Highlights
-
•
Pain symptoms in adolescents are associated with default mode network connectivity.
-
•
More frequent pain is associated with more connectivity to the superior frontal gyrus.
-
•
Higher pain intensity is associated with less connectivity to the cerebellum.
Keywords: Resting state, Default mode network, Superior frontal gyrus, Adolescence, Pain
Abstract
Pain during adolescence is common and is associated with future pain chronicity and mental health in adulthood. However, understanding of the neural underpinnings of chronic pain has largely come from studies in adults, with recent studies in adolescents suggesting potentially unique neural features during this vulnerable developmental period. In addition to alterations in the pain network, resting state functional magnetic resonance imaging studies in adults suggest alterations in the default mode network (DMN), involved in internally-driven, self-referential thought, may underlie chronic pain; however, these findings have yet to be examined in adolescents. The current study sought to investigate associations between pain frequency and intensity, and disruptions in DMN connectivity, in adolescents. Adolescents (ages 12–20) with varying levels of pain frequency and intensity, recruited from a pediatric pain clinic and the local community (n = 86; 60% female), underwent resting state functional magnetic resonance imaging. Using independent components analysis, the DMN was identified and correlated voxel-wise to assess associations between pain frequency and intensity and DMN connectivity. Findings revealed that adolescents with greater pain frequency demonstrated greater DMN to superior frontal gyrus connectivity, while adolescents with greater pain intensity demonstrated lesser DMN to cerebellum (lobule VIII) connectivity, during rest. These findings suggest that increasing levels of pain are associated with potential desegregation of the DMN and the prefrontal cortex, important for cognitive control, and with novel patterns of DMN to cerebellum connectivity. These findings may prove beneficial as neurobiological targets for future treatment efforts in adolescents.
1. Introduction
Chronic pain, or pain lasting at least 3–6 months (Merskey and Bogduk, 1994), is a debilitating health condition that effects up to a third of adolescents (King et al., 2011). Most research efforts to understand the neural etiology of chronic pain have focused on adults, despite knowledge that many chronic pain conditions first occur in adolescence, a time during which the brain and behavior are undergoing substantial development and many adult disease models do not hold. Thus, a lack of understanding of the neural underpinnings of adolescent pain may serve as a potential barrier in our effective treatment of pediatric pain. This is particularly pertinent as neural alterations associated with chronic pain may help explain why adolescent pain persists into young adulthood (Knook et al., 2012) and is associated with emergence of mental health disorders (Noel et al., 2016).
Functional magnetic resonance imaging (MRI) research in healthy adults, using pain-inducing paradigms, has demonstrated a robust pain network that includes primary and secondary somatosensory cortices, insula, thalamus, anterior cingulate cortex, and prefrontal cortex (Morton et al., 2016), and many of these regions show greater pain-related activity in adults with chronic pain compared to healthy controls (Farmer et al., 2011, Giesecke et al., 2006, Giesecke et al., 2004, Kobayashi et al., 2009). While findings in adolescents have been limited, we previously demonstrated pain-related decreases in brain activation in the primary somatosensory and motor cortices, posterior parietal lobe, and precuneus (Jones et al., 2019). Further, adolescents with chronic pain show greater pain-related activity, compared to healthy controls, in the posterior cingulate cortex (Jones et al., 2019). These results suggest that adolescents demonstrate a developmentally unique pattern of neural responsivity to pain.
Resting-state functional connectivity MRI (rsfcMRI), which correlates spontaneous fluctuations in brain activity between brain regions, or networks, in the absence of task-related stimulation, has also been used to compare individuals with and without chronic pain. One of the most robust and well-studied brain networks is the default mode network (DMN). The DMN includes regions of the prefrontal cortex, posterior cingulate, hippocampus and parahippocamal cortex, and lateral parietal and temporal cortices, and is typically deactivated during external attention-demanding task-based processes, but activated during internally-driven and self-referential processes (Andrews-Hanna et al., 2014). Numerous studies in adults with chronic pain have demonstrated alterations in connectivity between the DMN and the pain network (particularly the insula) (Farmer et al., 2012).
To date, rsfcMRI studies in adolescents have been limited. Two studies used an a priori region-of-interest approach to demonstrate altered amygdalar connectivity in adolescents with complex regional pain syndrome (Linnman et al., 2013, Simons et al., 2014). Meanwhile, alterations in the sensorimotor network, and reduced anti-correlations (thought to reflect less network segregation) between the posterior cingulate cortex (a key hub in the DMN) and the prefrontal cortex, were demonstrated in adolescents with irritable bowel syndrome (Bhatt et al., 2019, Hubbard et al., 2016). The current study sought to expand upon these studies by investigating continuous associations between DMN network functional connectivity and frequency and intensity of pain in a heterogeneous population of adolescents with varying levels of pain. Based on previous findings, we hypothesized greater pain frequency and intensity would be associated with greater connectivity between the DMN and regions of the pain network (e.g. insula and prefrontal cortex).
