Abstract
Cancer patients have a 2 times higher prevalence of insomnia than healthy populations and cancer-related insomnia has received minimal attention while insomnia can aggravate the rehabilitation of cancer patients. Cheonwangbosimdan is a Korean herbal medicine generally used to relieve sleep deprivation, however, few studies presented the effects of Cheonwangbosimdan on cancer-related insomnia. The purpose of study is to examine the feasibility of Cheonwangbosimdan treatments for cancer patients. Twenty-two participants were allocated into a Cheonwangbosimdan or cognitive-behavioral therapy for insomnia (CBT-I) control group by equal number. The intervention group took Cheonwangbosimdan liquid once in a day and attend visits once a week for 4 weeks. The CBT-I group underwent individualized behavioral therapy 4 times in 4 weeks. The primary outcome is changes in the Insomnia Severity Index (ISI) from baseline to the end of the trial. Responses to the Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale (ESS), Zung Self-Rating Anxiety Scale (SAS), Brief Fatigue Inventory (BFI), Euroqol-5 Dimensions-5 Levels (EQ-5D-5L), and Eastern Cooperative Oncology Group Performance Status (ECOG-PS) were secondary outcomes used to evaluate the quality of sleep. Outcomes were measured at a follow-up visit (visit 5) in the fifth week of the trial. There is no difference between 2 groups, but both groups showed tendency to alleviate cancer insomnia symptoms. SAS-K showed significant difference between the 2 groups (P < .001), as treatment group score was highly lowered than control group score. The study can contribute to more attentive care for insomnia in cancer patients.
Keywords: insomnia, cancer-related insomnia, Korean medicine, Cheonwangbosim liquid, cognitive-behavioral therapy, comparative effectiveness
Introduction
Insomnia is generally defined as having difficulties to sleep although the patient has enough opportunity.1 Sleep disturbance and insomnia are concomitantly used terms in insomnia researches,2 but sleep disturbance encompasses a wider range of sleep disorders, including sleep maintenance, and dysfunctions related to sleep.3
On an epidemiologic view, insomnia has 4 definitions: insomnia symptoms, symptoms consequent with daytime function, dissatisfaction of sleep quality, and insomnia diagnosis.4 Insomnia symptoms are mostly selected criteria used to measure research subjects’ sleep status.4 The Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV), and the International Classification of Sleep Disorders (ISCD) define the criteria of insomnia.5 A sleep disturbance that occurs more than 3 times weekly for 1 month and related to daytime impairment can be regarded as insomnia diagnosis. About one third of the general population has insomnia symptoms, which is the most common sleep disturbance in people.4 Insomnia exacerbates physical conditions and psychological health, including cardiovascular6 and mental disorders,7 and diabetes,8 leading to extreme reductions in the quality of life (QOL).9
Global studies have shown that the prevalence of insomnia was 10% to 60%10 and sleep disturbance tends to be continued. Chronic insomnia, sustained at least for 1 month, occurred in 31% to 75% of insomnia patients, with more than two thirds of patients suffering from longer than 1 year.11,12 In South Korea, 17% of the population had sleep deprivation more than 3 times a week. In 2012, the total direct treatment costs for sleeping difficulties had increased to 1.81 times the costs in 2008.13
Cancer-related insomnia has a significantly higher prevalence than insomnia in the general population,14 ranging from 17% to 70%.15,16 In an 18-month cohort study in the United States, cancer patients’ recurrence rates of insomnia were higher than those of noncancer patients. Furthermore, 20% of cancer patients who had a history of insomnia recurrences have suffered from frequent sleep deprivation relapses.14
Insomnia in cancer is hard to categorize into one type. Cancer-related insomnia is composed of primary disorders, secondary insomnia comorbid with other diseases, and insomnia associated with cancer.17,18 Sleeping difficulties in cancer patients occur before and after cancer therapy, and there are many factors involved. The etiology of insomnia in patients with cancer is complex and involves factors ranging from demographic characteristics to precipitating and perpetuating factors of sleep deprivation.19-21 Insomnia, fatigue, pain, and depression, also called the symptom cluster of cancer patients, occur with concurrent multiple symptoms.22,23 However, sleep problems are rarely addressed during cancer treatment.24 In cancer care programs, insomnia is not included, and cancer patients do not typically receive psychological treatments.25,26 Moreover, patients are unaware of their sleep deprivation and regard it as a transient symptom due to the cancer treatment steps.16,25 Insomnia in patients with cancer lowers survival rate,27 impairs daytime function, and aggravates the symptoms that result from cancer treatments, such as chronic fatigue and depression.28,29
Cognitive-behavioral therapy for insomnia (CBT-I) is the first recommendation for cancer patients with insomnia to help relieve sleep deprivation,30 and it is utilized when patients suffer from recurrent insomnia.31 Compared with the pharmacotherapy, adherence rates of CBT-I are reduced and dropout rates were increased according to the systematic reviews of CBT-I.30,32 Long-term adherence to CBT-I is difficult.33 Pharmacotherapy is not the first recommended guideline to treat insomnia, but it is the most easily accessible treatment for cancer patients with insomnia.34 Despite their generalizability, hypnotic drugs are only recommended for acute insomnia that lasts less than 4 weeks.35 In addition, cancer patients are reluctant to use additional hypnotic drugs and antipsychotics because they have already taken chemotherapeutic drugs and received high doses of radiation therapies.36 Complementary and alternative medicine has gradually been utilized to care for cancer-related insomnia, using herbal medicine treatments.
Cheonwangbosimdan, first recorded in Effective Formulae Handed Down for Generations (世醫得效方; Shi Yi De Xiao Fang) in 1337,37,38 is a herbal medicine that has been continuously utilized in Chinese medicine and Korean medicine (KM). It alleviates insomnia, anxiety, and palpitation by furnishing energy and stabilizing patients’ mind.39 Cheonwangbosimdan can mitigate primary insomnia and secondary insomnia in patients with hyperthyroidism and menopause without severe side effects.40-42 However, there is no previous study that has examined the effectiveness of Cheonwangbosimdan on cancer patients. This is the first study to evaluate the feasibility of using Cheonwangbosimdan to treat cancer-related insomnia through a comparison with CBT-I.
Methods
Study Design
This study was a randomized, parallel-group, controlled pilot trial. After screening, 22 eligible subjects who finished cancer therapies were randomly allocated to the Cheonwangbosimdan group or CBT-I control group in a 1:1 ratio. Although this was an open-label trial due to the nature of the treatment, which the subjects could not be blind to, the assessor and clinical investigators independently performed the study. The study flowchart is listed in Figure 1, and timeline of participants is presented in Figure 2. This study was approved by the Institutional Review Board of Kyung Hee University Hospital at Gangdong (Approval Number KHNMC OH 2017-04-002-001), and each participant submitted an informed consent.
Figure 1.
Study flow chart.
Figure 2.
