Abstract
The present study assessed the effect of nearby construction activity on the responses of rat middle cerebral arteries (MCA) to the endothelium-dependent vasodilator acetylcholine and the NO donor sodium nitroprusside (SNP) and the activity of MaxiK potassium channels in MCA smooth muscle cells from male Sprague–Dawley rats. Two monitoring systems were used to assess vibrations in the animal rooms during and immediately after construction activities near the research building where the animal facility is located. One was a commercially available system; the other was a Raspberry-Pi (RPi)–based vibration monitoring system designed in our laboratory that included a small computing unit attached to a rolling sensor (low sensitivity) and a piezoelectric film sensor (high sensitivity). Both systems recorded increased levels of vibration during construction activity outside the building. During the construction period, vasodilator responses to acetylcholine and SNP were abolished, and MaxiK single-channel current opening frequency and open-state probability in cell-attached patches of isolated MCA myocytes were dramatically decreased. Recovery of acetylcholine- and SNP-induced dilation was minimal in MCA from rats studied after completion of construction but housed in the animal facility during construction, whereas responses to acetylcholine and SNP were intact in rats purchased, housed, and studied after construction. Baseline levels of vibration returned after the completion of construction, concomitant with the recovery of normal endothelium-dependent vasodilation to acetylcholine and of NO sensitivity assessed by using SNP in MCA from animals obtained after construction. The results of this study indicate that the vibration associated with nearby construction can have highly disruptive effects on crucial physiologic phenotypes.
Abbreviations: MCA, middle cerebral artery; NPo, open state probability; RPi, Raspberry-Pi; SNP, sodium nitroprusside
During the period of October 2017 through May 2018, our institution undertook several substantial construction projects near or within research buildings in which animal facilities are located. Prior to the construction period, we were concerned about the potential effects of activities on animal research, in light of an earlier study40 showing that previous construction activities our institution had a deleterious effect on physiologic responses such as cortisol secretion. Other studies similarly have shown deleterious effects of noise and vibration on physiologic phenotypes in research animals.5,7,32,42,46,55,56,58 The effects of noise and vibration on research animals have been examined in a report from a leading cancer research institute,29 and AALAS has developed a valuable webinar presentation on minimizing the disruptive effects of noise and vibration on research animals.50 Despite existing knowledge of potential deleterious effects of construction activities on rodent phenotypes, the project at our institution was initiated without adequate notification to researchers and without plans to isolate animals housed in the animal facility from the noise and vibration resulting from the intense construction activities.
The ability of blood vessels to relax in response to acetylcholine is a ‘gold standard’ for normal endothelial function and arterial reactivity, and the loss of acetylcholine-induced dilation is a universal indicator of endothelial dysfunction.8,9,12,14,51 As a result, acetylcholine-induced relaxation is a standard test used in numerous laboratories throughout the world and has been an essential and reliable phenotype incorporated into the design of NIH-funded experiments by our research group.10,28,38,49,53,54 Normally, the loss of endothelium-dependent dilation to acetylcholine is the consequence of a reduction in the availability of endothelium-derived NO due to impaired NO release, destruction of NO by reactive oxygen species— especially superoxide—or uncoupling of endothelial nitric oxide synthase, resulting in superoxide production rather than NO production.11,45 However, to definitively establish that loss of acetylcholine-induced vascular relaxation is due to reduced availability of NO, it is essential to verify that the vascular smooth muscle retains its sensitivity to the vasodilator effects of NO. The latter objective is accomplished by verifying that the blood vessels being tested exhibit normal relaxation in response to exogenous NO donors, such as sodium nitroprusside (SNP).
Because of the sudden loss of our established phenotype to verify normal endothelial function in control animals (acetylcholine-induced dilation in rat middle cerebral artery [MCA]) and our concern regarding the potential effects of vibration, noise, and construction activity on physiologic phenotypes including endothelial function,32,55,56 we hypothesized that increased levels of vibration would be present in the animal holding room during construction activity and that this elevated vibration would be associated with the loss of crucial physiologic mechanisms involved in the normal relaxation of vascular smooth muscle cells in cerebral arteries. Mechanisms tested in the current study included endothelium-dependent vasodilation in response to acetylcholine and the activation of the large conductance Ca2+-activated MaxiK channels in isolated arterial smooth muscle cells. The present study had 2 goals: 1) to identify and quantify at the cage level any vibrations caused by construction activities and 2) to determine whether those vibrations affected the cellular and ionic mechanisms regulating active tone and vascular relaxation in arterial smooth muscle cells.
To achieve those goals, we measured vibration levels in the animal facility by using 2 different methods. One was a commercially available monitoring system, and the other was a vibration monitoring system that designed in our own laboratory and based on a small computing unit attached to low- and high-sensitivity vibration detectors. Endothelial function was assessed by evaluating vascular responses to acetylcholine, and vascular NO sensitivity was assessed by using the NO donor SNP in isolated MCA from Sprague–Dawley rats obtained from the same source as for our previous studies and housed in the laboratory animal facility during the construction activities. In other experiments, the activity of the large-conductance Ca2+-activated K+ channels (MaxiK channels) was evaluated by using patch-clamp methodology in vascular smooth muscle cells isolated from MCA to determine whether construction-related activities have direct effects on a crucial cellular ionic mechanism regulating active tone and vascular relaxation in smooth muscle cells.
Materials and Methods
Animals.
The present experiments involved male Sprague–Dawley rats (age, 8 to 12 wk on arrival; Envigo, Indianapolis, IN), which had been extensively used in our laboratory in prior studies to assess vascular regulation in normotensive rats. All protocols used in the present experiments were approved by the Medical College of Wisconsin IACUC.
The rats were maintained on a commercial chow (Teklad 5001 global diet [0.2% NaCl], Envigo) from the time of arrival until the day of the experiment and did not receive any treatments. The experiments reported herein arose because of a sudden loss of basic vascular phenotypes, notably endothelium-dependent dilation to acetylcholine in the Envigo Sprague–Dawley rats used as control animals for a variety of experiments in our laboratory. The 16 rats in which endothelium-dependent dilation to acetylcholine was completely lost in this study (see following section) were housed in the animal facility for periods ranging from 2 to 3 wk (6 rats), 4 wk (7 rats), or 5 wk (3 rats).
Animal housing and husbandry.
All animals used for testing MCA responses to acetylcholine and SNP in this study were housed in a single holding room within an animal facility of conventional design. Rats used for studies of MaxiK channel currents in MCA myocytes were housed in a holding room immediately adjacent to the one housing the rats used in the studies of MCA reactivity to acetylcholine and SNP. Throughout the facility, walls are constructed of concrete block and finished with epoxy paint. Floors are concrete slab with a troweled epoxy finish. Animal room ceilings are drywall coated with epoxy paint, and corridor ceilings are fiberglass-reinforced panels in a suspended frame. Animal room doors are of steel construction finished with epoxy paint. Controlled animal room environmental parameters included temperature (72 ± 2 °F [22.2 °C ± 1.1°]), relative humidity (30% to 70%), air exchange rate (approximately 16 air changes hourly, negative pressure relative to corridor), and light cycle (12:12-h light:dark).
Rats were housed in pairs in IVC (model no. RS10147U40MVSPSHR-R, Allentown Caging, Allentown, NJ). HEPA-filtered air was supplied to cages via a blower (model SB4100, Allentown Caging) mounted on the top of the cage rack. A similarly mounted exhauster (model EP5000E, Allentown Caging) HEPA-filtered cage exhaust air prior to discharge into the room. Rats were fed Laboratory Rodent Diet 5001 (LabDiet, St Louis, MO) and received drinking water treated by reverse osmosis, chlorinated to 3 ppm, and distributed via an automatic watering system (Edstrom Industries, Waterford, WI). Cages contained hardwood bedding (Sani-Chips, PJ Murphy, Montville, NJ), and a paper towel was added to each cage for nesting material. Cages and caging supplies were sanitized in mechanical washers prior to use. Cage changing was performed approximately weekly, in a laminar-flow cage-changing station (model NU-S612-400, Nuaire, Plymouth, MN). Animal rooms were swept and then mopped by using a detergent-based cleaner (GP100, Sanitation Strategies, Holt, MI) daily, except on weekends and holidays. Animal health monitoring used sentinel rats (CRL:CD, Charles River Laboratories, Wilmington, MA) exposed to dirty bedding from the cages housing study animals. Sentinels were negative for Sendai virus, pneumonia virus of mice, sialodacryoadenitis virus, Kilham rat virus, Toolan H1 virus, rat parvovirus, rat minute virus, reovirus 3, rat theilovirus, Mycoplasma pulmonis, Pneumocystis carinii, pinworms, and fur mites.
