Abstract
Background:
Epstein–Barr virus-associated gastric cancer (EBVaGC) has unique clinicopathologic features and our present understanding of its treatment outcome is limited. Here, we investigated the clinical outcomes of resectable and metastatic EBVaGC cases with regards to their respective treatment.
Methods:
We retrieved the data of EBVaGC patients treated at our center from October 2014 to June 2019. The primary endpoint was overall survival (OS). Secondary endpoints were disease-free survival (DFS) for stage I–III patients, progression-free survival (PFS) and objective response rate (ORR) for stage IV patients.
Results:
Patients classified as stage I–III accounted for 83.7% of the total 197 cases analyzed. Two patients had mismatched repair-deficiency. The 5-year OS rate of the entire cohort was 63.51% [95% (confidence interval (CI): 52.31–72.76%]. Tumor-node-metastasis (TNM) stage and gastric stump cancer were identified as independent prognostic factors for OS. The 3- and 5-year DFS rate for stage I–III patients were 83.72% (95% CI: 75.86–89.19%) and 73.83% (95% CI: 60.39–83.32%), respectively. TNM stage III, neural invasion, lymphovascular invasion, and baseline plasma EBV-DNA positive were correlated with shorter DFS. The ORR and disease control rate (DCR) for metastatic EBVaGC patients to first-line therapy were 29.0% and 90.3% (median PFS: 9.8 months), respectively, and to second-line therapy were 25.0% and 75.0%, respectively. Seven patients received anti-PD1 therapy and had an ORR of 28.5% and a median PFS of 2.8 months.
Conclusions:
EBVaGC patients have few metastases, long DFS, and high DCR. TNM stage and gastric stump cancer were independent prognostic factors for OS.
Keywords: disease-free survival (DFS), Epstein–Barr virus, gastric cancer, prognosis, progression-free survival (PFS), response rate (RR)
Introduction
The Cancer Genome Atlas (TCGA) Research Network has identified four distinct molecular subtypes of gastric cancer (GC): chromosomal instability, genomically stable, Epstein–Barr virus (EBV)-positive, and microsatellite instable (MSI).1 The incidence rate of EBV-associated gastric cancer (EBVaGC) is 9% according to the TCGA study, and ~5% in all gastric cancer cases in China.2–4 EBVaGC is generally defined by the presence of EBV in GC cells by in situ hybridization (ISH) targeting EBV-encoded RNA (EBER).5 Based on the available literature and compared with EBV negative GC (EBVnGC), EBVaGC patients were observed to be younger, of male predominance, and often developed in the gastric stump.6–8 Overall, EBVaGCs were considered to have better prognoses compared with EBVnGC.9,10
After anti-programmed death 1 (PD-1) therapy was approved by the United States Food and Drug Administration (FDA) for advanced/metastatic GC (mGC), great efforts are being made to seek clinically reliable biomarkers to predict treatment response.11 In June 2018, Panda et al. reported that one EBVaGC patient who had low tumor mutation burden (TMB) and microsatellite stable (MSS) obtained partial response (PR) after treatment with programmed cell death 1 ligand 1 (PD-L1) inhibitor,12 and another study reported a 100% objective response rate (ORR) in EBV-positive mGC patients to PD-1 inhibitor but the sample size was relatively small (N = 6).13 However, recently, there have been two studies showing that the ORR to PD-1 inhibitor in EBVaGC was not as initially promising as thought, with an obtained ORR of only 25%.14,15
Due to the low incidence rate of EBVaGC, most previous studies on EBVaGC were of small sample size (N < 100).16–19 To date, little is known on the clinical outcome of EBVaGC, particularly its response to treatment, and corresponding outcomes such as disease-free survival (DFS), ORR to chemotherapy, and progression-free survival (PFS).
Since October 2014, after the release of TCGA GC data, EBER detection based on tumor tissue became a routine examination for GC patients at our cancer center. In this present study, we described the clinical and survival outcomes of EBVaGC patients to treatment based on an observation cohort.
Patients and methods
This study was conducted at the Sun Yat-sen University Cancer Center (Guangzhou, China). It was performed in accordance with the Declaration of Helsinki protocols and was approved by our local Ethics Committee (No. B2018-058-01).