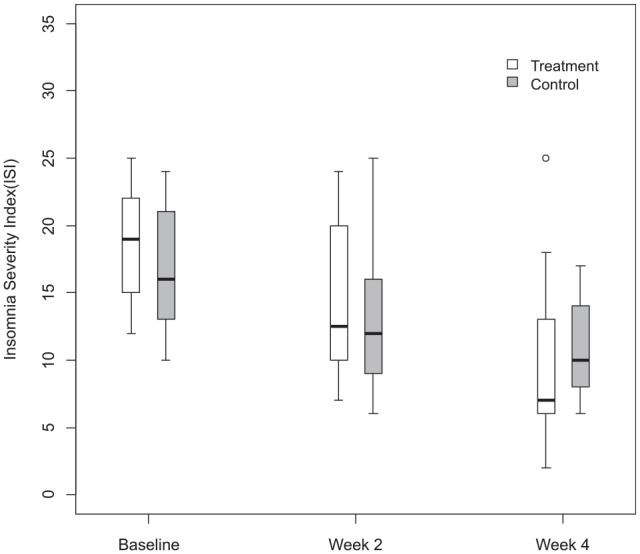
Insomnia Severity Score difference.
Inclusion and Exclusion Criteria
Cancer patients who visited the institution with insomnia and who met the following criteria were included:
Patients who were older than 19 years (either sex)
Patients who have been diagnosed with a malignant tumor (any type of cancer or tumor nodes metastasis [TNM] stage)
Patients who have undergone direct cancer treatment within a month to 5 years
Eastern Cooperative Oncology Group Performance Status (ECOG–PS) score was 2 or below
Patients whose Insomnia Severity Index (ISI) score is at least 8
Patients who have suffered sleep deprivation for more than a month
Patients who met any of the following criteria were excluded:
Patients who had insomnia induced by any type of pain, nausea or vomiting, ascites, pleural effusion, or hydrothorax
Patients who had a history of restless legs syndrome
Patients who had sleep apnea
Shift workers
Patients who had substance use disorders diagnosed by the DSM-5
Patients who had any type of psychological disorder
Patients who have experienced any type of seizure
Patients who have taken drugs or health supplements related to insomnia within a week
Patients who have undergone CBT-I within a month
Aspartate aminotransferase (AST) or alanine aminotransferase (ALT) scores were greater than or equal to 2 times the upper normal limit (UNL)
Patients whose creatinine levels were greater than 1.5 times the upper or lower limits of the normal range during screening
Patients pregnant at the time of the screening, breastfeeding women, or subjects who plan to be pregnant
Patients who did not provide informed consent
Patients determined by physicians to be unlikely to undergo treatments, outpatient assessments, or answer the surveys required for the protocol
Randomization and Allocation Concealment
A statistician not involved in the trial assessment allocated subjects to an intervention or control group using block randomization. Sealed envelopes contained allocation information in a sequence. Participants who had passed the screening test opened the envelops in order, and they were allocated to a group based on the information in the envelope. The researchers who created the blocks and envelopes were blind to the intervention. The randomization information was not opened without specific reasons. Patients in this trial were nonblinded, as the interventions for the 2 groups are innately different. Pharmacists, clinical investigators, and assessors were blind to each other’s tasks to minimize biases that may exaggerate the effect sizes of the interventions and increase the dropout rate of the study.
Interventions
Subjects who had undergone the screening test received the sleep hygiene guidelines and followed the instructions. Korean version of the “Top Sleep Hygiene Tips” produced by the American Sleep Association was utilized for sleep hygiene guidelines.43 Each participant received individual sleep hygiene guidance focused on the patient’s insomnia severity.
Cheonwangbosimdan is a KM herbal formula effective at relieving anxiety, sleep deprivation, and palpitations, and it is approved by the Korea Ministry of Food and Drug and manufactured by the Jung Woo Pharmaceutical Co, Ltd. We selected liquid-type drug for treatment to enhance the acceptance rate of oral administration and convenience of patients. Detailed components are listed in Table 1. Subjects received Cheonwangbosimdan liquid at each visit for 4 weeks. Patients took the liquid once a day between meals. This group underwent a 4-week treatment protocol, written in a sleep diary, and maintained sleep hygiene guidelines. On the final visit (visit 5), the assessor evaluated the participants’ final results.
Table 1.
Cheonwangbosimdan Liquid Composition (20 mL).
| Chinese name | Scientific name | Content (mg) |
|---|---|---|
| Danggui | Angelica gigas root | 125 |
| Tianmendong | Asparagus tuber | 125 |
| Huanglian | Coptis rhizome | 250 |
| Renshen | Ginseng | 62.5 |
| Fuling | Hoelen | 62.5 |
| Jiegeng | Platycodon root | 62.5 |
| Yuanzhi | Polygala root | 62.5 |
| Shengdihuang | Rehmannia root | 500 |
| Danshen | Salvia miltiorrhiza root | 62.5 |
| Wuweizi | Schisandra fruit | 125 |
| Xuanshen | Scrophularia root | 62.5 |
| Baiziren | Thujae semen | 125 |
| Suanzaoren | Ziziphus seed | 125 |
Participants underwent CBT-I once per week for 4 weeks with a therapist who had over 5 years of clinical experience. Each participant took personalized therapy based on his/her insomnia conditions using the following 5 interventions31:
Sleep hygiene: advice on general items including environmental, mental, and behavioral factors that induce qualified sleep
Relaxation: restricted awareness and relaxing therapies that can reduce muscle tension (meditation, breathing, gradual muscle relaxation, guided imagery)
Sleep restrictions: limiting the sleep onset
Stimulus control: strengthening the correlation between sleep and bedding, keeping the patients from engaging in activities irrelevant to sleeping while in bed
Cognitive therapies: examining the attitudes toward sleep and rectifying negative feelings
Prohibited Drugs
Psycholeptics (ATC Code N05) and psychotonic agents (ATC Code N06) were prohibited during the clinical trial.
Outcomes
Primary Clinical Outcomes
The primary clinical outcome was the ISI, a self-reported questionnaire that evaluated the features, severity, and effects of insomnia on QOL, composed of 7 questions.31 The Korean version of the ISI was developed in 2014.44 The ISI is a validated method of assessing primary sleep deprivation and insomnia in cancer patients. An ISI score of 8 was the criteria to diagnose a sleep deficiency with adequate sensitivity and specificity. The recall period of the ISI was 2 weeks, and participants completed the ISI once per week during the 4-week interventions.