2. Methods
2.1. Inclusionary and exclusionary criteria
Ninety-eight adolescents participating in two separate neuroimaging studies were included in the current study. The larger studies were an ongoing study of adolescent neurodevelopment (Jones et al., 2019) and a study investigating neural response to pain in adolescents (Jones et al., 2019). Participants in both studies were recruited either via community advertisements or by fliers distributed in clinical settings. In addition to exclusion criteria below, to be included in the current study, adolescents needed to be 12–20 years old, have completed a pain questionnaire (described in pain assessment below), and have resting-state functional neuroimaging data of acceptable quality (see imaging processing below). The current analyses are distinct from any previously published or planned analyses for the larger studies.
During recruitment, adolescents and their parents provided written consent and assent, respectively, and exclusionary criteria included: left handedness, head trauma or chronic health condition (e.g. juvenile idiopathic arthritis, Crohn’s Disease), intellectual or learning disabilities, non-English speaking, prenatal drug or alcohol exposure, current use of psychotropic or prescription pain medications, and MRI contraindications.
Following recruitment and completion of the study protocol, six participants were excluded due to invalid reporting on the pain questionnaire (i.e. reporting a value of 0 for pain frequency but a non-zero value for pain intensity, or vice versa), and six participants were excluded due to insufficient usable amounts of resting state data (see below). This resulted in a final sample of 86 adolescents. Study procedures were approved by the Oregon Health & Science University Institutional Review Board.
2.2. Sample characteristics
In additional to providing general demographics (age, sex, and ethnicity/race), adolescents completed the two-subtest version of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999) to estimate intelligence (IQ). Further, to obtain an indication of socioeconomic status, adolescents’ parents reported total household income and highest level of education for each parent. Household income was measured using either a continuous measure (in $1000 increments), or on a categorical scale ranging from 1 (less than $20 thousand) to 6 (more than $90 thousand), with all continuous data subsequently binned using the categorical scale. Highest level of education, for each parent, was reported on a categorical scale ranging from 1 (graduate/professional school) to 7 (<7 years of school).
2.3. Pain assessment
To assess symptoms of recent pain, adolescents competed a questionnaire reporting on pain frequency, pain intensity, and location where adolescents experienced the “most problems with aches or pains,” in the last month. Pain frequency was reported on a categorical scale ranging from 0 (not at all) to 5 (daily), and pain intensity was reported on an 11-point numerical rating scale from 0 (no pain) to 10 (worst pain possible). Adolescents’ self-reports of most painful part of the body were classified into the following categories: head (n = 11), musculoskeletal (including neck, shoulder, back and limb; n = 48), stomach (n = 11), and no pain (n = 12). Four participants reported pain in multiple body parts, but failed to report on the most painful part of their body, and were thus excluded from post-hoc analyses (see below).
2.4. Image acquisition
Participants were scanned on either a 3T Siemens Magnetom Tim Trio using a 12-channel head coil (n = 40) or 3T Siemens Magnetom Prisma using a 20-channel head/neck coil (n = 46). For all participants, a whole-brain T1-weighted MPRAGE structural image was acquired in the sagittal plane (inversion time (TI) = 900 ms, flip angle = 10°, echo time (TE) = 3.58/3.61 ms (Tim Trio/Prisma), repetition time (TR) = 2300 ms, field of view (FOV) = 256 × 240, resolution 1 × 1 × 1.1 mm). Whole-brain resting state functional T2*-weighted echo-planer images were collected axially, parallel to the anterior-posterior commissure line, in two runs (TR = 2500 ms, TE = 30 ms, flip angle = 90°, resolution = 3.8 × 3.8 × 3.8 mm, FOV = 240 mm2, slices = 36, measurements = 100 or 124 TRs, acquisition time, for each run = 4:17 or 5:17). During resting state scans, participants were instructed to remain awake, with their eyes open and focus on white fixation cross on a black background, projected onto a mirror on the head coil.
2.5. Image processing
Functional images were visually inspected for artifacts, and preprocessing was conducted using Analysis of Functional NeuroImages (AFNI, Cox, 1996). Preprocessing included the removal of the first 3 volumes in the time series collected pre-steady state, despiking, and slice time correction. Functional images underwent motion correction to register each volume to the minimum outlier in the time series and to register all functional volumes to the structural image. Nonlinear registration was used to register the structural image to a template in Montreal Neurological Institute (MNI) space. All of these spatial transformations were combined and applied in one-step to minimize interpolation error. Functional images were also smoothed using a 4-mm full-width half maximum Gaussian kernel and normalized to mean of 100. FreeSurfer (Dale et al., 1999) was used to generate ventricular; white-matter, and whole-brain masks from the structural images. Structural masks were transformed to MNI space using the registration parameters calculated with AFNI and applied to the functional data in order to extract average time courses. Linear regression was applied to functional data to remove ventricular, white-matter, and global signal; to covary for motion parameters and their first order temporal derivatives; to censor volumes with a framewise displacement (FD) >0.3 or segments of data with <5 consecutive volumes without excessive motion (Power et al., 2014); and to conduct bandpass filtering (0.01–0.1 Hz). Participants were required to have >5 min of uncensored data for inclusion. Lastly, to remove areas from the analyses where there was substantial dropout, subject-specific masks excluding areas with significant dropout (Peer et al., 2016) were generated. Then, a group-level mask was created that contained all voxels where at least 50% of participants demonstrated reliable levels of signal, and used in all subsequent analyses.
