Highlights
-
•
Isolated loss of smell without nasal obstruction is an early red-flag of COVID-19.
-
•
These patients should adopt all the preventive measures and a lockdown.
-
•
Olfactory/gustative dysfunction had high predictive value to identify COVID-19.
-
•
Olfactory/gustative dysfunction had high specificity to identify COVID-19.
-
•
Self-reported loss of smell, among other symptoms, could help to screen COVID-19.
Keywords: COVID-19, SARS-CoV-2, Anosmia, Dysguageusia, Loss of smell, Positive predictive value, Viral load
Abstract
Objectives
To determine the frequency of SARS-CoV-2 positive samples in a subset of patients consulting for primarily isolated acute (<7 days) loss of smell and to assess the diagnostic accuracy of olfactory/gustatory dysfunction for COVID-19 diagnosis in the overall population tested for COVID-19 in the same period.
Methods
Prospective multicentric cohort study in four olfactory ENT units and a screening center for COVID-19.
Results
i) Among a subset of 55 patients consulting for primarily recent loss of smell, we found that 51 (92.7%) had a COVID-19 positive test (median viral load of 28.8 cycle threshold). Loss of smell was mostly total (anosmia), rarely associated with nasal obstruction but associated with a taste disorder in 80%. Olfactory dysfunction occurred suddenly, either as first complaint or preceded by mild symptoms occurring a median of 3 days. The majority of patients (72.9%) partially recovered the sense of smell within 15 days. ii) In a population of 1824 patients tested for COVID-19, the positive predictive value and the specificity of loss of smell and/or taste were 78.5% and 90.3% respectively (sensitivity (40.8%), negative predictive value (63.6%)).
Conclusions
Self-reported loss of smell had a high predictive positive value to identify COVID-19. Making this sign well known publicly could help to adopt isolation measures and inform potential contacts.
- 2019-nCoV
novel coronavirus 2019
- APHP
Assistance Publique des Hôpitaux de Paris
- ARDS
acute respiratory distress syndrome
- COVID-19
Coronavirus Disease 2019
- CT
Cycle Threshold
- ENT
Ear Nose Throat
- HCoV
Human coronavirus
- MERS
Middle East respiratory syndrome
- OSN
Olfactory sensory neurons
- PPV
positive predictive value
- PVOD
post viral olfactory dysfunction
- RT-PCR
real time polymerase chain reaction
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus-2
- UTM-RT
universal transport medium- room temperature
- TLR
Toll Like Receptor
Introduction
Since December 2019, SARS-COV-2 has rapidly spread to become a world pandemic with alarming mortality and morbidity.1 SARS-COV-2 infection, COVID-19, is a new human disease presenting as a spectrum of symptoms. Some are characteristic of influenza-like disease (i.e. cough, headaches, myalgia, asthenia and eventually dyspnea) whereas other symptoms such as olfactory or taste dysfunction were evidenced along with the development of the pandemic.2 In the presence of suggestive symptoms, the definite diagnosis of COVID-19 mostly relies on positive real-time polymerase chain reaction (RT-PCR) on nasopharyngeal samples. RT-PCR also allows measuring viral load which could be linked to the disease severity and treatment outcome.3 However, due to the limited diagnostic test available, all patients suspected of COVID-19 are unlikely to be tested for the presence of the virus and the positive predictive value of each symptom is thus of importance in clinical practice. Self-reported olfactory impairment and gustatory dysfunction have recently been reported, in many patients with COVID-19 and may be an important predictor of clinical outcome.4 , 5 The few cohort studies dealing with these questions have reported a variable prevalence of loss of smell in patients with confirmed COVID-19 (from 5% to 85% according to studies and settings).4, 5, 6, 7 These studies being either retrospective5 , 7 or specifically conducted on laboratory-confirmed positive COVID-19 patients,4 , 6 , 7 the follow-up of anosmic/hyposmic patients, the link between loss of smell and nasal viral load were not described and the positive predictive value of loss of smell for COVID-19 infection rarely studied. Our first objective was to determine the frequency of SARS-CoV-2 positive samples in a subset of patients consulting for primarily recent (inferior or equal to 7 days) isolated acute loss of smell and to document the link between olfactory dysfunction and the viral load in rhinopharyngeal swabs. A secondary objective was to assess the diagnostic accuracy of olfactory/gustatory dysfunction for the diagnosis of COVID-19 (sensitivity, specificity, Positive and Negative Predictive Value) in the overall population tested for COVID-19 in the same period.