Eligibility
Eligibility criteria were as follows: histopathologically confirmed diagnosis of gastric adenocarcinoma; aged 18 years or older; EBER positivity; Eastern Cooperative Oncology Group performance status (PS) of 0–2; adequate organ function; and provision of written informed consent. Exclusion criteria were as follows: lymphoepithelioma-like gastric cancer; active infection; congestive heart failure (New York Heart Association Functional Classification III-IV); uncontrolled diabetes or hypertension; interstitial pneumonia or pulmonary fibrosis; symptomatic brain metastasis; liver cirrhosis or active hepatitis, and pregnancy. All patients were staged based on the seventh edition of the American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) 7th staging system.20
Study design
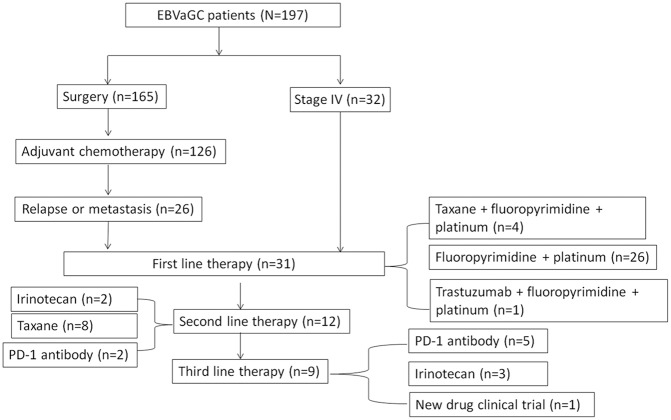
From October 2014 to October 2019, 197 EBVaGC patients were enrolled to this study. The enrollment process is illustrated in Figure 1. The patients were treated based on the investigators’ decision. A total of 165 patients received curative D2 gastrectomy; of these, 20 patients received neoadjuvant chemotherapy with the xelox (oxaliplatin 130 mg/m2, QD, d1 and capecitabine 1000 mg/m2, bid, QD, d1-14) or sox regimen [oxaliplatin 130 mg/m2, QD, d1 and S1 whose dose was based on the body surface area (BSA) as follows: BSA <1.25 m2, 80 mg/day; 1.25 m2 ⩽ BSA <1.5 m2, 100 mg/day; and BSA ⩾1.5 m2, 120 mg/day,d1-14], repeated every 3 weeks, for a total of three cycles. Stage II and III patients received perioperative chemotherapy, among them, 22 patients had 6–12 months of S1, 19 patients had 4–6 months of the sox regimen, and 85 patients had 4–6 months of the xelox regimen.
Figure 1.
Case grouping and treatment undergone of this observation cohort.
EBVaGC, Epstein–Barr virus-associated gastric cancer, PD-1, programmed death 1.
A total of 32 patients were diagnosed as stage IV at the time of diagnosis and 26 patients developed recurrence or metastasis after curative resection. Among these 58 patients, 31 received first-line therapy, 12 underwent second-line therapy, and 9 received third-line therapy. Seven patients received anti-PD1 therapy as a second or third line, with four patients receiving 200 mg of camrelizumab (also known as SHR1210), every 2 weeks, and 3 receiving 240 mg of toripalimab (also known as JS001), every 3 weeks. Both camrelizumab and toripalimab are PD-1 antibodies.
Follow up
As a routine clinical practice, patients had hematological and biochemistry tests and underwent assessments of clinical symptoms and signs at least once during each cycle of chemotherapy. For patients who finished adjuvant chemotherapy, imaging studies, including ultrasonography and computed tomography (CT) were scheduled at intervals of 6 months or less for the first 3 years, and at 1-year interval thereafter until 5 years after surgery. For patients who received palliative treatment, they underwent imaging studies every 6–8 weeks. Treatment response or relapse evaluation was confirmed by CT and classified using the Response Evaluation Criteria In Solid Tumors (RECIST) version 1.1 based on the investigators’ review. The last date of follow up was 30 October 2019.
Tumor tissue Epstein–Barr virus analysis
EBER detection was performed manually on formalin-fixed, paraffin-embedded (FFPE) samples by an in situ hybridization kit (ISH-7001, Zhongtian Jinqiao Biotechnology Co., Ltd., Beijing, China) using steps as described in one of our prior studies.2 EBER-positive excluding lymphoepithelioma-like gastric cancer, was defined as EBVaGC.
Quantitative analysis of EBV-DNA load using real-time quantitative polymerase chain reaction
DNA from plasma samples were extracted using the Qiamp Blood Kit (Qiagen, Hilden, Germany) following the manufacturer’s protocol. The cutoff value for plasma EBV DNA-positive was set as 100 copies/ml as our previous report.2
Statistical analysis
The primary endpoint was overall survival (OS). Secondary endpoints include DFS for stage I–III patients, PFS and ORR for patients who received palliative treatment. OS was defined as the time from the date of initial diagnosis to the date of death from any cause or last follow up (30 October 2019). DFS was defined as the time from the date of curative resection to the date when recurrence or metastasis was confirmed, death from any cause, or last follow up, whichever came first. PFS was defined as the time from the date of palliative treatment to the date when disease progression was confirmed, death from any cause, or last follow up, whichever came first.
Differences in clinical features between patients with and without recurrence were compared by Student’s t test for continuous variables and χ2 test (or Fisher exact test) for categorical variables. OS, DFS, and PFS were calculated by using the Kaplan–Meier (K-M) method, and the 95% confidence intervals (CIs) were reported. Cox proportional hazard models were used to estimate the clinical factors associated with OS, and factors with a p < 0.05 in univariate analysis were involved in the multivariate model. All analyses were performed using the Intercooled Stata software, version 13.0 (Stata Corporation, College Station, TX, USA). A p < 0.05 (two-sided) was considered statistically significant.
Results
Basic features of patients
The basic features of EBVaGC patients are listed in Table 1. EBVaGC patients were of male predominance. About 10% of the patients were gastric stump cancer who received gastric resection for benign diseases over 5 years ago. Maybe this is caused by localization since both EBVaGC and stump are proximal tumors. About half of the patients were diagnosed at stage III. Most of the primary tumors of stage I–III were bulky tumors with a median size of 4.2 cm (range: 1.0–10.5 cm). Only 16.3% of the patients were diagnosed at stage IV. The most common metastatic sites were distant lymph nodes, followed by peritoneum, liver, lung and bone.
Table 1.
