Abstract
Untreated articular cartilage damage normally results in osteoarthritis and even disability that affects millions of people. However, both the existing surgical treatment and tissue engineering approaches are unable to regenerate the original structures of articular cartilage durably, and new strategies for integrative cartilage repair are needed. Gene therapy provides local production of therapeutic factors, especially guided by biomaterials can minimize the diffusion and loss of the genes or gene complexes, achieve accurate spatiotemporally release of gene products, thus provideing long-term treatment for cartilage repair. The widespread application of gene therapy requires the development of safe and effective gene delivery vectors and supportive gene-activated matrices. Among them, polymeric biomaterials are particularly attractive due to their tunable physiochemical properties, as well as excellent adaptive performance. This paper reviews the recent advances in polymeric biomaterial-guided gene delivery for cartilage repair, with an emphasis on the important role of polymeric biomaterials in delivery systems.
Keywords: Cartilage repair, Gene therapy, Polymeric biomaterials, Delivery vectors, Gene-activated matrices
Graphical abstract
Highlights
-
•
Comprehensive overview on recent advancements of polymeric biomaterials-based gene delivery for cartilage repair.
-
•
The important role of polymeric biomaterials in gene delivery systems for cartilage repair has been discussed.
-
•
The challenges and opportunities for the engineering of functional cartilage tissues using gene activated polymeric biomaterials and clinical transformation problems were highlighted.
1. Introduction
Articular cartilage provides a low friction interface and mechanical support for articular joints, which is necessary to maintain normal human activities [1,2]. Nowadays, the incidence of cartilage destruction caused by trauma, degenerative diseases is increasing, and if it is left untreated, osteoarthritis (OA) can be resulted, leading to great pain, motor dysfunction and even disability that affects millions people [3]. However, articular cartilage possesses poor self-healing capacity due to the lack of blood vessels and connection with the subchondral bone, thus the chondral defects unable to be accessed by bone-resident stem cells [4].
At present, the clinical treatment strategies for cartilage defects include bone marrow stimulation techniques, autologous/allograft, periosteum transplantation and others [5,6]. However, these treatments are often accompanied by surgical trauma and poor long-term efficacy. Besides, there are some other issues such as the limited availability of donor sites and immune rejection. All these drawbacks led to the emergence of tissue engineered cartilage grafts, which have given many promising results and tremendous progress. However the generation of ideal biomimetic synthetic chondral and osteochondral replacements is still elusive, and in fact, many challenges remain [7,8]. For example, 1) the recombinant proteins used in tissue engineering are expensive and have a short half-life; 2) the unstable generation of chondrocyte phenotypes and the mechanical properties of engineered cartilage are hardly consistent with peripheral cartilage, which may vary greatly with age, weight, or tissue location; 3) it is difficult to achieve simultaneous differentiation into bone and cartilage, resulting in ineffective integration with surrounding normal tissue and subchondral bone.
Physiological gene therapy might overcome these barriers because it allows to continuously express therapeutic gene products, generate intricate structure of the osteochondral unit at the defect and functionally promote the formation of new cartilage [9,10]. However, direct injection of genes or gene complexes into the joint is limited by the fixed action sites, dilution of synovial fluid and apoptosis of transfected cells [8,11]. Biomaterials-guided gene delivery thus has been adapted to improve the spatiotemporal effects of gene products, as biomaterials can protect the therapeutic genes from degrading of enzymes, also allow them being delivered gradually and controllably. In recent years, subcategories of biomaterials used in gene delivery systems have been developed, typically including inorganic nanoparticles, liposomes and cationic polymers, of which the latter two are often regarded as the gold standards for gene delivery. Although not as extensively utilized as lipid systems, polymeric biomaterials have exhibited considerable potential to be used as both delivery vectors and supporting scaffolds to enhance the processes of cartilage repair by regulating endogenous chondrogenesis spatiotemporally [12].
This review thus focuses on the current progresses on application of polymeric biomaterial-guided gene delivery for cartilage repair. In particular, the design strategies and important roles of polymer materials on gene therapy for cartilage repair are highlighted. Finally, we discussed the current challenges and opportunities for the engineering of functional cartilage tissues using gene activated polymeric biomaterials.
2. Polymeric gene delivery vectors for cartilage repair
Because nucleic acids require effective cell uptake to pass through cell membrane and cytoplasm for efficient transfection and expression, vectors are required for effectively delivering gene of interest for cartilage regeneration [8]. As a bridge of exogenous target genes enter cells to induce cartilage regeneration in situ, the vectors in delivery system directly determine the success or failure of treatment. According to a recent study about intrinsic effects of gene delivery vectors including cationic polymers (polyethylenimine, PEI), inorganic nanoparticles (nanohydroxyapatite, nHA) and amphipathic peptides (RALA peptide) on mesenchymal stem cells (MSC) differentiation, although similar transfection efficiencies were shown, different vectors had significantly different effects on MSC viability, cytoskeleton morphology, and differentiation [13]. Earlier studies have also demonstrated that the selection of vectors has an effect on the activity of gene products [14]. And inappropriate delivery vectors can cause undesired phenotypic changes and off-target effects, leading to the failure of gene therapy or even more serious consequences. The intrinsic properties of vectors are the key factors of effective gene delivery, based on which they can be divided into a variety of categories.
Gene delivery vectors are generally divided into virus-guided or synthetic non-viral [15]. Viruses are commonly used for gene delivery because of their inherent ability to transfer genes efficiently and to express foreign genes continuously [16]. At present, lentiviral and adenoviral vectors are commonly used in cartilage gene therapy, although other viral vectors such as herpes simplex virus and retrovirus are also reported [7]. However, their clinical application is restricted due to low carrying capacity, high cost of large-scale production, and safety concerns including strong immunogenicity and carcinogenicity [17]. In comparison to viral gene delivery, the synthetic non-viral approach can be more advantageous due to temporal transfection, ease of production, safety and low immunogenicity [18]. At present, there are mainly subcategories of non-viral gene vectors: inorganic nanoparticle, liposome-based and synthetic polymer-based vectors. Nanosized metal or inorganic vectors with large surface area have some advantages including facile production, functionalization and stability. However, metal nanoparticles can easily accumulate in cells and affect cell growth due to their stability, while inorganic vectors are limited by the low transfection efficiency and potential cytotoxicity [19]. Cationic lipids have a wide size range, high DNA loading capacity and storage stability [20]. Many lipid-based transfection reagents are commercially available, typically lipofectamine 2000 [21]. However, the short-term transgene expression levels and cytotoxicity they have limit their applications [22]. Compared to liposomes, polymersomes have enhanced variability and stability through the regulation of synthesis process [23]. Polymeric vectors are easy to manufacture and modify to achieve desirable physiochemical properties. There are growing evidences that gene delivery using synthetic polymers for cartilage repair is a promising option for cartilage repair (Table 1). More details about gene delivery non-polymeric vectors can refer to related reviews [[17], [18], [19],[24], [25], [26]]. In the following section, we briefly introduce the polymeric vectors involved in gene delivery for cartilage repair, with more focus on polymer-mediated.
Table 1.
Polymeric gene delivery vectors used for cartilage repair.
| Forms | Polymer vectors | Transfected cells | Approach | Size /nm |
Genes | Ref |
|---|---|---|---|---|---|---|
| Nanoparticles | HA/CS | Chondrocytes | vitro | 100–300 | TGF-β1 pDNA | [27] |
| pmPPY | MEFs | vitro | 76.64 | SOX9 -pDNA | [28] | |
| PEI/PLGA | hMSCs | vitro | 80 | SOX9 pDNA plus cbfa-1 siRNA | [29] | |
| PEI/PLGA | hMSCs | vitro | 139.5 | Polycistronic SOX5,SOX6, and SOX9 | [30] | |
| CAP-PEI | Rats | intra-articular injection | 50 | Luciferase gene | [31] | |
| TMC | rBMSCs | vitro | 120 | TGF-β1 pDNA | [32] | |
| TMC | ASCs | vitro | 110 | TGF-β1 pDNA | [33] | |
| Nanogels | HA/PEI | hMSCs | vitro | 70–150 | SOX9 pDNA | [34] |
| Nanomicelles | PEG-PAsp(DET) | Mouse | intra-articular injection | 50 | RUNX1 mRNA | [35] |
| PEO-b-PLL | rBMSCs | vitro | 30 | TGF-β1 pDNA | [36] |
Abbreviations: HA, hyaluronic acid; CS, chitosan; PEI, polyethylenimine; pmPPY, PEI-modified polysaccharides of porphyra yezoensis; PLGA, poly(lactide-co-glycolic acid); CAP, cartilage-specific peptide; TMC, N,N,N-trimethyl chitosan chloride; PEG, polyethylene glycol; PAsp(DET), polyamino acid (Poly{N-[N′-(2-aminoethyl)-2-aminoethyl]aspartamide}); PEO, poly(ethylene oxide); PLL, poly (l-lysine); MEFs, mouse embryonic fibroblasts; hMSCs, human mesenchymal stem cells; ASCs, adipose-derived stem cells; rBMSCs, rabbit bone marrow mesenchymal stem cells; TGF-β1,transforming growth factor-β1; SOX, sex-determining region Y-type high-mobility group box; cbfa-1, core-binding factor alpha 1; RUNX1, runt-related transcription factor 1; pDNA, plasmid deoxyribonucleic acid; siRNA, small interfering ribonucleic acid; mRNA, messenger ribonucleic acid.
Cationic polymers are powerful gene vectors, which can bind to genes by electrostatic interaction and promote cell endocytosis [24]. Among them, PEI with various architectures and chain lengths has been widely employed and is often considered as the gold standard in non-viral gene transfection [26]. Sex-determining region Y-type high-mobility group box 9 (SOX9) gene has been widely used for cartilage repair, which exerts its functions by activating cartilage-specific genes such as type-II collagen and inhibiting terminal differentiation and hypertrophy, thus inducing chondrogenesis and MSC chondrodifferentiation [[37], [38], [39]]. However, PEI can cause cell membrane and cytoskeleton alterations, which could directly affect the differentiation of MSCs leading to adipogenesis, as well as potential cytotoxicity [13]. Park et al. added hyaluronic acid (HA) anion shield to the PEI/pDNA complexes containing the enhanced green fluorescent protein (EGFP)-tagged SOX9 gene to decrease toxicity while improving the gene expression efficiency [34]. HA is the core molecule of keratin sulfate and chondroitin sulfate to form aggrecan in native cartilage [40]. HA-shielded PEI/pDNA nanogels were easily internalized by interacting with HA and the specific receptor CD44 located at the plasma membranes of hMSCs, and without eliciting cytotoxic effects. Besides, the connection between CD44 and HA is helpful to stimulate the differentiation of stem cells into chondrocytes. The internalization can be enhanced by increasing the PEI: HA ratio, and the results showed that hMSCs easily differentiated into chondrocytes and the gene expression was enhanced following transfection of the complex. As a bioactive molecule, the efficacy of HA to enhance cartilage reconstruction has also been demonstrated in HA/CS/pDNA hybrid nanoparticles fabricated by Lu et al. [27]. In particular, they investigated the effect of composition on polymer particles, and generally with increasing HA there was an increase in size and a decrease in the surface charge, which can minimize nonspecific interactions with serum proteins to improve the transfection efficiency [41]. In fact, the capacity and migration of the plasmid, which are closely related to the shape and surface charge of polymeric nanoparticles, also affect the transfection efficiency. Yu stated that there is always a weight ratio range between cationic polymer and plasmid DNA to form the smallest nanoparticles, showing the best gene transfer efficiency [28]. In the first polysaccharide-based delivery system for chondrogenesis they established, pSox9/pmPPY (PEI-modified polysaccharides of porphyra yezoensis) nanoparticles with a weight ratio of 50:1 were the most prominent. Interestingly, the plasmids complexed to nanoparticles exist in a condensed form, not only allowing more efficient uptake by stem cells, but may also protect them from degradation [29], which is similar to the effect of nanomicelles self-assembled from block copolymers with plasmid-containing core. However, polyplex nanomicelles provide higher stability of cargos and more effective prevention of inflammation under physiological conditions because of the denser polymer outer layer [35,36].