2.6. Independent components analysis
Preprocessed functional data were entered into group independent component analysis (ICA) using FSL’s MELODIC (Beckmann et al., 2005, Beckmann and Smith, 2004). As it is difficult to determine a priori the number the number of “true” components that exist in a dataset, a data driven approach was taken, and the number of components generated was not restricted. The DMN was identified via visual inspection of all components output by MELIDOC and confirmed by cross correlating the spatial maps of our group-average independent components with the DMN network of a previous, publicly available (https://www.fmrib.ox.ac.uk/datasets/brainmap+rsns/), resting-state template (Smith et al., 2009). Dual regression was used to map the group-average DMN onto single subjects and to extract the average timecourse from each subject (Nickerson et al., 2017).
2.7. Voxel-wise analysis
In order to limit the need for additional multiple comparisons corrections and maintain adequate power, we decided a priori to only investigate the connectivity of the DMN (and not other networks) following ICA. The resulting DMN timeseries for each individual was correlated voxel-wise with participants’ processed resting-state data to create whole-brain connectivity maps. These connectivity maps were normalized using Fisher’s Z-transformation prior to group-level analysis. To adjust for differences in DMN connectivity as a function of scanner platform, a voxel-wise correction factor was applied using the ComBat function in R (v. 3.5.3) (Johnson et al., 2007), with pain frequency and intensity as biological covariates. It has been demonstrated that ComBat can successfully remove scanner effects in both structural and functional imaging data and is particularly useful in developmental samples (Fortin et al., 2017, Yu et al., 2018).
Due to the ordinal nature of the pain frequency and intensity measures, a rank-based, nonparametric test, the Jonckheere-Terpstra test (Jonckheere, 1954, Terpstra, 1952), was used to assess for a statistically significant trend between pain frequency/intensity and DMN connectivity. Using Neuropointillist (http://github.com/IBIC/neuropointillist) in conjunction with the ‘clinfun’ package in R (v 3.5.3), separate Jonckheere-Terpstra tests, with a monotonic (two-sided) hypothesis order, were fit voxel-wise. The resulting maps were then voxel (p < 0.001) and cluster (α < 0.05; 17 contiguous voxels) corrected using AFNI’s 3dClustSim, after estimating the blur in the functional dataset using the auto-correlation function (ACF) parameters obtained from the “uncleaned” timeseries, following recommendations outlined previously (Cox et al., 2017).
Following whole-brain analysis, exploratory post-hoc analyses were conducted to investigate how all primary findings differed based on adolescent self-report of location of most pain problems. For each region identified in voxel-wise analyses, mean connectivity values were extracted, Jonckheere-Terpstra tests were rerun in each of the three pain location subgroups (musculoskeletal, stomach, and head), and p-values were Bonferroni-corrected for multiple comparisons (3 tests for each region of interest). Further, to aid future studies, effect sizes have been reported for all post-hoc tests in pain location subgroups. These effect sizes are reported as Cohen’s d and were obtained by first estimating Kendall’s Tau correlation coefficients, and then converting to Cohen’s d via equations outlined previously (Walker, 2003). Four participants were missing from post-hoc analyses due to errors in reporting most painful location of the body.
3. Results
3.1. Sample characteristics
The sample included adolescents 12–20 years of age, who were 60% female, predominately white, non-Hispanic/Latino (86%). Sample characteristics, including pain characteristics are presented in Table 1. Pain frequency and intensity did not differ based on age, pubertal status, sex, IQ, household income or highest level of parental education. Pain frequency, pain intensity and all demographic variables also did not differ based on reported location of most pain problems.
Table 1.
Sample characteristics.
| Min | Median/Mean (SD) | Max | |
|---|---|---|---|
| Age | 12.89 | 16.19 (1.76) | 20.76 |
| IQ | 82 | 111 (11) | 136 |
| Pubertal development | early puberty | post-pubertal | post-pubertal |
| Household incomea | < $20 thousand | > $90 thousand | > $90 thousand |
| Highest parental education b | high school | graduate/professional school | graduate/professional school |
| Pain frequency | none | weekly | daily |
| Pain intensity | 0 | 3 | 7 |
8 missing/unreported; b5 missing/unreported.