Basic features of EBVaGC patients.
| Characters | N (%) |
|---|---|
| Gender | |
| Male | 175 (88.8) |
| Female | 22 (11.2) |
| Age | |
| Median | 59 |
| Mean ± SD | 58.1 ± 12.0 |
| Remnant stomach | |
| Yes | 21 (10.7) |
| No | 176 (90.3) |
| AJCC T 7th stage (n = 186) | |
| T1 | 25 (13.4) |
| T2 | 28 (15.1) |
| T3 | 64 (34.4) |
| T4 | 69 (37.1) |
| AJCC N 7th stage (n = 186) | |
| N0 | 53 (28.5) |
| N1 | 22 (11.8) |
| N2 | 28 (15.1) |
| N3a | 46 (24.7) |
| N3b | 37 (19.9) |
| AJCC TNM 7th stage | |
| I | 39 (19.8) |
| II | 32 (16.2) |
| III | 94 (47.7) |
| IV | 32 (16.3) |
| Metastasis sites (n = 32) | |
| Distant lymph nodes | 20 (62.5) |
| Peritoneal | 13 (40.6) |
| Liver | 7 (21.9) |
| Lung | 5 (15.6) |
| Bone | 2 (6.3) |
| Size (cm) | |
| Median | 4.2 |
| Mean ± SD | 4.8 ± 2.3 |
| MMR (n = 162) | |
| dMMR | 2 (1.2) |
| pMMR | 160 (98.8) |
| Lauren classification (n = 172) | |
| Diffuse | 52 (30.2) |
| Intestinal | 51 (29.7) |
| Mix | 69 (40.1) |
AJCC, American Joint Committee on Cancer; dMMR, mismatch repair deficiency; EBVaGC, Epstein–Barr virus-associated gastric cancer; MMR, mismatch repair; pMMR, mismatch repair proficient; SD, standard deviation; TNM, tumor-node-metastasis.
As shown in Table 1, only 162 patients had reports of their mismatch repair (MMR) status. Lauren’s classification was recorded in 172 patients. There were two cases of MMR-deficiency (dMMR). Plasma EBV-DNA was found to be elevated in 14.2% (28/197) of the patients, including 1 (2.56%), 3 (9.38%), 13 (13.83%), and 11 (34.38%) with stage I, II, III, and IV disease.
Survival analysis
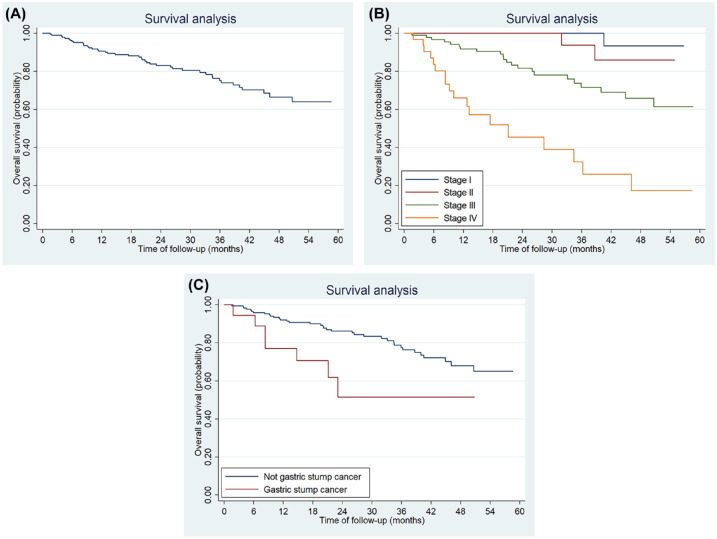
Survival analyses were performed for all patients. After a median follow up of 28.2 months, 45 patients died. The 5-year OS for the entire cohort was 63.51% (95% CI: 52.31–72.76%) (Figure 2A). The 5-year OS were 90.72% (95% CI: 63.77–97.91%) for stage I, 85.77% (95% CI: 53.33–96.32%) for stage II, 61.43% (95% CI: 45.18–74.17%) for stages III and 17.05% (95% CI: 3.51–39.27%) for stage IV disease, respectively, p < 0.001 (Figure 2B). The median survival for stage IV patients was 21.1 months (95% CI: 9.2–36.2 months).
Figure 2.
Kaplan–Meier plots of median overall survival for the entire study cohort (A), TNM stage (B), and gastric stump lesion status (C).
TNM, tumor-node-metastasis.
Univariate analyses showed that stump gastric, tumor size, Lauren classification, and TNM stage were all significantly related to OS (Table 2). Multivariate analyses showed that both stump gastric and TNM stage were independent prognostic factors for OS. The 5-year OS rate for gastric stump cancer and not gastric stump cancer patients were 47.91% (95% CI: 20.03–71.41%) and 64.96% (95% CI: 52.76–74.75%), respectively (p = 0.02) (Figure 2C).
Table 2.
