Abstract
Tricaine methanesulfonate (MS222) is widely used for the anesthesia and euthanasia of laboratory zebrafish. Fresh solutions have been recommended for each use; however, researchers often mix and store concentrated stock solutions for convenience and to reduce occupational exposure and environmental waste. While this is common practice, published guidelines are often inconsistent. Thus, the objective of this study was to evaluate the stability and anesthetic efficacy of MS222 after long-term storage and to develop specific storage parameters. Stock solutions (100 mg/mL MS222) were mixed and stored in amber jars at 4 °C and -20 °C for 2- and 6-mo. Stability of the solutions was analyzed using liquid chromatography-ion trap mass spectrometry and compared with fresh MS222. Fifty adult (30 male, 20 female) wildtype AB zebrafish (Danio rerio) were randomly anesthetized with 150 mg/L of one of the following MS222 solutions to evaluate anesthetic efficacy: 1) freshly prepared (0m); 2) 2 mo at 4 °C (2m4); 3) 2 mo at -20 °C (2m-20); 4) 6 mo at 4 °C (6m4); 5) 6 mo at -20 °C (6m-20). Time to cessation of swimming, loss of equilibrium, lack of response to von Frey (VF) stimulation, return of equilibrium, and resumption of swimming were compared between groups. Two fish from each group were euthanized at 24-h and 2-wk after anesthesia, and histopathology was performed. All solutions were determined to be stable under all storage conditions. No clinically significant differences were observed between the fresh and stored stock groups during anesthetic testing. No evidence of anesthetic-related histologic changes were noted in the gills, skin, kidneys, muscle, and central nervous system. Hepatic megalocytosis and a reduction in hepatic vacuolation were seen to varying degrees across all groups, but did not follow a treatment-related trend. Therefore, 100 mg/mL solutions of MS222 can be stored in amber jars at 4 °C or -20 °C for 6 mo and still used to effectively anesthetize zebrafish.
Abbreviation: MS222, Tricaine Methanesulfonate
Tricaine methanesulfonate (MS222) is the most commonly used anesthetic agent in fish and other aquatic species.13,14,28,29 It is used in research for sample collection, surgical procedures, implanting of radio transmitters or sampling devices, and euthanasia. In aquaculture, it is used for sorting, weighing and measuring, labeling, transporting, gamete collection, and health monitoring.29 MS222 is an ester-type local anesthetic agent that acts systemically when absorbed through the gills or skin.7 Once absorbed, it is distributed throughout the body via the blood to act on the muscle,16 as well as the peripheral and central nervous systems.29 It is highly lipid soluble and readily crosses the cell membrane, where it blocks sodium channels, limiting membrane excitability and action potential transmission.7,26,29 It is primarily excreted unchanged through the gills,29 but is also rapidly metabolized through acetylation7 by the liver, kidneys,7 blood, muscle,16 and/or gills depending on the species.29
A common practice is to prepare and store concentrated stock solutions of MS222 that can be diluted and buffered immediately prior to use.7 This practice reduces time and labor, occupational exposure,7,14,29 and waste. Tricaine-S (Western Chemical, which was used in this study, is now Syndel USA, Ferndale, WA) is currently the only FDA-approved formulation of MS222 for aquaculture.31 Western Chemical recommends storing 10% solutions for up to 3 d in a cool place protected from light.33 They fail to give more specific storage parameters and historically little guidance has been available on the proper storage of MS222.29 Most manufacturers recommend making concentrated stock solutions7 (10 mg/mL to 100 mg/mL)1,3,7,27-29,33that can be further diluted to a specific concentration with water from the fish's environment. Like Western Chemical, many recommend storing solutions in a cool, dark place or opaque container and discarding stock solutions after several days. All manufactures report that solutions are photosensitive and quickly change from clear to yellow or brown when exposed to light.3,7,21,24,27,29,33 Despite this, most claim the solution is stable.3,7,29,33 Others describe the formation of an oily substance on the surface of the solution that forms when water is buffered prior to the addition of the MS222 powder or when the stock solution is exposed to light.28 Cloudiness, darkening, or the presence of an oily substance on the stock solution surface indicate that the solution should be replaced.28 The expiration date of concentrated stock solutions is also confusing. Many report that a 10% solution can be stored at room temperature for up to 3 d with no significant loss of potency, but after 10 d, a brown color and 5% decrease in activity are observed.3,7,29,33Others suggest that solutions may be stable for at least one month1 and potentially effective for up to 3 mo.21 Finally, some suggest that the shelf life of stock solutions can be extended by refrigeration or freezing.28 Despite all of the claims, all manufacturers recommend preparing fresh solutions before use.3,7,24,29,33
These conflicting recommendations make it difficult to establish optimal procedures for the storage of MS222. At our institution, approximately 75% of all approved protocols involving aquatic species use MS222 as an anesthetic or euthanasia agent. Out of 16 protocols approved to use zebrafish, all but one use MS222 in some capacity. The veterinary staff also use it to euthanize sick aquatic species. Thus, the objective of this study was to determine whether MS222 was stable after long-term storage and to identify specific storage parameters for the stock solutions. Chemical analysis using liquid chromatography and mass spectrometry with subsequent anesthetic testing and histologic examination of zebrafish was performed to assess stability. Stored MS222 solutions were compared against freshly prepared solution. We hypothesized that MS222 is chemically stable and remains an effective anesthetic agent when stored for up to 6 mo at less than or equal to 4 °C.
Materials and Methods
Animals and housing.