Description and location of construction activity.
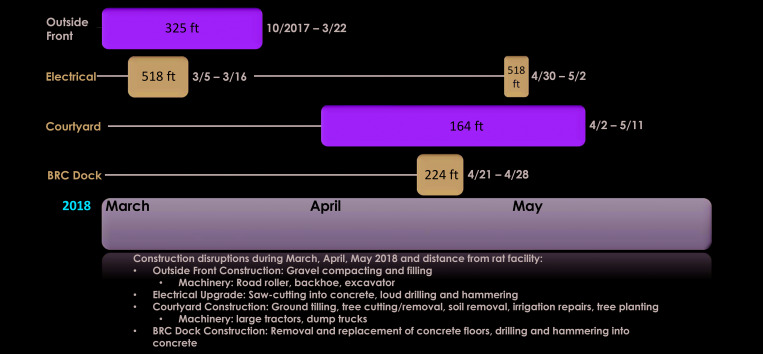
Construction activities in the vicinity of the animal facility, their approximate distance from the animal housing areas, and the dates of the individual construction activities are summarized in Figure 1. Replacement of the existing heating system at our institution began on 2 October 2017 and started immediately on one side of the facility near the animal quarters. Construction work continued with tunneling operations immediately in front of the main building. The project involved substantial vibration and extensive use of heavy equipment (steam shovels, bulldozers, dump trucks, front-end loaders, road roller, backhoe, excavator, etc.). The initial portion of the project was completed on 5 April 2018. The estimated distance between the construction work and the animal housing rooms was approximately 325 ft. After completion of that portion of the project, additional construction in front of the building continued until 31 May 2018.
Figure 1.
Nature and timeline of specific construction activities that occurred during the present study, with approximate distances to the animal housing areas.
Other construction activity at our institution (5 to 16 March 2018) included an electrical upgrade involving saw-cutting in concrete, drilling, and hammering. The estimated distance between the electrical upgrade work and animal housing was approximately 518 ft. Additional electrical work occurred between 30 April and May 2, 2018. Construction work in a courtyard near the animal facility (approximately 164 ft from the animal rooms) involved large tractors and dump trucks and occurred between 2 April and 11 May 2018. That activity included ground tilling, tree cutting and removal, soil removal, irrigation repairs, and tree planting. Construction work on the loading dock for the animal facility (21 to 28 April 2018) involved removal and replacement of concrete floors, drilling, and hammering into concrete. The estimated distance from the loading dock construction activities to the animal quarters was 224 ft.
Vibration monitoring.
Two vibration monitoring systems were used in this study. One sensor was a commercially available monitoring system (VivAlarm 24-Hour Vibration Monitor, Turner Scientific, Jacksonville, IL), which quantified vibrations as G force. Literature included with the VivAlarm system indicated that measurements of 0.050 G and higher are known to cause a stress response in rodents. Low-level alarms were set to notify users when values between 0.025 and 0.050 G were recorded, and high-level alarms were set to notify users when values of 0.050 G or higher were recorded. The sensor for the VivAlarm24 system was placed on the side of a standard mouse cage on a positive pressure rack and in several other locations within the animal facility at various times. Vibration levels were monitored on the VivAlarm24 system from 25 April to 7 May 2018.
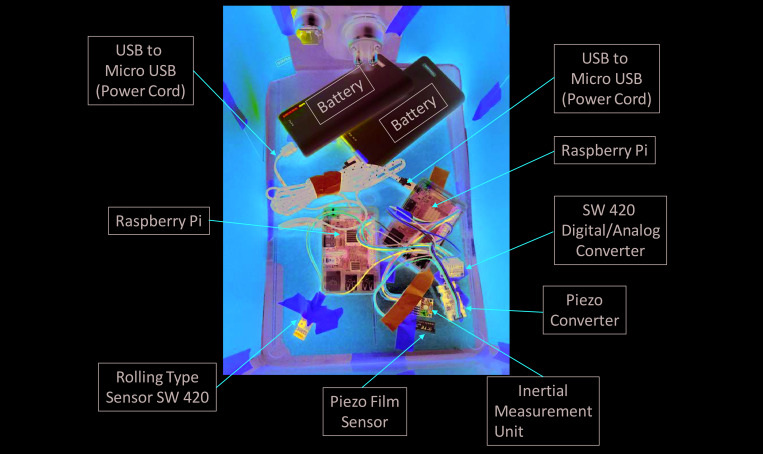
The other vibration monitor was a Raspberry Pi (RPi)-based system that was designed in our laboratory to provide an inexpensive and easily portable system to measure vibrations in multiple locations within the facility. The RPi system consisted of a small computing unit attached to a rolling-type vibration sensor (low sensitivity) and a piezoelectric film sensor (high sensitivity); these sensors are connected to an analog-to-digital converter wired to a central processing unit powered by a portable battery (Figure 2). Both sensors work by converting vibrations to electricity. In the piezo sensors, vibrations cause a closing of the detection circuit, triggering an electrical impulse proportional to the magnitude of the vibration, expressed as a portion of the maximum voltage of the circuit. The rolling sensor is maintained in the closed position until vibration is detected, at which point the closed circuit is disturbed, and the total voltage of the system drops below maximum. The reading on each computing system (in volts) is then compared with a standard relationship (determined individually depending on sensor and system) to express the detected signal in G forces. The RPi system monitored vibration levels from 16 March through 31 May 2018, corresponding to the time of ongoing construction activities; additional monitoring was conducted after the completion of construction activities.
Figure 2.
RPi vibration detection unit constructed from 3 different types of vibration sensors (rolling, piezoelectric film, and inertial measurement unit), with signals passed through digital-to-analog converters, processed by a Raspberry Pi computer, and emailed wirelessly to a desired destination. Power is supplied by large-capacity portable batteries.
The RPi unit, battery, and rolling and piezo film sensors were placed in an empty rat cage that was with other rat cages on an actively used rack in one of the standard rat rooms. The rolling and piezo film sensors were placed near each other and taped to the bottom of a cage devoid of bedding in a manner that allowed a free angle of rotation. This arrangement allowed all vibrations transmitted to the cage to be detected without static build-up from bedding, which could short the circuits. Vibration responses on the laboratory-designed monitoring system were initially quantified in hertz. As data were collected, they were stored in SQLite database files and transferred via email to a designated inbox for further aggregation and off-site analysis. Any lapses in coverage of the RPi system were due to limitations imposed by using rechargeable batteries.
All human activity in the room during this time was self-monitored by a sign-in sheet that enabled us to subtract regularly occurring vibration events due to animal facility staff activity from other vibrations detected by the sensors. At the time of analysis, these data points were manually subtracted from data files. At the end of data collection, the sensors were calibrated by using a combination of an oscilloscope and a wave generator to determine a standard relationship of hertz to volts and to establish thresholds of detection.
Studies involving isolated vessels.
Responses to acetylcholine and SNP were evaluated in MCA isolated from rats that were housed in the animal facility during the construction period and studied either before or after cessation of construction activity. In other experiments, the responses to acetylcholine and SNP were determined in MCA from newly obtained rats that were purchased, housed in the same animal room, and studied after cessation of the construction activity.
On the day of the experiment, the rats were anesthetized with 5% isoflurane and euthanized, and the brain was removed. MCA were isolated and cannulated with micropipettes. The vessels were maintained at a transmural pressure of 80 mm Hg and perfused and superfused with bicarbonate-buffered physiologic salt solution maintained at 37 °C and equilibrated by using a gas mixture (O2, 21%; CO2, 5%; N2, 74%) as described in previous studies by our laboratory.13,53,54 Endothelial function was assessed by adding increasing concentrations of the classic endothelium-dependent vasodilator acetylcholine (10−10 to 10−6 M) to the vessel chamber and vascular smooth muscle sensitivity to NO was assessed by adding increasing concentrations of the NO donor SNP (10−10 to 10−5 M) to the vessel chamber, as described previously.53,54 Active resting tone (%) in the vessels was calculated as [(Dmax – Drest) / Dmax] × 100%, where Drest is the diameter after equilibration in normal physiologic salt solution, and Dmax is the diameter in Ca2+-free physiologic salt solution at the control transmural pressure of 80 mm Hg.
Enzymatic isolation of rat MCA myocytes.
