultures of individual NSCs from the SEZ also showed asymmetric molecular segregations or activations. Specifically, the asymmetric segregation of the Dual-specificity tyrosine-phosphorylated and regulated kinase Dyrk1A at NSC division stabilizes EGFR and Notch signaling, biasing daughter cell fate (Ferron et al., 2010). Overexpressed Delta1-eGFP fusion protein also distributes asymmetrically upon NSC division, and marks the daughter cell fated to neuronal commitment (Kawaguchi et al., 2013). Finally, PEDF signaling from the niche can locally activate Notch in one NSC daughter (Ramírez-Castillejo et al., 2006; Andreu-Agulló et al., 2009). Parallels to these pioneer mechanistic works are currently lacking in vivo, and in zebrafish.
Stemness-related fate choices are key determinants of NSC population homeostasis, i.e., to the maintenance of a constant number of NSCs over time. Two mechanisms can in theory account for such homeostasis: invariant asymmetric cell fate, and “population asymmetry” (a combination of individual stochastic fate choices, balanced at the population level) (Simons and Clevers, 2011; Blanpain and Simons, 2013). In the mouse brain, NSC dynamics remains controversial (Table 4). As mentioned above, the privileged morphology of the zebrafish pallial germinal zone made it possible to combine complementary approaches and extract a unified model of adult NSC dynamics (Figure 4C). This resolved discrepancies between works describing an expansion (Rothenaigner et al., 2011) or a consumption (Barbosa et al., 2015b) of the NSC population. The current model (Than-Trong et al., 2020) includes both expansion and consumption but attributes these behaviors to distinct subpopulations of NSCs, and to stochastic fate choices. Further, it shows for the first time that both invariant asymmetric stem cell fate and population asymmetry can co-exist in an assembly of subpopulations hierarchically organized to account for NSC maintenance and physiological neuronal output.
TABLE 4.
NSC population dynamics assessed by long-term lineage tracing and clonal analysis in mouse.
| NSC niche | Methods | Chase time | Self-renewal capacity | Population dynamics | Population dynamic mechanism | References |
| SGZ | Nestin:CreERT2 lineage tracing + BrdU labeling | Up to 30 days | Limited | Progressive consumption of the NSC pool (the corresponding interpretation is hence referred to as the “disposable stem-cell model”) | ND | Balordi and Fishell, 2007; Encinas et al., 2011 |
| SGZ | Nestin:CreERT2 lineage tracing | Up to 1 year | Substantial | Maintenance of NSCs in clones over a year | ND | Balordi and Fishell, 2007; Bonaguidi et al., 2011 |
| SGZ | Ascl1:CreRT2 + live-imaging (chronic imaging through a window in the overlying cortex) | Up to 2 months | Limited | Progressive consumption of the NSC pool, no return to quiescence upon activation. Compatible with the disposable stem-cell model | ND | Pilz et al., 2018 |
| SGZ | Hopx:CreERT2 | Up to 12 months | substantial | Quiescent NSCs biased toward neurogenic fates once activated | ND | Berg et al., 2019 |
| SEZ | Glast:CreERT2 + confetti reporter mice | 4–6 months | Limited | Progressive consumption of the NSC pool with a specific sequence of divisions: few rounds of asymmetric self-renewing divisions symmetric differentiating division | Population asymmetry to maintain neuronal production at the expense of the NSC pool | Calzolari et al., 2015 |
| SEZ | Replication-incompetent avian RCAS-GFP retrovirus injected into hGFAP:Tva mice | Up to 4 weeks | Limited | Progressive consumption of the NSC pool: 20% of symmetric self-renewing divisions (with return to long-term quiescence), 80% of symmetric differentiating divisions, no asymmetric divisions | Population asymmetry paradigm to balance self-renewal and differentiation of NSCs at the population level | Obernier et al., 2018 |
| SEZ | Troy: GFPiresCreER and Ki67: GFPiresCreER lineage tracing | Up to 8 months | Substantial self-renewal capacity | NSC fates are chosen stochastically with probabilities inversely correlated with the number of surrounding NSCs | Population asymmetry driven by sensing niche occupancy | Basak et al., 2018 |
This study raises key questions pertaining to stemness-related fate choices. First, given the relatively uniform generation of neurons across the germinal zone surface and uniform expansion of the NSC population itself, it suggests that NSCs of the different sub-populations are interspersed, neighboring each other across the germinal sheet. This would argue against these different behaviors being controlled exclusively by different extrinsic local cues (such as different local niches), and rather stress the existence of intrinsic control mechanisms encoding one or the other asymmetry behavior. Second, it now pushes to search for molecular signatures of these heterogeneities. In the zebrafish pallium and in the mouse SEZ, it has long been emphasized that NSCs form a very heterogeneous population (Kriegstein and Götz, 2003; März et al., 2010a; Chaker et al., 2016). In the mouse brain and with the recent explosion of scRNA sequencing data, detailed NSC heterogeneities and clusters start to be described (Llorens-Bobadilla et al., 2015; Dulken et al., 2017; Mizrak et al., 2019). The significance and the role of NSC heterogeneities for NSC cell fate choice is not understood, and it will be important to try and overlap this information to transcriptionally identify the distinct NSC pools and directly track their relative contribution to NSC population homeostasis.
Physiological and Pathological Modulations of Zebrafish Adult Neurogenesis
Adult Neurogenesis in Zebrafish Responds to and Relays Environmental and Systemic Stimuli
Sensory Stimuli, Nutrition, and Stress Exert Parallel Effects on Adult Neurogenesis in Zebrafish and Rodents
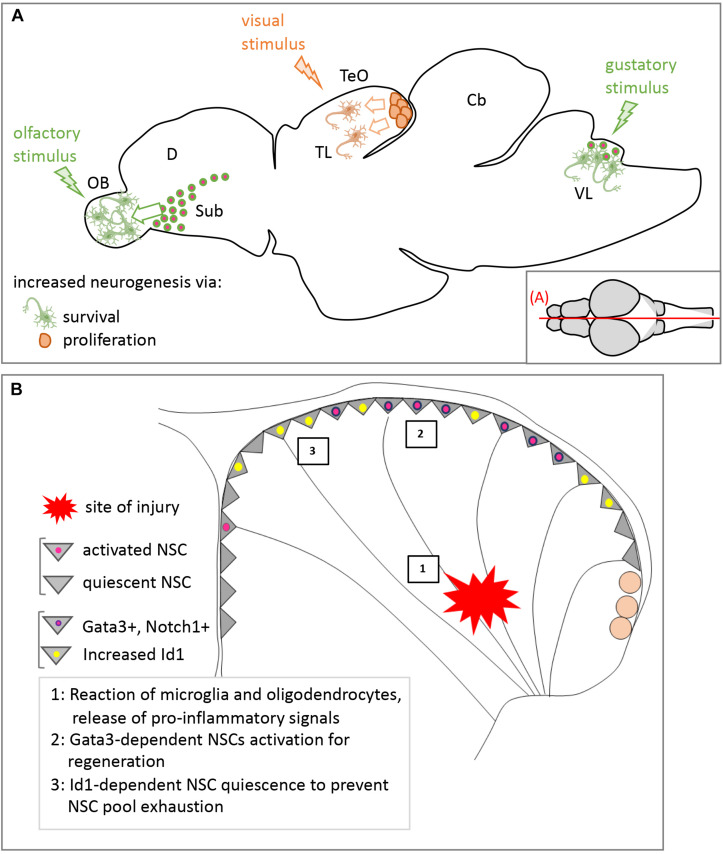
Environmental and systemic factors play an extensive role in modulating neurogenesis. For example, odorant stimuli can be integrated to tune neurogenic output from the SEZ niche in mice. Indeed, there are more newborn neurons in the OB, but not in the hippocampus, of mice reared in an odor-enriched environment (Rochefort et al., 2002), without an increase in proliferation in the SEZ. This suggests that simple sensory stimulation of adult neurogenesis is niche-specific and, in the case of this example, relies on an increase in newborn neuron survival. In teleosts, several neurogenic niches are in regions involved in sensory processing. Among them, the vagal lobe involved in gustation and the olfactory bulb get new neurons from RG NSCs, whereas the caudal periventricular gray zone of the optic tectum and the torus longitudinalis, both involved in visual processing, receive theirs from NE stem cells (Alunni et al., 2010; Sato et al., 2017). Presenting fish with stimuli processed in one of these niches leads to an increase in newborn neurons only in the respective niche and this increase is mediated differently in RG and NE niches: in NE niches, increased neurogenesis relies on an increase in proliferation, while in RG niches it involves an increase in newborn neurons survival (Lindsey et al., 2014), similarly, to OB neurogenesis in mice (Figure 8A).