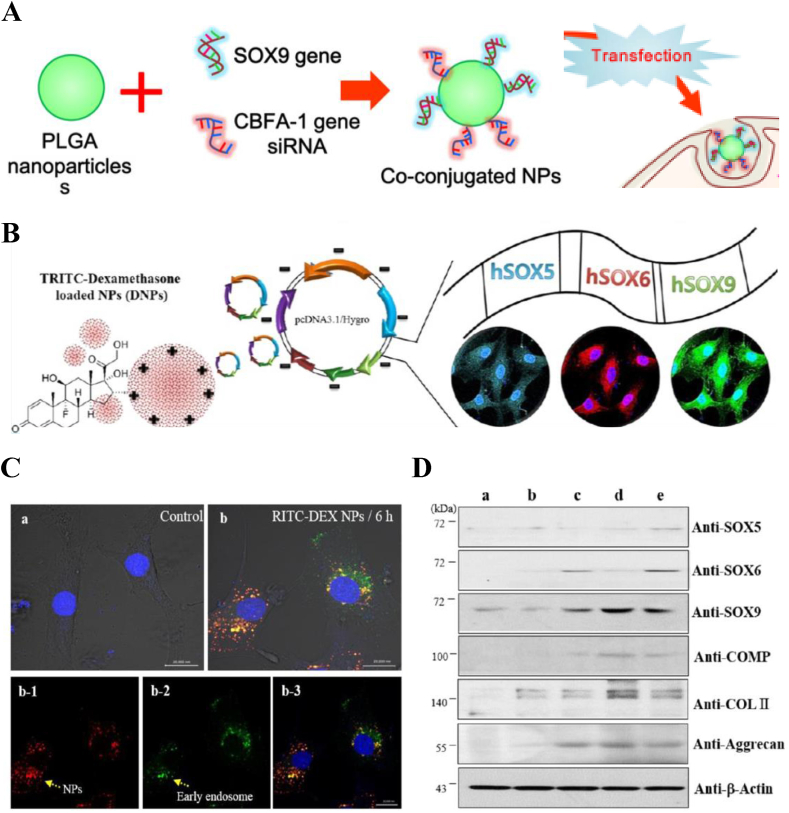
The wide application of polymeric vectors in delivery system also benefits from the facile of manufacture and modification platform. Park and his team decorated PEI onto poly(lactide-co-glycolic acid) (PLGA) nanoparticles'surface and achieved co-transfection in hMSCs by conjugating SOX9 gene or core-binding factor alpha (1) (cbfa-1) small interfering RNA (siRNA) via ion interactions [29] (Fig. 1A). Contrary to the function of chondrogenic SOX9 gene, the high mobility group domain transcription factor cbfa-1 gene is expressed during osteogenesis, and overexpression can support bone growth thus retarding neocartilage formation [42]. Therefore, the use of siRNA to block the expression of cfba-1 gene in the process of chondrogenesis of hMSCs is of great significance. The results showed that co-transfection markedly increased the expression of genes associated with chondrogenesis by hMSCs compared to single gene delivery systems, while that of osteoblasts did not. It is suggested that SOX9 gene promotes expression of genes involved in chondrogenesis while cfba-1 siRNA inhibits undesired gene expression, thereby enhancing the specific differentiation of hMSCs. To maximize the efficiency of chondrogenic differentiation, they also employed a polycistronic gene delivery system, in which three genes, SOX5, SOX6, and SOX9 were encoded into a single plasmid (Fig. 1B) [30]. This has important implications for gene therapy in cartilage repair, as the process of cartilage tissue regeneration is actually regulated by complex gene networks. SOX5 and SOX6 genes enable driving efficient chondrogenesis by enhancing the transcriptional activity of SOX9 gene [43]. The uptake process of polycistronic SOX pDNA-coated DEX-loaded PLGA nanoparticles including internalization, encapsulation and endosomal escape, is clearly demonstrated by flow cytometry as shown in Fig. 1C. And chondrocyte-related genes were successfully expressed (Fig. 1D), in which the expression of SOX9 in polycistronic trio genes lasted for 96 h.
Fig. 1.
Diagram of nanoparticles A) SOX9 pDNA plus cbfa-1 siRNA coated PEI-modified biodegradable PLGA NP and B) Polycistronic SOX pDNA-coated DEX-loaded PLGA NP. (C) The uptake of DEX-loaded PLGA nanoparticles, a: hMSCs without treatment of polycistronic SOX genes, b: hMSCs treated with TRITC-conjugated DEX-loaded PLGA nanoparticles for 6 h. Bars, 20 μm. b-1: Detection of TRITC-conjugated DEX-loaded PLGA nanoparticles in the cytosol of hMSCs. b-2: Early endosome staining of hMSCs. b-3: Merged images Bars, 20 μm. (D) Western blot analyses of chondrogenic differentiation of hMSCs transfected with polycistronic SOX pDNA-coated DEX-loaded PLGA nanoparticles in cell pellet culture system. a: hMSCs with nontreatment, b: mock pDNA-coated DEX-loaded PLGA nanoparticles, c: polycistronic SOX plasmid complexed with PEI, d: polycistronic SOX pDNA-coated DEX-loaded PLGA nanoparticles, and e: single SOX trio genes-coated PLGA nanoparticles. Panel A is reproduced with permission from Refs. [29], Biomaterials. Panel B is reproduced with permission from Refs. [30], ACS Applied Materials & Interfaces. Copyright (2017) American Chemical Society.
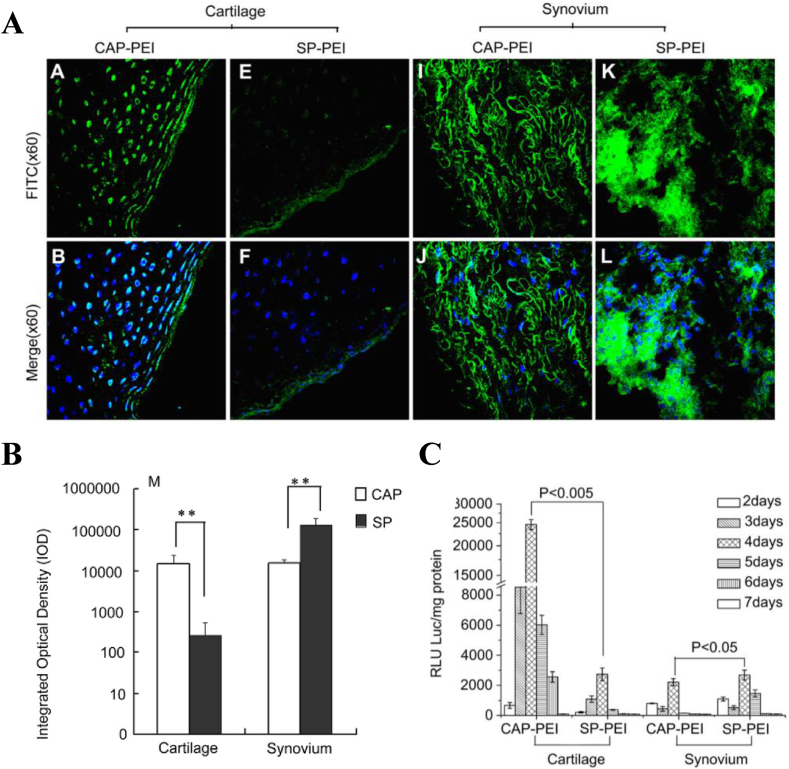
Polymeric vectors provide a variety of possibilities for enhancing gene expression in cartilage and play an increasingly important role in gene therapy delivery systems. By optimizing the chemical composition and structure of the polymeric vectors, a safe, stable and non-immunogenic system with modest transfer efficiencies (5–40%) can be obtained [7]. An effective gene delivery system not only needs to protect the therapeutic genes in the cytosol from degradation, but also requires to overcome many intracellular and extracellular barriers while delivering genes directly to the cells of the target tissue, thus avoiding off-target toxicity and negative host responses [7,19]. To address this issue, an efficient and specific delivery system was constructed by Pi et al. (Fig. 2) [31]. They used phage display technology to identify a cartilage-specific peptide (CAP) and conjugated it with PEI. The fluorescein-5-isothiocyanate (FITC)-labeled CAP-PEI/DNA complexes were injected directly into the rabbit knee joints to verify the cartilage-targeting property. The results showed that CAP-modified PEI can deliver gene specifically to the chondrocytes, resulting in enhanced transfection efficiency compared to a randomly scrambled peptide (SP)-modified vector in vivo. Unfortunately, the non-specific absorption of synovium can not be completely eradicated.
Fig. 2.
In vivo validation of cartilage-targeting property of CAP or SP conjugated PEI/DNA complexes. (A) FITC-labeled CAP or SP-PEI/DNA complexes, in weight ratio of 2, were injected into the knee joints of rabbits, the fluorescence distribution were observed after 48h injection. (B) The amount of CAP-PEI or SP-PEI in the cartilage and synovium were quantified by Image-Pro Plus version 6.0 software. (C) The luciferase activities of cartilage and synovium were measured at different time point after injection. Reproduced from Ref. [31], Biomaterials.
3. Polymeric biomaterial-based gene-activated matrices for cartilage repair
It should be noted that injecting the vectors directly into the joint space is often imprecise and transient because the small carriers are diluted by the joint fluid and fail to reach the target lesion area [7]. The rapid dispersion of vectors from the joint space prevents effective transduction. What's worse, the transgene expression at undesired sites can cause harmful side effects, such as immune responses and synovial chondrogenesis [44]. An indirect approach based on the use of transgenic cells emerged, which involves releasing genetically modified cells directly or with the help of biodegradable scaffolds [45]. However, either approach requires complex cell operations in vitro, and the long-term expression of transgenic products in vivo is limited due to the migration and apoptosis of implanted cells.
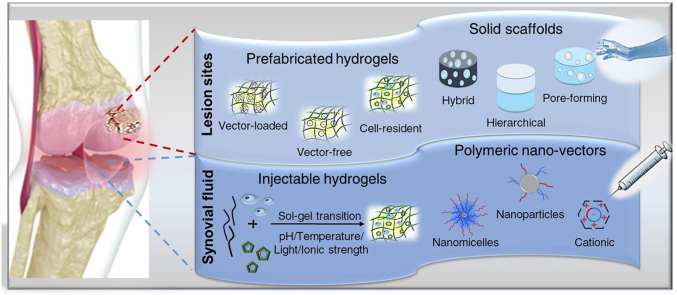
Another alternative strategy for prolonged expression is to directly load the gene complexes into the scaffolds by encapsulation (incorporating vectors during scaffold preparation) or by immobilization (incorporating vectors into a fabricated scaffold), which are known as gene-activated matrices (GAMs). The delivery system based on GAMs can protect cargos from rapid degradation and phagocytosis in the synovial fluid while offering a spatial confinement of the delivered genes that cells are transfected in situ [46]. These platforms provide a more precise, controlled, and sustained release of therapeutic genes without the limitations of local injection or genetically engineering cells [8,47]. In addition to gene vectors, supportive biomaterials as another basic component in the construction of GAMs can be fabricated in diversified forms, including solid scaffolds and hydrogels [10].
