Abstract
The Glasgow Outcome Scale, Pediatric Revision (GOSE-P) is an assessment of “global outcome” designed as a developmentally appropriate version of the Glasgow Outcome Scale-Extended for use in clinical trials of children with traumatic brain injury (TBI). Initial testing describes validity across a wide age and injury severity spectrum, yet the GOSE-P's utility for monitoring children with milder injuries is less clear. We examined the level of agreement between the GOSE-P and the Health and Behavior Inventory (HBI), a TBI-related symptom checklist used to assess children with mild TBI for clinical and research purposes. Participants included children and adolescents 3–16 years of age (n = 50) who presented to two level 1 trauma centers within 24 h of injury, with a GCS of 13–15, who underwent clinical neuroimaging. Outcome was assessed 2 weeks and 3 months following injury. We examined the severity of TBI-related symptoms across disability categories identified using the GOSE-P, and the level of agreement between the two measures in identifying deficits 2 weeks following injury and improvement from 2 weeks to 3 months. Using the GOSE-P, 62% had deficits at 2 weeks, and 42% improved from 2 weeks to 3 months. Agreement between the GOSE-P and HBI was fair 2 weeks after TBI (k = 0.24–0.33), and poor for identifying subsequent improvement (k = 0.10–0.16). Modest agreement between the GOSE-P and the HBI may reflect restricted participation from diverse causes, including TBI, other bodily injuries, and prescribed activity restrictions, and highlights the need for multi-dimensional outcome batteries.
Keywords: brain concussion; brain injuries, traumatic; head injuries, closed; outcome assessment (healthcare); pediatrics
Introduction
The Glasgow Outcome Scale (GOS) and the Glasgow Outcome Scale-Extended (GOSE) are the most commonly used outcome measures for traumatic brain injury (TBI) clinical trials.1–5 The GOS and GOSE are brief, require minimal examiner training, and can be administered via multiple modalities (i.e., in person, phone, mail), attributes that are hypothesized to contribute to high follow-up rates and utility for large clinical trials and outcome studies.5–7 Currently, the GOSE is the only measure currently recommended for use across all adult TBI studies as a core measure of “global outcome” per the National Institute of Neurologic Disorders and Stroke Common Data Elements (CDE).5,8,9 The more recently developed Glasgow Outcome Scale Extended-Pediatric Revision (GOSE-P), was designed as a developmentally appropriate version of the GOSE, for use in clinical trials of children and adolescents with TBI.10 Initial validation of the GOSE-P suggests adequate concurrent and predictive validity across a wide age and injury spectrum; however, the utility of the GOSE-P to detect and monitor deficits in children with injuries at the milder end of the severity spectrum remains unclear.8,10
To be a useful end-point for observational studies or clinical trials that include children with mild TBI that aim to inform diagnosis and treatment of children with mild TBI, the GOSE-P should reflect outcomes that are clinically relevant in the management of children with mild TBI. The presence and persistence of TBI-related symptoms are critical to the clinical management of children with mild TBI.11–13 Acutely, symptoms are used for diagnosis of TBI and may help identify those at risk for prolonged recovery.11,14–18 Symptom severity is used to grade activity as children return to school and play.19,20 Finally, the severity of symptoms may have functional implications if the symptoms interfere with academic performance or return to previously enjoyed activities.21,22
The objective of this study was to investigate the utility of the GOSE-P for assessing and monitoring the recovery of children with mild TBI by examining the relationship between the GOSE-P and the severity of TBI-related symptoms in a group of children and adolescents who presented to the emergency department following a mild TBI.23,24 Initial validation testing of the GOSE-P included children 1 month old to adolescents 17 years and 0 months old.10 Given our focus on TBI-related symptoms, which have been studied in children of pre-school age and older, we focused our analysis on children 3 years of age to adolescents <17 years at the time of injury.17,24–26 Specifically, we examined the severity of deficits identified by the GOSE-P and the severity of TBI-related symptoms 2 weeks and 3 months following injury. We hypothesized that children with more severe deficits identified by the GOSE-P would have more severe TBI-related symptoms. Additionally, we examined agreement between the GOSE-P and a TBI-related symptoms checklist for identifying children exhibiting deficits 2 weeks following injury and exhibiting improvement from 2 weeks to 3 months following injury. We hypothesized that the GOSE-P would demonstrate good agreement with the TBI-related symptom checklist for identifying children with deficits 2 weeks following injury, and in identifying children who improved from 2 weeks to 3 months following injury. Finally, given that both measures may reflect the consequences of non-brain injuries, we examined the impact of excluding those with other bodily injuries on the level of agreement between the measures.12,27,28
Methods
Participants
Participants were enrolled in the pediatric branch of the Transforming Research and Clinical Knowledge in TBI (TRACK-TBI) study from March of 2014 to March of 2018. Inclusion criteria for TRACK-TBI were presentation to a study emergency department (ED) within 24 h after sustaining at least a mild TBI per the American College of Rehabilitation Medicine definition,29 for which neuroimaging was required on clinical grounds. As part of standard clinical practice, computed tomography (CT) scan use was generally guided by clinical decision rules aimed at reducing unnecessary radiation.30 However, some children underwent magnetic resonance imaging (MRI) scans that were available in the ED, which had no risk of radiation.30 Children with significant polytrauma or spinal cord injury or significant baseline neurodevelopmental deficits were not enrolled. Children in state custody were not enrolled because of the complexities of obtaining consent within 24 h of injury and the need for caregivers with intimate knowledge of the child's pre-injury history and availability for longitudinal follow-up. This study included TRACK-TBI participants 3–16 years of age with a Glasgow Coma Scale (GCS) of 13–15 in the ED, and with no need for neurosurgical intervention. Informed consent was obtained from parents/guardians and, when not precluded by the severity of the injury, directly from adolescents 14–16 years of age, with assent obtained for children 7–13 years of age All data were collected in agreement with local institutional review boards.
A total of 76 subjects met study criteria, of which 50 (66%) had complete GOSE-P and Health and Behavior Inventory (HBI) data 2 weeks and 3 months following injury and were included in the analyses. There were no differences in age (p = 0.52) or the proportion of subjects with GCS of 13–14 (p = 1.00) between those with and without complete outcome data. A higher proportion of subjects with incomplete data were Hispanic and/or non-white (p < 0.01), female (p = 0.02), had multi-system versus single-system extracranial injuries (p < 0.01), or had motor vehicle-related injuries (p < 0.01). Higher rates of motor vehicle-related trauma were noted in subjects who were Hispanic or non-white (p < 0.01) and in girls (p = 0.04).
Included participants (n = 50) had a mean age of 11.4 (standard deviation [SD] = 3.9) years, were predominantly boys (78%), white/non-Hispanic (64%), and had high levels of maternal education (64% of mothers had a college degree). A history of attention-deficit disorder (ADD) or attention-deficit/hyperactivity disorder (ADHD) was reported in 20% of subjects. Falls were the most common mechanism of injury (56%), followed by motor vehicle accidents (22%), and sports injuries (16%). Loss of consciousness (LOC) was reported in 46% of the subjects, and 76% had a worst GCS of 15 in the ED. Extracranial injuries were observed in 16% of the sample. Trauma-related abnormalities visible on clinical neuroimaging (CT or MRI) were noted in 32% of subjects. (Table 1).
Table 1.