Material and methods
First part of the study: COVID-19 status of a subset of patients self-reporting smell loss as a main symptom
Study design and cohort description
We conducted an observational, prospective multicenter cohort study from March 17th to 25th, 2020 in five centers members of the Group of Greater Paris Hospitals (APHP): four ENT clinics and a Paris emergency screening center for COVID-19 based for health professionals and government employees. In order to document the link between olfactory dysfunction and the presence of SARS-CoV-2, we included consecutive patients consulting (or referred by our ENT colleagues) for a recent (inferior or equal to 7 days) loss of smell, as primary reason for consultation, either isolated or associated with mild symptoms of COVID-19 (such as headaches, low grade fever, myalgia, nose sneezing.) according to previous reports.8 All patients were tested for SARS-COV-2 by real-time polymerase chain reaction (RT-PCR) assay in nasopharyngeal swabs. Patients were not included if their olfactory symptoms had lasted for more than 7 days (to ensure that testing occurred during the replicative phase of the disease), if symptoms for COVID-19 were typical or predominant over olfactory symptoms (high fever, cough, dyspnea, pneumonia…) or if the SARS-COV-2 RT-PCR assay could not be performed. Presence of another obvious cause of anosmia was also an exclusion criterion.
Data collection
The following demographic data were collected at baseline: age, gender, weight, height, occupation, and tobacco use. Medical history included: respiratory allergy, chronic disease, asthma, immunosuppression, diabetes, long-term corticosteroid treatment, obesity, rhinologic history (head trauma, chronic rhinosinusitis, olfactory dysfunction history), acute anosmia history (date and onset (sudden or progressive), severity of associated olfactory symptoms (parosmia, phantosmia), taste disorders (ageusia/dysgeusia, sweet, salty, sour, bitter taste recognition), duration and treatment received (nasal irrigation, local or general corticosteroids, antibiotics…); other ENT symptoms. Data specifically covering COVID-19 included history and chronology of symptoms; severity (hospitalization/outpatient management) and treatment; Results for SARS-COV-2 by RT-PCR assay on nasopharyngeal swabs and contact with a confirmed case (by positive RT-PCR) as previously described.9 , 10
Patients were followed by a phone interview performed by a physician at day 7 (+/−2 days) repeated at day 15 (+/−2 days) to follow the features of their olfactory dysfunction as well as the evolution of COVID-19 disease (and by a 24-hour telephone platform)11. All patients provided informed consent. The local Institutional Review Board of Henri-Mondor Hospital (Ethics Committee number 00,011,558) granted ethics approval (Approval number 2020_056). A declaration to the National Commission of Informatics and Liberty (CNIL) was performed (CNIL-MR004).
SARS-COV-2 reverse transcription polymerase chain reaction (RT-PCR) on nasopharyngeal swabs
Samples were collected in Copan UTM-RT (universal transport medium- room temperature) nasopharyngeal swabs and were then inactivated in a Biosafety Level (BSL) 2 level laboratory. Five RT-PCR assays – the Roche, Altona, Elitech assay or the in house: IP2 (Institut Pasteur Paris, unpublished) and Coreman-Drosten– were used, depending on the center's routine practice, to detect SARS-CoV-2 RNA.10 The Roche assay (CobasⓇ SARS-CoV-2) is a qualitative assay based on fully automated sample preparation on a high throughput 8800 system (nucleic acid extraction and purification) followed by PCR amplification and detection. Two genes are targeted: the ORF1/a disordered region that is unique to SARS-CoV-2; and the E-gene of a conserved region in the structural protein envelope (pan-Sarbecovirus detection). The Elitech assay (GeneFinder™ COVID-19 PLUS RealAmp Kit) is a one-step reverse transcription RT-PCR. The protocol is based on viral polymerase (RdRp), envelope (E), and nucleoprotease (N) genes by RT-PCR amplification. Altona assay is based on the E and S genes detection. For all these tests, viral loads are expressed by Cycle Threshold (CT) values to give a semi quantitative assessment of the SARS-COV-2 viral load: the lower the CT value, the higher the viral load.
Second part of the study: diagnostic accuracy of self-reported loss of smell or taste for COVID-19 diagnosis on the whole cohort
For the second part of the study that assessed diagnostic accuracy of self-reported smell loss and/or taste for the diagnosis of COVID-19, all consecutive patients who were tested for SARS-COV-2 by real-time polymerase chain reaction (RT-PCR) during the same period in Paris screening center for COVID-19, were included and systematically assessed during the usual medical symptom's screening, about their potential olfactory and gustatory dysfunction.
Statistical analysis
Baseline demographic and medical characteristics (symptoms, history of the disease, ENT and general history) of the patients were described by n (%) for categorical data and mean (standard deviation SD) or median (inter-quartile range IQR) for continuous data as appropriate. We determined the proportion of patients with positive SARS-COV-2 RT-PCR status among patients with acute (<7 days) decreased sense of smell. 95% Confidence Intervals are displayed using binomial assumption.
For diagnostic accuracy of self-reported symptoms smell loss and/or taste for COVID-19 diagnostic, sensitivity, specificity, Positive and Negative Predictive Value and their Confidence Intervals were calculated and compared to that of other symptoms (cough, headache, sore throat) based all the population of all patients screened for COVID-19 during the same period in the Paris-based screening center for health professionals and government employees. Diagnostic accuracy of symptoms were also compared according to single or associated symptoms used as diagnostic criterion (only self-reported smell loss, only self-reported taste loss, presence of both, or non-exclusive association (self-reported smell loss and/or taste). Two-by-two comparisons (with other symptoms) were done using MacNemar test and Bonferroni correction, the threshold being p<0.017. Analysis was performed using Stata SE v15.0 (College Station, TX, USA).