FIGURE 8.
Modulation of adult neurogenesis by external stimuli in zebrafish. (A) Influence of sensory stimuli. Representation on a schematic sagittal section of the effect of the different sensory modalities studied to date, which can module either neuronal survival (green) or proliferation of NE progenitors (orange). (B) Influence of a mechanical injury on pallial neurogenesis. Representation on a schematic pallial cross-section of the sequence of events following injury (1–3) and the changes in NSC state and gene expression (color-coded). See text for references. Cb, cerebellum; D, dorsal part of the telencephalon; OB, olfactory bulb; Sub, subpallium; TeO, tectum opticum; TL, torus longitudinalis; VL, vagal lobe.
Nutritional factors also affect rates of neurogenesis in both rodents and zebrafish. Because most experiments involving individual foods were conducted only in mice, we focus here on experimental schemes investigating the effect of global changes of diet on neurogenesis. A high-fat diet and hyperglycemia have generally been linked to decreased hippocampal neurogenesis in rats and mice (Dorsemans et al., 2017a). Similarly, chronic high caloric intake and hyperglycemia lead to diminished proliferation in the forebrain neurogenic niches in zebrafish (Dorsemans et al., 2017b). On the other hand, caloric restriction through intermittent fasting increases the number of BrdU-positive cells after a 4-week chase in the hippocampus. However, equivalent schemes have not been worked out so far in zebrafish, while a global caloric restriction is generally assumed to lead to a decrease in proliferation. An important confounding factor is that the adult zebrafish body and brain keep on growing (Than-Trong et al., 2020), that brain growth notably occurs through the addition of neurons by NSCs, and that this growth is dependent on the quantity of food they receive. This makes it much harder in zebrafish than in rodents to ascertain whether changes in proliferation rates of NSCs are due to a specific regulation of NSC behavior or dictated by organism growth when these changes go in the same direction. Therefore, while the zebrafish can be used to investigate metabolic control of neurogenesis due to conservation of physiological responses to metabolic imbalances (Craig and Moon, 2011), there is still a need to work out experimental conditions before using it as a model for interventions that can interfere with its growth.
Finally, factors influencing emotional states also have consequences on neurogenesis. In particular, chronic high stress induced by social isolation decreases proliferation in the hippocampus of mice (Ibi et al., 2008) and non-human primates (Cinini et al., 2014) as well as in the forebrain of zebrafish (Tea et al., 2019).
These results together illustrate that adult neurogenesis in zebrafish is sensitive to environmental cues. Whether and how newly generated neurons relay some important measure of these stimuli, or convey some physiological response, remains to be shown.
Hormonal Regulation of Zebrafish Adult Neurogenesis
The environmental cues discussed above can be relayed to NSCs or adult newborn neurons via the activity of neurons contacting germinal zones. In many cases, however, they are also mediated by hormones. Among them, steroid hormones have been the subject of much focus. Glucocorticoids are elevated in response to stress as well as under high-fat, high-sugar and hyper caloric diets, and appear to be the cause of the reduced neurogenesis in these cases. Of note, in many species including amphibians, rodents and birds, the main glucocorticoid is corticosterone whereas in humans and teleost fish the main glucocorticoid is cortisol (which differs from corticosterone by the presence of one additional hydroxyl group), making zebrafish a particularly attractive model to study the effects of glucocorticoids on adult neurogenesis.
Sex steroids and in particular estrogens have also been extensively studied for their role in modulating adult neurogenesis. In the end, the nature of their involvement seems to not be conserved between species, even among rodents (Tanapat et al., 1999, 2005; Ormerod and Galea, 2001; Ormerod et al., 2003; Brock et al., 2010). In zebrafish, experimentally increasing estradiol levels decreases proliferation in the subpallium and some pallial subdivisions (Dl but not Dm) (Diotel et al., 2013; Makantasi and Dermon, 2014). An important peculiarity of teleosts when it comes to estrogen signaling is the duplication of the cyp19a1 gene coding for aromatase. In mammals, aromatase is expressed in the gonads and in a few neurons, whereas in zebrafish, aromatase A is expressed in the gonads while aromatase B is expressed at high levels in radial glia (Pellegrini et al., 2007). There, it could contribute to local estrogen synthesis, and this has been proposed to actively suppress proliferation in some neurogenic niches, in particular at the junction between olfactory bulbs and telencephalon and in the pallial region (Diotel et al., 2013).
Several other hormones are known to regulate neurogenesis in rodents such as Ghrelin, Thyroid hormones, Adiponectin and Androgens, however, their action has not yet been investigated in zebrafish. Mapping the expression of their receptors in the brain could constitute a first step in determining whether these regulations are conserved across species (Rastegar et al., 2019).
Zebrafish Adult Pallial NSCs Contribute Actively to Neuronal Regeneration
Pallial NSCs Are Activated for Regeneration Upon Mechanical Lesion
Perhaps the most well-known feature of zebrafish adult neurogenesis is its ability to contribute to neuronal regeneration after a brain injury contrary to mammals. In rodents and non-human primates, traumatic or excitotoxic brain injuries can increases NSC proliferation. However, neuron generation is inefficient: a fraction of NSC divisions is gliogenic and generates astrocytes, neuroblasts often fail to migrate toward the site of injury, and the neurogenesis process is usually not followed by functional integration of the new neurons (Skaggs et al., 2014) -although with a few exceptions (Nakatomi et al., 2002; Sirko et al., 2013; Magnusson et al., 2014)-. Upon injury in mammals, inflammation triggers the activation of reactive astrocytes and the formation of a glial scar which prevents regeneration (März et al., 2011). On the opposite, in the mechanically injured zebrafish pallium, nearby RG cells in the ventricular zone quickly increase their proliferation rate, reaching a peak at 7 days post-injury (dpi) before progressively returning to baseline. Genetic or BrdU-mediated tracing indicated that this allows for the production of newborn neurons that migrate to the site of injury and get synaptically connected. These neurons can survive for at least 3 months, leading to wound closure without formation of a glial scar (Kroehne et al., 2011; März et al., 2011; Baumgart et al., 2012). Their identity, however, remains to be described – notably to determine whether it matches that of the lesioned neurons-. Functional recovery also needs to be assessed, although this may prove a difficult task given that functional pallial neuroanatomy is not precise at this point. Overall, a huge efforts needs to be made to map functional circuits and their markers in adult zebrafish.
Similarly, the initial response to pallial injury in zebrafish is an activation of microglia and oligodendrocytes surrounding the site of injury, together with pro-inflammatory signals, but contrary to the situation in rodents, they act to promote regeneration. Expression of cysteinyl leukotriene receptor 1 (cysltr1) is upregulated especially close to the site of injury and, upon binding its ligand CysLT1, triggers the expression of Gata3 (Kyritsis et al., 2012), a transcription factor which is normally not (or lowly) expressed under physiological condition (Kizil et al., 2012c). Expression of gata3 is necessary for injury-induced NSC proliferation in the pallium, and experimental stimulation of CysLTr1 is in turn sufficient and necessary to induce gata3 and increase NSC proliferation (Figure 8B). Other proinflammatory signals probably also play a role as expression of cxcr5 is also increased after injury and its blockade reduces the regenerative response (Kizil et al., 2012a). Moreover, while inflammation plays an essential role in initiating the regenerative response, other signaling pathways are also necessary for it to reach its full extent. Indeed, blocking FGF signaling after injury reduces the upregulation of gata3 and proliferation of nearby NSCs (Kizil et al., 2012c). Regulatory mechanisms involved in controlling neurogenesis in physiological conditions are also essential in response to injury. After injury, BDNF is upregulated in the surrounding newborn and mature neurons for up to 15 days and acts through its receptor TrkB to promote NSC proliferation. Likewise, the expression of proteins involved in the Notch signaling pathway is modified upon lesion, and non-selectively inhibiting it with the gamma-secretase inhibitor DAPT decreases the magnitude of the response in NSCs. Moreover, division modes upon injury favor a more neurogenic fate at the expense of self-renewal, which risks leading to depletion of the NSC pool (Barbosa et al., 2015a). The upregulation of Notch1 or Notch3, which promote stemness and quiescence (Alunni et al., 2013; Than-Trong et al., 2018) could be a way to counteract this depletion. This was formally demonstrated to be a function of Id1, which is also upregulated upon injury independently of inflammatory signals and of Notch signaling and mitigates the proliferation of NSCs upon injury (Rodriguez-Viales et al., 2015).
The improved regeneration in zebrafish thus appears to be in part due to the absence of parenchymal astrocytes that generate a glial scar and a different management of the inflammation response that recedes faster in zebrafish (Kizil et al., 2012b). In addition, the “protection” of a subset of NSCs from the regenerative response might be relevant to also maintain physiological neurogenesis post-lesion. Getting a full picture of those differences represents a promising avenue to better understand how to promote neuronal regeneration for therapeutic purposes.