Survival analysis.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| 5-year OS (95% CI) | p value | HR (95% CI) | p value | |
| Gender | – | |||
| Male | 62.1 (49.7–72.2) | |||
| Female | 75.2 (50.2–88.9) | 0.56 | ||
| Age | – | |||
| <59 | 64.9 (49.4–76.7) | |||
| >58 | 63.1 (46.9–75.5) | 0.98 | ||
| Remnant | ||||
| No | 65.0 (52.8–74.8) | Reference | ||
| Yes | 47.9 (20.0–71.4) | 0.02 | 2.99 (1.22–7.36) | 0.017 |
| Size (cm) | ||||
| <4.3 | 68.9 (45.3–84.0) | Reference | ||
| >4.2 | 59.7 (46.6–70.6) | 0.03 | 1.25 (0.58–2.67) | 0.57 |
| Lauren | ||||
| Diffuse + Mix | 58.2 (43.6–70.3) | Reference | ||
| Intestine | 88.6 (71.9–95.7) | 0.03 | 2.69 (0.93–7.83) | 0.068 |
| HER2 | – | |||
| Positive | 50.0 (11.1–80.4) | |||
| Negative | 62.0 (48.8–72.7) | 0.21 | ||
| TNM stage | ||||
| I | 90.7 (63.8–97.9) | Reference | ||
| II | 85.8 (53.3–96.3) | 2.54 (0.23–28.39) | ||
| III | 61.4 (45.2–74.2) | 8.72 (1.13–67.09) | ||
| IV | 17.1 (3.5–39.3) | <0.001 | 37.98 (4.88–295.34) | <0.001 |
–: not involved in the multivariate analysis.
CI, confidence interval; HER2, human epidermal growth factor receptor 2; OS, overall survival; TNM, tumor-node-metastasis.
Disease-free survival
Among 165 stage I–III patients, 26 developed recurrence or metastasis after curative resection (2 from stage I, 3 from stage II and 21 from stage III). Table 3 shows the differences of clinical and pathological features between patients with and without recurrence. The results showed that patients in stage T4, stage III, large-sized tumor, diffuse subtype, neural invasion, lymphovascular invasion, and higher baseline plasma EBV-DNA load had higher percentage of recurrence.
Table 3.
Comparison of clinical features between patients with and without recurrence.
| Recurrent/metastasis (n = 26) | Not recurrent (n = 139) | p value | |
|---|---|---|---|
| Gender | |||
| Male | 24 (92.3) | 121 (87.1) | 0.45 |
| Female | 2 (7.7) | 18 (12.9) | |
| Age | |||
| median | 56 | 60 | 0.84 |
| Mean ± SD | 58.16 ± 13.60 | 58.11 ± 11.98 | |
| AJCC T stage | |||
| T1 | 0 (0) | 25 (18.0) | 0.006 |
| T2 | 2 (7.7) | 25 (18.0) | |
| T3 | 9 (34.6) | 51 (36.7) | |
| T4 | 15 (57.7) | 38 (27.3) | |
| AJCC N stage | |||
| N0 | 4 (15.4) | 49 (35.3) | 0.16 |
| N1 | 3 (11.5) | 16 (11.5) | |
| N2 | 4 (15.4) | 23 (16.5) | |
| N3 | 15 (57.7) | 51 (36.7) | |
| AJCC TNM stage | |||
| I | 2 (7.7) | 37 (26.6) | 0.025 |
| II | 3 (11.5) | 29 (20.9) | |
| III | 21 (80.8) | 73 (52.5) | |
| Neural invasion | |||
| No | 3 | 49 | |
| Yes | 23 | 90 | 0.017 |
| Lymphovascular invasion | |||
| No | 3 | 63 | |
| Yes | 23 | 76 | 0.001 |
| Size (cm) | |||
| Median | 5 | 4 | |
| Mean ± SD | 5.52 ± 2.44 | 4.48 ± 2.16 | 0.04 |
| MMR (n = 131) | |||
| dMMR | 0 (0) | 2 (1.9) | |
| pMMR | 25 (100) | 104 (98.1) | 0.13 |
| Lauren classification (n = 145) | |||
| Diffuse | 11 (52.4) | 32 (25.8) | 0.045 |
| Intestinal | 5 (23.8) | 40 (32.3) | |
| Mix | 5 (23.8) | 52 (41.9) | |
| EBV-DNA (copies/ml) | |||
| Median | 25250 | 0 | 0.003 |
Data were present as n (%) unless specified.
AJCC, American Joint Committee on Cancer; dMMR, mismatch repair deficiency; MMR, mismatch repair; pMMR, mismatch repair proficient; SD, standard deviation; TNM, tumor-node-metastasis.
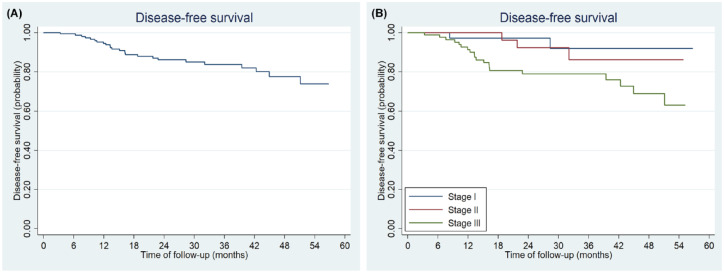
Results of K-M analysis showed that the 3- and 5-year DFS rate was 83.72% (95% CI: 75.86–89.19%) and 73.83% (95% CI: 60.39–83.32%), respectively (Figure 3A). Both univariate analyses and multivariate analyses showed that TNM stage, neural invasion, and lymphovascular invasion were all significantly related to DFS (Table 4). The 5-year DFS rates were 92.29% (95% CI: 71.29–98.11%) for stage I, 86.08% (95% CI: 61.63–95.46%) for stage II and 63.09% (95% CI: 44.28–77.06%) for stage III disease, respectively (Log-rank = 7.76, p = 0.021; Figure 3B).
Figure 3.
Kaplan–Meier plots of median DFS for all the patients who had curative resection (A) and by TNM stage (B).
DFS, disease-free survival; TNM, tumor-node-metastasis.
