Abstract
Vertebral heart scoring (VHS) is a semiquantitative method to assess the presence and severity of cardiomegaly by using thoracic radiographs. VHS in rhesus macaques (Macaca mulatta) has not been validated or used routinely in the clinical or research setting. We hypothesized that rhesus macaques with cardiac disease diagnosed by using echocardiography would have higher VHS than animals without cardiac disease. A total of 150 rhesus macaques were enrolled in this study. All animals underwent echocardiography and thoracic radiography (right lateral [RL], dorsoventral [DV], and ventrodorsal [VD] views). According to echocardiography, 121 rhesus macaques had no cardiac disease and were used to establish reference intervals for VHS. The remaining 29 macaques had hypertrophic cardiomyopathy (n = 20) or other cardiac disease (n = 9). Results showed that VHS of RL and VD views were significantly higher in macaques with any of the identified cardiac diseases and in the cardiac disease group that excluded hypertrophic cardiomyopathy. VHS of animals with HCM was not significantly different than that of control animals. In the RL view, VHS was moderately accurate for predicting the presence of cardiac disease, with an AUC of 0.71 and an optimal cut-off value of 10.25 (sensitivity: 62%, specificity: 77%). In the VD view, VHS was a mildly accurate test for cardiac disease, with an AUC of 0.654 and an optimal cut-off value of 10.65 (sensitivity, 66%; specificity, 63%). Study results indicated that VHS could be a useful screening tool for clinically identifying rhesus macaques with cardiac disease. However, VHS is unlikely to replace echocardiographic examination for determining the presence, type, and severity of cardiac disease in this species.
Abbreviations: ACD, all cardiac disease; DV, dorsoventral; HCM, hypertrophic cardiomyopathy; OCD, other cardiac disease; ROC, receiver operating characteristic; RL, right lateral; VD, ventrodorsal; VHS, vertebral heart score
Cardiac disease such as hypertrophic cardiomyopathy (HCM) has been reported in captive rhesus macaques.13,18,24,30,31 Cardiac diseases that compromise hemodynamic status can significantly affect animal health. In addition, cardiac abnormalities can compromise research findings in laboratory animals. Therefore, cardiovascular examination is recommended prior to enrolling animals into both cardiovascular or noncardiovascular studies using rhesus macaques.
Echocardiography is commonly used as a noninvasive, antemortem, diagnostic tool in veterinary medicine and can assess both cardiac structure and function.5 Information obtained through echocardiographic examination enables clinicians and researchers to make decisions regarding medical management and surgical interventions. For both cardiovascular and noncardiovascular research, fully assessing the health status of animals, including cardiac structure and function, prior to conducting experiments is extremely important. However, echocardiographic examination has several limitations. First, echocardiography is highly operator-dependent and requires considerable training to accurately evaluate cardiac structure and function.28 Second, echocardiographic equipment is not available in many clinical and research facilities due to its expense and a lack of fully trained operators. Therefore, alternative modalities for diagnosing the presence and severity of cardiac diseases in rhesus macaques for clinical and research purposes would be useful for veterinarians and researchers.
Vertebral heart scoring (VHS) is a semiquantitative assessment tool that has been used in various veterinary patients, including dogs and cats, to detect the presence and severity of various cardiac diseases.6 The systematic approach uses thoracic radiographs, from which the cardiac silhouette is measured along its short and long axes; these measurements are then compared with the number of thoracic vertebrae present within these measured distances. In contrast to echocardiography, VHS is less operator-dependent than echocardiography and is an inexpensive assessment tool to determine cardiac disease status. The ease of use and accessibility can make VHS an attractive method that might enable early identification of cardiac disease and follow-up of disease progression in rhesus macaques. VHS has been established and shown to be useful in determining the presence and severity of cardiac disease in various species, including some NHP.4,9,11,14,15,19-23,25-27,29,32,34 However, to our knowledge, no study of VHS in rhesus macaques has been published.
In the current study, VHS reference intervals were established in clinically healthy rhesus macaques without significant cardiac disease. We then compared VHS in rhesus macaques with and without various cardiac diseases, including HCM, with echocardiographic findings to determine the usefulness of VHS to identify cardiac disease. We hypothesized that rhesus macaques with cardiac disease diagnosed through echocardiography would have higher VHS than healthy animals without cardiac disease. Establishing VHS as a screening test for cardiac diseases could help clinicians and researchers to identify animals warranting additional cardiovascular examination, including prioritizing those that should undergo echocardiography.
Materials and Methods
Ethics statement
The California National Primate Research Center is a USDA-registered and AAALAC-accredited institution that receives NIH funding and maintains a Public Health Services Assurance. The University of California–Davis IACUC provides oversight and approval of all research protocols. All study procedures and methods were approved in advance by the IACUC before procedures were performed. All NHP at the California National Primate Research Center are cared for in accordance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals, and institutional standard operating procedures.2,3,16
Animal housing and management
All macaques of various ages and sex are socially housed with others of the same barrier status (SPF or conventional). SPF animals are those from which selected viral pathogens—such as herpes B virus, simian T lymphotrophic virus, SIV, simian retrovirus, simian foamy virus, rhesus cytomegalovirus, and rhesus rhadinovirus—have been eliminated from breeding programs through repeated testing and elimination. Conventional animals did not test negative for one or more of the listed SPF viruses. All outdoor macaques were socially housed (both sexes and all age groups) in field pens or corn cribs, with maximal capacities of 180 and 30 animals, respectively. Outdoor routine husbandry includes cleaning of readily accessible fixture surfaces (feed pads, feeding boxes, perches or anywhere animals may sit, etc.) and enrichment items (toys, barrels, etc.) by using standard sanitization methods at least twice each month. Field pens were steam-cleaned quarterly or more often as needed, and corn cribs were pressure-washed and then steam-cleaned twice each month, or more often as needed. After being steam-cleaned, perches were scrubbed with a 10% disinfecting solution and rinsed with water from a hose. Entire cages were fan-raked or spot-cleaned once each week, by removing feces, soiled areas, and waste, including leftover chow. All cages were provided protection from the elements. Lawns in field pens are mowed as needed.