3.2. Default mode network connectivity
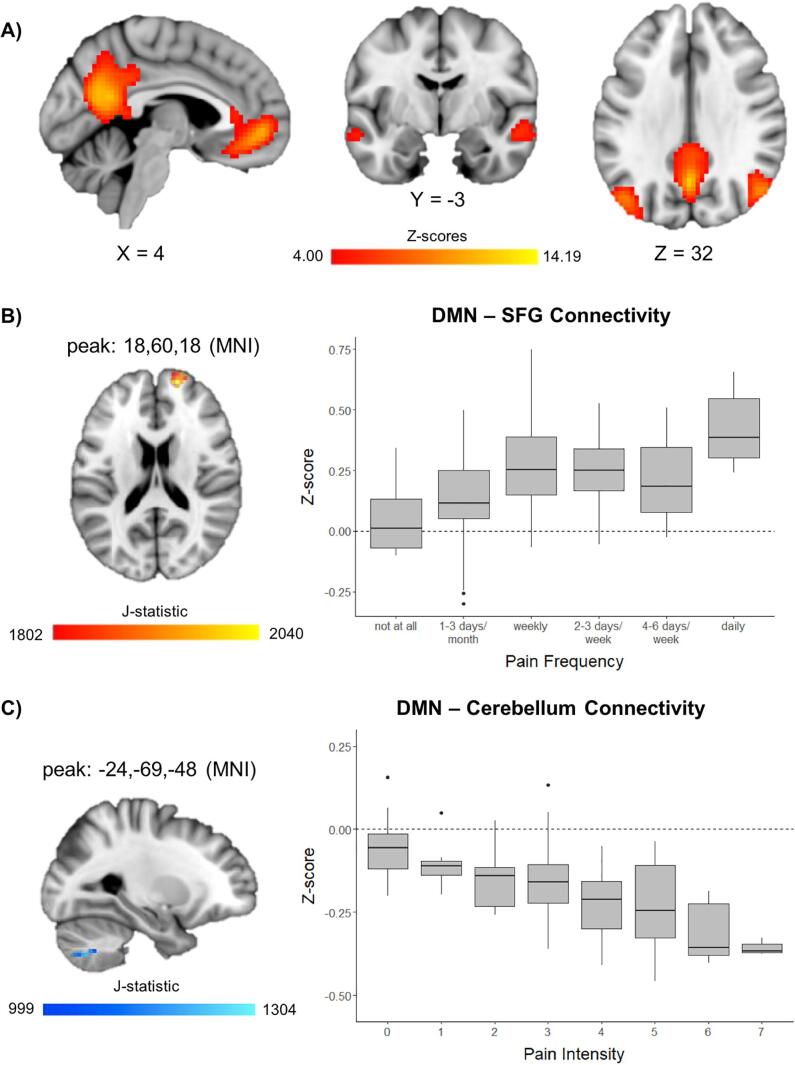
Results from the ICA returned a total of 28 independent components, including a robust DMN that included both anterior and posterior aspects of the DMN (Fig. 1A), which correlated highly with a previous DMN template (r = 0.56) (Smith et al., 2009). An additional component corresponded with posterior aspects of the DMN, but was not utilized for further analysis because it did not include several key DMN regions (e.g. anterior cingulate, and lateral temporal cortices; r = 0.50). All other ICA components showed lower correlation with the DMN template (r < 0.3). Voxel-wise Jonckheere-Terpstra tests for ordered alternatives showed that there was significantly higher median DMN connectivity in the superior frontal gyrus (SFG; 22 contiguous voxels) with higher levels of pain frequency (Fig. 1B) and lower median DMN connectivity in the cerebellum (largely, lobule VIII; 21 contiguous voxels) with higher levels of pain intensity (voxel-wise p < 0.001, cluster-corrected α < 0.05) (Fig. 1C). Post-hoc analyses looking at most painful body location reported by adolescents found that these findings remained significant in adolescents with musculoskeletal pain (SFG: TJT = 596, z = 3.016, corrected p < 0.01, d = 1.13; cerebellum: TJT = 298, z = −2.594, corrected p < 0.05, d = 0.94) and in the cerebellum (TJT = 8, z = −2.557, corrected p < 0.05, d = 2.83) but not the SFG (TJT = 32, z = 2.208, d = 2.37) in those with stomach pain. There was no significant association between pain frequency/intensity and DMN connectivity in those with head pain (SFG: TJT = 20, z = 0.640, d = 0.50; cerebellum: TJT = 15, z = 1.292, d = 1.04).
Fig. 1.
Voxel-wise connectivity of the default mode network (DMN). A) Z-scores (thresholded at z ≥ 4.00) for the DMN from independent components analysis. B) Significant association between pain frequency and connectivity (z-score) between the DMN and the superior frontal gyrus (SFG). C) Significant association between pain intensity and connectivity (z-score) between the DMN and the cerebellum. Box and whisker plots represent medians and first and third quartiles for each level of pain frequency/intensity.
