Abstract
Oral lubrication mediated by mucin and protein containing salivary conditioning films (SCFs) with strong water retainability can get impaired due to disease such as xerostomia, that is, a subjective dry mouth feel associated with the changed salivary composition and low salivary flow rate. Aberrant SCFs in xerostomia patient cause difficulties in speech, mastication, and dental erosion while the prescribed artificial saliva is inadequate to solve the complications on a lasting basis. With the growing aging population, it is urgently needed to propose a new strategy to restore oral lubrication. Existing saliva substitutes often overwhelm the aberrant SCFs, generating inadequate relief. Here we demonstrated that the function of aberrant SCFs in a patient with Sjögren syndrome can be boosted through mucin recruitment by a simple mucoadhesive, chitosan-catechol (Chi-C). Chi-C with different conjugation degrees (Chi-C7.6%, Chi-C14.5%, Chi-C22.4%) was obtained by carbodiimide chemistry, which induced a layered structure composed of a rigid bottom and a soft secondary SCF (S-SCF) after reflow of saliva. The higher conjugation degree of Chi-C generates a higher glycosylated S-SCF by mucin recruitment and a lower friction in vitro. The layered S-SCF extends the “relief period” for Sjögren patient saliva over 7-fold, measured on an ex vivo tongue-enamel friction system. Besides lubrication, Chi-C-treated S-SCF reduces dental erosion depths from 125 to 70 μm. Chi-C shows antimicrobial activity against Streptococcus mutans. This research provides a new key insight in restoring the functionality of conditioning film at articulating tissues in living systems.
Keywords: oral lubrication enhancement, mucin, dry mouth, Sjögren syndrome, conditioning film, chitosan-catechol
Introduction
Reduced saliva secretion and altered salivary composition are associated with xerostomia, that is, a subjective dry mouth feel, which seriously decreases the quality of patient life. Sjögren syndrome (von Bültzingslöwen et al. 2007; Castro et al. 2013), head and neck radiation therapy (Jellema et al. 2007), and use of medication (Tan et al. 2018) cause xerostomia (dry mouth). Glycoproteins, that is, mucins (Coles et al. 2010) in salivary condition films (SCFs), retain water and yield unmatchable hydration lubrication on the oral surface (Lee and Spencer 2008). However, the aberrant SCFs of xerostomia patients with limited mucins attributed to the changed salivary composition and low salivary flow rate yield poor lubrication associated with dental erosion and dental caries (Liu et al. 2018). To mellow the symptoms, xerostomia patients use saliva substitutes, which contain either food-grade thickeners extracted from animal and plant sources or lubricant molecules like porcine gastric mucins (PGMs), often masking and overwhelming the native SCFs. A Cochrane collaboration (Furness et al. 2011), in which 1,597 patients were included, concluded that topical delivery of saliva substitutes is ineffective in relieving dry mouth symptoms, which is also confirmed by Vinke et al. with the help of a tongue-enamel friction model (Vinke et al. 2018, 2019).
In an actual dry oral cavity with limited saliva, endogenous glycoproteins, including mucins, are available and could potentially be used as a part of treatment instead of being disregarded. Thus, an alternate strategy could be where instead of overwhelming the dry oral cavity with exogenous molecules, we work together and make mucins part of the solution. Intrigued by the fact that cationic polyelectrolytes (Salomaki and Kankare 2009) can improve the mechanical strength of polysaccharide multilayers, we tested their ability to act as an additive to improve oral lubrication by enhancing the SCFs. Chitosan (Kim et al. 2013, 2015) is a nature-derived cationic mucoadhesive with strong electrostatic interactions and a large amount of hydrogen bonding (Neto et al. 2014), especially after being modified with catechol (Chi-C), which endows it with water solubility at a neutral pH (Kim et al. 2015). Chi-C shows a high affinity to glycoprotein, and its oxidized derivative can bioconjugate with amines and cysteine residues of protein or glycoprotein through Michael addition or Schiff bases formation (Mizrahi et al. 2013; Yang et al. 2014; Neto et al. 2016; Ye et al. 2016), while its potential to stabilize SCF has never been investigated. We hypothesize that Chi-C will bind to and absorb on the limited SCF; meanwhile, sessile Chi-C then attracts and recruits glycoproteins from saliva through electrostatic attraction and chemical binding to boost SCFs, which can enhance oral lubrication and resist dental erosion.
Here we tested the above hypothesis based on Chi-C with increasing conjugation degree (Chi-C7.6%, Chi-C14.5%, Chi-C22.4%). The kinetics of SCF formation, the modification with Chi-C, and the formation of secondary SCF (S-SCF) during reflow of saliva were monitored by quartz crystal microbalance with dissipation (QCM-D). The composition alteration in SCFs was determined by X-ray photoelectron spectroscopy (XPS). Lubrication properties of S-SCF with Chi-C treatment at the nanoscale were investigated by colloidal probe atomic force microscopy (AFM), and saliva from a patient with Sjögren syndrome who had Chi-C treatment was evaluated on an ex vivo tongue-enamel friction system (Vinke et al. 2018) at the macroscale. The antimicrobial efficacy of Chi-C and the ability of Chi-C-treated S-SCF to resist dental erosion were also tested.