3.1. Solid scaffold-based gene-activated matrices for cartilage repair
3.1.1. Polymeric scaffold-based gene-activated matrices for cartilage repair
Polymers similar to cartilage tissue composition and properties are most often selected as matrix materials, such as collagen [[48], [49], [50]], alginate [51,52], fibrin [32,53,54] or chitosan (CS) [27,46,55]. The degradation products of them are non-toxic, and have advantages to regenerate cartilage in vivo. Gelatin and CS were fabricated to porous scaffolds with appropriate porosity and mechanical property by Guo et al. [46]. And the plasmid DNA encoding transforming growth factor TGF-β1 was incorporated into the scaffolds by surface adsorption and electric affinity. TGF-β1 has been proved to facilitate effective cartilage regeneration by promoting both chondrogenesis of MSCs and the synthesis of specific ECMs for chondrocyte proliferation [27]. The results showed that the transgene was stably expressed for 3 weeks in the CS-gelatin based GAMs, where the round phenotype of chondrocytes was maintained. Interestingly, a burst release of therapeutic genes was observed in the early stage. Although they stated that this may be beneficial because the higher protein concentration and short-term protein expression are needed in the initial stage of cartilage regeneration, though there is a potential risk of overexpression. The rapid release of plasmids that physically interact with scaffolds can be prevented by cross-linking, resulting in vectors release controlled by scaffold degradation. In the study of Capito and Spector [50], the pDNA encoding insulin-like growth factor (IGF)-1 or pIGF-1/lipid chemically cross-linked collagen-glycosaminoglycan (CG) scaffolds have higher initial load and plasmid retention than those combined by soaking, showing a slower release kinetics. And the prolonged and elevated expression of therapeutic IGF-1 led to enhanced cartilage formation. GAMs as bioactive depots for gene delivery can limit the distribution and overcome the rapid removal of nano-vectors from the delivery site thus reducing cytotoxic effects. In the first non-viral, non-lipid collagen-GAM platform for microRNA(miRNA)-based gene therapy established by Castaño et al., the nHA with Dy547-tagged scr. reporter miRNAs (nanomiRs) complexes were distributed uniformly along the surface of scaffolds, and the scaffolds supported cell infiltration without impairing viability of hMSCs, indicating the safety and biocompatibility of this system [48]. After cultivating hMSCs on the miRNA-activated collagen scaffolds over 7 days, the internalization efficiencies of nanomiR-mimics and nanoantagomiRs were increased by 2.6% and 48.8% respectively compared to hMSCs in monolayer. miRNAs are a class of endogenous non-coding small RNAs that can regulate overexpression or inhibition of multiple proteins by using mimics or antagomiRs respectively, allowing for enhanced tissue regeneration [49]. To verify the interference function of miRNA after transfection of hMSCs, the functional reporter nanomiR-mimic and nanoantagomiR complexes were designed to target mRNA of the housekeeping glyceraldehyde phosphate dehydrogenase (GAPDH) and miR-16 respectively. And the highly functional interference was shown after internalization: ~90% functionality of nanomiR-mimic silenced GAPDH expression and ~99% functionality of antagomiR decreased miR-16 expression.
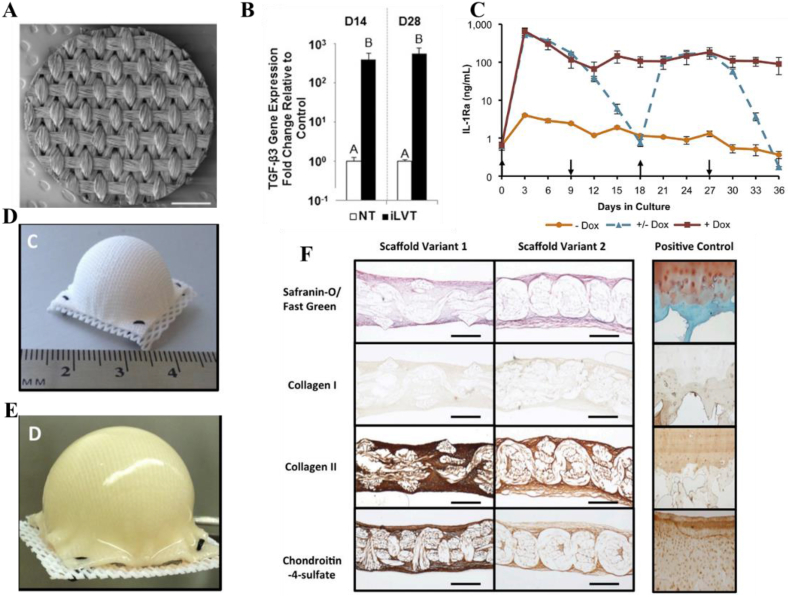
Although the natural polymeric scaffolds mentioned above have good degradability and biocompatibility, the weak compression properties and the poor shape-persistency do not match the requirements of neo-cartilage tissue regeneration. However, appropriate mechanical properties are needed to facilitate the cartilage formation and the functional restoration of a joint before eventual recovery. From the perspective of mechanical support, polycaprolactone (PCL) with controlled anisotropy and nonlinear mechanical properties seems to be a better choice for biomaterial-guided gene delivery in situ tissue engineering. Guilak and his colleagues developed a series of PCL-based GAMs after they first tested that poly-l-lysine (PLL) could immobilize lentiviral vectors (LV) to PCL films via charge interaction [38,[56], [57], [58]]. In their initial work, they showed that PLL treatment does not change the porosity, topography, or hydrophobicity of orthogonal braided PCL films (Fig. 3A), and the PLL-coated PCL with immobilized TGF-β3 LV (iLVT) can efficiently transduct hMSCs nearly 28 days, resulting in massive cartilage specific matrix accumulation (Fig. 3B) [57]. Based on this GAM, tunable immunomodulatory properties were obtained by inducing overexpression of interleukin-1 receptor antagonist (IL-1Ra) in MSCs (Fig. 3C). IL-1Ra can inhibit the expression of pro-inflammatory cytokines IL1, and enabling MSCs chondrogenesis even in an inflammatory environment which is particularly useful for long-term cartilage repair therapy [58]. In addition, PCL-based GAMs can also provide a template for the formation of cartilage, allowing reconstruction of the entire articular surface. An anatomically shaped GAM immobilized with doxycycline (dox)-inducible LV containing EGFP or IL-1Ra coding sequences was then fabricated (Fig. 3D). After culturing with human adipose stem cells (ASCs) for 28 d, the GAM had developed uniform tissue and biomimetic cartilage properties as well as inducing ASCs synthesized a uniform ECM completely filled their internal pore spaces (Fig. 3E–F). Crucially, the dimension of hemispherical scaffolds remains stable over time to resist the contractile forces generated by growing tissue while facilitating cell adhesion, survival, and differentiation. This work may enhance the long-term success of tissue engineering approaches to cartilage repair and shows promising prospects as a treatment for end-stage OA or other joint diseases.
Fig. 3.
(A) Scanning electron microscopy (SEM) image of a 3D woven PCL scaffold 5 mm disk. Scalebar ¼ 1 mm. (B) Quantitative RT-PCR assessing expression of the TGF-β3 transcript from samples in the iLVT group at D14 and D28. (C) IL-1Ra secretion from engineered cartilage constructs into media every 72 h over 36 days of chondrogenesis (mean SEM, n ¼ 3). þ Dox indicates dox induction at 1 mg/mL for 36 days. Dox indicates the baseline IL-1Ra expression in the absence of dox. Dox indicates that dox (1 mg/mL) was switched on and off every 9 days. Upward arrows show time points at which dox was induced and downward arrows show the withdrawal of dox. (D) Hemispherical-shaped 3D woven PCL scaffold before seeding with human ASCs and (E) after 38 d of culture. (F) Histology and IHC of hemispherical-shaped constructs at day 38 (cross-sectional views). Human osteochondral tissue (Right column) was used as a positive control for all staining protocols. (Scale bar, 0.5 mm). Panel A and C are reproduced with permission from Ref. [58], Biomaterials. Panel B is reproduced with permission from Ref. [57], Proceedings of the National Academy of Sciences of the United States of America, Copyright (2014), The National Academy of Sciences. Panel D,E and F are reproduced with permission from Refs. [53], Proceedings of the National Academy of Sciences of the United States of America, Copyright (2016), The National Academy of Sciences.
Recently, 3D-woven PCL scaffolds have also been used in the rAAV-mediated gene delivery. Human bone marrow aspirates within these scaffolds can be modified by rAAV vectors and transduced for at least 75 days, and transgene SOX9 expression at least 21 days [38]. Especially, the effective expression of SOX9 (up to 94.4%) was achieved in highly bioactive PCL films grafted with poly(sodium styrene sulfonate) (pNaSS) [12,39]. What is most heartening is that PCL has been approved by Food and Drug Administration (FDA) for clinical application and a number of commercial fabrications have been successfully developed for 3D tissue culture, which provide a promising polymer platform for the production of long-term degradable implants suitable for specific anatomical sites [59].
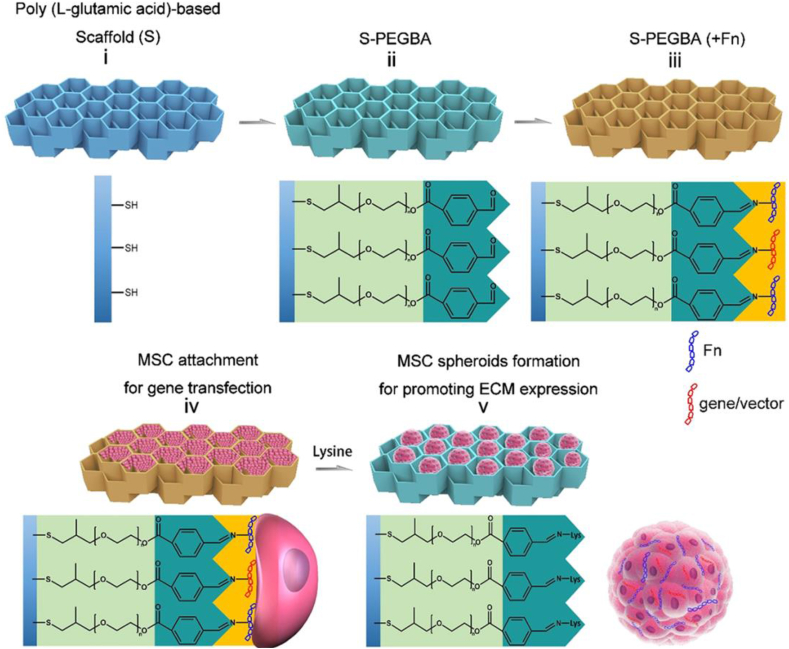
The stiffness of solid scaffolds can enhance the efficiency of gene transfection by promoting cell adhesion and spreading. However, the limited cell surface originated from the aggregation of cells in the process of cartilage formation exhibits a barrier to effective gene transfection. Zhang et al. developed a functionalized poly(l-glutamicacid)-based porous scaffold which provides an effective surface-mediated gene transfection before MSC spheroids form [33]. The tunable inner surface of the scaffold can realize cell-scaffold attachment, detachment, and in situ spheroid formation via reversible linkage between fibronectin (Fn) and the functionalized surface with aromatic aldehyde modification (Fig. 4). The extensive cell spreading and the subsequent spheroid formation after cellular detachment from the scaffold via lysine treatment, promote gene transfer between ASCs and N, N, N-trimethyl chitosan chloride (TMC)/DNA complexes which immobilized on the surface, thus amplifying the TGF-β1 expression and promoting chondrogenic differentiation of ASCs.
