Abstract
Peritoneal loose bodies (PLBs) have been sparingly documented within the surgical and radiologic literature, with 38 cases reported to date. A 67-year-old male presented to urology for the management of an asymmetric prostatic nodule. Imaging incidentally identified a well-circumscribed mass of low T2 signal intensity with a small fatty core in the left lower quadrant close to the sigmoid colon; malignancy was in the differential. The mass grew slightly over the next year. A diagnostic laparoscopy retrieved a free floating 4 × 4 cm benign mass from the pelvis, identified as necrotic fat with areas of dystrophic calcifications. PLBs are often a diagnostic dilemma without surgical intervention. Here we present a diagnostic algorithm based on a comprehensive literature review and our case to help better identify unknown abdominal and pelvic fatty masses and to avoid surgery strictly for diagnosis, especially for patients that are not ideal surgical candidates. Using this algorithm, the mass in the patient presented here could have been accurately characterized without invasive diagnostic measures.
Keywords: Peritoneal loose bodies, Hylanized fat, Radiologic differentiation
Introduction
Peritoneal loose bodies (PLBs) have been sparingly documented within the surgical and radiologic literature, with 38 cases reported to date [1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19]. It is the goal of this paper not only to demonstrate a case of a PLB that was not differentiated on imaging and was removed surgically but also to provide a radiologic algorithm for better noninvasive characterization of such masses in the future.
Case report
A 67-year-old male initially presented to urology for a consultation regarding the management of an asymmetric prostatic nodule on physical examination by his primary care physician. A multiparametric MRI was completed to further evaluate the prostate for potential malignancy. MRI demonstrated a small focus of intermediate suspicion at the junction of the transitional and peripheral zones of the prostate on the left, measuring 8 mm in diameter, categorized as PI-RADS3. Incidentally, a 3.3 × 3.1 cm well-circumscribed mass of low T2 signal intensity with a small fatty core in the left lower quadrant was noted close to the sigmoid colon, possibly arising from the soft tissues contiguous with the sigmoid colon (Fig. 1a-c). A follow-up CT was performed 6 months later, revealing a 3.1 × 2.9 cm well-defined round mass within the pelvis, now to the right of midline, suspected to be arising from or related to the adjacent loop of small bowel (Fig. 2a and b). No other changes from the previous MRI were noted. The mass was believed to represent a benign lesion comprised of soft tissue, fat, and calcifications, suggestive of a dermoid or fibroma. Surveillance CT was recommended and performed 6 months later, demonstrating a small increase in the size of the lesion (3.5 × 3.2 cm) without a change in morphology. The patient was referred to colorectal surgery for evaluation.
Fig. 1.
MRI Prostate with gadolinium. (a) T1 fat suppression sequence. Well marginated 3 cm mass in the left pelvis adjacent to the sigmoid colon with intermediate T1 signal. A focus of central intralesional fat is indicated by fat suppression. (b) Axial T2 sequence. Three cm parasigmoid mass with centralized fat indicated by low T2 signal. (c) Coronal T2 sequence. Left pelvic mass with low T2 signal intensity and located adjacent to the sigmoid colon and small bowel.
Fig. 2.
CT Abdomen Pelvis with intravenous contrast. (a) coronal. Well-defined 3.4 cm soft tissue mass in the pelvis, now located to the right of midline. Note the central focus of calcification and fat. (b) axial. Attenuation of the mass is equivalent to adjacent skeletal muscle with a small focus of central fat and calcification. It is adjacent to a loop of small bowel and clearly separate from the adjacent colon, urinary bladder, prostate, and seminal vesicles. The imaging findings suggestive of a benign lesion such as a dermoid.
A colonoscopy was performed, which demonstrated right colon tubular adenomatous polyps, but no correlate for the mass seen on CT and MRI. The prostate biopsy 2 months later revealed benign tissue.
Due to an increase in the size of the mass, diagnostic laparoscopy was performed by colorectal surgery. During this procedure, a free floating 4 × 4 cm mass found in the pelvis (Fig. 3). Retrieval was completed without complication. An intraoperative pathological consultation suggested cellular hyalinized material with necrotic fat. The final pathologic examination of the mass revealed a 3.5 × 3.1 × 2.9 cm tan-yellow circumscribed nodule with a firm, smooth surface, and a central area of necrotic material (Fig. 4). Microscopically this was composed of necrotic fat with areas of dystrophic calcifications.
Fig. 3.
Laparoscopic image of the mass. Loose peritoneal body found free floating in the pelvis on diagnostic laparoscopy.
Fig. 4.