Materials and Methods
Chi-C Synthesis, Formation of SCF, Chi-C Treatment of SCF, Formation of S-SCF, Change in SCF Composition, and Lubrication at the Nanoscale
Chi-C was synthesized using carbodiimide chemistry (Kim et al. 2013; Wang, Song, et al. 2017; Wang, Li, et al. 2017) and details are presented in the Appendix. A standard protocol (Veeregowda et al. 2012) was followed to collect and prepare stimulated whole saliva (SWS) from both healthy and patient volunteers. SWS from 4 healthy individuals (healthy saliva [HS]) and 4 patients (patient saliva [PS]) with Sjögren syndrome was collected for tongue-enamel friction measurement. All saliva collection and use were performed under the approval of the Medical Ethics Review Board of the University Medical Center Groningen (approval numbers M17.217043, M09.069162, M17.2157256, and UMCG IRB #2008109). A QCM-D device (E-4; Q-sense) was used to study the structural softness and formation kinetics of SCFs in vitro with or without Chi-C treatment. Briefly, saliva was allowed to flow over a cleaned QCM-D sensor with 50 µL/min at 25°C for 2 h, corresponding with a shear rate of 3 s−1, followed by perfusion with buffer or 0.05% w/v of Chi-C for 2 min, and finally saliva was allowed to reflow for another 2 h to form a secondary SCF (S-SCF). In between each step, the chamber was perfused with buffer for 15 min to remove unattached molecules from the tubes, chamber, and crystal surface. The crystals coated by a S-SCF with or without Chi-C treatment were taken out of the QCM-D for surface composition analysis by X-ray photoelectron spectroscopy (details in the Appendix) and nano-lubricity evaluation by colloidal probe AFM (details in the Appendix).
Tongue-Enamel Friction System
The friction measurements at macroscale were performed in a reciprocating sliding between the tongue and enamel using the same protocol as Vinke et al. (2018) with a universal mechanical tester (UMT-3; CETR, Inc.) under a normal force of 0.25 N at a sliding velocity of 4 mm/s and sliding distance of 20 mm. The ratio of measured friction force and applied normal force was taken as the coefficient of friction (COF).
First, the enamel was slid against the tongue for 10 cycles to obtain dry baseline and mimic dry mouth (Vinke et al. 2018). Then, a drop of 20 µL SWS from healthy controls or patients with Sjögren syndrome was placed at the tongue-enamel interface and rubbed for 4 cycles to spread the saliva on the tongue surface and form the SCF, followed by another 20 µL of buffer or Chi-C22.4% and 4 cycles of rubbing (step 3). Finally, another 20 µL of healthy or patient SWS was added to form the S-SCFs and the sliding was continued. The ratio between COF measured in dry baseline and wet S-SCFs was designated as “relief” using equation (1). The duration for which the COF remained low was designated as the “relief period.”
| (1) |
Dental Erosion Protection and Antimicrobial and Biocompatibility of Chi-C
Dental erosion was tested using an established protocol (Jager et al. 2008, 2012) on the bovine incisors, and the detailed protocol is presented in the Appendix. The antimicrobial efficacy of Chi-C was tested on Streptococcus mutans, and safety of Chi-C-treated S-SCF use was tested using the mouse fibroblastic cell line (L929) and human kidney epithelial cells with the help of the XTT assay (Zhou et al. 2017) and microscopic examination of the cells. The protocols are described in detail in the Appendix.
Statistical Analysis
All data are expressed as mean ± SD. Statistical differences between groups were determined with a 2-tailed Student’s t test, with significance set at P < 0.05.
Results
Preparation and Characterization of Chi-C
Chi-C with 3 conjugation degrees was successfully synthesized and respectively called Chi-C7.6%, Chi-C14.5%, and Chi-C22.4%. H-NMR (nuclear magnetic resonance) and UV-Vis spectrophotometry helped prove conjugation (Appendix Fig. 1) and calculation of the conjugation degree; detailed protocol and results are described in the Appendix.
In Vitro Modification of the SCF Due to Chi-C
After 2 h of salivary flow on the bare QCM-D sensor, a large amount of salivary protein adsorption took place, as shown by a large frequency shift (∆f3) of −70 ± 10 Hz and a dissipation (∆D3) greater than 10−5 (Fig. 1). Exposure of SCF to buffer (Fig. 1A) yielded a small change in ∆f3 and ∆D3, while exposure to Chi-C solutions (0.5 mg/mL) with 3 conjugation degrees provided a significant increase in ∆f3 (Fig. 1E) and a decrease in ∆D3/∆f3 (Fig. 1B–D) (dotted bars in Fig. 1F), suggesting a Chi-C-induced compaction of the SCF with hydrogen bonding, electrostatic attraction, and covalent bonding irrespective of the conjugation degree. To mimic the oral situation, saliva was reintroduced in the QCM-D, which caused renewed adsorption of salivary proteins and the formation of S-SCF (Fig. 1A–D). The higher conjugate degree of Chi-C14.5% (–160 ± 15 Hz) and Chi-C22.4% (–170 ± 8.6 Hz) led to larger frequency shifts and higher ∆D3/∆f3 (striped bars in Fig. 1F) as compared to Chi-C7.6% (–130 ± 10 Hz) and buffer (–75 ± 10 Hz) exposure.
Figure 1.
