Abstract
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults and carries a discouraging prognosis. Its aggressive and highly infiltrative nature renders the current standard treatment of maximal surgical resection, radiation, and chemotherapy relatively ineffective. Identifying the signaling pathways that regulate GBM migration/invasion and resistance is required to develop more effective therapeutic regimens to treat GBM. Expression of TROY, an orphan receptor of the TNF receptor superfamily, increases with glial tumor grade, inversely correlates with patient overall survival, stimulates GBM cell invasion in vitro and in vivo, and increases resistance to temozolomide and radiation therapy. Conversely, silencing TROY expression inhibits GBM cell invasion, increases sensitivity to temozolomide, and prolongs survival in a preclinical intracranial xenograft model. Here, we have identified for the first time that TROY interacts with JAK1. Increased TROY expression increases JAK1 phosphorylation. In addition, increased TROY expression promotes STAT3 phosphorylation and STAT3 transcriptional activity that is dependent upon JAK1. TROY-mediated activation of STAT3 is independent of its ability to stimulate activity of NF-κB. Inhibition of JAK1 activity by ruxolitinib or knockdown of JAK1 expression by siRNA significantly inhibits TROY-induced STAT3 activation, GBM cell migration, and decreases resistance to temozolomide. Taken together, our data indicate that the TROY signaling complex may represent a potential therapeutic target with the distinctive capacity to exert effects on multiple pathways mediating GBM cell invasion and resistance.
Abbreviations: GBM, Glioblastoma; TNFR, tumor necrosis factor receptor; JAK, Janus kinase; STAT, signal transducer and activator of transcription; TNF, tumor necrosis factor
Keywords: TROY, JAK1, STAT3, Glioblastoma, Migration
Introduction
Glioblastoma (GBM) is the most common primary malignant brain tumor in adults with a median overall survival of approximately 14.6 months after diagnosis [1], [2]. The overall survival of GBM patients remains poor and has improved only minimally over the past decade indicating the limited efficacy of the current standard of care treatment regimen of surgical resection, chemotherapy, and radiation therapy. GBMs are characterized by aggressive growth with diffuse infiltration which greatly complicates the complete surgical removal of the primary tumor [3]. Furthermore, the invading cells that have escaped resection exhibit significant resistance to chemotherapeutic agents virtually assuring tumor recurrence. Recurrent tumors are especially aggressive and median survival following recurrence drops to approximately 8 months [4]. Therefore, improved treatment strategies for this disease will require a detailed understanding of the signaling pathways responsible for glioma cell invasion and resistance and the identification of critical signaling effectors that could serve as potential therapeutic targets.
TROY (TNFRSF19), a type I transmembrane receptor, is an orphan member of the TNF receptor superfamily. TROY is widely expressed during embryonic development, but postnatal expression is more restricted [5], [6], [7], [8]. Elevated aberrant TROY expression has been reported in a number of invasive cancers, including colorectal cancer, lung cancer, melanoma, nasopharyngeal carcinoma, prostate cancer and GBM [9], [10], [11], [12], [13], [14], [15]. In GBM, we have shown that expression of TROY increases with increasing glial tumor grade and negatively correlates with patient survival [13]. Increased expression of TROY stimulates GBM cell invasion in vitro and in vivo and increases therapeutic resistance to temozolomide (TMZ) and radiation therapy. In contrast, silencing TROY expression inhibits GBM cell invasion, increases sensitivity to TMZ and prolongs survival in an intracranial xenograft model [14]. Collectively, these results position TROY expression and signaling as a potential target to inhibit GBM cell invasion and decrease therapeutic resistance. We have demonstrated that increased expression of TROY leads to self-oligomerization and the assembly of a multi-protein signaling complex (signalsome). We have previously reported that proteins contained in the TROY interactome include the non-receptor tyrosine kinase Pyk2 [13] and the epidermal growth factor receptor (EGFR) [16]. In addition, we have also identified the Rho guanine nucleotide exchange factor PDZ-RhoGEF (ARHGEF11) as a component of the signalsome and a downstream effector for TROY signaling in GBM [17]. Together, these results indicate that the TROY signalsome is uniquely positioned at a point of convergence to coordinate the activity of survival and growth signaling pathways.
Signal Transducer and Activator of Transcription 3 (STAT3) is a transcription factor that regulates expression of genes that modulate important processes including the anti-apoptotic response, cell proliferation, and angiogenesis [18]. Constitutive activation of STAT3 has been observed in a number of human cancers including breast, lung, ovarian, pancreatic, skin, prostate cancers, myeloma, and GBMs [19], [20]. Increased STAT3 phosphorylation is frequently observed in gliomas tissues, but is not typically observed in normal brain tissue or low-grade astrocytomas [21]. The JAK/STAT pathway has been implicated in the progression of human gliomas and high activity of this pathway serves as a negative prognostic factor for patients [22]. Silencing of STAT3 inhibits diffuse infiltration of murine glioma cells Tu-2449 and prolongs survival in an orthotopic syngeneic mouse glioma model [23]. Janus kinase 1 (JAK1) and JAK2, non-receptor tyrosine kinases critical for STAT3 activation, have also been reported to be aberrantly activated in human GBM tissues and xenografts [22], [24], [25].
In this study, we sought to further characterize the diversity of the cellular signaling pathways involved in TROY-mediated GBM cell migration and resistance. We have identified that JAK1 is a component of the TROY signalsome linking TROY to activation of STAT3. Increased expression of TROY promotes the phosphorylation of STAT3, whereas inhibition of JAK1 or knockdown of JAK1 by siRNA significantly inhibits TROY-induced STAT3 activation, GBM cell migration, and decreases resistance to TMZ. These results support a role for the TROY-JAK1 complex in GBM cell migration and support TROY and its signaling effectors as potential therapeutic targets to improve the efficacy of current treatment regimens for glioblastoma.
Methods
Antibodies and reagents
Antibodies to HA (C29F4, #3724), EGFR (D38B1, #4267), JAK1 (D1T6W, #50996), JAK2 (D2E12, #3230), Tyk2 (D4I5T, #14193), pY705-STAT3 (D3A7, #9145), STAT3 (124H6, #9139), GAPDH (D16H11, #5174), and tubulin (DM1A, #3873) were obtained from Cell Signaling Technology (Beverly, MA). A rabbit polyclonal antibody against a peptide in TROY N-terminus was prepared by Cocalico Biologicals (Reamstown, PA) and has previously been described [17]. The mouse anti-FLAG monoclonal antibody (clone M2, #F3165) was obtained from Sigma (St. Louis, MO). The goat anti-HA polyclonal antibody (#A190-138A) was obtained from Bethyl Laboratories (Montgomery, TX). Ruxolitinib (#11609) was purchased from Cayman Chemical (Ann Arbor, MI). Trypsin-EDTA, DMEM, penicillin–streptomycin were purchased from GIBCO (GIBCO Life Technologies, Grand Island, NY). Transfection Reagent Effectene was purchased from Qiagen (Valencia, CA). Collagen was obtained from Advanced Biomatrix (San Diego, CA). Temozolomide (#T2577) was purchased from Sigma (St. Louis, MO). EGF and bFGF were obtained from GIBCO.
Cell culture
Human glioblastoma cell line T98G and the human embryonic kidney cell line HEK293 were obtained from American Type Culture Collection. T98G cells with increased expression of an HA epitope-tagged TROY (T98G/TROY-HA), T98G cells overexpressing an HA-epitope-tagged TROY variant lacking the extracellular domain (T98G/TROYΔECD-HA), T98G cells with stable knockdown of TROY (T98G/shTROY), and HEK293 cells expressing a NF-κB response element-driven firefly luciferase reporter (HEK293/NF-κB-luc) have previously been described [16]. All cell lines were maintained in Dulbecco’s modified Eagle medium (DMEM) (GIBCO) supplemented with 10% heat-inactivated FBS (Invitrogen), 1% nonessential amino acids, 2 mmol/L glutamine, and 1% antibiotics (penicillin and streptomycin) at 37 °C under a humidified atmosphere containing 5% CO2. GBM43 and GBM10 are primary GBM patient-derived xenografts (PDX) obtained from the Mayo Clinic Brain SPORE [26]. Fresh flank GBM43 and GBM10 tumors were resected, processed to single cell suspension by mechanical dissociation, and cultured in neurosphere media (DMEM/F12 supplemented with 2% B-27, 20 ng/ml bFGF, and 20 ng/ml EGF).
Expression constructs
The STAT3 driven luciferase reporter plasmid (pGL4.47/SIE/Luc2P, #E404A) was obtained from Promega. The STAT3 reporter plasmid contains the firefly luciferase gene (luc2P) driven by five copies of the sis-inducible element (SIE), a canonical binding site for STAT3. Expression constructs encoding 3X HA epitope-tagged TROY, TROYΔECD, and TROY-TRAFm have been previously described [13], [16]. The cDNAs for the TROY cytoplasmic deletion constructs TROY-Q209, TROY-D240, and TROY-M254, each with a C-terminal 3X HA epitope, were amplified by PCR and subcloned into the pcDNA3 expression vector. The TROY variant designated TROY-αv containing the cytoplasmic domain of integrin αv substituted for the TROY cytoplasmic domain was generated using splice overlap extension PCR [27]. The coding sequence for human JAK1 was amplified from a JAK1 cDNA containing plasmid obtained from the DNASU Plasmid Repository (plasmid ID: HsCD00829787) [28]. The amplified fragment was cloned into the p3XFLAG-CMV expression vector (Sigma) using the NEBuilder HiFi DNA Assembly kit (New England Biolabs) according to the manufacturer’s instruction. Similarly, the sequence encoding the JAK1 FERM and SH2 domains was amplified by PCR and cloned into the p3XFLAG-CMV expression vector. Integrity of all constructs was verified by direct DNA sequencing.