There were no significant associations between DMN connectivity and any demographic variables. Further, DMN connectivity in the SFG and cerebellum was not associated with the amount of resting-state data (in minutes) or average motion (root-mean-squared; rms) after motion censoring. Given, previous associations between DMN connectivity and age in adolescents (Sherman et al., 2014) and sex effects in adults (Bluhm et al., 2008), we repeated the previous analyses after regressing out age and sex from the DMN connectivity maps. In this analysis, all previous findings remained significant, and no additional associations with pain frequency or intensity were present.
4. Discussion
The goal of the current study was to investigate the association between pain intensity and frequency and DMN connectivity in adolescents. As hypothesized, we found that greater DMN-SFG connectivity was associated with higher levels of pain frequency; however, there was no association between pain intensity/frequency and DMN connectivity in any other region of the pain network (most notably the insula). Additionally, we found lower DMN-cerebellar connectivity was associated with higher levels of pain intensity. This is the first study, to our knowledge, to demonstrate an association between pain symptoms and DMN connectivity in adolescents.
The role of the prefrontal cortex in pain response and pain processing has been demonstrated repeatedly in both clinical and pre-clinical work (for recent review, see Ong et al., 2019). However, the exact mechanism of the prefrontal cortex in pain circuitry is unclear, as there is evidence to support its role in both the suppression of pain, as well as pain detection (for review, see Seminowicz and Moayedi, 2017). The superior frontal gyrus is often thought to belong to the executive control network, important for responding to emotionally salient, arousing, or attention-demanding stimuli. Typically, a significant anti-correlation (i.e. negative correlation) between these networks is present and is thought to represent strong network segregation. To support this, a previous study of adolescents with IBS demonstrated that greater posterior cingulate to dorsal prefrontal cortex connectivity, compared to controls (Hubbard et al., 2016), was driven by the typical anti-correlation between these two regions in control subjects, but not those with IBS. Our findings extend this finding, by suggesting greater pain frequency is associated with greater connectivity, in the positive direction, between the DMN and the SFG, and thus may be representative of less segregation (i.e. desegregation) of these networks.
Interpretation of these DMN to SFG findings remains difficult. One possibility is that increased connectivity of the DMN to prefrontal cortex may represent a shift in the role of the prefrontal cortex from cognitive control to more interoceptive or internally-driven processes. While engagement of the prefrontal cortex is often thought to occur in response to allocation of attention to external stimuli, it has also been demonstrated to respond to internal physiological states and is involved in internal awareness (e.g. Critchley et al., 2004). As such, it is possible that monitoring of physiological aspects of pain may require both recruitment of the default mode network, as well as the prefrontal cortex to suppress aspects (particularly emotional aspects) of the pain response. Thus, in the current study, adolescents with greater pain frequency may require greater utilization of their prefrontal cortex to suppress the emotional aspects of the pain response, subsequently strengthening the functional connectivity between the DMN and SFG. This would be in line with the mechanism suggesting the prefrontal cortex as important for the suppression of pain (Seminowicz and Moayedi, 2017). Further, when taken in light of the developmental nature of the sample, increased connectivity between the DMN and SFG may more simply suggest impairments or delays in typical developmental processes by which there is increased intrinsic refinement of the DMN and increased segregation from the executive control network (Anderson et al., 2011, Sherman et al., 2014). Future longitudinal work will be necessary to gain a further understanding of pain and adolescent neurodevelopment.
The cerebellum has been suggested to play an integrative role in processing emotional aspects of pain, sensorimotor processing, and pain modulation (Moulton et al., 2010). Previous studies in adolescents suggest that functional connectivity between the cerebellum and the amygdala (a region important for encoding salience and emotional aspects of pain) is greater in those with chronic pain compared to controls (Linnman et al., 2013, Simons et al., 2014). While adolescent studies have yet to demonstrate DMN to cerebellum connectivity in association with pain, previous studies in adults suggest that DMN to cerebellum connectivity increases with increased pain intensity (Napadow et al., 2010). However, it is important to note that the cerebellum is a very complex structure with region-specific functional connectivity with several brain networks (Sang et al., 2012). In the current study, we demonstrated a negative association between pain intensity and functional connectivity between the DMN and cerebellar lobule VIII, a region shown to be primarily functionally connected to other regions of the DMN (Bernard et al., 2012, Sang et al., 2012). It is possible the lack of functional connectivity between these regions in our sample may indicate a decrease in the overall intrinsic connectivity in the DMN, a finding that has been demonstrated previously in adults with chronic pain, both in response to painful stimuli and during rest (Alshelh et al., 2018, Baliki et al., 2008, Baliki et al., 2014, Tessitore et al., 2013). Lower intrinsic connectivity of the DMN may also represent a neurodevelopmental delay in adolescents, as intrinsic connectivity within the DMN is thought to increase across adolescence (Sherman et al., 2014). Further, reduced intrinsic connectivity of the DMN has recently been linked to recurrent depression in a large study of adults (Yan et al., 2019); thus, the findings of this study may help to explain the relation between adolescent pain and future mental health disorders (Noel et al., 2016).