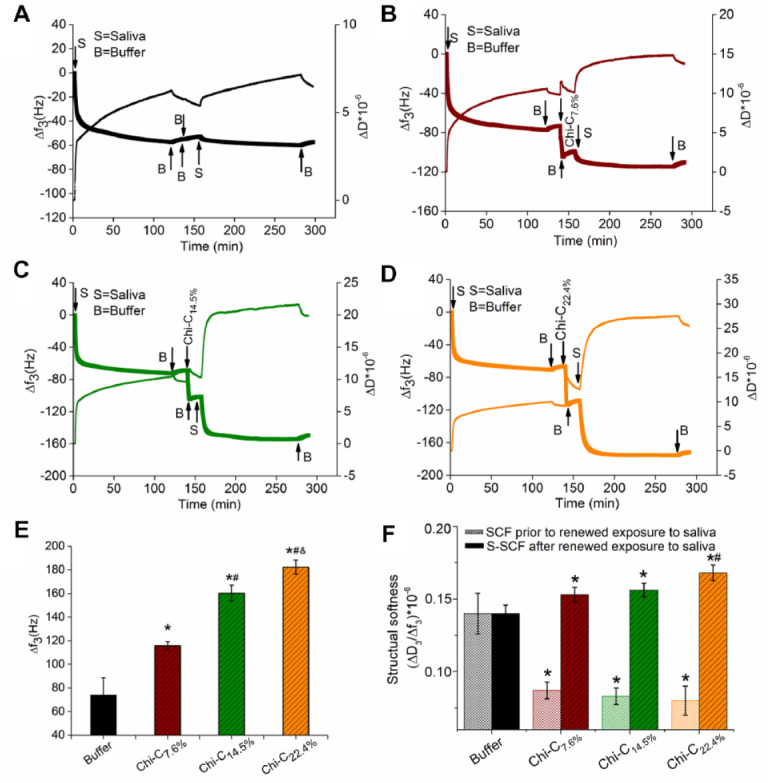
Kinetics of salivary conditioning film (SCF) formation, chitosan-catechol (Chi-C) adsorption to SCF, and renewed exposure to saliva to get S-SCF using the quartz crystal microbalance with dissipation (QCM-D). The mass adsorption was quantified by frequency shift and structural softness by calculating the ratio between dissipation and frequency shift. SCF treated with buffer (A), Chi-C7.6% (B), Chi-C14.5% (C), and Chi-C22.4% (D), respectively. (E) Frequency shift after renewed exposure to saliva and (F) structural softness of SCF with and without (buffer) Chi-C adsorption and renewed exposure to saliva. Error bar represents the standard deviation over 3 independent measurements. *Statistically significant (P < 0.05, 2-tailed Student’s t test) differences in softness and frequency compared to control film. #Significant differences in frequency or softness of S-SCF with Chi-C22.4% treatment compared to S-SCF with Chi-C7.6% treatment. &Significant difference in frequency between S-SCF with Chi-C14.5% and Chi-C22.4% treatment.
In Vitro Changes in Topography, Lubrication, and Composition of S-SCFs Induced by Chi-C
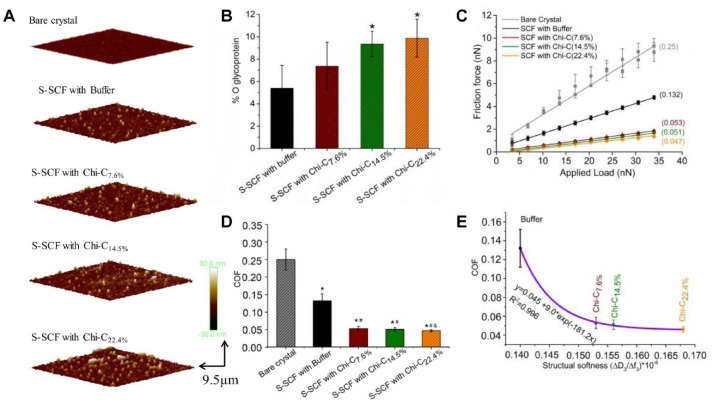
Salivary protein adsorption was also evident from the morphology of the S-SCFs as investigated by AFM and shown in Figure 2A. Bare sensor shows a smooth surface, but uneven, globular structures appeared after the adsorption of salivary protein with heights of about 23 ± 4 nm. Numerous similar 30 ± 7 nm high structures were observed in S-SCF with Chi-C7.6%, 38 ± 5 nm in S-SCF with Chi-C14.5%, and 37 ± 7 nm in S-SCF with Chi-C22.4%. Mucins in the SCFs are believed to be adsorbed in the form of loops and trains on the surface (Coles et al. 2010), and higher globular structures were found in S-SCFs with Chi-C, especially in Chi-C22.4% and Chi-C14.5%, which could be caused by a large amount of mucin recruitment on the top layer.
Figure 2.
Secondary salivary conditioning film (S-SCF) change in composition, measured using X-ray photoelectron spectroscopy (XPS), in topography and nanofriction, measured by atomic force microscopy (AFM) and colloidal probe. (A) Surface topography of bare Au-coated crystal and S-SCF treated with buffer, Chi-C7.6%, Chi-C14.5%, and Chi-C22.4%, respectively. (B) The amount of glycoprotein (%O) in S-SCF with buffer or different conjugate degree Chi-C treatment was obtained from decomposition of O1s photoelectron peak in XPS. (C) Friction force versus applied load curves of bare quartz crystal microbalance with dissipation (QCM-D) crystal and S-SCF treated with buffer or Chi-C, respectively. (D) Coefficient of friction (COF) of each S-SCF calculated by slope of the linear fitting. (E) Correlation between structural softness of S-SCF and COF, and the higher structural softness of S-SCF the lower COF of S-SCF in vitro with colloidal probe AFM was achieved. *Statistically significant differences (P < 0.05) in the content of glycoprotein in S-SCF with Chi-C treatment with respect to S-SCF with buffer treatment in (B), or in COF between S-SCF and bare crystal in (D). #Statistically significant (P < 0.05) difference in COF of S-SCF treated with Chi-C respect to S-SCF treated with buffer. &Significant difference in COF between S-SCF with Chi-C22.4.% and Chi-C7.6% treatment, respectively. Chi-C, chitosan-catechol.