Immunoblotting and immunoprecipitation
Immunoblotting of cell lysates and protein determination were done as described [16]. Briefly, cells were harvested by scraping and lysed in RIPA buffer (50 mM Tris, pH 8.0, 135 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, and 5% glycerol) supplemented with a cocktail of protease and phosphatase inhibitors (Thermo Scientific). The lysates were clarified by centrifugation and the protein concentration of the lysates was determined by BCA protein assay (Pierce). Equal amounts of protein were subjected to SDS-PAGE (Bio-Rad) and transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked in 5% bovine serum albumin (BSA) in Tris-Buffered Saline with 0.05% Tween (TBST) and then sequentially incubated with the appropriate primary antibodies. Protein detection was performed using IRDye-conjugated secondary antibodies with the Odyssey CLx Infrared Imaging System (LI-COR). Densitometric analysis was conducted using Image Studio (LI-COR) and all expression values were normalized to GAPDH or tubulin values.
For immunoprecipitation experiments, cells were lysed 24 h after transfection with RIPA buffer containing protease and phosphatase inhibitors. Equivalent amounts of cell lysates (600 μg) were precleared with protein G-agarose beads (Millipore) for 1 h at 4 °C. Precleared lysates were incubated with appropriate antibodies overnight followed by incubation with protein G-agarose beads for 1 h. The precipitates were washed five times with ice-cold RIPA buffer, resuspended in 2X SDS sample buffer, boiled in the presence of 2-mercaptoethanol, and separated by SDS-PAGE. Immunoblotting of resolved immunoprecipitates was conducted as described above.
Small interfering RNA transfection
Commercially available, validated small interfering RNA (siRNA) molecules for knockdown of JAK1 (#1027417) were from Qiagen (Valencia, CA). The negative control nontargeting siRNA (#AM4611) was purchased from Ambion (Austin, TX). Cells were plated and allowed to attach for 16 h and then transfected with siRNAs using Lipofectamine RNAiMax (Invitrogen) according to the manufacturer’s instructions. Two or three days after transfections, the knockdown of JAK1 was confirmed by immunoblotting with an anti-JAK1 antibody.
STAT3 luciferase reporter assay
HEK293 cells were plated in complete DMEM media in six-well plates for overnight growth. The cells were co-transfected in triplicate with the STAT3 reporter plasmid and 3X HA epitope-tagged TROY or TROY variants using Effectene (Qiagen). Twenty-four hours after transfection, the transfected cells were 0.1% serum starved for 16 h and then lysed in Reporter Lysis Buffer (Promega). Luciferase activity in the cell lysates was determined by luminometry using the assay system according to the manufacturer’s protocols.
NF-κB luciferase reporter assay
HEK293/NF-κB-luc cells were seeded in complete DMEM media in six-well plates. The cells were transfected in triplicate with 3X HA epitope-tagged TROY or TROY variants using Effectene (Qiagen). Twenty-four hours after transfection, the transfected cells were 0.1% serum starved in the absence and presence of 1 μM ruxolitinib for 16 h and then lysed in Reporter Lysis Buffer (Promega). Luciferase activity in the cell lysates was determined by luminometry using the assay system according to the manufacturer’s protocols.
Cell migration assay
After indicated treatments or transfections, cells were harvested, resuspended in DMEM with 0.1% BSA (1 × 105 cells/200 μl), seeded in triplicate into the top chamber of collagen-coated transwell inserts (8-μm pore size) (Falcon), and allowed to migrate towards DMEM with 10% serum in the bottom chamber. After incubation for 24 h at 37 °C, cells on the top of the membrane were wiped off using a cotton swab. The migrated cells on the lower surface of the membrane were fixed with 4% paraformaldehyde (Affymetrix) and stained with ProLong Gold Antifade reagent with DAPI (Invitrogen). For each experiment, five random high-power fields (HPF) were counted.
Colony formation assay
A colony formation assay was used to assess cell survival after TMZ treatment or in combination with ruxolitinib as described previously [29]. Briefly, T98G cells or T98G cells with increased expression of TROY (T98G/TROY-HA), GBM10, or GBM43 PDX cells were pretreated with 1 μM ruxolitinib for 2 h and then TMZ (500 µM) was added for an additional 24 h. The cells were then harvested, re-seeded in triplicate in six-well plates (1500 cells/well), and cultured for 10 days until colony formation was observed. The media for the ruxolitinib groups was changed every two days until the experiments were done. Colonies were fixed with a 10% acetic acid + 10% methanol solution and stained with 0.5% crystal violet (Sigma). The number of colonies consisting of ≥50 cells was counted manually. Results are normalized to cells treated with vehicle and depicted as mean +/− SD.
Statistical analysis
Statistical analyses were conducted by the two-sample t-test using GraphPad Prism 8.0 (GraphPad, Inc). A p value less than 0.05 was considered significant.
Results
TROY associates with JAK1
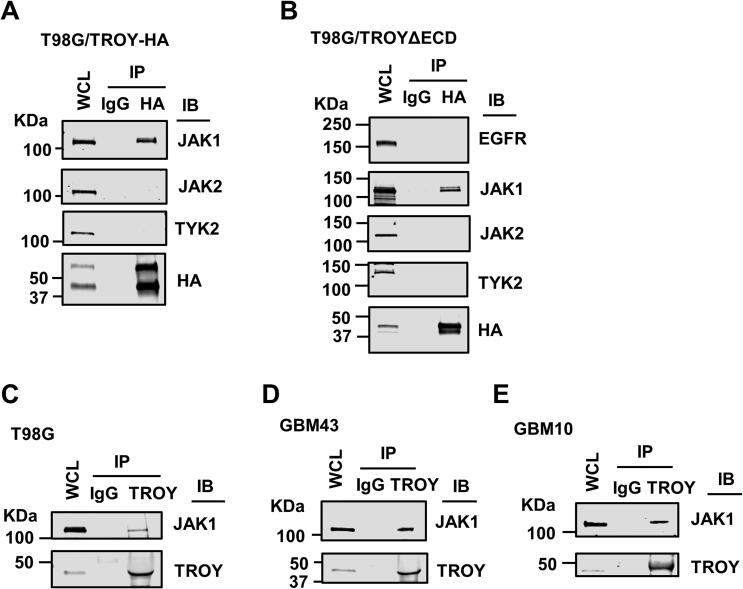
Our previous studies have indicated a role for signaling from the TROY receptor complex in GBM cell migration, invasion, and therapeutic resistance to TMZ and radiation therapy [13], [14]. To identify proteins that interact with TROY and potentially mediate TROY signaling, we immunoprecipitated TROY from T98G glioma cells and analyzed the precipitates using MALDI-TOF mass spectrometry [13]. Among the proteins identified in the TROY immunoprecipitate was JAK1. Co-immunoprecipitation analysis confirmed the interaction of TROY with JAK1 in T98G cells expressing HA epitope-tagged TROY (T98G/TROY-HA). Interestingly, the related family members JAK2 and TYK2 did not immunoprecipitate with TROY although western blotting indicated both were present in cell lysates (Fig. 1A). Consistent with our previous results [13], [14], immunoblotting for C-terminal HA epitope-tagged TROY often results in two bands. The lower molecular weight band likely corresponds to TROY isoform 3 (UniProtKB: Q9NS68-3) that results from translation initiation at a downstream ATG resulting in an isoform that lacks amino acids 1–132 of the canonical TROY.
Fig. 1.
TROY associates with JAK1. (A) T98G cells expressing HA epitope-tagged TROY (T98G/TROY-HA) or (B) T98G cells expressing a HA epitope-tagged TROY variant lacking the extracellular domain (T98G/TROYΔECD) were lysed, immunoprecipitated with anti-HA antibody, and the precipitates immunoblotted (IB) with the indicated antibodies. WCL, whole cell lysates. (C–E) TROY was immunoprecipitated from T98G (C), primary GBM xenograft lines GBM43 (D) and GBM10 (E) and precipitates were immunoblotted with the indicated antibodies. WCL, whole cell lysates.
We previously reported that TROY associates with EGFR through its extracellular domain [16]. As JAK1 is known to associate with EGFR [30], the observed immunoprecipitation of JAK1 with TROY could be secondary to TROY’s association with EGFR. In order to examine the involvement of EGFR in the TROY-JAK1 interaction, we utilized T98G cells expressing the TROY variant, TROYΔECD, which we have demonstrated does not associate with EGFR [16]. Co-immunoprecipitation analysis in this cell line confirmed that TROYΔECD did not co-immunoprecipitate with EGFR but maintained its association with JAK1 (Fig. 1B). Similar to wild-type TROY, TROYΔECD did not immunoprecipitate with the related family members JAK2 and TYK2 (Fig. 1B). These results indicate that the interaction of TROY with JAK1 is independent of EGFR. Co-immunoprecipitation analysis also showed that JAK1 interacted with endogenous TROY in T98G cells (Fig. 1C). To further confirm the association of TROY and JAK1 observed in the cultured glioma cells, we utilized two patient-derived GBM xenografts (PDX) [31]. Primary GBM xenografts GBM43 and GBM10 were lysed, immunoprecipitated with anti-TROY antibody, and the immunoprecipitates were immunoblotted with an anti-JAK1 antibody. Consistent with the results obtained with the cultured cell line, co-immunoprecipitation analysis showed that JAK1 associates with TROY in GBM43 and GBM10 cells (Fig. 1D and E). Taken together, the results show that TROY associates with JAK1 in glioma cells.