Pallial NSCs Are Activated for Regeneration Upon Neuronal Alzheimer-Like Degeneration
One of the regions affected early in Alzheimer’s disease (AD) is the hippocampus, and this hippocampal degeneration is thought to underlie the memory loss symptoms as well as the visuo-spatial disorientation that appear from the early stages of the disease. Stimulating hippocampal neurogenesis in order to regenerate the lost neurons and rescue hippocampal function is thus considered a potential therapy to alleviate the disease. In mouse models of AD where the disease is replicated by inducing the formation of amyloid beta plaques in the brain, and in samples from AD patients, the production of newborn neurons appears increased (Jin et al., 2004; Gan et al., 2008; Unger et al., 2016). However, the NSCs themselves also seem affected by the disease, leading to depletion of the NSC pool (Moreno-Jiménez et al., 2019). Recently the Kizil group proposed a zebrafish AD model using amyloid-b42 injections into the adult pallium (Bhattarai et al., 2016). While the relevance of such a model to the human disease still needs to be fully validated, the results suggest that NSCs proliferate in response to amyloidopathies through IL4 signaling but that not all NSCs respond similarly (Bhattarai et al., 2016; Cosacak et al., 2019). Understanding the bases for these differences will be an important point for future studies.
Conclusion
The location and efficiency of adult neurogenesis domains, under physiological or pathological conditions, vary greatly between vertebrate species. The mechanistic reasons for these differences largely remain to be understood, and comparative approaches are powerful ways toward this goal. As illustrated in this review, the zebrafish adult pallium offers novel perspectives to dive into the fundamental properties of adult telencephalic NSCs. These are linked in particular with unprecedented possibilities to record the behavior of NSCs in their niche (such as intravital imaging methods), and with the existence of unique physiological contexts (such as regeneration), associated with a vast repertoire of NSC properties that can be mechanistically matched or contrasted with rodent NSCs. Princeps discoveries on adult NSC quiescence and population dynamics were obtained in zebrafish with strong applicability potential to rodents, and continuation of such synergistic work will undoubtedly help progress in the field.
Author Contributions
ML, LM, DM, and LB-C wrote the review. LB-C coordinated the process. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all members of the LB-C team for critical reading of the manuscript and for generating the data that support many ideas of this review. Figure 1 includes panels more specifically generated by N. Dray, L. Dirian, and I. Foucher.
Footnotes
Funding. Work in the LB-C laboratory was funded by the ANR (Labex Revive), Centre National de la Recherche Scientifique, Institut Pasteur, the European Research Council (AdG 322936) and the Ligue Nationale Contre le Cancer. LM was recipient of a Ph.D. student 4th year fellowship from the Fondation pour la Recherche Médicale (FRM). DM was recipient of a Ph.D. student fellowship from the Ecole Normale Supérieure. ML, LM, and DM were Ph.D. students at Sorbonne Université.
References
- Adolf B., Chapouton P., Lam C. S., Topp S., Tannhäuser B., Strähle U., et al. (2006). Conserved and acquired features of adult neurogenesis in the zebrafish telencephalon. Dev. Biol. 295 278–293. 10.1016/j.ydbio.2006.03.023 [DOI] [PubMed] [Google Scholar]
- Aguirre A., Rubio M. E., Gallo V. (2010). Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature 467 323–327. 10.1038/nature09347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J., Das G. D. (1965). Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 124 319–335. 10.1002/cne.901240303 [DOI] [PubMed] [Google Scholar]
- Alunni A., Hermel J. M., Heuzé A., Bourrat F., Jamen F., Joly J. S. (2010). Evidence for neural stem cells in the Medaka optic tectum proliferation zones. Dev. Neurobiol. 70 693–713. 10.1002/dneu.20799 [DOI] [PubMed] [Google Scholar]
- Alunni A., Krecsmarik M., Bosco A., Galant S., Pan L., Moens C. B., et al. (2013). Notch3 signaling gates cell cycle entry and limits neural stem cell amplification in the adult pallium. Development 140 3335–3347. 10.1242/dev.095018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker C., Hen R. (2017). Adult hippocampal neurogenesis and cognitive flexibility-linking memory and mood. Nat. Rev. Neurosci. 18 335–346. 10.1038/nrn.2017.45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand S. K., Mondal A. C. (2017). Cellular and molecular attributes of neural stem cell niches in adult zebrafish brain. Dev. Neurobiol. 77 1188–1205. 10.1002/dneu.22508 [DOI] [PubMed] [Google Scholar]
- Andersen J., Urbán N., Achimastou A., Ito A., Simic M., Ullom K., et al. (2014). A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron 83 1085–1097. 10.1016/j.neuron.2014.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu-Agulló C., Morante-Redolat J. M., Delgado A. C., Farias I. (2009). Vascular niche factor PEDF modulates notch-dependent stemness in the adult subependymal zone. Nat. Neurosci. 12 1514–1523. 10.1038/nn.2437 [DOI] [PubMed] [Google Scholar]
- Bai G., Sheng N., Xie Z., Bian W., Yokota Y., Benezra R., et al. (2007). Id sustains hes1 expression to inhibit precocious neurogenesis by releasing negative Autoregulation of Hes1. Dev. Cell 13 283–297. 10.1016/j.devcel.2007.05.014 [DOI] [PubMed] [Google Scholar]
- Balordi F., Fishell G. (2007). Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J. Neurosci. 27 14248–14259. 10.1523/jneurosci.4531-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa J. S., Sanchez-gonzalez R., Di Giaimo R., Baumgart E. V., Theis F. J., Ninkovic J. (2015a). Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science 65 61–65. [DOI] [PubMed] [Google Scholar]
- Barbosa J. S., Sanchez-Gonzalez R., Di Giaimo R., Baumgart E. V., Theis F. J., Götz M., et al. (2015b). Neurodevelopment. Live imaging of adult neural stem cell behavior in the intact and injured zebrafish brain. Science 348 789–793. 10.1126/science.aaa2729 [DOI] [PubMed] [Google Scholar]
- Basak O., Giachino C., Fiorini E., MacDonald H. R., Taylor V. (2012). Neurogenic Subventricular zone stem/progenitor cells are notch1-dependent in their active but not Quiescent State. J. Neurosci. 32 5654–5666. 10.1523/jneurosci.0455-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak O., Krieger T. G., Muraro M. J., Wiebrands K., Stange D. E., Frias-Aldeguer J., et al. (2018). Troy+ brain stem cells cycle through quiescence and regulate their number by sensing niche occupancy. Proc. Natl. Acad. Sci. U.S.A. 115 E610–E619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baser A., Skabkin M., Kleber S., Dang Y., Gülcüler Balta G. S., Kalamakis G., et al. (2019). Onset of differentiation is post-transcriptionally controlled in adult neural stem cells. Nature 566 100–104. 10.1038/s41586-019-0888-x [DOI] [PubMed] [Google Scholar]
- Bast L., Calzolari F., Strasser M. K., Hasenauer J., Theis F. J., Ninkovic J., et al. (2018). Increasing neural stem cell division asymmetry and quiescence are predicted to contribute to the age-related decline in neurogenesis. Cell Rep. 25 3231–3240. [DOI] [PubMed] [Google Scholar]
- Baumgart E. V., Barbosa J. S., Bally-cuif L., Götz M., Ninkovic J. (2012). Stab wound injury of the zebrafish telencephalon: a model for comparative analysis of reactive gliosis. Glia 60 343–357. 10.1002/glia.22269 [DOI] [PubMed] [Google Scholar]
- Bayer S. A. (1985). Neuron production in the hippocampus and olfactory bulb of the adult rat brain: addition or replacement? Ann. N. Y. Acad. Sci. 457 163–172. 10.1111/j.1749-6632.1985.tb20804.x [DOI] [PubMed] [Google Scholar]
- Berberoglu M. A., Dong Z., Li G., Zheng J., Trejo Martinez L. D. C. G., Peng J., et al. (2014). Heterogeneously expressed fezf2 patterns gradient notch activity in balancing the quiescence, proliferation, and differentiation of adult neural stem cells. J. Neurosci. 34 13911–13923. 10.1523/jneurosci.1976-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D. A., Kirkham M., Beljajeva A., Knapp D., Habermann B., Ryge J., et al. (2010). Efficient regeneration by activation of neurogenesis in homeostatically quiescent regions of the adult vertebrate brain. Development 137 4127–4134. 10.1242/dev.055541 [DOI] [PubMed] [Google Scholar]