Experiments evaluating MaxiK channel current in MCA myocytes used male Sprague–Dawley rats (age, 8 to 19 wk) housed in an animal room adjacent to that housing the rats used for the acetylcholine and SNP experiments. The initial series of measurements of MaxiK channel currents was conducted between 17 July and 25 September 2017 (prior to the construction activity). The subsequent series of experiments evaluating MaxiK channel function was conducted between 11 December 2017 and 12 January 2018, when construction was in progress.
MCA myocytes were freshly isolated by enzymatic dissociation as previously described.16 On the day of the experiment, rats were anesthetized by using 4% isoflurane and then decapitated. Brains were quickly removed and placed on a dissecting dish containing ice-cold, low-calcium arterial myocyte dissociation solution comprising 134 mM NaCl, 5.2 mM KCl, 1.2 mM MgSO4 • 7 H2O, 1.18 mM KH2PO4, 0.05 mM CaCl2, 24 mM NaHCO3, 11 mM glucose, and 10 mM HEPES (pH 7.4). MCA segments were isolated under a dissecting microscope and placed in a vial containing 2 mL of a solution of bovine serum albumin (0.5 mg/mL) in low-calcium arterial myocyte dissociation solution for 10 min at room temperature. The isolated arterial segments were dissociated to single cerebral arterial myocytes by enzymatic digestion at 37 °C.16 The myocytes were kept on ice and used within 6 h for patch-clamp recording of MaxiK single-channel current.
Patch-clamp recording of MaxiK single-channel current.
MaxiK single-channel currents were recorded at a patch potential of +40 mV from cell-attached patches by using symmetrical 145 mM K+ recording solution as previously described.16,20 The recording pipette solution contained 145 mM KCl, 1.8 mM CaCl2, 1.1 mM MgCl2, 5 mM HEPES, and 5 mM EGTA, with the final pH adjusted to 7.2 with KOH. The bath solution was composed of 145 mM KCl, 1.8 mM CaCl2, 1.1 mM MgCl2, 5 mM HEPES, and 5 mM EGTA, with pH adjusted to 7.2 with KOH and resulting in a calculated final bath Ca2+ concentration of 10−7 M. The openings of MaxiK single-channel currents were analyzed by using the pClamp software package (version 10.4, Molecular Devices, San Jose, CA) to determine opening frequency, mean current amplitude, and the probability of channel opening (Po). The mean open state probability (NPo) was expressed as NPo = I/i, where I is the time averaged current, N is the number of channels, i is the amplitude of the single-channel K+ current, and Po is the probability of a channel opening.16,20
Statistical analysis.
All data are presented as mean ± SEM. Active resting tone (%) in vessels was calculated as [(Dmax – Drest) / Dmax] × 100%, where Drest is the diameter after equilibration in normal physiologic salt solution, and Dmax is the diameter in Ca2+-free physiologic salt solution at the control transmural pressure of 80 mm Hg. Two-way ANOVA followed by Bonferroni testing and multiple t tests followed by the Holm–Sidak method were used to test the significance of the differences between vasodilator response in different groups. In all experiments, a P value less than 0.05 was considered to indicate a significant difference. The Prism statistical software program (version 7.03, GraphPad Prism Software, San Diego, CA) was used to perform the statistical analysis.
Results
Diameter and active tone of MCA.
Table 1 summarizes the mean resting diameter, maximum diameter, and active resting tone in MCA from the different groups of animals. There were no significant differences in any of the measured parameters between the 3 groups of animals, indicating that construction activity did not affect spontaneous resting tone or result in remodeling of vessel structure, as indicated by a difference in the passive diameter of the maximally relaxed arteries.
Table 1.
Resting diameters, maximum diameters, and active resting tone in MCA from Sprague–Dawley rats during and after construction activities
| During construction (inhouse rats) n = 16 | After construction (inhouse rats) n = 9 | After construction (new rats) n = 5 | |
| Maximal diameter (μm) | 211 ± 6 | 224 ± 10 | 221 ± 6 |
| Resting diameter (μm) | 127 ± 9 | 124 ± 7 | 112 ± 11 |
| Active resting tone (%)a | 40 ± 4 | 45 ± 2 | 50 ± 4 |
Values are mean ± SEM
Active resting tone (%) = [(Dmax – Drest)/Dmax] × 100%.
Responses to acetylcholine and SNP.
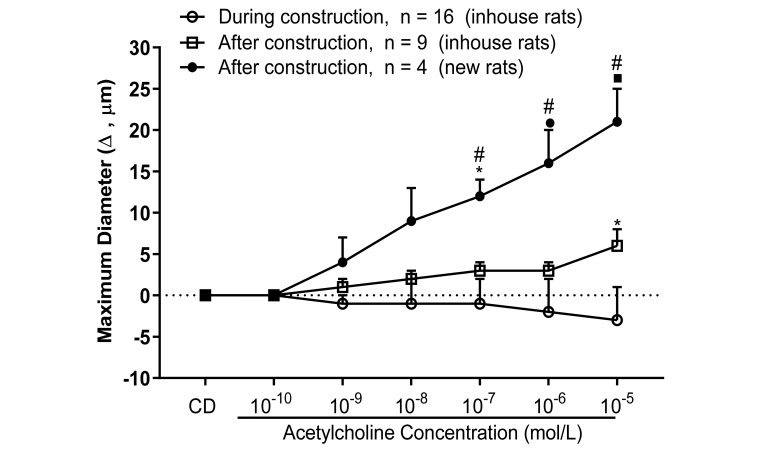
Figure 3 compares the responses to acetylcholine (10−10 to 10−6 M) in MCA from: 1) rats housed and studied during the construction period (1 October 2017 to 30 April 2018); 2) rats studied immediately after cessation of construction but housed in the animal facility during the period of construction; and 3) rats obtained and studied after completion of the construction activity. MCA from rats housed and studied during the construction period failed to dilate in response to acetylcholine. Arteries from animals studied after the construction period but present during construction exhibited little or no dilation in response to acetylcholine. Responses of MCA to acetylcholine in animals obtained after cessation of construction activity had returned and were consistent with normal historical levels, i.e., an approximately 15-µm increase from resting diameter in response to 10−6 M acetylcholine in studies from 2002 until 2016.10,28,38,49
Figure 3.
Response to acetylcholine (10−10 to 10−6 M) in isolated pressurized MCA from Sprague–Dawley rats during and after construction activities. Data are expressed as change from resting control diameter (CD) (µm; mean ± SEM). Value significantly different in new rats compared with responses during construction (inhouse rats; *, P < 0.05; ●, P < 0.0005; ■, P < 0.0001) or in new rats compared with inhouse rats studied after construction (#, P < 0.05).
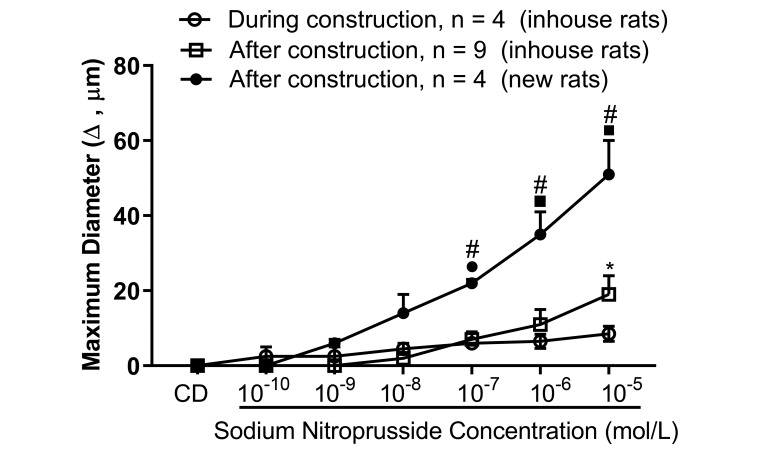
Figure 4 compares vessel responses to the NO donor SNP (10−10 to 10−5 M) in rats obtained and studied after cessation of construction compared with animals that were studied either during or after construction activity but housed in the same animal room prior to the cessation of construction. Similar to the acetylcholine responses, vasodilator responses to SNP were: 1) absent in MCA from animals housed and studied during construction; 2) minimal to absent in MCA from animals present in the animal facility during construction but studied after completion of construction; and 3) intact and consistent with normal historical levels in MCA from newly obtained animals studied after completion of the construction activities.
Figure 4.
Response to sodium nitroprusside (10−10 – 10−5 M) in isolated pressurized MCA from Sprague–Dawley rats during and after construction activities. Data are expressed as change from resting control diameter (CD) (µm; mean ± SEM). Value significantly different in new rats compared with responses during construction (inhouse rats; *, P < 0.05; ○, P < 0.005; ■, P < 0.0001) or in new rats compared with inhouse rats studied after construction (#, P < 0.05).