Secondary Clinical Outcomes
1. Insomnia survey questionnaire outcomes (PSQI, ESS, SAS, BFI, EQ-5D-5L)
a. Pittsburgh Sleep Quality Index (PSQI): The PSQI is a self-reported survey composed of 19 questions in 7 sections that includes sleep latency, subjective sleep quality, sleep consistency, habit of sleep efficiency, sleep disturbances, use of drugs, and daytime dysfunction. The Korean version of the PSQI was created in 2012.45 Given the 4 weeks of recall for this assessment, the PSQI was to be completed at visits 1 and 5.
b. Epworth Sleepiness Scale (ESS): The ESS is a self-reported questionnaire that assesses the probability of falling asleep during specific daily life situations. The Korean version was designed in 2011. This test measures the disability of daily life caused by sleep deprivation. The ESS was completed at visits 1 and 5 during the clinical trial.46
c. Zung Self-Rating Anxiety Scale (SAS): SAS is a self-reported questionnaire that measures anxiety levels and is composed of 20 questions. Various anxiety symptoms can be measured from emotional and psychological perspectives. The Korean version was developed in 1996.47,48 Patients completed the SAS during visits 1 and 5 during the trial period.
d. Brief Fatigue Inventory (BFI): Sleep disorders and fatigue are intimately correlated with each other. The BFI was developed to measure the severity and influence of fatigue associated with tumors and consists of 9 items. The Korean version was developed in 2005.49,50 The BFI was completed during visits 1 and 5 of the trial period.
e. Euroqol-5 Dimensions-5 Levels (EQ-5D-5L): EQ-5D-5L is a survey that assesses the status of 5 topics (movability, self-care, daily practices, pain/discomfort, and anxiety/depression). Participants answered questions in 5 levels and evaluated the utility weight of health-related quality of life (HRQoL). The validated Korean version of the EQ-5D-5L51 was completed during visits 1 and 5 during the trial.
2. Eastern Cooperative Oncology Group Performance Status score (ECOG-PS): ECOG-PS is a scoring system that measures the effects of daily practices on cancer patients’ diseases.52 To minimize confounding factors, patients confined to bed were excluded from the trial. The ECOG-PS score was assessed once during the screening phase.
3. Sleep patterns tested via Fitbit One (activity tracker device): Fitbit One is a wearable device for the general population, and it can be used to track physical activity, sleep, and energy consumption. This device was used to gather observational data on patients’ sleep durations. Although polysomnography is primarily recommended to assess sleep, this method is inappropriate for assessing sleep durations in daily life. Although actigraphy has been utilized in clinics, the Fitbit One has a higher accuracy in measuring sleep duration than actigraphy, and it is relatively inexpensive compared with polysomnography.53 The following data collected from the Fitbit One were utilized to assess the quality of sleep and the sleep diary adherence rate, and used as an alternative source of data missing from the sleep diary:
a. Sleep and wake times: Comparisons with the sleep diary to assess the compliance rate
b. Sleep onset: Hours of sleep
c. Frequency of awakening: Number of awakenings during sleep per day
d. Actual sleeping time: Daily actual sleeping times of each participant were recorded and used to primarily judge the quality of sleep
e. Sleep diary and drug administration diaries: To evaluate the quality of sleep and compare the outcomes from identical factors in the Fitbit One data, the sleeping time, awakening time, sleep onset, reason for sleep deprivation, and feelings of fatigue when waking up were recorded in sleep diaries. The Korean translated versions of the morning records from the Sleep Diary version 6.0 created by the National Sleep Foundation were used. Drug administration diaries were compared with Fitbit One data.
Safety Assessment Outcomes
Blood tests and the vital signs of patients were collected to observe the safety of the interventions and select eligible patients for clinical trials. The adherence rates for the Cheonwangbosimdan liquid and CBT-I groups were assessed by descriptive statistics.
Sample Size
The primary outcome of study, ISI, is a continuous outcome used to calculate the required sample size. To confirm clinical differences between groups whose levels were higher than moderate, the mean difference was set to a score of 7 based on prior studies. The noninferiority stage was 50% of the required clinical difference score (ISI score of 3.5). Due to the lack of primary experimental studies assessing Cheonwangbosimdan and cancer patients with insomnia, the standard deviation was set to 3.18, following results of a clinical trial for cancer patients with insomnia that used different interventions from the current study and a study of Cheonwangbosimdan on primary insomnia patients.54,55
Following the equation below, when considering 1-sided tests, 80% power, a 5% significance level, and a 20% dropout rate, 22 participants were required and identically divided into 2 groups:
Statistical Analysis
Hypothesis
Null hypothesis (H0): There is no difference between the effects of Cheonwangbosimdan liquid and CBT-I.
Alternative hypothesis (H1): The effects of Cheonwang-bosimdan liquid are superior to those of CBT-I.
Analysis Group
The data from all participants were analyzed with a full analysis set using an intention-to-treat method, except for participants who met the following criteria:
Subjects who violated the inclusion/exclusion criteria
Intervention group: subjects who did not take the drug
Control group: subjects who did not undergo CBT-I
Subjects who did not provide any clinical data after screening
General Rules of Data Analysis
All statistical analyses in the trial were 2-sided sets, and P value was 5%. The continuous data from subjects were depicted by descriptive statistics, such as means and 95% confidence intervals (CIs), and categorical data were described using percentages and frequencies. If the outcomes did not satisfy the normal distribution requirements, Mann-Whitney tests were used. To verify the effectiveness of the intervention and changes in means for each group after the 4-week intervention, linear mixed models for repeated measures (MMRM) were utilized. SAS Version 9.4 (SAS Institute Inc) was used to analyze the data.
Adverse Events (AEs)
The clinical investigator assessed AEs for patients at each visit during the study period. Clinical adverse effects were judged based on the Common Terminology Criteria for Adverse Events version 4.03 (CTCAE V4.03) by the National Cancer Institute. The severity of the assessments was categorized into 5 grades.
AE Evaluations and Information
The clinical investigator assessed AEs during the drug utilization period at each visit. Reported AEs and their relationship with interventions were documented in an “Adverse Events Record Card.” The clinical investigator also educated participants on how to self-report AEs and related information. The researcher identified AEs through medical examinations at every visit. When an AE was revealed, the director recorded details of the appearance date; disappearance date; severity; results; actions related to side effects; association with drugs, suspected drugs, or interventions except the interventions in the trial; and whether the treatment for AE was taken or not. Clinical AEs were classified based on the CTCAE V4.03.
Data Handling
Access to Data
Following the clinical trial phases, only authorized personnel was able to access the data through a pre-described access process. Any access was reported. Security systems were to be maintained to deny access to data from unauthorized personnel.
Data Protection and Preservation
Research data and records were separately protected in a secure depository. For the final report, the storage manager kept the research documents for 10 years from the end date of the study. After the trial had finished, the case report form and the original versions of the data collected in the trial were submitted to the research sponsor (Korea Institute of Oriental Medicine) within 1 month and stored separately, per institutional review board regulations.
Results
Twenty-four participants were screened, and 22 subjects were randomized by 1:1 ratio. One participant in the intervention group ceased the trial after visit 2 as the patient waived the informed consent. Another in control group refused to follow the staff’s instruction and stopped participating in the trial. Based on full analysis set on intention-to-treat analysis, 20 patients divided into 10 subjects in each group were analyzed. On the baseline, there were no difference between the 2 groups, except for the education level.
