Abstract
Context
Concerns about the adequacy of pain management among older adults are increasing, particularly with restrictions on opioid prescribing.
Objectives
To examine associations between prescription pain medication receipt and patient-reported pain interference in older adults with and without cancer.
Methods
Using the 2007–2012 Surveillance Epidemiology and End Results (SEER)-Medicare Health Outcomes Survey (MHOS) database linked to Medicare Part D prescription claims, we selected MHOS respondents (N = 15,624) aged ≥ 66 years, ≤ 5 years of a cancer diagnosis (N = 9105), or without cancer (N = 6519). We measured receipt of opioids, non-steroidal antiinflammatory drugs, and antiepileptics, and selected antidepressants within 30 days prior to survey. Patient-reported activity limitation due to pain (pain interference) within the past 30 days was summarized as severe, moderate, or mild/none. Logistic regression using predictive margins estimated associations between pain interference, cancer history, and pain medication receipt, adjusting for socio-demographics, chronic conditions, and Part D low-income subsidy.
Results
Severe or moderate pain interference was reported by 21.3% and 46.1%, respectively. Pain medication was received by 21.5%, with 11.6% receiving opioids. Among adults reporting severe pain interference, opioid prescriptions were filled by 27.0% versus 23.8% (p = 0.040) with and without cancer, respectively. Over half (56%) of adults reporting severe pain in both groups failed to receive any prescription pain medication.
Conclusions
Older adults with cancer were more likely to receive prescription pain medications compared with adults without cancer; however, many older adults reporting severe pain interference did not receive medications. Improved assessment and management of pain among older adults with and without cancer is urgently needed.
Keywords: Pain treatment, Opioids, Pain interference, Cancer, Medicare part D, Medicare health outcomes study
Background/introduction
The U.S. population is aging, and the prevalence of chronic health conditions, including cancer, is increasing. Older adults are susceptible to acute and chronic pain, with approximately 29% of U.S. adults aged ≥ 60 years reporting persistent pain [1]. Chronic non-cancer pain may result from a broad array of medical concerns including joint problems, orthopedic trauma including osteoporotic fractures, or the result of conditions such as heart failure [2]. Cancer may contribute further to the burden of pain, due to the disease itself, or sequelae of cancer-directed therapies [3]. Adequate pain management is essential to sustain quality of life and may reduce emotional distress and improve survival [4–6].
Understanding patterns and predictors of medication therapy for pain has become a critical research focus, given competing concerns about undertreatment and the potential for adverse medication side effects, longer-term dependence, and misuse. Opioids are the most commonly prescribed medications used to treat pain, prescribed for as many as two-thirds of those reporting severe pain [7]. Recent studies have reported higher opioid use rates for adults with, compared with without cancer [8–11], but few population-based studies have examined associations between pain medication use and pain severity [7, 12] and whether it differs for older adults with and without cancer.
In this study, we use a unique linkage between survey data detailing pain interference with usual activities, with cancer registry data and Medicare prescription drug (Part D) claims. We test the hypothesis that pain medication receipt will differ for older adults with and without cancer overall and across different levels of pain interference. We also examine whether beneficiary sociodemographic characteristics are associated with pain medication receipt when controlling for reported pain interference. Improving our understanding of these relationships is essential to address barriers to pain management for older adults both with and without cancer.
Methods
Data
We used the Surveillance, Epidemiology and End Results (SEER)-Medicare Health Outcomes Survey (MHOS) with a novel linkage to Medicare Part D claims. SEER provides detailed cancer registry data from selected U.S. regions including cancer type(s), stage, histology, and diagnosis date. The Centers for Medicare & Medicaid Services (CMS) uses MHOS to collect self-reported responses to questions on the Veteran’s Rand 12 item survey (VR-12), in addition to selected other questions related to health status. [13] The survey is administered annually to randomly sampled Medicare beneficiaries enrolled in Medicare Advantage (MA) plans. The SEER-MHOS represents a linkage for beneficiaries with a SEER-reported cancer who responded to a MHOS [13, 14]. The SEER-MHOS is augmented by a 5% sample of MHOS respondents without cancer but residing in SEER regions [14]. Medicare Part D prescription drug claims for the period from 2007 to 2014 were linked for Part D-enrolled MHOS respondents.
Sample selection
We selected beneficiaries in the SEER-MHOS dataset with only one cancer primary, any invasive disease or Stage 0 breast cancer, and diagnosed between January 2003 and December 2012. Beneficiaries had to complete at least one MHOS between January 2008 and December 2012 and within 5 years after their cancer diagnosis, a period during which respondents may receive active or adjuvant therapy or experience late or long-term effects of cancer treatment. The non-cancer comparison group completed at least one MHOS between January 2008 and December 2012 and did not self-report cancer on any MHOS surveys. We excluded observations if the cancer diagnosis month was unknown; diagnosis was first reported on death certificate or autopsy. To ensure complete claims data, we excluded beneficiaries age ≤ 65 years at MHOS date, and without continuous enrollment in Medicare Parts A, B, MA, and Part D during the 12 months pre-post the MHOS month or until death [Appendix Fig. 2].
Key measures
The key outcome was receipt of prescription medications for treatment of pain as of the MHOS date. We categorized pain medications as opioids, non-opioid analgesic (non-steroidal antiinflammatory drugs (NSAIDs), local anesthetics), and adjuvant pain medications (antiepileptics and selected antidepressants). We did not include steroids because they are prescribed for multiple clinical conditions, including part of chemotherapy regimens, and we were unable to distinguish the indication for use. We identified relevant individual medications for pain based on input from the literature [9, 15], and clinical guidelines [16]. Once we identified the medications, we searched Part D claims to identify relevant generic drug names, including combination medications. Finally, we searched for evidence of drug supply on the MHOS date or the 30 days prior. For opioid users, we quantified days supplied in the prior 60 days, categorized into ranges. We calculated the morphine equivalent daily dose (MEDD) as of the MHOS date or the closest prior date with opioid supply, using morphine equivalencies published by the Centers for Disease Control and Prevention (CDC) [17] and modified by CMS [18]. We generated an indicator for high MEDD (≥ 90 mg) based on thresholds designated as high-risk for overdose [19]. All medication measurement decisions were reviewed by palliative care clinicians and pharmacists on the research team.
Key independent variables were pain interference with function and cancer history. Pain interference was measured based on responses to the MHOS question “During the past 4 weeks, how much did pain interfere with your normal work (include both work outside the home and housework)?” We grouped the responses into three levels: mild/none (responses: not at all, a little bit); moderate; and severe (responses: quite a bit, extremely). Our analyses included adjustment for beneficiary demographics (age, race/ethnicity, sex, marital status, educational attainment), health status (indicators from a chronic condition checklist, and time from MHOS survey to death, (< 6 months compared with ≥ 6 months)), zip code level percentage of the population living in poverty, region (Northeast, South, Midwest, West), MA plan type (HMO or PPO/other), and receipt of the Part D low-income subsidy (LIS, full or partial versus none).