Results
First part of the study: COVID-19 status of a subset of patients self-reporting smell loss as a main symptom
General characteristics of the participants to the descriptive study
Our study subgroup was 55 patients with self-reported acute loss of smell or taste as main symptom (13 patients referred by the four ENT centers and 42 from the COVID-19 screening center). Median age was 34 years (range 22–61), 56.4% were female and 61.1% were healthcare professionals. Nineteen patients (34.5%) reported a history of allergic rhinitis, mainly seasonal pollen-induced allergic rhinitis. Twelve patients (21.8%) were current smokers and four (7.4%) had a history of head trauma. Nine patients (16.4%) reported a history of intermittent olfactory dysfunction but none had a history of chronic olfactory dysfunction. Two-thirds (6/9, 66%) of the patients with a history of intermittent anosmia had a history of allergic rhinitis or rhino-pharyngitis or sinusitis, higher than in patients without (13/33; 28%; p = 0.05). Patient characteristics are summarized in Table 1 .
Table 1.
Demographic characteristics and clinical history of the 55 patients presenting with olfactory dysfunction as main symptom of COVID-19.
| Characteristics | Available data | Patients |
|---|---|---|
| Clinical characteristics | ||
| Age, years | 55 | 34 [28–43] |
| Male, No. (%) | 55 | 24 (43.6) |
| Profession | 54 | |
| Healthcare worker, No. (%) | 33 (61.1) | |
| Medico-social or administrative worker, No. (%) | 4 (7.4) | |
| Other, No. (%) | 17 (31.5) | |
| BMI, kg/m² | 53 | 22.0 [20.3–24.1] |
| BMI, kg/m² | 53 | |
| <18.5, No. (%) | 4 (7.5) | |
| [18.5;25[, No. (%) | 41 (77.4) | |
| [25;30[, No. (%) | 4 (7.5) | |
| ≥30, No. (%) | 4 (7.5) | |
| Immunosupression, No. (%) | 55 | 0 |
| Smoking, No. (%) | 55 | 12 (21.8) |
| Diabetes, No. (%) | 55 | 0 |
| Arterial hypertension, No. (%) | 55 | 2 (3.6) |
| Asthma, No. (%) | 55 | 1 (1.8) |
| Respiratory allergy, No. (%)* | 55 | 23 (41.8) |
| Pollen | 13 | |
| Dust mite | 3 | |
| Olfactory risk factors | ||
| Head trauma, No. (%) | 54 | 4 (7.4) |
| History of allergic rhinitis No. (%) | 55 | 19 (34.5) |
| History of Olfactory Dysfunction No,(%) | 9 (16.4) | |
| Chronic Olfactory Dysfunction (n = 8) | 0 |
One patient had a missing data for the type of respiratory allergy.
Values are median [inter-quartile range] or number (percentage%). BMI: Body Mass Index; COVID-19: Coronavirus Disease 2019; No.: number.
SARS-COV-2 RT-PCR results
RT-PCR assay was positive in 51/55 patients (92.7%) [95% CI: 82.4–98.0]. Among the four patients with negative PCR, one patient had a contact with a confirmed case for SARS -COV-2. CT values ranged from 20.84 to 38.1 for the RdRP gene, and from 21.14 to 36.97 for the E-gene, corresponding to a moderate level of viral load. The median CT value for E gene was 28.83 [27.55–32.72] (supplementary Table 5).
Characteristics of anosmia and other ENT symptoms
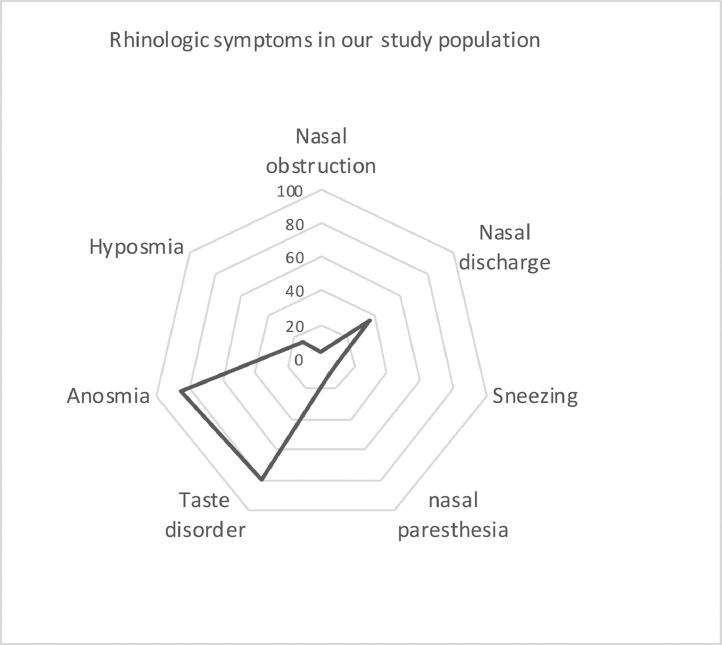
Characteristics of baseline olfactory dysfunction and other ENT symptoms are presented in Table 2 and in Fig. 1 . Loss of smell was the initial sign of COVID-19 infection in 16 patients (29.1%). For the others (n = 39, 70.9%), it was preceded by the occurrence of other mild symptoms of COVID-19 infection with loss of smell occurring a median of 3 days (2–4) after the first symptoms. In the whole group, olfactory dysfunction onset was sudden in 47 patients (88.7%). Forty-seven patients (85.5%) reported anosmia and eight (14.5%) hyposmia. Loss of smell associated a sensation of loss of taste in 46 patients (83.6%): partial loss in 47.3%, and ageusia in 36.4%. Among the patients with taste disorder, failure in salt taste recognition was the most self-reported taste recognition dysfunction.