Demographic features of the 2 groups are described in Table 2.
Table 2.
Demographic Baseline Feature of Patients.
| Characteristics | Treatment group | Control group | P |
|---|---|---|---|
| Gender (male/female) | 7 (63.6%)/4 (36.4%) | 4 (36.4%)/7 (63.6%) | .2008 |
| Age (year) | 63.0 [53.0-71.0] | 63.0 [54.0-67.0] | .8965 |
| BMI | .9999 | ||
| Underweight | 1 (9.1%) | 0 (0.0%) | |
| Normal | 5 (45.5%) | 5 (45.5%) | |
| Overweight | 3 (27.3%) | 4 (36.4%) | |
| Obese | 2 (18.2%) | 2 (18.2%) | |
| Education level | .0460* | ||
| Middle school | 3 (27.3%) | 1 (9.1%) | |
| High school | 2 (18.2%) | 8 (72.7%) | |
| University | 6 (54.5%) | 2 (18.2%) | |
| Primary cancer type | .4481 | ||
| Lung | 3 (27.3%) | 1 (9.1%) | |
| Breast | 0 (0.0%) | 3 (27.3%) | |
| Liver | 1 (9.1%) | 1 (9.1%) | |
| Gastric | 1 (9.1%) | 2 (18.2%) | |
| Esophagus | 2 (18.2%) | 0 (0.0%) | |
| Prostate | 1 (9.1%) | 1 (9.1%) | |
| Others | 3 (27.3%) | 3 (27.3%) | |
| Working status (yes/no)† | 6 (54.5%)/5(45.5%) | 3 (27.3%)/8 (72.7%) | .3870 |
| Insomnia period | 14.0 [6.0-28.0] | 27.0 [4.0-36.0] | .5602 |
| ECOG-PS (0/1) | 7 (63.6%)/4 (36.4%) | 7 (63.6%)/4 (36.4%) | .9999 |
| TNM stage | .3579 | ||
| I | 5 (45.5%) | 1 (9.1%) | |
| II | 4 (36.4%) | 6 (54.5%) | |
| III | 1 (9.1%) | 1 (9.1%) | |
| IV | 1 (9.1%) | 2 (18.2%) | |
| NA | 0 (0.0%) | 1 (9.1%) | |
| Usage of hypnotics (yes/no) | 2 (18.2%)/9 (81.8%) | 3 (27.3%)/8 (72.7%) | .9999 |
| ISI score | 18.0 [15.0-22.0] | 18.0 [14.0-22.0] | .6034 |
Abbreviations: BMI, body mass index; ECOG-PS, Eastern Cooperative Oncology Group–Performance Status score; TNM, tumor nodes metastasis; ISI, Insomnia Severity Index.
P < .05.
Wilcoxon rank sum test to analyze with non-parametric statistics.
The primary outcome is the difference of ISI between baseline and after the treatment. Although there is no significant difference of ISI between the 2 groups, both 2 interventions decreased the ISI score and the mean difference of treatment group is higher than that of the control group. Other variables including PSQI, ESS, and BFI were not significantly different between week 2 and week 4 within the group.
There is a significant difference between the treatment group and control group of SAS-K, with no difference in the baseline score (Table 3). Other variables, including ISI, were not statistically different with the control group. Fitbit One data were not included in the result due to insufficient patient sleep data.
Table 3.
Outcome Difference After Treatment.
| Outcome | Treatment group (N = 11) | Control group (N = 11) | P a |
|---|---|---|---|
| ISI | |||
| Baseline | 18.0 [15.0 to 22.0] | 16.0 [13.0 to 21.0] | |
| Week 2 | 12.5 [10.0 to 20.0] | 12.0 [9.0 to 16.0] | |
| Difference | −4.0 [−7.0 to −2.0] | −3.0 [−6.0 to 0.0] | .5507 |
| Pb | .0137 | .0430 | |
| Week 4 | 7.0 [6.0 to 13.0] | 10.0 [8.0 to 14.0] | |
| Difference | −8.5 [−12.0 to −5.0] | −6.5 [−10.0 to −3.0] | .2266 |
| Pb | .0059* | .0059* | |
| PSQI | |||
| Baseline | 15.0 [10.0 to 17.0] | 13.0 [12.0 to 17.0] | |
| Week 4 | 7.0 [4.0 to 12.0] | 10.5 [8.0 to 13.0] | |
| Difference | −7.5 [−9.0 to −5.0] | −3.5 [−5.0 to −2.0] | .0782 |
| Pb | .0059* | .0039* | |
| ESS | |||
| Baseline | 10.0 [5.0 to 16.0] | 6.0 [3.0 to 13.0] | |
| Week 4 | 5.0 [3.0 to 7.0] | 4.0 [3.0 to 5.0] | |
| Difference | −4.0 [−9.0 to 1.0] | −1.0 [−3.0 to 4.0] | .4578 |
| Pb | .0879 | .7266 | |
| SAS | |||
| Baseline | 38.0 [36.0 to 47.0] | 38.0 [32.0 to 44.0] | |
| Week 4 | 33.0 [29.0 to 38.0] | 39.0 [34.0 to 45.0] | |
| Difference | −7.5 [−9.0 to −4.0] | 2.0 [−2.0 to 5.0] | .0069* |
| Pb | .0059* | .2070 | |
| BFI | |||
| Baseline | 4.8 [3.0 to, 5.1] | 3.8 [3.3 to 4.9] | |
| Week 4 | 2.1 [1.6 to 3.6] | 2.5 [2.0 to 4.1] | |
| Difference | −1.5 [−2.3 to −1.2] | −0.8 [−1.3 to −0.1] | .0796 |
| Pb | .0020* | .0840 | |
| EQ-5D | |||
| Baseline | 0.86 [0.83 to 0.88] | 0.83 [0.80 to 0.87] | |
| Week 4 | 0.87 [0.83 to 1.00] | 0.85 [0.80 to 0.87] | |
| Difference | 0.00 [−0.03 to 0.05] | 0.00 [−0.13 to 0.01] | .3893 |
| Pb | .6719 | .6875 | |
Abbreviations: ISI, Insomnia Severity Index; PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth Sleepiness Scale; SAS, Zung Self-Rating Anxiety Scale; BFI, Brief Fatigue Inventory; EQ-5D, Euroqol-5 Dimensions.
Wilcoxon rank sum test.
Wilcoxon signed rank test Median [Q1-Q3].
P < .05.
Discussion
The study is conducted to explore the feasibility of Cheonwangbosimdan in cancer patients with insomnia, known as traditional Korean herbal medicine effective for allaying insomnia. Cancer patients with insomnia were cared with Cheonwangbosimdan or CBT-I, primarily recommended treatment in insomnia patients.
Both groups presented reducing insomnia symptoms and enhancing the quality of cancer patients’ life with significant difference between before and after treatment, although the result did not present the considerable difference of ISI and other insomnia outcomes between the 2 groups.