Fig. 4.
Design and function of the PLGA-based anti-cellular adhesive (non-fouling) porous scaffold. Reproduced from Refs. [33], ACS Applied Materials & Interfaces. Copyright (2018) American Chemical Society.
3.1.2. Composite/hybrid scaffold-based gene-activated matrices for cartilage repair
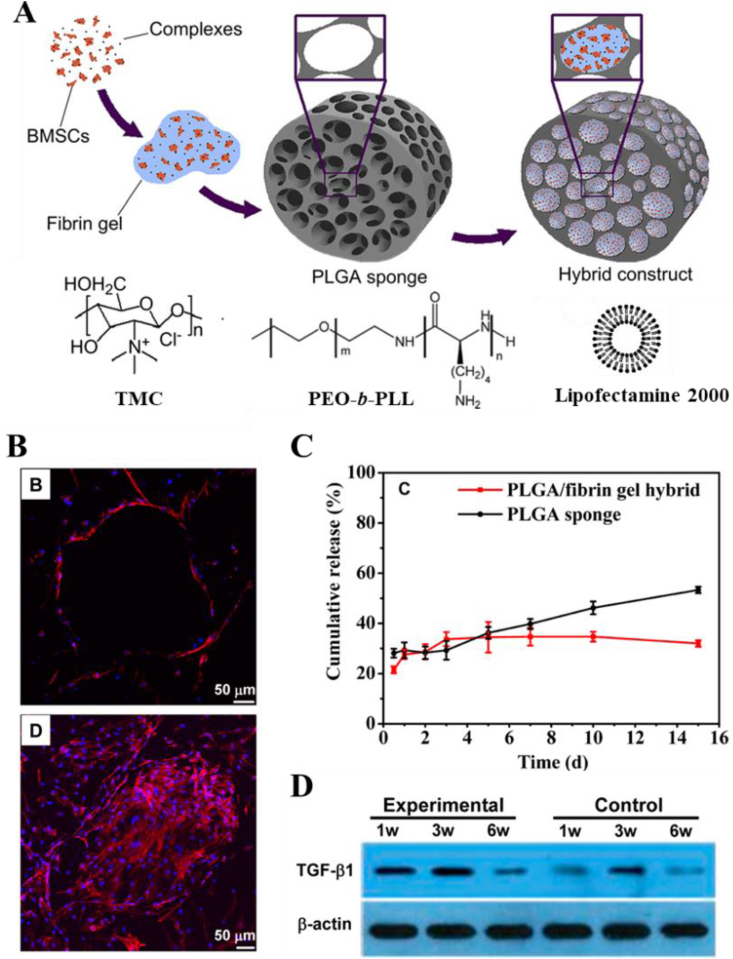
Actually, cartilage is a biphasic material, in which the porous solid phase is composed of cross-linked collagen fibers and hydrophilic proteoglycans, while the liquid phase consists of water and electrolytes [60]. In this regard, composite scaffolds may be more suitable for biomaterials-guided gene therapy as a cartilage mimetic tissue [57]. Gao and his team combined a porous poly(lactide-co-glycoside) (PLGA) sponge with genetically activated fibrin gel to better mimic cartilage tissue, in while gene cmplexes and BMSCs loaded simultaneously. They applied these composite scaffold-based GAMs for cartilage repair using different vectors including lipofectamin [47], poly(ethylene oxide)-b-poly (l-lysine) (PEO-b-PLL) [36] and TMC (Fig. 5A) [32]. Among all three systems, the strategie using a combination of BMSCs, PLGA sponge/fibrin gel scaffolds, and vector/gene complexes exhibited a higher therapeutic effect than the respective controls. As bioactive storages, the constructs can accommodate more gene vectors with a uniform distribution and allow higher cell density to facilitate the formation of cartilage extracellular matrix (Fig. 5B). Porous PLGA sponges provide sufficient adsorption sites and mechanical support, while fibrin gel filled in sponges helps genes retention, thus providing more opportunities for cellular uptake and significantly prolonged release time of gene complexes (Fig. 5C). The sustained gene release dramatically improved transgenic expression both in vitro and in vivo (Fig. 5D). What's more, all the components of the constructs are widely adopted in the biomedical field, with the advantages of cost-effective and FDA approved, ensuring the practice of the method for future application [61].
Fig. 5.
(A) Schematic illustration to show the fabricating procedures of the composite construct by filling BMSCs, lipofectamine/PEO-b-PLL/TMC with DNA complexes and fibrin gel into a PLGA sponge and chemical structure of TMC. pDNA-TGF-β1 was used in the in vivo experiment. (B) More number of cells with cluster morphology was found inside the PLGA/fibrin gel hybrids than inside the PLGA sponges. (C) Cumulative release of genes from PLGA sponge and PLGA/fibrin gel hybrid as a function of time, respectively. (D) Western blotting analyses of 9 expressions of mouse TGF-β1 in full-thickness cartilage defects after being treated by the experimental and control constructs for different time, respectively. 18S rRNA was used as the internal control gene for qRT-PCR analysis. Panel A is reproduced with permission from Refs. [32], Biomaterials. Panel B,C and D are reproduced with permission from Ref. [47], Molecular Pharmaceutics, Copyright (2014) American Chemical Society.
Composite and hybrid scaffolds are considered to be robust vehicles for gene delivery. In addition to enhancing the loading capacity and sustained transgenic expression, they can also integrate the advantages of each component and provide an effective strategy for the regeneration of highly organized complex cartilage tissues [57].
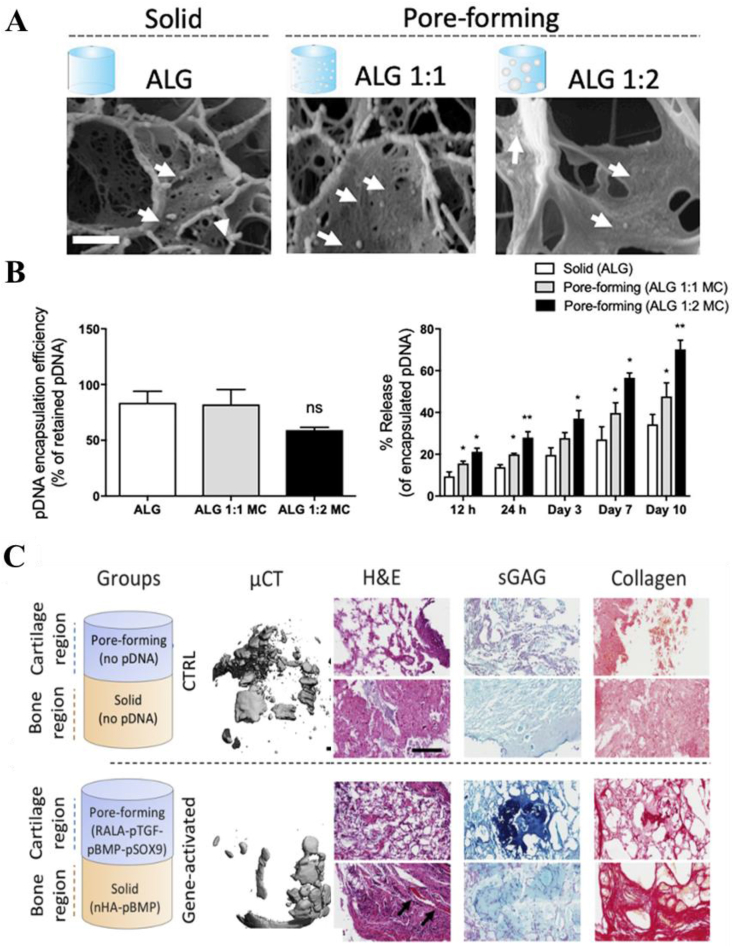
Cartilage damage often involves subchondral bone lesion, resulting in bone-cartilage defects. The integration of bone and cartilage repair is the key to ensure the balanced load distribution and stable mechanical conduction of the entire joint. However, this remains a significant challenge because cartilage and subchondral bone have significantly different physiological structures and functions. Hybrid scaffold-based GAMs provide a promising opportunity for anisotropic cartilage reconstruction and the integration of osteochondral repair. Hybrid scaffolds have a wider range of modulations, especially the template structures used for cell infiltration and tissue formation can be adaptively adjusted for direct tissue development in vivo. Chen et al. utilized stratified designs to fabricate an osteochondral gene-activated scaffold, where chitosan-gelatin layer was activated by plasmid TGF-β1 for chondrogenesis while hydroxyapatite/chitosan-gelatin layer was activated by plasmid bone morphogenetic protein 2 (BMP-2) for osteogenesis [62]. Porous scaffolds with chitosan cations can not only deliver pDNA but also maintain the sustained localized production of proteins. The spatial limitation allows the creation of specific tissues with different structures and chemical properties in each layer and shows significant cell proliferation as well as high expression of respective proteins. However, these bilayer scaffolds were joined by fibrin glue, which resulted in weak interfacial force and poor mechanical stability. A mechanically reinforced strategy was presented by Gonzalez et al. [11]. They fabricated a bilayered construct with mechanical enhancement of PCL where gene-activated bioinks deposited spatially to produce zonally gradients of cell populations. Meanwhile, they modulated gene transfection temporally through the pore-forming of alginate bioinks created by methylcellulose. The larger pores were obtained with increasing concentrations of methylcellulose thus enhanced the release rate and accelerated the transfection. After four weeks in vivo implantation, expressions of cartilage-anabolic markers and cellular aggregates were produced in the cartilage region while mineralization was spatially restricted to the bone region of these implants (Fig. 6).
Fig. 6.
Characterization of gene activated pore-forming bioinks. (A) CryoSEM images of the encapsulated RALA-pDNA complexes in the solid alginate and pore-forming ALG-MC (1:1 and 1:2) gels. Scale bar = 200 nm. White arrows point to RALA-pDNA complexes. (B) Encapsulation efficiency of the RALA-pDNA complexes in the different gels. (C) Percentage of the released RALA-pDNA complexes in the different gels at 12 h, 24 h, day 3, 7 and 10d. Spatial therapeutic gene delivery in mechanically reinforced bioinks after four weeks of in vivo implantation. MicroCT, Histological analysis of H&E, GAG, collagen and calcium staining. Reproduced from Refs. [11], Journal of Controlled Release.