Together, the results of this study provide important information regarding the neurobiological underpinnings of pain in adolescents. However, it should be noted that in the current study, participants were only asked to report on pain symptomology occurring in the last month. In contrast, in previous pediatric studies, which focused exclusively on pediatric chronic pain samples, altered amygdalar and sensorimotor connectivity was demonstrated in chronic pain disease states that had persisted for on average 19 months (Simons et al., 2014) to 3.62 years (Hubbard et al., 2016). Additionally, alterations in amygdalar connectivity have been shown to persist even after remittance of pain symptoms (Linnman et al., 2013). Whether the current findings show a similar pattern of persistence and are associated with chronic pain, or predictive of transition from acute to chronic pain, warrants further exploration.
While this study is the first to look at continuous associations between pain symptomology and DMN connectivity in adolescents, several limitations warrant discussion. First, the adolescents in the current sample were predominately white, with higher than average IQ and socioeconomic status. This sampling bias has been shown to be a potential confound in developmental neuroimaging studies (LeWinn et al., 2017), and future studies will be required to assess the generalizability of these results to other demographics. Second, the cross-sectional nature of this study makes it impossible to infer causality from our findings. While it is probable that previous pain experiences have altered adolescent neurocircuitry or caused delays in typical neurodevelopment, it is also possible that differential DMN connectivity as a function of pain frequency/intensity predates emergence of pain symptomology. Future longitudinal studies will be necessary to address this question. Finally, while the heterogeneity in pain locations in the sample helped to improve the generalizability of these findings to multiple pain conditions, given the sample size, this study was not powered to assess differential connectivity based on pain conditions. While post-hoc analyses found that results held for those with musculoskeletal and stomach pain, but not head pain, these findings should be considered preliminary. Given the small sample size in the subgroup of participants with head pain, it is unclear whether the current findings are indeed specific to certain pain locations, or if we were simply underpowered to detect these effects in all subgroups. While effect sizes for the null post-hoc results in the head pain subgroup are medium to large, and comparable to those observed in the musculoskeletal pain subgroup, it must be noted that obtaining effect sizes post-hoc in this way is circular in nature (i.e. re-running analyses with values deemed to already be significant via whole-brain analyses) and should be interpreted with caution.
In conclusion, results of this study demonstrate that pain frequency and intensity is associated with alterations in DMN connectivity during rest in adolescents with varying levels of pain. Extending prior work, we demonstrated that increasing pain frequency is associated with desegregation of the DMN with regions of the prefrontal cortex, typically thought to be involved in cognitive control. Further, our findings highlight a novel association between pain and DMN-cerebellar connectivity. In addition to shedding light on the underlying neurocircuitry associated with pain in adolescents, the results of this study may provide neurobiological targets for future pain treatment strategies in adolescents.
CRediT authorship contribution statement
Scott A. Jones: Conceptualization, Software, Formal analysis, Data curation, Writing - original draft, Writing - review & editing, Visualization. Angelica M. Morales: Software, Formal analysis, Data curation, Writing - original draft, Writing - review & editing. Amy L. Holley: Conceptualization, Writing - original draft, Writing - review & editing, Funding acquisition. Anna C. Wilson: Conceptualization, Writing - original draft, Writing - review & editing. Bonnie J. Nagel: Conceptualization, Writing - original draft, Writing - review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Past and present members of the Developmental Brain Imaging Lab are thanked for assisting in participant scheduling and data collection. This work was supported by R01 AA017664 (Nagel), K01 DA046649 (Morales), and an OHSU Medical Research Foundation Grant (Holley).