Histology of the mass. Fibrous connective tissue at the bottom left. Note the areas of fat necrosis.
Throughout diagnostic workup, there was low suspicion for malignant neoplasm, but it was not verified until the patient underwent surgery, and the tumor was processed by pathology. It is our goal to assess for pattern recognition based upon the imaging findings in this case as well as multiple case reports in the literature across various specialties to develop a diagnostic algorithm and potentially avoid surgical intervention when appropriate.
To date, the patient has had no complications or recurrence associated with the lesion and remains asymptomatic.
Literature review
From 1992 until 2017, there have been 38 case reports documented characterizing free PLBs. In 2004, a pictorial essay in the American Journal of Radiology discussed CT findings of various fatty abdominal and pelvic lesions and their tissue densities. However, a diagnostic algorithm was not developed since only single cases of each of the respective masses were presented [3]. In 2005, a compendium of extrahepatic fatty masses of the abdomen and pelvis was published, serving as a reference for the characterization of these lesions based upon CT and MR imaging [1]. A diagnostic algorithm for characterizing fatty abdominal masses was proposed in 2010; however, the decision tree was contingent upon knowing the origin of the lesion [2]. Nevertheless, this paper set the groundwork for diagnosing abdominal and retroperitoneal fatty masses systematically. A 2011 case report and literature review described various instances of mobile, calcified masses in the abdomen with associated CT findings [4]. For PLBs, they are located most often near the adnexa in females or pericolonic in either gender, as well as other areas of the peritoneal cavity depending on the patient's positioning during imaging [4,5].
A comprehensive literature review of all case reports available in native language or translation revealed that 63% (24/38) of cases had indeterminate diagnoses following CT. Of these, 25% (6/24) also performed an MRI in an attempt to form a differential diagnosis. Of the 4 chemical-shift techniques in MRI, In-Phase/Out-of-Phase imaging was the most widely referenced over CHESS and Water excitation [5,6]. Chemical shift helps to delineate water from fat in a magnetic field. Water molecules in fat are more heavily shielded and thus resonate at lower frequencies. Out-of-phase imaging demonstrates a drop-out of fat if it is in the same voxel as water. Therefore, if macroscopic fat is not visualized in-phase, signal loss in the out-of-phase sequence indicates microscopic fat. Further classification is accomplished through interval growth, presence of calcifications, and location. Only a small number of studies in our literature review indicated MRI as the preferred imaging modality. Nonetheless, in-phase/out-of-phase MRI techniques have been proven to aid in the accurate classification of fatty lesions. [1,5,6,16]
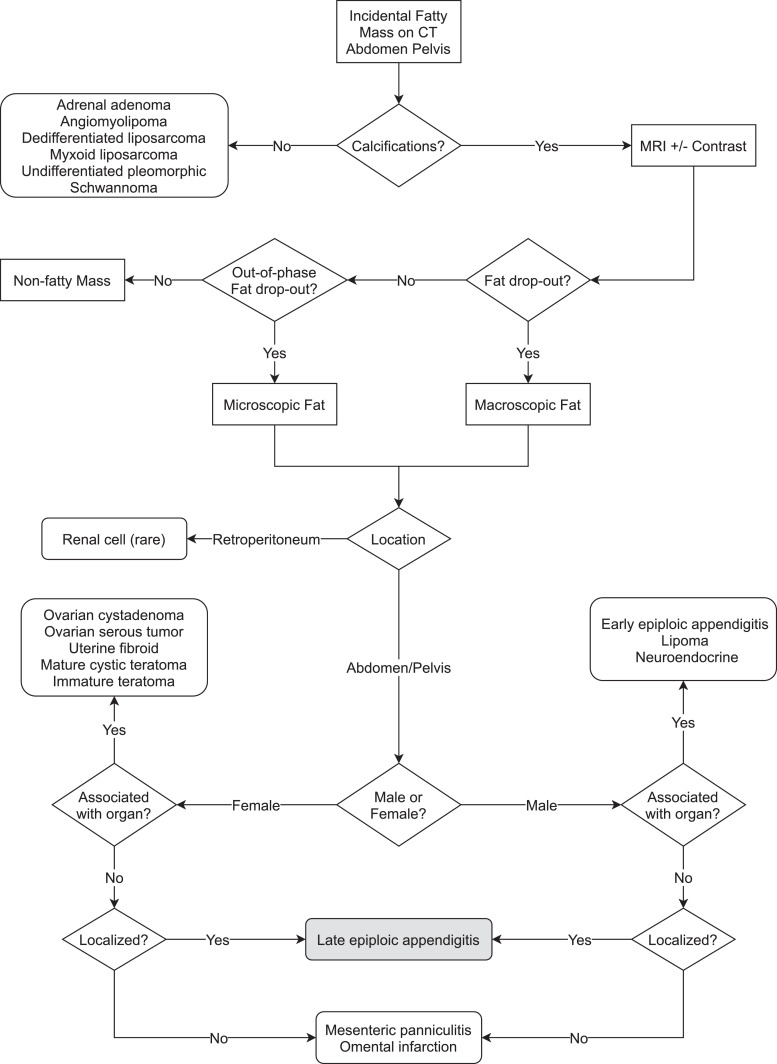
Given the frequency of indeterminate differentials following imaging for PBLs, we focused on the development of a simple algorithm to identify these rare manifestations (Fig. 5) [7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. Based on the literature review, MRI early in the diagnostic pathway is beneficial. Regarding our case, the mass was pericolonic but was mobile as subsequent scans demonstrated its location changing between subsequent scans. The mass contained macroscopic fat, was located in the peritoneal cavity, and was not associated with an adjacent organ. Thus, the differential diagnosis narrowed to late-stage epiploic appendigitis, mesenteric panniculitis, or omental infarction. The latter 2 diagnoses are unlikely given the discrete mass that was created had internal calcifications and was spherical in shape. Mesenteric panniculitis and omental infarction are usually amorphous.