Table 2.
Characteristics of baseline olfactory dysfunction (OD) and other symptoms of COVID-19 infection (n = 55).
| Variables | Number of aptients | N (%) |
|---|---|---|
| Characteristics of clinical Olfactory Dysfunctions (OD) | ||
| Chronology | 53 | |
| Sudden, No. (%) | 47 (88.7) | |
| Progressive, No. (%) | 6 (11.3) | |
| Initial symptom, No. (%) | 55 | 16 (29.1) |
| Time between the beginning of occurrence of COVID symptoms and the beginning of OD occurrence, days (if non-initial OD) | 39 | 3 |
| Anosmia, No. (%) | 55 | 47 (85.5) |
| Hyposmia, No. (%) | 55 | 8 (14.5) |
| Phantosmia, No. (%) | 55 | 2 (3.6) |
| Parosmia, No. (%) | 55 | 2 (3.6) |
| Rhinologic symptoms | ||
| Nasal discharge, No. (%) | 55 | 20 (36.4) |
| Nasal obstruction, No. (%) | 55 | 2 (3.6) |
| Sneezing, No. (%) | 55 | 6 (10.9) |
| Intranasal painful paresthesia, No. (%) | 55 | 6 (10.9) |
| Other rhinologic symptoms, No. (%) | 55 | 2 (3.6) |
| Taste disorders | ||
| No taste disorder, No. (%) | 55 | 9 (16.4) |
| Dysgeusia, No. (%) | 55 | 26 (47.3) |
| Ageusia, No. (%) | 55 | 20 (36.4) |
| Salty taste recognition, No. (%)* | 46 | 22 (47.8) |
| Sweet taste recognition, No. (%)* | 43 | 15 (34.9) |
| Bitter taste recognition, No. (%)* | 40 | 15 (37.5) |
| Acidic taste recognition, No. (%)* | 39 | 18 (46.2) |
| Other symptoms | ||
| Absence of other symptoms of COVID, No. (%) | 55 | 11 (20.0) |
| Presence of minor symptoms of COVID, No. (%) | 55 | 40 (72.7) |
| Presence of concomitant typical symptoms of COVID, No. (%) | 55 | 4 (7.3) |
| Low grade Fever, No. (%) | 55 | 16 (29.1) |
| Mild Cough, No. (%) | 55 | 22 (40.0) |
| Headache, No. (%) | 55 | 38 (69.1) |
| Asthenia, No. (%) | 50 | 28 (56.0) |
| Myalgia, No. (%) | 55 | 17 (30.9) |
| Dyspnea, No. (%) | 54 | 2 (3.7) |
| Odynophagia, No. (%) | 55 | 8 (14.5) |
| Diarrhea, No. (%) | 55 | 16 (29.1) |
| Conjunctivitis, No. (%) | 55 | 4 (7.3) |
| Laryngitis, No. (%) | 55 | 1 (1.8) |
| Skin hyperesthesia, No. (%) | 55 | 5 (9.1) |
Among patients with taste disorder (dysgeusia/ageusia) values are median [inter-quartile range] or number (percentage%). COVID-19: Coronavirus Disease 2019; No.: number; OD: Olfactory Dysfunction.
Fig. 1.
Frequency of rhinologic symptoms in the study population (n = 55 patients)
Values are percentage of patients. COVID-19: Coronavirus Disease 2019.
Only two patients (3.6%) reported concomitant nasal obstruction. Other rhinologic symptoms were: nasal discharge (36.4%), sneezing (10.9%), endonasal burning sensations (10.9%). Finally, eight patients (14.5%) reported odynophagia.
Associated clinical symptoms of COVID-19
Thirty-nine patients presented mild clinical symptoms of COVID-19 that usually appeared a few days before the onset of the loss of smell (Table 2). These included: headaches (69.1%), asthenia (56%), mild cough (40%), myalgia (30.9%), diarrhea (29.1%) and low-grade fever (29.1%). Cutaneous hyperesthesia with a root pathway on various parts of the body was reported by 9.1% of the patients.