Analysis
We described sample characteristics overall and stratified by cancer history, using chi-squared statistics to compare by cancer history. We calculated percentages receiving pain medications (any, by type, in combinations). Medication use was compared by cancer history and level of pain interference, with comparisons using chi-squared analysis, or Student’s T test. We estimated multivariable logistic regressions to test for associations between pain interference, cancer history, and the interaction between them with receipt of (a) any pain medication and (b) any opioid, adjusting for socio-demographics, health status, and plan characteristics as previously described. We report predictive margins and 95% confidence intervals (CI), which represent the predicted percentage with medication use, by cancer history, pain severity, and for selected covariates, adjusted for all other socio-demographic, health status, and plan characteristics. All comparisons were two-sided with alpha = .05. Analyses were conducted using SAS version 9.4 (SAS Institute Inc., Cary, NC) and Stata 15 (Statacorp, College Station, Tx). The Yale Human Investigations Committee granted exempt status to this project.
Results
The sample of 15,624 beneficiaries (9105 with cancer; 6519 without cancer) was 49% female and 66.4% white, non-Hispanic (Table 1). The sample came disproportionately from the western region (54.7%), with 20.6% receiving the Part D LIS; 2.9% died within 6 months of survey. Severe pain interference was reported by 21.3%, with 46.1% reporting moderate levels. The characteristics of beneficiaries with cancer differed from those without cancer on almost all dimensions except for marital status, LIS receipt, and plan type. Death occurred within 6 months for 4.2% and 1.0% of those with and without cancer, respectively. More beneficiaries with cancer (22.6%) reported severe pain interference than those without cancer (19.5%, p < 0.001). (Cancer type, stage at diagnosis and initial treatment modality is reported in Appendix Table 5.)
Table 1.
Sample characteristics
| Sample N’s | Overall | Cancer | No CANCER | ||||
|---|---|---|---|---|---|---|---|
| 15,624 | 9105 | 6519 | |||||
| N | % | N | % | N | % | p valuea | |
| Level of pain interference | < 0.001 | ||||||
| Severe | 3322 | 21.3 | 2054 | 22.6 | 1268 | 19.5 | |
| Moderate | 7201 | 46.1 | 4190 | 46.0 | 3011 | 46.2 | |
| Mild/none | 5101 | 32.7 | 2861 | 31.4 | 2240 | 34.4 | |
| Sex | < 0.001 | ||||||
| Male | 7968 | 51.0 | 5059 | 55.6 | 2909 | 44.6 | |
| Female | 7656 | 49.0 | 4046 | 44.4 | 3610 | 55.4 | |
| Age group | < 0.001 | ||||||
| 66–69 | 4387 | 28.1 | 2323 | 25.5 | 2064 | 31.7 | |
| 70–74 | 4447 | 28.5 | 2556 | 28.1 | 1891 | 29.0 | |
| 75–79 | 3328 | 21.3 | 2048 | 22.5 | 1280 | 19.6 | |
| 80+ | 3462 | 22.2 | 2178 | 23.9 | 1284 | 19.7 | |
| Race/ethnicity | < 0.001 | ||||||
| White, non-Hispanic | 10,370 | 66.4 | 6142 | 67.5 | 4228 | 64.9 | |
| Black, non-Hispanic | 1474 | 9.4 | 911 | 10.0 | 563 | 8.6 | |
| Hispanic | 1673 | 10.7 | 878 | 9.6 | 795 | 12.2 | |
| Asian, other | 2107 | 13.5 | 1174 | 12.9 | 933 | 14.3 | |
| Marital status | 0.547 | ||||||
| Currently married | 9181 | 58.8 | 5321 | 58.4 | 3860 | 59.2 | |
| Formerly married | 5823 | 37.3 | 3426 | 37.6 | 2397 | 36.8 | |
| Never married | 620 | 4.0 | 358 | 3.9 | 262 | 4.0 | |
| Education | < 0.001 | ||||||
| < High school diploma | 3868 | 24.8 | 2266 | 24.9 | 1602 | 24.6 | |
| High school diploma | 4850 | 31.0 | 2791 | 30.7 | 2059 | 31.6 | |
| > High school diploma | 6906 | 44.2 | 4048 | 44.5 | 2858 | 43.8 | |
| Has a doctor ever told you that you have… | |||||||
| Coronary artery disease | 2144 | 13.7 | 1331 | 14.6 | 813 | 12.5 | < 0.001 |
| Stroke | 1299 | 8.3 | 827 | 9.1 | 472 | 7.2 | < 0.001 |
| Pulmonary disease | 2440 | 15.6 | 1538 | 16.9 | 902 | 13.8 | < 0.001 |
| Diabetes | 4072 | 26.1 | 2428 | 26.7 | 1644 | 25.2 | 0.042 |
| Depression | 2255 | 14.4 | 1399 | 15.4 | 856 | 13.1 | < 0.001 |
| Arthritis | 6109 | 39.1 | 3535 | 38.8 | 2574 | 39.5 | 0.405 |
| Sciatica | 3388 | 21.7 | 1919 | 21.1 | 1469 | 22.5 | 0.029 |
| Poverty rates | < 0.001 | ||||||
| Low (0 to < 5%) | 3055 | 19.6 | 2265 | 24.9 | 790 | 12.1 | |
| 5 to < 10% | 4384 | 28.1 | 2428 | 26.7 | 1956 | 30.0 | |
| 10 to < 20% | 5099 | 32.6 | 2725 | 29.9 | 2374 | 36.4 | |
| High (20 to 100%) | 3086 | 19.8 | 1687 | 18.5 | 1399 | 21.5 | |
| Region | < 0.001 | ||||||
| Northeast | 1945 | 12.5 | 1258 | 13.8 | 687 | 10.5 | |
| South | 1427 | 9.1 | 848 | 9.3 | 579 | 8.9 | |
| Central | 3699 | 23.7 | 2098 | 23.0 | 1601 | 24.6 | |
| West | 8548 | 54.7 | 4901 | 53.8 | 3647 | 55.9 | |
| Part D low income subsidy | 0.284 | ||||||
| Full/partial | 3218 | 20.6 | 1902 | 20.9 | 1316 | 20.2 | |
| None | 12,406 | 79.4 | 7203 | 79.1 | 5203 | 79.8 | |
| Plan type | 0.118 | ||||||
| HMO | 14,451 | 92.5 | 8396 | 92.2 | 6055 | 92.9 | |
| Other plan type | 1173 | 7.5 | 709 | 7.8 | 464 | 7.1 | |
| Proximity of death | < 0.001 | ||||||
| < 6 months from MHOS survey | 446 | 2.9 | 378 | 4.2 | 68 | 1.0 | |
| ≥ 6 months from MHOS survey | 15,178 | 97.1 | 8727 | 95.8 | 6451 | 99.0 | |
| MHOS survey year | < 0.001 | ||||||
| 2008 | 2907 | 18.6 | 1771 | 19.5 | 1136 | 17.4 | |
| 2009 | 3951 | 25.3 | 2338 | 25.7 | 1613 | 24.7 | |
| 2010 | 3237 | 20.7 | 1882 | 20.7 | 1355 | 20.8 | |
| 2011 | 2884 | 18.5 | 1680 | 18.5 | 1204 | 18.5 | |
| 2012 | 2645 | 16.9 | 1434 | 15.7 | 1211 | 18.6 | |
p value reported for comparison of distribution for cancer and non-cancer samples. HMO, health maintenance organization; MHOS, Medicare Health Outcomes Survey. Source: SEER-MHOS-Part D linked data, 2008–2012
Overall, pain medication was received by 21.5% of the total sample, with 11.6% receiving an opioid, 17.0% a non-opioid analgesic, and 15.8% an adjuvant pain medication (Table 2). Medication rates overall and for each individual category were higher for beneficiaries with cancer versus without, for example, 22.5% versus 20.1% (p < 0.001) received any pain medication, and 13.1% versus 9.5% (p < 0.001) received opioid pain medication. The percentage receiving pain medication also varied by level of pain interference, with those reporting severe pain most likely to report medication use, overall (44.4%) and in each medication category, with levels decreasing with lower levels of pain interference. Among those receiving pain medications (Appendix Table 6), 46.1% received only non-opioid analgesics, while the remainder received both non-opioids and opioids. A larger percentage of adults with a cancer history received medication combinations (55.8%) compared with the non-cancer subgroup (44.9%, p < 0.001), who were more likely to receive only non-opioid analgesics.