Demographics and Injury Characteristics
| Included subjects n = 50 | Subjects excluded because of incomplete outcome data n = 26 | Included subjects without another bodily injury n = 42 | |
|---|---|---|---|
| Age | |||
| Median (IQR) | 12.5 (8–15) | 14 (8–15) | 13 (8–15) |
| Mean (SD) | 11.4 (3.9) | 11.5 (4.8) | 11.3 (4.0) |
| Range | 3–16 | 3–16 | 3–16 |
| Male, n (%) | 39 (78%) | 13 (50%) | 35 (83%) |
| Race/Ethnicity | |||
| White non-Hispanic, n (%) | 32 (64%) | 4 (15%) | 26 (62%) |
| Non-white, non-Hispanic, n (%) | 9 (18%) | 9 (34%) | 7 (17.0%) |
| Hispanic or Latino, n (%) | 9 (18%) | 12 (46%) | 9 (21%) |
| Insurance | |||
| Employer/Exchange, n (%) | 36 (72%) | 9 (35%) | 29 (69%) |
| Medicaid/Uninsured, n (%) | 14 (28%) | 10 (38%) | 13 (31%) |
| Maternal education level | |||
| Less than high school, n (%) | 5 (10%) | 2 (8%) | 5 (12%) |
| High school grad.-some college, n (%) | 13 (26%) | 10 (38%) | 11 (26%) |
| College grad. or above, n (%) | 32 (64%) | 9 (35%) | 26 (62%) |
| Medical historya | |||
| Reported ADD or ADHD | 10 (20%) | 5 (19%) | 10 (24%) |
| Severity indicators | |||
| LOC, n (%) | 23 (46%) | 19 (45%) | 21 (50%) |
| GCS in ED of 15, n (%) | 38 (76%) | 20 (77%) | 18 (93%) |
| GCS in ED of 13 or 14, n (%) | 12 (24%) | 6 (23%) | 3 (7%) |
| Intracranial lesion (CT or MRI) | 16 (32%) | 7 (27%) | 13 (31%) |
| Other injury | 8 (16%) | 7 (27%) | NA |
| Orthopedic fracture, n (%) | 6 (12%) | 6 (23%) | NA |
| Other bodily injury, n (%) | 2 (4%) | 6 (23%) | NA |
| Mechanism of injury | |||
| Fall, n (%) | 28 (56%) | 7 (27%) | 24 (57%) |
| Sports, n (%) | 8 (16%) | 2 (8%) | 8 (19%) |
| Motor vehicle, n (%) | 11 (22%) | 16 (61%) | 8 (19%) |
| Other, n (%) | 3 (6%) | 1 (4%) | 2 (5%) |
| Hospital unit | |||
| ED only, n (%) | 6 (12%) | 3 (12%) | 6 (14%) |
| Hospital no ICU, n (%) | 24 (48%) | 14 (54%) | 20 (48%) |
| Hospital ICU, n (%) | 20 (40%) | 9 (35%) | 16 (38%) |
| GOSE-P scores at 2 weeks, n (%) | |||
| 1-Upper Good Recovery | 19 (38%) | 18 (43%) | |
| 2-Lower Good Recovery | 8 (16%) | 8 (19%) | |
| 3-Upper Moderate Disability | 9 (18%) | - | 7 (17%) |
| 4-Lower Moderate Disability | 4 (8%) | 2 (5%) | |
| 5-Upper Severe Disability | 4 (8%) | 2 (5%) | |
| 6-Lower Severe Disability | 6 (12%) | 5 (12%) |
Medical history is based on parent or self-report.
Includes the three most common mechanisms of injury.
IQR, interquartile range; SD, standard deviation; ADD, attention deficit disorder; ADHD, attention- deficit/hyperactivity disorder; LOC, loss of consciousness; GCS, Glasgow Coma Score; ED, emergency department; CT, computed tomography; MRI, magnetic resonance imaging; ICU, intensive care unit; GOSE-P, Glasgow Outcome Scale-Extended, Pediatric Revision.
Injury variables
Injury data, including GCS scores and medical interventions, were extracted from electronic medical records (EMR) by trained research staff. Abbreviated Injury Scale (AIS) scores were used to identify extracranial injuries.31,32 Any injury scored as “moderate severity” (AIS 2) or worse to a body region other than the face or head was considered a bodily injury. Demographic information and medical history were collected via participant or parent interview.
Measures
GOSE-P
The GOSE-P includes a structured interview of parents and/or guardians regarding changes in function following injury across seven areas: consciousness, independence in the home, independence outside the home, school/work, social and leisure activities, family and friendships, and return to normal life.10 Children are scored into one of eight disability categories based on parental responses to the structured interview prompts; overall disability categories are described in Table 2.
Table 2.
Collapsed GOSE-P Categories with Sample Behaviors
| GOSE-P categories | Study group | Restriction examples |
|---|---|---|
| 1) Upper Good Recovery | No functional deficit | No problems relating to the injury affecting daily life |
| 2) Lower Good Recovery | Mild functional deficit | School: reduced capacity Social/Leisure: Participates a bit or much less Family/Friendships: occasional problems or frequent but tolerable problems |
| 3) Upper Moderate Disability | ||
| 4) Lower Moderate Disability | Significant functional deficit | School: attends school for severely injured children, tutored at home, unable to attend school Social/Leisure: rarely engages or is unable to participate Family/Friendships: constant problems or intolerable problems Home: increased dependence on caregivers |
| 5) Upper Severe Disability | ||
| 6) Lower Severe Disability | ||
| 7) Vegetative statea | ||
| 8) Deatha |
No subjects scored a 7 or 8 on the GOSE-P.
Given the focus of the study on mild TBI, in which it would be expected that few children would have severe post-injury deficits,33,34 and given our sample size, we elected to collapse the GOSE-P disability categories from eight to three categories based on the descriptions of behaviors in the GOSE-P structured interview prompts. We conceptualized three functional deficit groups that included children with “No Functional Deficits,” “Mild Functional Deficits,” and “Significant Functional Deficits.” The “Upper Good Recovery” category (GOSE-P score of 1) was considered the “No Functional Deficit” group.35 The “Lower Good Recovery” and “Upper Moderate Disability” groups (GOSE-P scores of 2 and 3) were collapsed into a “Mild Functional Deficit” group, reflecting continued participation in activities but with some limitations. The “Lower Moderate Disability” or worse groups (GOSE-P scores of 4–8) were collapsed into a “Significant Functional Deficit” group reflecting severely limited participation in activities, disabling disruptive behavior, or decreased independence with activities of daily living (ADLs). The collapsed categories and sample behaviors from the structured interview prompts are described in Table 2.
To examine the level of agreement between the GOSE-P and the HBI in identifying children with post-injury deficits, the GOSE-P scores were collapsed into two groups. Participants rated as GOSE-P 1 (“Upper Good Recovery”) were considered to have “no deficits,” whereas those scored as GOSE-P 2-8 (“Lower Good Recovery” or worse) were considered to have “deficits.” To examine agreement between the GOSE-P and the HBI in identifying children who improved from 2 weeks to 3 months, children who improved by at least one GOSE-P category were considered “improved,” whereas those scoring the same or worse were “not improved.”
HBI
The HBI is a 20-item scale used to assess the severity of cognitive and somatic TBI-related symptoms in children.23,24 The HBI has demonstrated adequate construct validity, internal consistency reliability, and the ability to monitor children's symptoms longitudinally, and is used clinically as part of the Child Sport Concussion Assessment Tool-5.24,28,36,37 The HBI includes a child-rated version and a parent-rated version; the latter was used for this analysis. In the parent-rated version, parents rate the frequency that their child experienced specific somatic and cognitive symptoms during the previous week and the 4 weeks before injury via the HBI.24 The severity of somatic, cognitive, and total symptoms is then calculated by summing the frequency scores in the respective domain. To examine agreement with the GOSE-P, HBI scores were dichotomized. At 2 weeks, those exhibiting a reliable increase in symptoms compared with pre-injury ratings were considered to have deficits. At 3 months, children exhibiting a reliable decrease in symptoms since the 2 week time point were considered to have improved.38 Our reliable change methodology is described further below.