All outdoor animals had a physical examination every 6 mo that includes tuberculosis blood testing. Indoor macaques (both sexes or same-sex pairs and all age groups) were housed in stainless steel cages. Routine husbandry of indoor animals included: controlled temperature, humidity, ventilation; 12:12-h light:dark cycles; and daily routine cleaning procedures. All macaques received commercial primate chow twice daily (LabDiet Monkey Diet 5045 or 5047, Purina Mills International, St Louis, MO), free-choice water through automatic watering devices, biweekly supplementation with fruit and vegetables, and species-appropriate environmental enrichment, such as manipulanda. All macaques underwent physical exam and a tuberculosis test (usually semiannually for outdoor animals and annually for indoor animals). In addition, indoor animals were weighed approximately every 2 mo. All geriatric animals or animals with clinical problems were evaluated further through CBC counts and serum biochemistry performed. Macaques with any chronic health issues were excluded from this study.
Study population
All rhesus macaques enrolled in this study had a complete physical examination including assessment of heart rate, respiratory rate, body condition score, and body weight as well as echocardiographic and radiographic examination during the same sedation event or 2 separate sedations within 1 mo of each other.8 In addition, 3 mL of venous blood was collected for evaluation of cardiac biomarkers prior to echocardiographic examination during the same sedation event. Echocardiographic examination was performed on all animals immediately or within 1 mo before the VHS study to determine the presence, type, and severity of any cardiac diseases. Rhesus macaques enrolled in this study were divided into 3 groups based on complete echocardiographic examination: control animals, which lacked any significant cardiac abnormalities; macaques with HCM; and animals with cardiac diseases other than HCM (OCD group). In addition, rhesus macaques with HCM or OCD were combined into a single group (all cardiac disease [ACD]).
Sedation protocol
All animals were fasted 6 h in the morning and sedated by using ketamine hydrochloride (10 mg/kg IM; Ketaject, Phoenix Pharmaceutical, St Joseph, MO) immediately prior to echocardiography and blood pressure examination (if performed). After the completion of echocardiography with or without blood pressure assessment, additional ketamine hydrochloride (10 mg/kg IM) with or without dexmedetomidine hydrochloride (0.025 to 0.075 mg/kg IM; Dexdomitor, Zoetis, Parsippany, NJ) was administered for radiographic examination. When dexmedetomidine was given, atipamezole hydrochloride (Antisedan, Zoetis Services) was administered intramuscularly for sedation reversal after all examinations were performed. After all study procedures were completed, animal care staff monitored macaques every 15 min until the animal was sitting in its cage.
Echocardiographic examination
Rhesus macaques were placed in right and left lateral recumbency for obtaining all echocardiographic images. Images were obtained by using a 4- to 12- mHz sector-array transducer (S12-4) with color and spectral Doppler capabilities (Affiniti 50, Phillips, Best, Netherlands). Echocardiography was performed by a board-certified cardiologist (JAS) or a veterinarian (YU) trained and directly supervised by a board-certified cardiologist. Still images and video were evaluated by a single cardiologist (JAS). Data analysis was completed by using standard offline analysis software (Syngo Dynamics, Siemens, Erlangen, Germany).
HCM was diagnosed based on echocardiographic evidence of thickened left ventricular walls with the presence of concurrent diastolic dysfunction. HCM was diagnosed when the thickness of the left ventricular posterior wall during diastole or interventricular septum during diastole (or both) exceeded 6.5 mm in rhesus macaques 9 y of age or younger. In rhesus macaques older than 9 y, HCM was diagnosed when the left ventricular posterior wall during diastole exceeded 7.4 mm or when the interventricular septum during diastole was more than 8.8 mm in thickness.30 Diastolic dysfunction was diagnosed when the ratio of the peak early ventricular transmitral filling velocity to that of the late ventricular transmitral filling velocity was 0.9 or less or when the medial or lateral peak mitral annular velocity during early and late filling was 0.9 or less.13,30
All macaques observed to have left ventricular concentric hypertrophy according to the stated diagnostic criteria underwent oscillometric systemic blood pressure assessment at the time of echocardiography. Briefly, diastolic, mean, and systolic blood pressures were measured by using an automated oscillometric system (Cardell 9401, Midmark, Versailles, OH) on the left forelimb while the animal was in right lateral recumbency. Blood pressure was measured 3 times; the heart rate displayed on the blood pressure measurement device was confirmed to match heart rate obtained on echocardiography. The average of 3 measures was used to identify animals with a mean blood pressure greater than 110 mm Hg or systolic blood pressure greater than 160 mmHg; these macaques were excluded from the study to eliminate phenocopies of HCM.1,17 Due to the sequence of events, the blood pressure measurement was always and only assessed under ketamine sedation.
The OCD group comprised rhesus macaques with various congenital or acquired cardiac diseases, including ventricular septal defect, systolic dysfunction, and moderate to severe aortic, mitral, or tricuspid regurgitation. Systolic dysfunction was diagnosed when fractional shortening of the left ventricle was less than 25% or the left ventricular ejection fraction was less than 50% (or both). Aortic regurgitation was described as moderate to severe when the ratio of jet height to left ventricular outflow tract width was greater than 25%. Mitral valve regurgitation was quantified as moderate to severe when jets occupied greater than 30% of the left atrial area. Tricuspid valve regurgitation was deemed moderate to severe when dilation of the right ventricle, right atrium, or vena cava (or their combinations) was observed. Rhesus macaques categorized into the control group were free from these abnormalities, including HCM; however, they could have trace to mild valve regurgitation without chamber enlargement, because these symptoms represent normal variations in sedated patients in various species.10 Furthermore, geriatric rhesus macaques (i.e., older than 16 y) also could have diastolic dysfunction, because this can be an age-related change in this species.30
VHS.