Structural basis of the interaction between TROY and JAK1
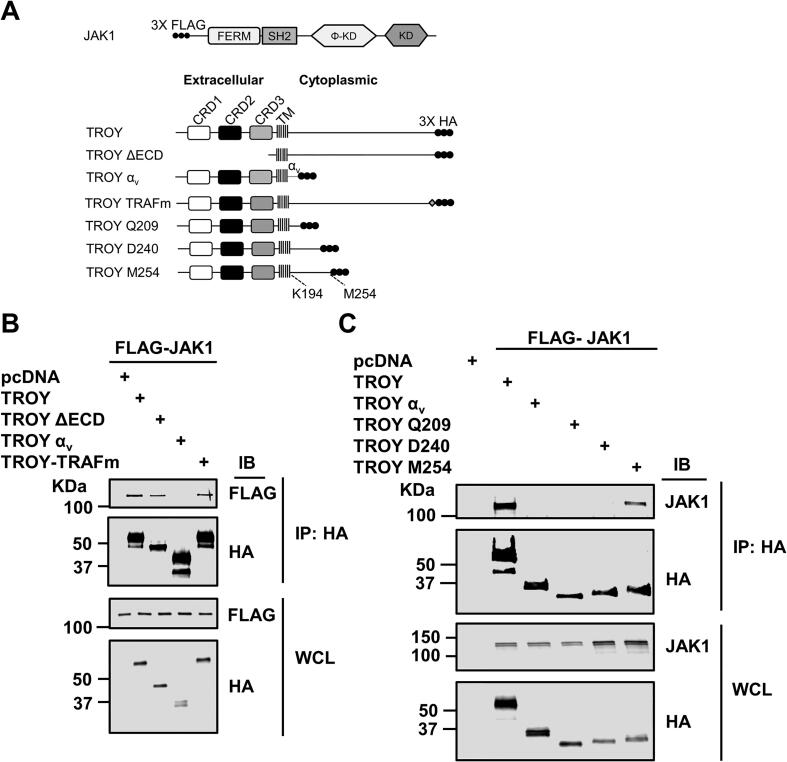
To determine the domain of TROY responsible for its interaction with JAK1, we generated a set of TROY variants containing substitutions or truncations in the TROY cytoplasmic domain (Fig. 2A). We co-transfected HEK293 cells with these HA epitope-tagged TROY variants and FLAG epitope-tagged JAK1. Twenty-four hours after transfection, the cells were lysed, immunoprecipitated with an anti-HA antibody, and the precipitates immunoblotted with an anti-FLAG antibody. The results showed that JAK1 was present in the precipitates of HA-tagged TROY WT and TROYΔECD, but not in the precipitates of TROY-αv, a TROY variant containing the cytoplasmic domain of integrin αv substituted for the TROY cytoplasmic domain, indicating that the cytoplasmic domain of TROY was required for its association with JAK1 (Fig. 2B). JAK1 was also present in the precipitates of the TROY variant (TROY-TRAFm) containing a mutation of the TRAF binding site (S413LQE416 → SLAA) in the TROY cytoplasmic domain, indicating that the TRAF binding site was not required for the TROY-JAK1 interaction (Fig. 2B).
Fig. 2.
TROY interacts with JAK1 through its cytoplasmic domain. (A) Schematic representation of the expression constructs encoding JAK1, TROY WT, and TROY variants. Φ-KD, pseudokinase domain; KD, Kinase domain; αv, integrin αv cytoplasmic domain; TRAFm, mutation of TRAF binding domain. The extracellular domain, the transmembrane (TM) domain, and cytoplasmic domains of TROY are indicated and the 3X HA epitope tag is shown. (B) HEK293 cells were co-transfected with FLAG epitope-tagged JAK1 and the indicated TROY construct, lysed 24 h after transfection, and immunoprecipitated with an anti-HA antibody. Immunoprecipitates or whole cell lysates (WCL) were IB with the indicated antibodies. (C) HEK293 cells were co-transfected with FLAG epitope-tagged JAK1 and the indicated TROY construct, lysed 24 h after transfection, and immunoprecipitated with an anti-HA antibody. Immunoprecipitates or WCL were IB with the indicated antibodies.
To narrow down the region of TROY cytoplasmic domain required for TROY-JAK1 interaction, we generated a set of TROY variants containing sequential C-terminal truncations of the cytoplasmic domain (Fig. 2A) for co-immunoprecipitation analysis. Results show that JAK1 was present in the immunoprecipitates of full-length TROY and TROY-M254, but not in the immunoprecipitates of TROY-Q209 and TROY-D240, suggesting that the region of residues between D240 and M254 is required for the interaction of TROY with JAK1 (Fig. 2C).
STAT3 is part of the TROY-JAK1 complex
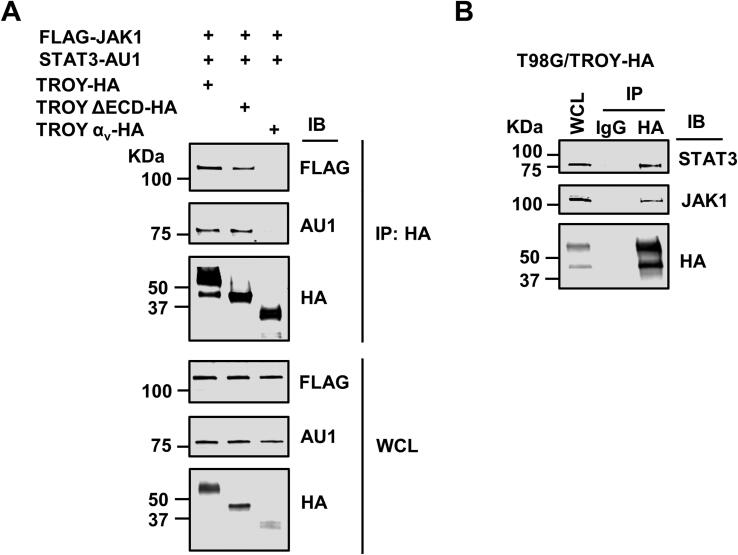
As STAT3 is a substrate for JAK1, we examined whether we could detect STAT3 in the TROY immunoprecipitates along with JAK1. HEK293 cells were co-transfected with HA epitope-tagged TROY variants, FLAG epitope-tagged JAK1, and AU1 epitope-tagged STAT3. The transfected cells were lysed 24 h after transfection, lysates immunoprecipitated with an anti-HA antibody, and the precipitates immunoblotted with appropriate anti-epitope antibodies. The results showed that both FLAG epitope-tagged JAK1 and AU1 epitope-tagged STAT3 were present in the precipitates of HA-tagged TROY WT and TROYΔECD, but not in the precipitates of TROY-αv, suggesting that STAT3 is a component of TROY-JAK1 signaling complex (Fig. 3A). To validate the results of the transfection studies, T98G/TROY-HA cells were lysed, immunoprecipitated with anti-HA antibody, and the immunoprecipitates were immunoblotted with anti-JAK1 antibody and anti-STAT3 antibody. The results showed that both endogenous JAK1 and STAT3 were present in TROY precipitates (Fig. 3B) confirming they are components of the assembled TROY signalsome.
Fig. 3.
STAT3 is part of TROY-JAK1 complex. (A) HEK293 cells were co-transfected with FLAG epitope-tagged JAK1, AU1 epitope-tagged STAT3, and the indicated TROY construct. Cells were lysed 24 h after transfection and immunoprecipitated with an anti-HA antibody. Immunoprecipitates or WCL were immunoblotted (IB) with the indicated antibodies. (B) Cell lysates of T98G/TROY-HA cells were immunoprecipitated with anti-HA antibody and the precipitates immunoblotted (IB) with the indicated antibodies. WCL, whole cell lysates.