The GOSE-P and HBI were completed at 2 weeks (± 4 days) and 3 months (± 7 days) post-injury. At 2 weeks, assessments were typically completed in person, but 3 (6%) participants were unable to attend in person, so they completed the assessments via phone. At 3 months, all assessments were completed via phone.
Statistical analysis
All analyses were performed using SPSS 25.0 (SPSS Inc., Armonk, NY). Medians, interquartile ranges (IQR), means, SDs, and percentages were used to describe the sample. Fisher's exact and Mann–Whitney U tests were used to examine differences between children included versus those excluded because of missing outcome data. Kruskal–Wallis tests were used to determine differences in cognitive, somatic, and total symptom severity between the three collapsed GOSE-P categories at each time point (2 weeks and 3 months) and any differences in pre-injury symptom severity among the GOSE-P categories. For significant group differences, post-hoc analyses were performed using Dunn's test with a Bonferroni correction for multiple comparisons.39 For each analysis, the E2 estimate of effect size was calculated.40 Agreement between the HBI and the GOSE-P for identifying both deficit and improvement was assessed using Cohen's κ. Values of <0.20 were considered to have “poor,” those of 0.21–0.40 were considered to have “fair,” those of 0.41–0.60 were considered to have “moderate,” those of 0.61–0.80 were considered to have “good,” and those of >0.81 were considered to have “very good” agreement.41 Each analysis was completed, both including and excluding those with extracranial injuries, and differences in effect sizes were compared.
Reliable change confidence intervals (CI) were calculated to determine if each child showed a statistically reliable increase or decrease in symptoms. Reliable change CI are used to determine if changes in psychological test scores represent change beyond measurement error.42–51 A CI is created by multiplying the standard error of the difference (SEdiff) by the z score associated with the desired level of confidence to create an estimate of measurement error surrounding the difference score between repeated measurements. When test–retest data are available, the SEdiff is calculated using the standard error of measurement (SEM) calculated for each time point as follows: SEM = (where SD is standard deviation for the respective time point and r12 is the test–retest reliability coefficient). SEdiff is calculated as follows: SEdiff = If retest data are not available, an “estimated” SEdiff has been recommended by multiplying the squared SEM for time 1 by 2, and taking the square root (i.e., ).45,52,53 For the HBI, test–retest data have not been published for a sample that would be appropriate to calculate reliable change estimates for the present study. Therefore, we used the estimated SEdiff formula calculated from the parent ratings of the 50 subjects in the present study obtained 2 weeks post-injury.
Results
Two weeks following injury, 38% of the sample had a GOSE-P score of 1 (“Upper Good Recovery”), 16% had a GOSE-P score of 2 (“Lower Good Recovery”), and 46% had GOSE-P scores of 3–6 indicating “Upper Moderate Disability” to “Lower Severe Disability.” Details of GOSE-P scores at 2 weeks are described in Table 1. No differences in pre-injury HBI-determined symptom severity were noted among the three collapsed GOSE-P functional deficit categories (No, Mild, or Significant Functional Deficit). The severity of somatic symptoms differed among the GOSE-P outcome categories at 2 weeks (χ2 [2] = 9.33 p < 0.01) and 3 months (χ2 [2] = 8.27 p = 0.02), and for total symptoms at 2 weeks following injury (χ2 [2] = 7.34 p = 0.03). Post-hoc analyses revealed more severe symptoms in the “Mild Functional Deficit” group than in the “No Deficit” group for somatic symptoms at 2 weeks (χ2 [2] = -14.29 p = 0.01) and 3 months (χ2 [2] = -12.35 p = 0.03), and for total symptoms at 2 weeks (χ2 [2] = -12.56 p = 0.03). Comparisons of symptom severity across functional disability categories are detailed in Table 3.
Table 3.
Symptom Severity (HBI) by Functional Deficit Category (GOSE-P)
| |
|
2 weeks |
3 months |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | GOSE-P groups | n | Mediana (IQR) | Meana (SD) | E2 | p value | n | Medianb (IQR) | Meanb (SD) | E2 | p value |
| HBI pre-injury cognitive score | No functional deficit | 19 | 7 (0–16) | 8.3 (7.5) | 0.03 | 0.43 | 29 | 13 (3–16) | 11.0 (8.1) | <0.01 | 0.88 |
| Mild functional deficit | 17 | 11 (7–17) | 11.5 (7.5) | 15 | 13 (7–17) | 11.9 (6.0) | |||||
| Significant functional deficit | 14 | 9 (3–17) | 9.9 (7.5) | 6 | 9(2–19) | 10.2 (9.1) | |||||
| HBI cognitive score | No functional deficit | 19 | 9 (1–17) | 9.3 (7.9) | 0.07 | 0.18 | 29 | 12 (2–16) | 11.0 (8.7) | 0.03 | 0.43 |
| Mild functional deficit | 17 | 16 (6–21) | 14.1 (9.2) | 15 | 14 (8–18) | 13.5 (6.8) | |||||
| Significant functional deficit | 14 | 16 (7–28) | 13.7 (6.5) | 6 | 14 (8–21) | 15.0 (8.7) | |||||
| HBI pre-injury somatic score | No functional deficit | 19 | 0 (0–1) | 1.1 (2.4) | 0.09 | 0.12 | 29 | 2 (0–3) | 2.0 (2.4) | 0.09 | 0.09 |
| Mild functional deficit | 17 | 2 (0–5) | 2.8 (3.0) | 15 | 3 (2–4) | 3.1 (1.7) | |||||
| Significant functional deficit | 14 | 1 (0–4) | 2.1 (2.6) | 6 | 2 (0–5) | 2.2 (2.4) | |||||
| HBI somatic score | No functional deficit | 19 | 2 (0–5)* | 3.1 (3.9) | 0.19 | <0.01 | 29 | 2 (0–5)* | 3.3 (4.2) | 0.16 | 0.02 |
| Mild functional deficit | 17 | 6 (4–14)* | 8.1 (5.9) | 15 | 5 (4–8)* | 6.1 (3.9) | |||||
| Significant functional deficit | 14 | 7 (2–10) | 6.1 (4.4) | 6 | 2 (0–6) | 2.5 (2.8) | |||||
| HBI pre-injury total score | No functional deficit | 19 | 10 (1–16) | 9.4 (7.8) | 0.06 | 0.25 | 29 | 14 (5–20) | 13.1 (8.9) | 0.02 | 0.57 |
| Mild functional deficit | 17 | 16 (7–21) | 14.2 (8.6) | 15 | 18 (9–19) | 15.0 (6.5) | |||||
| Significant functional deficit | 14 | 11 (3–18) | 11.9 (8.6) | 6 | 17 (2–23) | 12.3 (10.2) | |||||
| HBI total score | No functional deficit | 19 | 10 (5–19)* | 12.3 (9.2) | 0.15 | 0.03 | 29 | 14 (6–22) | 14.3 (10.3) | 0.08 | 0.13 |
| Mild functional deficit | 17 | 25 (7–33)* | 22.1 (13.0) | 15 | 19 (15–25) | 19.7 (9.1) | |||||
| Significant functional deficit | 14 | 21 (16–26) | 19.8 (8.3) | 6 | 18 (10–26) | 17.5 (8.7) | |||||
Pre-injury symptom level rated at 2 weeks.
Pre-injury symptom level rated at 3 months.
Indicates significant group differences at α = 0.05 from post-hoc test.