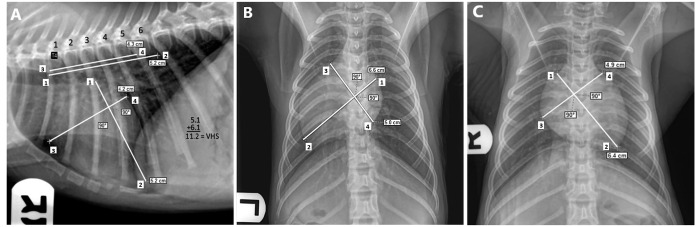
All animals underwent right lateral (RL), dorsoventral (DV), and ventrodorsal (VD) thoracic radiography (Innovet Select, Summit Industries, Niles, IL) using a 20 KH2 high-frequency beam of 65 to 80 kVp and 5 to 9 mA. VHS was calculated from digitally processed, stored images by using Sound-eklin eFilm Workstation 3.3 software (Merge Healthcare 2010, Mississauga, Ontario, Canada). VHS was measured as described in the previous study for dogs.6 Figure 1 demonstrates the measurement of VHS in the RL, DV, and VD radiographic views of normal rhesus macaques by using the long axis (A–B) and short axis (C–D) measurements of the cardiac silhouette. In the RL view, the long axis is measured from the ventral aspect of the carina to the base of the cardiac silhouette. The short axis is perpendicular to the long axis in the area of the coronary groove at both edges of the cardiac silhouette. In the VD and DV views, the long axis is the maximal distance from the base of the heart to the apex edge; the short axis is perpendicular to the long axis at the maximal length of the silhouette. For all 3 views, the 2 separately measured axes are aligned parallel to the fourth thoracic vertebra (T4) on the ventrocranial aspect but only in the RL view. Vertebral bodies crossed by the axes are counted, and the sum of these 2 numbers (to the nearest tenth) determines the VHS.
Figure 1.
(A) RL, (B) DV, and (C) VD radiographic views of the thorax in a healthy rhesus macaque without significant cardiac disease (control). Each view illustrates the measurements of long (point 1 to point 2) and short (point 3 to point 4) axes of the cardiac silhouette. Regardless of the radiographic view, both axes are aligned with the fourth thoracic vertebrae (T4) in the RL view. Vertebral bodies are counted for each axis dimension, and their sum is the VHS. RL, right lateral; DV, dorsoventral; VD, ventrodorsal; VHS, vertebral heart score.
Statistical analysis
Statistical analyses were performed by using commercially available software packages (MedCalc version 16.4.3, MedCalc Software, Ostend, Belgium, and Prism version 8.2.1, GraphPad Software, La Jolla, CA). Descriptive statistics (mean, median, standard deviation, range, and interquartile range) were calculated for VHS parameters in all animals. After outliers were omitted, the double-sided 95% reference interval was constructed by using the Tukey test. The 90% confidence intervals for the reference limits were estimated by using bootstrap methods in accordance with reference interval guidelines.10
Patient characteristics (sex, age, body weight, body condition score, heart rate, and respiratory rate) were compared among the groups with different cardiac conditions. Normality testing for continuous values was performed by using the D'Agostino–Pearson test in each group. Descriptive statistics were obtained and reported as mean ± 1 SD for parametric values and median and interquartile range for nonparametric values. Continuous variables with 2 groups were compared by using nonpaired Student t tests for parametric variables and Mann–Whitney U tests for nonparametric variables. Three groups were compared by using ordinary one-way ANOVA with Holm–Sidak posthoc comparisons for parametric variables and Kruskal–Wallis testing with Dunn posthoc comparisons for nonparametric variables. Categorical variables (e.g., sex) were compared by using χ2 and Fisher exact tests.
Correlations between various patient characteristics and the VHS values from the 3 radiographic views were assessed by using the Pearson correlation method. Receiver operating characteristic (ROC) analysis was performed to evaluate diagnostic accuracy and determine optimal cut-off values of VHS for diagnosing cardiac diseases in rhesus macaques; the AUC was used to evaluate the diagnostic accuracy of VHS analysis. Accuracy was described as low at 0.5 ≤ AUC < 0.7, moderate with 0.7 ≤ AUC < 0.9, or high with AUC ≥ 0.9. The cut-off values were reported with statistical parameters including sensitivity, specificity, and likelihood ratio. The VHS value with the maximal Youden index (J = specificity + sensitivity – 1) was chosen as a cut-off value to distinguish between rhesus macaques with and without cardiac diseases. For all statistical tests, a P value of less than 0.05 was considered significant.
Results
A total of 150 rhesus macaques (age: range, 1.3 to 26.8 y; median, 6.6 y; IQR, 3.7 to 12.7 y) were enrolled in the study; 83 animals were female and 67 were male. Overall, body weight ranged from 2.4 to 20.1 kg (median, 8.7 kg; IQR, 6.5 to 11.3 kg), and body condition score ranged from 1.5 to 5 (median, 3; IQR, 3 to 3.5; Table 1). According to echocardiographic examination, 121 rhesus macaques were free of any significant cardiac disease and were categorized as the control group. Of the remaining 29 macaques, 20 were diagnosed with HCM, and the remaining 9 animals were diagnosed with OCD. None of the 20 macaques with HCM were excluded due to systemic hypertension (mean blood pressure, less than 110 mm Hg; mean systolic blood pressure, less than 160 mm Hg). Of the 9 rhesus macaques with OCD, 3 had moderate to severe systolic dysfunction, 3 had moderate mitral valve regurgitation, 2 had moderate tricuspid regurgitation, one had moderate aortic valve regurgitation, one had a ventricular septal defect, and one had dilated cardiomyopathy.
Table 1.
Characteristics of rhesus macaques in the control, HCM, and OCD groups
| Group | P | |||||||
| Overall | Control | HCM | OCD | Overall | Control compared with HCM | Control compared with OCD | HCM compared with OCD | |
| Sex (male/female) | 67/83 | 52/69 | 10/10 | 5/4 | 0.67 | |||
| Age (y) | 6.6 (3.7–12.6) | 5.7 (3.6–10.9) | 12.8 (6.9–16) | 11.6 (6.7 –16) | 0.0001a | 0.0005a | 0.07 | >0.99 |
| Body weight (kg) | 8.1 (6.1–10.4) | 8.1 (6.1–10.3) | 11.4 (9.8–14.7) | 12.9 (9.6–14) | 0.0001a | <0.0001a | 0.009a | >0.99 |
| BCS (0 to 5) | 3.0 (3–3.4) | 3.1 ± 0.6 | 3.8 ± 0.9 | 3.6 ± 0.7 | 0.0001a | <0.0001a | 0.063 | 0.72 |
| Heart rate (bpm) | 120 (100–121) | 115 ± 22 | 128 ± 24 | 130 ± 24 | 0.015a | 0.051 | 0.12 | 0.96 |
| Respiratory rate (breaths per minute) | 36 (24–36) | 32 (28–36) | 30 (24–36) | 36 (24–36) | 0.5 | |||
BCS, body condition score; HCM, hypertrophic cardiomyopathy; OCD, other cardiac disease
Parameters were first compared among the control, HCM, and OCD groups; when parameters were significantly different overall, they then were compared between groups.