Increased TROY expression activates JAK1/STAT3 pathway
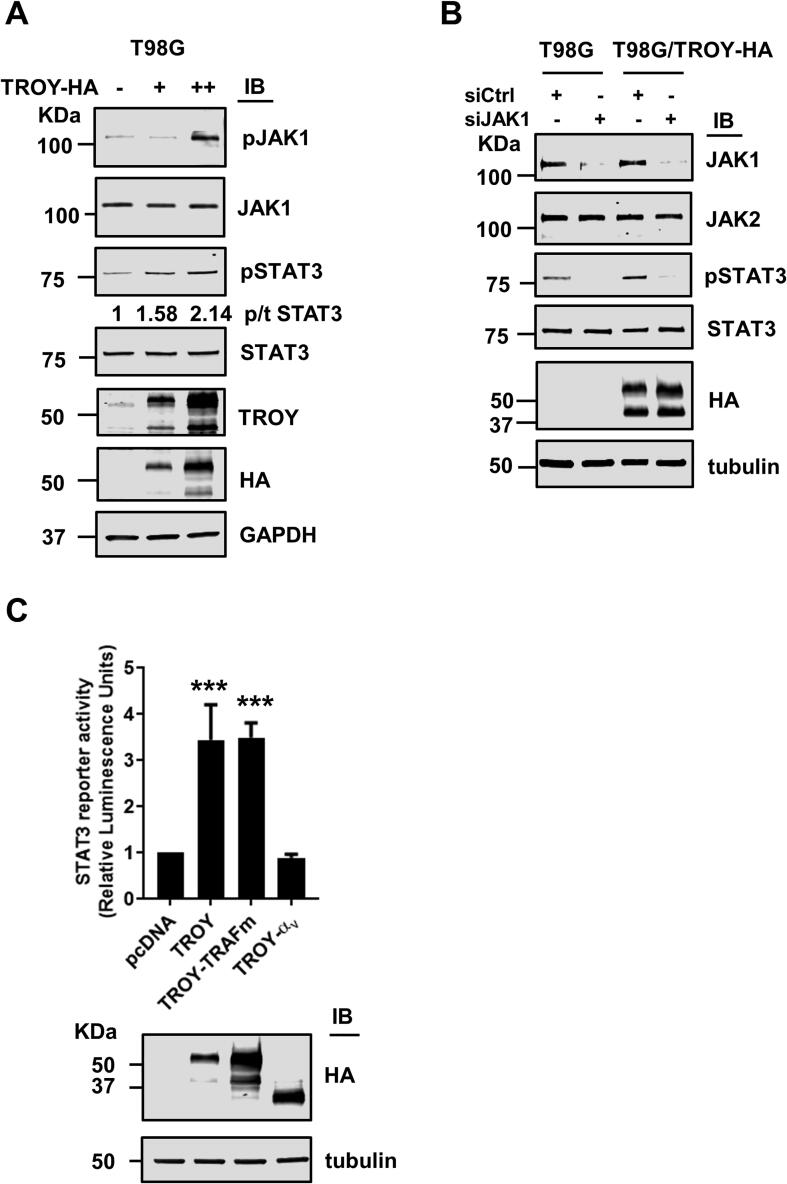
Activation of JAKs enables recruitment and phosphorylation of downstream STAT effectors [32]. As both JAK1 and STAT3 were present in the TROY signalsome, we examined the effect of TROY expression on the activity of the JAK1/STAT3 pathway. T98G cells transiently transfected with increasing amounts of TROY were lysed and lysates immunoblotted with antibodies against phospho-JAK1 and phospho-STAT3. Results showed that increased TROY expression produces a significant increase in the staining for phosphorylated JAK1 (Fig. 4A). The increase in staining for phosphorylated JAK1 was accompanied by a two-fold increase in staining for phosphorylated STAT3. To validate the role of JAK1 in TROY-induced STAT3 activation, we silenced JAK1 expression using validated siRNA in T98G and T98G/TROY-HA cells. Forty-eight hours after transfection with siRNA, cells were serum starved overnight, lysed, and cell lysates immunoblotted (Fig. 4B). Transfection of cells with a control, non-targeting siRNA did not alter JAK1 or STAT3 expression levels. Transfection of cells with a siRNA targeting JAK1 significantly reduced expression levels of JAK1 while JAK2 expression levels were unaffected indicating specificity of the knockdown. Knockdown of JAK1 markedly decreased TROY-induced STAT3 phosphorylation indicating that TROY-induced STAT3 activation is dependent upon JAK1. To examine whether increased TROY expression induced STAT3 transcriptional activity, we used a cell-based STAT3 reporter assay. HEK293 cells were co-transfected with a STAT3 reporter plasmid and either a plasmid encoding wild-type TROY, the TROY-TRAFm variant, or the TROYαv variant. The reporter assay showed that increased TROY expression significantly increased STAT3 activation (Fig. 4C). Similarly, expression of the TROY-TRAFm variant, which is unable to activate NF-κB [16], retained the ability to activate STAT3 indicating that interaction of TROY with TRAFs is not required for STAT3 activation further highlighting the diversity of TROY signaling. In contrast, co-transfection of the STAT3 reporter with the TROYαv variant which fails to interact with JAK1, failed to activate STAT3.
Fig. 4.
Increased TROY expression activates JAK1/STAT3 pathway. (A) Immunoblots of lysates of T98G cells transfected with increasing amounts of TROY plasmid. Cells were transfected with HA-tagged TROY plasmid for 24 h followed by overnight serum starvation. The values for p/t STAT3 represent the ratio of phosphorylated STAT3 to total STAT3 and were normalized to GAPDH. (B) T98G and T98G/TROY-HA cells were transfected with siRNA targeting JAK1 (siJAK1) or control siRNA (siCtrl). Forty-eight hours after transfection the cells were serum starved overnight, lysed, and immunoblotted with the indicated antibodies. (C) A cell based STAT3 reporter assay indicated STAT3 activation following expression of TROY and TROY-TRAFm variant but not the TROY-αv variant. Immunoblots for these samples show the equivalent expression of TROY and its variants. ***, p < 0.001.
Knockdown of JAK1 or inhibition of JAK1 reduces TROY-induced GBM cell migration
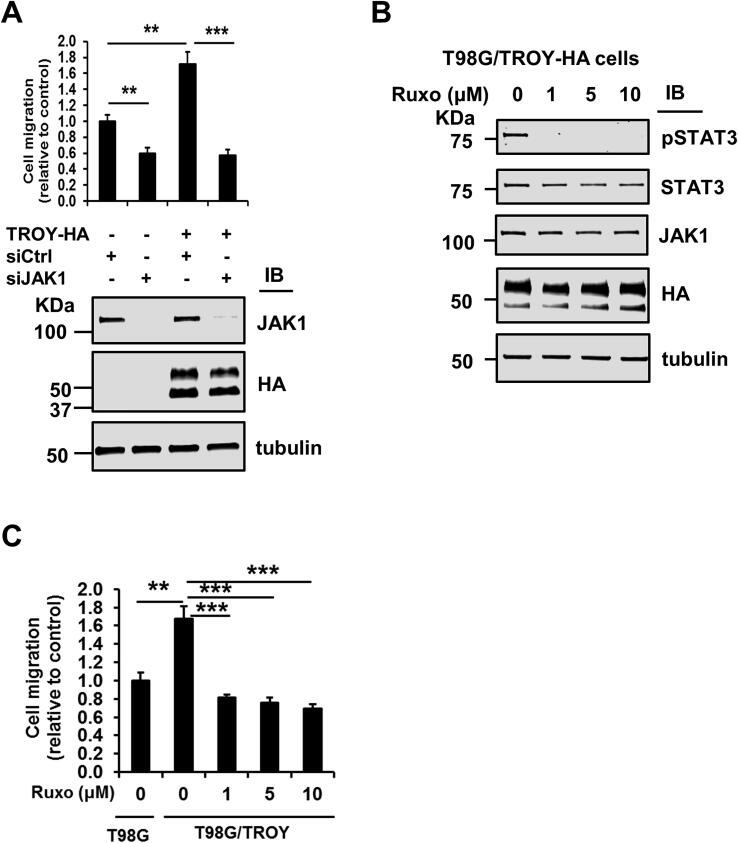
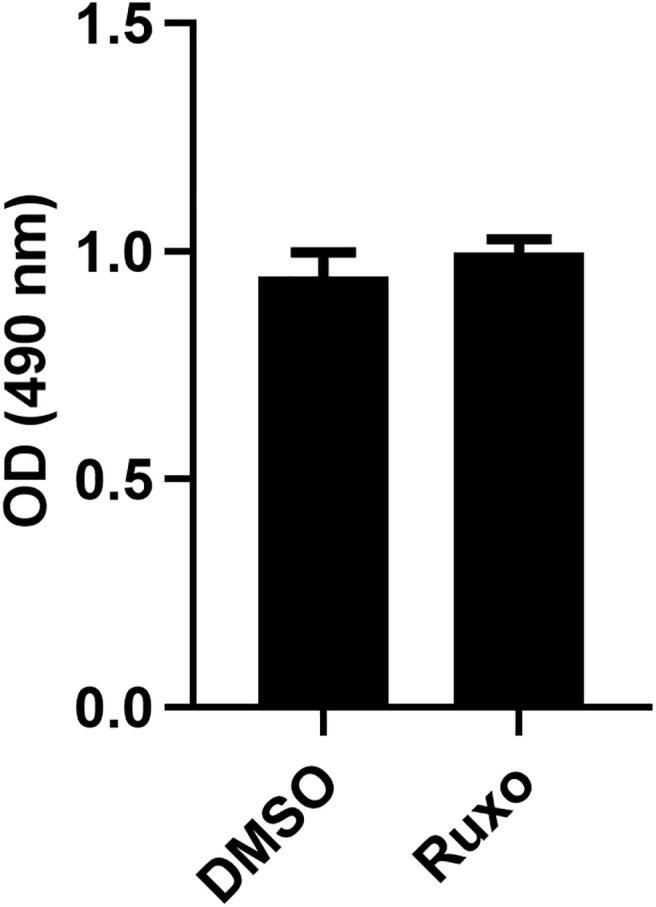
To examine the role of JAK1 in TROY-induced GBM cell migration, T98G and T98G/TROY-HA cells were transfected with a validated siRNA targeting JAK1 or a control nontargeting siRNA and migration analyzed by transwell assay. Consistent with previous results [13], increased expression of TROY significantly increased the migration of cells (Fig. 5A). Knockdown of JAK1 by siRNA significantly inhibited the migration of both parental T98G cells as well as the migration of T98G cells with increased TROY expression (Fig. 5A). To test whether inhibition of JAK1 has the same effect as knockdown of JAK1 expression, we treated T98G/TROY-HA cells with increasing concentrations of the JAK inhibitor ruxolitinib [33]. Immunoblotting analysis showed that ruxolitinib treatment potently blocked the TROY-mediated phosphorylation of STAT3, confirming the importance of JAK1 in TROY-induced STAT3 activation (Fig. 5B). Moreover, the results of a transwell migration assay showed that the increased migration induced by increased TROY expression was significantly inhibited by treatment with ruxolitinib (Fig. 5C), consistent with the effects of JAK1 knockdown on TROY-induced cell migration. To ensure this migration difference was not a result of concomitant changes in cell proliferation, we examined the effects of ruxolitinib on cell proliferation. We found that treatment with ruxolitinib did not change the proliferation of glioma cells as assessed by MTS assay (Suppl. Fig. 1). Thus, knockdown of JAK1 expression by siRNA or inhibition of JAK1 activity reduced TROY-mediated GBM cell migration.