HBI, Health and Behavior Inventory. GOSE-P, Glasgow Outcome Scale-Extended, Pediatric Revision. No functional deficit, GOSE-P score of “Upper Good Recovery”; Mild functional deficit, GOSE-P scores of “Lower Good Recovery” or “Upper Moderate Disability”; Significant functional deficit, GOSE-P scores of “Lower Moderate Disability” or below; IQR, interquartile range; SD, standard deviation; E2, epsilon-squared estimate of effect size.
Examining reliable change for individual children on the HBI parent ratings
The reliable change methodology was used to examine increases and decreases in symptoms for each child based on parent HBI ratings. The internal consistency reliability coefficients for the HBI parent ratings at 2 weeks were as follows: cognitive = 0.92, somatic = 0.85, and total score = 0.91. The standard deviations for the HBI parent ratings at 2 weeks were as follows: cognitive = 8.16, somatic = 5.14, and total score = 11.12. The estimated SEdiff was 3.26 for the cognitive scale, 2.82 for the somatic scale, and 4.72 for the total score (i.e., ). Each SEdiff was multiplied by 1.28 to create the 80% CI for estimating measurement error, yielding the following: 4.18 for the cognitive scale, 3.60 for the somatic scale, and 6.04 for the total score. Therefore, a child's score had to improve or worsen by 5, 4, and 6 points before concluding that the change was statistically reliable for the respective domain.
Comparison of subacute deficits identified with the GOSE-P and HBI
At the 2 week time point, 31 (62%) participants had GOSE-P scores of ≥2 and therefore were identified as having deficits per the GOSE-P. Using the HBI, 14 (28%) subjects had a reliable increase in cognitive symptoms, 21 (42%) had a reliable increase in somatic symptoms, and 21 (42%) had a reliable increase in total symptoms compared with retrospective pre-injury ratings, and therefore were considered to have deficits. Absolute agreement between the two measures in identifying children with post-injury deficits was 58% for cognitive symptoms, 64% for somatic symptoms, and 68% for total symptoms. There was fair statistical agreement between the GOSE-P and the HBI at identifying deficits 2 weeks following injury for cognitive (k = 0.24, 95% CI: 0.04–0.44, p = 0.03), somatic (k = 0.31, 95% CI: 0.07–0.55, p = 0.02), and total symptoms (k = 0.38, 95% CI: 0.15–0.61, p < 0.01). When the measures differed, children were more commonly identified as having deficits using the GOSE-P compared with the HBI. For example, 13 children had GOSE-P scores of ≤2 (i.e., had deficits) and had no reliable increase in total symptoms from pre-injury, whereas only 3 subjects who had a reliable increase in total symptoms had a GOSE-P score of 1 (i.e., had no deficits). When subjects with extracranial injuries were excluded, absolute agreement increased slightly to 62% for cognitive symptoms and to 71% for somatic and total symptoms. Statistical agreement also slightly increased with the level of agreement increasing from “fair” to “moderate” for somatic (κ = 0.44, 95% CI: 0.18–0.70, p < 0.01) and total symptoms (κ = 0.44, 95% CI: 0.18–0.70, p < 0.01). Agreement at the individual participant level is described in Table 4.
Table 4.
HBI (Parent) and GOSE-P Raw Scores and Change in Scores by Subject 2 Weeks and 3 Months after Injury
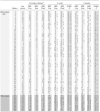 |
GOSE-P, Glasgow Outcome Scale-Pediatric Revision (1, “Upper Good Recovery”- 6, “Lower Severe Disability”); HBI, Health and Behavior Inventory (parent-rated, a higher value indicates more severe symptoms); Pre-injury ratingsa = Retrospective ratings of pre-injury symptoms collected 2 weeks after injury; Cog, cognitive. Som, somatic. At 2 weeks: ↑, improvement from pre-injury (reliable decrease in symptoms) ↓, decline from pre-injury (reliable increase in symptoms or any deficit identified on the GOSE-P). 3 months: ↑, improvement from 2 week rating (reliable decrease in symptoms or improved function per the GOSE-P score by ≥1 category) ↓, decline from 2 week rating (reliable increase in symptoms or decreased function per the GOSE-P by ≥1 category).
Improvement Identified with the GOSE-P and HBI
At 3 months, 21 (42%) participants had a ≥1 point improvement on the GOSE-P, and therefore were identified as having improved since the 2 week time point. Using the HBI, 13 (26%) participants had a reliable decrease in cognitive symptoms, 14 (28%) had a reliable decrease in somatic symptoms, and 17 (34%) subjects had a reliable decrease in total symptoms since the 2 week time point and therefore were considered to have improved. Absolute agreement between the two measures for identifying children exhibiting improvement from 2 weeks to 3 months was 60% for cognitive symptoms, 58% for somatic symptoms, and 64% for total symptoms. Agreement was poor agreement for identifying which children had improved for cognitive (κ = 0.13, 95% CI: -0.13–0.39, p = 0.31), somatic (κ = 0.10, 95% CI: -0.17–0.37, p = 0.48), and fair for total (κ = 0.24, 95% CI: -0.03–0.51, p = 0.08) symptoms. When subjects with extracranial injuries were excluded, absolute agreement increased slightly to 67%, 62%, and 69% for cognitive, somatic, and total symptoms respectively. Kappa values also increased slightly for cognitive (κ = 0.25, 95% CI: -.05–0.55, p = 0.10), and total symptoms (κ = 0.32, 95% CI: 0.02–0.62, p = 0.04). Agreement at the individual participant level is described in Table 4.
Discussion
The objective of this study was to evaluate the utility of the GOSE-P to assess and monitor outcome in children with mild TBI by comparing the severity of post-injury deficits identified using the GOSE-P to the severity of TBI-related symptoms identified using the HBI, a TBI-related symptom checklist used clinically to assess and monitor recovery of children following mild TBI.24,28,37 At 2 weeks, nearly two thirds (62%) of our sample had functional deficits documented by the GOSE-P. As hypothesized, children with more severe deficits identified on the GOSE-P also had more severe somatic symptoms (2 weeks and 3 months) and total symptoms (2 weeks), although no difference in cognitive symptom severity was observed across the three GOSE-P deficits groups. However, our hypothesis that the measures would demonstrate at least “good” agreement in identifying children exhibiting deficits and recovery was not supported. The two measures demonstrated “fair” agreement in identifying those with deficits, supporting a limited relationship between GOSE-P scores and the severity of cognitive and somatic TBI-related symptoms. The GOSE-P identified more children with deficits than the HBI. It is important to note that the children included in this study all had GCS scores of 13–15, but were required to present to the hospital and undergo clinical neuroimaging. In our sample, 32% had abnormal neuroimaging, 40% were admitted to the intensive care unit (ICU), and 54% were hospitalized >24 h. Therefore, our results only apply to children who presented to the hospital with presentations that warranted neuroimaging, presumably excluding those with the mildest acute presentations.
Distinctions between the HBI and GOSE-P may help to explain the modest level of agreement between the two measures. Whereas the GOSE-P is an assessment of functional status and measures participation, as defined by the International Classification of Functioning Disability and Health (ICF), in school, community, and recreational activities, the HBI measures impairments, reflected as cognitive and somatic symptoms.54–56 The HBI includes cognitive and somatic symptoms, which can impact participation. However, other types of restrictions, and behavioral or emotional symptoms, included in some symptom checklists but not the HBI, may also influence a child's return to activities.21,36,57–60 Finally, in this study, both the HBI and the GOSE-P were rated by parents, but parents may rate symptoms differently than children, particularly symptoms that are not easily observable.61,62 Participation restrictions measured by the GOSE-P may be more observable to parents than symptoms. Future work that includes child-ratings of symptoms may provide additional insight into the relationship between the GOSE-P and clinically used symptom measures. Our results do not indicate that the GOSE-P is more sensitive to the effects of mild TBI than the HBI, but that differences between the measures reflect the additional depth gained via multi-domain outcome assessments that include measures across domains of function and the ICF.