Adult wildtype AB zebrafish (Danio rerio) (n = 50, 30 males and 20 females; 5-mo-old; Zebrafish International Resource Center, University of Oregon, Eugene, OR) were used for this study. Fish were raised from embryos in Stanford's aquatic facility. Postlarval fish were housed in mixed sex groups of no more than 5 fish per liter in 9.5-L tanks on a recirculating system (Aquaneering, San Diego, CA). System filtration included a 10 µm dacron prefilter, a fluidized bed biofilter with fine sand media, 2 granular activated carbon filters, and a 40 W UV sterilizer (Aquaneering, San Diego, CA). System water was municipal water treated with acid washed granular activated carbon filters (Evoqua Vantage PTC carbon filters), cation resin bed ion exchange water softener (Evoqua Vantage PTC Twin Softeners), and reverse osmosis (Vantage M41 General Purpose Series Reverse Osmosis Units, Evoqua Water Technologies, Pittsburgh, PA). Sodium bicarbonate and sea salt (Instant Ocean, Blacksburg, VA) were added automatically to the system as needed. A 10% water change was performed daily. Water chemistry was maintained at 26 to 28 °C, pH 7.2 to 7.6, conductivity 500 to 900 μS/cm, ammonia less than 0.02 ppm, nitrite less than 0.05 ppm, nitrate less than or equal to 50 ppm, alkalinity 50 to 135 ppm, hardness 75 to 120 ppm, and relative dissolved oxygen (RDO) ≥ 95% in a room with a 14:10 h light:dark cycle. Twice a day fish were fed a diet of brine shrimp (Artemia spp.) and irradiated pellets (Adult Zebrafish Irradiated Diet, Zeigler Bros., Gardners, PA). Although we do not have a formal direct animal disease screening program, both environmental samples from the recirculating system and sentinel fish tested positive for Mycobacterium chelonae and M. fortuitum at the time of the study. Animals were housed in an AAALAC-accredited facility in compliance with the Guide for the Care and Use of Laboratory Animals.11 All animal research was approved by Stanford's Administrative Panel on Laboratory Animal Care (IACUC).
MS222 solution preparation and storage.
Tricaine methanesulfonate (Tricaine-S, Western Chemical; Syndel USA, Ferndale, WA.) 10 mL (100 mg/mL) stock solutions (dissolved in Sterile Water for Irrigation, USP; Baxter Healthcare Corp., Deerfield, IL) were prepared under a chemical fume hood 6- and 2-mo prior to experimental testing. Solutions were prepared in duplicate for each time-point and stored in amber glass jars (Thermo Scientific, Waltham, MA) at 4 °C and -20 °C. Twenty-four hours prior to anesthetic testing, samples stored at -20 °C were thawed at 4 °C. All samples were warmed at room temperatures (24 to 28 °C) 2 h prior to testing. A fresh stock solution was also prepared prior to testing. Thus, the 5 experimental stock solutions included fresh MS222 (0m); MS222 stored for 2 mo at 4 °C (2m4) and -20 °C (2m-20); and MS222 stored for 6 mo at 4 °C (6m4) and -20 °C (6m-20).
Stock solutions were diluted with recirculating system water to a final concentration of 150 mg/L and buffered with sodium bicarbonate USP (Greenbrier International, INC. Chesapeake, VA) to a pH of 7.2 to 7.6 immediately prior to anesthetic testing. Water temperature (°C), pH, conductivity (µS/cm), and RDO (%) were measured with a multiple-parameter portable meter (Orion Star A329, Thermo Fisher Scientific, Waltham, MA). The portable meter was calibrated daily prior to the experiment. All parameters were assessed immediately prior to testing and after every fifth fish.
MS222 chemical analysis and stability.
Liquid chromatography-ion trap mass spectrometry (LC/MS) was performed on all stock solutions after storage by the Vincent Coates Foundation Mass Spectrometry Laboratory at Stanford University (Stanford, CA) prior to any anesthetic testing. A calibration curve was constructed from a freshly prepared MS222 standard stock solution of 3.175 mg/mL salt equivalent (2 mg/mL free base) in water (Sterile Water for Irrigation, USP; Baxter Healthcare Corp., Deerfield, IL). For LC/MS, all MS222 concentrations are expressed as the salt equivalent. The standard stock solution was serially diluted with water to obtain a series of standard calibration solutions that were used to generate the 5-point calibration curve. The curve range was from 3.97 µg/mL to 79.37 µg/mL.
The MS222 stored stock samples (2m4, 2m-20, 6m4, and 6m-20) were serially diluted 5000-fold with water to yield 20 µg/mL solutions. Samples were analyzed by a unified LC-ion trap mass spectrometry method, using an 1100 series high-performance liquid chromatography (HPLC) (Agilent Technologies, Santa Clara, CA) integrated with a LTQ XL ion trap mass spectrometer (Thermo Fisher Scientific, Waltham, MA). MS used heated electrospray ionization (HESI) in positive mode. MS acquisition used full (m/z 100 to 400) and data dependent sacs in dynamic exclusion mode. LC was performed on a 2.1 × 100 mm Hypersil Gold column, 3 µm particle size, with gradient elution. Flow rate was 0.25 mL/min and column temperature was 40 °C. Mobile phases consisted of A: 0.1% formic acid in water and B: 0.1% formic acid in acetonitrile. The elution profile consisted of an initial hold at 10% B for one minute, followed by a gradient of 10% to 95% in 8 min, and a hold at 95% for one minute. Total run time was 12 min. Injection volume was 10 µL. Blank samples were run intermittently during sample analysis.
Quantitative analysis was performed using Thermo Xcalibur Quan Browser (Thermo Fisher Scientific, Waltham, MA) by means of the external standard method. Extracted ion chromatogram (EIC) peak areas (m/z = 166) were used for quantification. The calibration curve was linear (R > 0.99) over the concentration range using a weighted factor of 1/X2 where X is the concentration. The back-calculated standard concentrations were within ± 10% of nominal values for all calibration points.
All stock samples were tested in duplicate after storage. Change in concentration of solution after storage was calculated, [(final concentration-initial concentration)/initial concentration] x 100. The initial concentration was prepared to be 100 mg/mL, but concentration was not confirmed with LC/MS prior to storage. A final concentration of ± 10% was considered stable.
Experimental design.