Fig. 5.
Knockdown of JAK1 expression or inhibition of JAK1 inhibits TROY-induced GBM cell migration. (A) Migration assay for T98G and T98G/TROY-HA cells transfected for siJAK1. Cells were transfected with siRNA targeting JAK1 (siJAK1) or control siRNA (siCtrl) for 24 h. Cells were harvested and plated in DMEM with 0.1% serum into the top chamber of collagen-coated transwell inserts with DMEM containing 10% serum in the bottom chamber. Twenty-four hours later, the migrated cells were fixed and counted. Immunoblots show the knockdown efficiency of JAK1 siRNA. **, p < 0.01; ***, p < 0.001. (B) Immunoblots for the lysates of serum starved T98G/TROY-HA treated with increasing concentration of ruxolitinib (Ruxo) show potent inhibition of STAT3 activation. (C) Migration assay for T98G and T98G/TROY-HA cells treated with ruxolitinib. Serum starved T98G/TROY-HA cells were treated with ruxolitinib for 24 h. Cells were harvested and plated in DMEM with 0.1% serum into the top chamber of collagen-coated transwell inserts with DMEM containing 10% serum in the bottom chamber. Twenty-four hours later, the migrated cells were fixed and counted. T98G cells were used a control. Results indicate that increased TROY expression significantly increased cell migration which was inhibited by treatment with ruxolitinib (Ruxo). **, p < 0.01; ***, p < 0.001.
TROY-mediated activation of STAT3 is independent of its ability to stimulate activity of NF-κB
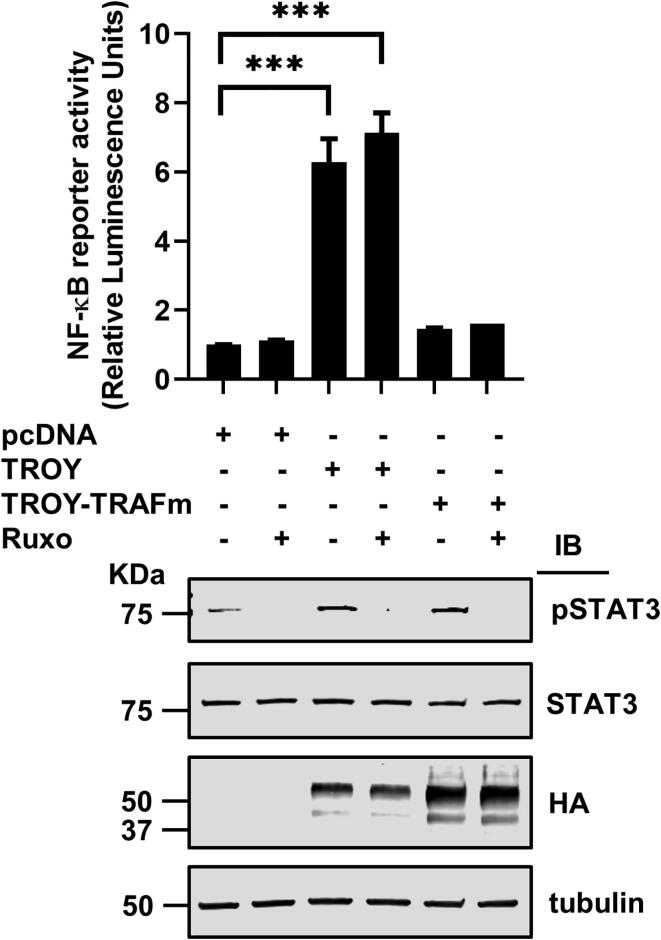
We have previously demonstrated that increased expression of TROY results in activation of NF-κB [14]. Co-operation and crosstalk between STAT3 and NF-κB signaling pathways have been reported to promote the development and progression of several cancers [34]. Since we observed that increased TROY expression can activate STAT3 activity through JAK1, we examined whether TROY-induced NF-κB signaling and TROY-induced STAT3 signaling can be independently modulated. HEK293 cells expressing an NF-κB reporter were transfected with wild-type TROY or the TROY-TRAFm variant and the effect of NF-κB activity determined in the presence or absence of ruxolitinib. Although TROY-induced increased phosphorylation of STAT3 was completely blocked by ruxolitinib (Fig. 5B), TROY-mediated activation of NF-κB activity was not inhibited by ruxolitinib (Fig. 6). Additionally, while mutation of the TRAF binding site in the TROY cytoplasmic domain (TROY TRAFm) was not required for TROY-mediated STAT3 activation (Fig. 4C), this mutation potently inhibited TROY-mediated activation of NF-κB. Taken together, the results showed that TROY-induced NF-κB activity and TROY-stimulated STAT3 activation can be independently modulated.
Fig. 6.
Inhibition of JAK1 by ruxolitinib does not inhibit TROY-induced NF-κB activation. A cell based NF-κB reporter assay indicated that TROY mediated NF-κB activation which was not inhibited by ruxolitinib treatment. The immunoblots (lower panel) of these samples showed the activation of STAT3 by TROY and TROY-TRAFm variant as well as the equivalent expression of TROY and TROY-TRAFm variant. ***, p < 0.001.
Ruxolitinib sensitizes T98G/TROY-HA cells to TMZ treatment
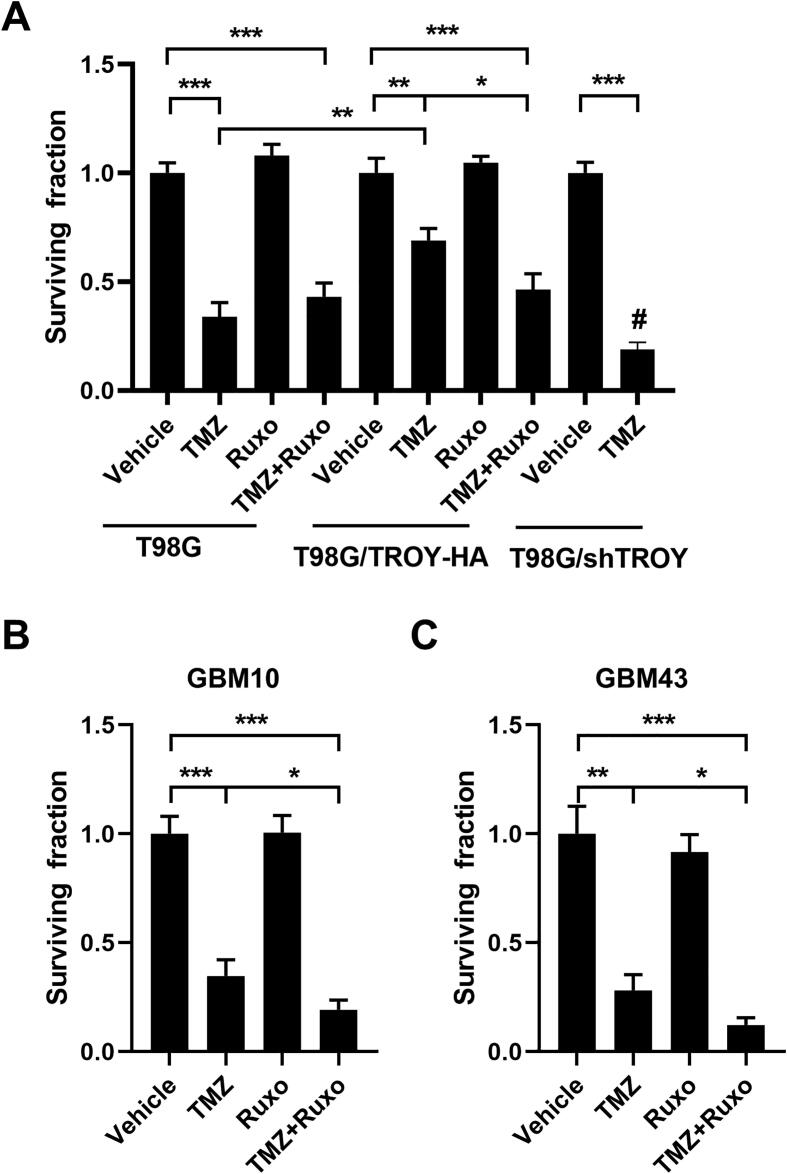
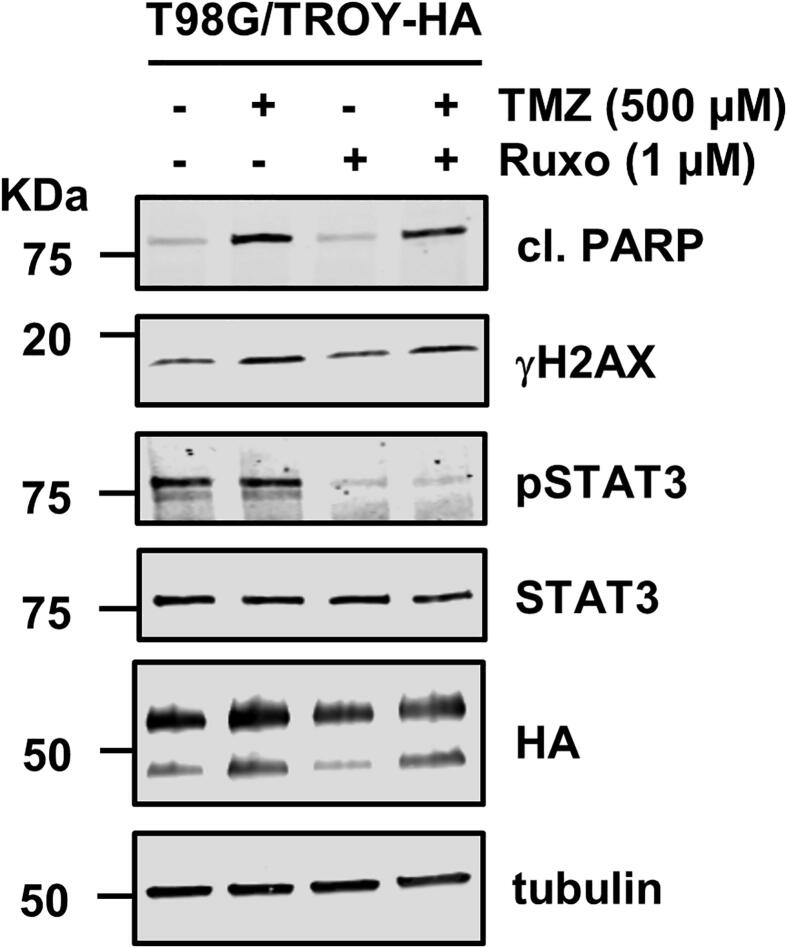
We have previously shown that increased expression of TROY results in increased resistance to TMZ [14]. To examine the effect of inhibition of JAK1 on resistance of glioma cells to TMZ, T98G and T98G/TROY-HA cells were treated with vehicle, ruxolitinib alone, TMZ alone, or pretreated with ruxolitinib for 2 h followed by TMZ for 24 h. After 24 h, cells were harvested and the effect of treatments was determined by colony formation assay. Consistent with previous results showing that knockdown of TROY expression sensitized cells to TMZ [14], the results of the colony formation assay showed that increased expression of TROY increased the resistance to TMZ as indicated by a greater surviving fraction relative to the parental cells (Fig. 7A). Treatment with ruxolitinib alone did not alter colony growth of either the T98G and T98G/TROY-HA cells relative