P < 0.05.
VHS in the control group was used for establishing the reference intervals for each radiographic view (RL, DV, and VD; Table 2 and Figure 2). The patient characteristics (sex, age, body weight, body condition score, respiratory rate, and heart rate) of the rhesus macaques in the control group were assessed. VHS did not differ significantly between male and female macaques in all 3 radiographic views. Significant correlations were noted between age and VHS (r = –0.3, P < 0.0001) and between body weight and VHS in the DV view only (r = –0.18, P = 0.02). No significant correlations were noted between any patient characteristic and VHS in the RL and VD views (Table 3).
Table 2.
Reference intervals of VHS in 121 healthy rhesus macaques without significant cardiac disease
| View | Minimum | Maximum | Mean | Median | Standard deviation | Lower limit | 90% CI | Upper limit | 90% CI |
| Right lateral | 7.2 | 11.6 | 9.79 | 9.8 | 0.71 | 8.39 | 8.21–8.58 | 11.19 | 11.00–11.37 |
| Dorsoventral | 7.1 | 11.6 | 10.2 | 10.3 | 0.71 | 8.8 | 8.61–8.99 | 11.62 | 11.43–11.80 |
| Ventrodorsal | 8.2 | 12 | 10.4 | 10.4 | 0.67 | 9.13 | 8.96–9.30 | 11.75 | 11.58–11.92 |
Figure 2.
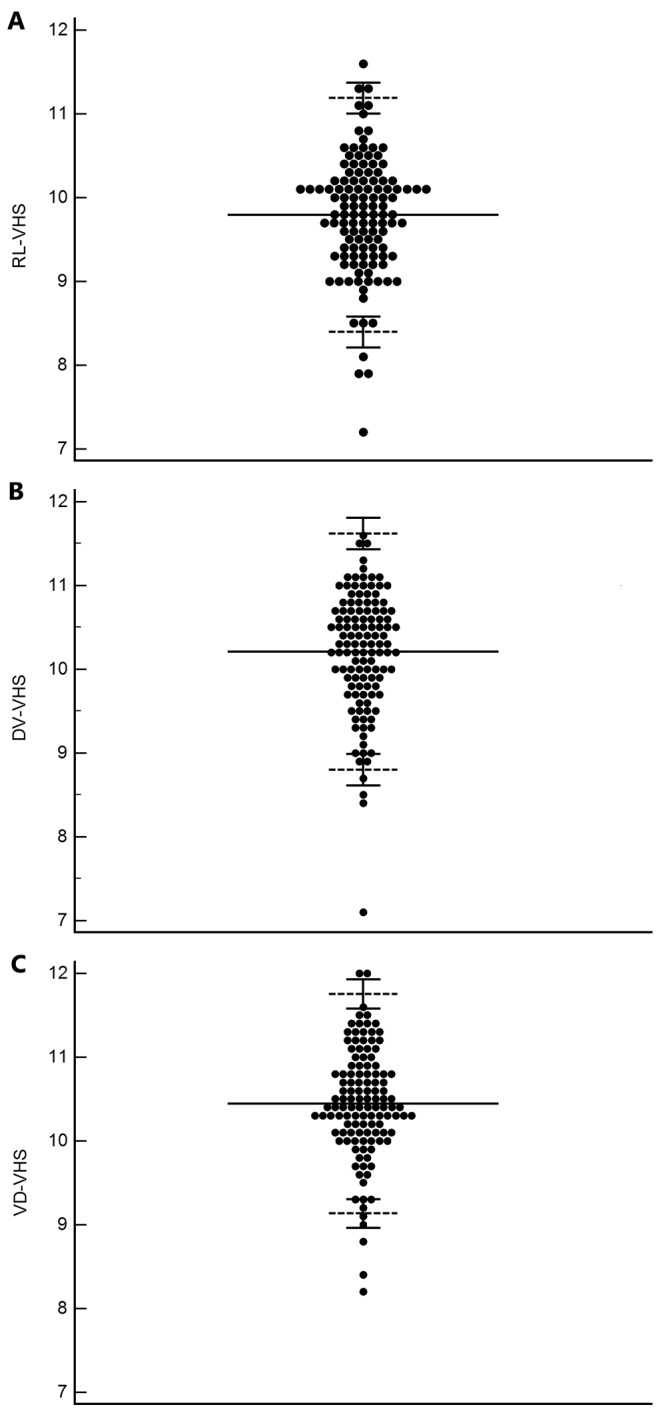
VHS in the (A) RL, (B) DV, and (C) VD radiographic views. Each dot indicates the VHS from each rhesus macaque. Mean (solid line) and upper and lower limits (short dashed lines) are shown. The short solid lines above and below the upper and lower limits indicate the 90% confidence intervals. DV, dorsoventral; VD, ventrodorsal; VHS, vertebral heart score.
Table 3.