Treatment and follow-up
Over half of the patients (30 patients, 54.5%) had received an initial treatment for their olfactory dysfunction: nasal irrigation with saline (n = 17/30, 56.7%), a local corticosteroid (n = 6/30, 20%), or an oral corticosteroid (n = 1/30, 3.3%). Olfactory training was prescribed to 18 patients (n = 18/30, 60%). One patient received a 5-day treatment of hydroxychloroquine and azithromycin as an anti COVID-19 therapy and a second one received a 10-day treatment of hydroxychloroquine associated with a 5-day treatment of azithromycin.
Among the 55 patients, 50 had a follow-up at Day 7 (+/−2 days) and at Day 15 (+/−3 days). At Day 7, 33/50 (66%) had a beginning of smell recovery, partial for the majority (Table 3 ). At Day 15, 48/51 (94.1%) had a beginning of smell recovery but only complete for 13 (27.1%) (Table 3). The only patient who received oral corticosteroid therapy (for 2 days) completely recovered his sense of smell in less than 7 days and did not develop severe COVID-19 form. During follow-up, no patients were hospitalized or developed severe complications of COVID-19.
Table 3.
Short-term follow-up of clinical course and management of acute olfactory disorder (OD) in the subset of 55 patients with acute loss of smell as main reason of consultation in olfactory ENT units or referred by ENT collegues in the screening center for COVID-19.
| Variables | Available data | patients (%) |
|---|---|---|
| Olfactory treatment | ||
| Received treatment for OD, No. (%) | 55 | 30 (54.5) |
| Local treatment, No. (%)* | 30 | 18 (60.0) |
| Local corticosteroids, No. (%)* | 30 | 6 (20.0) |
| Nasal irrigation with saline solution, No. (%)* | 30 | 17 (56.7) |
| Anti-COVID treatment (hydroxychloroquine/azithromycine) | 2 | |
| General corticosteroids, No. (%)* | 30 | 1 (3.3) |
| Olfactive training, No. (%)* | 30 | 18 (60.0) |
| Olfactory recovery | ||
| Olfactory recovery at Day 7 (+/−2 days) | 50 | |
| None, No. (%) | 17 (34) | |
| Type of recovery at Day 7(+/−2 days) n = 32 Partial, No. (%) |
28 (87.5) | |
| Complete, No. (%) | 4 (12.5) | |
| Olfactory recovery at Day 15 (+/−2 days) None, No. (%) |
51 | 3 (5.9) |
| Type of recovery at Day 15 (+/−2 days) n = 48 Partial, No. (%) |
35 (72.9) | |
| Complete, No. (%) | 13 (27.1) | |
| Progression of COVID-19 at Day 15 | ||
| Community care management No. (%) | 55 | 55 (100) |
| Hospitalization | 55 | 0 |
| Anti-COVID treatment No. (%) | 55 | 2 (3.6) |
| Severe form (complication such as organ failure or acute respiratory distress) | 55 | 0 |
| Death | 54 | 0 |
Among treated patients
Values are median [inter-quartile range] or number (percentage%).
Second part of the study: diagnostic accuracy of self-reported loss of smell and/or taste for COVID-19 diagnosis on the whole cohort
During the period of inclusion, 1824 consecutive patients were tested for SARS-COV-2 in the Paris-based screening center for COVID-19. Out of these, 849/1824 (46.5%) patients had a positive SARS-COV-2 RT-PCR in nasopharyngeal swabs. Among positive patients for COVID-19, 40.8% reported loss of smell and/or loss of taste. The positive predictive value (PPV) of olfactory and/or gustatory dysfunction was 78.5% [CI95% :76.6- 80.3], the sensitivity was 40.8% [CI95% :38.5-43.0], the specificity was 90.3% [CI95% :88.9-91.6] and the negative predictive value (NPV) 63.6% [CI95% :61.4- 65.8] (supplementary Table 6). The best diagnostic accuracy was obtained with the non-exclusive association of both symptoms: loss of smell and/or loss of taste (Table 4 ). The diagnostic accuracy of self-reported loss of smell and/or taste was much higher than that of cough, headache and sore throat while sensitivity of cough and headache were higher than loss of smell/taste (Table 4b ).
Table 4a.
Diagnostic accuracy of SINGLE or ASSOCIATED symptoms of self-reported loss of smell and/or taste for SARS COV2 diagnosis in a monocentric population of 1824 patients screened for SARSCoV-2 according to the choice of symptom criterion:4a: Diagnostic accuracy single or associated symptoms of self-reported loss of smell and/or taste.
| Loss of smell onlyN = 128 | Loss of taste onlyN = 136 | Loss of smell and tasteN = 313 | Loss of smell and/or loss of taste N = 441 | |
|---|---|---|---|---|
| Sensibility [CI95] | 17.5% | 13.7% | 37.0% | 40.8% |
| [15.5–19.5] | [11.9–15.6] | [34.6–39.4] | [38.5–43.0] | |
| Specificity [CI95] | 95.8% | 92.4% | 93.2% | 90.3% |
| [94.7–96.8] | [91.0–93.8] | [92.0–94.5] | [88.9–91.6] | |
| Positive Predictive Value [CI95] | 71.9% | 50.7% | 81.2% | 78.5% |
| [69.5–74.3] | [48.1–53.4] | [79.1–83.2] | [76.6–80.3] | |
| Negative Predictive Value [CI95] | 65.3% | 65.2% | 65.2% | 63.6% |
| [62.7–67.8] | [62.7–67.8] | [62.7–67.8] | [61.4–65.8] |
Table 4b.