Mucin recruitment was confirmed by the increased glycosylation of the S-SCFs with different Chi-C treatment (Fig. 2B, Appendix Fig. 3, Table, Appendix Table 1) measured using X-ray photoelectron spectroscopy. The result from the Table showed a different relative content of C, O, N. The C1s spectra of each surface could be deconvoluted into 4 different peaks: C-(C,H), C-N/C-O, and O-C-O/ O=C-O, and their percentages for S-SCF with or without Chi-C treatment were different, as shown in Appendix Figure 3 and the Table, suggesting different proteins were present on the surface. In S-SCF with Chi-C treatment, the relative content of C-C was slightly decreased while the C-N was increased in the Table, which may be attributed to the protein or glycoprotein recruited to the surface. As for the O1s spectra, they could be deconvoluted into 2 components: O=C-N and O=C-O, considered the O from the protein and glycol group, respectively. The relative contents of glycoprotein could be calculated by the integral of O1s at 532.7ev in Figure 2B and Appendix Figure 2. The higher amount of O1s at 532.7ev (Veeregowda et al. 2012), that is, about 9.88 ± 1.6 and 9.35 ± 1.3, was achieved in SCF with Chi-C22.4% and Chi-C14.5% modification, respectively, while only 5.39 ± 2.25 and 7.04 ± 2.6 were detected in SCF with buffer and Chi-C7.6% treatment, respectively. This indicates that Chi-C can recruit glycoprotein on the SCF surface to increase the glycosylation, which is in agreement with the higher ∆D3/∆f3 measured by QCM-D (Fig. 1F). The QCM-D crystals with S-SCFs were mounted under the colloidal probe AFM for lubrication evaluation. On the bare gold (Au), Ff increased linearly with Fn, corresponding to a COF of 0.25 ± 0.03 (Fig. 2C, D), which is consistent with the literature (Veeregowda et al. 2013). Formation of S-SCF with an intermediate exposure to buffer deceased the COF to 0.132 ± 0.021 (Fig. 2C, D). S-SCFs with intermediate exposure to Chi-C further decreased the COF to 0.053 ± 0.0052 with Chi-C7.6% and to 0.051 ± 0.0053 with Chi-C14.5%, and the extremely low COF was observed on S-SCFs with intermediate exposure to Chi-C22.4%, about 0.047 ± 0.0031. A clear correlation between the increasing structural softness and decreasing COF was obtained (Fig. 2E). A plateau with respect to COF was achieved with Chi-C22.4%, indicating that any further increase in the conjugation degree would probably not provide any further decrease in friction. The lowest COF was detected for S-SCF with Chi-C22.4% treatment, corresponding to the highest mass adsorption (Fig. 1E), highest structural softness (Fig. 1F), and highest glycosylation (Fig. 2D). Since Chi-C caused rigidification (Fig. 1F) of the lower layer (SCF) irrespective of the Chi-C conjugation degree, the decrease in friction can be mainly attributed to increased softness of the top layer (S-SCF) due to mucin recruitment.
Table.
Surfaces Chemical Bonding of S-SCF with or without (PBS) Chi-C Treatment.
| C1s Binding Energy and Relative Area (%) | O1s Binding Energy and Relative Area (%) | |||||
|---|---|---|---|---|---|---|
| Samples | C-C | C-N | O-C-O | O-C=O | N-C=O | O=C-O |
| S-SCF-PBS | 61.6 | 22.4 | 12.3 | 3.7 | 66.3 | 33.7 |
| S-SCF-Chi-C7.6% | 52.2 | 30.2 | 14.9 | 2.7 | 49.9 | 50.1 |
| S-SCF-Chi-C14.5% | 56.3 | 27.5 | 13.8 | 2.4 | 48.7 | 51.3 |
| S-SCF-Chi-C22.4% | 58.5 | 30.5 | 7.7 | 3.3 | 43.3 | 56.7 |
Chi-C, chitosan-catechol; PBS, phosphate-buffered saline; S-SCF, secondary salivary conditioning film.
Translation of an In Vitro Observation to the Ex Vivo Stage and for Patient Saliva
Chi-C22.4% was chosen to explore its potential lubrication enhancement efficacy with saliva from patients with Sjögren syndrome (an etiology of xerostomia) on the tongue-enamel system (Vinke et al. 2018; Vinke et al., unpublished data) using real biological tissue, which can provide information for relief and relief period. Consistent with the observations in previous studies, a COFdry of around 2.5 was observed (Fig. 3). A sharp drop in COF was observed after formation of initial SCF with an introduction of 20 µL saliva (called COFwet). From Figure 3C and D (black up arrow), a slight increase in COFwet is clearly visible both for PS and HS immediately after the interaction of Chi-C22.4%, with the SCF indicating a very strong stabilization and compaction due to the hydrogen bond, irreversible covalent formation, and electrostatic attraction (Fig. 1F). Upon reexposure to 20 µL saliva, the COF again decreases, which is caused by the formation of a softer S-SCF by recruiting salivary glycoproteins (Fig. 2C). No significant difference was found in the relief between PS and HS after treatment with either buffer (5.2 ± 1.2-fold and 4.9 ± 1.2-fold, respectively) or Chi-C22.4% (5.1 ± 1.1-fold and 5.0 ± 1.3-fold, respectively). The relief period (Fig. 3F) for Chi- C22.4% treated S-SCFs was drastically extended both for PS (25 ± 4.8 min) and HS (36 ± 3.3 min) compared to buffer-treated SCFs, that is, PS (3.3 ± 1.3 min) and HS (7.2 ± 0.3 min). The longer relief period is attributed to Chi-C22.4%, which stabilized the SCF and recruited salivary glycoproteins to form a very soft S-SCF (Figs. 1F and 3G).
Figure 3.
