Abstract
The factor VIII (FVIII)-neutralizing antibody (inhibitor) seen in 25%–30% of patients with severe haemophilia A (SHA). Vaccination is a non-genetic risk factor of inhibitor development as ‘danger signal’ which may provide a pro-inflammatory microenvironment to increase FVIII immunogenicity. We reported a previously treated SHA patient postponed the first vaccination to 15-month age received diphtheria-pertussis-tetanus intramuscularly. At 18-month age, the patient received Hepatitis A intramuscularly and Varicella Zoster Virus subcutaneously with 2 weeks interval and FVIII infusion was given <24 h prior for each. Successive bleedings occurred 1 week later with inefficacy of FVIII replacement. High-titre inhibitor was tested at 117 exposure days. This case suggested that continuous vaccinations in close proximity to FVIII could induce inhibitor. The relationship between vaccination and FVIII immunogenicity still needs to be revealed by further study.
Keywords: severe haemophilia A, previously treated patient, vaccination, inhibitor, FVIII immunogenicity
Introduction
The development of factor VIII (FVIII) neutralizing antibodies, known as inhibitor, is the most serious treatment-related complication for hemophilia A (HA) and is detected in 25%–30% of patients.1 Inhibitor development is a multifactorial process involving genetic and non-genetic risk factors. Non-genetic risk factors include severe bleeding, infections, and vaccinations around the time of FVIII treatment based on the ‘danger theory’.2 However, some evidences showed vaccinations do not increase inhibitor risk.3,4 We reported a severe haemophilia A (SHA) patient who have been treated with FVIII for more than 100 exposure days (EDs) also termed previously treated patient (PTP) developed high-titre inhibitor after concurrent vaccinations.
Case presentation
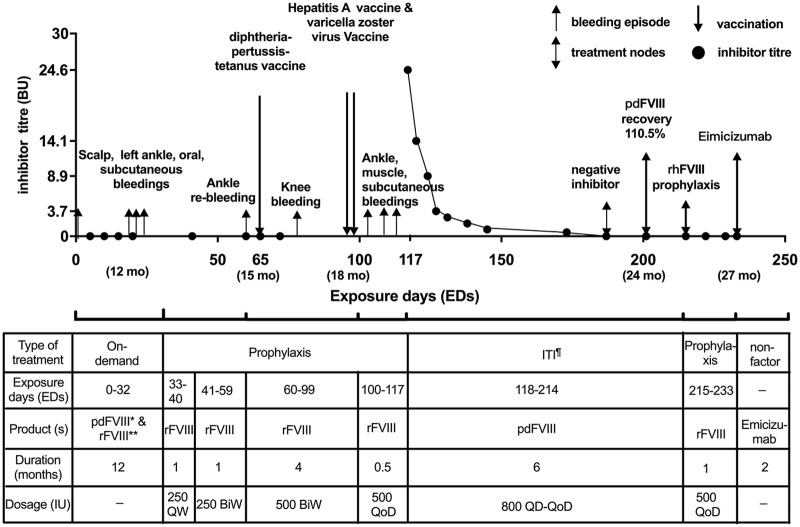
The patient was born on 40th week gestational age, suffered scalp hematoma caused by vacuum during vaginal delivery, then diagnosed with SHA (FVIII activity < 1%). He had a positive family history of F8 gene inversions of intron 22 but without inhibitor history. He received the first FVIII infusion with recombinant FVIII (rFVIII) (Advate® and Kogenate®) and plasma-derived FVIII (pdFVIII) (Greencross®) 200–250 IU/QD for 23 EDs because of the scalp hematoma at birth with negative inhibitor (<0.6 Bethesda Unit (BU) tested every 5 EDs. He then received on-demand treatment prior to 12-month age. Three bleeding episodes happened during this period, rFVIII (Kogenate) 250 IU/QD for 3–4 EDs were given for each. The patient started prophylaxis at 13 month age (34EDs) with rFVIII (Kogenate) 250 IU/QW and 250 IU/BiW for 1 month respectively, then escalated to 500 IU/BiW after a joint bleeding. Inhibitor was negative at 41, 60, and 65 EDs. By then, the patient was no received any vaccination.
At 15-month age, the patient was first vaccinated with inactivated diphtheria-pertussis-tetanus intramuscularly which caused an intramuscular bleeding and recovered by rFVIII replacement. Inhibitor was negative detected 2 weeks later (72 EDs). At 18-month age, the patient received the second vaccination of inactivated Hepatitis A intramuscularly with factor prophylaxis 12h prior (96 EDs) and the third vaccination of live-attenuated Varicella Zoster Virus subcutaneously with 2 weeks interval and received prophylactic factor 20h prior (100 EDs). One week later, three bleedings occurred successively in the next 2 weeks: one left thigh muscle bleeding and one left ankle bleeding were treated with repeatedly rFVIII. One post-venipuncture subcutaneous bleeding was controlled by recombinant factor VIIa [NovoSeven®]. Then positive inhibitor (24.6 BU) was tested at 19.5-month age (117 EDs).