- Berg D. A., Su Y., Jimenez-Cyrus D., Patel A., Huang N., Morizet D., et al. (2019). A common embryonic origin of stem cells drives developmental and adult neurogenesis. Cell 177 654–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattarai P., Thomas A. K., Cosacak M. I., Papadimitriou C., Mashkaryan V., Froc C., et al. (2016). IL4/STAT6 signaling activates neural stem cell proliferation and neurogenesis upon amyloid-β42 aggregation in Adult Zebrafish brain. Cell Rep. 17 941–948. 10.1016/j.celrep.2016.09.075 [DOI] [PubMed] [Google Scholar]
- Blanpain C., Simons B. D. (2013). Unravelling stem cell dynamics by lineage tracing. Nat. Rev. Mol. Cell Biol. 14 489–502. 10.1038/nrm3625 [DOI] [PubMed] [Google Scholar]
- Blažek R., Polačik M., Reichard M. (2013). Rapid growth, early maturation and short generation time in African annual fishes. EvoDevo 4:24. 10.1186/2041-9139-4-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomfield I. M., Rocamonde B., del Mar Masdeu M., Mulugeta E., Vaga S., van den Berg D. L. C., et al. (2019). Id4 promotes the elimination of the pro-activation factor ascl1 to maintain quiescence of adult hippocampal stem cells. eLife 8:e48561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boareto M., Iber D., Taylor V. (2017). Differential interactions between Notch and ID factors control neurogenesis by modulating Hes factor autoregulation. Development 144 3465–3474. 10.1242/dev.152520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi M. A., Peng C.-Y., McGuire T., Falciglia G., Gobeske K. T., Czeisler C., et al. (2008). Noggin expands neural stem cells in the adult hippocampus. J. Neurosci. 28 9194–9204. 10.1523/jneurosci.3314-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi M. A., Song J., Ming G. L., Song H. (2012). A unifying hypothesis on mammalian neural stem cell properties in the adult hippocampus. Curr. Opin. Neurobiol. 22 754–761. 10.1016/j.conb.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaguidi M. A., Wheeler M. A., Shapiro J. S., Stadel R. P., Sun G. J., Ming G. L., et al. (2011). In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 145 1142–1155. 10.1016/j.cell.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock O., Keller M., Veyrac A., Douhard Q., Bakker J. (2010). Short term treatment with estradiol decreases the rate of newly generated cells in the subventricular zone and main olfactory bulb of adult female mice. Neuroscience 166 368–376. 10.1016/j.neuroscience.2009.12.050 [DOI] [PubMed] [Google Scholar]
- Byrd C. A., Brunjes P. C. (2001). Neurogenesis in the olfactory bulb of adult zebrafish. Neuroscience 105 793–801. 10.1016/s0306-4522(01)00215-9 [DOI] [PubMed] [Google Scholar]
- Calegari F., Huttner W. B. (2003). An inhibition of cyclin-dependent kinases that lengthens, but does not arrest, neuroepithelial cell cycle induces premature neurogenesis. J. Cell Sci. 116 4947–4955. 10.1242/jcs.00825 [DOI] [PubMed] [Google Scholar]
- Calzolari F., Michel J., Baumgart E. V., Theis F., Götz M., Ninkovic J. (2015). Fast clonal expansion and limited neural stem cell self-renewal in the adult subependymal zone. Nat. Neurosci. 18 490–492. 10.1038/nn.3963 [DOI] [PubMed] [Google Scholar]
- Castro D. S., Martynoga B., Parras C., Ramesh V., Pacary E., Johnston C., et al. (2011). A novel function of the proneural factor Ascl1 in progenitor proliferation identified by genome-wide characterization of its targets. Genes Dev. 25 930–945. 10.1101/gad.627811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaker Z., Codega P., Doetsch F. (2016). A mosaic world: puzzles revealed by adult neural stem cell heterogeneity. Wiley Interdiscip. Rev. Dev. Biol. 5 640–658. 10.1002/wdev.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C. D., Ottavio L., Travali S., Lipson K. E., Baserga R. (1990). Transcriptional and posttranscriptional regulation of the proliferating cell nuclear antigen gene. Mol. Cell. Biol. 10 3289–3296. 10.1128/mcb.10.7.3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapouton P., Skupien P., Hesl B., Coolen M., Moore J. C., Madelaine R., et al. (2010). Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J. Neurosci. 30 7961–7974. 10.1523/jneurosci.6170-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Hartman A., Guo S. (2015). Choosing cell fate through a dynamic cell cycle. Curr. Stem Cell Rep. 1 129–138. 10.1007/s40778-015-0018-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinini S. M., Barnabe G. F., Galvão-Coelho N., de Medeiros M. A., Perez-Mendes P., Sousa M. B. C., et al. (2014). Social isolation disrupts hippocampal neurogenesis in young non-human primates. Front. Neurosci. 8:45. 10.3389/fnins.2014.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codega P., Silva-Vargas V., Paul A., Maldonado-Soto A. R. R., DeLeo A. M. M., Pastrana E., et al. (2014). Prospective identification and purification of quiescent adult neural stem cells from their in vivo niche. Neuron 82 545–559. 10.1016/j.neuron.2014.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coolen M., Labusch M., Mannioui A., Bally-Cuif L. (2020). Mosaic heterochrony in neural progenitors sustains accelerated brain growth and neurogenesis in the Juvenile Killifish N. furzeri. Curr. Biol. 30 736–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosacak M. I., Bhattarai P., Reinhardt S., Petzold A., Dahl A., Zhang Y., et al. (2019). Single-cell transcriptomics analyses of neural stem cell heterogeneity and contextual plasticity in a Zebrafish brain model of amyloid toxicity. Cell Rep. 27 1307–1318. [DOI] [PubMed] [Google Scholar]
- Craig P. M., Moon T. W. (2011). Fasted zebrafish mimic genetic and physiological responses in mammals: a model for obesity and diabetes? Zebrafish 8 109–117. 10.1089/zeb.2011.0702 [DOI] [PubMed] [Google Scholar]
- Dambroise E., Simion M., Bourquard T., Bouffard S., Rizzi B., Jaszczyszyn Y., et al. (2017). Postembryonic fish brain proliferation zones exhibit neuroepithelial-type gene expression profile. Stem Cells 35 1505–1518. 10.1002/stem.2588 [DOI] [PubMed] [Google Scholar]
- de Oliveira-Carlos V., Ganz J., Hans S., Kaslin J., Brand M. (2013). Notch receptor expression in neurogenic regions of the adult Zebrafish brain. PLoS One 8:e73384. 10.1371/journal.pone.0073384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diotel N., Beil T., Strähle U., Rastegar S. (2015). Differential expression of id genes and their potential regulator znf238 in zebrafish adult neural progenitor cells and neurons suggests distinct functions in adult neurogenesis. Gene Expr. Patterns 19 1–13. 10.1016/j.gep.2015.05.004 [DOI] [PubMed] [Google Scholar]
- Diotel N., Vaillant C., Gabbero C., Mironov S., Fostier A., Gueguen M. M., et al. (2013). Effects of estradiol in adult neurogenesis and brain repair in zebrafish. Horm. Behav. 63 193–207. 10.1016/j.yhbeh.2012.04.003 [DOI] [PubMed] [Google Scholar]
- Dirian L., Galant S., Coolen M., Chen W., Bedu S., Houart C., et al. (2014). Spatial regionalization and heterochrony in the formation of adult pallial neural stem cells. Dev. Cell 30 123–136. 10.1016/j.devcel.2014.05.012 [DOI] [PubMed] [Google Scholar]
- Doe C. Q. (2017). Temporal patterning in the Drosophila CNS. Annu. Rev. Cell Dev. Biol. 33 219–240. 10.1146/annurev-cellbio-111315-125210 [DOI] [PubMed] [Google Scholar]
- Dong Z., Yang N., Yeo S.-Y., Chitnis A., Guo S. (2012). Intralineage directional Notch signaling regulates self-renewal and differentiation of asymmetrically dividing radial glia. Neuron 74 65–78. 10.1016/j.neuron.2012.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsemans A. C., Couret D., Hoarau A., Meilhac O., Lefebvre d’Hellencourt C., Diotel N. (2017a). Diabetes, adult neurogenesis and brain remodeling: new insights from rodent and zebrafish models. Neurogenesis 4:e1281862. 10.1080/23262133.2017.1281862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsemans A. C., Soulé S., Weger M., Bourdon E., Lefebvre d’Hellencourt C., Meilhac O., et al. (2017b). Impaired constitutive and regenerative neurogenesis in adult hyperglycemic zebrafish. J. Comp. Neurol. 525 442–458. 10.1002/cne.24065 [DOI] [PubMed] [Google Scholar]