Effect of construction activity on MaxiK single-channel currents in isolated MCA smooth muscle cells.
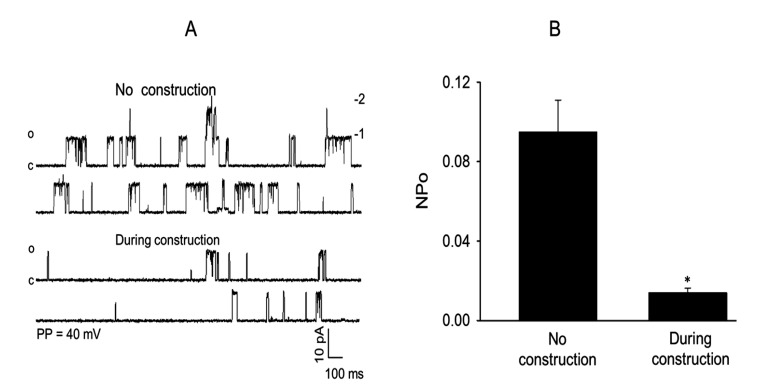
The effect of ongoing construction activity on the frequency of MaxiK single-channel current opening and NPo of MaxiK channels in isolated rat MCA myocytes studied by using the patch clamp ion channel current recording technique is shown in Figure 5. In these experiments, the opening frequency (Figure 5 A) and NPo (Figure 5 B) of the MaxiK single-channel currents measured by using the cell-attached mode and symmetrical 145 mM KCl recording solution were markedly reduced during ongoing construction work compared with similar measurements obtained from rats prior to the start of construction.
Figure 5.
Effect of nearby construction activity on the opening frequency and open state probability (NPo) of MaxiK+ single-channel currents recorded from cell-attached patches of isolated MCA myocytes from Sprague–Dawley rats by using a symmetrical 145-mM KCl recording solution at a patch potential of +40 mV. The presence of nearby construction activity caused (A) a striking reduction in the frequency of opening of MaxiK+ channels and (B) a significant decrease in mean open state probability (NPo) for MaxiK+ channels compared with that recorded in the absence of construction activity (n = 10–12; *, P < 0.05).
Activity detected by commercial and laboratory-designed vibration sensors.
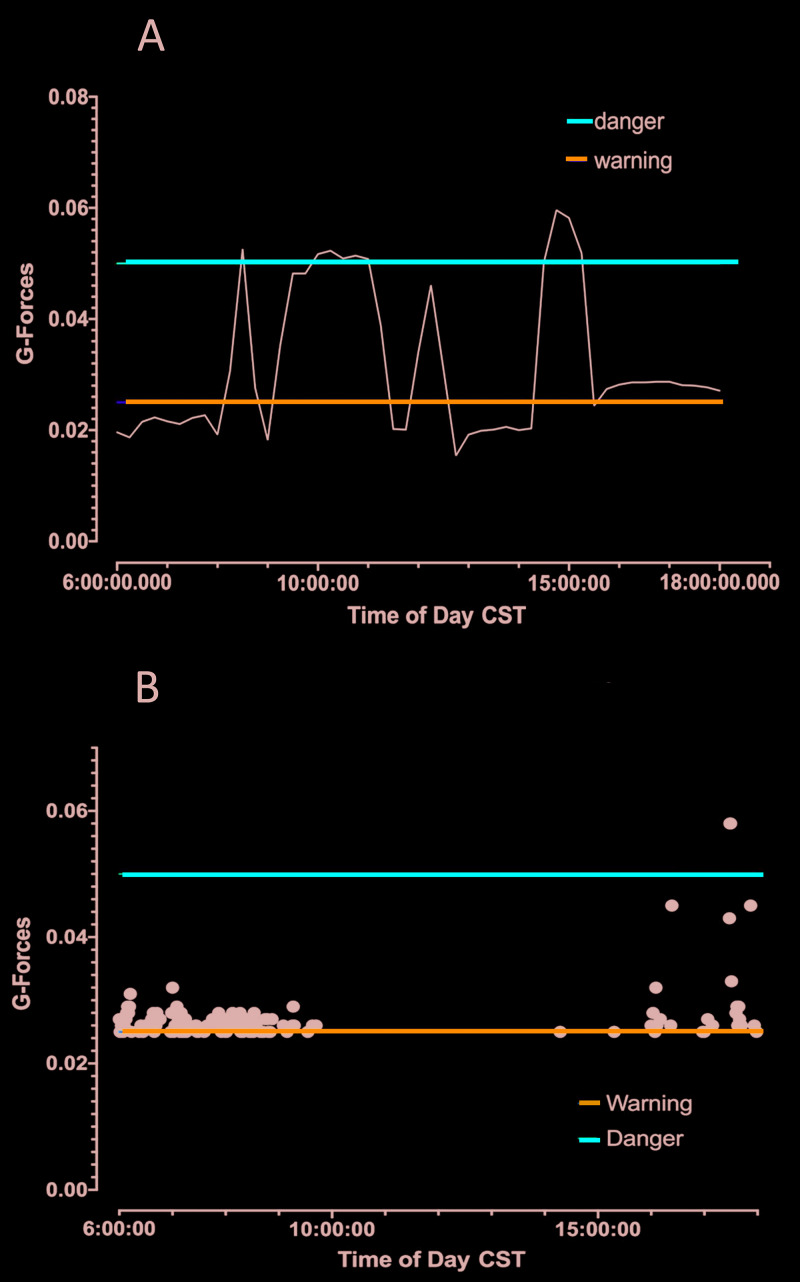
Examples of the vibrations detected by the 2 vibration sensor systems are shown in Figures 6 and 7. The recording from the commercial system (Figure 6 A) was obtained during construction activity on 26 April 2018. In that figure, the horizontal blue line indicates the threshold for vibrations that are sufficiently large to cause a disturbance to laboratory animals, as determined in a previous study,29 and the red horizontal line indicates the vibration levels deemed sufficient to trigger stress response pathways in rats. On 26 April 2018, there were 3 sustained periods between 0900 and 1600 when vibration levels exceeded the threshold for triggering stress response pathways. These vibration levels corresponded to experiments in which isolated MCA failed to respond to acetylcholine (Figure 3). Over the period of monitoring, measurements from the commercial sensor were below the threshold for a stress response in rodents (0.05 G and higher) on each day that we measured. However, we did obtain measurements in the 0.025- to 0.049-G range (close to levels that cause activation of stress pathways) on each day that construction occurred and the device was working. We consistently recorded measurements above 0.05 G when construction was occurring. However, on 22 May 2018, cosmetic courtyard renovations were being conducted which involved much less vibration than previous operations (and were likely affected by low outdoor temperatures and rain). On that day, vibration levels recorded by the commercial system were much lower, except for a few disturbances later in the day, (Figure 6 B).
Figure 6.
(A) VivAlarm data during courtyard renovations on 26 April 2018. Multiple periods had spikes that exceeded the warning level (blue line), and 3 periods had spikes higher the danger level (red line). (B) VivAlarm data sent via email during cosmetic renovation of courtyard on 22 May 2018 during reduced construction activity and a brief disturbance between 1600 and 1800. Data are expressed as G-force.
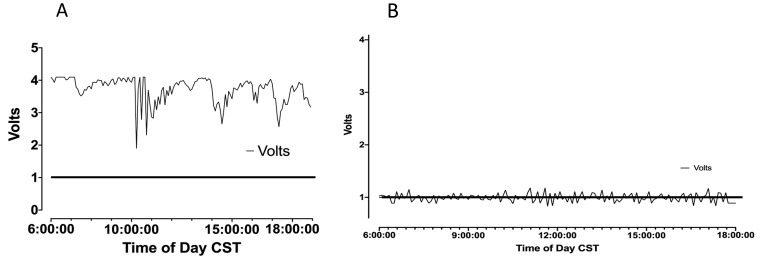
Figure 7.
(A) RPi data collected during construction activities above the vivarium on 27 April 2018. Many collections of spikes exceed the minimum for detection; data are expressed as volts. MCA of exposed rats were not acetylcholine-responsive. (B) RPi data collected during cosmetic courtyard work on 2 May 2018, with several spikes that exceed the minimum. MCA responses to acetylcholine and SNP were still compromised compared with historical levels and with animals obtained and studied after the completion of construction.