The results highlight the benefits of incorporating the GOSE-P into a multi-domain outcome assessment, but also identify potential challenges and considerations for future use of the GOSE-P. First, as expected, we noted that the “Mild Functional Deficit” group had more severe somatic and total symptoms than the “No Functional Deficit” group, but found no difference in symptom severity between the “Significant Functional Deficit” group and the “No Functional Deficit” group. Although patients with more severe injuries may report fewer symptoms than those with milder deficits,63 the specific scoring scheme of the GOSE-P also may result in overestimation of “severe” disability in children with milder injuries. The scoring reflects the alignment of the GOSE-P with the adult GOSE but does not fully account for developmental differences in expected behaviors between children and adults.10 For example, if a child is more dependent on caregivers following injury, including requiring additional prompting for ADLs, the GOSE-P scores the child as having “severe” disability. Adults are expected to be independent in the home and community, so an increased need for assistance or prompting reflects significant disability.
In contrast, children normally require some assistance based on developmental level; the increased need for assistance in the home may reflect deficit, but not necessarily “severe” disability. In our sample, 8 of the 14 children with “Significant Functional Deficit” 2 weeks post-injury were categorized based on decreased independence in the home, without significant deficits in other domains. Whereas impairment in home activities results in an “automatic” severe disability categorization, decreased participation in social or leisure activities can only be scored as “Lower Good Recovery” to “Lower Moderate Disability” but cannot be characterized as “severe” disability on that basis alone. Therefore, allowable scoring within GOSE-P functional domains may limit assessment of overall post-injury abilities in children across the injury severity and developmental spectrum.
Second, following TBI, recommendations for return to school often include a graduated process, and full return to sports is only allowed after a subsequent, similarly graduated, stepwise protocol.19,64 Recommendations for return to activity are based on symptoms and activity tolerance, but guidelines also advise caution and close monitoring of children for several weeks.22,65 GOSE-P scores 2 weeks post-injury may reflect restricted activity because of recommendations for limited activity, rather than the severity of ongoing problems per se.
Finally, as noted in the adult GOSE, the GOSE-P likely reflects functional deficits and disability from multiple causes, not exclusively brain injury.27,35 We noted that exclusion of subjects with extracranial injuries resulted in increased agreement between the GOSE-P and the HBI, suggesting that in some cases functional status was likely impacted by other injuries (i.e., upper extremity fracture) versus the severity of TBI-related symptoms. Details regarding symptoms and functional status for those with other bodily injuries compared with those without are described in Table 4.
The results should be interpreted in light of several limitations. First, of otherwise eligible participants, only 66% had complete outcome data at the 2 week and 3 month time points. Additionally, differences were noted between those with and without complete follow-up data in terms of the proportion of motor vehicle injuries, representation of racial and ethnic minorities, female participants, and those with multiple extracranial injuries. Given the higher rates of motor vehicle-related injuries among groups with lower follow-up rates, we suspect that consequences of these injuries may account for the differences, such as complexities of follow-up care after multi-system trauma and/or loss of a vehicle. However, lower follow-up rates among racial or ethnic minorities and females limits the generalizability of the findings.
Our interpretation of change also has limitations. For the HBI, we used reliable change methodology, which identifies statistically reliable but not necessarily clinically meaningful change.38 With the GOSE-P's ordinal scale, we defined improvement as a change of one category. However, it is unknown if a one category change is statistically reliable or clinically meaningful. Additionally, at 2 weeks post-injury, we identified children with post-injury deficits using a retrospective rating of pre-injury symptoms, which are commonly used but prone to recall biases, and may result in overestimation of post-injury deficits.66,67
Finally, this investigation was preliminary and limited by small sample size and restriction of range such that few subjects exhibited improvement, potentially contributing to low κ values.68,69 We were also limited in our ability to analyze potentially important subgroups because of the sample size. Parent ratings of symptoms may differ based on the child's age, as symptoms can manifest differently in pre-school versus older children and adolescents.25,26,70 We completed a sensitivity analysis excluding children under the age of 5, and the level of agreement between the HBI and the GOSE-P remained “fair.” However, given differences in symptom manifestation between older and younger subjects, and the identified concerns regarding the GOSE-P scoring scheme particularly in younger children, additional investigation with a larger sample that allows for analysis within specific age groups and other clinically relevant subgroups is warranted. Finally, collapsing the GOSE-P was a practical decision based on the limitations of our sample size; however, this impacted our examination of differences in symptoms across all GOSE-P categories and the scoring scheme on the overestimation of disability.
Future investigations into statistically reliable and clinically meaningful change in GOSE-P will help determine its utility for longitudinal outcome assessment. Additionally, further refinement of the scoring scheme to account for extracranial injuries, and perhaps age-appropriate estimation of true disability in young children, may improve the GOSE-P's utility to assess participation-level outcome after TBI. Overall, the findings highlight the value of the GOSE-P as an assessment of functional status. However, the differences between the measures also support the need for comprehensive multi-dimensional assessment that includes measures of specific functional domains, not captured in a single hierarchical measure of global outcome, to fully understand functional outcome following TBI in children and adolescents.
Acknowledgments
The authors thank research coordinators Scott Haire, Vianca Caridad Diaz, Carla Fortes-Monteiro, and Frederique Wittkampf, and lab members Beth Costine-Bartell, Eleanor Crawford, George Price, John Shen, Madeline Perlewitz, Natalie Escobar, Jacqueline Andrews, Scott Henderson, Zoe Silsby, Andrew Bourque, and Madeline Karsten for their contributions.
Contributor Information
Collaborators: the TRACK-TBI Investigators, Opeolu Adeoye, Neeraj Badjatia, Kim Boase, Yelena Bodien, M. Ross Bullock, Randall Chesnut, John D. Corrigan, Karen Crawford, Ramon Diaz-Arrastia, Sureyya Dikmen, Richard Ellenbogen, V Ramana Feeser, Adam R. Ferguson, Brandon Foreman, Raquel Gardner, Etienne Gaudette, Joseph Giacino, Dana Goldman, Luis Gonzalez, Shankar Gopinath, Rao Gullapalli, J Claude Hemphill, Gillian Hotz, Sonia Jain, Frederick K. Korley, Joel Kramer, Natalie Kreitzer, Harvey Levin, Chris Lindsell, Joan Machamer, Christopher Madden, Geoffrey T Manley, Alastair Martin, Thomas McAllister, Michael McCrea, Randall Merchant, Pratik Mukherjee, Lindsay Nelson, Laura B. Ngwenya, Florence Noel, David Okonkwo, Eva Palacios, Daniel Perl, Ava Puccio, Miri Rabinowitz, Claudia Robertson, Jonathan Rosand, Angelle Sander, Gabriella Satris, David Schnyer, Seth Seabury, Sabrina Taylor, Nancy Temkin, Arthur Toga, Alex Valadka, Mary Vassar, Paul Vespa, Kevin Wang, John K. Yue, Esther Yuh, and Ross Zafonte
TRACK-TBI Investigators
Opeolu Adeoye, Neeraj Badjatia, Kim Boase, Yelena Bodien, M. Ross Bullock, Randall Chesnut, John D. Corrigan, Karen Crawford, Ramon Diaz-Arrastia, Sureyya Dikmen, Richard Ellenbogen, V Ramana Feeser, Adam R. Ferguson, Brandon Foreman, Raquel Gardner, Etienne Gaudette, Joseph Giacino, Dana Goldman, Luis Gonzalez, Shankar Gopinath, Rao Gullapalli, J Claude Hemphill, Gillian Hotz, Sonia Jain, Frederick K. Korley, Joel Kramer, Natalie Kreitzer, Harvey Levin, Chris Lindsell, Joan Machamer, Christopher Madden, Geoffrey T Manley, Alastair Martin, Thomas McAllister, Michael McCrea, Randall Merchant, Pratik Mukherjee, Lindsay Nelson, Laura B. Ngwenya, Florence Noel, David Okonkwo, Eva Palacios, Daniel Perl, Ava Puccio, Miri Rabinowitz, Claudia Robertson, Jonathan Rosand, Angelle Sander, Gabriella Satris, David Schnyer, Seth Seabury, Sabrina Taylor, Nancy Temkin, Arthur Toga, Alex Valadka, Mary Vassar, Paul Vespa, Kevin Wang, John K. Yue, Esther Yuh, Ross Zafonte.