Correlations between patient characteristics and VHS
| View | Age | Body weight | Body condition score | Heart rate | Respiratory rate | |
| Right lateral | r | −0.12 | −0.007 | −0.015 | 0.12 | 0.047 |
| P | 0.14 | 0.93 | 0.85 | 0.15 | 0.57 | |
| Dorsoventral | r | −0.3 | −0.18 | −0.11 | −0.11 | 0.079 |
| P | <0.0001a | 0.02a | 0.17 | 0.19 | 0.34 | |
| Ventrodorsal | r | −0.051 | 0.097 | 0.016 | −0.021 | −0.094 |
| P | 0.52 | 0.21 | 0.84 | 0.8 | 0.25 |
P < 0.05
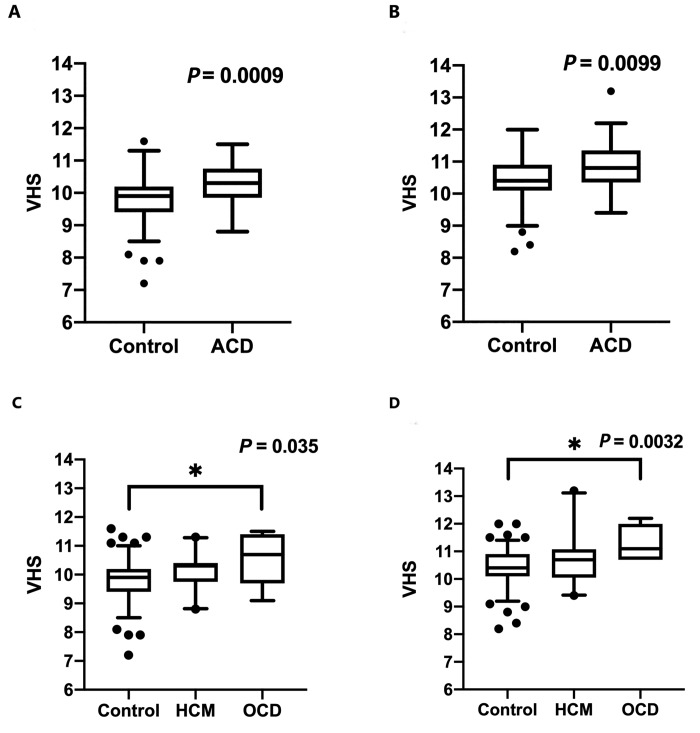
VHS was significantly higher in the ACD group than in controls when measured on the RL (P = 0.0009) and VD (P = 0.0099) views but not in the DV view (P = 0.64; Table 4 and Figure 3). When the rhesus macaques with ACD were categorized into HCM and OCD groups, VHS was significantly higher in animals with OCD than in the control group in the RL (P = 0.036) and VD (P = 0.0032) views but not the DV view (P = 0.18). VHS did not differ between the HCM and control or HCM and OCD groups in any radiographic view (Table 4).
Table 4.
Median (interquartile range) of VHS of rhesus macaques in the control, HCM, and OCD groups
| Cardiac disease status | P | |||||||
| View | Control | ACD | HCM | OCD | Control compared with ACD | Control compared with HCM | Control compared with OCD | HCM compared with OCD |
| Right lateral | 9.9 (9.4–10.2) | 10.3 (9.85–10.75) | 10.3 (9.75–10.4) | 10.7 (9.7–11.4) | 0.0009a | 0.13 | 0.036a | >0.99 |
| Dorsoventral | 10.3 (9.9–10.7) | 10.4 (9.8–10.75) | 10.05 (9.72–10.6) | 10.7 (10.15–11.45) | 0.64 | >0.99 | 0.18 | 0.14 |
| Ventrodorsal | 10.4 (10.1–10.9) | 10.8 (10.35–11.35) | 10.7 (10.05–11.08) | 11.1 (10.7-12.0) | 0.0099a | 0.84 | 0.0032a | 0.09 |
ACD, all cardiac disease; HCM, hypertrophic cardiomyopathy; OCD, other cardiac disease
P < 0.05
Figure 3.
Box-and-whisker plots for VHS in the (A and C) RL and (B and D) VD views. Rhesus macaques were divided into (A and B) control and ACD groups or (C and D) control, HCM, and OCD groups. ACD, all cardiac disease; HCM, hypertrophic cardiomyopathy, OCD other cardiac diseases; RL, right lateral; VD, ventrodorsal.
The patient characteristics (sex, age, body weight, body condition score, respiratory rate, and heart rate) were compared among different cardiac disease groups. No significant differences were detected in sex distribution (P = 0.67) or respiratory rate (P = 0.5) among the control, HCM, and OCD groups. Age, body weight, body condition score, and heart rate were significantly different overall, between the control and HCM groups, and between the control and OCD groups (Table 1); these patient characteristics did not differ significantly between the HCM and OCD groups. No significant sex-associated differences in VHS were noted for any radiographic view or group, except for the DV view in the OCD group (male, 10.4; female 11.5; P = 0.016).
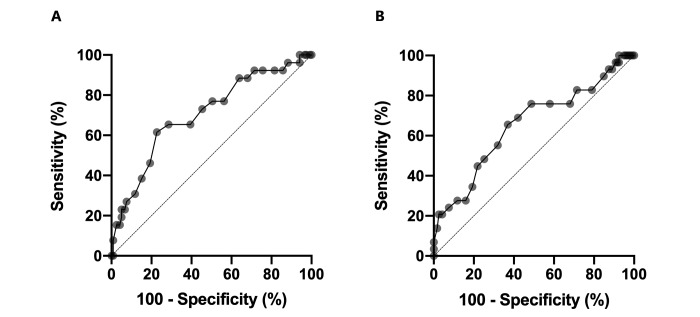
ROC analysis was performed for each radiographic view to determine the accuracy of the VHS test in predicting the presence of cardiac disease in rhesus macaques. For the RL view, AUC was 0.71 (95% CI, 0.59 to 0.82), and VHS was considered to be moderately accurate for predicting the presence of cardiac disease (P = 0.001; Table 5 and Figure 4). The optimal cut-off value of RL VHS was 10.25 according to the maximal Youden index (0.39), with a sensitivity of 62% (95% CI, 43% to 78%) and specificity of 77% (95% CI, 69% to 84%; Supplemental Table S1 and Supplemental Table S2). In the VD view, AUC was 0.65 (95% CI, 0.54 to 0.77), and VHS was considered to be mildly accurate for predicting cardiac disease (P = 0.01). The optimal cut-off value of VD VHS was 10.65 according to the maximal Youden index (0.29), with a sensitivity of 66% (95% CI, 47% to 80%) and specificity of 63% (95% CI, 54% to 71%). VHS in the DV view was not accurate for predicting the presence of cardiac disease in rhesus macaques.
Table 5.
Results of ROC analysis for VHS
| View | AUC | SEM | 95% CI | P |
| Right lateral | 0.71 | 0.058 | 0.59–0.82 | 0.0011a |
| Dorsoventral | 0.53 | 0.062 | 0.41–0.65 | 0.63 |
| Ventrodorsal | 0.65 | 0.06 | 0.54–0.77 | 0.011a |
Figure 4.
ROC analysis of VHS in the control and OCD groups. (A) The sensitivity and specificity of VHS in the RL view at the cut-off value of 10.25 were 65% and 77%, respectively. (B)The sensitivity and specificity of VHS in the VD view at the cut-off value of 10.75 was 66% and 63%, respectively. OCD, other cardiac disease; RL, right lateral; ROC, receiver operating characteristics; VD, ventrodorsal; VHS, vertebral heart score.