Fish (n = 50, 30 males, 20 females) were randomly assigned to 5 experimental groups for anesthetic evaluation—0m, 2m4, 2m-20, 6m4, and 6m-20. Sex was not controlled among anesthetic groups and was not evaluated.
Researchers were blind to the experimental groups. The fish were fasted 12 h prior to anesthesia to reduce the risk of regurgitation. All fish were assessed between 0900 and 1300. Each fish underwent only a single anesthetic event. Approximately 1 L of anesthetic solution (150 mg/L MS222) was placed in a static 1.4 L induction tank (Aquaneering, San Diego, CA). A similar static recovery tank was filled with 1 L of system water and replaced between experimental groups. The water temperature, pH, conductivity (µS/cm), and RDO (%) were measured as previously described, in both induction and recovery tanks before testing and after every fifth fish. All fish were video recorded (2016 iPad Pro, Apple, Cupertino, CA) during anesthetic induction and recovery for later evaluation. Zebrafish were moved from their home tank to the induction tank, and then to the recovery tank once a light plane9 of anesthesia was achieved. Fish were monitored throughout induction for signs of distress, such as erratic swimming, increased opercular rate, regurgitation, and piping (gulping air at the top of the tank). Fish in distress were removed from the anesthetic tank and allowed to recover or euthanized. On completion of testing, fish were housed with their experimental groups in 2.8 L tanks on the recirculating system for the remainder of the study.
Anesthesia evaluation.
Zebrafish video recordings were analyzed independently by 4 researchers who were blind to the anesthetic groups. Their scores were then averaged for statistical analysis. The time of all variables was determined from the video timestamp. Once zebrafish were placed in the induction chamber, they were evaluated for the initial opercular rate (operculum movement per minute); time (seconds) to stop swimming, loss of equilibrium, and lack of response to von Frey (VF) monofilament; and the final opercular rate. The initial and final opercular rates were the rates immediately after entry and prior to removal from the induction tank, respectively. They were estimated by counting opercular movement over a period of 1 to 6 s. Time to stop swimming was defined as cessation of all forward purposeful movement. Loss of equilibrium or righting reflex was when the fish was unable to maintain a normal orientation with the dorsal fin upright. A 10 g VF monofilament (Neuropen, Owen Mumford, Marietta, GA) was applied to the caudal fin every 10 s after fish stopped swimming and were resting on the bottom of the tank. Pressure was briefly applied to the fin until the monofilament bent. VF testing was selected to assess anesthetic depth because it provides consistent and reproducible mechanical stimulation. The time at which 2 consecutive VF tests resulted in no response was recorded. Deep pain was not assessed. Therefore, the lack of response to VF, loss of equilibrium, cessation of swimming, and reduced opercular rate was considered a light plane of anesthesia.9 At this time, fish were transferred to the recovery tank. From these recordings, the initial opercular rate was determined in a similar manner as well as the time (seconds) to neutral buoyancy and commencement of swimming. Neutral buoyancy, or the return of the righting reflex, was defined as the ability to maintain normal posture with the dorsal fin up. Time (seconds) to start swimming was when the fish began purposeful, continued forward motion.
Euthanasia and Histopathology.
To evaluate the potential adverse effects associated with the use of stored MS222, all fish were euthanized via rapid cooling, in accordance with the AVMA Guidelines for the Euthanasia of Animals,2 and necropsied. Five fish per group were euthanized at timepoints of either 24-h or 2-wk after anesthesia to evaluate acute and chronic effects, respectively. Five naïve fish from the same cohort were euthanized in a similar manner at the acute timepoint for comparison. After euthanasia, 3 fish per group were stored at -20 °C for ancillary toxicity testing, if warranted. The remaining 2 fish from each group were immersion-fixed in formalin (10% Buffered Formalin Acetate, Fisher Chemical, Fair Lawn, NJ) for 72 h and decalcified for 2 h using Cal-ExII (Fisher Chemical, Fair Lawn, NJ). Zebrafish were sectioned sagitally; sections were processed routinely, embedded in paraffin, sectioned at 5 µm, and stained with hematoxylin and eosin. Select sections were stained with acid-fast (Ziehl-Neelsen) and Gomori's methanamine silver (GMS) stains. Histologic samples were examined by a board-certified veterinary pathologist who was blind to the MS222 experimental groups. See Figure 1 for descriptions of histologic grading criteria.
Figure 1.
Histologic scoring criteria for zebrafish exposed to MS222.
Statistical Analysis.
Anesthetic and water quality variables were averaged and analyzed using one-way ANOVA (RStudio 1.0.136, Boston, MA). For the induction tank, time to stop swimming and time to loss of equilibrium were transformed using natural logarithm. Time until no response to 2 VF was transformed using square root. For the recovery tank, natural logarithm was used to transform the time to neural buoyance. Summary statistics are expressed as the mean ± SEM. A P-value of less than 0.05 was considered statistically significant.
Results
MS222 chemical analysis and stability.
All stock solutions were within 6% of their initial concentrations (Table 1) and therefore were considered stable and safe for subsequent anesthetic testing. No chemical metabolites or contamination was detected with LC/MS and therefore, no additional chemical analysis was pursued.
Table 1.
LC/MS concentrations of MS222 stock solutions after storage for 2- and 6-mo at 4 °C and -20 °C. Prepared concentrations of all stock solutions were initially 100 mg/mL. The change in concentration is the [(mean final concentration-initial concentration) / initial concentration] x 100.
| Sample | Storage conditions | Final measured concentration (mg/mL) | Mean final concentration (mg/mL) | Change in concentration (%) |
| 1 | 2m4 | 105 | 104 | +4% |
| 2 | 103 | |||
| 3 | 2m-20 | 107 | 106 | +6% |
| 4 | 106 | |||
| 5 | 6m4 | 108 | 106 | +6% |
| 6 | 105 | |||
| 7 | 6m-20 | 106 | 106 | +6% |
| 8 | 106 |
Anesthesia evaluation.