Similar to the commercial system, our inhouse-designed sensor detected significant elevations in vibration levels during the daylight hours on days when construction activity occurred. Figure 7 A shows examples of vibration levels detected by our system on 27 April 2018, when concrete flooring work occurred in the basic science building housing the vivarium and when drilling was conducted on the dock of an adjacent building. The normal background vibration level for the laboratory-designed sensor is 8000 Hz, corresponding to 1 V output. On that date, vibration levels throughout the day were continuously elevated and remained at 25,000 to 32,000 Hz throughout the day (output of 3 to 4 V output through most of the day, with only 4 very brief periods when sensor output fell to between 2 and 3V [still far in excess of the 1 V normal output for vibration]). Responses of the isolated cannulated MCA to acetylcholine and SNP were absent during this time period. During the period of active construction in various locations near and within the basic science building, vibration levels revealed by our system were either continuously elevated throughout the day or were elevated through the majority of the day, with only a few, brief reductions.
By contrast, periods of reduced construction activity during late April and early May 2018 (28 April to 2 May and 6 to 10 May) were generally associated with low levels of vibration, with very few periods of high vibration levels. Figure 7 B shows vibration levels recorded on 2 May 2018, when the cosmetic renovation of the courtyard and irrigation repairs—which included digging, soil removal, and the addition of soil—were conducted, with much decreased vibration. Throughout that day, vibration levels were near baseline values. After the completion of construction activity on 1 June 2018, baseline levels of vibration had returned, and the responses of MCA to acetylcholine and SNP in rats obtained after the construction period and housed in the same animal room had recovered to normal levels (Figures 3 and 4).
Discussion
The results of this study strongly indicate that ongoing construction activity associated with noise and vibration near animal housing areas has profound detrimental effects on crucially important physiologic phenotypes in the MCA of normal Sprague–Dawley rats. These include vascular relaxation in response to acetylcholine (a ‘gold standard’ indicator of normal function of the vascular endothelium) and vascular relaxation in response to the endothelium-independent NO donor SNP, a standard indicator of vascular smooth muscle NO sensitivity. These deleterious effects of construction activity on endothelial function and vascular NO sensitivity are not readily reversible, given that they were still present in MCA from rats studied after cessation of construction activity but present in the animal facility during the construction activity. In addition, the present study indicates that ongoing construction activity directly suppresses the activity of the high-conductance Ca2+-activated K+ channel (MaxiK channel) that plays an essential role in regulating active tone and mediating the relaxation of cerebral artery smooth muscle cells in response to vasodilator factors.
Noise exposure has been shown to have adverse effects on endothelial function in healthy adults33 and in humans with or at high risk for coronary artery disease.43 Aircraft noise causes a reduction in flow-mediated dilation in healthy human subjects; this reduction in dilation is abrogated by the antioxidant vitamin C (indicating the presence of vascular oxidative stress).44 Other studies have found that traffic noise leads to an increased risk of stroke.19,47 Ultimately, the deleterious effects of noise exposure in humans with links to cardiovascular disease, heart disease, arterial hypertension, myocardial infarction, stroke, obesity, and cardiometabolic diseases such as type 2 diabetes place a substantial burden on health care systems,21,23,33,48 underscoring the importance of obtaining more precise knowledge of the effects of noise and other forms of stress on physiologic processes.23
The known effects of noise on human health have naturally led to animal studies to evaluate noise-related effects on physiologic responses in laboratory animals. Studies of the effects of noise and vibration on laboratory animals generally show patterns that are similar to those reported in humans, including increased blood pressure, endothelial dysfunction, and oxidative stress.2,26,42 Although studies of the effects of lower and more common noise levels on the cardiovascular system of animals are sparse,33 one investigation32 used a simulated aircraft noise exposure model to show that mice exposed to lower levels of noise (peak sound levels of less than 85 dBA and mean sound pressure levels of 72 dBA) and shorter exposure times (1 to 4 d) that do not cause auditory damage still exhibited impaired dilation in response to both acetylcholine and the NO donor nitroglycerin. In that study,32 vascular NO production was reduced, vascular superoxide production was elevated, and the elevated vascular superoxide production was inhibited by L-NAME, indicative of nitric oxide synthase uncoupling. The authors32 also noted increased NADPH oxidase activity, an increase in serum proteins positive for 3-nitrotyrosine, and enhanced 3-nitrotyrosine staining throughout the vasculature. However, animals exposed to equal sound pressure levels of white noise did not exhibit those changes. Another potential contributor to vascular dysfunction with noise exposure could be scavenging of NO by superoxide to form the vasoconstrictor peroxynitrite—a known culprit of vascular dysfunction.6,18
The hearing range of rats (200 to 90,000 Hz) is much higher than that in humans and extends into the ultrasonic spectrum. One group of investigators41 concluded that mice hear less noise due to construction equipment than do humans. In the present study, it is unlikely that ultrasonic noise would travel far enough for the rats to hear it, whereas vibrations can be propagated through the earth and substrates. This conclusion is consistent with the findings of 2 studies,35,41 which indicated that vibration due to construction could be a greater concern than the associated noise. However, an important problem is that physiologic phenotypes in laboratory animals can be affected by noise levels and vibrations that are inaudible or imperceptible to humans in the animal housing areas, as noted by colleagues40 during earlier construction activity near our institution that was much less severe than that encountered during the present study period. In the case of less severe construction activity, noise levels generated within (or close to the vivarium) could be affecting animal phenotypes with no warning signals that are perceptible to investigators. In addition, construction-related noise generated within the building housing the vivarium during the current study (for example, electrical upgrade work [5 to 16 March 2018]) and work on the animal facility loading dock (21 to 28 April 2018) could adversely affect a multitude of physiologic phenotypes in laboratory animals.
In contrast to their insensitivity to construction-related noise levels external to the building, rodents are very sensitive to vibrations in the earth. Although rodents can hear ultrasonic sounds that are above our hearing range, ultrasonic sounds do not travel very far, and they are easily buffered and eliminated by barriers. Because the vast majority of our vibrational sources were external to the building and propagated through soil, buildings, floors, etc., we believe that it is unlikely that any sound produced by the construction equipment outside the building would have been heard by the rats. However, although the same cannot be said for some of the work within the building.
A previous study15 reported that the initiation of vibration at certain levels led to behavioral responses in awake mice, including freeze responses (a fear response) and startle reflexes and that sleeping mice were alerted by vibration. In addition, vibration leads to a persistent increase in heart rate in awake mice.27 Another study34 found that vibration led to a reduction in microvessel density in the mouse soleus muscle, and another group15 emphasized that, due to the known and unknown effects of vibration on animals, care should be taken to minimize all sources of vibration in animal facilities. Consistent with that conclusion, other studies35,39 have indicated that animals likely perceive vibrations at higher frequencies than humans and that construction activity may expose rodents to more vibration than that perceived by humans. Because the current configuration of the RPi system does not measure vibration frequency, one limitation of the present study was the lack of vibration frequency data for the vibrations encountered during the construction activity. An important addition to future studies investigating this problem is to obtain measurements of the predominant frequency ranges of vibrations, especially high-magnitude vibrations. Nonetheless, previous findings by other investigators and those of the current study regarding the effect of vibration on various phenotypes suggest the need to qualify the animal facility environment in terms of noise and vibration, as is currently practiced for factors such as lighting, temperature, humidity, cleanliness, and air exchange rates.
One question that arises is whether the effects of noise on animals are different from those in humans and, therefore, not representative of effects in humans.33 However, the immense importance of animal models in investigating molecular and physiologic mechanisms of human disease strongly emphasizes the need to understand how noise and vibration affect physiologic mechanisms in animals because noise- and vibration-related artifacts could confound the conclusions of carefully designed and extramurally funded studies seeking to investigate specific physiologic and molecular mechanisms of human disease. Implicit in this concern is the need for institutions to be aware of the potential effects of construction-related noise and vibration on investigators using animal models and to develop effective communication plans to protect researchers from the potentially confounding effects of construction-related noise and vibration on their research.
One dangerous assumption is that molecular studies, genomic studies, and other reductionist approaches will be immune from the effects of construction activity that affect physiologic responses at the whole-animal level. However, oxidative stress and redox regulation can strongly affect gene regulation, stress adaptation processes, and other essential pathways.30 Relevant to those concerns, one group32 reported that noise exposure led to changes in many important genes that are at least partially responsible for the regulation of vascular function, vascular remodeling, and cell death. Especially noteworthy in this regard is the conclusion that environmental disturbances very likely produce marked effects on the results of investigators who collect tissues for measurements of transcription factors and other measures of specific gene activity.7
In addition to noise and vibration generated in the animal quarters by human activity or sources such as ventilation equipment, etc., noise and vibration can easily arise as a result of nearby construction activity.40 The growing importance of specialized animal genetic models and increasingly powerful methods to analyze widespread effects of experimental perturbations or genetic factors on gene expression (e.g., RNAseq)36,37 strongly emphasize the need to avoid any effects of extraneous factors on genetic and homeostatic mechanisms in laboratory animals. In the case of laboratory animals, one extremely important extraneous factor that can affect these mechanisms is the influence of nearby construction activity, which can involve not only noise but, more importantly, significant levels of vibration.