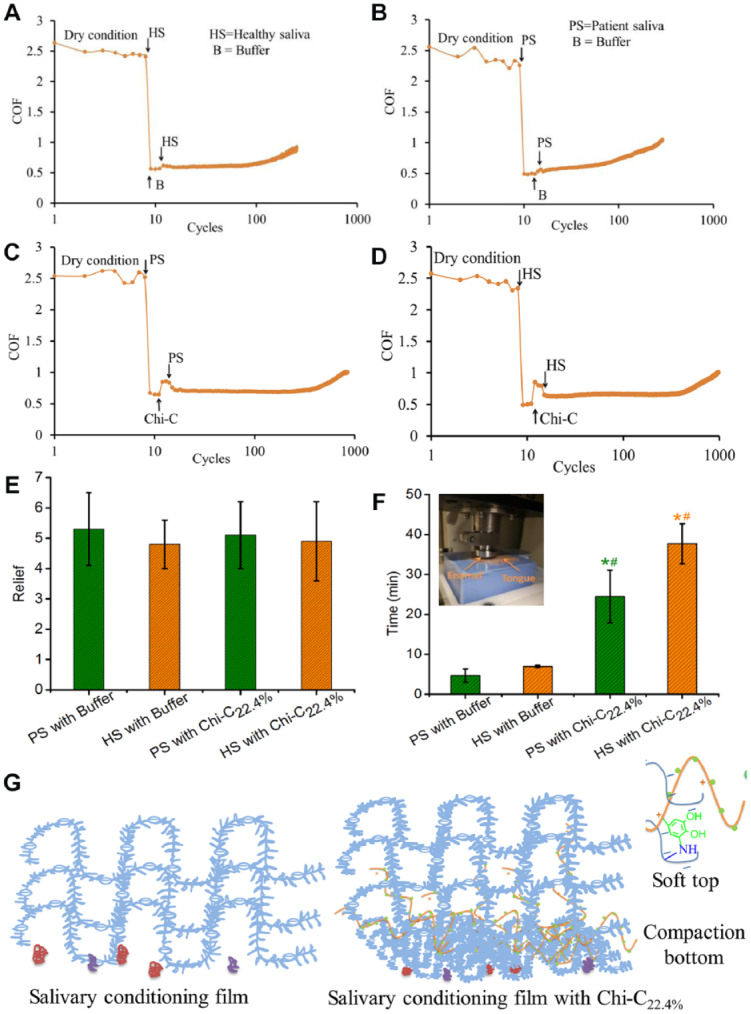
Relief and relief period of the secondary salivary conditioning film (S-SCF) with patient saliva (PS) and healthy saliva (HS) in an ex vivo tongue-enamel friction system. The stimulated saliva from 4 healthy volunteers (a flow rate of 3.36, 1.76, 1.04, 1.02 mL/min) and 4 patients with Sjögren syndrome (an etiology of xerostomia) with a reduced flow rate (0.48, 0.72, 0.45, 0.98 mL/min) was collected to transfer this strategy to a real biological tissue at macroscale. (A) Healthy S-SCF treated with buffer and (B) patient S-SCF treated with buffer. (C) Patient S-SCF and (D) healthy S-SCF treated with Chi-C22.4%, respectively. (E) Relief induced by patient and healthy S-SCF with buffer and Chi-C22.4% treatment. (F) Relief period of patient and healthy S-SCF with buffer and Chi-C22.4% treatment. (G) Schematic of Chi-C interaction with salivary mucin and forming a softer S-SCF. Error bar represents the standard deviation over 3 independent measurements. *Statistically significant (P < 0.05, 2-tailed Student’s t test) differences in relief period of healthy S-SCF with buffer and healthy S-SCF with Chi-C22.4% and patient S-SCF with Chi-C22.4% with respect to patient S-SCF with buffer. #Significant differences in relief period of healthy S-SCF with Chi-C22.4% and patient S-SCF with Chi-C22.4% with respect to healthy S-SCF with buffer. Chi-C, chitosan-catechol.
Chi-C Is Antimicrobial, and Chi-C-Treated S-SCF Decreases Dental Erosion and Remains Biocompatible
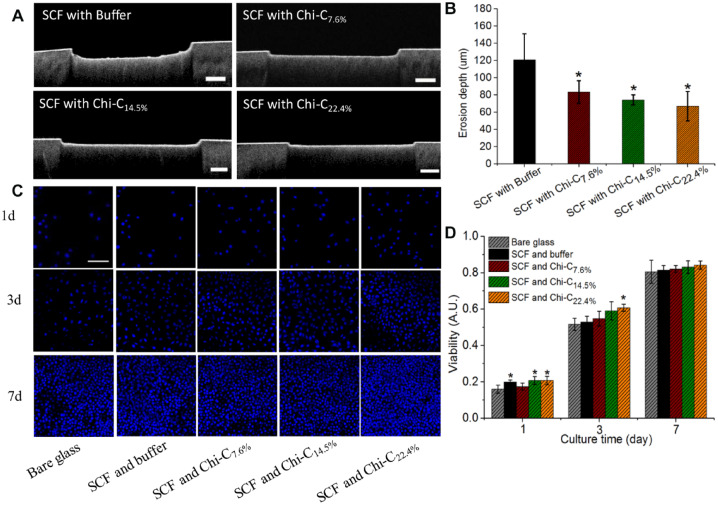
The ability of S-SCF to resist dental erosion increases after the modification with Chi-C, as shown in Figure 4. The erosion depth of enamel with buffer-treated (control) S-SCF was about 125 ± 24 µm, which decreased to 83 ± 19 µm with Chi-C7.6% treatment, 76 ± 11 µm with Chi-C14.5%, and 70 ± 15 µm with Chi-C22.4% treatment. Even if there was no significant difference in erosion depths for different conjugation densities of Chi-C, Chi-C22.4% treatment S-SCF caused a significant drop by 44% in the erosion depth as compared to buffer treatment. The erosion prevention could be due to obstruction in citric acid diffusion toward the enamel or Ca2+ diffusion outward. Both these effects are related to an increase in the thickness of the S-SCF (Fig. 1) caused by Chi-C-induced mucin recruitment and a decrease in the negative charge density within the S-SCF due to the chitosan molecule.
Figure 4.