- Dranovsky A., Picchini A. M., Moadel T., Sisti A. C., Yamada A., Kimura S., et al. (2011). Experience dictates stem cell fate in the adult hippocampus. Neuron 70 908–923. 10.1016/j.neuron.2011.05.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray N., Bedu S., Vuillemin N., Alunni A., Coolen M., Krecsmarik M., et al. (2015). Large-scale live imaging of adult neural stem cells in their endogenous niche. Development 142 3592–3600. 10.1242/dev.123018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulken B. W., Leeman D. S., Boutet S. C., Hebestreit K., Brunet A., Blurb E. (2017). Single cell transcriptomic analysis defines heterogeneity and transcriptional dynamics in the adult neural stem cell lineage. Cell Rep. 1712 777–790. 10.1016/j.celrep.2016.12.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehm O., Göritz C., Covic M., Schäffner I., Schwarz T. J., Karaca E., et al. (2010). RBPJkappa-dependent signaling is essential for long-term maintenance of neural stem cells in the adult hippocampus. J. Neurosci. 30 13794–13807. 10.1523/jneurosci.1567-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encinas J. M., Michurina T. V., Peunova N., Park J. H., Tordo J., Peterson D. A., et al. (2011). Division-coupled astrocytic differentiation and age-related depletion of neural stem cells in the adult hippocampus. Cell Stem Cell 8 566–579. 10.1016/j.stem.2011.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A., Rolando C., Giachino C., Saotome I., Erni A., Brien C., et al. (2018). Notch2 signaling maintains NSC quiescence in the murine ventricular-Subventricular zone. Cell Rep. 22 992–1002. 10.1016/j.celrep.2017.12.094 [DOI] [PubMed] [Google Scholar]
- Eriksson P. S., Perfilieva E., Björk-Eriksson T., Alborn A. M., Nordborg C., Peterson D. A., et al. (1998). Neurogenesis in the adult human hippocampus. Nat. Med. 4 1313–1317. [DOI] [PubMed] [Google Scholar]
- Fan X., Xiong Y., Wang Y. (2019). A reignited debate over the cell(s) of origin for glioblastoma and its clinical implications. Front. Med. 13:531–539. 10.1007/s11684-019-0700-1 [DOI] [PubMed] [Google Scholar]
- Ferron S. R., Pozo N., Laguna A., Aranda S., Porlan E., Moreno M., et al. (2010). Regulated segregation of kinase Dyrk1A during asymmetric neural stem cell division is critical for EGFR-mediated biased signaling. Cell Stem Cell 7 367–379. 10.1016/j.stem.2010.06.021 [DOI] [PubMed] [Google Scholar]
- Folgueira M., Bayley P., Navratilova P., Becker T. S., Wilson S. W., Clarke J. D. W. (2012). Morphogenesis underlying the development of the everted teleost telencephalon. Neural Dev. 7:32. 10.1186/1749-8104-7-32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba L. C., Obernier K., Alvarez-Buylla A. (2012). Adult neural stem cells bridge their niche. Cell Stem Cell 10 698–708. 10.1016/j.stem.2012.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlan G., Cuccioli V., Vuillemin N., Dirian L., Muntasell A. J., Coolen M., et al. (2017). Life-long neurogenic activity of individual neural stem cells and continuous growth establish an outside-in architecture in the Teleost Pallium. Curr. Biol. 27 3288–3301.e3. 10.1016/j.cub.2017.09.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutachi S., Miya H., Watanabe T., Kawai H., Yamasaki N., Harada Y., et al. (2015). Slowly dividing neural progenitors are an embryonic origin of adult neural stem cells. Nat. Neurosci. 18 657–665. 10.1038/nn.3989 [DOI] [PubMed] [Google Scholar]
- Gan L., Qiao S., Lan X., Chi L., Luo C., Lien L., et al. (2008). Neurogenic responses to amyloid-beta plaques in the brain of Alzheimer’s disease-like transgenic (pPDGF-APPSw,Ind) mice. Neurobiol. Dis. 29 71–80. 10.1016/j.nbd.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganz J., Kaslin J., Hochmann S., Freudenreich D., Brand M. (2010). Heterogeneity and Fgf dependence of adult neural progenitors in the zebrafish telencephalon. Glia 58 1345–1363. 10.1002/glia.21012 [DOI] [PubMed] [Google Scholar]
- Ganz J., Kroehne V., Freudenreich D., Machate A., Geffarth M., Braasch I., et al. (2015). Subdivisions of the adult zebrafish pallium based on molecular marker analysis. F1000Res. 520 633–655. 10.1002/cne.22757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giachino C., Taylor V. (2014). Notching up neural stem cell homogeneity in homeostasis and disease. Front. Neurosci. 8:32. 10.3389/fnins.2014.00032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman S. A., Nottebohm F. (1983). Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain (learning/neurogenesis/neuronal death/glial cells/endothelial cells). Proc. Nati. Acad. Sci. U.S.A. 80 2390–2394. 10.1073/pnas.80.8.2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz M., Nakafuku M., Petrik D. (2016). Neurogenesis in the developing and adult brain-similarities and key differences. Cold Spring Harb. Perspect. Biol. 8:a018853. 10.1101/cshperspect.a018853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandel H., Kaslin J., Ganz J., Wenzel I., Brand M. (2006). Neural stem cells and neurogenesis in the adult zebrafish brain: origin, proliferation dynamics, migration and cell fate. Dev. Biol. 295 263–277. 10.1016/j.ydbio.2006.03.040 [DOI] [PubMed] [Google Scholar]
- Guesmi K., Abdeladim L., Tozer S., Mahou P., Kumamoto T., Jurkus K., et al. (2018). Dual-color deep-tissue three-photon microscopy with a multiband infrared laser. Light Sci. Appl. 7 12 10.1017/s1551929512000430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris L., Zalucki O., Clément O., Fraser J., Matuzelski E., Oishi S., et al. (2018). Neurogenic differentiation by hippocampal neural stem and progenitor cells is biased by NFIX expression. Development 145:dev155689. 10.1242/dev.155689 [DOI] [PubMed] [Google Scholar]
- Hevner R. F. (2019). Intermediate progenitors and Tbr2 in cortical development. J. Anat. 235 616–625. 10.1111/joa.12939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochgerner H., Zeisel A., Lönnerberg P., Linnarsson S. (2018). Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat. Neurosci. 21 290–299. 10.1038/s41593-017-0056-2 [DOI] [PubMed] [Google Scholar]
- Huttner W. B., Kosodo Y. (2005). Symmetric versus asymmetric cell division during neurogenesis in the developing vertebrate central nervous system. Curr. Opin. Cell Biol. 17 648–657. 10.1016/j.ceb.2005.10.005 [DOI] [PubMed] [Google Scholar]
- Ibi D., Takuma K., Koike H., Mizoguchi H., Tsuritani K., Kuwahara Y., et al. (2008). Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J. Neurochem. 105 921–932. 10.1111/j.1471-4159.2007.05207.x [DOI] [PubMed] [Google Scholar]
- Imayoshi I., Sakamoto M., Yamaguchi M., Mori K., Kageyama R. (2010). Essential roles of notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 30 3489–3498. 10.1523/jneurosci.4987-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K., Peel A. L., Mao X. O., Xie L., Cottrell B. A., Henshall D. C., et al. (2004). Increased hippocampal neurogenesis in Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 101 343–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen C. (2018). Adult mammalian neurogenesis and motivated behaviors. Integr. Zool. 16 655–672. 10.1111/1749-4877.12335 [DOI] [PubMed] [Google Scholar]
- Kalamakis G., Brüne D., Ravichandran S., Bolz J., Fan W., Ziebell F., et al. (2019). Quiescence modulates stem cell maintenance and regenerative capacity in the aging brain. Cell 176 1407–1419.e14. 10.1016/j.cell.2019.01.040 [DOI] [PubMed] [Google Scholar]
- Katz S., Cussigh D., Urbán N., Blomfield I., Guillemot F., Bally-Cuif L., et al. (2016). A nuclear role for miR-9 and argonaute proteins in balancing quiescent and activated neural stem cell states. Cell Rep. 17 1383–1398. 10.1016/j.celrep.2016.09.088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi D., Furutachi S., Kawai H., Hozumi K., Gotoh Y. (2013). Dll1 maintains quiescence of adult neural stem cells and segregates asymmetrically during mitosis. Nat. Commun. 4:1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai H., Kawaguchi D., Kuebrich B. D., Kitamoto T., Yamaguchi M., Gotoh Y., et al. (2017). Area-specific regulation of quiescent neural stem cells by Notch3 in the adult mouse subependymal zone. J. Neurosci. 37 11867–11880. 10.1523/jneurosci.0001-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto N., Alfaro-Cervello C., Shimizu K., Asakawa K., Urasaki A., Nonaka S., et al. (2011). Migration of neuronal precursors from the telencephalic ventricular zone into the olfactory bulb in adult zebrafish. J. Comp. Neurol. 519 3549–3565. 10.1002/cne.22722 [DOI] [PubMed] [Google Scholar]