References
- Alshelh Z., Marciszewski K.K., Akhter R., Di Pietro F., Mills E.P., Vickers E.R., Peck C.C., Murray G.M., Henderson L.A. Disruption of default mode network dynamics in acute and chronic pain states. NeuroImage Clin. 2018;17:222–231. doi: 10.1016/j.nicl.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J.S., Ferguson M.A., Lopez-Larson M., Yurgelun-Todd D. Connectivity gradients between the default mode and attention control networks. Brain Connect. 2011;1(2):147–157. doi: 10.1089/brain.2011.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J.R., Smallwood J., Spreng R.N. The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014;1316:29–52. doi: 10.1111/nyas.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Geha P.Y., Apkarian A.V., Chialvo D.R. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J. Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Mansour A.R., Baria A.T., Apkarian A.V. Functional reorganization of the default mode network across chronic pain conditions. PLoS ONE. 2014;9(9):e106133. doi: 10.1371/journal.pone.0106133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., DeLuca M., Devlin J.T., Smith S.M. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R Soc. Lond. B Biol. Sci. 2005;360(1457):1001–1013. doi: 10.1098/rstb.2005.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Bernard J.A., Seidler R.D., Hassevoort K.M., Benson B.L., Welsh R.C., Wiggins J.L., Jaeggi S.M., Buschkuehl M., Monk C.S., Jonides J., Peltier S.J. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front. Neuroanat. 2012;6:31. doi: 10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt R.R., Gupta A., Labus J.S., Zeltzer L.K., Tsao J.C., Shulman R.J., Tillisch K. Altered brain structure and functional connectivity and its relation to pain perception in girls with irritable bowel syndrome. Psychosom. Med. 2019;81(2):146–154. doi: 10.1097/PSY.0000000000000655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm R.L., Osuch E.A., Lanius R.A., Boksman K., Neufeld R.W., Théberge J., Williamson P. Default mode network connectivity: effects of age, sex, and analytic approach. NeuroReport. 2008;19(8):887–891. doi: 10.1097/WNR.0b013e328300ebbf. [DOI] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput. Biomed. Res. Int. J. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox R.W., Chen G., Glen D.R., Reynolds R.C., Taylor P.A. FMRI clustering in AFNI: false-positive rates redux. Brain Connect. 2017;7(3):152–171. doi: 10.1089/brain.2016.0475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nat. Neurosci. 2004;7(2):189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. NeuroImage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Farmer M.A., Baliki M.N., Apkarian A.V. A dynamic network perspective of chronic pain. Neurosci. Lett. 2012;520(2):197–203. doi: 10.1016/j.neulet.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer M.A., Chanda M.L., Parks E.L., Baliki M.N., Apkarian A.V., Schaeffer A.J. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J. Urol. 2011;186(1):117–124. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortin J.-P., Parker D., Tunç B., Watanabe T., Elliott M.A., Ruparel K., Roalf D.R., Satterthwaite T.D., Gur R.C., Gur R.E. Harmonization of multi-site diffusion tensor imaging data. NeuroImage. 2017;161:149–170. doi: 10.1016/j.neuroimage.2017.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesecke, T., Gracely, R., Clauw, D., Nachemson, A., Dück, M., Sabatowski, R., Gerbershagen, H., Williams, D., Petzke, F., 2006. Central pain processing in chronic low back pain. Evidence for reduced pain inhibition. Schmerz (Berlin, Germany) 20(5): 411–414, 416–417. [DOI] [PubMed]
- Giesecke T., Gracely R.H., Grant M.A., Nachemson A., Petzke F., Williams D.A., Clauw D.J. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis Rheumatism: Off. J. Am. College Rheumatol. 2004;50(2):613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- Hubbard C.S., Becerra L., Heinz N., Ludwick A., Rasooly T., Wu R., Johnson A., Schechter N.L., Borsook D., Nurko S. Abdominal pain, the adolescent and altered brain structure and function. PLoS ONE. 2016;11(5):e0156545. doi: 10.1371/journal.pone.0156545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W.E., Li C., Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics (Oxford, England) 2007;8(1):118–127. doi: 10.1093/biostatistics/kxj037. [DOI] [PubMed] [Google Scholar]
- Jonckheere A.R. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41(1/2):133–145. [Google Scholar]
- Jones S., Cooke H., Wilson A.C., Nagel B.J., Holley A. A pilot study examining neural response to pain in adolescents with and without chronic pain. Front. Neurol. 2019;10:1403. doi: 10.3389/fneur.2019.01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones S.A., Morales A.M., Nagel B.J. Resilience to risk for psychopathology: the role of white matter microstructural development in adolescence. Biol. Psychiatr. Cogn. Neurosci. Neuroimaging. 2019;4(2):180–189. doi: 10.1016/j.bpsc.2018.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King S., Chambers C.T., Huguet A., MacNevin R.C., McGrath P.J., Parker L., MacDonald A.J. The epidemiology of chronic pain in children and adolescents revisited: a systematic review. Pain. 2011;152(12):2729–2738. doi: 10.1016/j.pain.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Knook L., Lijmer J.G., Konijnenberg A.Y., Taminiau B. The course of chronic pain with and without psychiatric disorders: a 6-year follow-up study from childhood to adolescence and young adulthood. J. Clin. Psychiatr. 2012;73(1):e134–139. doi: 10.4088/JCP.10m06751. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y., Kurata J., Sekiguchi M., Kokubun M., Akaishizawa T., Chiba Y., Konno S.-i., Kikuchi S.-i. Augmented cerebral activation by lumbar mechanical stimulus in chronic low back pain patients: an FMRI study. Spine. 2009;34(22):2431–2436. doi: 10.1097/BRS.0b013e3181b1fb76. [DOI] [PubMed] [Google Scholar]