Discussion
The present study established reference intervals for VHS in RL, DV, and VD thoracic radiographic views of healthy rhesus macaques. Study strengths include a large sample size for control animals, which permits establishing the reference intervals. In addition, VHS was compared between rhesus macaques with and without cardiac diseases, and VHS for the RL and VD views was significantly higher in rhesus macaques with cardiac disease than in controls. When rhesus macaques with cardiac diseases were categorized into HCM and OCD groups and then compared with controls, RL and VD VHS remained significantly higher in macaques with OCD but not HCM. VHS did not differ between the HCM and OCD groups in any of the radiographic views. Although age, body weight, body condition score, and heart rate were significantly higher in the macaques with HCM or OCD than in controls, no significant correlations were noted between RL or VD VHS and any of these patient characteristics.
The method we chose to measure VHS in rhesus macaques was the same as described for dogs because of their similar chest conformation and position of the heart in lateral radiographs. In addition, VHS has been measured in other NHP species by using this same method.7,14,15,19,20,22,25,26,32,34 The reference intervals obtained in our current study closely resemble those for VHS in other NHP species, including owl monkeys (Aotus spp.) common marmosets (Callithrix jacchus), spider monkeys (Ateles fusciceps), goeldi monkeys (Callimico goeldii), tufted capuchins (Cebus apella), African green vervet monkeys (Chlorocebus sabaeus), howler monkeys (Aloutta guariba clamitans), Japanese macaques (Macaca fuscata), and squirrel monkeys (Saimiri spp.).7,14,15,19,20,22,25,26,32,34 These similarities indicate that the conformations of the thoracic wall and cavity and cardiac structures are similar among various NHP species. However, most of these previous studies only reported VHS for the RL radiographic view. Here we reported the reference intervals for VHS measured in the RL, VD, and DV views, because this additional information may be useful to diagnose the presence of cardiac disease in other veterinary species, as in dogs.6 Our findings differ from those identified for Japanese macaques.14 In that previous publication, differences in VHS emerged depending on sex, weight, and age. These differences may reflect species-specific findings or—perhaps more likely—a smaller sample size or younger animals in the previous study. Regardless, the differences previously identified in Japanese macaques, although statistically significant, had very weak correlation values that translated to little or no effect size.14
In our current study, VHS measured on the RL and VD views—but not the DV view—were significantly higher in rhesus macaques with cardiac disease than animals without cardiac disease. The AUC values indicated that the VHS measured in the RL and VD views had a significant accuracy to distinguish rhesus macaques with and without cardiac disease. In the RL and VD views, VHS values of 10.25 and 10.65, respectively, were selected, according to the maximal Youden index, as optimal cut-off values to distinguish between animals with or without cardiac disease. However, the sensitivities and specificities of these thresholds are not sufficiently robust to avoid a high probability of false-negative and false-positive results. Therefore, RL and VD VHS cannot be used as isolated screening tools to identify rhesus macaques needing further cardiovascular examination, including echocardiographic assessment. In the clinical setting, VHS could be used as a screening tool by choosing a lower cut-off than the optimal values to decrease the false-negative rate. In addition, considering other patient data during interpretation of the VHS will help improve the accuracy of VHS in distinguishing between rhesus macaques with or without cardiac disease. For example, clinical signs of cardiac disease or heart murmurs can be expected to improve the sensitivity and specificity of VHS to identify animals with true cardiac disease.
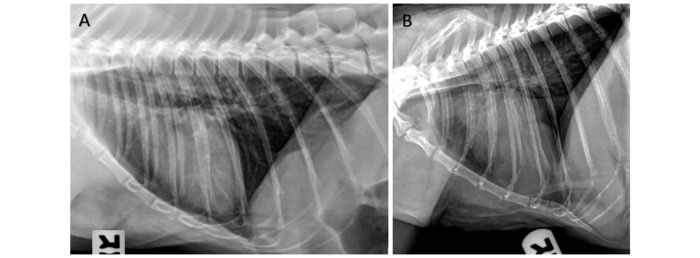
Because VHS does not distinguish between types of cardiac disease, such as HCM and others, it cannot replace echocardiographic assessment. In addition, VHS was not significantly different between rhesus macaques with HCM and control animals. We presume this is because HCM is generally associated with pathologic cardiomyocyte remodeling and concentric hypertrophy; these pathologies do not increase the size of the cardiac silhouette in the radiographic images until disease is severe and marked chamber enlargement is present. An example of this situation is shown in Figure 5, where the RL radiographic projection of an animal with HCM is compared with that of an animal with OCD; the HCM animal shows a lack of heart enlargement as compared with the readily apparent enlargement in the OCD macaque. In cats, VHS identifies patients with severe HCM once they develop concomitant left atrial enlargement.12 In light of these findings, VHS is not a suitable screening test for occult HCM in rhesus macaques but may be helpful in screening for other cardiac diseases, including ventricular septal defect, dilated cardiomyopathy, systolic dysfunction, and moderate to severe valve regurgitation.
Figure 5.
RL radiographic projections of study animals diagnosed with (A) HCM or (B) OCD. The VHS for the HCM animal is 9.5; that for the OCD macaque is 11.1. HCM, hypertrophic cardiomyopathy; OCD, other cardiac disease; RL, right lateral; VHS, vertebral heart score.
In our current study, various patient characteristics, including age, body weight, body condition score, and heart rate, were significantly associated with cardiac disease in rhesus macaques. This association may result because cardiac diseases, including HCM and valve degeneration, are more common in older animals, with their larger body size and higher body condition score. In human patients, obesity is a known risk factor for the development of cardiovascular diseases, and this association may also be true for rhesus macaques. However, it is beyond the scope of the present study to determine the association between the development of cardiac disease and various patient characteristics such as age, body weight and body condition score. Indeed, we did not obtain any significant correlations between various patient characteristics and RL or VD VHS. Therefore, the VHS is not likely to be truly influenced by these patient characteristics, and an adjustment based on these covariates is not necessary when using VHS in the clinical setting. In addition, various aspects of the cardiovascular system are known to differ between men and women.33 Because we noted no significant sex-associated difference in RL or VD VHS according to cardiac status, VHS analysis can be performed in the same manner for both male and female rhesus macaques.