Table 2.
Medication therapy for pain management by type, overall, by cancer history, and level of pain interference (unadjusted)
| Overall | By cancer history | By level of pain interference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | No Cancer | Severe | Moderate | Mild/none | ||||||||||
| N | % | N | % | N | % | p valueb | N | % | N | % | N | % | p value | |
| By typea | ||||||||||||||
| Any pain medication | 3361 | 21.5 | 2048 | 22.5 | 1313 | 20.1 | < 0.001 | 1475 | 44.4 | 1412 | 19.6 | 474 | 9.3 | < 0.001 |
| Opioid | 1811 | 11.6 | 1195 | 13.1 | 616 | 9.5 | < 0.001 | 983 | 29.6 | 646 | 9.0 | 182 | 3.6 | < 0.001 |
| Non-opioid analgesic | 2663 | 17.0 | 1635 | 18.0 | 1028 | 15.8 | < 0.001 | 1206 | 36.3 | 1098 | 15.3 | 359 | 7.0 | < 0.001 |
| Adjuvant pain medications | 2475 | 15.8 | 1499 | 16.5 | 976 | 15.0 | 0.01 | 953 | 28.7 | 1068 | 14.8 | 454 | 8.9 | < 0.001 |
Categories not mutually exclusive. Ns may sum to > 100%
p values reported for chi-squared comparison of medication use rates by cancer history and pain interference
Source: SEER-MHOS-Part D linked data, 2008–2012
After adjusting for all characteristics, the percentage receiving any pain medication or any opioid varied by cancer history and severity of pain interference (Fig. 1). The percentage receiving any pain medication was higher for those with cancer versus the non-cancer comparison group for those with severe (cancer 38.7%; 95% CI 36.5–40.9 versus non-cancer 37.1%; 95% CI 34.4–39.8 p = 0.048) or little/no pain interference (cancer 12.6%; 95% CI 11.3–14.0 versus non-cancer 9.6%;95% CI 8.3–10.9, p = 0.001). Opioid receipt was higher for individuals with cancer compared with the non-cancer group across all three levels of pain interference. For example, among the cancer sample reporting severe pain interference, 26.4% (95% CI 24.4–28.4) received opioids versus 23.8% (95% CI 21.5–26.2, p = 0.003) in the non-cancer group. Receipt of any pain medication was also higher for women compared with men (23.1% (95% CI22.2–24.0) versus 19.8% (95% CI 18.9–20.7) p < 0.001), decreased with increasing age, and was higher for individuals with the Part D LIS compared with those without, and higher for those with proximal death (Table 3). Paralleling receipt of any pain medication, opioid receipt was higher for women versus men, with the Part D LIS, proximal death, and decreased with increasing age. In addition, opioid receipt was higher for non-Hispanic whites compared with all other race/ethnic groups, with lower educational attainment, and with higher poverty rates.
Fig. 1.
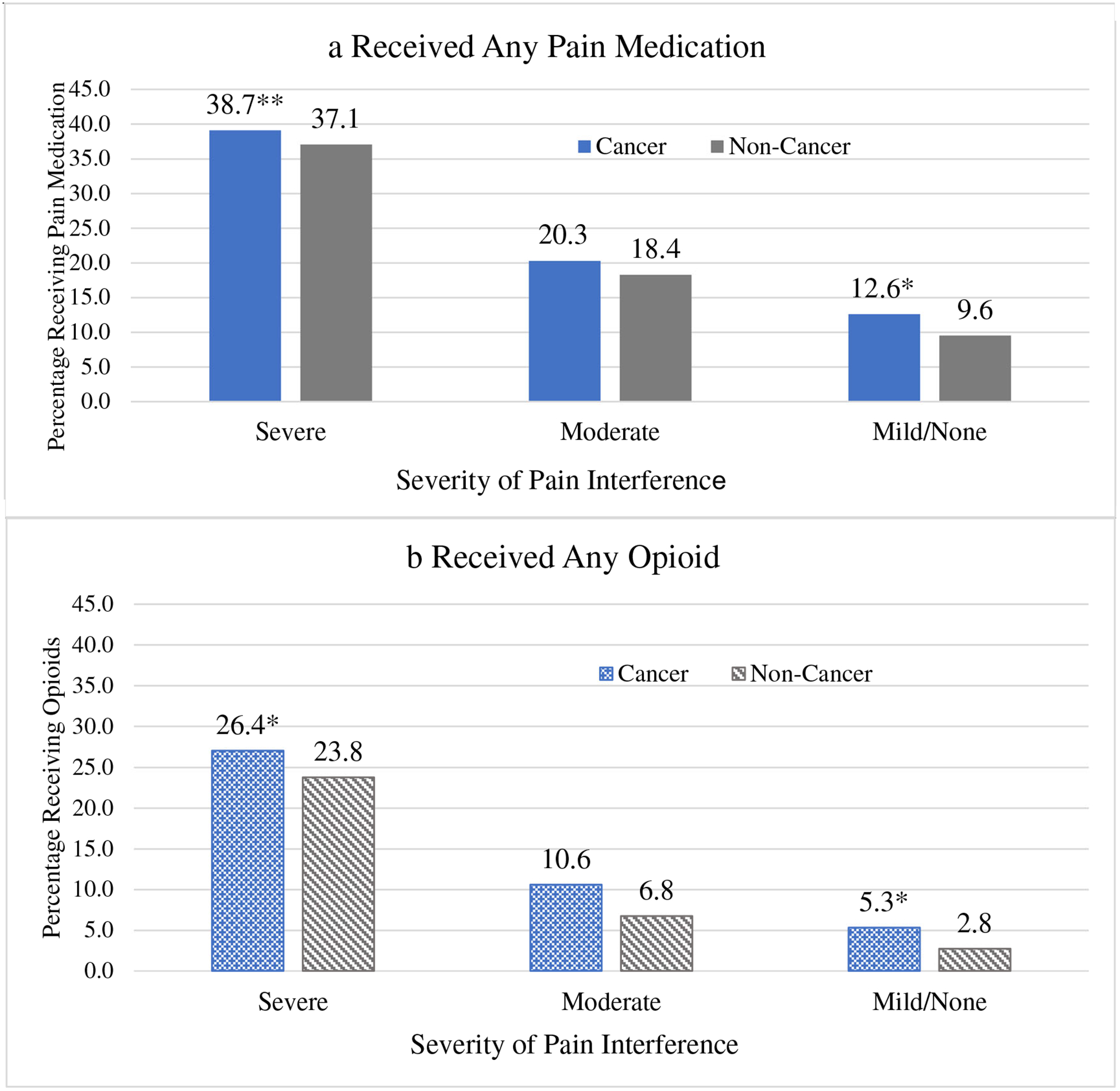
Adjusted Levels (predictive margins) for pain medication receipt by severity of pain interference and cancer history. Definition: color graphic with shading
Models adjust for age, sex, race/ethnicity, marital status, education, comorbidities, area poverty rates, region of the country, Part D LIS, and plan type. Levels of any pain medication use (Figure 1a) or any opioid use (Figure 1b) differ by cancer history when stratified by pain severity at * p<.01; **p<.05. Abbreviations: CA, cancer. Source: SEER-MHOS-Part D linked data, 2008–2012.