The hierarchical structure of hybrid scaffolds provides an opportunity to replicate the spatial complexity of osteochondral units, and can simultaneously avoid interference from some promoters. For example, SOX 5, 6, and 9 (SOX trio) and Runt-related transcription factor 2 (RUNX2) play an essential role in chondrogenesis and osteogenesis respectively, and was confirmed that they have inhibitory effects on each other when applied to the same cells in vitro and in vivo. Needham et al. loaded combination of RUNX2 and SOX trio DNA to distinct zones of oligo[poly(ethylene glycol) fumarate]/carboxymethyl cellulose (OPF/CMC) hybrid hydrogels. They found tissue generation and quality were significantly improved compared to those empty or either factor alone. It indicated that loading gene complexes spatially into bi-layered scaffolds achieved dual delivery of genetic materials without negative interference, and the effective simultaneous expression of multiple genes greatly improved the therapeutic effect [63].
3.2. Hydrogel-based gene-activated matrices for cartilage repair
Although the prefabricated solid scaffolds allow facile and controllable delivery of genes and provide mechanical support that facilitate cell growth and tissue formation [[64], [65], [66]], they are relatively structured static biomaterials [67]. This static characteristic is not conducive to filling the defects, but also increases the difficulty of clinical operation. More importantly, it renders the low survival rate of cells and can hardly adapt to the growth of new tissues. In native cartilage, chondrocytes are resident in dynamic ECM that consists of 80% water content and exhibits high viscoelasticity [2,68]. Hydrogels seem to be potential candidates as ECM mimics, which have a 3D network structure that enable to absorb large amounts of water. Better than solid scaffolds, hydrogels can provide a suitable environment for maintaining cell phenotypes, and other unique features they have such as injectability and inherent mechanical tunability are all particularly attractive for GAMs [69].
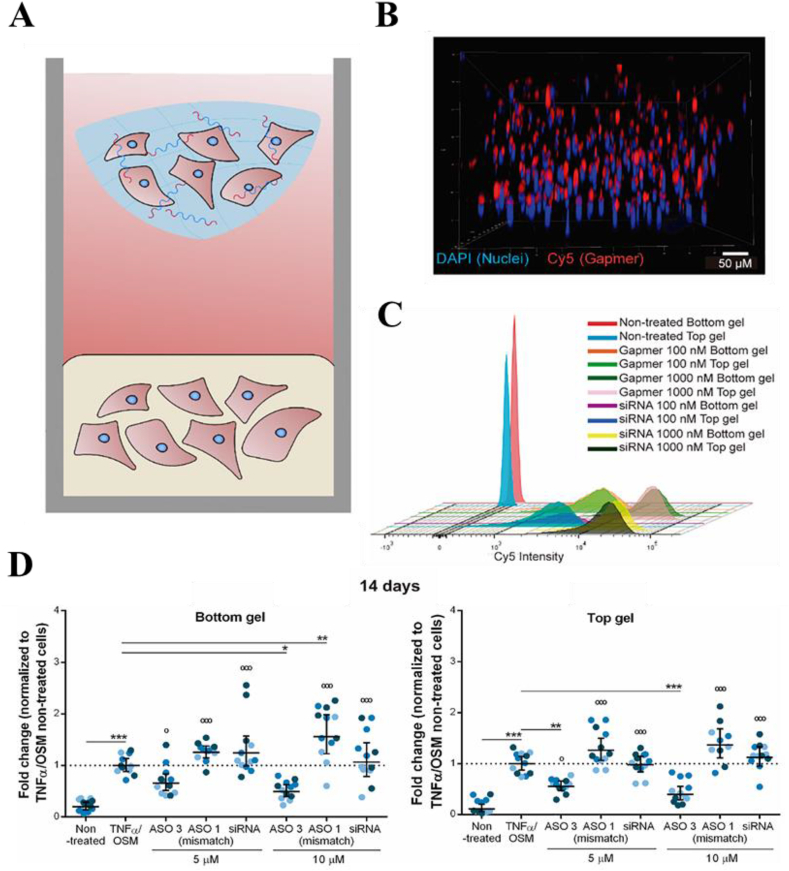
Tomas et al. encapsulated MSCs and nHA complexed with plasmid into alginate hydrogels [51], and the results showed that the developed gene-activated alginate hydrogels were able to deliver genes and sustain overexpression of the transgenes over 14 days without negatively impacting cell viability, thus supporting an effective transfection of encapsulated MSCs. In addition to co-embedded cells, the delivery of hydrogels is also effective to transfect resident cells. Garcia et al. developed a novel hierarchical fibrin-hyaluronic acid hydrogel culture model in which the top hydrogel containing antisense oligonucleotides (ASOs) and OA chondrocytes was used to mimic therapeutically “delivered cells”, while the bottom one only containing OA chondrocytes was uesd to mimic “resident cells” (Fig. 7A) [70]. The ASO sequences also called gapmers were designed and screened to inhibit the expression of ADAMTS (a disintegrin and metallo proteinase with thrombospondin motifs) which lead to the loss of proteoglycans during cartilage degeneration in OA. They found that the sustained release of the incorporated gapmers and the efficient ADAMTS5 silencing were shown up to 14 days in both co-embedded chondrocytes and chondrocytes in a neighboring gapmer-free hydrogel (Fig. 7B–D). In the research of Lolli et al., the fibrin-hyaluronic acid hydrogel only with therapeutic genes was futher shown to be effective in controlled delivery in a osteochondral defect model in vivo [71]. This cell-free delivery strategy can in situ guide cartilage regeneration by endogenous cells while avoiding the operational, cost, and regulatory issues associated with cell manipulation, and has an easier path to clinical practice.
Fig. 7.
A novel in vitro system mimicking chondrocyte delivery for cartilage resurfacing and its interaction with surrounding native joint tissues exposed to a pro-inflammatory environment. (A) Scheme of two-hydrogel in vitro model. Top hydrogel containing ASOs and OA chondrocytes, and bottom hydrogel containing OA chondrocytes only. Gapmer diffusion and cellular association: (B) 3D reconstruction of 350 μm of the bottom hydrogel 72 h after culture with Cy5-labeled gapmer in the top hydrogel. Blue: DAPI, Red: Cy5; and (C) Representative flow cytometric analysis of OA chondrocytes cultured in the bottom and top hydrogels in the presence of 100 and 1000 nM gapmer or siRNA. (D) Long-term gapmer-mediated ADAMTS5 knockdown in 3D cell constructs. Reproduced from Ref. [70], Journal of Controlled Release.
In clinical practice, the efficient, simple and minimally invasive treatment has been largely pursued of cartilage repair [72]. Injectable hydrogels are attractive, because the flexible sol-gel transition allows accurate minimally invasive maneuvers and adaptive defect filling [73]. Self-assembling peptides that can form stable hydrogels under physiological pH and ionic strength have been proved to be effectively deliver rAAV vectors to transduct hMSCs in a sustained, controlled manner [74]. The high transduction efficiency up to 80% was obtained in RAD16-I peptide hydrogels combined with HA, and the transgenic expression of hMSCs as well as chondrogenic differentiation can last for at least 21 days.
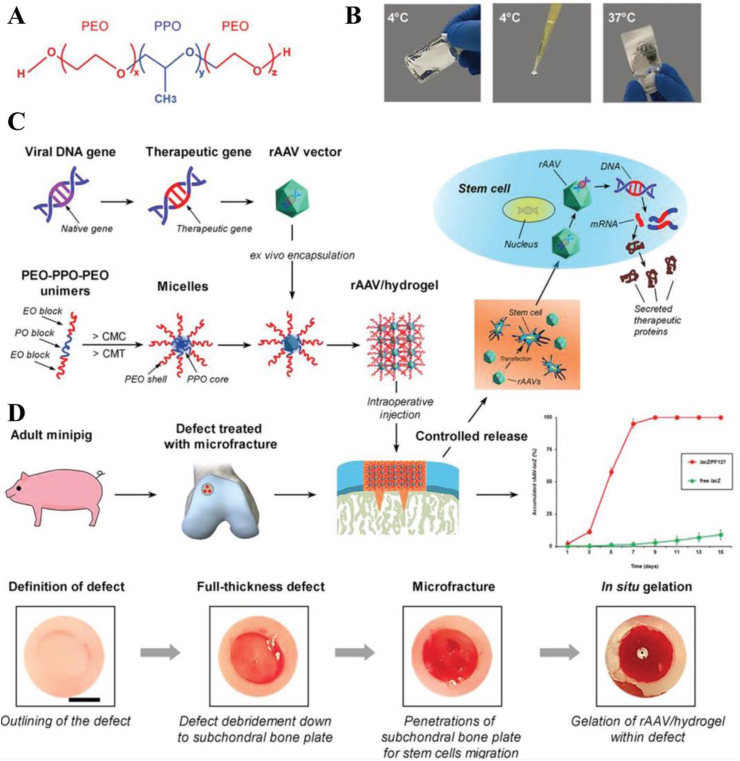
In addition, physically crosslinked thermosensitive hydrogels have been widely described as potential candidates in gene delivery for cartilage repair and have made some advances [75]. Recently, an injectable and thermosensitive hydrogel based on poly(ethylene oxide)-poly(propylene oxide)-poly(ethylene oxide) (PEO-PPO-PEO) was developed for effective gene therapy of cartilage defects by Cucchiarini and colleagues [76].The triblock copolymers PEO-PPO-PEO exhibit a sol-gel transition around 37 °C, enabling a controlled and minimally invasive delivery (Fig. 8A & B). The effective protection of PEO-PPO-PEO polymeric micelles on vectors and gene transfer was demonstrated in their early work, in which the concentration, stability, and bioactivity of the vectors were enhanced compared with levels in vector-free treatment [37,77,78]. Furthermore, they displayed the potential of the injectable and thermosensitive hydrogel to stimulate the natural reparative processes in places of injured tissue in large animal models of cartilage damage (Fig. 8C) [76]. The hydrogel protected against potentially destructive host immune responses and the subchondral bone plate from early bone loss, which significantly improved the repair of full-thickness chondral defects four weeks postoperatively (Fig. 8D). Significantly, this is the first report of the successful use of biomaterial mediated GAMs in the treatment of cartilage in a large animal model, representing an important step in the clinical translation of this system in cartilage repair.
Fig. 8.
Study design. (A) Structure of the PEO-PPO-PEO (PF127) block copolymer. (B) Thermo-sensitive characteristics of the copolymer (liquid form at 4 °C, solid form at 37 °C). (C) Flowchart of generation of the rAAV/hydrogel systems for the controlled release of rAAV with implantation in knee full-thickness chondral defects in minipigs following microfracture. The accumulated controlled release pattern of rAAV from PF127 is presented relative to free rAAV. (D) Intraoperative view of the full-thickness chondral defect creation and treatment with microfracture augmented with in situ gelation of the rAAV/hydrogels. The defects were outlined in the superior region of the lateral trochlear facets of both knees with a biopsy punch and debrided down to subchondral bone plate after removal of the entire calcified cartilage layer. Three microfracture holes were always introduced per defect in a standardized manner. Then, the PEO–PPO–PEO systems carrying rAAV-FLAG-hsox9 (sox9/hydrogel) or rAAV-lacZ (lacZ/hydrogel) were directly applied into the treated cartilage defects, allowing for in situ gelation. Reproduced from Ref. [76], Advanced Materials, Copyright (2020), WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Although the enhanced repair of full-thickness chondral defects has shown here, hurdles still need to be overcome for successful clinical application. The obvious point is that the self-assembled PEO-PPO-PEO hydrogels would soon be eroded due to partial hydrophilicity. A possible strategy for extending residence times was developed by Rey-Rico and colleagues, they added alpha-cyclodextrin (αCD) to Pluronic1F68 (PF68) or Tetronic1908 (T908) dispersions containing HA or CS to form a series of supramolecular polypseudorotaxane gels with different formulations [79]. αCD can thread along copolymer chains and interact with those of adjacent polypseudorotaxanes, endowing hydrogels reduced solubility and enhanced viscoelasticity that allow durably deliver rAAV vectors for applications in cartilage regeneration. Using the spacing effect, Yang et al. developed a more stable cell support and local delivery platform by adding anionic clays to poly (N-isopropylacrylamide) (PNIPAAm) hydrogels [80]. PNIPAAm-based hydrogel is another extensively investigated injectable hydrogel, which undergo sol-gel transition around physiological temperature due to the hydrophobic interactions [81]. The addition of clays not only improved the precipitation of PNIPAA caused by instability, but also proved to be effective in delivering siRNA to transfect chondrocytes from diseased tissue. siRNA can specifically interfer the expression of several negative tissue homeostasis regulators in cartilage tissue, and in their report, the cellular uptake of siRNA (~75%) in hybrid hydrogel constructs was significantly enhanced compare to the clay-free hydrogels. Therefore, it might be a more effective strategy for delivering siRNA and provides great hope for the in suit treatment of cartilage tissue degeneration.