All 50 fish were safely anesthetized to a light plane of anesthesia and recovered without complication. None of the fish were removed from the anesthetic tank prematurely or exhibited any signs of distress. Evaluation of the videos for both initial and final opercular rates indicated that the recordings were not captured at a frame rate necessary for such high opercular rates. Post-acquisition software processing further reduced the frame rates, which varied among all videos captured. Therefore, initial and final opercular rates could not reliably be determined and were not analyzed.
Evaluation of the other anesthetic parameters showed that the time to stop responding to 2 VF tests in zebrafish anesthetized with MS222 that was stored for 2m4 was significantly longer than that stored for 2m-20 or for 6m-20. In addition, the time to neutral buoyancy was significantly longer with MS222 stored for 2m4 than for 6m-20 (Table 2). No statistically significant differences were detected for water temperature, pH, conductivity, or RDO in the induction tank or recovery tank for all MS222 treatment groups (data not shown). All fish remained clinically normal after anesthesia.
Table 2.
Anesthetic evaluation. Fish were anesthetized in solutions of MS222 that were diluted to a concentration of 150 mg/L and buffered with sodium bicarbonate. Anesthetic solutions were prepared from stock solutions that were stored for 2- and 6-mo at 4 °C and -20 °C. A fresh solution was also prepared (0m). Fish were evaluated for time (s) to stop swimming (cessation of forward purposeful movement), loss of equilibrium (dorsal fin no longer upright), and no response to 2 consecutive von Frey (VF) applications to the tail fin. Fish were considered under a light plane of anesthesia and transferred to a recovery tank with fresh water. Time (seconds) to neutral buoyance (dorsal fin upright) and to start swimming were measured. All times expressed in mean ± SEM. *, aStatistically significant (P-value <0.05).
| MS222 Treatment | |||||||
| Time (s) | 0m (n = 10) | 2m4 (n = 10) | 2m-20 (n = 10) | 6m4 (n = 10) | 6m-20 (n = 10) | P | |
| Induction Tank | Stop swimming | 21.0 ± 18.9 | 19.9 ± 13.1 | 14.4 ± 9.5 | 21.68 ± 8.84 | 13.7 ± 7.6 | 0.30 |
| Loss of equilibrium | 28.6 ± 10.4 | 29.5 ± 8.7 | 27.4 ± 8.5 | 27.9 ± 5.2 | 26.3 ± 7.5 | 0.916 | |
| No response to 2 VF | 44.1 ± 14.5 | 54.9 ± 9.7* | 40.0 ± 8.2* | 45.6 ± 6.7 | 39.6 ± 7.70* | 0.006 | |
| Recovery Tank | Neutral buoyance | 49.2 ± 10.7 | 90.8 ± 48.4a | 43.9 ± 40.8 | 56.3 ± 32.4 | 34.7 ± 34.6a | 0.007 |
| Start swimming | 125.5 ± 37.6 | 133.8 ± 28.7 | 118.7 ± 58.9 | 110.7 ± 22.0 | 96.9 ± 24.7 | 0.228 | |
Histopathology.
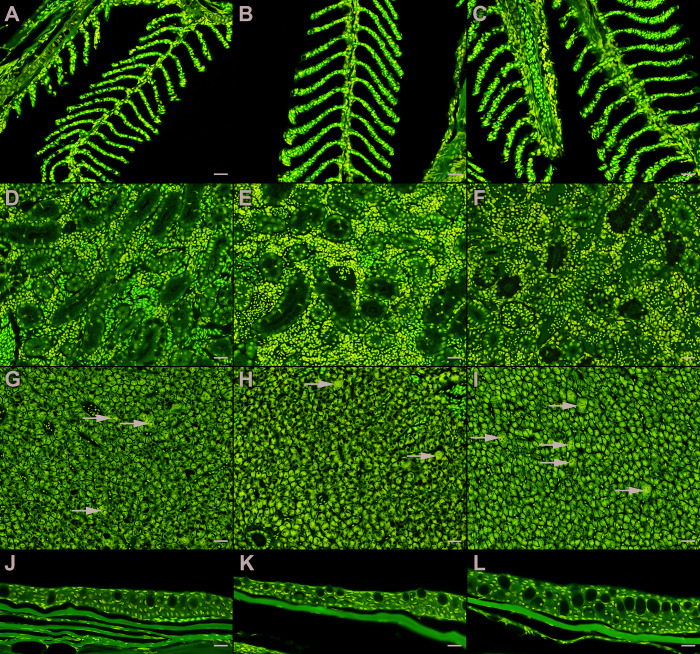
Twenty-two zebrafish (8 males, 14 females) were examined histologically for acute and chronic effects of MS222 anesthesia after various storage conditions. None of the zebrafish exhibited histologic lesions in the gills or skin, which are the sites of MS222 absorption. Furthermore, no histologic lesions were found in the kidneys (site of metabolism), muscle (site of metabolism and target organ), or central nervous system (target organ) (Figure 2).
Figure 2.
Histopathology of select MS222 target-organs from zebrafish. Hematoxylin and eosin, scale bar = 20 µm. Gills (A through C), kidneys (D through F), liver (G through I), and ventral coelomic skin (J through L) of naïve zebrafish (A, D, G, J) were histologically indistinguishable from zebrafish exposed to fresh MS222 (B, E, H, K) and MS222 stored at -20 °C for 6 mo (C, F, I, L). Hepatic megalocytosis (arrows) was seen across all treatment groups and did not follow a treatment-related trend.
Varying degrees of hepatic megalocytosis were seen across all groups and euthanasia timepoints (Table 3); however, severity did not follow a treatment-related trend. In total, 20/22 zebrafish exhibited some degree of hepatic megalocytosis, including control fish. The two unaffected fish were in the 2m4 and 6m4 treatment groups. Hepatic megalocytosis was defined as increased nuclear (karyomegaly) and cell (cytomegaly) size. In addition, a reduction in hepatocellular vacuolation was seen in 12 of the 20 anesthetized zebrafish. Of those 12, only one lacked a concurrent histologic abnormality, as described below.