Dental erosion prevention and safety of chitosan-catechol (Chi-C). (A) Erosion depth under different conditions by optical coherence tomography images. (B) The erosion depths were quantified from 3 different samples coated with secondary salivary conditioning film (S-SCF) treated with buffer, Chi-C7.6%, Chi-C14.5%, and Chi-C22.4%, respectively. (C) Chi-C treatment of salivary conditioning film (SCF) caused higher L929 proliferation. Fluorescent images of L929 cells were stained with DAPI at days 1, 3, and 7. (D) Cell metabolic activity measured by XTT. Statistical differences are marked by *P < 0.05.
The safety of Chi-C use was tested on rat L292 and human kidney epithelial cells. The result is shown in Figure 4C, D and Appendix Figure 7. The overview images on each surface clearly display more cells presented over culture time. After 3 d of proliferation, the SCF modified with Chi-C22.4% even showed a higher metabolic activity, which may have been caused by the S-SCF surface with a certain roughness (Fig. 2E and Appendix Fig. 7) or a higher softness (Fig. 1F). The cytotoxicity of Chi-C, tested on 2 cell lines, in all conjugation degrees was negligible, indicating that Chi-C is completely safe for biomedical applications.
Catechol conjugation of chitosan increased its antimicrobial efficacy on S. mutans UA159, as shown in Appendix Table 2 and Appendix Figure 5.
Discussion
An urgent need exists to develop a new strategy to restore oral lubrication for xerostomia patients. Most of the current artificial saliva focuses on optimizing the viscosity, although it has been shown that there is only little correlation between viscosity and ability to lubricate the oral cavity (Hahnel et al. 2009; Furness et al. 2011). Unlike the existing saliva substitutes, which overwhelm the oral cavity with exogenous molecules, we propose to work with the highly evolved natural salivary lubrication system, howsoever aberrant due to disease. Chi-C demonstrates the ability to stabilize SCF by the formation of a layered structure with a rigid lower layer due to physical and chemical attraction and followed by formation of the very soft top layer (S-SCF) in Figure 1. The ratio between dissipation and frequency shift (∆D3/∆f3) was larger than 10−6, indicating that a hydrated, soft SCF formed on the QCM-D crystal. This observation is consistent with the findings of Veeregowda et al. (2012). The ∆D3/∆f3 for Chi-C-treated S-SCF increased irrespective of the conjugation degree while no obvious changes were found with buffer treatment. Chi-C7.6% with a low conjugation degree performed less efficiently in salivary protein recruitment than a higher conjugation degree, indicating that electrostatic attraction does not play a major role in salivary protein recruitment when Chi-C is involved. A similar phenomenon was found by Kim et al. (2015), who reported that a higher conjugation degree of Chi-C resulted in effective association of Chi-C to glycoprotein. The soft top layer is composed of mucins recruited from the saliva, as shown by the increase in glycosylation (Fig. 2B) and layer softness (Fig. 1F). This layered structure of S-SCF decreased the friction when measured on a nanoscale (Fig. 2), but at macroscale (ex vivo) with real tissue and saliva from patients with Sjögren syndrome, no difference in friction (i.e., relief; Fig. 3E) was observed for S-SCFs with Chi-C treatment as compared to control. Often it is difficult to bridge the gap between macro- and micro/nanoscale friction (Tirrell and Meyer 2001; Ren et al. 2003; Stoyanov and Chromik 2017). The sliding speeds, applied loads, and sliding surfaces during the measurements on colloidal probe AFM and tongue-enamel are different, yielding different results.
The advantage of Chi-C treatment of S-SCF was the enhancement of the relief period (Fig. 3F) for both HS and PS. The relief period for PS was significantly lower as compares to HS after treatment with Chi-C. Chaudhury et al. (2015) have shown that although the concentration of mucins MUC5B and MUC7 was similar between patients with dry mouth and controls, a comparison of protein and glycan staining identified a reduction in mucin glycosylation, especially in MUC7, for patients with Sjögren syndrome (Chaudhury et al. 2016). ATR-FTIR (attenuated total reflectance Fourier-transform infrared spectroscopy) measurements (Song et al. 2016) on the saliva used in our study (Appendix Fig. 4) confirmed lower glycosylation in the 4 PS samples as compared to HS. Chi-C is able to recruit mucins from PS on the SCF, but due to lower glycosylation, the S-SCF still has lower water-holding ability, which results in a lower relief period.
Based on the result from the tongue-enamel friction system, where the total amount of fluid is the same but the relief period is different, the surface-bound molecules take precedence in lubrication, that is, attracting mucin on SCF yields a long-lasting relief period. Treatment with Chi-C gives rise to a softer (Fig. 1F) and thicker (Fig. 1E) top layer, which is able to hold the water for a longer period of time and hence the long relief period. A similar mechanism was found by Singh et al. (2014), in which hyaluronan binding peptides were able to restore the lubrication of degraded cartilage. Besides lubrication, the presence of SCFs is essential to reduce dental erosion (Busscher et al. 2007; Santos et al. 2010). Our results (Fig. 4A, B) show that the layered S-SCFs formed due to Chi-C treatment decrease dental erosion. Yet another problem of xerostomia patients is the high risk of developing oral infections. Chitosan is known for its broad-spectrum antibiotic activity, but this activity is limited due to its low solubility at neutral pH. Conjugation with catechol enhances chitosan solubility (Kim et al. 2013) and causes a 4-fold reduction in its minimal inhibitory concentration (MIC) for Staphylococcus epidermidis (Amato et al. 2018). We tested the antibacterial activity of Chi-C on the more relevant S. mutans UA159. Both the MIC (0.5 mg/mL) and the minimal bactericidal concentration (MBC) (1 mg/mL) for S. mutans were reduced with Chi-C compared to the Chi with a higher MIC (1 mg/mL) and MBC (2 mg/mL) (Appendix Table 2 and Appendix Fig. 5). The promising ex vivo results obtained for the use of Chi-C indicate that this molecule is a strong candidate for human trials.