to cells treated with vehicle. The combination of ruxolitinib and TMZ did not reduce the colony number compared to TMZ alone in the parental T98G cells. In contrast, combination treatment of T98G/TROY-HA cells significantly reduced colony growth relative to TMZ treatment alone. These data suggest that ruxolitinib could enhance the chemotherapeutic efficacy of TMZ in GBM cells with increased expression of TROY. We also examined the effect of each treatment on apoptosis. After three days of treatment, immunoblotting analysis showed that treatment with TMZ induced apoptosis as evidenced by an increase in cleaved PARP while treatment with ruxolitinib alone did not induce an increase in cleaved PARP (Suppl. Fig. 2). We also examined the effect of ruxolitinib treatment on the sensitivity of two primary PDX cell lines to TMZ. Treatment of cells with TMZ or ruxolitinib, alone or in combination, did not decrease the interaction of TROY with JAK1 relative to cells treated with vehicle (data not shown). Consistent with the results obtained from T98G/TROY-HA cells, treatment with ruxolitinib alone did not alter colony growth of GBM10 and GBM43 relative to cells treated with vehicle, while combination treatment of GBM10 and GBM43 cells significantly reduce colony growth relative to TMZ treatment alone (Fig. 7B and C).
Fig. 7.
Ruxolitinib sensitizes T98G/TROY-HA, GBM10, and GBM43 cells to TMZ treatment. T98G and T98G/TROY-HA cells (A), GBM10 cells (B), and GBM43 cells (C) were treated with vehicle, TMZ (500 μM), or ruxolitinib (ruxo, 1 μM) for 24 h. The TMZ+ruxo group was pretreated with ruxo for 2 h before addition of TMZ. T98G cells with stable knockdown of TROY (T98G/shTROY) were used as a control, and treated with or without TMZ. After 24 h, cells from all groups were harvested, 1500 cells were replated in triplicate, and the surviving fraction was measured by colony formation 10 days later. *, p < 0.05; **, p < 0.01; ***, p < 0.001. #, p < 0.05, compared to parental cells treated with TMZ.
Discussion
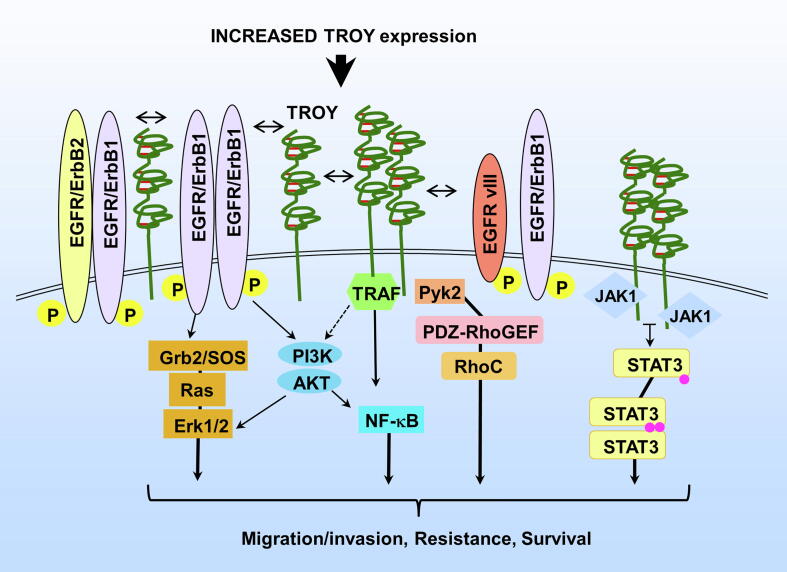
In the present study, we identify further diversity in the signaling pathways activated by the multi-protein TROY signalsome and describe a role for the novel TROY-JAK1 interaction in glioma cell migration. The major findings of this study are as follows: (1) TROY associates with JAK1 and its interaction is independent of its association with EGFR; (2) Residues between D240 and M254 of the TROY cytoplasmic domain are required for its interaction with JAK1; (3) Increased TROY expression promotes STAT3 phosphorylation and STAT3 transcriptional activity that is dependent upon JAK1; (4) TROY-mediated activation of STAT3 and its stimulation of NF-κB are independent; (5) Knockdown of JAK1 expression or inhibition of JAK1 by ruxolitinib inhibits TROY-stimulated GBM cell migration; (6) Ruxolitinib enhances the chemotherapeutic efficacy of TMZ in GBM cells with increased expression of TROY. Together, these results demonstrate that the TROY signalsome may represent a unique therapeutic target to inhibit multiple signaling pathways mediating GBM cell invasion and resistance (Fig. 8).
Fig. 8.
The TROY signalsome activates multiple signaling pathways.
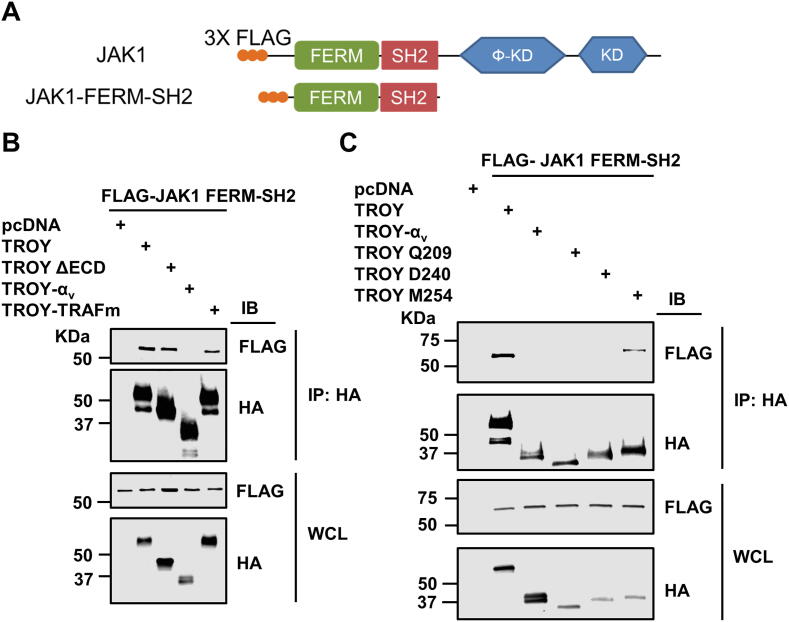
This study is the first to identify JAK1 as a component of the TROY signalsome. In addition to TROY, other members of TNF receptor superfamily have been reported to be involved in JAK/STAT3 signaling. For instance, TNFR1 has been shown to activate JAK/STAT3 signaling in murine 3T3-L1 adipocytes [35]. In addition, TNFR1 has been shown to co-immunoprecipitate with JAK1 in human B cells after TNF-α treatment [36]. JAK1 consists of N-terminal FERM and SH2 domains followed by the pseudokinase domain and the kinase domain [37]. The FERM and SH2 domains of JAK1 have been demonstrated to be required for mediating the JAK1 – cytokine receptors interaction [37]. Similar to the interaction of JAK1 with cytokine receptors, co-immunoprecipitation analysis showed that the FERM and SH2 domains of JAK1 are sufficient to interact with TROY (Suppl. Fig. 3).