Table 3.
Histologic changes for each MS222 storage condition for acute (24 h) and chronic (2 wk) timepoints. Hepatic megalocytosis, 0 = absent; 1 = minimal (<10 hepatocytes); 2 = mild (10 to 19 hepatocytes); 3 = moderate (20 to 50 hepatocytes); 4 = severe (>50 hepatocytes). Liver vacuolation, 0 = within normal limits; 1 = >75% reduction in vacuolation. Egg-associated inflammation, 0 = absent; 1 = mild (<25% of ovary); 2 = moderate (25 to 50% of ovary); 3 = severe (>50% of ovary).
| Fish | Sex | MS222 storage conditions | Euthanasia timepoint | Hepatic megalocytosis | Reduced hepatic vacuolation | Egg-associated inflammation | Granulomatous inflammation | Acid fast stain |
| 1 | F | 0m | acute | 1 | 1 | 2 | — | negative |
| 2 | F | 0m | acute | 2 | 1 | 2 | — | negative |
| 3 | F | 0m | chronic | 2 | 0 | 1 | — | negative |
| 4 | M | 0m | chronic | 1 | 1 | — | — | — |
| 5 | F | 2m4 | acute | 3 | 1 | 3 | — | negative |
| 6 | M | 2m4 | acute | 0 | 0 | — | — | — |
| 7 | F | 2m4 | chronic | 1 | 1 | 2 | coelom, pancreas, intestine [severe] | negative |
| 8 | M | 2m4 | chronic | 2 | 0 | — | — | — |
| 9 | F | 2m-20 | acute | 1 | 1 | 3 | — | negative |
| 10 | M | 2m-20 | acute | 1 | 1 | — | pancreas [mild] | negative |
| 11 | M | 2m-20 | chronic | 1 | 0 | — | — | — |
| 12 | F | 2m-20 | chronic | 2 | 0 | 0 | — | — |
| 13 | F | 6m4 | acute | 0 | 1 | 3 | — | negative |
| 14 | F | 6m4 | acute | 1 | 0 | 1 | — | negative |
| 15 | M | 6m4 | chronic | 1 | 0 | — | pancreas [mild] | negative |
| 16 | F | 6m4 | chronic | 1 | 0 | 3 | — | negative |
| 17 | F | 6m-20 | acute | 1 | 1 | 3 | — | negative |
| 18 | M | 6m-20 | acute | 3 | 1 | — | coelom [mild] | positive |
| 19 | F | 6m-20 | chronic | 2 | 1 | 1 | — | negative |
| 20 | F | 6m-20 | chronic | 3 | 1 | 1 | — | negative |
| 21 | M | naive | acute | 1 | 0 | — | — | — |
| 22 | F | naïve | acute | 3 | 0 | 2 | — | negative |
Concurrent histologic abnormalities were seen within all treatment groups, but did not follow a treatment-related trend. Of the female fish examined across all anesthetic groups, 13/14 had egg-associated inflammation of the ovary (EAI). Egg-associated inflammation was defined as a combination of atretic or degenerative oocytes, granulomatous infiltrate, and/or fibroplasia. Four fish had granulomatous inflammation (mild n = 3; severe n = 2) of at least one of the following organs: pancreas, coelom, or intestine. Slides from all zebrafish with EAI or granulomatous inflammation were subsequently stained with acid fast (Ziehl-Neelsen) and GMS stains. One zebrafish (6m-20) was acid-fast positive, which is consistent with Mycobacterium spp infection. None of the fish were GMS-positive. Detritus samples from the recirculating system that previously housed the fish were submitted for pathogen testing by PCR and found to be positive for M. chelonae and M. fortuitum and negative for M. abscessus, M. haemophilum, M. marinum, and M. peregrinum (Mycobacterium Panel, Charles River Laboratories, Portland, ME).
Discussion
Our data shows that MS222 is a stable and effective agent for zebrafish anesthesia when stored at a concentration of 100 mg/mL in opaque, glass jars at 4 °C and -20 °C for up to 6 mo. This is evident in the chemical analysis, which showed that all stock solutions were within 6% of the initial concentration after storage. Fish were safely and effectively anesthetized (150 mg/L) with all stored MS222 solutions without evidence of histologic toxicity.
LC/MS analysis showed that all stock samples increased in concentration on average by 6%. However, one sample increased up to 8%. Because they were considered stable, the minimal increase in concentration was not accounted for when preparing the anesthetic solutions for testing. The stock solutions ranged from 103 to 108 mg/mL. Thus, the concentration of the diluted anesthetic solution was actually 154 to 162 mg/L. This is below the lethal concentration 50% (LD50) of zebrafish, which varies from 171 ± 7 mg/L to 216 ± 4 mg/L.23
The apparent increase in concentration is likely the result of the different analytical scales used to weigh MS222 powder. The same scale was used for preparation of the stock solutions. MS222 was measured in triplicate to within 0.5 mg. Fresh samples used to generate the standard curve were prepared by the Mass Spectrometry Laboratory using different and more precise equipment. This difference illustrates the importance of precise measurement in anesthetic preparation. Samples were stored in sealed glass jars and immediately frozen after preparation and thawed in a uniform manner prior to use; however minor evaporation or sublimation cannot be ruled out. To definitively determine whether the concentration increased with storage would require new samples prepared using the same equipment, with LC/MS chemical analysis performed on the same sample both before and after storage.
Zebrafish that were anesthetized in MS222 stored for 2 mo at 4 °C (55 ± 10 s) took a significantly longer time to reach a light plane of anesthesia than did fish anesthetized with MS222 stored for 2 mo at -20 °C (40 ± 8 s) or for 6 mo at -20 °C (40 ± 8 s). On average, fish in the latter 2 groups required 15 s longer to stop responding to VF. Fifteen seconds longer is clinically insignificant when anesthetizing fish. As expected, the 2m4 cohort was slower to recover due to the prolonged exposure to MS222. Time to neutral buoyancy was significantly longer in the 2m4 cohort than in the cohort anesthetized with MS222 stored for 6 mo at -20 °C. The difference between the 2 cohorts on average is 56.1 s slower for the 2m4 group.