Table 3.
Adjusted levels (predictive margins) for any pain medication or any opioid receipt by cancer history, severity of pain interference, and selected respondent characteristics
| Any pain medication | Any opioid | |||||||
|---|---|---|---|---|---|---|---|---|
| Predictive margin | 95% CI margin | p value* | Predictive margin | 95% CI margin | p value* | |||
| Pain interference | ||||||||
| Severe | 38.1 | 36.3 | 39.9 | < 0.001 | 25.3 | 23.7 | 27.0 | < 0.001 |
| Moderate | 19.5 | 18.6 | 20.4 | < 0.001 | 9.0 | 8.3 | 9.7 | < 0.001 |
| Mild/none | 11.3 | 10.4 | 12.3 | 4.2 | 3.6 | 4.9 | ||
| Cancer history | ||||||||
| Cancer | 22.4 | 21.6 | 23.2 | 0.002 | 9.8 | 9.1 | 10.5 | < 0.001 |
| No cancer | 20.3 | 19.4 | 21.3 | 12.9 | 12.2 | 13.5 | ||
| Cancer history, stratified by pain interference | ||||||||
| Severe | ||||||||
| Cancer | 38.7 | 36.5 | 40.9 | 0.048 | 26.4 | 24.4 | 28.4 | 0.003 |
| No cancer | 37.1 | 34.4 | 39.8 | 23.8 | 21.5 | 26.2 | ||
| Moderate | ||||||||
| Cancer | 20.3 | 19.1 | 21.5 | 0.105 | 10.6 | 9.6 | 11.5 | 0.292 |
| No cancer | 18.4 | 17.0 | 19.8 | 6.8 | 5.9 | 7.7 | ||
| Mild/none | ||||||||
| Cancer | 12.6 | 11.3 | 14.0 | 0.001 | 5.3 | 4.4 | 6.2 | < 0.001 |
| No cancer | 9.6 | 8.3 | 10.9 | 2.8 | 2.0 | 3.5 | ||
| Sex | ||||||||
| Male | 19.8 | 18.9 | 20.7 | 11.0 | 10.3 | 11.7 | ||
| Female | 23.1 | 22.2 | 24.0 | < 0.001 | 12.2 | 11.5 | 12.8 | 0.034 |
| Age group | ||||||||
| 66–69 | 23.3 | 22.1 | 24.6 | 13.0 | 12.0 | 14.0 | ||
| 70–74 | 22.6 | 21.4 | 23.7 | 0.353 | 12.5 | 11.6 | 13.5 | 0.598 |
| 75–79 | 20.7 | 19.5 | 22.0 | 0.004 | 11.3 | 10.3 | 12.3 | 0.023 |
| 80+ | 18.9 | 17.7 | 20.2 | < 0.001 | 9.4 | 8.5 | 10.3 | < 0.001 |
| Race/ethnicity | ||||||||
| White, non-Hispanic | 22.6 | 21.7 | 23.4 | 13.0 | 12.3 | 13.6 | ||
| Black, non-Hispanic | 20.5 | 18.6 | 22.5 | 0.078 | 10.8 | 9.4 | 12.3 | 0.03 |
| Hispanic | 22.0 | 20.1 | 23.9 | 0.621 | 9.8 | 8.5 | 11.1 | 0.001 |
| Asian, other | 17.0 | 15.5 | 18.6 | < 0.001 | 7.9 | 6.7 | 9.0 | < 0.001 |
| Currently married | ||||||||
| Married | 21.0 | 20.1 | 21.8 | 0.068 | 10.8 | 10.1 | 11.4 | 0.001 |
| Not married | 22.2 | 21.2 | 23.2 | 12.6 | 11.8 | 13.3 | ||
| Education | ||||||||
| < High school diploma | 22.4 | 21.1 | 23.7 | 12.8 | 11.8 | 13.8 | ||
| High school diploma | 21.5 | 20.4 | 22.6 | 0.291 | 11.6 | 10.7 | 12.4 | 0.091 |
| > High school diploma | 20.9 | 20.0 | 21.9 | 0.087 | 10.8 | 10.0 | 11.5 | 0.001 |
| Has a doctor ever told you that you have… | ||||||||
| Coronary artery disease | 20.6 | 19.1 | 22.2 | 0.235 | 11.7 | 10.5 | 12.9 | 0.878 |
| Stroke | 22.9 | 20.9 | 24.9 | 0.15 | 10.3 | 9.0 | 11.7 | 0.057 |
| Pulmonary disease | 22.5 | 21.1 | 24.0 | 0.134 | 12.8 | 11.7 | 13.9 | 0.048 |
| Diabetes | 23.7 | 22.5 | 24.9 | < 0.001 | 12.0 | 11.2 | 12.9 | 0.12 |
| Depression | 22.2 | 20.7 | 23.7 | 0.305 | 11.8 | 10.7 | 12.8 | 0.895 |
| Arthritis | 24.3 | 23.4 | 25.2 | < 0.001 | 12.6 | 12.0 | 13.2 | < 0.001 |
| Sciatica | 24.3 | 23.0 | 25.6 | < 0.001 | 12.8 | 11.9 | 13.8 | 0.001 |
| Poverty rates | ||||||||
| Low (0 to < 5%) | 21.3 | 19.8 | 22.7 | 0.326 | 11.4 | 10.2 | 12.5 | 0.054 |
| 5 to < 10% | 21.0 | 19.9 | 22.2 | 0.175 | 11.0 | 10.1 | 11.9 | 0.004 |
| 10 to < 20% | 21.5 | 20.5 | 22.6 | 0.369 | 11.1 | 10.3 | 11.9 | 0.007 |
| High (20 to 100%) | 22.3 | 20.9 | 23.7 | 13.1 | 12.0 | 14.2 | ||
| Region | ||||||||
| Northeast | 17.8 | 16.2 | 19.4 | 9.1 | 7.9 | 10.3 | ||
| South | 22.8 | 20.7 | 24.8 | < 0.001 | 12.6 | 11.0 | 14.2 | 0.001 |
| Central | 22.1 | 20.8 | 23.4 | < 0.001 | 11.6 | 10.7 | 12.6 | 0.003 |
| West | 21.9 | 21.0 | 22.8 | < 0.001 | 12.0 | 11.3 | 12.7 | 0.001 |
| Part D Low income subsidy | ||||||||
| Full/partial | 25.3 | 23.8 | 26.8 | < 0.001 | 13.6 | 12.5 | 14.7 | < 0.001 |
| None | 20.3 | 19.6 | 21.1 | 10.9 | 10.3 | 11.4 | ||
| Plan type | ||||||||
| HMO | 21.6 | 20.9 | 22.2 | 11.5 | 11.0 | 12.0 | ||
| Other plan type | 20.8 | 18.7 | 23.0 | 0.512 | 12.5 | 10.8 | 14.2 | 0.363 |
| Proximity of death | ||||||||
| < 6 months from MHOS survey | 26.4 | 22.8 | 30.1 | 0.004 | 17.4 | 14.5 | 20.3 | < 0.001 |
| ≥ 6 months from MHOS survey | 21.3 | 20.7 | 22.0 | 11.4 | 10.9 | 11.8 | ||
p values reported for comparison of predictive margins for each attribute compared with a reference category. HMO, health maintenance organization; MHOS, Medicare Health Outcomes Survey. Source: SEER-MHOS-Part D linked data, 2008–2012
Approximately half (45.7%) of adults receiving opioids had less than a 7-day supply during the 30-day observation period, while 38.4% received opioids for 30 days or longer (Table 4), with no difference by cancer history. Hydrocodone, oxycodone, and tramadol were the mostly commonly received opioids overall (Appendix Table 7). The median MEDD was 30.0 mg, with a mean of 55.7 mg (standard error (S.E.) 2.0). Mean MEDD was higher for adults with cancer (58.3 mg, S.E. 2.7) versus without (50.7 mg, S.E. 2.6, p = 0.044). Overall, 14.5% had a high or very high MEDD (90 mg or greater), with no difference by cancer history.