In summary, the strategy to work together with an impaired but highly evolved natural lubrication system is promising. A simple mucoadhesive, Chi-C, is able to bind to the SCF and recruit mucins from the saliva by both physisorption and chemisorption to form a nanocomposite S-SCF (rigid bottom and soft top) to enhance oral lubrication. These structural and compositional adjustments in the S-SCF extend the relief period at the macroscale with saliva from patients with Sjögren syndrome and decrease dental erosion. Thus, Chi-C22.4% is a strong candidate molecule as an additive to future artificial saliva formulations.
Author Contributions
H. Wan, contributed to conception, design, data acquisition, analysis, and interpretation, drafted the manuscript; A. Vissink, contributed to conception, critically revised the manuscript; P.K. Sharma, contributed to conception, design, and data interpretation, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034520917675 for Enhancement in Xerostomia Patient Salivary Lubrication Using a Mucoadhesive by H. Wan, A. Vissink and P.K. Sharma in Journal of Dental Research
Acknowledgments
We are thankful to all healthy volunteers and patients with Sjögren syndrome who donated SWS. The UMT-3 tribometer (Bruker) setup was purchased thanks to grant ZonMW91112026 from the Netherlands Organization for Health Research and Development. We also thank the China Scholarship Council for a 4-y scholarship to Dr. H. Wan to pursue her PhD in the Netherlands.
Footnotes
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
A supplemental appendix to this article is available online.
ORCID iD: P.K. Sharma  https://orcid.org/0000-0002-8342-8939
https://orcid.org/0000-0002-8342-8939
References
- Amato A, Migneco LM, Martinelli A, Pietrelli L, Piozzi A, Francolini I. 2018. Antimicrobial activity of catechol functionalized-chitosan versus Staphylococcus epidermidis. Carbohydr Polym. 179:273–281. [DOI] [PubMed] [Google Scholar]
- Busscher HJ, White DJ, Atema-Smit J, van der Mei HC. 2007. Efficacy and mechanisms of non-antibacterial, chemical plaque control by dentifrices—an in vitro study. J Dent. 35(4):294–301. [DOI] [PubMed] [Google Scholar]
- Castro I, Sepúlveda D, Cortés J, Quest AF, Barrera MJ, Bahamondes V, Aguilera S, Urzúa U, Alliende C, Molina C, et al. 2013. Oral dryness in Sjögren’s syndrome patients: not just a question of water. Autoimmun Rev. 12(5):567–574. [DOI] [PubMed] [Google Scholar]
- Chaudhury NM, Shirlaw P, Pramanik R, Carpenter GH, Proctor GB. 2015. Changes in saliva rheological properties and mucin glycosylation in dry mouth. J Dent Res. 94(12):1660–1667. [DOI] [PubMed] [Google Scholar]
- Chaudhury NMA, Proctor GB, Karlsson NG, Carpenter GH, Flowers SA. 2016. Reduced mucin-7 (muc7) sialylation and altered saliva rheology in Sjögren’s syndrome associated oral dryness. Mol Cell Proteomics. 15(3):1048–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles JM, Chang DP, Zauscher S. 2010. Molecular mechanisms of aqueous boundary lubrication by mucinous glycoproteins. Curr Opin Colloid Interface Sci. 15(6):406–416. [Google Scholar]
- Furness S, Worthington HV, Bryan G, Birchenough S, McMillan R. 2011. Interventions for the management of dry mouth: topical therapies. Cochrane Database Syst Rev. 8(12):CD008934. [DOI] [PubMed] [Google Scholar]
- Hahnel S, Behr M, Handel G, Bürgers R. 2009. Saliva substitutes for the treatment of radiation-induced xerostomia—a review. Support Care Cancer. 17(11):1331–1343. [DOI] [PubMed] [Google Scholar]
- Jager DH, Vieira AM, Ruben JL, Huysmans MC. 2008. Influence of beverage composition on the results of erosive potential measurement by different measurement techniques. Caries Res. 42(2):98–104. [DOI] [PubMed] [Google Scholar]
- Jager DH, Vieira AM, Ruben JL, Huysmans MC. 2012. Estimated erosive potential depends on exposure time. J Dent. 40(12):1103–1108. [DOI] [PubMed] [Google Scholar]
- Jellema AP, Slotman BJ, Doornaert P, Leemans CR, Langendijk JA. 2007. Impact of radiation-induced xerostomia on quality of life after primary radiotherapy among patients with head and neck cancer. Int J Radiat Oncol Biol Phys. 69(3):751–760. [DOI] [PubMed] [Google Scholar]
- Kim K, Ryu JH, Lee DY, Lee H. 2013. Bio-inspired catechol conjugation converts water-insoluble chitosan into a highly water-soluble, adhesive chitosan derivative for hydrogels and LbL assembly. Biomater Sci. 1(7):783–790. [DOI] [PubMed] [Google Scholar]
- Kim K, Kim K, Ryu JH, Lee H. 2015. Chitosan-catechol: a polymer with long-lasting mucoadhesive properties. Biomaterials. 52:161–170. [DOI] [PubMed] [Google Scholar]
- Lee S, Spencer ND. 2008. Materials science. Sweet, hairy, soft, and slippery. Science. 319(5863):575–576. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ren Y, Li Y, Su L, Zhang Y, Huang F, Liu Jinjian, Liu Jianfeng, van Kooten TG, An Y, et al. 2018. Nanocarriers with conjugated antimicrobials to eradicate pathogenic biofilms evaluated in murine in vivo and human ex vivo infection models. Acta Biomater. 79:331–343. [DOI] [PubMed] [Google Scholar]