The identification of the interaction of JAK1 with TROY further increases the diversity of the composition of the TROY signalsome and the complexity of signaling pathways potentially modulated by this complex. We have previously shown that TROY associates with the non-receptor tyrosine kinase Pyk2 and depletion of Pyk2 inhibits TROY-induced Rac1 activity, resulting in inhibition of TROY-mediated GBM cell migration [13]. We have also shown that TROY forms a complex with EGFR and can modulate EGFR signaling [16]. Furthermore, we have demonstrated that the TROY signalsome includes PDZ-RhoGEF [17]. PDZ-RhoGEF co-immunoprecipitates with TROY and Pyk2 and can be phosphorylated by Pyk2. Silencing of PDZ-RhoGEF expression inhibits TROY-mediated GBM cell migration, increases sensitivity to TMZ, and prolongs survival in an orthotopic xenograft model [17]. Therefore, the assembly of the TROY signalsome in cells with aberrant TROY overexpression suggests that these cells may have an added survival benefit due to facilitated activation of multiple cellular signaling pathways stimulating growth, migration, and resistance.
It is well appreciated that JAK/STAT3 signaling pathway plays a key role in the growth and development of many human cancers [38]. STAT3 tyrosine phosphorylation has been shown to correlate with a poor outcome in GBM [39]. Therefore, targeting JAK/STAT3 pathway could represent a potential promising approach for the treatment of GBM. Ruxolitinib, a small molecular inhibitor for JAK1/2, has been approved by FDA to treat myelofibrosis and has been evaluated for the treatment of chronic lymphocytic leukemia in a phase II clinical trial [33], [40]. In addition, Tavallai et al. demonstrated that ruxolitinib synergistically interacted with dual ERBB1/2/4 inhibitors to kill breast, lung, ovarian, and brain cancer cells in vitro [41]. Dietary curcumin, a polyphenol found in turmeric, has been reported to inhibit glioma growth by inhibition of the JAK/STAT3 signaling pathway in a syngeneic mouse model [42]. In this study, we found TROY-induced STAT3 activation and GBM cell migration can be inhibited by ruxolitinib. We have previously shown that increased expression of TROY results in therapeutic resistance in GBM [14]. Although ruxolitinib did not increase the sensitivity of cells without increased TROY expression to TMZ, inhibition of the JAK/STAT3 pathway by ruxolitinib could enhance the efficacy of TMZ in GBM cells with increased expression of TROY. Notably, ruxolitinib was found to penetrate the brain-blood barrier when systemically administered in mice [43]. Thus, utilizing ruxolitinib in combination with TMZ warrants further investigation in murine patient-derived xenograft models to determine its potential as a therapeutic regimen for GBM patients with increased TROY expression.
We have previously shown that increased expression of TROY results in activation of NF-κB [14]. Cooperation and crosstalk between STAT3 and NF-κB signaling pathways has been reported in a number of cancers [34]. Both of these transcription factors are involved not only in the regulation of cellular processes including proliferation, apoptosis, angiogenesis, and metastasis, but also in the development of therapeutic resistance. In addition to binding to adjacent sites in the control regions of target genes shared with STAT3, several NF-κB family members, in particular RelA/p65 and p50, have been found to physically interact with STAT3 [44], [45]. Moreover, activated STAT3 can maintain NF-κB activity in tumors by prolonging the nuclear retention of NF-κB [45]. In this study, we showed that TROY-TRAFm variant, which is unable to activate NF-κB, retained the ability to activate STAT3. In addition, TROY-induced NF-κB activation was not inhibited by ruxolitinib. Therefore, GBM cells with increased expression of TROY can activate NF-κB through a classical TRAF mediated pathway and facilitate JAK1-mediated STAT3 activation stimulating the transcription of STAT3 target genes and potentially maintaining or amplifying NF-κB activity. These results underscore that single modality treatment of GBM cells with increased expression of TROY that targets either pathway individually is unlikely to have strong therapeutic efficacy. Thus, activation of NF-κB and STAT3 by TROY, as well as the capacity of TROY to stimulate EGFR signaling, positions the TROY signalsome as an emerging molecular hub (Fig. 8).
In summary, the current data establish an important role for the JAK1/STAT3 pathway in signaling from the TROY signalsome. The results indicate that TROY-stimulated STAT3 activation and glioma cell migration are regulated by JAK1. Overall, these findings suggest that TROY signalsome may represent an appealing therapeutic target with the distinctive capacity to exert effects on multiple signaling pathways regulating cell invasion, resistance, and survival in malignant glioma.
In brief
An improved understanding of the mechanisms responsible for glioblastoma (GBM) cell invasion and resistance is critical for developing therapies against GBM. Ding et al. identify further diversity in the signaling pathways activated by the multi-protein TROY signalsome and describe a role for the novel TROY-JAK1 interaction in glioma cell migration.
Grant support
The work was funded by National Institutes of Health grants R01 NS086853, R01 NS092955, and U01 CA220378-01 (J.C.L. & N.L.T.).
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.neo.2020.06.005.
Contributor Information
Nhan L. Tran, Email: Tran.Nhan@mayo.edu.
Joseph C. Loftus, Email: Loftus.Joseph@mayo.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Supplementary Fig. 1.
Supplementary Fig. 2.
Supplementary Fig. 3.
References
- 1.Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Giese A., Bjerkvig R., Berens M.E., Westphal M. Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol. 2003;21:1624–1636. doi: 10.1200/JCO.2003.05.063. [DOI] [PubMed] [Google Scholar]
- 4.Wong E.T., Hess K.R., Gleason M.J., Jaeckle K.A., Kyritsis A.P., Prados M.D., Levin V.A., Yung W.K. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol. 1999;17:2572–2578. doi: 10.1200/JCO.1999.17.8.2572. [DOI] [PubMed] [Google Scholar]
- 5.Hu S., Tamada K., Ni J., Vincenz C., Chen L. Characterization of TNFRSF19, a novel member of the tumor necrosis factor receptor superfamily. Genomics. 1999;62:103–107. doi: 10.1006/geno.1999.5979. [DOI] [PubMed] [Google Scholar]
- 6.Pispa J., Mikkola M.L., Mustonen T., Thesleff I. Ectodysplasin, Edar and TNFRSF19 are expressed in complementary and overlapping patterns during mouse embryogenesis. Gene Expr Patterns. 2003;3:675–679. doi: 10.1016/s1567-133x(03)00092-9. [DOI] [PubMed] [Google Scholar]
- 7.Hisaoka T., Morikawa Y., Kitamura T., Senba E. Expression of a member of tumor necrosis factor receptor superfamily, TROY, in the developing mouse brain. Brain Res Dev Brain Res. 2003;143:105–109. doi: 10.1016/s0165-3806(03)00101-9. [DOI] [PubMed] [Google Scholar]
- 8.Hisaoka T., Morikawa Y., Kitamura T., Senba E. Expression of a member of tumor necrosis factor receptor superfamily, TROY, in the developing olfactory system. Glia. 2004;45:313–324. doi: 10.1002/glia.10323. [DOI] [PubMed] [Google Scholar]
- 9.Schon S., Flierman I., Ofner A., Stahringer A., Holdt L.M., Kolligs F.T., Herbst A. Beta-catenin regulates NF-kappaB activity via TNFRSF19 in colorectal cancer cells. Int J Cancer. 2014;135:1800–1811. doi: 10.1002/ijc.28839. [DOI] [PubMed] [Google Scholar]
- 10.Spanjaard R.A., Whren K.M., Graves C., Bhawan J. Tumor necrosis factor receptor superfamily member TROY is a novel melanoma biomarker and potential therapeutic target. Int J Cancer. 2007;120:1304–1310. doi: 10.1002/ijc.22367. [DOI] [PubMed] [Google Scholar]
- 11.Bei J.X., Li Y., Jia W.H., Feng B.J., Zhou G., Chen L.Z., Feng Q.S., Low H.Q., Zhang H., He F. A genome-wide association study of nasopharyngeal carcinoma identifies three new susceptibility loci. Nat Genet. 2010;42:599–603. doi: 10.1038/ng.601. [DOI] [PubMed] [Google Scholar]
- 12.Deng C., Lin Y.X., Qi X.K., He G.P., Zhang Y., Zhang H.J., Xu M., Feng Q.S., Bei J.X., Zeng Y.X. TNFRSF19 Inhibits TGFbeta signaling through interaction with TGFbeta receptor Type I to promote tumorigenesis. Cancer Res. 2018;78:3469–3483. doi: 10.1158/0008-5472.CAN-17-3205. [DOI] [PubMed] [Google Scholar]