Although few studies describe the effects of nearby construction on research animals to date, the deleterious effects of construction activities on experimental animals has been noted by several authors5,7,40,42,46,58 For example, one group7 reported that nearby construction activity altered crucial phenotypes (hyperphagic responses and weight gain) associated with a change in the background genotype (knockout of the 5HT2C receptor gene) in mice and that the mutant mice did not eat or grow as well as those in a more protected room that was similar in regard to cleanliness, lighting, and diet. In addition, the authors7 noted that jackhammering on the roof (performed late in the day to avoid disturbances to people in the building) abruptly reduced food intake and caused weight loss in a sentinel rat. Construction activity also caused a reduction in nocturnal food intake and an increase in diurnal food intake in a sentinel rat—a response characteristic of stressed rats. As shown in Figure 7 A, jackhammering and heavy equipment use produced significant levels of vibration in animal housing rooms in our institution as well.
Significant effects on physiologic phenotypes have been demonstrated during construction activity even in the absence of changes in arterial blood pressure and body weight and without changes in well-known drivers of stress responses, such as plasma renin activity. One study40 investigated the effects of nearby construction activity at MCW that had many similarities to the current construction activity, that is, buildout, remodeling, and landscaping outside the animal facility. Basal HPA and renin–aldosterone activity was evaluated in rats before, during, and after the construction activities. In that study,40 plasma ACTH, corticosterone, and aldosterone levels were approximately doubled during construction compared with pre- and postconstruction levels. The magnitude of the increases in plasma ACTH and corticosterone were similar to those known to be induced by stressors in laboratory rodents1 and comparable to those seen in response to mild acute stressors, such as open-field or elevated plus-maze tests.22,31,52 However, in that study, body weight was unaffected during construction—in contrast to another study,7 which showed weight loss in mice during construction.
One previous study40 reported that plasma renin activity did not increase during the construction activity. This failure of PRA to increase40 is important in light of other studies showing significant increases in plasma angiotensin II levels (as well as oxidative stress and nitric oxide synthase uncoupling) in animals exposed to noise and vibration,32 providing evidence for a very narrow window for angiotensin II levels to maintain normal function57 and the potentially powerful effects of both low and high levels of angiotensin II on vascular function. For example, previous studies3 have shown that 2 kidney–1 clip hypertension, which increases plasma angiotensin II levels, restores endothelium-dependent dilation to acetylcholine, which is normally impaired or absent in Sprague–Dawley rats fed a high-salt diet, due to salt-induced suppression of plasma angiotensin II levels. In a separate study,4 diet-induced obesity, which increases plasma angiotensin II levels, restored acetylcholine-induced dilation in MCA of normotensive Dahl salt-sensitive (SS) rats fed a low-salt (0.4% NaCl) diet (which normally exhibit endothelial dysfunction due to chronically low plasma ANG II levels). In that study,4 diet-induced obesity also eliminated acetylcholine-induced dilation in low-salt–fed SS.13BN consomic rats, which are 98% identical to the SS rats genetically but exhibit normal plasma angiotensin II levels at rest due to the presence of the Brown Norway renin gene in the Dahl SS genetic background. Although not tested directly, the loss of acetylcholine-induced dilation in the consomic rats in that study4 would be consistent with the prooxidative effects of elevated plasma angiotensin II levels.
Comparison of the results of previous studies32,40 and studies comparing different phenotypes, for example, body weight,7,40 clearly demonstrate that construction activity and other sources of noise and vibration can have different effects on the same phenotype. The existence of such striking differences in the effect of noise, vibration, and construction activity on the same physiologic phenotype underscores the dangers of assessing the potentially detrimental effects of construction activity based on one specific parameter.
In one report,40 the effects of construction activity were evident even in the absence of noise and vibration that were perceptible to humans in the area. The authors noted that an important limitation of their study was that noise and vibration levels were not monitored in their experiments, due to the unexpected onset of subtle noise and vibration influences on physiologic responses. Those authors40 stressed the importance of actual monitoring of noise and vibration levels in animal quarters to gain a more precise indication of specific factors that can adversely affect physiologic responses.
During our study, we considered the importance of monitoring vibration levels in animal housing areas by using continuous monitoring of vibration with 2 different systems. One was a commercially available monitoring system, which has an accelerometer that measures forces in one plane. Although the commercial system provided very valuable information, measurement accuracy would be improved using an accelerometer that measures forces in multiple planes. The other monitoring device used in the present study was a small, low-cost, and easily deployed monitoring system of our own design (Figure 2), which provided continuous monitoring of vibration levels and could be easily placed in cages on standard racks in different animal rooms. Both of these monitoring systems enabled us to evaluate the levels of construction-related vibrations present in the animal housing areas. Vibrations were monitored during times when individual experiments showed either a loss of crucial phenotypes (acetylcholine-induced and SNP-induced dilation) or a recovery of vasodilator responses in arteries of rats that were obtained and studied after the completion of the construction activity.
Both monitoring systems showed dramatic increases in vibration in animal housing areas during the period of construction (when acetylcholine- and SNP-induced dilation of the MCA were lost and MaxiK single-channel current activity was significantly reduced). Vibration levels recovered to baseline values after completion of the construction activity. Consistent with previous reports in the literature32 regarding other physiologic phenotypes, such as impaired vasodilation to acetylcholine and the NO donor nitroglycerin (as well as NOS uncoupling and enhanced constriction to norepinephrine and endothelin 1), we found that acetylcholine-induced dilation, SNP-induced dilation, and MaxiK single-channel channel activity were eliminated or dramatically suppressed during the construction period. Although not reported here, endothelium-dependent dilation to acetylcholine was lost in other normotensive animals housed in the facility and likewise appeared to be related to the onset of construction activities in the surrounding area.
Vasodilator responses to acetylcholine and SNP were minimal or absent in MCA from animals that were present during construction activity but studied after the completion of construction. However, in animals obtained and studied after cessation of construction activity, MCA responses to acetylcholine and SNP were intact and consistent with historical levels.10,28,38,49 Taken together, these findings indicate that the effects of construction-related vibration on crucial vascular functions are not immediately reversible and persist even after the construction activity is completed. The precise mechanisms responsible for the persistent impairment of endothelium-dependent vasodilation and reduced NO sensitivity in animals studied after construction but housed in the facility during construction are unclear. However, one possible mechanism for the impaired responses to acetylcholine and SNP in these animals is prolonged exposure to vascular oxidative stress (e.g., superoxide radicals in particular) during construction. Such prolonged exposure to superoxide radicals would not only degrade endothelium-derived NO but could also impair soluble guanylate cyclase function, as previously reported by others,17,24,25 leading to reduced formation of cGMP in response to NO and subsequent impairment of the vascular relaxation in response to the NO donor. Although not tested in the present study, vascular oxidative stress and elevated levels of reactive oxygen species such as superoxide could lead to an impaired response to vasodilator stimuli (for example prostacyclin) that relax vascular smooth muscle cells directly by activating membrane K+ channels to hyperpolarize the cell membrane, as previously reported for Sprague–Dawley rats fed a high-salt diet.53,54
Two areas of substantial concern are apparent. First, construction-related noise and vibration levels that occur in housing areas and that affect the animals may not be perceptible to humans in the area. Second, planners, construction workers, and institutional officials often erroneously assume that construction-related activities will not affect the results of ongoing experiments.5 The findings of the current study emphasize the importance of monitoring vibration levels in animal facilities and carefully assessing the validity of phenotypes measured in animals exposed to construction activities (or any other source of stress) prior to the study. In addition, our current findings strongly emphasize the need for institutions to provide adequate notification of construction activities to vivarium directors and investigators engaged in animal-related research projects and to take steps to protect research animals from the potential influences of noise and vibration during such activities. In this regard, careful planning is essential—not only as a gesture of courtesy and respect for investigators and vivarium directors but also to avoid substantial amounts of lost time, unnecessary expenses (often derived from extramural funding), and potentially erroneous findings in important research studies.