One limitation of the present study is the small sample size for various cardiac diseases. Furthermore, these affected rhesus macaques were deemed to be healthy, without apparent clinical cardiac signs, thus effectively testing VHS for its ability to identify occult cardiac disease only. The small sample size and mild phenotypes of various cardiac diseases could result in type II error with a high probability of false-negative results. In future studies, VHS assessment should be evaluated in a large population of rhesus macaques with more severe forms of various cardiac diseases. In addition, we did not aim to correlate echocardiographic measures with VHS values; therefore, our study does not address which specific echocardiographic changes are driving the increases in VHS. Finally, our study criteria identified 20 animals as having HCM. We attempted to rule out phenocopies of HCM, such as the effect of systemic hypertension, by ensuring no gross evidence of systemic hypertension. However, these blood pressure assessments were performed under ketamine sedation, which could hinder the identification of animals with mild systemic hypertension. Given the known heritable HCM identified at our facility31 and the lack of significant hypertension in the patients diagnosed within the current study, we believe these macaques to be a cohort of animals with true HCM.
In summary, the present study established reference intervals of VHS for RL, DV, and VD radiographic views. These reference intervals may be useful in future clinical and research settings. VHS in the RL and VD views were significantly higher in rhesus macaques with various cardiac diseases than in control animals. However, regardless of the radiographic projection, VHS failed to discriminate between macaques with HCM and control animals or between those with HCM compared with OCD. These results indicate that VHS could be a valuable screening method for cardiac disease but is not likely to be useful for screening for occult HCM or other diseases resulting in concentric left ventricular hypertrophy in rhesus macaques. Our findings are similar to those in various other species, including dogs, suggesting that VHS could be a valuable screening tool in clinical settings to aid in the diagnosis of cardiac disease in rhesus macaques, as in other species. In addition, the results of our current study might facilitate future cardiovascular research in rhesus macaques. However, VHS does not substitute for the added value of echocardiographic examination for determining the presence, type, and severity of cardiac diseases in rhesus macaques.
Supplementary Material
VHS cut-off values with sensitivity, specificity, and likelihood ratio for RL radiographic view in rhesus macaques with cardiac disease (ACD group).
VHS cut-off values with sensitivity, specificity, and likelihood ratio in the VD radiographic view of rhesus macaques with cardiac diseases (ACD group).
Acknowledgments
We thank Kami Elliot, the California National Primate Research Center, the Primate Medicine staff, and, most of all, the rhesus macaques that have contributed to this project.
References
- 1.Acierno MJ, Brown S, Coleman AE, Jepson RE, Papich M, Stepien RL, Syme HM. 2018. ACVIM consensus statement: Guidelines for the identification, evaluation, and management of systemic hypertension in of veterinary internal medicine. J Vet Intern Med 32:1803–1822. doi: 10.1111/jvim.15331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Animal Welfare Act as Amended. 2013. 7 §2131–2156. [Google Scholar]
- 3.Animal Welfare Regulations. 2013. 9 CFR §3.129. [Google Scholar]
- 4.Black PA, Marshall C, Seyfried AW, Bartin AM. 2011. Cardiac assessment of African hedgehogs (Atelerix albiventris). J Zoo Wildl Med 42:49–53. 10.1638/2010-0012.1. [DOI] [PubMed] [Google Scholar]
- 5.Boon JA, Boon JA. 2011. Veterinary echocardiography, 2nd ed Ames (IA): Wiley–Blackwell. [Google Scholar]
- 6.Buchanan JW. 2000. Vertebral scale system to measure heart size in radiographs. Vet Clin North Am Small Anim Pract 30:379–393. 10.1016/S0195-5616(00)50027-8. [DOI] [PubMed] [Google Scholar]
- 7.Charlier MGS, Filippi MG, Girotto CH, Ribeiro VL, Teixeira CR, Lourenço MLG, Vulcano LC. 2018. Morphometric and morphologic parameters of the heart in healthy Alouatta guariba clamitans (Cabrera, 1940). J Med Primatol 47:60–66. 10.1111/jmp.12320. [DOI] [PubMed] [Google Scholar]
- 8.Clingerman KJ, Summers L. 2005. Development of a body condition scoring system for nonhuman primates using Macaca mulatta as a model. Lab Anim (NY) 34:31–36. 10.1038/laban0505-31. [DOI] [PubMed] [Google Scholar]
- 9.de Moura CR, das Neves Diniz A, da Silva Moura L, das Chagas Araújo Sousa F, Baltazar PI, Freire LD, Guerra PC, de Sousa JM, Giglio RF, Pessoa GT, de Sá RP, Alves FR. 2015. Cardiothoracic ratio and vertebral heart scale in clinically normal black-rumped agoutis (Dasyprocta prymnolopha, Wagler 1831). J Zoo Wildl Med 46:314–319. 10.1638/2014-0038R.1. [DOI] [PubMed] [Google Scholar]
- 10.Friedrichs KR, Harr KE, Freeman KP, Szladovits B, Walton RM, Barnhart KF, Blanco-Chavez J. American Society for Veterinary Clinical Pathology. 2012. ASVCP reference interval guidelines: determination of de novo reference intervals in veterinary species and other related topics. Vet Clin Pathol 41:441–453. 10.1111/vcp.12006. [DOI] [PubMed] [Google Scholar]
- 11.Garcia EB, Eshar D, Thomason JD, Harkin KR, Biller D. 2016. Cardiac assessment of zoo-kept, black-tailed prairie dogs (Cynomys ludovicianus) anesthetized with isoflurane. J Zoo Wildl Med 47:955–962, 958. [DOI] [PubMed] [Google Scholar]