- Mizrahi B, Shankarappa SA, Hickey JM, Dohlman JC, Timko BP, Whitehead KA, Lee JJ, Langer R, Anderson DG, Kohane DS. 2013. A stiff injectable biodegradable elastomer. Adv Funct Mater. 23(12):1527–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto AI, Cibrão AC, Correia CR, Carvalho RR, Luz GM, Ferrer GG, Botelho G, Picart C, Alves NM, Mano JF. 2014. Nanostructured polymeric coatings based on chitosan and dopamine-modified hyaluronic acid for biomedical applications. Small. 10(12):2459–2469. [DOI] [PubMed] [Google Scholar]
- Neto AI, Vasconcelos NL, Oliveira SM, Ruiz-Molina D, Mano JF. 2016. High-throughput topographic, mechanical, and biological screening of multilayer films containing mussel-inspired biopolymers. Adv Funct Mater. 26(16):2745–2755. [Google Scholar]
- Ren S, Yang S, Zhao Y. 2003. Micro- and macro-tribological study on a self-assembled dual-layer film. Langmuir. 19(7):2763–2767. [Google Scholar]
- Salomaki M, Kankare J. 2009. Influence of synthetic polyelectrolytes on the growth and properties of hyaluronan-chitosan multilayers. Biomacromolecules. 10(2):294–301. [DOI] [PubMed] [Google Scholar]
- Santos O, Lindh L, Halthur T, Arnebrant T. 2010. Adsorption from saliva to silica and hydroxyapatite surfaces and elution of salivary films by SDS and delmopinol. Biofouling. 26(6):697–710. [DOI] [PubMed] [Google Scholar]
- Singh A, Corvelli M, Unterman SA, Wepasnick KA, McDonnell P, Elisseeff JH. 2014. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat Mater. 13(10):988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Hou J, van der Mei HC, Veeregowda DH, Busscher HJ, Sjollema J. 2016. Antimicrobials influence bond stiffness and detachment of oral bacteria. J Dent Res. 95(7):793–799. [DOI] [PubMed] [Google Scholar]
- Stoyanov P, Chromik RR. 2017. Scaling effects on materials tribology: from macro to micro scale. Materials (Basel). 10(5). pii: E550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan ECK, Lexomboon D, Sandborgh-Englund G, Haasum Y, Johnell K. 2018. Medications that cause dry mouth as an adverse effect in older people: a systematic review and metaanalysis. J Am Geriatr Soc. 66(1):76–84. [DOI] [PubMed] [Google Scholar]
- Tirrell M, Meyer E. 2001. Discussion forum report: bridging the gap between macro- and micro/nanoscale adhesion and friction. In: Bhushan B, editor. Fundamentals of tribology and bridging the gap between the macro- and micro/nanoscales. Dordrecht (Netherlands): Springer; p. 355–357. [Google Scholar]
- Veeregowda DH, Busscher HJ, Vissink A, Jager DJ, Sharma PK, van der Mei HC. 2012. Role of structure and glycosylation of adsorbed protein films in biolubrication. PLoS One. 7(8):e42600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veeregowda DH, Kolbe A, van der Mei HC, Busscher HJ, Herrmann A, Sharma PK. 2013. Recombinant supercharged polypeptides restore and improve biolubrication. Adv Mater. 25(25):3426–3431. [DOI] [PubMed] [Google Scholar]
- Vinke J, Kaper HJ, Vissink A, Sharma PK. 2018. An ex vivo salivary lubrication system to mimic xerostomic conditions and to predict the lubricating properties of xerostomia relieving agents. Sci Rep. 8(1):9087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinke J, Kaper HJ, Vissink A, Sharma PK. 2019. Saliva substitutes capable of modifying the existing salivary condition film by adsorption improves oral lubrication. Clin Oral Investig. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bültzingslöwen I, Sollecito TP, Fox PC, Daniels T, Jonsson R, Lockhart PB, Wray D, Brennan MT, Carrozzo M, Gandera B, et al. 2007. Salivary dysfunction associated with systemic diseases: systematic review and clinical management recommendations. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 103(Suppl):S57.e1–15. [DOI] [PubMed] [Google Scholar]
- Wang R, Li J, Chen W, Xu T, Yun S, Xu Z, Xu Z, Sato T, Chi B, Xu H. 2017. A biomimetic mussel-inspired ε-poly-l-lysine hydrogel with robust tissue-anchor and anti-infection capacity. Adv Funct Mater. 27(8):1604894. [Google Scholar]
- Wang R, Song X, Xiang T, Liu Q, Su B, Zhao W, Zhao C. 2017. Mussel-inspired chitosan-polyurethane coatings for improving the antifouling and antibacterial properties of polyethersulfone membranes. Carbohydr Polym. 168:310-319. [DOI] [PubMed] [Google Scholar]
- Yang C, Ding X, Ono RJ, Lee H, Hsu LY, Tong YW, Hedrick J, Yang YY. 2014. Brush-like polycarbonates containing dopamine, cations, and PEG providing a broad-spectrum, antibacterial, and antifouling surface via one-step coating. Adv Mater. 26(43):7346–7351. [DOI] [PubMed] [Google Scholar]
- Ye H, Xia Y, Liu Z, Huang R, Su R, Qi W, Wang L, He Z. 2016. Dopamine-assisted deposition and zwitteration of hyaluronic acid for nanoscale fabrication of low-fouling surfaces. J Mater Chem B. 4(23):4084–4091. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Castañeda Ocampo O, Guimarães CF, Kühn PT, van Kooten TG, van Rijn P. 2017. Screening platform for cell contact guidance based on inorganic biomaterial micro/nanotopographical gradients. ACS Appl Mater Interfaces. 9(37):31433–31445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034520917675 for Enhancement in Xerostomia Patient Salivary Lubrication Using a Mucoadhesive by H. Wan, A. Vissink and P.K. Sharma in Journal of Dental Research