Funding Information
This project was supported by the National Institutes of Health (NIH), National Institute of Neurological Disorders and Stroke (NINDS), and Department of Health and Human Services, through grant U01NS086090-0. NIH had no role in the design or conduct of the study, including collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Author Disclosure Statement
Emily Evans received support from the Center on Health Services Training and Research (CoHSTAR). Grant Iverson has received research support from test publishing companies including Psychological Assessment Resources, Inc. and CNS Vital Signs in the past (not in the past 5 years). He acknowledges unrestricted philanthropic support from ImPACT Applications, Inc., the Mooney-Reed Charitable Foundation, the Heinz Family Foundation, and the Spaulding Research Institute. He receives royalties for one neuropsychological test (Wisconsin Card Sorting Test-64 Card Version). No other conflict of interests or competing financial interests exist.
References
- 1. Jennett B., and Bond M. (1975). Assessment of outcome after severe brain damage. Lancet 1, 480–484 [DOI] [PubMed] [Google Scholar]
- 2. Wilson J.T., Pettigrew L.E., and Teasdale G.M. (1998). Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J. Neurotrauma 15, 573–585 [DOI] [PubMed] [Google Scholar]
- 3. Levin H.S., Boake C., Song J., Mccauley S., Contant C., Diaz-Marchan P., Brundage S., Goodman H., and Kotrla K.J. (2001). Validity and sensitivity to change of the extended Glasgow Outcome Scale in mild to moderate traumatic brain injury. J. Neurotrauma 18, 575–584 [DOI] [PubMed] [Google Scholar]
- 4. Teasdale G.M., Pettigrew L.E., Wilson J.T., Murray G., and Jennett B. (1998). Analyzing outcome of treatment of severe head injury: a review and update on advancing the use of the Glasgow Outcome Scale. J. Neurotrauma 15, 587–597 [DOI] [PubMed] [Google Scholar]
- 5. McMillan T., Wilson L., Ponsford J., Levin H., Teasdale G., and Bond M. (2016). The Glasgow Outcome Scale – 40 years of application and refinement. Nat. Rev. Neurol. 12, 477–485 [DOI] [PubMed] [Google Scholar]
- 6. Pettigrew L.E., Wilson J.T., and Teasdale G.M. (2003). Reliability of ratings on the Glasgow Outcome Scales from in-person and telephone structured interviews. J. Head Trauma Rehabil. 18, 252–258 [DOI] [PubMed] [Google Scholar]
- 7. Wilson J.T., Edwards P., Fiddes H., Stewart E., and Teasdale G.M. (2002). Reliability of postal questionnaires for the Glasgow Outcome Scale. J. Neurotrauma 19, 999–1005 [DOI] [PubMed] [Google Scholar]
- 8. Hicks R., Giacino J., Harrison-Felix C., Manley G., Valadka A., and Wilde E.A. (2013). Progress in developing common data elements for traumatic brain injury research: version two—the end of the beginning. J. Neurotrauma 30, 1852–1861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuh E.L., Cooper S.R., Mukherjee P., Yue J.K., Lingsma H.F., Gordon W.A., Valadka A.B., Okonkwo D.O., Schnyer D.M., Vassar M.J., Maas A.I., Manley G.T., and TRACK-TBI Investigators (2014). Diffusion tensor imaging for outcome prediction in mild traumatic brain injury: a TRACK-TBI study. J. Neurotrauma 31, 1457–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Beers S.R., Wisniewski S.R., Garcia-Filion P., Tian Y., Hahner T., Berger R.P., Bell M.J., and Adelson P.D. (2012). Validity of a pediatric version of the Glasgow Outcome Scale–Extended. J. Neurotrauma 29, 1126–1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lumba-Brown A., Yeates K.O., Sarmiento K., Breiding M.J., Haegerich T.M., Gioia G.A., Turner M., Benzel E.C., Suskauer S.J., Giza C.C., Joseph M., Broomand C., Weissman B., Gordon W., Wright D.W., Moser R.S., McAvoy K., Ewing-Cobbs L., Duhaime A.C., Putukian M., Holshouser B., Paulk D., Wade S.L., Herring S.A., Halstead M., Keenan H.T., Choe M., Christian C.W., Guskiewicz K., Raksin P.B., Gregory A., Mucha A., Taylor H.G., Callahan J.M., DeWitt J., Collins M.W., Kirkwood M.W., Ragheb J., Ellenbogen R.G., Spinks T.J., Ganiats T.G., Sabelhaus L.J., Altenhofen K., Hoffman R., Getchius T., Gronseth G., Donnell Z., O'Connor R.E., and Timmons S.D. (2018). Centers for Disease Control and Prevention Guideline on the Diagnosis and Management of Mild Traumatic Brain Injury Among Children. JAMA Pediatr. 172, e182853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McNally K.A., Bangert B., Dietrich A., Nuss K., Rusin J., Wright M., Taylor H.G., and Yeates K.O. (2013). Injury versus noninjury factors as predictors of postconcussive symptoms following mild traumatic brain injury in children. Neuropsychology 27, 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeates K.O., Taylor H.G., Rusin J., Bangert B., Dietrich A., Nuss K., and Wright M. (2012). Premorbid child and family functioning as predictors of post-concussive symptoms in children with mild traumatic brain injuries. Int. J. Dev. Neurosci. 30, 231–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schatz P., Pardini J.E., Lovell M.R., Collins M.W., and Podell K. (2006). Sensitivity and specificity of the ImPACT Test Battery for concussion in athletes. Arch. Clin. Neuropsychol. 21, 91–99 [DOI] [PubMed] [Google Scholar]
- 15. Gioia G.A., Schneider J.C., Vaughan C.G., and Isquith P.K. (2009). Which symptom assessments and approaches are uniquely appropriate for paediatric concussion? Br. J. Sports Med. 43, i13–22 [DOI] [PubMed] [Google Scholar]
- 16. Babikian T., Satz P., Zaucha K., Light R., Lewis R.S., and Asarnow R.F. (2011). The UCLA longitudinal study of neurocognitive outcomes following mild pediatric traumatic brain injury. J. Int. Neuropsychol. Soc. 17, 886–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chrisman S.P., Rivara F.P., Schiff M.A., Zhou C., and Comstock R.D. (2013). Risk factors for concussive symptoms 1 week or longer in high school athletes. Brain Inj. 27, 1–9 [DOI] [PubMed] [Google Scholar]
- 18. Zemek R., Barrowman N., Freedman S.B., Gravel J., Gagnon I., McGahern C., Aglipay M., Sangha G., Boutis K., Beer D., Craig W., Burns E., Farion K.J., Mikrogianakis A., Barlow K., Dubrovsky A.S, Meeuwisse W., Gioia G., Meehan W.P., 3rd, Beauchamp M.H., Kamil Y., Grool A.M., Hoshizaki B., Anderson P., Brooks B.L., Yeates K.O., Vassilyadi. M., Klassen T., Keightley M., Richer L., DeMatteo C., Osmond M.H., and Pediatric Emergency Research Canada (PERC) Concussion Team (2016). Clinical risk score for persistent postconcussion symptoms among children with acute concussion in the ED. JAMA 315, 1014–1025 [DOI] [PubMed] [Google Scholar]