Table 4.
Detailed characteristics of opioid use, overall and by cancer status
| Overall | Cancer | Non-cancer | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | col% | N | col% | N | col% | p valuea | ||||
| N opioid users | 1811 | 100.0 | 1195 | 100.0 | 616 | 100.0 | ||||
| Duration of opioid use prior to MHOS date | 0.44 | |||||||||
| < 7 days | 827 | 45.7 | 553 | 46.3 | 274 | 44.5 | ||||
| 7–29 days | 288 | 15.9 | 195 | 16.3 | 93 | 15.1 | ||||
| 30+ days | 696 | 38.4 | 447 | 37.4 | 249 | 40.4 | ||||
| MEDD (continuous) | Median | Mean | SE | Median | Mean | SE | Median | Mean | SE | p value |
| Overall by pain interference | 30.0 | 55.7 | 2.0 | 31.3 | 58.3 | 2.7 | 30.0 | 50.7 | 2.6 | 0.04 |
| Severe | 30.0 | 56.5 | 2.7 | 30.0 | 59.4 | 3.7 | 30.0 | 51.5 | 3.3 | 0.12 |
| Moderate | 30.0 | 54.1 | 3.5 | 31.3 | 57.4 | 4.6 | 30.0 | 47.2 | 4.4 | 0.11 |
| Mild/none | 36.6 | 57.0 | 6.4 | 37.5 | 56.3 | 8.2 | 32.1 | 59.0 | 9.9 | 0.83 |
| MEDD—% high or very high (MEDD > 90 mgs) | % | % | % | |||||||
| Overall by pain interference | 14.5 | 15.2 | 13.1 | 0.50 | ||||||
| Severe | 15.6 | 16.6 | 13.8 | 0.46 | ||||||
| Moderate | 13.6 | 14.6 | 11.6 | 0.43 | ||||||
| Mild/none | 12.1 | b | b | |||||||
p values reported for comparison of cancer and non-cancer samples
Value suppressed because cell size n < 11
MHOS, Medicare Health Outcomes Survey; MEDD, morphine equivalent daily dose. Source: SEER-Medicare-Part D linked data, 2008–2012
Discussion/conclusion
In this first, large, U.S. population-based study, we documented patterns of pain medication use associated with patient-reported pain interference and compared patterns for older adults with and without cancer. We found a strong association between increasing severity of pain interference and the likelihood of receiving any prescription pain medication, particularly opioids. In addition, older adults with a cancer history were more likely to receive pain medications compared with older adults without a cancer history; however, the magnitude of difference was small. Finally, we found that despite national efforts to increase the availability of supportive and palliative care services, many older adults who reported severe pain interference did not receive any prescription pain medication. This is concerning as pain is an important contributor to diminished quality of life and function, across health conditions, and particularly in older age.
During our 30-day observation period, over one-fifth of older adults received prescription pain medications and approximately half of those received an opioid. Our results parallel those of Barbera et al. [7], which used the Edmonton Symptom Assessment Scale to characterize pain intensity for older adults with cancer [20], and then assessed opioid use during the 30 days prior and 7 days after pain assessment. The results from that study indicate that 26.3% who reported severe pain received opioids during the 30-day pre-period, which matches the 27.0% reported in this study. Several other smaller studies that have assessed pain symptoms and medication management find higher opioid use rates, but these tend to be clinical cohorts of oncology patients undergoing active treatment or surveillance [12, 21], which are more homogeneous than our sample. While numerous other studies address pain medication use overall [22–27] or either for adults with [28–35] or without cancer [36, 37], few compare medication patterns across these groups within a single population setting [8, 9]; incorporation of patient-reported pain information is rare.
Our results suggest that older adults with cancer were more likely than adults without cancer to receive opioids. This finding is consistent with CDC guidelines on chronic pain management, which discourage opioid prescribing except for adults receiving active cancer therapy, palliative, or end-of-life care [19]. In contrast, opioids are seen as an important component of pain management for patients with cancer undergoing active therapy or at end-of-life [16]. In fact, we found that among adults receiving pain medication, a larger proportion of adults without cancer received non-opioid analgesics only, suggesting a focus on prescribing non-opioid analgesics instead of opioids. The relatively small magnitude of the difference in use rates may be explained by the heterogeneity of our sample of adults with cancer, with respect to the criteria mentioned in the CDC guidelines. While we observed slightly higher rates of proximal death, our dataset did not include information on active cancer therapy for all cancer types, so we could not test whether meeting the CDC criteria predicted an even higher likelihood of opioid use than observed.
Beginning in the 1980s, clinicians and patient advocacy groups placed increasing emphasis on pain assessment and treatment, which spurred increasing opioid use [9, 10, 27, 32]. Opioids were prescribed frequently and without careful oversight, resulting in prolonged dependence, misuse, or diversion. In response, over the past 5–7 years, states have added new or strengthened existing laws or regulations restricting opioid prescribing [38, 39]. This has the potential to reduce access to opioids for patients who need them, the so-called “chilling effect,” potentially increasing the problem of undertreated or untreated pain. This study spans the early transition period—when states were just beginning to respond by increasing restrictions. This may explain the failure to find time trends in the data. Furthermore, despite the perception of opioid overuse, we find that a large proportion of older adults had untreated pain, including 56% of those who reported severe pain interference and did not receive any pain medication, and 70% who did not receive an opioid. These results suggest that any increase in untreated pain associated with new restrictions will build on high baseline rates. Mechanisms to ensure access to opioids for individuals with severe pain are essential as overall opioid use restrictions and safety measures are implemented.
While some characteristics significantly predicted both non-opioid and opioid receipt, only the latter was associated with race/ethnicity, marital status, education, and poverty rates. Racial minorities and individuals in higher poverty and lower educational attainment areas were less likely to receive opioid prescriptions when compared with non-Hispanic whites, and those living in areas with higher socioeconomic status, consistent with prior research suggesting bias in prescribing opioids [24, 40]. In addition, adults receiving the Part D LIS were more likely to receive opioid and non-opioid medications. The LIS is associated with lower cost-sharing for prescriptions, and there is evidence that Part D enrollees respond to price variation, including that associated with the LIS [41–44]. The estimated effect of the LIS in this analysis of any pain medication may reflect physician prescribing of NSAIDS for low-income beneficiaries, which would bias estimates. However, that explanation would not hold for opioids, which are never obtained over-the-counter.