Table 4.
Disease free survival.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| 5-year DFS (95% CI) | p value | HR (95% CI) | p value | |
| Gender | – | |||
| Male | 71.9 (56.8–82.5) | |||
| Female | 87.8 (59.5–96.8) | 0.48 | ||
| Age | – | |||
| <59 | 65.5 (42.7–81.1) | |||
| >58 | 81.9 (66.8–90.5) | 0.25 | ||
| Plasma EBV-DNA (copies/ml) | ||||
| Negative | 77.3 (63.2–86.6) | Reference | ||
| Positive | 37.0 (7.3–68.6) | 0.0005 | 4.60 (1.79–11.80) | 0.001 |
| Remnant | – | |||
| No | 73.8 (59.1–83.9) | |||
| Yes | 72.2 (36.1–90.1) | 0.15 | ||
| Size (cm) | – | |||
| <4.3 | 77.4 (65.1–85.7) | |||
| >4.2 | 64.9 (34.2–84.0) | 0.29 | ||
| Lauren | – | |||
| Diffuse + Mix | 66.9 (45.5–81.4) | |||
| Intestine | 87.0 (71.5–94.4) | 0.75 | ||
| Differentiation | – | |||
| High + moderate | 87.5 (38.7–98.1) | |||
| Poor | 72.5 (58.1–82.7) | 0.56 | ||
| HER2 | – | |||
| Positive | 66.7 (5.4–94.5) | |||
| Negative | 67.3 (47.9–80.9) | 0.59 | ||
| Neural invasion | ||||
| No | 92.9 (73.5–98.3) | Reference | ||
| Yes | 75.0 (59.8–85.2) | 0.024 | 4.77 (1.08–21.06) | 0.039 |
| Lymphovascular invasion | ||||
| No | 92.9 (73.5–98.3) | Reference | ||
| Yes | 45.8 (13.9–73.4) | 0.002 | 7.21 (1.65–31.59) | 0.009 |
| TNM stage | ||||
| I | 92.3 (71.3–98.1) | Reference | ||
| II | 86.1 (61.6–95.5) | 1.81 (0.30–10.83) | 0.52 | |
| III | 63.1 (44.3–77.1) | 0.021 | 4.94 (1.15–21.10) | 0.031 |
–: not involved in the multivariate analysis.
CI, confidence interval; DFS, disease free survival; HER2, human epidermal growth factor receptor 2; TNM, tumor-node-metastasis.
Palliative treatment
As shown in Figure 1, the number of patients who received first-, second- and third-line therapy was 31, 12, and 9 respectively. The response to treatment was present in supplemental Table S1.
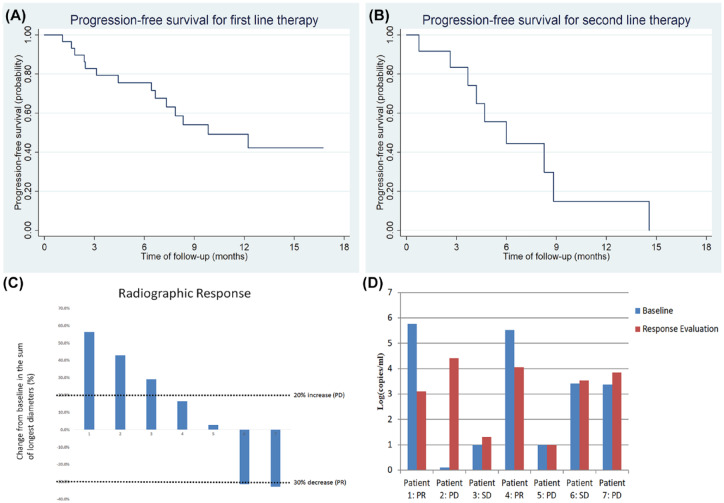
The ORR and DCR to first-line therapy was 29.0% and 90.3%, respectively. For second-line therapy, the ORR and DCR were 25.0% and 75.0%, respectively. The median PFS for patients who had first-line therapy was 9.8 months, and was 6.0 months for those with second-line therapy (Figure 4A and B).
Figure 4.
Kaplan–Meier plots of median progression-free survival for EBVaGC patients to first-line (A), and second-line (B) therapy. Maximal change of tumor size from baseline in target lesion(s) for EBVaGC patients who received anti-PD1 therapy, n = 7 (C). Dynamic monitoring of plasma EBV-DNA load in metastatic EBVaGC patients who received anti-PD1 therapy (D).
Seven patients received anti-PD1 single agent therapy, including two as second-line and five as a third-line treatment. All were diagnosed as MSS, and two had high TMB (the cutoff value of high TMB was set as 10/MB). The ORR was only 28.5% and median PFS was 2.8 months. The TMB for these two patients with PR was 12.25/MB and 1.58/MB. Tumor response to anti-PD1 therapy is shown in Figure 4C. Four of the seven patients had elevated plasma EBV-DNA before anti-PD1 therapy. After PD-1 therapy, plasma EBV-DNA load decreased for the two patients who had PR, and increased for those had progressive disease (PD) (Figure 4D).