Cheonwangbosimdan consists of herbs such as Ziziphus seed, Thujae Semen, and Polygala root that mitigate anxiety symptoms and significantly lowered the anxiety score of insomnia patients in this trial. Components of Cheonwangbosimdan act for heart and anxiety symptoms are related to heart function deficiency56 in traditional KM and Chinese medicine. Taiwan nationwide cohorts analyzed that Cheonwangbosimdan has been widely used for heart disease including ischemic heart diseases and chronic heart diseases.57,58
Prevalence of insomnia in cancer is somewhat different according to the type of cancer. Most studies observed that breast cancer patients showed the highest prevalence of insomnia, with 38% to 50% of prevalence.59,60 Prevalence of insomnia in lung (37%), gastrointestinal (32%), and gynecological (29% to 39%) cancer patients followed in sequence.61 In Korea, a national database retrospective study found that lung cancer patients mostly suffered from insomnia (15.2%), non-Hodgkin lymphoma and bladder cancer followed, contrast to prior researches.62 Although the prevalence of each cancer type is different and taking diverse measurement and subjects into consideration, the prevalence of insomnia in cancer patients is 3 times greater than the rate in the general population.
Despite the high prevalence, cancer-related insomnia is an overlooked disorder unlike psychological and physiological problems, as insomnia is regarded as a trivial side effect from both doctors and patients themselves. In the cancer patients’ point of view, insomnia is a less important symptom to cancer itself.
In addition, insomnia patients who are comorbid with anxiety or depression assume that therapy for mental disorder is enough to vanish insomnia symptoms. Deficient experiences of oncology staffs dealing with insomnia also cause the neglect of insomnia symptoms.59,63 Consequently, insomnia patients are reluctant to seek expert consultations.59,64
Treating insomnia in cancer patients is important to precipitate their recovery. Sustained insomnia increases the risk of chronic diseases, including cancer incidence and cancer-related death, by augmenting inflammatory responses.65-67 Sleep arousal activates NF-κB to release inflammatory and pro-inflammatory cytokines, which stimulates the onset of depression and fatigue.20 The effects of insomnia on cancer risk is parallel to that of other chronic diseases. An increased risk of stroke was observed in both the primary insomnia cohort study68 and European cancer cohort study with diabetes and myocardial infarction.69
Association of certain cancer types and insomnia has been partly researched. In breast cancer, pre- and posttreatment sequences can affect the worsening of insomnia symptoms.70 A Japanese cohort explained that hyperarousal augmented risk of prostate cancer.71 Postmenopausal women cohort also presented that extremely short- or long-time sleep were associated with colorectal cancer.72 Insomnia is one of the most common side effects of cancer treatment, with cancer-related fatigue.16,49,73 Cancer-related fatigue consequently occurs after sleep disturbance, and these 2 factors are reciprocally affecting each other, as measurements of insomnia and fatigue are overlapped.74,75
Insomnia presentations in cancer patients consists of primary insomnia, insomnia comorbid with pain or depression, and insomnia associated with cancer therapies and symptoms.76 For cancer patients, the diagnosis of cancer can induce arousal due to anxiety, and the pain of chemotherapy stimulates wakefulness.77
These intermingled factors for sleep disturbance in cancer patients require persistent and multidimensional sleep therapies. Hypnotic therapies can be hardly applied to chronic insomnia, with increasing dependence and tolerance over time requiring maintenance treatment78 and longitudinally decreasing effectiveness.79,80
Nonpharmacological studies such as sleep hygiene advices are also common.81 CBT-I is more effective than medications,82 and it is the first recommended treatment for insomnia according to national guidelines.83 However, limited accessibility to experts and the relatively high costs of face-to-face–delivered CBT-I make patients indecisive to care with CBT-I.84 Alternatively, nurse-led and internet CBT can be utilized with easy access to patients and higher adherence rate.85,86
Insomnia Severity Index is an appropriate outcome to observe the insomnia status with PSQI for evaluating the effectiveness of CBT-I.18,31 However, ISI is a more preferred scale for insomnia patients as PSQI can partially illustrate the longitudinal data because the survey only covers a 1-month period sleeping status, and ISI showed higher reliability with longitudinal data than PSQI.87,88 Consequently, we decided ISI to be the primary outcome and PSQI an additional scale to examine cancer patients’ sleep quality and difference in the time.
Complementary and alternative medicine can be utilized in insomnia with cancer. Movement therapies such as Yoga, reflexology, and Tai-Chi have been utilized and showed significant improvement.89-92 Traditional Chinese medicine also plays a role in caring for cancer-related insomnia. A systematic review that analyzed the effects of acupuncture on cancer-related insomnia concluded that the effect of acupuncture was similar to conventional drugs by measuring PSQI score.93 Chinese herbal medicines relieving fatigue and insomnia showed the feasibility of utilization on cancer-related insomnia, as PSQI score was significantly lower than the control group.94,95 Gamiguibi-tang, a traditional KM, also has been used to mitigate sleep disturbance represented efficacy and safety for cancer patients with insomnia.96
In this study, insomnia in cancer patients was scrutinized with various scales, which measure physiological and psychological symptoms, commonly comorbid with insomnia, and enhanced the sensitivity of trial. With a sleep diary and insomnia survey questions, the insomnia status of cancer patients was cross-checked. Fitbit One data were not analyzed in this trial, because the activity tracker was out of stock while conducting the trial, and the sleep status data of patients were deficient to analyze and compare with sleep diary and other insomnia variables. Alternatively, another wearable activity tracker device, actigraphy, has been widely utilized to collect the sleep quality data with stable production,97,98 and in the future studies, it can be also utilized given that the period of trial.
This trial has several limitations. First, this study could not associate the types and stages of cancer with insomnia severity, and small sample size. Second, the causality of insomnia and cancer could be hardly investigated in this study as the predisposing, precipitating, and perpetuating factors by patients’ information were not fully collected. Despite these limitations, this study was the first clinical trial to observe the feasibility of Cheonwangbosimdan treatments on insomnia in cancer patients and support the ability to conduct large-scale trial in future.
Acknowledgments
Authors appreciate Jungwoo Pharm. Co., Ltd for providing Cheonwangbosimdan liquid to conduct our clinical trial.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Korea Institute of Oriental Medicine (K18121). The funding source had no involvement in the study design; collection, analysis, and interpretation of data; writing of the report; or decision to submit the article for publication.