- Kizil C., Dudczig S., Kyritsis N., Machate A., Blaesche J., Kroehne V., et al. (2012a). The chemokine receptor cxcr5 regulates the regenerative neurogenesis response in the adult zebrafish brain. Neural Dev. 7:27. 10.1186/1749-8104-7-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizil C., Kaslin J., Kroehne V., Brand M. (2012b). Adult neurogenesis and brain regeneration in zebrafish. Dev. Neurobiol. 72 429–461. 10.1002/dneu.20918 [DOI] [PubMed] [Google Scholar]
- Kizil C., Kyritsis N., Dudczig S., Kroehne V., Freudenreich D., Kaslin J., et al. (2012c). Regenerative neurogenesis from neural progenitor cells requires injury-induced expression of Gata3. Dev. Cell 23 1230–1237. 10.1016/j.devcel.2012.10.014 [DOI] [PubMed] [Google Scholar]
- Knoblich J. A. (2008). Mechanisms of asymmetric stem cell division. Cell 132 583–597. 10.1016/j.cell.2008.02.007 [DOI] [PubMed] [Google Scholar]
- Kressmann S., Campos C., Castanon I., Fürthauer M., González-Gaitán M. (2015). Directional Notch trafficking in Sara endosomes during asymmetric cell division in the spinal cord. Nat. Cell Biol. 17 333–339. 10.1038/ncb3119 [DOI] [PubMed] [Google Scholar]
- Kriegstein A. R., Götz M. (2003). Radial glia diversity: a matter of cell fate. Glia 43 37–43. 10.1002/glia.10250 [DOI] [PubMed] [Google Scholar]
- Kroehne V., Freudenreich D., Hans S., Kaslin J., Brand M. (2011). Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development 138 4831–4841. 10.1242/dev.072587 [DOI] [PubMed] [Google Scholar]
- Kyritsis N., Kizil C., Zocher S., Kroehne V., Kaslin J., Freudenreich D., et al. (2012). Acute inflammation initiates the regenerative response in the adult zebrafish brain. Science 338 1353–1356. 10.1126/science.1228773 [DOI] [PubMed] [Google Scholar]
- Lal P., Tanabe H., Suster M. L., Ailani D., Kotani Y., Muto A., et al. (2018). Identification of a neuronal population in the telencephalon essential for fear conditioning in zebrafish. BMC Biol. 16:45. 10.1186/s12915-018-0502-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C., Rost F., Machate A., Reinhardt S., Lesche M., Weber A., et al. (2020). Single cell sequencing of radial glia progeny reveals the diversity of newborn neurons in the adult zebrafish brain. Development 147:dev185595. 10.1242/dev.185595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavado A., Oliver G. (2014). Jagged1 is necessary for postnatal and adult neurogenesis in the dentate gyrus. Dev. Biol. 388 11–21. 10.1016/j.ydbio.2014.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levison S. W., Goldman J. E. (1993). Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron 10 201–212. 10.1016/0896-6273(93)90311-e [DOI] [PubMed] [Google Scholar]
- Li G., Fang L., Fernández G., Pleasure S. J. (2013). The ventral hippocampus is the embryonic origin for adult neural stem cells in the Dentate Gyrus. Neuron 78 658–672. 10.1016/j.neuron.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D. A., Tramontin A. D., Trevejo J. M., Herrera D. G., García-Verdugo J. M., Alvarez-Buylla A. (2000). Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron 28 713–726. 10.1016/s0896-6273(00)00148-3 [DOI] [PubMed] [Google Scholar]
- Lindsey B. W., Di Donato S., Kaslin J., Tropepe V. (2014). Sensory-specific modulation of adult neurogenesis in sensory structures is associated with the type of stem cell present in the neurogenic niche of the zebrafish brain. Eur. J. Neurosci. 40 3591–3607. 10.1111/ejn.12729 [DOI] [PubMed] [Google Scholar]
- Lindsey B. W., Hall Z. J., Heuzé A., Joly J. S., Tropepe V., Kaslin J. (2018). The role of neuro-epithelial-like and radial-glial stem and progenitor cells in development, plasticity, and repair. Prog. Neurobiol. 170 99–114. 10.1016/j.pneurobio.2018.06.004 [DOI] [PubMed] [Google Scholar]
- Liu X., Wang Q., Haydar T. F., Bordey A. (2005). Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 8 1179–1187. 10.1038/nn1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llorens-Bobadilla E., Zhao S., Baser A., Saiz-Castro G., Zwadlo K., Martin-Villalba A. (2015). Single-cell transcriptomics reveals a population of dormant neural stem cells that become activated upon brain injury. Cell Stem Cell 17 329–340. 10.1016/j.stem.2015.07.002 [DOI] [PubMed] [Google Scholar]
- Lugert S., Basak O., Knuckles P., Haussler U., Fabel K., Götz M., et al. (2010). Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 6 445–456. 10.1016/j.stem.2010.03.017 [DOI] [PubMed] [Google Scholar]
- Lugert S., Vogt M., Tchorz J. S., Müller M., Giachino C., Taylor V. (2012). Homeostatic neurogenesis in the adult hippocampus does not involve amplification of Ascl1 high intermediate progenitors. Nat. Commun. 3:670. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A. I., Nguyen C., Melendez E., Lin D. P., Teo J. L., Lai K. K. Y., et al. (2019). The mode of stem cell division is dependent on the differential interaction of β-catenin with the kat3 coactivators CBP or p300. Cancers 11:962. 10.3390/cancers11070962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson J. P., Göritz C., Tatarishvili J., Dias D. O., Smith E. M. K., Lindvall O., et al. (2014). A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science 346 237–241. 10.1126/science.346.6206.237 [DOI] [PubMed] [Google Scholar]
- Makantasi P., Dermon C. R. (2014). Estradiol treatment decreases cell proliferation in the neurogenic zones of adult female zebrafish (Danio Rerio) brain. Neuroscience 277 306–320. 10.1016/j.neuroscience.2014.06.071 [DOI] [PubMed] [Google Scholar]
- Martynoga B., Mateo J. L., Zhou B., Andersen J., Achimastou A., Urbán N., et al. (2013). Epigenomic enhancer annotation reveals a key role for NFIX in neural stem cell quiescence. Genes Dev. 27 1769–1786. 10.1101/gad.216804.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- März M., Chapouton P., Diotel N., Vaillant C., Hesl B., Takamiya M., et al. (2010a). Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 58 870–888. [DOI] [PubMed] [Google Scholar]
- März M., Schmidt R., Rastegar S., Strähle U. (2010b). Expression of the transcription factor Olig2 in proliferating cells in the adult zebrafish telencephalon. Dev. Dyn. 239 3336–3349. 10.1002/dvdy.22455 [DOI] [PubMed] [Google Scholar]
- März M., Schmidt R., Rastegar S., Strahle U. (2011). Regenerative response following stab injury in the adult zebrafish telencephalon. Dev. Dyn. 240 2221–2231. 10.1002/dvdy.22710 [DOI] [PubMed] [Google Scholar]
- Matarredona E. R., Pastor A. M. (2019). Neural stem cells of the subventricular zone as the origin of human glioblastoma stem cells. Therapeutic implications. Front. Oncol. 9:779. 10.3389/fonc.2019.00779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattar P., Ericson J., Blackshaw S., Cayouette M. (2015). A conserved regulatory logic controls temporal identity in mouse neural progenitors. Neuron 85 497–504. 10.1016/j.neuron.2014.12.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menn B., Garcia-Verdugo J. M., Yaschine C., Gonzalez-Perez O., Rowitch D., Alvarez-Buylla A. (2006). Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 26 7907–7918. 10.1523/jneurosci.1299-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle F. T., Fuentealba L. C., Sanders T. A., Magno L., Kessaris N., Alvarez-Buylla A. (2014). Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat. Neurosci. 17 207–214. 10.1038/nn.3610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle F. T., Mirzadeh Z., Alvarez-Buylla A. (2007). Mosaic organization of neural stem cells in the adult brain. Science 317 381–384. 10.1126/science.1144914 [DOI] [PubMed] [Google Scholar]
- Mira H., Andreu Z., Suh H., Chichung Lie D., Jessberger S., Consiglio A., et al. (2010). Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell 7 78–89. 10.1016/j.stem.2010.04.016 [DOI] [PubMed] [Google Scholar]