Fig. 5.
Diagnostic Algorithm. This reflects our suggested methodology for further categorizing an incidental fatty mass found on a CT of the abdomen and pelvis. MRI is the next suggested modality where the greatest differentiation of the mass can be completed. Our case report demonstrated a fatty mass that showed fat drop-out on phase shift in the MRI of the abdomen and pelvis representational of macroscopic fat. Calcifications had been identified on the aforementioned CT. The location of the mass was in the pelvis and demonstrated central distribution of the calcification without a Rokitansky nodule. The greatest purpose of this algorithm is to eliminate the need for surgical excision for diagnostic purposes. Another future purpose this algorithm may provide is to supply neural networks and artificial intelligence frameworks that power computer-aided identification of such lesions.
Conclusion
Although rarely described in the literature, PLBs are often a diagnostic dilemma without surgical intervention. With our algorithm that is based upon the current literature and our case, the mass in question in our patient was retrospectively identified as hyaline necrosis from epiploic appendigitis. We aim to have this algorithm serve in the future to help better identify unknown abdominal and pelvic fatty masses and to avoid surgery strictly for diagnosis. More specifically, for patients that are not ideal surgical candidates, imaging can be definitive for these individuals. Based upon our review of previous case studies in surgical journals, the unidentified PLBs were most likely late-stage epiploic appendigitis that had undergone fat necrosis based upon their pathology of centralized calcification and lamellar structure. The mass could have been accurately characterized by our novel algorithm proposed in this manuscript, without invasive diagnostic measures.
Footnotes
Dr. Devane has been a paid speaker Johnson and Johnson and is a consultant BTG, plc.
Conflicts of Interest: The authors report no conflicts of interest.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.radcr.2020.06.040.
Appendix A. Supplementary materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/
References
- 1.Pereira J.M., Sirlin C.B., Pinto P.S., Casola G. CT and MR imaging of extrahepatic fatty masses of the abdomen and pelvis: techniques, diagnosis, differential diagnosis, and pitfalls. Radiographics. 2005;25(1):69–85. doi: 10.1148/rg.251045074. [DOI] [PubMed] [Google Scholar]
- 2.Shin N.Y., Kim M.J., Chung J.J., Chung Y.E., Choi J.Y., Park Y.N. The differential imaging features of fat-containing tumors in the peritoneal cavity and retroperitoneum: the radiologic-pathologic correlation. Korean J Radiol. 2010;11(3):333–345. doi: 10.3348/kjr.2010.11.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Méndez-Uriburu L., Ahualli J., Méndez-Uriburu J., Méndez-Uriburu M., Fajre L., Méndez-Uriburu F. CT appearances of intraabdominal and intrapelvic fatty lesions. Am J Roentgenol. 2004;183(4):933–943. doi: 10.2214/ajr.183.4.1830933. [DOI] [PubMed] [Google Scholar]
- 4.Kamaya A., Federle M.P., Desser T.S. Imaging manifestations of abdominal fat necrosis and its mimics. Radiographics. 2011;31(7):2021–2034. doi: 10.1148/rg.317115046. [DOI] [PubMed] [Google Scholar]
- 5.Ikeno H., Sakai K., Imai H., Mizuta M., Nakagawa T., Goto M. Effects of different fat-suppression methods on T1 values in dynamic contrast-enhanced magnetic resonance imaging: a phantom study. Radiol Phys Technol. 2019;12(3):335–342. doi: 10.1007/s12194-019-00521-x. [DOI] [PubMed] [Google Scholar]