Limitations
Our study is subject to limitations typically associated with use of survey and administrative datasets. This dataset captures medication use patterns for beneficiaries enrolled in MA plans, which may undertake internal review of prescribing practices. As a result, the patterns observed in the MA setting may differ from fee-for-service enrolled populations with Part D plans. This analysis focused on prescription medications typically prescribed for pain management. Medications such as acetaminophen and NSAIDs are available over-the-counter and would not be captured with claims unless prescribed and covered by Part D. As a result, our estimates may under-report use of these medications. Recent estimates suggest that 8.7% of older adults purchase acetaminophen and 4.7% naproxen, but the study did not distinguish prescription from over-the-counter purchase [45]. We are unable to discern the prescriber’s intent from claims, so that some antidepressants or antiepileptics may have been prescribed for purposes other than pain management. Our measures cannot capture pharmacologic pain treatments typically administered in a physician office (or hospital), through an implanted device, or non-pharmacologic treatments for pain that may be covered by the MA plan. These might include acupuncture, massage therapy, or palliative radiation. Finally, our measures are based on prescriptions filled, but do not measure medications prescribed but not filled.
Despite these limitations, the study has substantial strengths, including a large population-based sample that includes older adults with and without a cancer history. The linkage between Part D claims and the SEER-MHOS allows us to examine the association between pain medication use and self-reported pain symptoms, specifically severity of pain interference with daily activities. Other studies examining associations between pain and medication use have used scales that report pain levels, such as the Edmonton Symptom Assessment Scale. By using pain interference, we can provide unique insights, as older adults commonly focus on functional status as a key contributor to quality of life. We were unable to assess the sensitivity of study results to the use of alternative pain measures because the linked dataset available for this study only included the pain interference measure.
In conclusion, older adults with cancer were more likely to receive prescription pain medications compared with adults without cancer, overall and when stratifying by severity of pain interference. Furthermore, a large proportion of both groups reporting severe pain interference did not receive medications for pain management, a special concern for older adults with cancer, for whom pharmacologic therapy is recommended. New ways to improve and standardize assessment and management of pain are urgently needed to limit functional impairment and enhance quality of life. Increasing clinician training, as well as building on quality metrics and electronic-health records, is a strategy that may ensure more routine capture and treatment of symptoms, including pain, among older adults with and without cancer.
Acknowledgments
This study used data from the SEER-MHOS linked data resource. The authors acknowledge the efforts of the National Cancer Institute; the Centers for Medicare & Medicaid Services; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-MHOS database.
Funding information This research was funded through a contract with the National Cancer Institute (Contract HHSN261201700690P).
Appendix
Fig. 2.
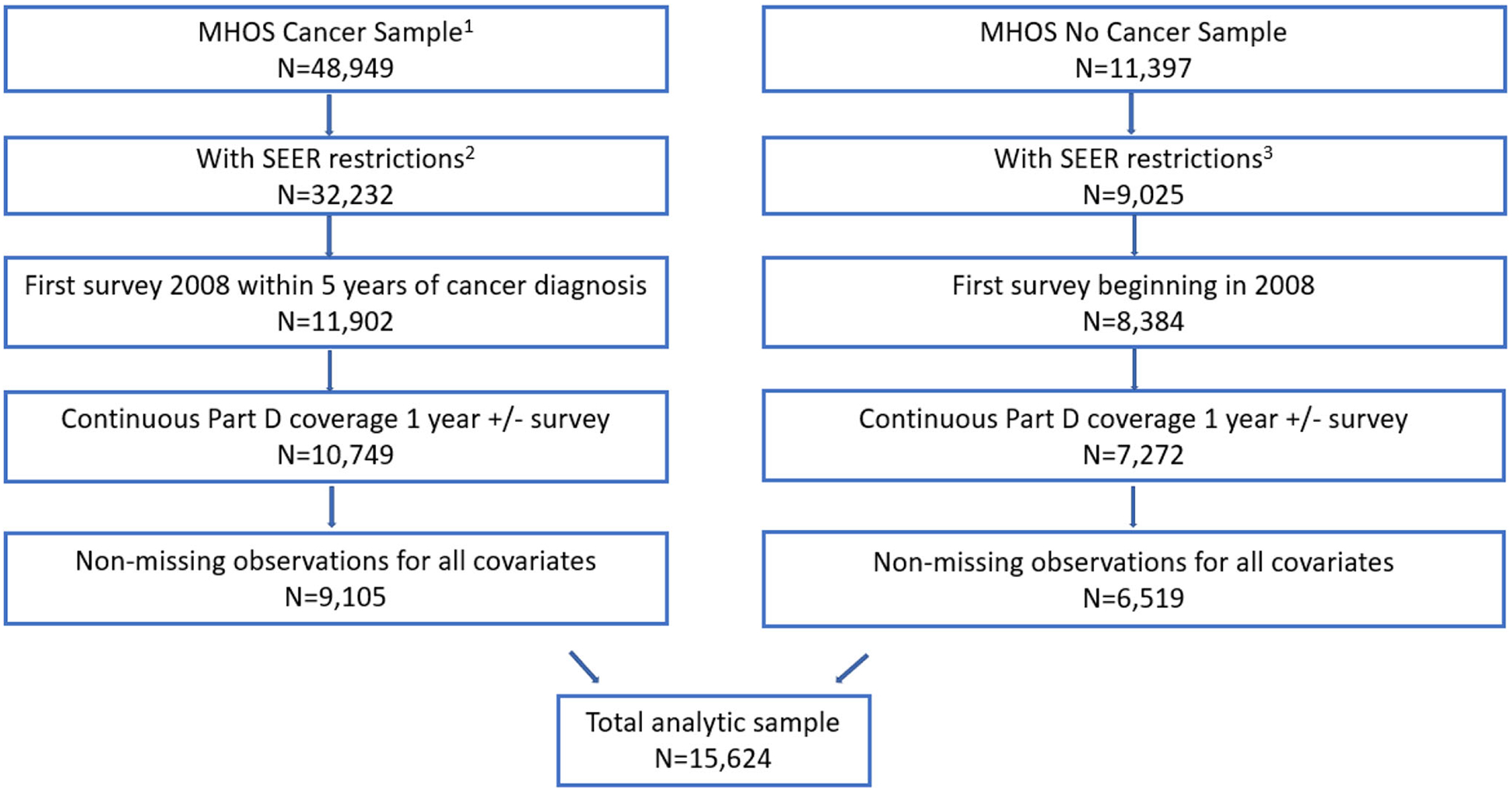
Sample selection steps and exclusions
1 Linked cancer sample with known date of cancer diagnosis and cancer diagnosis not at autopsy. (Without ANY restriction by cancer N=49,766)
2 Cancer sample has additional SEER restrictions: age > 65, only primary tumor and invasive disease
3 Non-cancer sample with SEER restrictions: age > 65, In SEER region at time of survey and cancer status=NO for all records
Table 5.
Cancer type, stage at diagnosis, and initial therapy
| Cancer | ||
|---|---|---|
| Sample N | 9105 | |
| N | % | |
| Cancer type | ||
| Breast | 1746 | 19.2 |
| Prostate | 2798 | 30.7 |
| Colorectal | 958 | 10.5 |
| Lung | 582 | 6.4 |
| Other | 3021 | 33.2 |
| Stage at diagnosis | ||
| I | 2324 | 25.5 |
| II | 3387 | 37.2 |
| III | 888 | 9.8 |
| IV | 516 | 5.7 |
| Unknown | 1990 | 21.9 |
| Initial therapy | ||
| Surgery | 5693 | 62.5 |
| Radiation | 2923 | 32.1 |
Source: SEER-MHOS-Part D linked data, 2008–2012
Table 6.
