Abstract
Although signal transduction pathways provide spatiotemporal control of cytokinesis, additional regulation likely occurs through complex cytoskeletal network interactions. In this issue of Developmental Cell, Mukhina et al. (2007) show that myosin-II modulates the cortical lifetime of the actin crosslinker α-actinin, which in turn tunes actin filament dynamics, thereby controlling furrow ingression.
Cytokinesis is the process of reshaping one cell into two daughter cells and is driven by actin, actin crosslinkers, and myosin-II. Classically, cytokinesis contractility is thought to occur through the constriction of a sarcomeric-like contractile ring (a pursestring) of actin and myosin-II filaments. However, contractile ring structure varies widely among different organisms, from a highly ordered ring in S. pombe to a more disordered actin network in some mammalian cell types (including the normal rat kidney [NRK] cells used in the study by Mukhina et al., [2007]). α-actinin is an actin crosslinker that localizes to the cleavage furrow region in a variety of cells, from yeast to mammals. Yet, until Mukhina et al. (2007), α-actinin’s function in cytokinesis in a cell type with a more disorganized contractile network had not been studied. Mukhina et al. (2007) discovered that α-actinin modulates furrow ingression dynamics and actin turnover and that myosin-II activity modulated the lifetime of α-actinin at the equatorial cortex.
To determine how α-actinin contributes to cytokinesis, Mukhina et al. (2007) studied how increasing or decreasing α-actinin expression levels influences the actin cortex and cytokinesis fidelity. When they overexpressed α-actinin, furrow ingression slowed down or reversed, leading to failure of cytokinesis. Under these conditions, the authors observed increased equatorial F-actin levels and slower actin turnover, which may explain the effects on furrow formation. Increased concentration of F-actin at the furrow likely increases viscoelasticity, slowing the removal of cytoskeleton and cytoplasm during furrow ingression. Higher concentrations of α-actinin may also enhance the stability of the polymerized actin network. Indeed, one function of myosin-II at the cleavage furrow is to increase actin turnover, facilitating furrow ingression (Murthy and Wadsworth, 2005).
Conversely, when Mukhina et al. (2007) reduced α-actinin expression by RNAi, ectopic furrows formed and furrow ingression rates increased. Again, the impact of lowering α-actinin levels on cytokinesis shape changes may be through α-actinin’s modulation of F-actin, since F-actin levels are reduced by α-actinin RNAi. These results suggest that inhibition of actin turnover by α-actinin may act as a brake to allow cytokinesis to proceed by controlled, stereotypical cell shape changes.
The ability of actin crosslinkers to act as cytokinesis brakes is not unprecedented; dynacortin slows the rate of furrow ingression and contributes to cortical tension and viscoelasticity (Girard et al., 2004; Zhang and Robinson, 2005). It is unclear from Mukhina et al. (2007) whether α-actinin also impacts the viscoelastic properties of the actin cortex. Using glass needles to push against cells attached to the substrate, α-actinin overexpression did not appear to significantly increase the stiffness of the cortex, although differences in cell-substrate adhesion complicate this assay. As overexpression or removal of dynacortin increases or decreases cortical viscoelasticity, respectively (Girard et al., 2004), it is possible that α-actinin does also, especially as the authors show that α-actinin overexpression has a drastic impact on F-actin dynamics and concentration.
Finally, Mukhina et al. (2007) used fluorescence recovery after photobleaching (FRAP) analysis to measure α-actinin lifetimes. They found that α-actinin has a shorter lifetime at the equatorial cortex than at the polar cortex, suggesting that cytoskeletal dynamics are different at these two regions of the dividing cell cortex. They then examined whether myosin-II can influence α-actinin’s lifetime, using blebbistatin, an inhibitor of myosin-II that blocks tight binding of the myosin-II motor to the actin filament. In this analysis, they discovered that myosin-II increases α-actinin recovery rates. In summary, all of the results in Mukhina et al. (2007) suggest an antagonistic interplay between myosin-II and α-actinin: α-actinin provides a braking function, slowing contractility, while myosin-II shortens α-actinin’s lifetime in the cleavage furrow network.
How might such an antagonistic interplay between myosin-II and α-actinin work to promote and control cytokinesis contractility? Early in vitro work found that the extent of crosslinking impacts myosin-II contraction of actin networks. In the absence of any crosslinkers, myosin-II does not contract the network (Janson et al., 1991). However, increasing the crosslinker: myosin-II ratio above a threshold also inhibits network contraction by promoting isometric rather than isotonic tension (Janson et al., 1992). Thus, some crosslinker binding is necessary to allow productive myosin-II force generation, but crosslinker release is required to allow contraction to proceed.