- 13.Paulino V.M., Yang Z., Kloss J., Ennis M.J., Armstrong B.A., Loftus J.C., Tran N.L. TROY (TNFRSF19) is overexpressed in advanced glial tumors and promotes glioblastoma cell invasion via Pyk2-Rac1 signaling. Mol Cancer Res. 2010;8:1558–1567. doi: 10.1158/1541-7786.MCR-10-0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loftus J.C., Dhruv H., Tuncali S., Kloss J., Yang Z., Schumacher C.A., Cao B., Williams B.O., Eschbacher J.M., Ross J.T. TROY (TNFRSF19) promotes glioblastoma survival signaling and therapeutic resistance. Mol Cancer Res. 2013;11:865–874. doi: 10.1158/1541-7786.MCR-13-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng A., Zhao S., FitzGerald L.M., Wright J.L., Kolb S., Karnes R.J., Jenkins R.B., Davicioni E., Ostrander E.A., Feng Z. A four-gene transcript score to predict metastatic-lethal progression in men treated for localized prostate cancer: development and validation studies. Prostate. 2019;79:1589–1596. doi: 10.1002/pros.23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ding Z., Roos A., Kloss J., Dhruv H., Peng S., Pirrotte P., Eschbacher J.M., Tran N.L., Loftus J.C. A novel signaling complex between TROY and EGFR mediates glioblastoma cell invasion. Mol Cancer Res. 2018;16:322–332. doi: 10.1158/1541-7786.MCR-17-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Z., Dhruv H., Kwiatkowska-Piwowarczyk A., Ruggieri R., Kloss J., Symons M., Pirrotte P., Eschbacher J.M., Tran N.L., Loftus J.C. PDZ-RhoGEF is a signaling effector for TROY-induced glioblastoma cell invasion and survival. Neoplasia. 2018;20:1045–1058. doi: 10.1016/j.neo.2018.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huynh J., Chand A., Gough D., Ernst M. Therapeutically exploiting STAT3 activity in cancer - using tissue repair as a road map. Nat Rev Cancer. 2019;19:82–96. doi: 10.1038/s41568-018-0090-8. [DOI] [PubMed] [Google Scholar]
- 19.Bromberg J. Stat proteins and oncogenesis. J Clin Invest. 2002;109:1139–1142. doi: 10.1172/JCI15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brantley E.C., Benveniste E.N. Signal transducer and activator of transcription- 3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6:675–684. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abou-Ghazal M., Yang D.S., Qiao W., Reina-Ortiz C., Wei J., Kong L.Y., Fuller G.N., Hiraoka N., Priebe W., Sawaya R. The incidence, correlation with tumor-infiltrating inflammation, and prognosis of phosphorylated STAT3 expression in human gliomas. Clin Cancer Res. 2008;14:8228–8235. doi: 10.1158/1078-0432.CCR-08-1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tu Y., Zhong Y., Fu J., Cao Y., Fu G., Tian X., Wang B. Activation of JAK/STAT signal pathway predicts poor prognosis of patients with gliomas. Med Oncol. 2011;28:15–23. doi: 10.1007/s12032-010-9435-1. [DOI] [PubMed] [Google Scholar]
- 23.Priester M., Copanaki E., Vafaizadeh V., Hensel S., Bernreuther C., Glatzel M., Seifert V., Groner B., Kogel D., Weissenberger J. STAT3 silencing inhibits glioma single cell infiltration and tumor growth. Neuro Oncol. 2013;15:840–852. doi: 10.1093/neuonc/not025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McFarland B.C., Ma J.Y., Langford C.P., Gillespie G.Y., Yu H., Zheng Y., Nozell S.E., Huszar D., Benveniste E.N. Therapeutic potential of AZD1480 for the treatment of human glioblastoma. Mol Cancer Ther. 2011;10:2384–2393. doi: 10.1158/1535-7163.MCT-11-0480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu S., Fu J., Dong Y., Yi Q., Lu D., Wang W., Qi Y., Yu R., Zhou X. GOLPH3 promotes glioma progression via facilitating JAK2-STAT3 pathway activation. J Neurooncol. 2018;139:269–279. doi: 10.1007/s11060-018-2884-7. [DOI] [PubMed] [Google Scholar]
- 26.Sarkaria J.N., Yang L., Grogan P.T., Kitange G.J., Carlson B.L., Schroeder M.A., Galanis E., Giannini C., Wu W., Dinca E.B. Identification of molecular characteristics correlated with glioblastoma sensitivity to EGFR kinase inhibition through use of an intracranial xenograft test panel. Mol Cancer Ther. 2007;6:1167–1174. doi: 10.1158/1535-7163.MCT-06-0691. [DOI] [PubMed] [Google Scholar]
- 27.Loftus J.C., Yang Z., Tran N.L., Kloss J., Viso C., Berens M.E., Lipinski C.A. The Pyk2 FERM domain as a target to inhibit glioma migration. Mol Cancer Ther. 2009;8:1505–1514. doi: 10.1158/1535-7163.MCT-08-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seiler C.Y., Park J.G., Sharma A., Hunter P., Surapaneni P., Sedillo C., Field J., Algar R., Price A., Steel J. DNASU plasmid and PSI: biology-materials repositories: resources to accelerate biological research. Nucleic Acids Res. 2014;42:D1253–1260. doi: 10.1093/nar/gkt1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franken N.A., Rodermond H.M., Stap J., Haveman J., van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–2319. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 30.Tong J., Taylor P., Jovceva E., St-Germain J.R., Jin L.L., Nikolic A., Gu X., Li Z.H., Trudel S., Moran M.F. Tandem immunoprecipitation of phosphotyrosine-mass spectrometry (TIPY-MS) indicates C19ORF19 becomes tyrosine-phosphorylated and associated with activated epidermal growth factor receptor. J Proteome Res. 2008;7:1067–1077. doi: 10.1021/pr7006363. [DOI] [PubMed] [Google Scholar]
- 31.Giannini C., Sarkaria J.N., Saito A., Uhm J.H., Galanis E., Carlson B.L., Schroeder M.A., James C.D. Patient tumor EGFR and PDGFRA gene amplifications retained in an invasive intracranial xenograft model of glioblastoma multiforme. Neuro Oncol. 2005;7:164–176. doi: 10.1215/S1152851704000821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Shea J.J., Schwartz D.M., Villarino A.V., Gadina M., McInnes I.B., Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med. 2015;66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mascarenhas J., Hoffman R. Ruxolitinib: the first FDA approved therapy for the treatment of myelofibrosis. Clin Cancer Res. 2012;18:3008–3014. doi: 10.1158/1078-0432.CCR-11-3145. [DOI] [PubMed] [Google Scholar]
- 34.Grivennikov S.I., Karin M. Dangerous liaisons: STAT3 and NF-kappaB collaboration and crosstalk in cancer. Cytokine Growth Factor Rev. 2010;21:11–19. doi: 10.1016/j.cytogfr.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guo D., Dunbar J.D., Yang C.H., Pfeffer L.M., Donner D.B. Induction of Jak/STAT signaling by activation of the type 1 TNF receptor. J Immunol. 1998;160:2742–2750. [PubMed] [Google Scholar]
- 36.Miscia S., Marchisio M., Grilli A., Di Valerio V., Centurione L., Sabatino G., Garaci F., Zauli G., Bonvini E., Di Baldassarre A. Tumor necrosis factor alpha (TNF-alpha) activates Jak1/Stat3-Stat5B signaling through TNFR-1 in human B cells. Cell Growth Differ. 2002;13:13–18. [PubMed] [Google Scholar]
- 37.Ferrao R., Lupardus P.J. The Janus Kinase (JAK) FERM and SH2 domains: bringing specificity to JAK-receptor interactions. Front Endocrinol (Lausanne) 2017;8:71. doi: 10.3389/fendo.2017.00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas S.J., Snowden J.A., Zeidler M.P., Danson S.J. The role of JAK/STAT signalling in the pathogenesis, prognosis and treatment of solid tumours. Br J Cancer. 2015;113:365–371. doi: 10.1038/bjc.2015.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Birner P., Toumangelova-Uzeir K., Natchev S., Guentchev M. STAT3 tyrosine phosphorylation influences survival in glioblastoma. J Neurooncol. 2010;100:339–343. doi: 10.1007/s11060-010-0195-8. [DOI] [PubMed] [Google Scholar]
- 40.Jain P., Keating M., Renner S., Cleeland C., Xuelin H., Gonzalez G.N., Harris D., Li P., Liu Z., Veletic I. Ruxolitinib for symptom control in patients with chronic lymphocytic leukaemia: a single-group, phase 2 trial. Lancet Haematol. 2017;4:e67–e74. doi: 10.1016/S2352-3026(16)30194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tavallai M., Booth L., Roberts J.L., Poklepovic A., Dent P. Rationally repurposing ruxolitinib (Jakafi ((R))) as a solid tumor therapeutic. Front Oncol. 2016;6:142. doi: 10.3389/fonc.2016.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weissenberger J., Priester M., Bernreuther C., Rakel S., Glatzel M., Seifert V., Kogel D. Dietary curcumin attenuates glioma growth in a syngeneic mouse model by inhibition of the JAK1,2/STAT3 signaling pathway. Clin Cancer Res. 2010;16:5781–5795. doi: 10.1158/1078-0432.CCR-10-0446. [DOI] [PubMed] [Google Scholar]
- 43.Haile W.B., Gavegnano C., Tao S., Jiang Y., Schinazi R.F., Tyor W.R. The Janus kinase inhibitor ruxolitinib reduces HIV replication in human macrophages and ameliorates HIV encephalitis in a murine model. Neurobiol Dis. 2016;92:137–143. doi: 10.1016/j.nbd.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lee H., Herrmann A., Deng J.H., Kujawski M., Niu G., Li Z., Forman S., Jove R., Pardoll D.M., Yu H. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida Y., Kumar A., Koyama Y., Peng H., Arman A., Boch J.A., Auron P.E. Interleukin 1 activates STAT3/nuclear factor-kappaB cross-talk via a unique TRAF6- and p65-dependent mechanism. J Biol Chem. 2004;279:1768–1776. doi: 10.1074/jbc.M311498200. [DOI] [PubMed] [Google Scholar]