The patient started immune tolerance induction (ITI) 2 weeks after inhibitor detected with pdFVIII (Greencross) 66.7 IU/kg QD to QoD each for 3 months. He achieved negative inhibitor at 4.6 months, and normal FVIII recovery (>66% of expected) at 5.5 months. Then pdFVIII was switched to rFVIII (Advate) ~40 IU/kg/QoD for another month. He finally used non-factor replacement (emicizumab) without inhibitor recurrence (Figure 1).
Figure 1.
Clinical manifestations of the case
*Plasma derived; **recombinant; ¶immune tolerance induction.
Discussion
According to “danger theory,” pathogen and danger-associated molecular patterns have been hypothesized as non-genetic risk factors of inhibitor development.5 Vaccines administered closely with FVIII exposure may provide a source of such danger signals.6 Study in mice implicated anti-FVIII immune response could be amplified by Toll-like receptor.7 Human dendritic cells could be activated by FVIII in a danger signal-dependent manner.8 In contrast, conflicting evidence showed that mice vaccinated in close proximity to FVIII exposure exhibited a decreased incidence of inhibitor attributed to antigenic competition which may divert immune resources away from anti-FVIII immune responses to the site of vaccination.9 In clinical observations, only a single case reported recurrence inhibitor is manifested simultaneously with allergenic vaccines against grass pollen.10 Other evidences showed no apparent associations with inhibitor development and the concomitant FVIII exposure in case control studies.3,11 A retrospective study from the PedNet Registry investigating 375 previously untreated SHA received vaccinations came up with the similar conclusion.4 Recently, vaccination was proposed no association with inhibitor formation.1 But the conscious of vaccination on inhibitor development has not declined for physician. More than 50% of the physicians in Germany recommend a time interval of >24h before vaccination.12
The inhibitor development in the case could be mainly attributed to the continuous vaccinations in close proximity to FVIII. In the study from PedNet Registry, patients received median 4 vaccinations from 1.9 months (the first vaccination) to 21.2 months (the last vaccination).4 While this case received more intensive vaccinations as 3 times from 15 to 18 months with FVIII infusion given <24h prior for each. Although PedNet study found no association between vaccinations and FVIII exposure,4 the stimulation by frequent antigen and adjuvant may provide a pro-inflammatory microenvironment and increase the immnue response to a certain amount of FVIII. In patients with haemophilia, the subcutaneous injections are usually given instead of intramuscular injections to avoid the risk for hematoma formation leading to FVIII exposure and the increasing inhibitor risk.13 Although some studies confirmed a similar vaccine efficacy by given intramuscularly or subcutaneously,14,15 the guideline from US states the deviation from the recommended route might reduce vaccine efficacy.16 The FVIII immunogenicity is also concerned to be influenced by the route of vaccination.6 In mice, intramuscular was found to lead a decreasing FVIII immunogenicity than intravenous.9 However, that study failed to compare the difference between intramuscular and subcutaneous, given the unsuccessful antigen immunization.9 The case we reported was injected intramuscularly for 2 times and subcutaneously for 1 time, while in PedNet Registry study the majority (74.0%) were injected subcutaneously.4 Therefore, this case may induce a stronger FVIII immunogenicity. In addition, immune system in neonate shows limited T-cell responses to vaccination with increased suppressive T-regulatory cells which secret IL-10 and TGF-β and inducing antigen presenting cells tolerogenicity.17 This case received vaccination later than PedNet Registry study,4 leading to a stronger immune response and a higher inhibitor risk. Furthermore, this case lost FVIII tolerance after 100 EDs and received low-dose ITI achieving negative inhibitor with a similar time to high-dose ITI.18 We could speculate that the inherent immunologic mechanism is different in typical inhibitor failed to tolerance at the outset and inhibitor induced by lost tolerance which may easier to tolerance.
Conclusion
This case suggested continuous vaccinations in close proximity to FVIII could induce anti-FVIII inhibitor in SHA patient based on the ‘danger theory’. Additional research on inhibitor risk especially in PTP received vaccinations is required to understand the relationship of vaccination and FVIII immunogenicity.
Footnotes
Author contributions: R.W. clinically treated the patient and wrote the manuscript; Z.L. performed the data collection and reviewed manuscript drafts; Z.C. and G.L. measured Factor VIII inhibitor; X.W., Y.Z., and X.C. was performed blood collection and study execution; and M.-C.P. provided manuscript drafts review.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethics approval: Ethical approval to report this case was obtained from Medical Ethics Committee of Beijing Children’s Hospital, Capital Medical University (2019-59).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Capitals Funds for Health Improvement and Research of China (grant no. CFH 2018-2-2094), Research on the application of clinical characteristics of the Beijing Municipal Science and Technology Commission (code Z181100001718182), the Beijing Natural Science Foundation of China (grant no. 7162059), and the National Science and Technology Key Projects (grant no. 2017ZX09304029004).