- 19. McCrory P., Meeuwisse W., Dvořák J., Aubry M., Bailes J., Broglio S., Cantu R.C., Cassidy D., Echemendia R.J., Castellani R.J., Davis G.A., Ellenbogen R., Emery C., Engebretsen L., Feddermann-Demont N., Giza C.C., Guskiewicz K.M., Herring S., Iverson G.L., Johnston K.M., Kissick J., Kutcher J., Leddy J.J., Maddocks D., Makdissi M., Manley G.T., McCrea M., Meehan W.P., Nagahiro S., Patricios J., Putukian M., Schneider K.J., Sills A., Tator C.H., Turner M., and Vos P.E. (2017). Consensus statement on concussion in sport–the 5th International Conference on Concussion in Sport held in Berlin October, 2016. Br. J. Sports Med. 51, 838–847 [DOI] [PubMed] [Google Scholar]
- 20. Halstead M.E., Walter K.D., Moffatt K.D., and Council on Sports Medicine and Fitnessn (2010). American Academy of Pediatrics, Clinical report—sport-related concussion in children and adolescents. Pediatrics 126, 597–615 [DOI] [PubMed] [Google Scholar]
- 21. Ransom D.M., Vaughan C.G., Pratson L., Sady M.D., McGill C.A., and Gioia G.A. (2015). Academic effects of concussion in children and adolescents. Pediatrics 135, 1043–1050 [DOI] [PubMed] [Google Scholar]
- 22. Halstead M.E., McAvoy K., Devore C.D., Carl R., Lee M., Logan K., Council on Sports Medicine and Fitness, and Council on School Health (2013). Returning to learning following a concussion. Pediatrics 132, 948–957 [DOI] [PubMed] [Google Scholar]
- 23. McCauley S.R., Wilde E.A., Anderson V.A., Bedell G., Beers S.R., Campbell T.F., Chapman S.B., Ewing-Cobbs L., Gerring J.P., Gioia G.A., Levin H.S., Michaud L.J., Prasad M.R., Swaine B.R., Turkstra L.S., Wade S.L., Yeates K.O., and Pediatric TBI Outcomes Workgroup (2012). Recommendations for the use of common outcome measures in pediatric traumatic brain injury research. J. Neurotrauma 29, 678–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ayr L.K., Yeates K.O., Taylor H.G., and Browne M. (2009). Dimensions of postconcussive symptoms in children with mild traumatic brain injuries. J. Int. Neuropsychol. Soc. 15, 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bernard C., McKinlay A., Krieser D., Testa R., and Ponsford A.J. (2017). Acute post-concussive symptoms in young children. Brain Inj. 31, 1414–1421 [DOI] [PubMed] [Google Scholar]
- 26. Suskauer S.J., Rane S., Reesman J., Slomine B.S. (2018). Caregiver-report of symptoms following traumatic brain injury in a small clinical sample of preschool-aged children. J. Pediatr. Rehabil. Med. 11, 7–14 [DOI] [PubMed] [Google Scholar]
- 27. Bodien Y.G., McCrea M., Dikmen S., Temkin N., Boase K., Machamer J., Taylor S.R., Sherer M., Levin H., Kramer J.H., Corrigan J.D., McAllister T.W., Whyte J., Manley G.T., Giacino J.T., and TRACK-TBI Investigators (2018). Optimizing outcome assessment in multicenter TBI trials: perspectives from TRACK-TBI and the TBI Endpoints Development Initiative. J. Head Trauma Rehabil. 33, 147–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yeates K.O., Taylor H.G., Rusin J., Bangert B., Dietrich A., Nuss K., Wright M., Nagin D.S., and Jones B.L. (2009). Longitudinal trajectories of postconcussive symptoms in children with mild traumatic brain injuries and their relationship to acute clinical status. Pediatrics 123, 735–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kay T. (1993). Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J. Head Trauma Rehabil. 8, 886–887 [Google Scholar]
- 30. Kuppermann N., Holmes J.F., Dayan P.S., Hoyle J.D. Jr, Atabaki S.M., Holubkov R., Nadel F.M., Monroe D., Stanley R.M., Borgialli D.A., Badawy M.K., Schunk J.E., Quayle K.S., Mahajan P., Lichenstein R., Lillis K.A., Tunik M.G., Jacobs E.S., Callahan J.M., Gorelick M.H., Glass T.F., Lee L.K., Bachman M.C., Cooper A., Powell E.C., Gerardi M.J., Melville K.A., Muizelaar J.P., Wisner D.H., Zuspan S.J., Dean J.M., Wootton-Gorges S.L., and Pediatric Emergency Care Applied Research Network (PECARN) (2009). Identification of children at very low risk of clinically-important brain injuries after head trauma: a prospective cohort study. Lancet 374, 1160–1170 [DOI] [PubMed] [Google Scholar]
- 31. Gennarelli T.A., and Wodzin E. (2006). AIS 2005: a contemporary injury scale. Injury 37, 1083–1091 [DOI] [PubMed] [Google Scholar]
- 32. Adelson P.D., Pineda J., Bell M.J., Abend N.S., Berger R.P., Giza C.C., Hotz G., Wainwright M.S., and Pediatric TBI Demographics and Clinical Assessment Working Group (2012). Common data elements for pediatric traumatic brain injury: recommendations from the working group on demographics and clinical assessment. J. Neurotrauma 29, 639–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jaffe K.M., Polissar N.L., Fay G.C., and Liao S. (1995). Recovery trends over three years following pediatric traumatic brain injury. Arch. Phys. Med. Rehabil. 76, 17–26 [DOI] [PubMed] [Google Scholar]
- 34. Light R., Asarnow R., Satz P., Zaucha K., McCleary C., and Lewis R. (1998). Mild closed-head injury in children and adolescents: behavior problems and academic outcomes. J. Consult. Clin. Psychol. 66, 1023–1029 [DOI] [PubMed] [Google Scholar]
- 35. Nelson L.D., Ranson J., Ferguson A.R., Giacino J., Okonkwo D.O., Valadka A., Manley G., and McCrea M. (2017). Validating multidimensional outcome assessment using the TBI common data elements: an analysis of the TRACK–TBI pilot sample. J. Neurotrauma [Epub a head of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yeates. K.O., Kaizar E., Rusin J., Bangert B., Dietrich A., Nuss K., Wright M., and Taylor H.G. (2012). Reliable change in postconcussive symptoms and its functional consequences among children with mild traumatic brain injury. Arch. Pediatr. Adolesc. Med. 166, 615–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Davis G.A., Purcell L., Schneider K.J., Davis G.A., Purcell L., Schneider K.J., Yeates K.O., Gioia G.A., Anderson V., Ellenbogen R.G., Echemendia R.J., Makdissi M., Sills A., Iverson G.L., Dvořák J., McCrory P., Meeuwisse W., Patricios J., Giza C.C., and Kutcher J.S. (2017). The Child Sport Concussion Assessment Tool 5th Edition (Child SCAT5): background and rationale. Br. J. Sports Med. 51, 859–861 [DOI] [PubMed] [Google Scholar]
- 38. Iverson G. (2011). Reliable change index, in: Encyclopedia of Clinical Neuropsychology. Kreutzer J. DeLuca J. and Caplan B.(eds.). Springer-Verlag: New York, pps. 158–203 [Google Scholar]
- 39. Dunn O.J. (1964). Multiple comparisons using rank sums. Technometrics 6, 241–252 [Google Scholar]
- 40. Tomczak M., and Tomczak E,. (2014). The need to report effect size estimates revisited. An overview of some recommended measures of effect size. Trends Sport Sci. 1, 19–25 [Google Scholar]