Discussion
Based on our observations of the 197 treated EBVaGC patients, the main findings of this study included: dMMR and EBV positivity was found to co-exist, but were rare; EBVaGC cases had a high 5-year DFS rates, even for stage III patients; patients with T4 disease, stage III, large-sized tumors, diffuse subtype, neural invasion, lymphovascular invasion, or higher baseline plasma EBV-DNA load had a higher risk of recurrence or metastasis; the ORR of metastatic EBVaGC patients to standard first-line and second-line therapy was not high, but the DCR was high; the ORR to PD-1 antibody for EBVaGC was not promising; both TNM stage and gastric stump were independent prognostic factors for EBVaGC patients.
According to the TCGA molecular subtypes of GC, MSI-H, and EBV positivity were two independent subgroups, and were thought to be exclusive.1 Kim et al. also reported that EBV-positivity was mutually exclusive with dMMR.21 However, in our study cohort we found the co-existence of both EBV positivity and dMMR in two patients. Both were female and negative for MLH1 and PMS2. Hewitt et al. also found that one patient had dMMR and EBV-positive tumor from an investigated cohort of 1063 GC patients,17 suggesting that further analysis to uncover the mechanism of coexistence of dMMR and EBV positive is deserved. There is another molecular subtype using p53, CIN, and e-cadherine as classifiers.22 We could not analyze the relationship of these factors with EBER since we did not collect sequence data in the present study.
Patients with lymphoepithelioma-like gastric cancer may also be EBER positive and have more favorable prognosis.23 To exclude the effect of lymphoepithelioma-like gastric cancer, we did not enroll this type of patient in the cohort.
Gastrectomy with D2 lymph node dissection is the standard treatment for curable GC in China.24 Postoperative adjuvant chemotherapy was recommended for stage II and III patients. The estimated 3- and 5-year DFS rate was 74% and 68% from the phase III CLASSIC trial,25,26 and for stage IIIA and IIIB patients, the estimated 5-year DFS rate was 58% and 52%.26 Due to the low incidence rate of EBVaGC, to date, there is no report on the DFS rate for EBVaGC patients. Our findings showed that the estimated 3- and 5-year DFS rate for stage I–III EBVaGC patients were 83.72% and 73.83%, respectively, and the 5-year DFS rate was 63.09% for stage III EBVaGC patients. Actually, the TNM staging system was different between the present study and that of the CLASSIC study, which used the AJCC TNM 6th staging system. Recently, a phase II study using adjuvant chemotherapy with S-1 plus docetaxel for stage III gastric cancer reported a 5-year DFS rate of 60%.27 EBVaGC patients had a high 5-year DFS rate after curative resection.
The preferred regimens for first-line systemic therapy in metastatic GC patients include the combination of fluoropyrimidine with either oxaliplatin or cisplatin. Taxane or irinotecan is recommended for second-line therapy. The ORR to first-line and second-line therapy was about 40–50% and 20–25%, respectively.28-31 In the present study, we found that the ORR to fluoropyrimidine and platin-based first-line therapy was only 29.0%, but the DCR was 90.3%. There has been no report of response rate of chemotherapies for advanced EBVGCs in clinical trials. EBVGC was reported to be resistant to 5-fluorouracil in a previous in vitro study.32 The ORR to second-line therapy was comparable with literature data.28,31 Both the mPFS for first-line (9.8 months) and second-line (6.0 months) therapy were longer than previous reports on unselected mGC patients.28–31 We realized that the number of metastatic EBVaGC patents who received systematic therapy was small, so larger sample prospective study is warranted to confirm this finding.
Previous studies indicated that EBV positivity was an effective response predictor to PD-1 antibody.12,13 However, another two studies showed the ORR to PD-1 inhibitor in EBVaGC was only 25%.14,15 In our present study, we found that the response to PD-1 inhibitor for metastatic EBVaGC patients was also only 28.5%. Two of the three patients who got disease progression had ascites and a PS score of 2 at the time of treatment. Subgroup analyses from the ATTRACTION-02 study indicated that patients with ascites or poor PS could not benefit from nivolumab.33 The value of EBV status to predict response to PD-1 antibody deserves further analysis, and, to answer this question, a prospective phase II study has been initiated at our cancer center [ClinicalTrials.gov identifier: NCT03755440].
Our previous study had found that EBVaGC patients had a better prognosis than EBVnGC patients.2 The 5-year OS was 63.51% in our cohort and 63.2% from another study.3 Findings from this present study can provide some clues to explain the superior prognosis observed in EBVaGC patients. First, over 80% of the patients were diagnosed at stage I–III, and, therefore, they had the opportunity to receive radical resection. Second, fewer patients developed recurrence or metastasis after radical resection and they had higher DFS rates. Third, even for metastatic EBVaGC patients, the mPFS for first-line and second-line chemotherapy was long. Molecular mechanisms to explore the reason for better prognosis in EBVaGC patients is warranted. In the present study, we further identified TNM stage and gastric stump cancer as independent prognostic factors for EBVaGC patients. A previous study found that EBV infection, together with long-standing inflammation, might cause gastric stump cancer.34 EBVaGC was correlated positively with gastric stump lesion.3,4 However, no studies have analyzed the prognostic value of the stump gastric in EBVaGC patients. We found that patients with gastric stump cancer had a worse prognosis than those with non-gastric stump cancer. However, the mechanism is still unknown.
The limitations of the present study are (i) a small number of patients for analysis of response to chemotherapy and PD-1 antibody; (ii) single-center study; (iii) it was an observation study, but not a clinical trial; (iv) being a retrospective analysis, the endpoint of DFS and PFS were not accurate enough, and not using central review for response.