Acknowledgment
We thank and appreciate Eric Jensen for his advice and assistance in the vibration monitoring experiments used in the present study.
References
- 1.Akana SF, Dallman MF, Bradbury MJ, Scribner KA, Strack AM, Walker CD. 1992. Feedback and facilitation in the adrenocortical system: unmasking facilitation by partial inhibition of the glucocorticoid response to prior stress. Endocrinology 131:57–68. 10.1210/endo.131.1.1319329. [DOI] [PubMed] [Google Scholar]
- 2.Altura BM, Altura BT, Gebrewold A, Ising H, Gunther T. 1992. Noise-induced hypertension and magnesium in rats: relationship to microcirculation and calcium. J Appl Physiol (1985) 72:194–202. 10.1152/jappl.1992.72.1.194. [DOI] [PubMed] [Google Scholar]
- 3.Beyer AM, Fredrich K, Lombard JH. 2013. AT1 receptors prevent salt-induced vascular dysfunction in isolated middle cerebral arteries of 2 kidney–1 clip hypertensive rats. Am J Hypertens 26:1398–1404. 10.1093/ajh/hpt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyer AM, Raffai G, Weinberg B, Fredrich K, Lombard JH. 2012. Dahl salt-sensitive rats are protected against vascular defects related to diet-induced obesity. Hypertension 60:404–410. 10.1161/HYPERTENSIONAHA.112.191551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blaustein JD. 2011. Nearby construction influences the physiology of research animals: beyond stress hormones. Endocrinology 152:1197–1198. 10.1210/en.2010-1499. [DOI] [PubMed] [Google Scholar]
- 6.Brzezinska AK, Gebremedhin D, Chilian WM, Kalyanaraman B, Elliott SJ. 2000. Peroxynitrite reversibly inhibits Ca2+-activated K+ channels in rat cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol 278:H1883–H1890. 10.1152/ajpheart.2000.278.6.H1883. [DOI] [PubMed] [Google Scholar]
- 7.Dallman MF, Akana SF, Bell ME, Bhatnagar S, Choi S, Chu A, Gomez F, Laugero K, Soriano L, Viau V. 1999. Warning! Nearby construction can profoundly affect your experiments. Endocrine 11:111–113. 10.1385/ENDO:11:2:111. [DOI] [PubMed] [Google Scholar]
- 8.Drexler H, Lu W. 1992. Endothelial dysfunction of hindquarter resistance vessels in experimental heart failure. Am J Physiol 262:H1640–H1645. [DOI] [PubMed] [Google Scholar]
- 9.Drexler H, Zeiher AM. 1991. Progression of coronary endothelial dysfunction in man and its potential clinical significance. Basic Res Cardiol 86 Suppl 2:223–232. [DOI] [PubMed] [Google Scholar]
- 10.Durand MJ, Raffai G, Weinberg BD, Lombard JH. 2010. Angiotensin-(1-7) and low-dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am J Physiol Heart Circ Physiol 299:H1024–H1033. 10.1152/ajpheart.00328.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Förstermann U, Münzel T. 2006. Endothelial nitric oxide synthase in vascular disease: from marvel to menace. Circulation 113:1708–1714. 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 12.Freiman PC, Mitchell GG, Heistad DD, Armstrong ML, Harrison DG. 1986. Atherosclerosis impairs endothelium-dependent vascular relaxation to acetylcholine and thrombin in primates. Circ Res 58:783–789. 10.1161/01.RES.58.6.783. [DOI] [PubMed] [Google Scholar]
- 13.Frisbee JC, Sylvester FA, Lombard JH. 2001. High-salt diet impairs hypoxia-induced cAMP production and hyperpolarization in rat skeletal muscle arteries. Am J Physiol Heart Circ Physiol 281:H1808–H1815. 10.1152/ajpheart.2001.281.4.H1808. [DOI] [PubMed] [Google Scholar]
- 14.Furchgott RF, Zawadzki JV. 1980. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 288:373–376. 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 15.Garner AM, Norton JN, Kinard WL, Kissling GE, Reynolds RP. 2018. Vibration-induced behavioral responses and response threshold in female C57BL/6 mice. J Am Assoc Lab Anim Sci 57:447–455. 10.30802/AALAS-JAALAS-17-00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gebremedhin D, Zhang DX, Weihrauch D, Uche NN, Harder DR. 2017. Detection of TRPV4 channel current-like activity in Fawn Hooded hypertensive (FHH) rat cerebral arterial muscle cells. PLoS One 12:1–23. 10.1371/journal.pone.0176796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerassimou C, Kotanidou A, Zhou Z, Simoes DC, Roussos C, Papapetropoulos A. 2007. Regulation of the expression of soluble guanylyl cyclase by reactive oxygen species. Br J Pharmacol 150:1084–1091. 10.1038/sj.bjp.0707179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzik TJ, West NE, Pillai R, Taggart DP, Channon KM. 2002. Nitric oxide modulates superoxide release and peroxynitrite formation in human blood vessels. Hypertension 39:1088–1094. 10.1161/01.HYP.0000018041.48432.B5. [DOI] [PubMed] [Google Scholar]
- 19.Halonen JI, Hansell AL, Gulliver J, Morley D, Blangiardo M, Fecht D, Toledano MB, Beevers SD, Anderson HR, Kelly FJ, Tonne C. 2015. Road traffic noise is associated with increased cardiovascular morbidity and mortality and all-cause mortality in London. Eur Heart J 36:2653–2661. 10.1093/eurheartj/ehv216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch 391:85–100. 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 21.Harding AH, Frost GA, Tan E, Tsuchiya A, Mason HM. 2013. The cost of hypertension-related ill-health attributable to environmental noise. Noise Health 15:437–445. 10.4103/1463-1741.121253. [DOI] [PubMed] [Google Scholar]
- 22.Herman JP, Dolgas CM, Carlson SL. 1998. Ventral subiculum regulates hypothalamo-pituitary-adrenocortical and behavioural responses to cognitive stressors. Neuroscience 86:449–459. 10.1016/S0306-4522(98)00055-4. [DOI] [PubMed] [Google Scholar]
- 23.Jansen G. 1968. Effects of noise on health. Ger Med Mon 13:446–448. [PubMed] [Google Scholar]
- 24.Kagota S, Tamashiro A, Yamaguchi Y, Nakamura K, Kunitomo M. 2002. High salt intake impairs vascular nitric oxide/cyclic guanosine monophosphate system in spontaneously hypertensive rats. J Pharmacol Exp Ther 302:344–351. 10.1124/jpet.302.1.344. [DOI] [PubMed] [Google Scholar]
- 25.Kagota S, Tamashiro A, Yamaguchi Y, Sugiura R, Kuno T, Nakamura K, Kunitomo M. 2001. Downregulation of vascular soluble guanylate cyclase induced by high salt intake in spontaneously hypertensive rats. Br J Pharmacol 134:737–744. 10.1038/sj.bjp.0704300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lauer AM, May BJ, Hao ZJ, Watson J. 2009. Analysis of environmental sound levels in modern rodent housing rooms. Lab Anim (NY) 38:154–160. 10.1038/laban0509-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Y, Rabey KN, Schmitt D, Norton JN, Reynolds RP. 2015. Characteristics of vibration that alter cardiovascular parameters in mice. J Am Assoc Lab Anim Sci 54:372–377. [PMC free article] [PubMed] [Google Scholar]
- 28.McEwen ST, Balus SF, Durand MJ, Lombard JH. 2009. Angiotensin II maintains cerebral vascular relaxation via EGF receptor transactivation and ERK1/2. Am J Physiol Heart Circ Physiol 297:H1296–H1303. 10.1152/ajpheart.01325.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MD Anderson. [Internet]. 2010. Noise, vibration, and ultrasound design guide. CSTI Report 640 Revision A. [Cited 08 November 2018]. Available at: https://www.mdanderson.org/documents/about-md-anderson/about-us/doing-business/owners-design-guidelines/supplemental-resources/MDACC_Noise_Vibration_Ultrasound_DG.pdf.