The statistically significant findings for the 2m4 cohort of fish is likely related to testing order. As previously described, all researchers were blind to treatment groups prior to anesthetic testing and video evaluation. The anesthetic solution tested wasrandomly selected; however, all fish within the same anesthetic cohort were tested in succession, so treatment was not randomized between each animal. Disclosing the anesthetic groups revealed that the 2m4 was the first group tested and scored. Data acquisition improved over time as researchers became more skilled at identifying stages of anesthesia, performing VF testing, and transferring fish between tanks. Therefore, the statistically significant effects in the 2m4 cohort are likely due to systematic error in our experimental design. All anesthetic solutions (fresh and stored) should have been prepared and both the fish and anesthetic solution selected at random. This method would have been much more cumbersome, but would have removed bias of testing order.
The latency to stop swimming and loss of equilibrium were more objective measures that could be reliably determined via video analysis. Those variables were statistically similar between fresh MS222 and all stored MS222 groups, supporting the hypothesis that the MS222 remained stable after storage and still consistently induced anesthesia.
The anesthetic induction and recovery variables for the stored MS222 solutions are consistent with those reported in the literature. A study using 150 mg/L of MS222 reported that the latency to loss of equilibrium was 111 ± 92 s and recovery time was 140 ± 51 s.9 This reported latency to loss of equilibrium appears prolonged compared to ours (Table 2); however, the loss of equilibrium was recorded after the fish remained inverted, with their ventrum oriented toward the surface, for more than 3 s.9 We marked the loss of equilibrium as the start at which the fish was unable to maintain an upright orientation, which by definition is shorter. In addition, those zebrafish were 3-mo-old and were anesthetized with Finquel MS222,9 which is no longer available. The time to recovery was defined as the time from which the fish was placed in the recovery tank of fresh tank water until it swam upright for at least 5 s.9 This is similar to our marker of time to start swimming.
Another study of 8-mo-old male AB zebrafish reports a similar latency to loss of equilibrium of 23 ± 4 s in fish anesthetized with Finquel at a concentration of 168 mg/L.18 Because chemical analysis showed that our fish were anesthetized with up to 162 mg/L of Tricaine-S, the findings from these 2 studies may be comparable. Anesthetic depth was determined by response to a tail pinch using 2 fingers, and they reported a latency of loss of response to tail pinch of 102 ± 27 s. The previous study reported a lack of response to tail-fin pinch at 252 ± 89 s.9 A deeper plane of anesthesia and longer latency would be necessary to abolish a response to tail pinch. Prolonged exposure required to achieve such a plane would lead to longer recovery times, which were 92 ± 54 s.18
Overall, no histologic lesions were detected in MS222-related organs including the gills, skin, kidney, muscle, and central nervous system. The main histologic findings included EAI of the ovary, hepatic megalocytosis, and a reduction in hepatic vacuolation. When present, these pathologic findings were distributed across all storage conditions and euthanasia time points and were also seen in naïve fish. Thus, they cannot be attributed to MS222, either fresh or stored.
All but one of the female fish examined by histology had EAI. EAI is the result of egg retention and degeneration that leads to chronic inflammation of the ovary with occasional extension into the visceral cavity.12,15 In a report of zebrafish ovarian pathology, EAI was found in 42% of 59 3-mo-old fish.22 Although EAI was higher in our fish (93%), this may be partly attributable to their greater age (5 mo). Moreover, the previous study used fish that were regularly bred.22
Hepatic megalocytosis, which varied in severity from minimal to moderate, was found in all but 2 fish, including naïve controls. Megalocytosis is the result of the failure of hepatocytes to mitotically divide, due to sublethal hepatocellular injury.34 It is a subclinical histologic diagnosis with a variety of potential underlying etiologies, including toxicity.12
Reduced hepatic vacuolation was noted in just over half of the anesthetized zebrafish (12/20). Fish hepatocytes are more vacuolated than those of mammalian species as the result of a relatively higher glycogen and/or lipid content. A common morphologic response of fish hepatocytes to toxicity is loss of glycogen and/or lipid, which manifests histologically as a loss of vacuolization. Such loss can be the direct result of intoxication or may occur secondary to poor body condition caused by inanition, stress, or concurrent disease. Further complicating interpretation, toxicity can also result in accumulations of lipid or glycogen in the liver.34
Reduction in hepatocellular vacuolation as a result of sublethal MS222 exposure cannot be definitively excluded, as it only occurred in anesthetized zebrafish. However, of the 12 anesthetized fish with reduced hepatic vacuolation, only one lacked other concurrent histologic disease (EAI, granulomatous disease, and/or mycobacteriosis). Furthermore, 3 of 4 fish exposed to fresh MS222 also had reduced vacuolation. The reduced hepatic vacuolation is likely the result of concurrent histologic disease (EAI, granulomatous disease, and/or mycobacteriosis). Environmental PCR screening was positive for both M. chelonae and M. fortuitum, and histology of an experimental fish was acid-fast positive, which is consistent for mycobacteria. M. chelonae can be transmitted between zebrafish and biofilm,8 which could have occurred in our fish. However, additional molecular and histologic analysis is required to better determine the overall health status of our fish.
Limitations of this study include evaluation of a low number of stored stock solutions. Samples were prepared in duplicate for storage and subsequent analysis, and insufficient samples were available to perform statistical analysis of the change in MS222 concentration after storage for each timepoint and parameter. However, the fact that all samples stored up to 6 mo remained stable supports the stability of the samples at 2 mo.
Another limitation is a short storage duration. Samples were only stored and tested up to 6 mo. Samples may remain stable for longer periods, but such conclusions cannot be determined without analyzing additional samples. Our findings are also limited in their application. Refrigeration to store stock solutions is readily available in the laboratory setting but is not practical for field work. Determining if solutions remained stable at higher temperatures, such as room temperature (20 to 25 °C), would provide greater flexibility to the user.