References
- 1. Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 suppl):S7-S10. [PMC free article] [PubMed] [Google Scholar]
- 2. Murray SE, Pathak PR, Schaefer SC, Chen H, Sippel RS. Improvement of sleep disturbance and insomnia following parathyroidectomy for primary hyperparathyroidism. World J Surg. 2014;38:542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Walker HK, Hall WD, Hurst JW. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Butterworths; 1990:398-403. [PubMed] [Google Scholar]
- 4. Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6:97-111. [DOI] [PubMed] [Google Scholar]
- 5. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. American Psychiatric Association; 1994. [Google Scholar]
- 6. Mallon L, Broman JE, Hetta J. Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med. 2002;251:207-216. [DOI] [PubMed] [Google Scholar]
- 7. Taylor DJ, Schmidt-Nowara W, Jessop CA, Ahearn J. Sleep restriction therapy and hypnotic withdrawal versus sleep hygiene education in hypnotic using patients with insomnia. J Clin Sleep Med. 2010;6:169-175. [PMC free article] [PubMed] [Google Scholar]
- 8. Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166:1768-1774. [DOI] [PubMed] [Google Scholar]
- 9. Léger D, Morin CM, Uchiyama M, Hakimi Z, Cure S, Walsh JK. Chronic insomnia, quality-of-life, and utility scores: comparison with good sleepers in a cross-sectional international survey. Sleep Med. 2012;13:43-51. [DOI] [PubMed] [Google Scholar]
- 10. Bhaskar S, Hemavathy D, Prasad S. Prevalence of chronic insomnia in adult patients and its correlation with medical comorbidities. J Fam Med Prim Care. 2016;5:780-784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morin CM, Benca R. Chronic insomnia. Lancet. 2012;379:1129-1141. [DOI] [PubMed] [Google Scholar]
- 12. Morin CM, Bélanger L, LeBlanc M, et al. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169:447-453. [DOI] [PubMed] [Google Scholar]
- 13. Health Insurance Review and Assessment Services. Sleep difficulty annually increased 12% in recent 5 years. https://www.hira.or.kr/bbsDummy.do?pgmid=HIRAA020041000100&brdScnBltNo=4&brdBltNo=9731&pageIndex=1. Accessed June 9, 2020.
- 14. Savard J, Ivers H, Villa J, Caplette-Gingras A, Morin CM. Natural course of insomnia comorbid with cancer: an 18-month longitudinal study. J Clin Oncol. 2011;29:3580-3586. [DOI] [PubMed] [Google Scholar]
- 15. Anderson KO, Getto CJ, Mendoza TR, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage. 2003;25:307-318. [DOI] [PubMed] [Google Scholar]
- 16. Davidson JR, MacLean AW, Brundage MD, Schulze K. Sleep disturbance in cancer patients. Soc Sci Med. 2002;54:1309-1321. [DOI] [PubMed] [Google Scholar]
- 17. Denlinger CS, Ligibel JA, Are M, et al. Survivorship: sleep disorders, version 1.2014. J Natl Compr Canc Netw. 2014;12: 630-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson JA, Rash JA, Campbell TS, et al. A systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy for insomnia (CBT-I) in cancer survivors. Sleep Med Rev. 2016;27:20-28. [DOI] [PubMed] [Google Scholar]
- 19. Berger AM. Update on the state of the science: sleep-wake disturbances in adult patients with cancer. Oncol Nurs Forum. 2009;36:E165-E177. [DOI] [PubMed] [Google Scholar]
- 20. Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: do fatigue, depression, and sleep disturbance share a common underlying mechanism? J Clin Oncol. 2011;29:3517-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bastien CH, Vallieres A, Morin CM. Precipitating factors of insomnia. Behav Sleep Med. 2004;2:50-62. [DOI] [PubMed] [Google Scholar]
- 22. Fan G, Filipczak L, Chow E. Symptom clusters in cancer patients: a review of the literature. Curr Oncol. 2007;14: 173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs. 2005;28:270-282. [DOI] [PubMed] [Google Scholar]
- 24. Howell D, Oliver TK, Keller-Olaman S, et al. Sleep disturbance in adults with cancer: a systematic review of evidence for best practices in assessment and management for clinical practice. Ann Oncol. 2014;25:791-800. [DOI] [PubMed] [Google Scholar]
- 25. Ancoli-Israel S. Recognition and treatment of sleep disturbances in cancer. J Clin Oncol. 2009;27:5864-5866. [DOI] [PubMed] [Google Scholar]
- 26. Davidson J, Feldman-Stewart D, Brennenstuhl S, Ram S. How to provide insomnia interventions to people with cancer: insights from patients. Psychooncology. 2007;16: 1028-1038. [DOI] [PubMed] [Google Scholar]
- 27. Mormont MC, Waterhouse J, Bleuzen P, et al. Marked 24-h rest/activity rhythms are associated with better quality of life, better response, and longer survival in patients with metastatic colorectal cancer and good performance status. Clin Cancer Res. 2000;6:3038-3045. [PubMed] [Google Scholar]
- 28. Stepanski EJ, Walker MS, Schwartzberg LS, Blakely LJ, Ong JC, Houts AC. The relation of trouble sleeping, depressed mood, pain, and fatigue in patients with cancer. J Clin Sleep Med. 2009;5:132-136. [PMC free article] [PubMed] [Google Scholar]
- 29. Banthia R, Malcarne VL, Ko CM, Varni JW, Sadler GR. Fatigued breast cancer survivors: the role of sleep quality, depressed mood, stage and age. Psychol Health. 2009;24: 965-980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arico D, Raggi A, Ferri R. Cognitive behavioral therapy for insomnia in breast cancer survivors: a review of the literature. Front Psychol. 2016;7:1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:191-204. [DOI] [PubMed] [Google Scholar]
- 32. Zhou ES, Suh S, Youn S, Chung S. Adapting cognitive-behavior therapy for insomnia in cancer patients. Sleep Med Res. 2017;8:51-61. [Google Scholar]
- 33. Lacks P, Morin CM. Recent advances in the assessment and treatment of insomnia. J Consult Clin Psychol. 1992;60:586-594. [DOI] [PubMed] [Google Scholar]
- 34. Derogatis LR, Feldstein M, Morrow G, et al. A survey of psychotropic drug prescriptions in an oncology population. Cancer. 1979;44:1919-1929. [DOI] [PubMed] [Google Scholar]
- 35. National Health Service Clinical Knowledge Summaries. Insomnia. National Health Service Clinical Knowledge Summaries; 2009. [Google Scholar]
- 36. Fiorentino L, Ancoli-Israel S. Sleep dysfunction in patients with cancer. Curr Treat Options Neurol. 2007;9:337-346. [PMC free article] [PubMed] [Google Scholar]
- 37. Kang RY, Kim HJ, Han HJ, et al. A pilot study about the effect of Chunwangbosim-dan (天王補心丹) on heart rate variability of healthy subjects. J Orient Neuropsychiatry. 2009;20:127-135. [Google Scholar]