- 30.Miguel V, Cui JY, Daimiel L, Espinosa-Diez C, Fernandez-Hernando C, Kavanagh TJ, Lamas S. 2018. The Role of MicroRNAs in environmental risk factors, noise-induced hearing loss, and mental stress. Antioxid Redox Signal 28:773–796. 10.1089/ars.2017.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mueller NK, Dolgas CM, Herman JP. 2004. Stressor-selective role of the ventral subiculum in regulation of neuroendocrine stress responses. Endocrinology 145:3763–3768. 10.1210/en.2004-0097. [DOI] [PubMed] [Google Scholar]
- 32.Münzel T, Daiber A, Steven S, Tran LP, Ullmann E, Kossmann S, Schmidt FP, Oelze M, Xia N, Li H, Pinto A, Wild P, Pies K, Schmidt ER, Rapp S, Kröller-Schön S. 2017. Effects of noise on vascular function, oxidative stress, and inflammation: mechanistic insight from studies in mice. Eur Heart J 38:2838–2849. 10.1093/eurheartj/ehx081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Münzel T, Sorensen M, Schmidt F, Schmidt E, Steven S, Kröller-Schön S, Daiber A. 2018. The adverse effects of environmental noise exposure on oxidative stress and cardiovascular risk. Antioxid Redox Signal 28:873–908. 10.1089/ars.2017.7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Murfee WL, Hammett LA, Evans C, Xie L, Squire M, Rubin C, Judex S, Skalak TC. 2005. High-frequency, low-magnitude vibrations suppress the number of blood vessels per muscle fiber in mouse soleus muscle. J Appl Physiol (1985) 98:2376–2380. 10.1152/japplphysiol.01135.2004. [DOI] [PubMed] [Google Scholar]
- 35.Norton JN, Kinard WL, Reynolds RP. 2011. Comparative vibration levels perceived among species in a laboratory animal facility. J Am Assoc Lab Anim Sci 50:653–659. [PMC free article] [PubMed] [Google Scholar]
- 36.Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. 2014. Identification of genes whose expression is altered by obesity throughout the arterial tree. Physiol Genomics 46:821–832. 10.1152/physiolgenomics.00091.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Padilla J, Jenkins NT, Thorne PK, Martin JS, Rector RS, Davis JW, Laughlin MH. 2014. Transcriptome-wide RNA sequencing analysis of rat skeletal muscle feed arteries. II. Impact of exercise training in obesity. J Appl Physiol (1985) 116:1033–1047. 10.1152/japplphysiol.01234.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Priestley JR, Kautenburg KE, Casati MC, Endres BT, Geurts AM, Lombard JH. 2016. The NRF2 knockout rat: a new animal model to study endothelial dysfunction, oxidant stress, and microvascular rarefaction. Am J Physiol Heart Circ Physiol 310:H478–H487. 10.1152/ajpheart.00586.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabey KN, Li Y, Norton JN, Reynolds RP, Schmitt D. 2015. Vibrating frequency thresholds in mice and rats: implications for the effects of vibrations on animal health. Ann Biomed Eng 43:1957–1964. 10.1007/s10439-014-1226-y. [DOI] [PubMed] [Google Scholar]
- 40.Raff H, Bruder ED, Cullinan WE, Ziegler DR, Cohen EP. 2011. Effect of animal facility construction on basal hypothalamic-pituitary-adrenal and renin-aldosterone activity in the rat. Endocrinology 152:1218–1221. 10.1210/en.2010-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reynolds RP, Kinard WL, Degraff JJ, Leverage N, Norton JN. 2010. Noise in a laboratory animal facility from the human and mouse perspectives. J Am Assoc Lab Anim Sci 49:592–597. [PMC free article] [PubMed] [Google Scholar]
- 42.Said MA, El-Gohary OA. 2016. Effect of noise stress on cardiovascular system in adult male albino rat: implication of stress hormones, endothelial dysfunction and oxidative stress. Gen Physiol Biophys 35:371–377. 10.4149/gpb_2016003. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt F, Kolle K, Kreuder K, Schnorbus B, Wild P, Hechtner M, Binder H, Gori T, Munzel T. 2014. Nighttime aircraft noise impairs endothelial function and increases blood pressure in patients with or at high risk for coronary artery disease. Clin Res Cardiol 104:23–30. 10.1007/s00392-014-0751-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt FP, Basner M, Kroger G, Weck S, Schnorbus B, Muttray A, Sariyar M, Binder H, Gori T, Warnholtz A, Munzel T. 2013. Effect of nighttime aircraft noise exposure on endothelial function and stress hormone release in healthy adults. Eur Heart J 34:3508–3514. 10.1093/eurheartj/eht269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schulz E, Jansen T, Wenzel P, Daiber A, Münzel T. 2008. Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 10:1115–1126. 10.1089/ars.2007.1989. [DOI] [PubMed] [Google Scholar]
- 46.Shepherd EJ, Helliwell PA, Mace OJ, Morgan EL, Patel N, Kellett GL. 2004. Stress and glucocorticoid inhibit apical GLUT2-trafficking and intestinal glucose absorption in rat small intestine. J Physiol 560:281–290. 10.1113/jphysiol.2004.072447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sørensen M, Hvidberg M, Andersen ZJ, Nordsborg RB, Lillelund KG, Jakobsen J, Tjonneland A, Overvad K, Raaschou-Nielsen O. 2011. Road traffic noise and stroke: a prospective cohort study. Eur Heart J 32:737–744. 10.1093/eurheartj/ehq466. [DOI] [PubMed] [Google Scholar]
- 48.Swinburn TK, Hammer MS, Neitzel RL. 2015. Valuing quiet: an economic assessment of US environmental noise as a cardiovascular health hazard. Am J Prev Med 49:345–353. 10.1016/j.amepre.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sylvester FA, Stepp DW, Frisbee JC, Lombard JH. 2002. High-salt diet depresses acetylcholine reactivity proximal to NOS activation in cerebral arteries. Am J Physiol Heart Circ Physiol 283:H353–H363. 10.1152/ajpheart.00127.2002. [DOI] [PubMed] [Google Scholar]
- 50.Turner J. [Internet]. 2018. AALAS webinar: Noise and vibration in the vivarium: Best practices for minimizing negative impacts on animals, ongoing research studies, and relationships with scientists. [Cited 11 October 2018]. Available at: https://www.aalas.org/store/detail?productId=9098929.
- 51.Vita JA, Treasure CB, Yeung AC, Vekshtein VI, Fantasia GM, Fish RD, Ganz P, Selwyn AP. 1992. Patients with evidence of coronary endothelial dysfunction as assessed by acetylcholine infusion demonstrate marked increase in sensitivity to constrictor effects of catecholamines. Circulation 85:1390–1397. 10.1161/01.CIR.85.4.1390. [DOI] [PubMed] [Google Scholar]
- 52.Watts AG, Tanimura S, Sanchez-Watts G. 2004. Corticotropin-releasing hormone and arginine vasopressin gene transcription in the hypothalamic paraventricular nucleus of unstressed rats: daily rhythms and their interactions with corticosterone. Endocrinology 145:529–540. 10.1210/en.2003-0394. [DOI] [PubMed] [Google Scholar]
- 53.Weber DS, Lombard JH. 2000. Elevated salt intake impairs dilation of rat skeletal muscle resistance arteries via ANG II suppression. Am J Physiol Heart Circ Physiol 278:H500–H506. 10.1152/ajpheart.2000.278.2.H500. [DOI] [PubMed] [Google Scholar]
- 54.Weber DS, Lombard JH. 2001. Angiotensin II AT1 receptors preserve vasodilator reactivity in skeletal muscle resistance arteries. Am J Physiol Heart Circ Physiol 280:H2196–H2202. 10.1152/ajpheart.2001.280.5.H2196. [DOI] [PubMed] [Google Scholar]
- 55.Wu CC, Chen SJ, Yen MH. 1992. Effects of noise on blood pressure and vascular reactivities. Clin Exp Pharmacol Physiol 19:833–838. 10.1111/j.1440-1681.1992.tb00422.x. [DOI] [PubMed] [Google Scholar]
- 56.Wu CC, Chen SJ, Yen MH. 1994. Attenuation of endothelium-dependent relaxation in mesenteric artery during noise-induced hypertension. J Biomed Sci 1:49–53. 10.1159/000456765. [DOI] [PubMed] [Google Scholar]
- 57.Yun J, Rocic P, Pung YF, Belmadani S, Carrao AC, Ohanyan V, Chilian WM. 2009. Redox-dependent mechanisms in coronary collateral growth: the “redox window” hypothesis. Antioxid Redox Signal 11:1961–1974. 10.1089/ars.2009.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zymantiene J, Zelvyte R, Pampariene I, Aniuliene A, Juodziukyniene N, Kantautaite J, Oberauskas V. 2017. Effects of long-term construction noise on health of adult female Wistar rats. Pol J Vet Sci 20:155–165. 10.1515/pjvs-2017-0020. [DOI] [PubMed] [Google Scholar]