- 41. Altman D.G. (1991). Some common problems in medical research: inter-rater agreement, in: Practical Statistics for Medical Research. Chapman & Hall: London, pps. 403–408 [Google Scholar]
- 42. Jacobson N.S., and Truax P. (1991). Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J. Consult. Clin. Psychol. 59, 12–19 [DOI] [PubMed] [Google Scholar]
- 43. Hageman W.J., and Arrindell W.A. (1999). Establishing clinically significant change: increment of precision and the distinction between individual and group level of analysis. Behav. Res. Ther. 37, 1169–1193 [DOI] [PubMed] [Google Scholar]
- 44. Hageman W.J., and Arrindell W.A. (1999). Clinically significant and practical! Enhancing precision does make a difference. Reply to McGlinchey and Jacobson, Hsu, and Speer. Behav. Res. Ther. 37, 1219–1233 [DOI] [PubMed] [Google Scholar]
- 45. Hageman W.J., and Arrindell W.A. (1993). A further refinement of the reliable change (RC) index by improving the pre-post difference score: introducing RCID. Behav. Res. Ther. 31, 693–700 [DOI] [PubMed] [Google Scholar]
- 46. Hsu L.M. (1999). A comparison of three methods of identifying reliable and clinically significant client changes: commentary on Hageman and Arrindell. Behav. Res. Ther. 37, 1195–1219 [DOI] [PubMed] [Google Scholar]
- 47. Speer D.C. (1992). Clinically significant change: Jacobson and Truax (1991) revisited. J. Consult. Clin. Psychol. 60, 402–408 [DOI] [PubMed] [Google Scholar]
- 48. Speer D.C., and Greenbaum P.E. (1995) Five methods for computing significant individual client change and improvement rates: support for an individual growth curve approach. J. Consult. Clin. Psychol. 63, 1044–1048 [DOI] [PubMed] [Google Scholar]
- 49. Hsu L.M. (1989). Reliable changes in psychotherapy: taking into account regression toward the mean. Behav. Assess. 11, 459–467 [Google Scholar]
- 50. Jacobson N.S., Roberts L.J., Berns S.B., and McGlinchey J.B. (1999). Methods for defining and determining the clinical significance of treatment effects: description, application, and alternatives. J. Consult. Clin. Psychol. 67, 300–307 [DOI] [PubMed] [Google Scholar]
- 51. Ogles B.M., Lambert M.J., and Masters K.S. (1996). Assessing Outcome in Clinical Practice. Allyn and Bacon: Boston [Google Scholar]
- 52. Iverson G.L. (1998). Interpretation of Mini-Mental State Examination scores in community-dwelling elderly and geriatric neuropsychiatry patients. Int. J. Geriatr. Psychiatry 13, 661–666 [DOI] [PubMed] [Google Scholar]
- 53. Iverson G.L. (2001). Interpreting change on the WAIS-III/WMS-III in clinical samples. Arch. Clin. Neuropsychol. 16, 183–191 [PubMed] [Google Scholar]
- 54. Grill E., Bronstein A., Furman J., Zee D.S., and Müller M(2012). International Classification of Functioning, Disability and Health (ICF) Core Set for patients with vertigo, dizziness and balance disorders. J. Vestib. Res. 22, 261–271 [DOI] [PubMed] [Google Scholar]
- 55. World Health Organization (2001). ICF: International Classification of Functioning, Disability and Health. World Health Organization: Geneva [Google Scholar]
- 56. Jette A.M. (2006). Toward a common language for function, disability, and health. Phys. Ther. 86 726–734 [PubMed] [Google Scholar]
- 57. Emery C.A., Barlow K.M., Brooks B.L., Max J.E., Villavicencio-Requis A., Gnanakumar V., Robertson H.L., Schneider K., and Yeates K.O. (2016). A systematic review of psychiatric, psychological, and behavioural outcomes following mild traumatic brain injury in children and adolescents. Can. J. Psychiatry 61, 259–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hawley C.A. (2004). Behaviour and school performance after brain injury. Brain Inj. 18, 645–659 [DOI] [PubMed] [Google Scholar]
- 59. Sady M.D., Vaughan C.G., and Gioia G.A. (2014). Psychometric characteristics of the postconcussion symptom inventory in children and adolescents. Arch. Clin. Neuropsychol. 29, 348–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. King N.S., Crawford S., Wenden F.J., Moss N.E., and Wade D.T. (1995). The Rivermead Post Concussion Symptoms Questionnaire: a measure of symptoms commonly experienced after head injury and its reliability. J. Neurol. 242, 587–592 [DOI] [PubMed] [Google Scholar]
- 61. Hajek C.A., Yeates K.O., Taylor H.G., Bangert B., Dietrich A., Nuss K.E., Rusin J., and Wright M. (2011). Agreement between parents and children on ratings of post-concussive symptoms following mild traumatic brain injury. Child Neuropsychol. 17, 17–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stevens P.K., Penprase B., Kepros J.P., and Dunneback J. (2010). Parental recognition of postconcussive symptoms in children. J. Trauma Nurs. 17, 178–182 [DOI] [PubMed] [Google Scholar]
- 63. Gordon W.A., Haddad L., Brown M., Hibbard M.R., and Sliwinski M. (2000). The sensitivity and specificity of self-reported symptoms in individuals with traumatic brain injury. Brain Inj. 14, 21–33 [PubMed] [Google Scholar]
- 64. Kirkwood M.W., Yeates K.O., and Wilson P.E. (2006). Pediatric sport-related concussion: a review of the clinical management of an oft-neglected population. Pediatrics 177, 1359–1371 [DOI] [PubMed] [Google Scholar]
- 65. Baker J.G., Rieger B.P., McAvoy K., Leddy J.J., Master C.L., Lana S.J., and Willer B.S. (2014). Principles for return to learn after concussion. Int. J. Clin. Pract. 68, 1286–1288 [DOI] [PubMed] [Google Scholar]
- 66. Brooks B.L., Kadoura B., Turley B., et al. (2014). Perception of recovery after pediatric mild traumatic brain injury is influenced by the “good old days” bias: tangible implications for clinical practice and outcomes research. Arch. Clin. Neuropsychol. 29, 186–193 [DOI] [PubMed] [Google Scholar]
- 67. Iverson G.L., Lange R.T., Brooks B.L., and Rennison V.L. (2010). “Good old days” bias following mild traumatic brain injury. Clin. Neuropsychol. 24, 17–37 [DOI] [PubMed] [Google Scholar]
- 68. Streiner D.L., Norman G.R., Cairney J(2015) Reliability, in: Health Measurement Scales: a practical guide to their development and use. Oxford University Press: Oxford, pps. 159–196 [Google Scholar]
- 69. McHugh M.L.(2012). Interrater reliability: the kappa statistic. Biochem. Med. (Zagreb) 22, 276–282 [PMC free article] [PubMed] [Google Scholar]
- 70. McKinlay A., Ligteringen V., and Than M. (2014). A comparison of concussive symptoms reported by parents for preschool versus school-aged children. J. Head Trauma Rehabil. 29, 233–238 [DOI] [PubMed] [Google Scholar]