Conclusion
To our knowledge, this is the largest sample size of EBVaGC patients investigated. We found that dMMR and EBV positivity could co-exist, but were rare; EBVaGC is a unique subtype of GC with few metastases, high DFS rate, and good prognosis. Further clinical research in EBVaGC is needed to confirm our findings.
Supplemental Material
Supplemental material, Supplementary_table_1_3 for Observational cohort study of clinical outcome in Epstein–Barr virus associated gastric cancer patients by Miao-Zhen Qiu, Cai-Yun He, Da-Jun Yang, Da-Lei Zhou, Bai-Wei Zhao, Xiao-Jian Wang, Li-Qiong Yang, Shi-Xun Lu, Feng-Hua Wang and Rui-Hua Xu in Therapeutic Advances in Medical Oncology
Acknowledgments
We gratefully thank the patients and their families for participating in this study.
Footnotes
Author contributions: MZQ,CYH and RHX designed the study, performed the analyses, contributed to the analysis of the results, and wrote the manuscript. YDJ, DLZ, BWZ, XJW and SXL performed the analyses, contributed to the interpretation of the results, and critically reviewed the manuscript. LQY and FHW designed the survival study, contributed to the interpretation of the results, and critically reviewed the manuscript. All authors approved the final format of the manuscript.
Availability of data and material: The key raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn), with the Approval Number of RDDB2019000762 and the datasets used in this study are publicly available.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Ethics approval and consent to participate: This study was conducted at the Sun Yat-sen University Cancer Center (Guangzhou, China). It was performed in accordance with the Declaration of Helsinki protocols and was approved by our local Ethics Committee (Sun yat-sen University Cancer Center, No.B2018-058-01). Written informed consent was obtained from participants.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Key R&D Program of China (grant number: 2018YFC1313300), National Natural Science Foundation of China (grant number. 81602066, 81602426 and 81772587), Guangdong Esophageal Cancer Institute Science and Technology Program (grant number M201809), CSCO-HengRui Oncology Research Fund (grant number Y-HR2018-184), the third outstanding young talents training plan and Medical Scientist program of Sun Yat-sen University Cancer Center.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Miao-Zhen Qiu, Department of Medical Oncology, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, P. R. China.
Cai-Yun He, Department of Molecular Diagnostics, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, P. R. China.
Da-Jun Yang, Department of Experimental Research, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, P. R. China.
Da-Lei Zhou, Department of Molecular Diagnostics, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, P. R. China.
Bai-Wei Zhao, Department of Gastric Surgery, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, 651 Dongfeng Road East, Guangzhou, P. R. China.
Xiao-Jian Wang, Department of Medical Oncology, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, P. R. China; Department of Medical Oncology, Ganzhou Cancer Hospital, Ganzhou, P. R. China.
Li-Qiong Yang, Department of Experimental Research, Sun Yat-Sen University Cancer Center, State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, P. R. China.
Shi-Xun Lu, Department of Pathology, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, P. R. China.
Feng-Hua Wang, Department of Medical Oncology, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou, P. R. China.
Rui-Hua Xu, Department of Medical Oncology, Sun Yat-Sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, 651 Dongfeng Road East, Guangzhou, 510060, P. R. China.
References
- 1. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014; 513: 202–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Qiu MZ, He CY, Lu SX, et al. Prospective observation: clinical utility of plasma Epstein-Barr virus DNA load in EBV-associated gastric carcinoma patients. Int J Cancer 2020; 146: 272–280. [DOI] [PubMed] [Google Scholar]
- 3. Yang Y, Liu YQ, Wang XH, et al. [Clinicopathological and molecular characteristics of Epstein-Barr virus associated gastric cancer: a single center large sample case investigation]. Beijing Da Xue Xue Bao Yi Xue Ban 2019; 51: 451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang SC, Ng KF, Yeh TS, et al. Subtraction of Epstein-Barr virus and microsatellite instability genotypes from the Lauren histotypes: combined molecular and histologic subtyping with clinicopathological and prognostic significance validated in a cohort of 1,248 cases. Int J Cancer 2019; 145: 3218–3230. [DOI] [PubMed] [Google Scholar]
- 5. Shinozaki-Ushiku A, Kunita A, Fukayama M. Update on Epstein-Barr virus and gastric cancer (review). Int J Oncol 2015; 46: 1421–1434. [DOI] [PubMed] [Google Scholar]
- 6. Jacome AA, Lima EM, Kazzi AI, et al. Epstein-Barr virus-positive gastric cancer: a distinct molecular subtype of the disease? Rev Soc Bras Med Trop 2016; 49: 150–157. [DOI] [PubMed] [Google Scholar]