- 12.Guglielmini C, Diana A. 2015. Thoracic radiography in the cat: identification of cardiomegaly and congestive heart failure. J Vet Cardiol 17 Suppl 1:S87–S101. 10.1016/j.jvc.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 13.Haertel AJ, Stern JA, Reader JR, Spinner A, Roberts JA, Christe KL. 2016. Antemortem screening for left ventricular hypertrophy in rhesus macaques (Macaca mulatta). Comp Med 66:333–342. [PMC free article] [PubMed] [Google Scholar]
- 14.Harada M, Koie H, Iwaki S, Sato T, Kanayama K, Taira M, Sakai T. 2010. Establishment of vertebral heart scale in the growth period of the Japanese macaque (Macaca fuscata). J Vet Med Sci 72:503–505. 10.1292/jvms.09-0328. [DOI] [PubMed] [Google Scholar]
- 15.Houdellier B, Liekens V, Smets P, Bouts T, Saunders JH. 2018. Thoracic radiography of healthy captive male and female squirrel monkey (Saimiri spp.). PLoS One 13:1–14. 10.1371/journal.pone.0201646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed, p 246 Washington (DC): National Academies Press. [Google Scholar]
- 17.Kang SC, Jampachaisri K, Pacharinsak C. 2019. Doppler and oscillometric mean blood pressure best represent direct blood pressure measurements in anesthetized rhesus macaques (Macaca mulatta). J Med Primitol 48:123–128. 10.1111/jmp.12397. [DOI] [PubMed] [Google Scholar]
- 18.Kanthaswamy S, Reader R, Tarara R, Oslund K, Allen M, Ng J, Grinberg C, Hyde D, Glenn DG, Lerche N. 2014. Large scale pedigree analysis leads to evidence for founder effects of hypertrophic cardiomyopathy in rhesus macaques (Macaca mulatta). J Med Primatol 43:288–291. 10.1111/jmp.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Knowlen GG, Weller RE, Perry RL, Baer JF, Gozalo AS. 2013. Hypertrophic cardiomyopathy in owl monkeys (Aotus spp.). Comp Med 63:279–287. [PMC free article] [PubMed] [Google Scholar]
- 20.Kubiak ML, Jayson SL, Saunders RA. 2015. Determination of vertebral heart score in Goeldi's monkeys (Callimico goeldii). J Med Primatol 44:183–186. 10.1111/jmp.12173. [DOI] [PubMed] [Google Scholar]
- 21.Lee MY, Lee SH, Lee SG, Park SH, Lee CY, Kim KH, Hwang SH, Lim SY, Ahn YK, Han HJ. 2007. Comparative analysis of heart functions in micropigs and conventional pigs using echocardiography and radiography. J Vet Sci 8:7–14. 10.4142/jvs.2007.8.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Makungu M, du Plessis WM, Barrows M, Groenewald HB, Koeppel KN. 2014. Radiographic thoracic anatomy of the ring-tailed lemur (Lemur catta). J Med Primatol 43:144–152. 10.1111/jmp.12102. [DOI] [PubMed] [Google Scholar]
- 23.Onuma M, Ono S, Ishida T, Shibuya H, Sato T. 2010. Radiographic measurement of cardiac size in 27 rabbits. J Vet Med Sci 72:529–531. 10.1292/jvms.09-0390. [DOI] [PubMed] [Google Scholar]
- 24.Reader JR, Canfield DR, Lane JF, Kanthaswamy S, Ardeshir A, Allen AM, Tarara RP. 2016. Left ventricular hypertrophy in rhesus macaques (Macaca mulatta) at the California national primate research center (1992–2014). Comp Med 66:162–169. [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha-Neto HJ, Moura Ld S, Pessoa GT, Ambrósio CE, Sousa FCA, Rodrigues RPS, Guerra PC, Alves FR. 2015. Cardiothoracic ratio and vertebral heart size (VHS) to standardize the heart size of the tufted capuchin (Cebus apella Linnaeus, 1758) in computerized radiographic images. Pesqui Vet Bras 35:853–858. 10.1590/S0100-736X2015001000006. [DOI] [Google Scholar]
- 26.Saunders RA, Kubiak M, Dobbs P. 2017. Determination of vertebral heart score in three species of spider monkey (Ateles fusciceps, A. hybridus and A. paniscus). J Med Primatol 47:51–54. 10.1111/jmp.12279. [DOI] [PubMed] [Google Scholar]
- 27.Silva-Filho OF, Pessoa GT, Sousa FCA, Rodrigues RPS, Moura LS, Ambrósio CE, Silva ABS, Alves FR. 2018. Assessment of the vertebral heart scale and cardiothoracic ratio to standardize the heart size of collared peccaries (Tayassu tajacu Linnaeus, 1758) restrained with ketamine and midazolam. Pesqui Vet Bras 38:1705–1711. 10.1590/1678-5150-pvb-5434. [DOI] [Google Scholar]
- 28.Sinha U, Sahay US, Athavale SA, Deopujari R, Kumar S. 2013. Comparative study of cardiac size by chest X-ray and echocardiography. J Anat Soc India 62:28–32. 10.1016/S0003-2778(13)80008-1. [DOI] [Google Scholar]
- 29.Stepien RL, Benson KG, Forrest LJ. 1999. Radiographic measurement of cardiac size in normal ferrets. Vet Radiol Ultrasound 40:606–610. 10.1111/j.1740-8261.1999.tb00886.x. [DOI] [PubMed] [Google Scholar]
- 30.Ueda Y, Gunther-Harrington CT, Cruzen CL, Roberts JA, Stern JA. 2017. Echocardiographic parameters of clinically normal geriatric rhesus macaques (Macaca mulatta). J Am Assoc Lab Anim Sci 56:361–368. [PMC free article] [PubMed] [Google Scholar]
- 31.Ueda Y, Stern JA. 2017. A one health approach to hypertrophic cardiomyopathy. Yale J Biol Med 90:433–448. [PMC free article] [PubMed] [Google Scholar]
- 32.Wagner WM, Kirberger RM. 2005. Radiographic anatomy of the thorax and abdomen of the common marmoset (Callithrix jacchus). Vet Radiol Ultrasound 46:217–224. 10.1111/j.1740-8261.2005.00044.x. [DOI] [PubMed] [Google Scholar]
- 33.Wingate S. 1997. Cardiovascular anatomy and physiology in the female. Crit Care Nurs Clin North Am 9:447–452. 10.1016/S0899-5885(18)30237-5 [DOI] [PubMed] [Google Scholar]
- 34.Young AN, du Plessis WM, Rodriguez D, Beierschmitt A. 2013. Thoracic radiographic anatomy in vervet monkeys (Chlorocebus sabaeus). J Med Primatol 42:310–317. 10.1111/jmp.12058. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
VHS cut-off values with sensitivity, specificity, and likelihood ratio for RL radiographic view in rhesus macaques with cardiac disease (ACD group).
VHS cut-off values with sensitivity, specificity, and likelihood ratio in the VD radiographic view of rhesus macaques with cardiac diseases (ACD group).