Despite the limitations in application, this study is important because it suggests that MS222 remains stable and effective after long-term storage. This is important to researchers in our facility because it reduces waste, preparation time, and occupational exposure. Solutions of MS222 are reported to be safe at commonly used concentrations.21,27However, the claim that the powder is retinotoxic7,29 is pervasive in the literature. A case report describes reversible retinal toxicity in an ichthyologist with over 30 y of occupational exposure from immersion of ungloved hands.5 He presented with decreased vision, photophobia, and photopsia. His ocular exam was unremarkable, but his electroretinogram (ERG) was similar to those of frogs’ eye cups exposed to MS222 in vitro.4,5,20 With discontinued use, both his symptoms and ERG markedly improved.4,5,20 A few reports claim that MS222 is a carcinogen,19,29 but the consensus in the literature is that it is not.1,6 These claims may have originated from carbisocaine, a similar ester-type local anesthetic agent that has genotoxic activity.6 According to the Globally Harmonized System (GHS) Classification and Labeling of Chemicals30 from 28 companies, MS222 may cause skin, eye, or respiratory irritation or serious damage.10,17It is not labeled as a carcinogen.17 Users should avoid breathing the aerosolized powder or vapors. Solutions should be prepared within chemical fume hoods or in well-ventilated areas such as outside. Proper use of PPE is important, especially when handling the powder and working with solutions. Recommended PPE includes nitrile rubber gloves or other impervious skin protection,25 eye25 and/or face protection, and N9532 masks or respirators.25,32
In conclusion, concentrated stock solutions (100 mg/mL) of MS222 (Tricaine-S) were found to be chemically stable and effective for the anesthesia of zebrafish when stored at 4 °C or -20 °C for up to 6 mo. The authors found no clinically significant differences between naïve zebrafish and those anesthetized with fresh or stored MS222. Likewise, no histologic signs of toxicity attributable to storage were detected. Although the best practice is to prepare fresh solutions, our findings indicate that 100 mg/mL stock solutions of Tricaine-S may be stored in amber or opaque glass containers for up to 6 mo at 4 °C or -20 °C. Dilute buffered anesthetic solutions should be prepared immediately prior to use.
Acknowledgments
The authors thank Dr Ludmila Alexandrova of the Vincent Coates Foundation Mass Spectrometry Laboratory at Stanford University for her chemical analysis and help in preparing this manuscript. We would also like to thank the Stanford APLAC for providing the statistics on protocols approved to use MS222. We thank Stanford Animal Histology Services for their help with preparation of histologic specimens. We also thank Sayed Ahmadi and the Stanford Veterinary Service Center animal care staff for their help with this study.
This work was supported in part by NIH P30 CA124435 utilizing the Stanford Cancer Institute Proteomics/Mass Spectrometry Shared Resource.
References
- 1.ALPHARMA Animal Health Ltd. [Internet]. 2001. MS222 (TRICAINE METHANE SULPHONATE). Technical bulletin. [Cited 9 April 2018]. Available at: http://www.tossehuset.dk/pdf/MS222.pdf.
- 2.American Veterinary Medical Association. [Internet]. 2013. Guidelines for the euthanasia of animals: 2013 Edition. [Cited 9 April 2018]. Available at: https://www.avma.org/KB/Policies/Pages/Euthanasia-Guidelines.aspx.
- 3.Argent Chemical Laboratories. 2014. FINQUEL MS-222. Package Insert for NADA 042-427.Redmond (WA): ARGENT Chemical Laboratories. [Google Scholar]
- 4.Bailey KM, Hempstead JE, Tobias JR, Borst LB, Clode AB, Posner LP. 2013. Evaluation of the effects of tricaine methanesulfonate on retinal structure and function in koi carp (Cyprinus carpio). J Am Vet Med Assoc 242:1578–1582. 10.2460/javma.242.11.1578. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein PS, Digre KB, Creel DJ. 1997. Retinal toxicity associated with occupational exposure to the fish anesthetic MS-222. Am J Ophthalmol 124:843–844. 10.1016/S0002-9394(14)71705-2. [DOI] [PubMed] [Google Scholar]
- 6.Braz MG, Karahalil B. 2015. Genotoxicity of anesthetics evaluated in vivo (animals). BioMed Res Int 2015:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carter KM, Woodley CM, Brown RS. 2011. A review of tricaine methanesulfonate for anesthesia of fish. Rev Fish Biol Fish 21:51–59. 10.1007/s11160-010-9188-0. [DOI] [Google Scholar]
- 8.Chang CT, Lewis J, Whipps CM. 2019. Source or sink: examining the role of biofilms in transmission of Mycobacterium spp. In laboratory zebrafish. Zebrafish 16:197–206. 10.1089/zeb.2018.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collymore C, Tolwani A, Lieggi C, Rasmussen S. 2014. Efficacy and safety of 5 anesthetics in adult zebrafish (Danio rerio). J Am Assoc Lab Anim Sci 53:198–203. [PMC free article] [PubMed] [Google Scholar]
- 10.European Chemicals Agency. [Internet]. 2015. Substance information: 3-ethoxycarbonylanilinium methanesulphonate. [Cited 17 April 2018]. Available at: https://echa.europa.eu/substance-information/-/substanceinfo/100.011.779.
- 11.Institute for Laboratory Animal Research. 2011. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press. [Google Scholar]
- 12.Kent ML, Spitsbergen JM, Mathews JM, Fournie JW, Murray KN, Westerfield M. [Internet]. 2016. Diseases of zebrafish in research facilities. [Cited 31 March 2019]. Available at: https://zebrafish.org/wiki/health/disease_manual/start.