What's more, the enhanced stability and mechanical properties can also overcome the disadvantage of most injectable hydrogels that the modulus they have are too low to match the surrounding cartilage tissue, thus driving the chondrogenic differentiation of MSCs and the formation of neochondral tissue [49]. The widely tunable mechanical properties can be easily achieved by regulating the formulation and fabrication of hydrogels that are also closely related to the release kinetics of gene delivery in vivo. The polypseudosilane hydrogels with different formulations mentioned above also exhibit different controlled-release capacities. The gel of CS (or HA) dispersion in PF68 rapidly released rAAV vectors while in T908 provided sustained release. It is worth noting that the release rate of gels was not always consistent with the level of transgenic expression. The rapid release of CS/PF68/αCD gels in the early stage effectively improves the initial levels of transgene expression, whereas in HA-based gels it was obtained through sustained release. They speculated that it is due to the difference of charge properties in different systems. Thus, a further conclusion is drawn that the effects of gene therapy are related to the composition, charge and mechanical properties of hydrogel scaffolds. In addition, the concentration of hydrogels has also been proved to affect transfection efficiency. In an early report, diluted fibrin hydrogels produced a more open network that can release more gene vectors, resulting in enhanced transduction efficiency and chondrogenic potential of hMSCs [44].
4. Conclusion and outlook
Cartilage repair has been hampered by complex multi-structural components and limited inherent ability for self-healing. Gene therapy enables cells to locally synthesize therapeutic gene products that enhance the internal repair mechanism, providing a promising approach to osteochondral regeneration. In particular, the adaptability of polymeric biomaterials can be customized for an effective gene delivery system. They can not only as non-viral vectors to directly protect and transport genes, but also as scaffold matrixs for precise spatiotemporal control and local sustained release. Unfortunately, although polymeric biomaterials offer the possibility to improve the efficiency of gene delivery and expression, no non-viral vector has yet achieved the extremely high transfection efficiency as a viral vector. It is still a primary task for researchers to maximize the transfection capacity of polymeric biomaterials in gene therapy under the premise of ensuring safety. In addition, even if polymeric biomaterials offer desired release kinetics and spatially defined architecture, the optimal release time and the dose of therapeutic genes required for effective cartilage repair are still unknown. The precise regulation of each stage matching to the process of cartilage formation is still hardly realized at present. Therefore, polymeric biomaterials-mediated gene delivery, which forms an efficient, well-defined regulated transduction system, represents the next frontier to enhance cartilage repair in vivo.
Another priority in manufacturing systems is the microenvironment provided by GAMs for cells infiltration and tissue development in vivo. In contrast, nanocarriers have relatively better biological activity because of the surface and volume effect, which are more conducive to genes loading and cell adhesion and proliferation, but the subsequent infiltration of cells is limited. Solid scaffolds can be fabricated into multifarious three-dimensional structures with appropriate porosity and high mechanical properties to adapt to cell adhesion and spreading as well as controllable release of gene complexes. They can also provide hierarchical regional limitations for the requirement of personalized treatment. However, the static structure of solid scaffolds increases the difficulty of clinical operation and reduces the adaptability to new tissue growth. Hydrogel scaffolds provide a suitable environment for new tissue growth. In particular, sol-gel transition allows filling irregular cartilage defects and achieving minimally invasive. However, the mechanical strength of single component hydrogel is generally poor, and the encapsulated cells are limited in their ability to bind and migrate. Polymeric biomaterials allow the combination of multiple constituents and forms, and the multi-material integration has become an emerging trend of biomaterial-mediated gene delivery. In addition to enhancing the efficiency of gene transfection, additional functions can also be achieved, and the integration of advantages of each component offers unlimited possibilities for enhanced gene therapy. However, the most appropriate, effective, and safe delivery system is still unknown, even if there are different degrees of regeneration in cartilage defects. Therefore, we need to further enhance the comprehension of the molecular mechanisms underlying cartilage reconstruction and the effects of polymeric biomaterials on cell fate. With the development of advanced biofabrication techniques, the combination of various biomaterials to construct composites with tailorable properties and spatially controlled biological function will be realized, and more personalized polymeric biomaterials-mediated gene delivery systems should be exploited to promote clinical application.
At present, the translational research of polymeric biomaterial-mediated gene therapy into human clinical practice remains a substantial challenge. Most researches are still limited to in vitro and small animal experiments, and the side effects caused by the degradation of polymeric biomaterials as well as related potential toxicity need more clinical verification. In addition, practical factors need to be taken into account when translating into clinical application, such as the patient's systemic health status, the cost of treatment, and regulatory restrictions. Encouragingly, the clinical trials related to gene therapy or mediated by polymeric biomaterials are exploding and more clinical approvals of the new therapeutics have been obtained under a clearer regulatory pattern. For instance, FDA approved MACI® (autologous cultured chondrocytes on porcine collagen membrane) in December 2016 for the repair of symptomatic cartilage damage of the adult knee. And the first cell-mediated gene therapy product, Tonogenchoncel-L (Invossa-K ®), for the treatment of knee osteoarthritis, was approved for listing in South Korea in July 2017, and phase III clinical trials have been completed in the United States. What's more, the new framework for identification and production of gene therapy system announced by FDA in July 2018 promises a bright future for the market.
We believe that with integrating principles of regenerative medicine, cell biology and material science, as well as the ongoing improvement of the framework for clinical review and approval of new treatment strategies, polymeric biomaterials-guided gene therapy will bring about more effective and translational advances for the treatment of traumatic cartilage injuries in near future.
CRediT authorship contribution statement
Ran Yang: Investigation, Writing - original draft, Writing - review & editing. Fei Chen: Writing - review & editing, Funding acquisition. Jinshan Guo: Writing - review & editing, Conceptualization, Funding acquisition. Dongfang Zhou: Conceptualization, Supervision, Writing - review & editing, Funding acquisition. Shifang Luan: Conceptualization, Supervision, Resources.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (Grant No. 51773198), the Open Research Fund of the State Key Laboratory of Polymer Physics and Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences (2019-12), and the SIAT Innovation Program for Excellent Young Researchers (Y9G075).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Dongfang Zhou, Email: dfzhou@smu.edu.cn, dfzhou@smu.edu.cn.
Shifang Luan, Email: sfluan@ciac.ac.cn.
References
- 1.Armiento A.R., Stoddart M.J., Alini M., Eglin D. Biomaterials for articular cartilage tissue engineering: learning from biology. Acta Biomater. 2018;65:1–20. doi: 10.1016/j.actbio.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 2.Camarero-Espinosa S., Rothen-Rutishauser B., Foster E.J., Weder C. Articular cartilage: from formation to tissue engineering. Biomater. Sci. 2016;4:734–767. doi: 10.1039/c6bm00068a. [DOI] [PubMed] [Google Scholar]
- 3.Li K.C., Hu Y.C. Cartilage tissue engineering: recent advances and perspectives from gene regulation/therapy. Adv. Healthc. Mater. 2015;4:948–968. doi: 10.1002/adhm.201400773. [DOI] [PubMed] [Google Scholar]
- 4.Freedman B.R., Mooney D.J. Biomaterials to mimic and heal connective tissues. Adv. Mater. 2019;31 doi: 10.1002/adma.201806695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee H.P., Gu L., Mooney D.J., Levenston M.E., Chaudhuri O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017;16:1243–1251. doi: 10.1038/nmat4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon H., Brown W.E., Lee C.A., Wang D., Paschos N., Hu J.C., Athanasiou K.A. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019;15:550–570. doi: 10.1038/s41584-019-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cucchiarini M., Madry H. Biomaterial-guided delivery of gene vectors for targeted articular cartilage repair. Nat. Rev. Rheumatol. 2019;15:18–29. doi: 10.1038/s41584-018-0125-2. [DOI] [PubMed] [Google Scholar]
- 8.Yan X., Chen Y.R., Song Y.F., Yang M., Ye J., Zhou G., Yu J.K. Scaffold-based gene therapeutics for osteochondral tissue engineering. Front. Pharmacol. 2019;10:1534. doi: 10.3389/fphar.2019.01534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raftery R.M., Walsh D.P., Blokpoel Ferreras L., Mencia Castano I., Chen G., LeMoine M., Osman G., Shakesheff K.M., Dixon J.E., O'Brien F.J. Highly versatile cell-penetrating peptide loaded scaffold for efficient and localised gene delivery to multiple cell types: from development to application in tissue engineering. Biomaterials. 2019;216:119277. doi: 10.1016/j.biomaterials.2019.119277. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Fernandez T., Kelly D.J., O'Brien F.J. Controlled non-viral gene delivery in cartilage and bone repair: current strategies and future directions. Adv. Ther. 2018;1:1800038. [Google Scholar]