Informed consent: Written informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iDs: Zekun Li  https://orcid.org/0000-0002-1421-4590
https://orcid.org/0000-0002-1421-4590
Xiaoling Cheng  https://orcid.org/0000-0002-0048-2658
https://orcid.org/0000-0002-0048-2658
References
- 1. Cormier M, Batty P, Tarrant J, et al. (2020) Advances in knowledge of inhibitor formation in severe haemophilia A. British Journal of Haematology 189(1): 39–53. [DOI] [PubMed] [Google Scholar]
- 2. Schep SJ, Boes M, Schutgens REG, et al. (2019) An update on the ‘danger theory’ in inhibitor development in hemophilia A. Expert Review of Hematology 12: 335–344. [DOI] [PubMed] [Google Scholar]
- 3. Santagostino E, Mancuso ME, Rocino A, et al. (2005) Environmental risk factors for inhibitor development in children with haemophilia A: A case-control study. British Journal of Haematology 130: 422–427. [DOI] [PubMed] [Google Scholar]
- 4. Platokouki H, Fischer K, Gouw SC, et al. (2018) Vaccinations are not associated with inhibitor development in boys with severe haemophilia A. Haemophilia; 24: 283–290. [DOI] [PubMed] [Google Scholar]
- 5. Delignat S, Rayes J, Russick J, et al. (2018) Inhibitor formation in congenital hemophilia A: An immunological perspective. Seminars in Thrombosis and Hemostasis 44: 517–530. [DOI] [PubMed] [Google Scholar]
- 6. Astermark J, Altisent C, Batorova A, et al. (2010) Non-genetic risk factors and the development of inhibitors in haemophilia: A comprehensive review and consensus report. Haemophilia 16: 747–766. [DOI] [PubMed] [Google Scholar]
- 7. Steinitz KN, van Helden PM, Binder B, et al. (2012) CD4+ T-cell epitopes associated with antibody responses after intravenously and subcutaneously applied human FVIII in humanized hemophilic E17 HLA-DRB1*1501 mice. Blood 119: 4073–4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miller L, Weissmuller S, Ringler E, et al. (2015) Danger signal-dependent activation of human dendritic cells by plasma-derived factor VIII products. Thrombosis and Haemostasis 114: 268–276. [DOI] [PubMed] [Google Scholar]
- 9. Lai JD, Moorehead PC, Sponagle K, et al. (2016) Concurrent influenza vaccination reduces anti-FVIII antibody responses in murine hemophilia A. Blood 127: 3439–3449. [DOI] [PubMed] [Google Scholar]
- 10. Bermejo N, Martin Aguilera C, Carnicero F, et al. (2012) Allergenic vaccines administration and inhibitor development in haemophilia. Haemophilia 18: e392–e393. [DOI] [PubMed] [Google Scholar]
- 11. Maclean PS, Richards M, Williams M, et al. 2011. Treatment related factors and inhibitor development in children with severe haemophilia A. Haemophilia 17: 282–287. [DOI] [PubMed] [Google Scholar]
- 12. Pfrepper C, Krause M, Sigl-Kraetzig M, et al. 2019. Vaccination in patients with haemophilia: Results from an online survey among haemophilia treatment centres in Germany. Haemophilia 25: e304–e306. [DOI] [PubMed] [Google Scholar]
- 13. Makris M, Conlon CP, Watson HG. (2003) Immunization of patients with bleeding disorders. Haemophilia 9: 541–546. [DOI] [PubMed] [Google Scholar]
- 14. Knuf M, Zepp F, Meyer CU, et al. (2010) Safety, immunogenicity and immediate pain of intramuscular versus subcutaneous administration of a measles-mumps-rubella-varicella vaccine to children aged 11-21 months. European Journal of Pediatrics 169: 925–933. [DOI] [PubMed] [Google Scholar]
- 15. Carpenter SL, Soucie JM, Presley RJ, et al. (2015) Hepatitis B vaccination is effective by subcutaneous route in children with bleeding disorders: A universal data collection database analysis. Haemophilia 21: e39–e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. National Center for Immunizatio and Respiratory Diseases (2011) General recommendations on immunization: Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommendations and Reports 60: 1–64. [PubMed] [Google Scholar]
- 17. Saso A, Kampmann B. (2017) Vaccine responses in newborns. Seminars Immunopathology 39: 627–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hay CR, DiMichele DM. and International Immune Tolerance S. (2012) The principal results of the International Immune Tolerance Study: A randomized dose comparison. Blood 119: 1335–1344. [DOI] [PubMed] [Google Scholar]