- 38. Hongling W, Min L. Brief analysis of academic thought about moxibustion therapy recorded in the book of effective formulae handed down for generations. J Clin Acupunct Moxibustion. 2017;9:64-66. [Google Scholar]
- 39. Yang XQ, Liu L, Ming SP, Fang J, Wu DN. Tian Wang Bu Xin Dan for insomnia: a systematic review of efficacy and safety. Evid Based Complement Alternat Med. 2019;2019:4260801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen W, Qian Y, Song X. Effects of Tian Wang Buxin Dan on hypothalamus—hypophysis—thyroid hormones of insomnia patients of fire hyperactivity caused by YIN deficiency. Hebei J Tradit Chin Med. 2012;34:1454-1456. [Google Scholar]
- 41. Han YC, Li YX, Chen HJ. Tian Wang Bu Xin Dan with Sanzaoren treatment on heart-liver yin deficiency type of insomnia: 27 cases. Glob Tradit Chin Med. 2013;6:620-622. [Google Scholar]
- 42. Suna L, Yang Y, Bing W, Liqi Q, Binli Z. Clinical observation of modified Tianwang Buxin pill in treating 120 cases with female insomnia at the perimenopausal period. Clin J Tradit Chin Med. 2016;12:1745-1747. [Google Scholar]
- 43. Cho YW. Sleep scale and sleep hygiene. J Korean Sleep Res Soc. 2004;1:12-23. [Google Scholar]
- 44. Ustinov Y, Lichstein KL, Wal GS, Taylor DJ, Riedel BW, Bush AJ. Association between report of insomnia and daytime functioning. Sleep Med. 2010;11:65-68. [DOI] [PubMed] [Google Scholar]
- 45. Sohn SI, Kim DH, Lee MY, Cho YW. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. 2012;16:803-812. [DOI] [PubMed] [Google Scholar]
- 46. Cho YW, Lee JH, Son HK, Lee SH, Shin C, Johns MW. The reliability and validity of the Korean version of the Epworth Sleepiness Scale. Sleep Breath. 2011;15:377-384. [DOI] [PubMed] [Google Scholar]
- 47. Lee JH. Development of the Korean form of Zung’s Self-Rating Anxiety Scale. Yeungnam Univ J Med. 1996;13:279-294. [Google Scholar]
- 48. Zung WW. A rating instrument for anxiety disorders. Psychosomatics. 1971;12:371-379. [DOI] [PubMed] [Google Scholar]
- 49. Trudel-Fitzgerald C, Savard J, Ivers H. Which symptoms come first? Exploration of temporal relationships between cancer-related symptoms over an 18-month period. Ann Behav Med. 2013;45:329-337. [DOI] [PubMed] [Google Scholar]
- 50. Yun YH, Wang XS, Lee JS, et al. Validation study of the Korean version of the brief fatigue inventory. J Pain Symptom Manage. 2005;29:165-172. [DOI] [PubMed] [Google Scholar]
- 51. Kim SH, Ahn J, Ock M, et al. The EQ-5D-5L valuation study in Korea. Qual Life Res. 2016;25:1845-1852. [DOI] [PubMed] [Google Scholar]
- 52. West H, Jin JO. Performance status in patients with cancer. JAMA Oncol. 2015;1:998-998. [DOI] [PubMed] [Google Scholar]
- 53. Ferguson T, Rowlands AV, Olds T, Maher C. The validity of consumer-level, activity monitors in healthy adults worn in free-living conditions: a cross-sectional study. Int J Behav Nutr Phys Act. 2015;12:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Poon MMK, Chung KF, Yeung WF, Yau VHK, Zhang SP. Classification of insomnia using the traditional Chinese medicine system: a systematic review. Evid Based Complement Alternat Med. 2012;2012:735078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32:449-457. [DOI] [PubMed] [Google Scholar]
- 56. Cheng J, Xiao S, Liu T. Analysis of active patents to investigate the frequency and patterns of Chinese herbal extract combinations claiming to treat heart disease. J Tradit Chin Med Sci. 2016;3:81-90. [Google Scholar]
- 57. Lin SK, Yan SH, Lai JN, Tsai TH. Patterns of Chinese medicine use in prescriptions for treating Alzheimer’s disease in Taiwan. Chin Med. 2016;11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hung YC, Tseng YJ, Hu WL, et al. Demographic and prescribing patterns of Chinese herbal products for individualized therapy for ischemic heart disease in Taiwan: population-based study. PLoS One. 2015;10:e0137058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Savard J, Morin CM. Insomnia in the context of cancer: a review of a neglected problem. J Clin Oncol. 2001;19:895-908. [DOI] [PubMed] [Google Scholar]
- 60. Bardwell WA, Profant J, Casden DR, et al. The relative importance of specific risk factors for insomnia in women treated for early-stage breast cancer. Psychooncology. 2008;17:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Palesh OG, Roscoe JA, Mustian KM, et al. Prevalence, demographics, and psychological associations of sleep disruption in patients with cancer: University of Rochester Cancer Center–Community Clinical Oncology Program. J Clin Oncol. 2010;28:292-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Park B, Youn S, Hann CWC, et al. Prevalence of insomnia among Patients with the ten most common cancers in South Korea: health insurance review and assessment service—National Patient Sample. Sleep Med Res. 2016;7:48-54. [Google Scholar]
- 63. Engstrom CA, Strohl RA, Rose L, Lewandowski L, Stefanek ME. Sleep alterations in cancer patients. Cancer Nurs. 1999;22:143-148. [DOI] [PubMed] [Google Scholar]
- 64. Morin CM, LeBlanc M, Belanger L, Ivers H, Merette C, Savard J. Prevalence of insomnia and its treatment in Canada. Can J Psychiatry. 2011;56:540-548. [DOI] [PubMed] [Google Scholar]
- 65. Lieberman MD, Eisenberger NI. Neuroscience. Pains and pleasures of social life. Science. 2009;323:890-891. [DOI] [PubMed] [Google Scholar]
- 66. Irwin MR, Olmstead RE, Ganz PA, Haque R. Sleep disturbance, inflammation and depression risk in cancer survivors. Brain Behavior Immun. 2013;30(suppl):S58-S67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Irwin MR, Cole SW. Reciprocal regulation of the neural and innate immune systems. Nat Rev Immunol. 2011;11:625-632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wu MP, Lin HJ, Weng SF, Ho CH, Wang JJ, Hsu YW. Insomnia subtypes and the subsequent risks of stroke: report from a nationally representative cohort. Stroke. 2014;45:1349-1354. [DOI] [PubMed] [Google Scholar]
- 69. von Ruesten A, Weikert C, Fietze I, Boeing H. Association of sleep duration with chronic diseases in the European Prospective Investigation into Cancer and Nutrition (EPIC)—Potsdam study. PLoS One. 2012;7:e30972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Costa AR, Fontes F, Pereira S, Goncalves M, Azevedo A, Lunet N. Impact of breast cancer treatments on sleep disturbances—a systematic review. Breast. 2014;23:697-709. [DOI] [PubMed] [Google Scholar]