- 11.Gonzalez-Fernandez T., Rathan S., Hobbs C., Pitacco P., Freeman F.E., Cunniffe G.M., Dunne N.J., McCarthy H.O., Nicolosi V., O'Brien F.J., Kelly D.J. Pore-forming bioinks to enable spatio-temporally defined gene delivery in bioprinted tissues. J. Contr. Release. 2019;301:13–27. doi: 10.1016/j.jconrel.2019.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Venkatesan J.K., Falentin-Daudre C., Leroux A., Migonney V., Cucchiarini M. Biomaterial-guided recombinant adeno-associated virus delivery from poly(sodium styrene sulfonate)-grafted poly(varepsilon-caprolactone) films to target human bone marrow aspirates. Tissue Eng. A. 2020;26:450–459. doi: 10.1089/ten.TEA.2019.0165. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez-Fernandez T., Sathy B.N., Hobbs C., Cunniffe G.M., McCarthy H.O., Dunne N.J., Nicolosi V., O'Brien F.J., Kelly D.J. Mesenchymal stem cell fate following non-viral gene transfection strongly depends on the choice of delivery vector. Acta Biomater. 2017;55:226–238. doi: 10.1016/j.actbio.2017.03.044. [DOI] [PubMed] [Google Scholar]
- 14.Raisin S., Belamie E., Morille M. Non-viral gene activated matrices for mesenchymal stem cells based tissue engineering of bone and cartilage. Biomaterials. 2016;104:223–237. doi: 10.1016/j.biomaterials.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Cucchiarini M. Human gene therapy: novel approaches to improve the current gene delivery systems. Discov. Med. 2016;21:495–506. [PubMed] [Google Scholar]
- 16.Evans C.H., Huard J. Gene therapy approaches to regenerating the musculoskeletal system. Nat. Rev. Rheumatol. 2015;11:234–242. doi: 10.1038/nrrheum.2015.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang D., Tai P.W.L., Gao G. Adeno-associated virus vector as a platform for gene therapy delivery. Nat. Rev. Drug Discov. 2019;18:358–378. doi: 10.1038/s41573-019-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 19.Qadir A., Gao Y., Suryaji P., Tian Y., Lin X., Dang K., Jiang S., Li Y., Miao Z., Qian A. Non-viral delivery system and targeted bone disease therapy. Int. J. Mol. Sci. 2019;20:565. doi: 10.3390/ijms20030565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madry H., Trippel S.B. Efficient lipid-mediated gene transfer to articular chondrocytes. Gene Ther. 2000;7:286–291. doi: 10.1038/sj.gt.3301086. [DOI] [PubMed] [Google Scholar]
- 21.Li J., Zhao X., Ye L., Coates P., Caton-Rose F. Multiple shape memory behavior of highly oriented long-chain-branched poly(lactic acid) and its recovery mechanism. J. Biomed. Mater. Res. A. 2019;107:872–883. doi: 10.1002/jbm.a.36604. [DOI] [PubMed] [Google Scholar]
- 22.Cucchiarini M., Rey-Rico A. Controlled gene delivery systems for articular cartilage repair. Adv. Biomater. Biomed. Appl. 2017:261–300. doi: 10.1155/2016/1215263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bleul R., Thiermann R., Maskos M. Techniques to control polymersome size. Macromolecules. 2015;48:7396–7409. [Google Scholar]
- 24.Santos J.L., Pandita D., Rodrigues J., Pego A.P., Granja P.L., Tomas H. Non-viral gene delivery to mesenchymal stem cells: methods, strategies and application in bone tissue engineering and regeneration. Curr. Gene Ther. 2011;11:46–57. doi: 10.2174/156652311794520102. [DOI] [PubMed] [Google Scholar]
- 25.Raftery R.M., Walsh D.P., Castano I.M., Heise A., Duffy G.P., Cryan S.-A., O'Brien F.J. Delivering nucleic-acid based nanomedicines on biomaterial scaffolds for orthopedic tissue repair: challenges, progress and future perspectives. Adv. Mater. 2016;28:5447–5469. doi: 10.1002/adma.201505088. [DOI] [PubMed] [Google Scholar]
- 26.Bono N., Ponti F., Mantovani D., Candiani G. Non-viral in vitro gene delivery: it is now time to set the bar! Pharmaceutics. 2020;12:565. doi: 10.3390/pharmaceutics12020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu H., Lv L., Dai Y., Wu G., Zhao H., Zhang F. Porous chitosan scaffolds with embedded hyaluronic acid/chitosan/plasmid-DNA nanoparticles encoding TGF-beta1 induce DNA controlled release, transfected chondrocytes, and promoted cell proliferation. PloS One. 2013;8 doi: 10.1371/journal.pone.0069950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu Q.T., Wang Y., Cao X., Deng W.W., Frimpong M.A., Yu J.N., Xu X.M. One-step formation of chondrocytes through direct reprogramming via polysaccharide-based gene delivery. Adv. Polym. Technol. 2019:1–2. 2019. [Google Scholar]
- 29.Jeon S.Y., Park J.S., Yang H.N., Woo D.G., Park K.H. Co-delivery of SOX9 genes and anti-Cbfa-1 siRNA coated onto PLGA nanoparticles for chondrogenesis of human MSCs. Biomaterials. 2012;33:4413–4423. doi: 10.1016/j.biomaterials.2012.02.051. [DOI] [PubMed] [Google Scholar]
- 30.Park J.S., Yi S.W., Kim H.J., Kim S.M., Kim J.H., Park K.H. Construction of PLGA nanoparticles coated with polycistronic SOX5, SOX6, and SOX9 genes for chondrogenesis of human mesenchymal stem cells. ACS Appl. Mater. Interfaces. 2017;9:1361–1372. doi: 10.1021/acsami.6b15354. [DOI] [PubMed] [Google Scholar]
- 31.Pi Y.B., Zhang X., Shi J.J., Zhu J.X., Chen W.Q., Zhang C.G., Gao W.W., Zhou C.Y., Ao Y.F. Targeted delivery of non-viral vectors to cartilage in vivo using a chondrocyte-homing peptide identified by phage display. Biomaterials. 2011;32:6324–6332. doi: 10.1016/j.biomaterials.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 32.Wang W., Li B., Li Y., Jiang Y., Ouyang H., Gao C. In Vivo restoration of full-thickness cartilage defects by poly(lactide-co-glycolide) sponges filled with fibrin gel, bone marrow mesenchymal stem cells and DNA complexes. Biomaterials. 2010;31:5953–5965. doi: 10.1016/j.biomaterials.2010.04.029. [DOI] [PubMed] [Google Scholar]
- 33.Zhang K., Fang H., Qin Y., Zhang L., Yin J. Functionalized scaffold for in situ efficient gene transfection of mesenchymal stem cells spheroids toward chondrogenesis. ACS Appl. Mater. Interfaces. 2018;10:33993–34004. doi: 10.1021/acsami.8b12268. [DOI] [PubMed] [Google Scholar]
- 34.Park J.S., Yi S.W., Kim H.J., Park K.H. Receptor-mediated gene delivery into human mesenchymal stem cells using hyaluronic acid-shielded polyethylenimine/pDNA nanogels. Carbohydr. Polym. 2016;136:791–802. doi: 10.1016/j.carbpol.2015.09.053. [DOI] [PubMed] [Google Scholar]
- 35.Aini H., Itaka K., Fujisawa A., Uchida H., Uchida S., Fukushima S., Kataoka K., Saito T., Chung U.I., Ohba S. Messenger RNA delivery of a cartilage-anabolic transcription factor as a disease-modifying strategy for osteoarthritis treatment. Sci. Rep. 2016;6:18743. doi: 10.1038/srep18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B., Yang J., Ma L., Li F., Tu Z., Gao C. Fabrication of poly(lactide-co-glycolide) scaffold filled with fibrin gel, mesenchymal stem cells, and poly(ethylene oxide)-b-poly(L-lysine)/TGF-beta1 plasmid DNA complexes for cartilage restoration in vivo. J. Biomed. Mater. Res. A. 2013;101:3097–3108. doi: 10.1002/jbm.a.34618. [DOI] [PubMed] [Google Scholar]
- 37.Rey-Rico A., Venkatesan J.K., Schmitt G., Speicher-Mentges S., Madry H., Cucchiarini M. Effective remodelling of human osteoarthritic cartilage by sox9 gene transfer and overexpression upon delivery of rAAV vectors in polymeric micelles. Mol. Pharm. 2018;15:2816–2826. doi: 10.1021/acs.molpharmaceut.8b00331. [DOI] [PubMed] [Google Scholar]
- 38.Venkatesan J.K., Moutos F.T., Rey-Rico A., Estes B.T., Frisch J., Schmitt G., Madry H., Guilak F., Cucchiarini M. Chondrogenic differentiation processes in human bone-marrow aspirates seeded in three-dimensional-woven poly(varepsilon-caprolactone) scaffolds enhanced by recombinant adeno-associated virus-mediated SOX9 gene transfer. Hum. Gene Ther. 2018;29:1277–1286. doi: 10.1089/hum.2017.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Venkatesan J.K., Meng W., Rey-Rico A., Schmitt G., Speicher-Mentges S., Falentin-Daudre C., Leroux A., Madry H., Migonney V., Cucchiarini M. Enhanced chondrogenic differentiation activities in human bone marrow apirates via sox9 overexpression mediated by pNaSS-grafted PCL film-guided rAAV gene transfer. Pharmaceutics. 2020;12:280. doi: 10.3390/pharmaceutics12030280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roughley P.J., Mort J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014;1:8. doi: 10.1186/s40634-014-0008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu H.D., Zhao H.Q., Wang K., Lv L.L. Novel hyaluronic acid-chitosan nanoparticles as non-viral gene delivery vectors targeting osteoarthritis. Int. J. Pharm. 2011;420:358–365. doi: 10.1016/j.ijpharm.2011.08.046. [DOI] [PubMed] [Google Scholar]
- 42.Byers B.A., Pavlath G.K., Murphy T.J., Karsenty G., Garcia A.J. Cell-type-dependent up-regulation of in vitro mineralization after overexpression of the osteoblast-specific transcription factor Runx2/Cbfal. J. Bone Miner. Res. 2002;17:1931–1944. doi: 10.1359/jbmr.2002.17.11.1931. [DOI] [PubMed] [Google Scholar]
- 43.Baroti T., Zimmermann Y., Schillinger A., Liu L., Lommes P., Wegner M., Stolt C.C. Transcription factors Sox5 and Sox6 exert direct and indirect influences on oligodendroglial migration in spinal cord and forebrain. Glia. 2016;64:122–138. doi: 10.1002/glia.22919. [DOI] [PubMed] [Google Scholar]
- 44.Lee H.H., Haleem A.M., Yao V., Li J., Xiao X., Chu C.R. Release of bioactive adeno-associated virus from fibrin scaffolds: effects of fibrin glue concentrations. Tissue Eng. A. 2011;17:1969–1978. doi: 10.1089/ten.tea.2010.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cucchiarini M., Madry H. Advances in gene therapy for cartilage repair. Ann. Joint. 2018;3 doi: 10.1177/1947603510392914. 97-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guo T., Zhao J., Chang J., Ding Z., Hong H., Chen J., Zhang J. Porous chitosan-gelatin scaffold containing plasmid DNA encoding transforming growth factor-beta1 for chondrocytes proliferation. Biomaterials. 2006;27:1095–1103. doi: 10.1016/j.biomaterials.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 47.Li B., Li F.F., Ma L., Yang J.Z., Wang C.F., Wang D.A., Gao C.Y. Poly(lactide-co-glycolide)/fibrin gel construct as a 3D model to evaluate gene therapy of cartilage in vivo. Mol. Pharm. 2014;11:2062–2070. doi: 10.1021/mp5000136. [DOI] [PubMed] [Google Scholar]