Diagnostic accuracies of loss of smell and/or taste, cough, headache, sore throat for thepresence of SARS COV2
| Loss of smell and/or tasteN = 441 | Cough N = 1247 | HeadacheN = 1242 | Sore throat N = 839 | |
|---|---|---|---|---|
| Sensibility [% ; 95%CI] | 40.8%1,2 | 70.4% | 71.0% | 40.1% |
| [38.5–43.0] | [68.3–72.5] | [68.9–73.1] | [37.8–42.3] | |
| Specificity [% ; 95%CI] | 90.3%3,4,5 | 32.4% | 34.4% | 48.9% |
| [88.9–91.6] | [30.2–34.5] | [32.2–36.6] | [46.6–51.2] | |
| Positive Predictive Value [% ; 95%CI] |
78.5% | 47.5% | 48.5% | 40.5% |
| [76.6–80.3] | [45.2–49.8] | [46.2–50.8] | [38.3–42.8] | |
| Negative Predictive Value [% ; 95%CI] |
63.6% | 55.7% | 57.7% | 48.4% |
| [61.4–65.8] | [53.5–58.0] | [55.5–60.0] | [46.1–50.7] |
95%CI indicated 95% confidence interval.
Significant difference of sensitivity of anosmia/ageusia versus Cough p<0.017 (Bonferroni correction).
Significant difference of sensitivity of anosmia/ageusia versus Headache p<0.017 (Bonferroni correction).
Significant difference of specificity of anosmia/ageusia versus Headache p<0.017 (Bonferroni correction).
Significant difference of specificity of anosmia/ageusia versus Headache p<0.017 (Bonferroni correction).
Significant difference of specificity of anosmia/ageusia versus Sore throat p<0.017 (Bonferroni correction).
Discussion
In the context of the French COVID-19 outbreak, among a subset of patients consulting for an acute and recent (inferior or equal to 7 days) loss of smell as main symptom of COVID-19 suspicion, we found that 51/55 (92.7%) had a positive test for SARS-COV2, thus confirming loss of smell as hallmark of COVID-19 disease. Loss of smell occurred mostly suddenly (within hours) and was the initial COVID-19 symptom in one third of the cases and was associated with taste disorders in 80%. Unlike the usual loss of smell during the common cold, only two patients complained of nasal obstruction.
On the overall population seen at the emergency screening center for COVID-19, by comparing patients self-reporting smell or gustative loss and those without such symptoms, we assessed the high predictive positive value of olfactory and/or gustative dysfunctions for COVID-19 diagnosis (78.5%) as well as its very high specificity (90%) as already observed in a recent shorter series.2 Concerning SARS-COV-2 viral loads, although only expressed semi-quantitatively as CTs, we noted that they were globally lower in our series than usually reported in severe forms of COVID.3
Viral upper respiratory infection is a major cause of olfactory dysfunction.12 While rhinoviruses are the most common pathogens found, CoVs are also known to induce post-viral olfactory dysfunction (PVOD).13 PVOD exhibits a seasonal pattern with a spring predominance.14 Most of the time, PVOD is temporary, and recovers partially or completely in 80% at one year,15 which corresponds to the time needed for neuroepithelium regeneration. The very short-term follow-up of 2 weeks in our cohort explains the low complete recovery rate (27.1% at Day 15). Although the pathophysiology of PVOD induced by SARS COV-2 is not well known,16 three pathophysiological pathways have been suggested. The first is a conductive dysfunction due to mucosal swelling of the olfactory cleft with common cold. In our cohort, only two patients complained of nasal obstruction. The inflammatory process could be localized in the olfactory cleft alone due to the neurologic tropism of this virus and this could explain the absence of nasal obstruction. Expression of SARS-CoV 2 entry factors (ACE 2 and TMPRSS2) have been proven in nasal epithelial cultured cells grown in liquid air interface, and specifically in olfactory epithelium.17 , 18 Secondly, PVOD can be a sensorineural dysfunction resulting from damage of the olfactory neuroepithelium. Histological analysis in patients with post-infectious olfactory loss shows acute inflammation and chronic neuroepithelial remodeling.19 Yet, SARS-CoV2 has been shown to have a neurologic tropism and neuroinvasive potential.20 The virus is known to induce a cytokine storm with release of pro-inflammatory cytokines, including interleukin and activation of the toll-like receptors in the respiratory tract.21 Moreover, it has been previously reported that neuroepithelium is more widely spread in the nasal cavity of younger people which could explain such a prevalence of smell loss in our population of young health professionals.22 Furthermore, we observed an atypically high frequency of neurogenic signs such as headaches (69.1%) usually described in around 10% of the patients in COVID-19 disease,23 endonasal burning sensations (10.9%) and cutaneous paresthesia (9.1%), which seems to argue in favor of neuroepithelium damage. The third pathway, less probable from the rapid beginning of recovery in a majority of the patients, involves central dysfunction resulting in damage of the olfactory central nervous system with one hypothesis for PVOD being that pathogens enter the brain directly through the olfactory nerve.24
The main strength of this study is our unique approach in the current literature of swabbing patients presenting with isolated anosmia (a very unusual symptom when not associated with nasal obstruction) during the COVID-19 pandemic. In the second part of our study we have included a large number of patients screened for COVID-19 of whom 46.5% had a positive RT-PCR which enable to determine the diagnostic accuracy of self-reported smell and taste loss for the diagnosis of COVID-19. The main limitation of the present study was a selection bias in the first part of the study as we focused on the inclusion of patients with recent loss of smell, which can lead to an overestimation of the predictive value. However, in the second part of the study performed in one of the participating center, we included all consecutive patients with a suspicion of COVID, with or without loss of smell and confirmed the high predictive value and specificity of self-reported smell and taste loss for the diagnosis of COVID-19, than other usual symptoms such as cough, headache and sore throat. No data was reported in the current literature about diagnostic value of loss of smell/taste or other symptoms of COVID-19.