- 7. Truong CD, Feng W, Li W, et al. Characteristics of Epstein-Barr virus-associated gastric cancer: a study of 235 cases at a comprehensive cancer center in U.S.A. J Exp Clin Cancer Res 2009; 28: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sivachandran N, Dawson CW, Young LS, et al. Contributions of the Epstein-Barr virus EBNA1 protein to gastric carcinoma. J Virol 2012; 86: 60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Camargo MC, Kim WH, Chiaravalli AM, et al. Improved survival of gastric cancer with tumour Epstein-Barr virus positivity: an international pooled analysis. Gut 2014; 63: 236–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gasenko E, Isajevs S, Camargo MC, et al. Clinicopathological characteristics of Epstein-Barr virus-positive gastric cancer in Latvia. Eur J Gastroenterol Hepatol 2019; 31: 1328–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Folprecht G. Tumor mutational burden as a new biomarker for PD-1 antibody treatment in gastric cancer. Cancer Commun (Lond) 2019; 39: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Panda A, Mehnert JM, Hirshfield KM, et al. Immune activation and benefit from avelumab in EBV-positive gastric cancer. J Natl Cancer Inst 2018; 110: 316–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim ST, Cristescu R. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat Med 2018; 24: 1449–1458. [DOI] [PubMed] [Google Scholar]
- 14. Wang F, Wei XL, Wang FH, et al. Safety, efficacy and tumor mutational burden as a biomarker of overall survival benefit in chemo-refractory gastric cancer treated with toripalimab, a PD-1 antibody in phase Ib/II clinical trial NCT02915432. Ann Oncol 2019; 30: 1479–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mishima S, Kawazoe A, Nakamura Y, et al. Clinicopathological and molecular features of responders to nivolumab for patients with advanced gastric cancer. J Immunother Cancer 2019; 7: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen JN, Ding YG, Feng ZY, et al. Association of distinctive Epstein-Barr virus variants with gastric carcinoma in Guangzhou, southern China. J Med Virol 2010; 82: 658–667. [DOI] [PubMed] [Google Scholar]
- 17. Hewitt LC, Inam IZ, Saito Y, et al. Epstein-Barr virus and mismatch repair deficiency status differ between oesophageal and gastric cancer: a large multi-centre study. Eur J Cancer 2018; 94: 104–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kijima Y, Ishigami S, Hokita S, et al. The comparison of the prognosis between Epstein-Barr virus (EBV)-positive gastric carcinomas and EBV-negative ones. Cancer Lett 2003; 200: 33–40. [DOI] [PubMed] [Google Scholar]
- 19. Nogueira C, Mota M, Gradiz R, et al. Prevalence and characteristics of Epstein-Barr virus-associated gastric carcinomas in Portugal. Infect Agent Cancer 2017; 12: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010; 17: 1471–1474. [DOI] [PubMed] [Google Scholar]
- 21. Kim HS, Shin SJ, Beom SH, et al. Comprehensive expression profiles of gastric cancer molecular subtypes by immunohistochemistry: implications for individualized therapy. Oncotarget 2016; 7: 44608–44620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cristescu R, Lee J, Nebozhyn M, et al. Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes. 2015; 21: 449–456. [DOI] [PubMed] [Google Scholar]
- 23. Ramos M, Pereira MA, Dias AR, et al. Lymphoepithelioma-like gastric carcinoma: clinicopathological characteristics and infection status. J Surg Res 2017; 210: 159–168. [DOI] [PubMed] [Google Scholar]
- 24. Wang FH, Shen L, Li J, et al. The Chinese Society of Clinical Oncology (CSCO): clinical guidelines for the diagnosis and treatment of gastric cancer. Cancer Commun (Lond) 2019; 39: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet 2012; 379: 315–321. [DOI] [PubMed] [Google Scholar]
- 26. Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 1389–1396. [DOI] [PubMed] [Google Scholar]
- 27. Fujitani K, Tamura S, Kimura Y, et al. Five-year outcomes of a phase II study of adjuvant chemotherapy with S-1 plus docetaxel for stage III gastric cancer after curative D2 gastrectomy (OGSG1002). Gastric Cancer 2019; 23: 520–530. [DOI] [PubMed] [Google Scholar]
- 28. Hironaka S, Ueda S, Yasui H, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013; 31: 4438–4444. [DOI] [PubMed] [Google Scholar]
- 29. Okines AF, Norman AR, McCloud P, et al. Meta-analysis of the REAL-2 and ML17032 trials: evaluating capecitabine-based combination chemotherapy and infused 5-fluorouracil-based combination chemotherapy for the treatment of advanced oesophago-gastric cancer. Ann Oncol 2009; 20: 1529–1534. [DOI] [PubMed] [Google Scholar]
- 30. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 2008; 358: 36–46. [DOI] [PubMed] [Google Scholar]
- 31. Sym SJ, Hong J, Park J, et al. A randomized phase II study of biweekly irinotecan monotherapy or a combination of irinotecan plus 5-fluorouracil/leucovorin (mFOLFIRI) in patients with metastatic gastric adenocarcinoma refractory to or progressive after first-line chemotherapy. Cancer Chemother Pharmacol 2013; 71: 481–488. [DOI] [PubMed] [Google Scholar]
- 32. Seo JS, Kim TG, Hong YS, et al. Contribution of Epstein-Barr virus infection to chemoresistance of gastric carcinoma cells to 5-fluorouracil. Arch Pharm Res 2011; 34: 635–643. [DOI] [PubMed] [Google Scholar]
- 33. Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017; 390: 2461–2471. [DOI] [PubMed] [Google Scholar]
- 34. Kaizaki Y, Hosokawa O, Sakurai S, et al. Epstein-Barr virus-associated gastric carcinoma in the remnant stomach: de novo and metachronous gastric remnant carcinoma. J Gastroenterol 2005; 40: 570–577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_table_1_3 for Observational cohort study of clinical outcome in Epstein–Barr virus associated gastric cancer patients by Miao-Zhen Qiu, Cai-Yun He, Da-Jun Yang, Da-Lei Zhou, Bai-Wei Zhao, Xiao-Jian Wang, Li-Qiong Yang, Shi-Xun Lu, Feng-Hua Wang and Rui-Hua Xu in Therapeutic Advances in Medical Oncology