Combinations of medication type for pain management among treated adults, overall, by cancer history and level of pain interference (unadjusted)
| Overall | By cancer history | By level of pain interference | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer | No cancer | p valueb | Severe | Moderate | Mild/none | p value | ||||||||
| N | % | N | % | N | % | N | % | N | % | N | % | |||
| By typea | < 0.001 | < 0.001 | ||||||||||||
| Opioids only | 79 | 2.3 | 53 | 2.5 | 27 | 2.0 | 44 | 2.9 | 26 | 1.8 | 11 | 2.1 | ||
| Non-opioids only | 1550 | 46.1 | 853 | 41.7 | 697 | 53.1 | 492 | 33.4 | 766 | 54.3 | 292 | 61.6 | ||
| Both | 1732 | 51.6 | 1142 | 55.8 | 589 | 44.9 | 939 | 63.7 | 620 | 44.0 | 171 | 36.3 | ||
Categories are mutually exclusive
p values reported for chi-squared comparison of medication use rates by cancer history and pain interference
Source: SEER-MHOS-Part D linked data, 2008–2012
Table 7.
Use of individual opioids conditional on opioid receipt, overall and by cancer history
| Overall | Cancer | Non-cancer | ||||
|---|---|---|---|---|---|---|
| Specific opioids received | N | % | N | % | N | % |
| Hydrocodone | 948 | 52.3 | 630 | 52.7 | 318 | 51.6 |
| Oxycodone | 363 | 20.0 | 269 | 22.5 | 94 | 15.3 |
| Tramadol | 333 | 18.4 | 197 | 16.5 | 136 | 22.1 |
| Codeine | 116 | 6.4 | 74 | 6.2 | 42 | 6.8 |
| Propoxyphene | 93 | 5.1 | 55 | 4.6 | 38 | 6.2 |
| Fentanyl | 74 | 4.1 | 64 | 5.3 | 10 | 1.6 |
| Morphine | 64 | 3.5 | 46 | 3.9 | 18 | 3.0 |
| Methadone | 18 | 1.0 | < 11 | < 11 | ||
| Hydromorphone | 15 | 0.8 | < 11 | < 11 | ||
| Meperidine | < 11 | < 11 | < 11 | |||
| Oxymorphone | < 11 | < 11 | < 11 | |||
| Sum | 2027 | 111.9 | 1357 | 113.5 | 669 | 108.7 |
Multiple opioids may be received; row categories are not mutually exclusive
Source: SEER-MHOS Part D linked data, 2008–2012
Footnotes
Conflict of interest EEK was a contractor for NCI and assisted with project oversight as well as contributing substantial intellectual input. AJD’s institution receives research funding from Celgene Corporation outside of the submitted project. CJP is a Paul Calabresi Scholar supported by the OSU K12 Training Grant for Clinical Faculty Investigators (K12 CA133250) and is a consultant for Potentia Metrics. SW’s institution receives funding from Genentech to support his research. No other author reports any conflict of interest to disclose. The research team had direct control of the data throughout the study project. We are unable to release the data to other researcher due to constraints of the Data Use Agreement with the NCI. However, we would be willing to provide SAS code for selection of medications identified in the analysis.
Publisher's Disclaimer: Disclaimer The interpretation and reporting of these data are the sole responsibility of the authors and do not reflect the positions of the National Cancer Institute, the Centers for Medicare & Medicaid Services, or the U.S. Department of Health and Human Services.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S (2014) Prevalence of persistent pain in the U.S. adult population: new data from the 2010 national health interview survey. J Pain 15:979–984 [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine (2011) Relieving pain in America: a blueprint for transforming prevention, care, education, and research. National Academies of Science and Medicine, Washington, DC: [PubMed] [Google Scholar]
- 3.Burton AW, Fanciullo GJ, Beasley RD, Fisch MJ (2007) Chronic pain in the cancer survivor: a new frontier. Pain Med 8:189–198 [DOI] [PubMed] [Google Scholar]
- 4.Deandrea S, Montanari M, Moja L, Apolone G (2008) Prevalence of undertreatment in cancer pain. A review of published literature. Ann Oncol 19:1985–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reis-Pina P, Lawlor PG, Barbosa A (2017) Adequacy of cancer-related pain management and predictors of undertreatment at referral to a pain clinic. J Pain Res 10:2097–2107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greco MT, Roberto A, Corli O, Deandrea S, Bandieri E, Cavuto S, Apolone G (2014) Quality of cancer pain management: an update of a systematic review of undertreatment of patients with cancer. J Oncol Pract 32:4149–4154 [DOI] [PubMed] [Google Scholar]
- 7.Barbera L, Seow H, Husain A, Howell D, Atzema C, Sutradhar R, Earle C, Sussman J, Liu Y, Dudgeon D (2012) Opioid prescription after pain assessment: a population-based cohort of elderly patients with cancer. J Oncol Pract 30:1095–1099 [DOI] [PubMed] [Google Scholar]
- 8.Barbera L, Sutradhar R, Chu A, Seow H, Howell D, Earle CC, O’Brien MA, Dudgeon D, Atzema C, Husain A, Liu Y, DeAngelis C (2018) Comparison of opioid prescribing among cancer and noncancer patients aged 18–64: analysis using administrative data. J Pain Symptom Manag 56:72–79 [DOI] [PubMed] [Google Scholar]
- 9.Barbera L, Sutradhar R, Chu A, Seow H, Howell D, Earle CC, O’Brien MA, Dudgeon D, Atzema C, Husain A, Liu Y, DeAngelis C (2017) Opioid prescribing among cancer and non-cancer patients: time trend analysis in the elderly using administrative data. J Pain Symptom Manag 54:484–492 [DOI] [PubMed] [Google Scholar]
- 10.Harrison JM, Lagisetty P, Sites BD, Guo C, Davis MA (2018) Trends in prescription pain medication use by race/ethnicity among US adults with noncancer pain, 2000–2015. Am J Public Health 108:788–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sutradhar R, Lokku A, Barbera L (2017) Cancer survivorship and opioid prescribing rates: a population-based matched cohort study among individuals with and without a history of cancer. Cancer 123:4286–4293 [DOI] [PubMed] [Google Scholar]
- 12.Moryl N, Dave V, Glare P, Bokhari A, Malhotra VT, Gulati A, Hung J, Puttanniah V, Griffo Y, Tickoo R, Wiesenthal A, Horn SD, Inturrisi CE (2018) Patient-reported outcomes and opioid use by outpatient cancer patients. J Pain 19:278–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ambs A, Warren JL, Bellizzi KM, Topor M, Haffer SC, Clauser SB (2008) Overview of the SEER–medicare health outcomes survey linked dataset. Health Care Financ Rev 29:5–21 [PMC free article] [PubMed] [Google Scholar]
- 14.Kent EE, Ambs A, Mitchell SA, Clauser SB, Smith AW, Hays RD (2015) Health-related quality of life in older adult survivors of selected cancers: data from the SEER-MHOS linkage. Cancer 121: 758–765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Check DK, Rosenstein DL, Dusetzina SB (2016) Early supportive medication use and end-of-life care among Medicare beneficiaries with advanced breast cancer. Support Care Cancer 24:3463–3472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denlinger CS, Ligibel JA, Are M, Baker KS, Demark-Wahnefried W, Friedman DL, Goldman M, Jones L, King A, Ku GH, Kvale E, Langbaum TS, Leonardi-Warren K, McCabe M, Melisko M, Montoya JG, Mooney K, Morgan MA, Moslehi JJ, O’Connor T, Overholser L, Paskett ED, Raza M, Syrjala KL, Urba SG, Wakabayashi MT, Zee P, McMillian N, Freedman-Cass D, National Comprehensive Cancer Network (2014) Survivorship: pain version 1.2014. J Natl Compr Cancer Netw 12:488–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention (2017) Calculating total daily dose. Centers for Disease Control and Prevention, Atlanta [Google Scholar]