- Mizrak D., Levitin H. M., Delgado A. C., Crotet V., Yuan J., Chaker Z., et al. (2019). Single-cell analysis of regional differences in adult V-SVZ neural stem cell lineages. Cell Rep. 26 394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Jiménez E. P., Flor-García M., Terreros-Roncal J., Rábano A., Cafini F., Pallas-Bazarra N. (2019). Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 25 554–560. 10.1038/s41591-019-0375-9 [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B., Decker L., Lachapelle F., Avellana-Adalid V., Bachelin C., Baron-Van Evercooren A. (1999). Progenitor cells of the adult mouse subventricular zone proliferate, migrate and differentiate into oligodendrocytes after demyelination. Eur. J. Neurosci. 11 4357–4366. 10.1046/j.1460-9568.1999.00873.x [DOI] [PubMed] [Google Scholar]
- Nakatomi H., Kuriu T., Okabe S., Yamamoto S., Hatano O., Kawahara N., et al. (2002). Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell 110 429–441. 10.1016/s0092-8674(02)00862-0 [DOI] [PubMed] [Google Scholar]
- Nelson B., Hodge R., Am Daza R., Tripathi P., Arnold S., Millen K., et al. (2020). Intermediate progenitors support migration of neural stem cells into Dentate Gyrus outer neurogenic niches. eLife 9:e53777. 10.7554/elife.53777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obernier K., Cebrian-Silla A., Thomson M., Parraguez J. I., Anderson R., Guinto C., et al. (2018). Adult neurogenesis is sustained by symmetric self-renewal and differentiation. Cell Stem Cell 22 221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogino T., Sawada M., Takase H., Nakai C., Herranz-Pérez V., Cebrián-Silla A., et al. (2016). Characterization of multiciliated ependymal cells that emerge in the neurogenic niche of the aged zebrafish brain. J. Comp. Neurol. 524 2982–2992. 10.1002/cne.24001 [DOI] [PubMed] [Google Scholar]
- Ormerod B. K., Galea L. A. M. (2001). Reproductive status influences cell proliferation and cell survival in the dentate gyrus of adult female meadow voles: a possible regulatory role for estradiol. Neuroscience 102 369–379. 10.1016/s0306-4522(00)00474-7 [DOI] [PubMed] [Google Scholar]
- Ormerod B. K., Lee T. T. Y., Galea L. A. M. (2003). Estradiol initially enhances but subsequently suppresses (via adrenal steroids) granule cell proliferation in the dentate gyrus of adult female rats. J. Neurobiol. 55 247–260. 10.1002/neu.10181 [DOI] [PubMed] [Google Scholar]
- Ortega F., Gascón S., Masserdotti G., Deshpande A., Simon C., Fischer J., et al. (2013). Oligodendrogliogenic and neurogenic adult subependymal zone neural stem cells constitute distinct lineages and exhibit differential responsiveness to Wnt signalling. Nat. Cell Biol. 15 602–613. 10.1038/ncb2736 [DOI] [PubMed] [Google Scholar]
- Otsuki L., Brand A. H. (2018). Cell cycle heterogeneity directs the timing of neural stem cell activation from quiescence. Science 360 99–102. 10.1126/science.aan8795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik J. H., Ding Z., Narurkar R., Ramkissoon S., Muller F., Kamoun W. S., et al. (2009). FoxOs cooperatively regulate diverse pathways governing neural stem cell homeostasis. Cell Stem Cell 5 540–553. 10.1016/j.stem.2009.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. (1974). A restriction point for control of normal animal cell proliferation. Proc. Natl. Acad. Sci. U.S.A. 71 1286–1290. 10.1073/pnas.71.4.1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini E., Mouriec K., Anglade I., Menuet A., Le Page Y., Gueguen M. M., et al. (2007). Identification of aromatase-positive radial glial cells as progenitor cells in the ventricular layer of the forebrain in zebrafish. J. Comp. Neurol. 501 150–167. 10.1002/cne.21222 [DOI] [PubMed] [Google Scholar]
- Pilz G. A., Bottes S., Betizeau M., Jörg D. J., Carta S., Simons B. D., et al. (2018). Live imaging of neurogenesis in the adult mouse hippocampus. Science 359 658–662. 10.1126/science.aao5056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platel J. C., Angelova A., Bugeon S., Wallace J., Ganay T., Chudotvorova I., et al. (2019). Neuronal integration in the adult mouse olfactory bulb is a non-selective addition process. eLife 8:e44830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G., Obernier K., Guinto C., Jose L., Bonfanti L., Alvarez-Buylla A. (2013). Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc. Natl. Acad. Sci. U.S.A. 110 E1045–E1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai K. S., Hattiangady B., Shetty A. K. (2007). Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur. J. Neurosci. 26 1765–1779. 10.1111/j.1460-9568.2007.05820.x [DOI] [PubMed] [Google Scholar]
- Raj B., Wagner D. E., McKenna A., Pandey S., Klein A. M., Shendure J., et al. (2018). Simultaneous single-cell profiling of lineages and cell types in the vertebrate brain. Nat. Biotechnol. 36 442–450. 10.1038/nbt.4103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramírez-Castillejo C., Sánchez-Sánchez F., Andreu-Agulló C., Ferrón S. R., Aroca-Aguilar J. D., Sánchez P., et al. (2006). Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat. Neurosci. 9 331–339. 10.1038/nn1657 [DOI] [PubMed] [Google Scholar]
- Rastegar S., Parimisetty A., Cassam Sulliman N., Narra S. S., Weber S., Rastegar M., et al. (2019). Expression of adiponectin receptors in the brain of adult zebrafish and mouse: links with neurogenic niches and brain repair. J. Comp. Neurol. 527 2317–2333. [DOI] [PubMed] [Google Scholar]
- Reimer M. M., Kuscha V., Wyatt C., Sorensen I., Frank R. E., Knuwer M., et al. (2009). Sonic hedgehog is a polarized signal for motor neuron regeneration in adult Zebrafish. J. Neurosci. 29 15073–15082. 10.1523/jneurosci.4748-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault V. M., Rafalski V. A., Morgan A. A., Salih D. A. M., Brett J. O., Webb A. E., et al. (2009). FoxO3 regulates neural stem cell homeostasis. Cell Stem Cell 5 527–539. 10.1016/j.stem.2009.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccio M., Schmitter D., Knobloch M., Okawa Y., Sage D., Lutolf M. P. (2013). Predicting stem cell fate changes by differential cell cycle progression patterns. Development 140 459–470. 10.1242/dev.086215 [DOI] [PubMed] [Google Scholar]
- Rochefort C., Gheusi G., Vincent J. D., Lledo P. M. (2002). Enriched odor exposure increases the number of newborn neurons in the adult olfactory bulb and improves odor memory. J. Neurosci. 22 2679–2689. 10.1523/jneurosci.22-07-02679.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez F., López J. C., Vargas J. P., Gómez Y., Broglio C., Salas C. (2002). Conservation of spatial memory function in the pallial forebrain of reptiles and ray-finned fishes. J. Neurosci. 22 2894–2903. 10.1523/jneurosci.22-07-02894.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viales R., Diotel N., Ferg M., Armant O., Eich J., Alunni A., et al. (2015). The helix-loop-helix protein Id1 controls stem cell proliferation during regenerative neurogenesis in the adult zebrafish telencephalon. Stem Cells 33 892–903. 10.1002/stem.1883 [DOI] [PubMed] [Google Scholar]
- Rothenaigner I., Krecsmarik M., Hayes J. A., Bahn B., Lepier A., Fortin G., et al. (2011). Clonal analysis by distinct viral vectors identifies bona fide neural stem cells in the adult zebrafish telencephalon and characterizes their division properties and fate. Development 138 1459–1469. 10.1242/dev.058156 [DOI] [PubMed] [Google Scholar]
- Sato Y., Yano H., Shimizu Y., Tanaka H., Ohshima T. (2017). Optic nerve input-dependent regulation of neural stem cell proliferation in the optic tectum of adult zebrafish. Dev. Neurobiol. 77 474–482. 10.1002/dneu.22423 [DOI] [PubMed] [Google Scholar]
- Schäffner I., Minakaki G., Khan M. A., Balta E. A., Schlötzer-Schrehardt U., Schwarz T. J., et al. (2018). FoxO function is essential for maintenance of autophagic flux and neuronal morphogenesis in adult neurogenesis. Neuron 99 1188–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B., García-Verdugo J. M., Collado-Morente L., McEwen B. S., Alvarez-Buylla A. (2004). Cell types, lineage, and architecture of the germinal zone in the adult dentate gyrus. J. Comp. Neurol. 478 359–378. 10.1002/cne.20288 [DOI] [PubMed] [Google Scholar]
- Shah P. T., Stratton J. A., Stykel M. G., Abbasi S., Sharma S., Mayr K. A., et al. (2018). Single-cell transcriptomics and fate mapping of ependymal cells reveals an absence of neural stem cell function. Cell 173 1045–1057. [DOI] [PubMed] [Google Scholar]