The positive predictive value is also impacted by the prevalence of COVID-19 which could evolve over time and settings. Finally, definite diagnosis of COVID-19 infection was only based on RT-PCR and it is possible that, if performed, chest CT-scan would have added more patients in the COVID-19 positive group. While the current gold standard for the diagnosis of COVID-19 infection is RT-PCR, this assay does not have a 100% sensitivity and it is associated with several potential vulnerabilities (incorrect sampling or preanalytical condition or weak viral excretion…).25 We minimized the risk by including patients with loss of smell onset inferior or equal to 7 days. Among the four patients with a negative RT-PCR out of 55 included in our subset, one had a PCR-confirmed case contact and the three others had a very typical picture of anosmia without nasal obstruction. Thus, we believe that the negative test results in these four patients could be false negatives. To note, false negative results were lower in this study than observed in others, probably because clinical presentation with loss of smell corresponds to a viral localization in nasopharynx.
In conclusion, the present study confirms the high diagnostic value of self-reported loss of smell for SARS-COV-2 infection which appears as an early sign of the disease. And this finding has potential implications at the individual and public health levels in the current COVID-19 outbreak. Our cohort study confirms that i) acute isolated self-reported loss of smell without nasal obstruction is an early red flag of COVID-19 and an additional criterion for identifying COVID-19 infected-patients and does not need further investigation. This symptom could be added to the list of warning symptoms for public to look out for. Making this sign well known publicly could help these patients, highly contagious despite otherwise mild symptoms, to self-isolate and inform potential contacts of potential sources of contamination. ii) The very high predictive value and specificity of self-reported loss of smell and/or taste, among other classical symptoms, could help to make a presumptive diagnosis of COVID-19. At the public health level, this sign could be easily contained in public health guidance for follow-up of the disease along with the fever, cough and breathlessness. Further studies are needed to better understand the physiopathology and define therapeutic approaches that could increase the recovery rate of the patients.
Ethics approval and consent to participate
All patients provided informed consent. The local Institutional Review Board of Henri-Mondor Hospital (Ethics Committee number 00011558) granted ethics approval (Approval number 2020_056). A declaration to the National Commission of Informatics and Liberty (CNIL) was performed (CNIL-MR004).
Consent for publication
Not applicable.
Availability of data and materials
All data generated or analysed during this study are included in this published article [and its supplementary information files].
Funding
This study was not sponsored by any external financial support
Authors' contributions
Conceptualization: DS, EB, JFP, AC, FB, YN, RL, FCP, ES and CH; Data curation: EB, ES, DS, SB, CH, YN, JN, ALH, YC, FCP, BV, SD, SHB, DC, AC, AB, NE, FR, RL, JFP Formal analysis : EB, DS, SB, CH, YN, JN, ALH, YC, FCP, BV, SD, SHB, DC, JFP, AC, FR, RL, ES Methodology :EB, ES, DS, SB, JFP, CH, FCP; Orginal draft: SB, EB, DS, JFP, SHB, JFP, AC, CH, YN, FCP, SHB, ES and RL; Supervision : DS, EB, JFP, CH, YN Validation, EB, DS, SB, CH, YN, JN, ALH, YC, FCP, BV, SD, SHB, DC, AC, AB, NE, FR, RL, JFP, ES Writing – original draft: SB, EB, DS, JFP, YN, CH, FCP, AC, SHB, ES and RL
Declaration of Competing Interest
All the authors have read and agreed with the paper's content. No authors have financial or personal conflicts. Neither the work nor any part of its essential substance, tables or figures have been or will be published or submitted to another scientific journal or are being considered for publication elsewhere.