- 13.Lidster K, Readman GD, Prescott MJ, Owen SF. 2017. International survey on the use and welfare of zebrafish Danio rerio in research. J Fish Biol 90:1891–1905. 10.1111/jfb.13278. [DOI] [PubMed] [Google Scholar]
- 14.Martins T, Valentim AM, Pereira N, Antunes LM. 2016. Anaesthesia and analgesia in laboratory adult zebrafish: A question of refinement. Lab Anim 50:476–488. 10.1177/0023677216670686. [DOI] [PubMed] [Google Scholar]
- 15.Matthews JL. 2004. Common diseases of laboratory zebrafish, p 617–643. In: Detrich HW, III, Westerfield M, Zon LI, editors. The zebrafish: genetics, genomics, and informatics: Methods in cell biology, vol 77, 2nd ed San Diego (CA): Academic Press. [DOI] [PubMed] [Google Scholar]
- 16.Matthews M, Varga ZM. 2012. Anesthesia and euthanasia in zebrafish. ILAR J 53:192–204. 10.1093/ilar.53.2.192. [DOI] [PubMed] [Google Scholar]
- 17.National Center for Biotechnology Information. [Internet]. 2020. Tricaine methanesulfonate. PubChem. [Cited 17 April 2018]. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/261501.
- 18.Nordgreen J, Tahamtani FM, Janczak AM, Horsberg TE. 2014. Behavioral effects of the commonly used fish anaesthetic tricaine methanesulfonate (MS-222) on zebrafish (Danio rerio) and its relevance for the acetic acid pain test. PLoS One 9:1–6. 10.1371/journal.pone.0092116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pirhonen J, Schreck CB. 2003. Effects of anaesthesia with MS-222, clove oil and CO2 on feed intake and plasma cortisol in steelhead trout (Oncorhynchus mykiss). Aquaculture 220:507–514. 10.1016/S0044-8486(02)00624-5. [DOI] [Google Scholar]
- 20.Rapp LM, Basinger SF. 1982. The effects of local anaesthetics on retinal function. Vision Res 22:1097–1103. 10.1016/0042-6989(82)90073-6. [DOI] [PubMed] [Google Scholar]
- 21.Ross LG, Ross B. 2008. Anaesthesia of fish: I. Inhalation anesthesia, p 69–126. In: Ross LG, Ross B, editors. Anaesthetic and sedative techniques for aquatic animals, 3rd ed. Ames (IA): Blackwell Publishing. [Google Scholar]
- 22.Rossteuscher S, Schmidt-Posthaus H, Schafers C, Teigeler M, Segner H. 2008. Background pathology of the ovary in a laboratory population of zebrafish Danio rerio. Dis Aquat Organ 79:169–172. 10.3354/dao01893. [DOI] [PubMed] [Google Scholar]
- 23.Sánchez-Vázquez FJ, Terry MI, Felizardo VO, Vera LM. 2011. Daily rhythms of toxicity and effectiveness of anesthetics (MS222 and Eugenol) in zebrafish (Danio rerio). Chronobiol Int 28:109–117. 10.3109/07420528.2010.538105. [DOI] [PubMed] [Google Scholar]
- 24.Sigma–Aldrich. [Internet]. 1996. 3-Aminobenzoic acid ethyl ester methanesulfonate sigma prod. no. A5040. Product information sheet. [Cited 10 April 2018] Available at: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma-Aldrich/Product_Information_Sheet/1/a5040pis.pdf
- 25.Sigma-Aldrich. [Internet]. 2016. Ethyl 3-aminobenzoate methanesulfonate. Safety data sheet. Version 4.4. [Cited 10 April 2018]. Available at: https://www.sigmaaldrich.com/MSDS/MSDS/PrintMSDSAction.do?name=msdspdf_1908223190208244
- 26.Spears J, Kamunde C, Stevens ED. 2014. Effect of TRIS and bicarbonate as buffers on anesthetic efficacy of tricaine methane sulfonate in zebrafish (Danio rerio). Zebrafish 11:590–596. 10.1089/zeb.2014.0975. [DOI] [PubMed] [Google Scholar]
- 27.Stetter MD. 2001. Fish and amphibian anesthesia. Vet Clin North Am Exot Anim Pract 4:69–82. 10.1016/S1094-9194(17)30052-X. [DOI] [PubMed] [Google Scholar]
- 28.Stoskopf M, Posner LP. 2008. Anesthesia and restraint of laboratory fish, p 519–534. In: Fish RE, Brown MJ, Danneman PJ, Karas AZ, editors. Anesthesia and analgesia in laboratory animals, 2nd ed Burlington (MA): Academic Press. [Google Scholar]
- 29.Topic Popovic N, Strunjak-Perovic I, Coz-Rakovac R, Barisic J, Jadan M, Persin Berakovic A, Sauerborn Klobucar R. 2012. Tricaine methane-sulfonate (MS-222) application in fish anaesthesia. J Appl Ichthyol 28:553–564. 10.1111/j.1439-0426.2012.01950.x. [DOI] [Google Scholar]
- 30.United Nations. 2011. Globally harmonized system of classification and labelling of chemicals (GHS), 7th ed New York (NY): United Nations. [Google Scholar]
- 31.US Food Drug Administration. [Internet]. 2019. Approved Aquaculture Drugs. [Cited 1 August 2019]. Available at: https://www.fda.gov/animal-veterinary/aquaculture/approved-aquaculture-drugs.
- 32.Western Chemical. [Internet]. 2015. TRICAINE-S. Safety data sheet. [Cited 9 April 2018]. Available at: https://www.syndel.com/downloads/dl/file/id/1/tricaine_s_sds.pdf.
- 33.Western Chemical. [Internet]. 2016. TRICAINE-S. Directions for use. [Cited 9 April 2018]. Available at: https://www.syndel.com/downloads/dl/file/id/3/tricaine_s_directions_for_use.pdf.
- 34.Wolf JC, Wolfe MJ. 2005. A brief overview of nonneoplastic hepatic toxicity in fish. Toxicol Pathol 33:75–85. 10.1080/01926230590890187. [DOI] [PubMed] [Google Scholar]