- 18.Center for Medicare & Medicaid Services (2017) Opioid oral morphine milligram equivalent (MME) conversion factors. Centers for Medicare and Medicaid Services, Baltimore [Google Scholar]
- 19.Dowell D, Haegerich TM, Chou R (2016) CDC guideline for prescribing opioids for chronic pain - United States, 2016. MMWR Recomm Rep 65:1–49 [DOI] [PubMed] [Google Scholar]
- 20.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K (1991) The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care 7:6–9 [PubMed] [Google Scholar]
- 21.Fisch MJ, Lee JW, Weiss M, Wagner LI, Chang VT, Cella D, Manola JB, Minasian LM, McCaskill-Stevens W, Mendoza TR, Cleeland CS (2012) Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Oncol Pract 30:1980–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown W (2008) Opioid use in dying patients in hospice and hospital, with and without specialist palliative care team involvement. Eur J Cancer Care (Engl) 17:65–71 [DOI] [PubMed] [Google Scholar]
- 23.Gomes T, Juurlink DN, Dhalla IA, Mailis-Gagnon A, Paterson JM, Mamdani MM (2011) Trends in opioid use and dosing among socio-economically disadvantaged patients. Open Med 5:e13–e22 [PMC free article] [PubMed] [Google Scholar]
- 24.Guy GP Jr, Zhang K, Bohm MK, Losby J, Lewis B, Young R, Murphy LB, Dowell D (2017) Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep 66:697–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosher HJ, Krebs EE, Carrel M, Kaboli PJ, Weg MWV, Lund BC (2015) Trends in prevalent and incident opioid receipt: an observational study in veterans health administration 2004–2012. J Gen Intern Med 30:597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schubert I, Ihle P, Sabatowski R (2013) Increase in opiate prescription in Germany between 2000 and 2010: a study based on insurance data. Dtsch Arztebl Int 110:45–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smolina K, Gladstone E, Morgan SG (2016) Determinants of trends in prescription opioid use in British Columbia, Canada, 2005–2013. Pharmacoepidemiol Drug Saf 25:553–559 [DOI] [PubMed] [Google Scholar]
- 28.Barbera L, Sutradhar R, Chu A, Seow H, Earle CC, O’Brien MA, Dudgeon D, DeAngelis C, Atzema C, Husain A, Liu Y, Howell D (2017) Has province-wide symptom screening changed opioid prescribing rates in older patients with cancer? J Oncol Pract 13:e927–e934 [DOI] [PubMed] [Google Scholar]
- 29.Berger A, Dukes E, Mercadante S et al. (2006) Use of antiepileptics and tricyclic antidepressants in cancer patients with neuropathic pain. Eur J Cancer Care (Engl) 15:138–145 [DOI] [PubMed] [Google Scholar]
- 30.Berger A, Dukes E, Smith M, Hagiwara M, Seifeldin R, Oster G (2003) Use of oral and transdermal opioids among patients with metastatic cancer during the last year of life. J Pain Symptom Manag 26:723–730 [DOI] [PubMed] [Google Scholar]
- 31.Gagnon B, Scott S, Nadeau L, Lawlor PG (2015) Patterns of community-based opioid prescriptions in people dying of cancer. J Pain Symptom Manag 49:36–44 [DOI] [PubMed] [Google Scholar]
- 32.Higginson IJ, Gao W (2012) Opioid prescribing for cancer pain during the last 3 months of life: associated factors and 9-year trends in a nationwide United Kingdom cohort study. J Oncol Pract 30: 4373–4379 [DOI] [PubMed] [Google Scholar]
- 33.Jarlbaek L, Hansen DG, Bruera E, Andersen M (2010) Frequency of opioid use in a population of cancer patients during the trajectory of the disease. Clin Oncol (R Coll Radiol) 22:199–207 [DOI] [PubMed] [Google Scholar]
- 34.Vardy J, Agar M (2014) Nonopioid drugs in the treatment of cancer pain. J Oncol Pract 32:1677–1690 [DOI] [PubMed] [Google Scholar]
- 35.Ziegler L, Mulvey M, Blenkinsopp A, Petty D, Bennett MI (2016) Opioid prescribing for patients with cancer in the last year of life: a longitudinal population cohort study. Pain 157:2445–2451 [DOI] [PubMed] [Google Scholar]
- 36.Campbell CI, Weisner C, Leresche L et al. (2010) Age and gender trends in long-term opioid analgesic use for noncancer pain. Am J Public Health 100:2541–2547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smolina K, Gladstone EJ, Rutherford K, Morgan SG (2016) Patterns and trends in long-term opioid use for non-cancer pain in British Columbia, 2005–2012. Can J Public Health 107:e404–e409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wickramatilake S, Zur J, Mulvaney-Day N, Klimo MC, Selmi E, Harwood H (2017) How states are tackling the opioid crisis. Public Health Rep 132:171–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patrick SW, Fry CE, Jones TF, Buntin MB (2016) Implementation of prescription drug monitoring programs associated with reductions in opioid-related death rates. Health Aff (Project Hope) 35: 1324–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cleeland CS, Gonin R, Baez L et al. (1997) Pain and treatment of pain in minority patients with cancer. The Eastern Cooperative Oncology Group Minority Outpatient Pain Study. Ann Intern Med 127:813–816 [DOI] [PubMed] [Google Scholar]
- 41.Doshi JA, Li P, Huo H, Pettit AR, Kumar R, Weiss BM, Huntington SF (2016) High cost sharing and specialty drug initiation under Medicare Part D: a case study in patients with newly diagnosed chronic myeloid leukemia. Am J Manag Care 22:s78–s86 [PubMed] [Google Scholar]
- 42.Dusetzina SB, Winn AN, Abel GA, Huskamp HA, Keating NL (2014) Cost sharing and adherence to tyrosine kinase inhibitors for patients with chronic myeloid leukemia. J Clin Oncol 32:306–311 [DOI] [PubMed] [Google Scholar]
- 43.Olszewski AJ, Dusetzina SB, Eaton CB, Davidoff AJ, Trivedi AN (2017) Subsidies for oral chemotherapy and use of immunomodulatory drugs among Medicare beneficiaries with myeloma. J Oncol Pract 35:3306–3314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wieder R, Delarosa N, Bryan M et al. (2014) Prescription coverage in indigent patients affects the use of long-acting opioids in the management of cancer pain. Pain Med 15:42–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qato DM, Wilder J, Schumm LP, Gillet V, Alexander GC (2016) Changes in prescription and over-the-counter medication and dietary supplement use among older adults in the United States, 2005 vs 2011. JAMA Intern Med 176:473–482 [DOI] [PMC free article] [PubMed] [Google Scholar]