- 48.Mencia Castano I., Curtin C.M., Shaw G., Murphy J.M., Duffy G.P., O'Brien F.J. A novel collagen-nanohydroxyapatite microRNA-activated scaffold for tissue engineering applications capable of efficient delivery of both miR-mimics and antagomiRs to human mesenchymal stem cells. J. Contr. Release. 2015;200:42–51. doi: 10.1016/j.jconrel.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 49.Carthew J., Donderwinkel I., Shrestha S., Truong V.X., Forsythe J.S., Frith J.E. In situ miRNA delivery from a hydrogel promotes osteogenesis of encapsulated mesenchymal stromal cells. Acta Biomater. 2020;101:249–261. doi: 10.1016/j.actbio.2019.11.016. [DOI] [PubMed] [Google Scholar]
- 50.Capito R.M., Spector M. Collagen scaffolds for nonviral IGF-1 gene delivery in articular cartilage tissue engineering. Gene Ther. 2007;14:721–732. doi: 10.1038/sj.gt.3302918. [DOI] [PubMed] [Google Scholar]
- 51.Gonzalez-Fernandez T., Tierney E.G., Cunniffe G.M., O'Brien F.J., Kelly D.J. Gene delivery of TGF-beta 3 and BMP2 in an MSC-laden alginate hydrogel for articular cartilage and endochondral bone tissue engineering. Tissue Eng. A. 2016;22:776–787. doi: 10.1089/ten.TEA.2015.0576. [DOI] [PubMed] [Google Scholar]
- 52.Cunniffe G.M., Gonzalez-Fernandez T., Daly A., Sathy B.N., Jeon O., Alsberg E., Kelly D.J. Three-dimensional bioprinting of polycaprolactone reinforced gene activated bioinks for bone tissue engineering. Tissue Eng. A. 2017;23:891–900. doi: 10.1089/ten.tea.2016.0498. [DOI] [PubMed] [Google Scholar]
- 53.Venkatesan J.K., Gardner O., Rey-Rico A., Eglin D., Alini M., Stoddart M.J., Cucchiarini M., Madry H. Improved chondrogenic differentiation of rAAV SOX9-modified human MSCs seeded in fibrin-polyurethane scaffolds in a hydrodynamic environment. Int. J. Mol. Sci. 2018;19:3359–3364. doi: 10.3390/ijms19092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hong H., Seo Y.B., Kim D.Y., Lee J.S., Lee Y.J., Lee H., Ajiteru O., Sultan M.T., Lee O.J., Kim S.H., Park C.H. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials. 2020;232:119679. doi: 10.1016/j.biomaterials.2019.119679. [DOI] [PubMed] [Google Scholar]
- 55.Lee J.E., Kim K.E., Kwon I.C., Ahn H.J., Lee S.H., Cho H., Kim H.J., Seong S.C., Lee M.C. Effects of the controlled-released TGF-beta 1 from chitosan microspheres on chondrocytes cultured in a collagen/chitosan/glycosaminoglycan scaffold. Biomaterials. 2004;25:4163–4173. doi: 10.1016/j.biomaterials.2003.10.057. [DOI] [PubMed] [Google Scholar]
- 56.Moutos F.T., Glass K.A., Compton S.A., Ross A.K., Gersbach C.A., Guilak F., Estes B.T. Anatomically shaped tissue-engineered cartilage with tunable and inducible anticytokine delivery for biological joint resurfacing. Proc. Natl. Acad. Sci. U. S. A. 2016;113:E4513–E4522. doi: 10.1073/pnas.1601639113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brunger J.M., Huynh N.P., Guenther C.M., Perez-Pinera P., Moutos F.T., Sanchez-Adams J., Gersbach C.A., Guilak F. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc. Natl. Acad. Sci. U. S. A. 2014;111:E798–E806. doi: 10.1073/pnas.1321744111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glass K.A., Link J.M., Brunger J.M., Moutos F.T., Gersbach C.A., Guilak F. Tissue-engineered cartilage with inducible and tunable immunomodulatory properties. Biomaterials. 2014;35:5921–5931. doi: 10.1016/j.biomaterials.2014.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mkhabela V.J., Ray S.S. Poly(epsilon-caprolactone) nanocomposite scaffolds for tissue engineering: a brief overview. J. Nanosci. Nanotechnol. 2014;14:535–545. doi: 10.1166/jnn.2014.9055. [DOI] [PubMed] [Google Scholar]
- 60.Huey D.J., Hu J.C., Athanasiou K.A. Unlike bone, cartilage regeneration remains elusive. Science. 2012;338:917–921. doi: 10.1126/science.1222454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gritsch L., Conoscenti G., La Carrubba V., Nooeaid P., Boccaccini A.R. Polylactide-based materials science strategies to improve tissue-material interface without the use of growth factors or other biological molecules. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;94:1083–1101. doi: 10.1016/j.msec.2018.09.038. [DOI] [PubMed] [Google Scholar]
- 62.Chen J., Chen H., Li P., Diao H., Zhu S., Dong L., Wang R., Guo T., Zhao J., Zhang J. Simultaneous regeneration of articular cartilage and subchondral bone in vivo using MSCs induced by a spatially controlled gene delivery system in bilayered integrated scaffolds. Biomaterials. 2011;32:4793–4805. doi: 10.1016/j.biomaterials.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 63.Needham C.J., Shah S.R., Dahlin R.L., Kinard L.A., Lam J., Watson B.M., Lu S., Kasper F.K., Mikos A.G. Osteochondral tissue regeneration through polymeric delivery of DNA encoding for the SOX trio and RUNX2. Acta Biomater. 2014;10:4103–4112. doi: 10.1016/j.actbio.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim H.J., Han M.A., Shin J.Y., Jeon J.H., Lee S.J., Yoon M.Y., Kim H.J., Choi E.J., Do S.H., Yang V.C., He H., Yang Y.I. Intra-articular delivery of synovium-resident mesenchymal stem cells via BMP-7-loaded fibrous PLGA scaffolds for cartilage repair. J. Contr. Release. 2019;302:169–180. doi: 10.1016/j.jconrel.2019.04.002. [DOI] [PubMed] [Google Scholar]
- 65.Sadtler K., Singh A., Wolf M.T., Wang X., Pardoll D.M., Elisseeff J.H. Design, clinical translation and immunological response of biomaterials in regenerative medicine. Nat. Rev. Mater. 2016;1:16040. [Google Scholar]
- 66.Lin D., Chai Y., Ma Y., Duan B., Yuan Y., Liu C. Rapid initiation of guided bone regeneration driven by spatiotemporal delivery of IL-8 and BMP-2 from hierarchical MBG-based scaffold. Biomaterials. 2019;196:122–137. doi: 10.1016/j.biomaterials.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 67.Rosales A.M., Anseth K.S. The design of reversible hydrogels to capture extracellular matrix dynamics. Nat. Rev. Mater. 2016;1:1–15. doi: 10.1038/natrevmats.2015.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dey K., Agnelli S., Sartore L. Dynamic freedom: substrate stress relaxation stimulates cell responses. Biomater. Sci. 2019;7:836–842. doi: 10.1039/c8bm01305e. [DOI] [PubMed] [Google Scholar]
- 69.Cabral J., Moratti S.C. Hydrogels for biomedical applications. Future Med. Chem. 2011;3:1877–1888. doi: 10.4155/fmc.11.134. [DOI] [PubMed] [Google Scholar]
- 70.Garcia J.P., Stein J., Cai Y., Riemers F., Wexselblatt E., Wengel J., Tryfonidou M., Yayon A., Howard K.A., Creemers L.B. Fibrin-hyaluronic acid hydrogel-based delivery of antisense oligonucleotides for ADAMTS5 inhibition in co-delivered and resident joint cells in osteoarthritis. J. Contr. Release. 2019;294:247–258. doi: 10.1016/j.jconrel.2018.12.030. [DOI] [PubMed] [Google Scholar]
- 71.Lolli A., Sivasubramaniyan K., Vainieri M.L., Oieni J., Kops N., Yayon A., van Osch G. Hydrogel-based delivery of antimiR-221 enhances cartilage regeneration by endogenous cells. J. Contr. Release. 2019;309:220–230. doi: 10.1016/j.jconrel.2019.07.040. [DOI] [PubMed] [Google Scholar]
- 72.Qi C., Liu J., Jin Y., Xu L.M., Wang G.B., Wang Z., Wang L. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials. 2018;163:89–104. doi: 10.1016/j.biomaterials.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 73.Li X., Ding J., Zhang Z., Yang M., Yu J., Wang J., Chang F., Chen X. Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl. Mater. Interfaces. 2016;8:5148–5159. doi: 10.1021/acsami.5b12212. [DOI] [PubMed] [Google Scholar]
- 74.Rey-Rico A., Venkatesan J.K., Frisch J., Schmitt G., Monge-Marcet A., Lopez-Chicon P., Mata A., Semino C., Madry H., Cucchiarini M. Effective and durable genetic modification of human mesenchymal stem cells via controlled release of rAAV vectors from self-assembling peptide hydrogels with a maintained differentiation potency. Acta Biomater. 2015;18:118–127. doi: 10.1016/j.actbio.2015.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Arya N., Forget A., Sarem M., Shastri V.P. RGDSP functionalized carboxylated agarose as extrudable carriers for chondrocyte delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;99:103–111. doi: 10.1016/j.msec.2019.01.080. [DOI] [PubMed] [Google Scholar]
- 76.Madry H., Gao L., Rey-Rico A., Venkatesan J.K., Muller-Brandt K., Cai X., Goebel L., Schmitt G., Speicher-Mentges S., Zurakowski D., Menger M.D., Laschke M.W., Cucchiarini M. Thermosensitive hydrogel based on PEO-PPO-PEO poloxamers for a controlled in situ release of recombinant adeno-associated viral vectors for effective gene therapy of cartilage defects. Adv. Mater. 2020;32 doi: 10.1002/adma.201906508. [DOI] [PubMed] [Google Scholar]
- 77.Rey-Rico A., Frisch J., Venkatesan J.K., Schmitt G., Rial-Hermida I., Taboada P., Concheiro A., Madry H., Alvarez-Lorenzo C., Cucchiarini M. PEO-PPO-PEO carriers for rAAV-mediated transduction of human articular chondrocytes in vitro and in a human osteochondral defect model. ACS Appl. Mater. Interfaces. 2016;8:20600–20613. doi: 10.1021/acsami.6b06509. [DOI] [PubMed] [Google Scholar]
- 78.Rey-Rico A., Venkatesan J.K., Frisch J., Rial-Hermida I., Schmitt G., Concheiro A., Madry H., Alvarez-Lorenzo C., Cucchiarini M. PEO-PPO-PEO micelles as effective rAAV-mediated gene delivery systems to target human mesenchymal stem cells without altering their differentiation potency. Acta Biomater. 2015;27:42–52. doi: 10.1016/j.actbio.2015.08.046. [DOI] [PubMed] [Google Scholar]
- 79.Rey-Rico A., Babicz H., Madry H., Concheiro A., Alvarez-Lorenzo C., Cucchiarini M. Supramolecular polypseudorotaxane gels for controlled delivery of rAAV vectors in human mesenchymal stem cells for regenerative medicine. Int. J. Pharm. 2017;531:492–503. doi: 10.1016/j.ijpharm.2017.05.050. [DOI] [PubMed] [Google Scholar]
- 80.Yang H.Y., van Ee R.J., Timmer K., Craenmehr E.G.M., Huang J.H., Oner F.C., Dhert W.J.A., Kragten A.H.M., Willems N., Grinwis G.C.M., Tryfonidou M.A., Papen-Botterhuis N.E., Creemers L.B. A novel injectable thermoresponsive and cytocompatible gel of poly(N-isopropylacrylamide) with layered double hydroxides facilitates siRNA delivery into chondrocytes in 3D culture. Acta Biomater. 2015;23:214–228. doi: 10.1016/j.actbio.2015.05.018. [DOI] [PubMed] [Google Scholar]
- 81.Park J.S., Yang H.N., Woo D.G., Jeon S.Y., Park K.H. Poly(N-isopropylacrylamide-co-acrylic acid) nanogels for tracing and delivering genes to human mesenchymal stem cells. Biomaterials. 2013;34:8819–8834. doi: 10.1016/j.biomaterials.2013.07.082. [DOI] [PubMed] [Google Scholar]