- Shin Y. J., Park S. K., Jung Y. J., Kim Y. N., Kim K. S., Park O. K., et al. (2015). Nanobody-targeted E3-ubiquitin ligase complex degrades nuclear proteins. Sci. Rep. 5:14269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons B. D., Clevers H. (2011). Strategies for homeostatic stem cell self-renewal in adult tissues. Cell 145 851–862. 10.1016/j.cell.2011.05.033 [DOI] [PubMed] [Google Scholar]
- Sirko S., Behrendt G., Johansson P. A., Tripathi P., Costa M., Bek S., et al. (2013). Reactive glia in the injured brain acquire stem cell properties in response to sonic hedgehog glia. Cell Stem Cell 12 426–439. 10.1016/j.stem.2013.01.019 [DOI] [PubMed] [Google Scholar]
- Skaggs K., Goldman D., Parent J. M. (2014). Excitotoxic brain injury in adult zebrafish stimulates neurogenesis and long-distance neuronal integration. Glia 62 2061–2079. 10.1002/glia.22726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn J., Orosco L., Guo F., Chung S. H., Bannerman P., Ko E. M., et al. (2015). The subventricular zone continues to generate corpus callosum and rostral migratory stream astroglia in normal adult mice. J. Neurosci. 35 3756–3763. 10.1523/jneurosci.3454-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H., Berg D. A., Bond A. M., Ming G. (2018). Radial glial cells in the adult dentate gyrus: What are they and where do they come from? F1000Res. 7:277. 10.12688/f1000research.12684.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J., Zhong C., Bonaguidi M. A., Sun G. J., Hsu D., Gu Y., et al. (2012). Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 489 150–154. 10.1038/nature11306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassky N., Merkle F. T., Flames N., Tramontin A. D., Garcia-Verdugo J. M., Alvarez-Buylla A. (2005). Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J. Neurosci. 25 10–18. 10.1523/jneurosci.1108-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer S. L., Cappell S. D., Tsai F. C., Overton K. W., Wang C. L., Meyer T. (2013). The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155 369–383. 10.1016/j.cell.2013.08.062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueda R., Imayoshi I., Harima Y., Kageyama R. (2019). High Hes1 expression and resultant Ascl1 suppression regulate quiescent vs. active neural stem cells in the adult mouse brain. Genes Dev. 33 511–523. 10.1101/gad.323196.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh H., Consiglio A., Ray J., Sawai T., D’Amour K. A., Gage F. H. (2007). In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell 1 515–528. 10.1016/j.stem.2007.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanapat P., Hastings N. B., Gould E. (2005). Ovarian steroids influence cell proliferation in the dentate gyrus of the adult female rat in a dose- and time-dependent manner. J. Comp. Neurol. 481 252–265. 10.1002/cne.20385 [DOI] [PubMed] [Google Scholar]
- Tanapat P., Hastings N. B., Reeves A. J., Gould E. (1999). Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J. Neurosci. 19 5792–5801. 10.1523/jneurosci.19-14-05792.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverna E., Huttner W. B. (2019). “The Golgi apparatus in polarized neuroepithelial stem cells and their progeny: canonical and noncanonical features,” in Results and Problems in Cell Differentiation, ed. Kloc M. (Berlin: Springer Verlag; ), 359–375. 10.1007/978-3-030-23173-6_15 [DOI] [PubMed] [Google Scholar]
- Tea J., Alderman S. L., Gilmour K. M. (2019). Social stress increases plasma cortisol and reduces forebrain cell proliferation in subordinate male zebrafish (Danio rerio). J. Exp. Biol. 222:jeb194894. 10.1242/jeb.194894 [DOI] [PubMed] [Google Scholar]
- Than-Trong E., Bally-Cuif L. (2015). Radial glia and neural progenitors in the adult zebrafish central nervous system. Glia 63 1406–1428. 10.1002/glia.22856 [DOI] [PubMed] [Google Scholar]
- Than-Trong E., Kiani B., Dray N., Ortica S., Simons B., Rulands S., et al. (2020). Lineage hierarchies and stochasticity ensure the long-term maintenance of adult neural stem cells. Sci. Adv. 6:eaaz5424. 10.1126/sciadv.aaz5424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than-Trong E., Ortica-Gatti S., Mella S., Nepal C., Alunni A., Bally-Cuif L. (2018). Neural stem cell quiescence and stemness are molecularly distinct outputs of the Notch3 signalling cascade in the vertebrate adult brain. Development 145:dev161034. 10.1242/dev.161034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toda T., Parylak S. L., Linker S. B., Gage F. H. (2019). The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 24 67–87. 10.1038/s41380-018-0036-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topp S., Stigloher C., Komisarczuk A. Z., Adolf B., Becker T. S., Bally-Cuif L. (2008). Fgf signaling in the zebrafish adult brain: association of Fgf activity with ventricular zones but not cell proliferation. J. Comp. Neurol. 510 422–439. 10.1002/cne.21802 [DOI] [PubMed] [Google Scholar]
- Tozer S., Baek C., Fischer E., Goiame R., Morin X. (2017). Differential routing of mindbomb1 via centriolar satellites regulates asymmetric divisions of neural progenitors. Neuron 93 542–551.e4. 10.1016/j.neuron.2016.12.042 [DOI] [PubMed] [Google Scholar]
- Turrero García M., Chang Y., Arai Y., Huttner W. B. (2016). S-phase duration is the main target of cell cycle regulation in neural progenitors of developing ferret neocortex. J. Comp. Neurol. 524 456–470. 10.1002/cne.23801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger M. S., Marschallinger J., Kaindl J., Höfling C., Rossner S., Heneka M. T. (2016). Early changes in hippocampal neurogenesis in transgenic mouse models for Alzheimer’s Disease. Mol. Neurobiol. 53 5796–5806. 10.1007/s12035-016-0018-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbán N., van den Berg D. L. C., Forget A., Andersen J., Demmers J. A. A., Hunt C., et al. (2016). Return to quiescence of mouse neural stem cells by degradation of a proactivation protein. Science 353 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Praag H., Christie B. R., Sejnowski T. J., Gage F. H. (1999). Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc. Natl. Acad. Sci. U.S.A. 96 13427–13431. 10.1073/pnas.96.23.13427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Trotha J. W., Vernier P., Bally-Cuif L. (2014). Emotions and motivated behavior converge on an amygdala-like structure in the zebrafish. Eur. J. Neurosci. 40 3302–3315. 10.1111/ejn.12692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb A., Pollina E., Vierbuchen T., Urbán N., Ucar D., Leeman D., et al. (2013). FOXO3 shares common targets with ASCL1 genome-wide and inhibits ASCL1-dependent neurogenesis. Cell Rep. 4 477–491. 10.1016/j.celrep.2013.06.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. W., Chang Y. T., Yu L., Chen H. I., Jen C. J., Wu S. Y., et al. (2008). Exercise enhances the proliferation of neural stem cells and neurite growth and survival of neuronal progenitor cells in dentate gyrus of middle-aged mice. J. Appl. Physiol. 105 1585–1594. 10.1152/japplphysiol.90775.2008 [DOI] [PubMed] [Google Scholar]
- Xing Y. L., Röth P. T., Stratton J. A. S., Chuang B. H. A., Danne J., Ellis S. L., et al. (2014). Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J. Neurosci. 34 14128–14146. 10.1523/jneurosci.3491-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambusi A., Ninkovic J. (2020). Regeneration of the central nervous system-principles from brain regeneration in adult zebrafish. World J. Stem Cells 12 8–24. 10.4252/wjsc.v12.i1.8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G., Ferg M., Lübke L., Takamiya M., Beil T., Gourain V., et al. (2020). Bone morphogenetic protein signaling regulates Id1-mediated neural stem cell quiescence in the adult zebrafish brain via a phylogenetically conserved enhancer module. Stem Cells. 10.1002/stem.3182 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Zhang R., Boareto M., Engler A., Louvi A., Giachino C., Iber D., et al. (2019). Id4 downstream of Notch2 maintains neural stem cell quiescence in the adult hippocampus. Cell Rep. 28 1485–1498. [DOI] [PubMed] [Google Scholar]
- Zhao M., Li D., Shimazu K., Zhou Y. X., Lu B., Deng C. X. (2007). Fibroblast growth factor receptor-1 is required for long-term potentiation, memory consolidation, and neurogenesis. Biol. Psychiatry 62 381–390. 10.1016/j.biopsych.2006.10.019 [DOI] [PubMed] [Google Scholar]