- 71. Kakizaki M, Inoue K, Kuriyama S, et al. Sleep duration and the risk of prostate cancer: the Ohsaki Cohort study. Br J Cancer. 2008;99:176-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Jiao L, Duan Z, Sangi-Haghpeykar H, Hale L, White DL, El-Serag HB. Sleep duration and incidence of colorectal cancer in postmenopausal women. Br J Cancer. 2013;108:213-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Roscoe JA, Kaufman ME, Matteson-Rusby SE, et al. Cancer-related fatigue and sleep disorders. Oncologist. 2007;12(suppl 1):35-42. [DOI] [PubMed] [Google Scholar]
- 74. Fiorentino L, Ancoli-Israel S. Insomnia and its treatment in women with breast cancer. Sleep Med Rev. 2006;10:419-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Donovan KA, Jacobsen PB. Fatigue, depression, and insomnia: evidence for a symptom cluster in cancer. Semin Oncol Nurs. 2007;23:127-135. [DOI] [PubMed] [Google Scholar]
- 76. Davis MP, Goforth HW. Long-term and short-term effects of insomnia in cancer and effective interventions. Cancer J. 2014;20:330-344. [DOI] [PubMed] [Google Scholar]
- 77. Morris BA, Thorndike FP, Ritterband LM, Glozier N, Dunn J, Chambers SK. Sleep disturbance in cancer patients and caregivers who contact telephone-based help services. Support Care Cancer. 2015;23:1113-1120. [DOI] [PubMed] [Google Scholar]
- 78. Riemann D, Perlis ML. The treatments of chronic insomnia: a review of benzodiazepine receptor agonists and psychological and behavioral therapies. Sleep Med Rev. 2009;13:205-214. [DOI] [PubMed] [Google Scholar]
- 79. Buysse DJ. Insomnia. JAMA. 2013;309:706-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wilt TJ, MacDonald R, Brasure M, et al. Pharmacologic treatment of insomnia disorder: an evidence report for a clinical practice guideline by the American College of Physicians. Ann Intern Med. 2016;165:103-112. [DOI] [PubMed] [Google Scholar]
- 81. Sivertsen B, Nordhus IH, Bjorvatn B, Pallesen S. Sleep problems in general practice: a national survey of assessment and treatment routines of general practitioners in Norway. J Sleep Res. 2010;19(1 pt 1):36-41. [DOI] [PubMed] [Google Scholar]
- 82. Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC Fam Pract. 2012;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Wilson SJ, Nutt DJ, Alford C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders. J Psychopharmacol. 2010;24:1577-1601. [DOI] [PubMed] [Google Scholar]
- 84. Thomas A, Grandner M, Nowakowski S, Nesom G, Corbitt C, Perlis ML. Where are the behavioral sleep medicine providers and where are they needed? A geographic assessment. Behav Sleep Med. 2016;14:687-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sandlund C, Hetta J, Nilsson GH, Ekstedt M, Westman J. Improving insomnia in primary care patients: a randomized controlled trial of nurse-led group treatment. Int J Nurs Stud. 2017;72:30-41. [DOI] [PubMed] [Google Scholar]
- 86. Okajima I, Komada Y, Inoue Y. A meta-analysis on the treatment effectiveness of cognitive behavioral therapy for primary insomnia. Sleep Biol Rhythms. 2011;9:24-34. [Google Scholar]
- 87. Smith MT, Wegener ST. Measures of sleep: the Insomnia Severity Index, Medical Outcomes Study (MOS) Sleep Scale, Pittsburgh Sleep Diary (PSD), and Pittsburgh Sleep Quality Index (PSQI). Arthritis Care Res. 2003;49(suppl 5):S184-S196. [Google Scholar]
- 88. Chen PY, Jan YW, Yang CM. Are the Insomnia Severity Index and Pittsburgh Sleep Quality Index valid outcome measures for cognitive behavioral therapy for insomnia? Inquiry from the perspective of response shifts and longitudinal measurement invariance in their Chinese versions. Sleep Med. 2017;35:35-40. [DOI] [PubMed] [Google Scholar]
- 89. Rao RM, Vadiraja HS, Nagaratna R, et al. Effect of yoga on sleep quality and neuroendocrine immune response in metastatic breast cancer patients. Indian J Palliat Care. 2017;23:253-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Handel DL, Neron S. Cancer palliation: layered hypnotic approaches mending symptoms, minding hope, and meaning. Am J Clin Hypn. 2017;60:33-49. [DOI] [PubMed] [Google Scholar]
- 91. Irwin MR, Olmstead R, Carrillo C, et al. Tai Chi Chih compared with cognitive behavioral therapy for the treatment of insomnia in survivors of breast cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2017;35: 2656-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tarrasch R, Carmel-Neiderman NN, Ben-Ami S, et al. The effect of reflexology on the pain-insomnia-fatigue disturbance cluster of breast cancer patients during adjuvant radiation therapy. J Altern Complement Med. 2018;24: 62-68. [DOI] [PubMed] [Google Scholar]
- 93. Choi TY, Kim JI, Lim HJ, Lee MS. Acupuncture for managing cancer-related insomnia: a systematic review of randomized clinical trials. Integr Cancer Ther. 2017;16:135-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kaiyin C. The Cohort Study of Bu Zhong Yi Qi Tang Improves Cancer Related Fatigue [dissertation]. Guangzhou University of Chinese Medicine; 2017. [Google Scholar]
- 95. G. Z. The clinical research of using the method of Yang Yin Yi Qi An Shen to treat the type of Qi Yin Liang Xu stage I-II non–small cell lung cancer-related insomnia. Chinese PLA General Hospital; 2017. http://new.oversea.cnki.net/KCMS/detail/detail.aspx?dbcode=CMFD&dbname=CMFD201801&filename=1017203737.nh&uid=WEEvREcwSlJHSldRa1FhdXNzY2Z2S3ZXWUdVNmhCbnlWZHYyL0liZzZyYz0=$9A4hF_YAuvQ5obgVAqNKPCYcEjKensW4IQMovwHtwkF4VYPoHbKxJw!!&v=MDU2NzMzcVRyV00xRnJDVVI3cWZZZWRzRnkzbVVMdkJWRjI2R2JHNEhkYlBxSkViUElSOGVYMUx1eFlTN0RoMVQ=. Accessed June 16, 2020. [Google Scholar]
- 96. Lee JY, Oh HK, Ryu HS, Yoon SS, Eo W, Yoon SW. Efficacy and safety of the traditional herbal medicine, Gamiguibi-tang, in patients with cancer-related sleep disturbance: a prospective, randomized, wait-list–controlled, pilot study. Integr Cancer Ther. 2018;17:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Werner H, Molinari L, Guyer C, Jenni OG. Agreement rates between actigraphy, diary, and questionnaire for children’s sleep patterns. Arch Pediatr Adolesc Med. 2008; 162: 350-358. [DOI] [PubMed] [Google Scholar]
- 98. Cruse D, Thibaut A, Demertzi A, et al. Actigraphy assessments of circadian sleep-wake cycles in the vegetative and minimally conscious states. BMC Med. 2013;11:18. [DOI] [PMC free article] [PubMed] [Google Scholar]