- 6.Ünal E., Karaosmanoğlu A.D., Akata D., Özmen M.N., Karçaaltıncaba M. Invisible fat on CT: making it visible by MRI. Diagnost Intervent Radiol. 2016;22(2):133. doi: 10.5152/dir.2015.15286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gayer G., Petrovitch I. CT diagnosis of a large peritoneal loose body: a case report and review of the literature. Brit J Radiol. 2011;84(1000):e83–e85. doi: 10.1259/bjr/98708052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murat F.J.L., Gettman M.T. Free-floating organized fat necrosis: rare presentation of pelvic mass managed with laparoscopic techniques. Urology. 2004;63(1):176–177. doi: 10.1016/j.urology.2003.09.080. [DOI] [PubMed] [Google Scholar]
- 9.Rajakannu M. Giant peritoneal loose body in a patient with haemorrhoids. Tropical Gastroenterol. 2010;31(2):132–133. [PubMed] [Google Scholar]
- 10.Ghabremani G.G., White E.M., Hoff F.L., Gore R.M., Miller J.W., Christ M.L. Appendices epiploicae of the colon: radiologic and pathologic feature. Radiographics. 1992;12:59–77. doi: 10.1148/radiographics.12.1.1734482. [DOI] [PubMed] [Google Scholar]
- 11.Van Zyl C., Davis R., Hurter D., Van der Westhuizen G. Giant peritoneal loose bodies. SA J Radiol. 2015;19(1) [Google Scholar]
- 12.Guo S., Yuan H., Xu Y., Chen P., Zong L. Giant peritoneal loose body: a case report. Biomed Rep. 2019;10(6):351–353. doi: 10.3892/br.2019.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng Y., Qu L., Li J., Wang B., Geng J., Xing D. Abdominal cocoon accompanied by multiple peritoneal loose body. Medicine. 2017;96(9) doi: 10.1097/MD.0000000000006185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obaid M., Gehani S. Deciding to remove or leave a peritoneal loose body: a case report and review of literature. Am J Case Rep. 2018;19:854. doi: 10.12659/AJCR.908614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elsner A., Walensi M., Fuenfschilling M., Rosenberg R., Mechera R. Symptomatic giant peritoneal loose body in the pelvic cavity: a case report. Int J Surg Case Rep. 2016;21:32–35. doi: 10.1016/j.ijscr.2016.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eberhardt S.C., Strickland C.D., Epstein K.N. Radiology of epiploic appendages: acute appendagitis, post-infarcted appendages, and imaging natural history. Abdom Radiol. 2016;41(8):1653–1665. doi: 10.1007/s00261-016-0757-0. [DOI] [PubMed] [Google Scholar]
- 17.Ghahremani G.G., White E.M., Hoff F.L., Gore R.M., Miller J.W., Christ M.L. Appendices epiploicae of the colon: radiologic and pathologic features. Radiographics. 1992;12(1):59–77. doi: 10.1148/radiographics.12.1.1734482. [DOI] [PubMed] [Google Scholar]
- 18.Hasbahceci M., Erol C., Seker M. Epiploic appendagitis: is there need for surgery to confirm diagnosis in spite of clinical and radiological findings? World J Surg. 2012;36(2):441–446. doi: 10.1007/s00268-011-1382-2. [DOI] [PubMed] [Google Scholar]
- 19.Takabe K., Greenberg J.I., Blair S.L. Giant peritoneal loose bodies. J Gastrointest Surg. 2006;10(3):465–468. doi: 10.1016/j.gassur.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Matsubara K., Takakura Y., Urushihara T., Nishisaka T., Itamoto T. Laparoscopic extraction of a giant peritoneal loose body: case report and review of literature. Int J Surg Case Rep. 2017;39:188–191. doi: 10.1016/j.ijscr.2017.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang H., Ling Y.Z., Cui M.M., Xia Z.X., Feng Y., Chen C.S. Giant peritoneal loose body in the pelvic cavity confirmed by laparoscopic exploration: a case report and review of the literature. World J Surg Oncol. 2015;13(1):118. doi: 10.1186/s12957-015-0539-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Raw Research Data. This is open data under the CC BY license http://creativecommons.org/licenses/by/4.0/