Acknowledgment
The authors would like to thank Pr Sebastien Gallien, Henri Mondor Hospital, Pr Stéphane Jaurreguiberry and Dr Tran Khai Nhung, Bicêtre Teaching Hospital, Le Kremlin Bicêtre France. We thank all patients who participated in the study and nurses/doctors who performed the nasopharyngeal samples. We thank Felicity Neilson for linguistic revision.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jinf.2020.07.005.
Appendix. Supplementary materials
References
- 1.Paules C.I., Marston H.D., Fauci A.S. Coronavirus infections-more than just the common cold. JAMA. 2020 doi: 10.1001/jama.2020.0757. [DOI] [PubMed] [Google Scholar]
- 2.Hopkins C., Surda P., Kumar N. Presentation of new onset anosmia during the COVID-19 pandemic. Rhinology. 2020 doi: 10.4193/Rhin20.116. [DOI] [PubMed] [Google Scholar]
- 3.Yu X., Sun S., Shi Y., Wang H., Zhao R., Sheng J. SARS-CoV-2 viral load in sputum correlates with risk of COVID-19 progression. Crit Care Lond Engl. 2020;24:170. doi: 10.1186/s13054-020-02893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lechien J.R., Chiesa-Estomba C.M., De Siati D.R. Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Oto-Rhino-Laryngol Off J Eur Fed Oto-Rhino-Laryngol Soc EUFOS Affil Ger Soc Oto-Rhino-Laryngol - Head Neck Surg. 2020 doi: 10.1007/s00405-020-05965-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bénézit F., Le Turnier P., Declerck C. Utility of hyposmia and hypogeusia for the diagnosis of COVID-19. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30297-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moein S.T., Hashemian S.M.R., Mansourafshar B., Khorram-Tousi A., Tabarsi P., Doty R.L. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yan C.H., Faraji F., Prajapati D.P., Ostrander B.T., DeConde A.S. Self-reported olfactory loss associates with outpatient clinical course in Covid-19. Int Forum Allergy Rhinol. 2020 doi: 10.1002/alr.22592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang C., Wang Y., Li X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet Lond Engl. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang D., Hu B., Hu C. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020 doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Corman V.M., Landt O., Kaiser M. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill Bull Eur Sur Mal Transm Eur Commun Dis Bull. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Épidémie Coronavirus (SARS-CoV-2) -Application Covidom : un télésuivi des patients porteurs ou suspectés de Covid-19 | service-public.fr. Available at:https://www.service-public.fr/particuliers/actualites/A13927. Accessed 26 March 2020.
- 12.Suzuki M., Saito K., Min W.-.P. Identification of viruses in patients with postviral olfactory dysfunction. Laryngoscope. 2007;117:272–277. doi: 10.1097/01.mlg.0000249922.37381.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seiden A.M. Postviral olfactory loss. Otolaryngol Clin North Am. 2004;37:1159–1166. doi: 10.1016/j.otc.2004.06.007. [DOI] [PubMed] [Google Scholar]
- 14.Konstantinidis I., Haehner A., Frasnelli J. Post-infectious olfactory dysfunction exhibits a seasonal pattern. Rhinology. 2006;44:135–139. [PubMed] [Google Scholar]
- 15.Lee D.Y., Lee W.H., Wee J.H., Kim J.-.W. Prognosis of postviral olfactory loss: follow-up study for longer than one year. Am J Rhinol Allergy. 2014;28:419–422. doi: 10.2500/ajra.2014.28.4102. [DOI] [PubMed] [Google Scholar]
- 16.Hummel T., Whitcroft K.L., Andrews P. Position paper on olfactory dysfunction. Rhinology. 2016;56:1–30. doi: 10.4193/Rhino16.248. [DOI] [PubMed] [Google Scholar]
- 17.Zou L., Ruan F., Huang M. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sungnak W., Huang N., Bécavin C. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020 doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamagishi M., Fujiwara M., Nakamura H. Olfactory mucosal findings and clinical course in patients with olfactory disorders following upper respiratory viral infection. Rhinology. 1994;32:113–118. [PubMed] [Google Scholar]
- 20.Li Y.-.C., Bai W.-.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet Lond Engl. 2020 doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holbrook E.H., Wu E., Curry W.T., Lin D.T., Schwob J.E. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121:1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L.-.Q., Huang T., Wang Y.-.Q. ovel coronavirus patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2019 n2020 doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Youngentob S.L., Schwob J.E., Saha S., Manglapus G., Jubelt B. Functional consequences following infection of the olfactory system by intranasal infusion of the olfactory bulb line variant (OBLV) of mouse hepatitis strain JHM. Chem Senses. 2001;26:953–963. doi: 10.1093/chemse/26.8.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lippi G., Simundic A.-.M., Plebani M. Potential preanalytical and analytical vulnerabilities in the laboratory diagnosis of coronavirus disease 2019 (COVID-19) Clin Chem Lab Med. 2020 doi: 10.1515/cclm-2020-0285. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article [and its supplementary information files].