- LeWinn K.Z., Sheridan M.A., Keyes K.M., Hamilton A., McLaughlin K.A. Sample composition alters associations between age and brain structure. Nat. Commun. 2017;8(1):874. doi: 10.1038/s41467-017-00908-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linnman C., Becerra L., Lebel A., Berde C., Grant P.E., Borsook D. Transient and persistent pain induced connectivity alterations in pediatric complex regional pain syndrome. PLoS ONE. 2013;8(3):e57205. doi: 10.1371/journal.pone.0057205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merskey H., Bogduk N. IASP Press; Seattle: 1994. Classification of Chronic Pain. [Google Scholar]
- Morton D.L., Sandhu J.S., Jones A.K. Brain imaging of pain: state of the art. J. Pain Res. 2016;9:613–624. doi: 10.2147/JPR.S60433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulton E.A., Schmahmann J.D., Becerra L., Borsook D. The cerebellum and pain: passive integrator or active participator? Brain Res. Rev. 2010;65(1):14–27. doi: 10.1016/j.brainresrev.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napadow V., LaCount L., Park K., As-Sanie S., Clauw D.J., Harris R.E. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62(8):2545–2555. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickerson L.D., Smith S.M., Ongur D., Beckmann C.F. Using dual regression to investigate network shape and amplitude in functional connectivity analyses. Front. Neurosci. 2017;11:115. doi: 10.3389/fnins.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noel M., Groenewald C.B., Beals-Erickson S.E., Gebert J.T., Palermo T.M. Chronic pain in adolescence and internalizing mental health disorders: a nationally representative study. Pain. 2016;157(6):1333–1338. doi: 10.1097/j.pain.0000000000000522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong W.Y., Stohler C.S., Herr D.R. Role of the prefrontal cortex in pain processing. Mol. Neurobiol. 2019;56(2):1137–1166. doi: 10.1007/s12035-018-1130-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer M., Abboud S., Hertz U., Amedi A., Arzy S. Intensity-based masking: a tool to improve functional connectivity results of resting-state fMRI. Hum. Brain Mapp. 2016;37(7):2407–2418. doi: 10.1002/hbm.23182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. NeuroImage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sang L., Qin W., Liu Y., Han W., Zhang Y., Jiang T., Yu C. Resting-state functional connectivity of the vermal and hemispheric subregions of the cerebellum with both the cerebral cortical networks and subcortical structures. NeuroImage. 2012;61(4):1213–1225. doi: 10.1016/j.neuroimage.2012.04.011. [DOI] [PubMed] [Google Scholar]
- Seminowicz D.A., Moayedi M. The dorsolateral prefrontal cortex in acute and chronic pain. J. Pain. 2017;18(9):1027–1035. doi: 10.1016/j.jpain.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L.E., Rudie J.D., Pfeifer J.H., Masten C.L., McNealy K., Dapretto M. Development of the default mode and central executive networks across early adolescence: a longitudinal study. Develop. Cogn. Neurosci. 2014;10:148–159. doi: 10.1016/j.dcn.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons L., Pielech M., Erpelding N., Linnman C., Moulton E., Sava S., Lebel A., Serrano P., Sethna N., Berde C. The responsive amygdala: treatment-induced alterations in functional connectivity in pediatric complex regional pain syndrome. PAIN®. 2014;155(9):1727–1742. doi: 10.1016/j.pain.2014.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Fox P.T., Miller K.L., Glahn D.C., Fox P.M., Mackay C.E., Filippini N., Watkins K.E., Toro R., Laird A.R., Beckmann C.F. Correspondence of the brain's functional architecture during activation and rest. PNAS. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terpstra T.J. The asymptotic normality and consistency of Kendall's test against trend, when ties are present in one ranking. Indagations Math. 1952;14:327–333. [Google Scholar]
- Tessitore A., Russo A., Giordano A., Conte F., Corbo D., De Stefano M., Cirillo S., Cirillo M., Esposito F., Tedeschi G. Disrupted default mode network connectivity in migraine without aura. J Headache Pain. 2013;14(1):89. doi: 10.1186/1129-2377-14-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D.A. JMASM9: converting Kendall’s tau for correlational or meta-analytic analyses. J. Modern Appl. Statist. Methods. 2003;2(2):26. [Google Scholar]
- Wechsler D. Psychological Corporation; San Antonio, TX: 1999. Wechsler Abbreviated Scale of Intelligence. [Google Scholar]
- Yan C.-G., Chen X., Li L., Castellanos F.X., Bai T.-J., Bo Q.-J., Cao J., Chen G.-M., Chen N.-X., Chen W. Reduced default mode network functional connectivity in patients with recurrent major depressive disorder. Proc. Natl. Acad. Sci. 2019;116(18):9078–9083. doi: 10.1073/pnas.1900390116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Linn K.A., Cook P.A., Phillips M.L., McInnis M., Fava M., Trivedi M.H., Weissman M.M., Shinohara R.T., Sheline Y.I. Statistical harmonization corrects site effects in functional connectivity measurements from multi-site fMRI data. Hum. Brain Mapp. 2018;39(11):4213–4227. doi: 10.1002/hbm.24241. [DOI] [PMC free article] [PubMed] [Google Scholar]