Another way α-actinin could contribute to contractility is by increasing myosin-II’s duty ratio (the ratio of bound motor heads to the total number of available motor heads). α-actinin can increase myosin-II’s Mg2+ATPase activity (Condeelis et al., 1984), which would enhance the proportion of actin-bound myosin-II heads. Myosin-II’s duty ratio is also increased by mechanical load, which locks the motor heads onto the actin filament by decreasing the ADP-off-rate. Therefore, α-actinin enrichment at the equator may increase the duty ratio of myosin-II by stimulating the myosin-II ATPase activity and/or by creating a crosslinked network that generates enough resistance (load) to slow ADP release. Either or both mechanisms would increase the tension at the furrow.
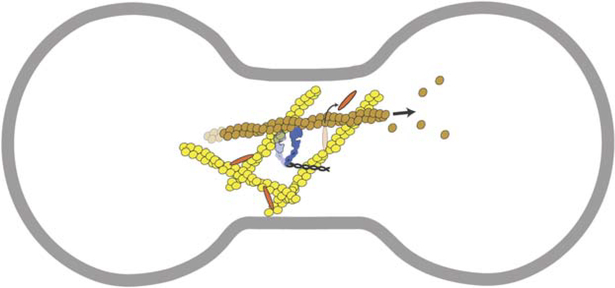
In contrast, myosin-II appears to modulate α-actinin binding, suggesting another way by which cytokinesis contractility may be regulated. The enhanced α-actinin dynamics at the equatorial cortex, as observed by Mukhina et al. (2007), may be due to strain induced by equatorially enriched myosin-II. By pulling on F-actin that is crosslinked by α-actinin, myosin-II may lead to an increased α-actinin off-rate, perhaps due to slip-bond behavior of α-actinin. Similar behaviors have been observed in vitro; for example, myosin-II can disperse filaments from fimbrin-crosslinked actin networks (Prassler et al., 1997). In the furrow, myosin-II may increase actin removal by increasing crosslinker off-rates, allowing filaments to be transported away from the furrow, or allowing access to the actin severing protein, cofilin, which is needed to maintain constant cleavage furrow actin levels as the furrow ingresses (Figure 1).
Figure 1.
Dynamic Interplay between Actin, Crosslinkers, and Myosin-II during Furrow Ingression
Cartoon of a dividing cell depicting how myosin-II, going through its working stroke, causes α-actinin to release from the network, allowing the filament to be pulled from the furrow cytoskeleton. Actin filaments, yellow; myosin-II, blue; α-actinin, orange; membrane, gray.
Although different tissue environments and cell compositions may result in different cellular mechanics, it is likely that the universally conserved cytokinesis machinery in eukaryea, consisting of actin, actin crosslinkers, and myosin-II, interacts in a similar manner to generate contractile force. Mukhina et al. (2007) propose that mammalian cleavage furrows contract by remodeling of the actin cortex rather than by constricting a sarcomeric contractile ring. Remodeling may occur through interplay of cytoskeletal components, wherein myosin-II alters crosslinker binding to F-actin, allowing the actin filaments to slide through the network or to be turned over. Additionally, the interdependent modulation of cytoskeletal dynamics may allow for feedback control of cell shape during mechanical perturbation (Effler et al., 2006). This self-regulation of the cytoskeletal components of the contracting network may make cytokinesis highly adaptable to the differing mechanical environments that cells might encounter.
REFERENCES
- Condeelis JS, Vahey M, Carboni JM, Demey J, and Ogihara S (1984). J. Cell Biol 99, 119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effler JC, Kee S, Berk JM, Tran MN, Iglesias PA, and Robinson DN (2006). Curr. Biol 16, 1962–1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard KD, Chaney C, Delannoy M, Kuo SC, and Robinson DN (2004). EMBO J. 23, 1536–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson LW, Kolega J, and Taylor DL (1991). J. Cell Biol 114, 1005–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janson LW, Sellers JR, and Taylor DL (1992). Cell Motil. Cytoskeleton 22, 274–280. [DOI] [PubMed] [Google Scholar]
- Murthy K, and Wadsworth P (2005). Curr. Biol 15, 724–731. [DOI] [PubMed] [Google Scholar]
- Mukhina S, Wang Y, and Murata-Hori M (2007). Dev. Cell 13, this issue, 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prassler J, Stocker S, Marriott G, Heidecker M, Kellermann J, and Gerisch G (1997). Mol. Biol. Cell 8, 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang WW, and Robinson DN (2005). Proc. Natl. Acad. Sci. USA 102, 7186–7191. [DOI] [PMC free article] [PubMed] [Google Scholar]