Abstract
Metabolic associated fatty liver disease (MAFLD) encompasses a broad spectrum of hepatic disorders, which include steatosis, nonalcoholic steatohepatitis (NASH), fibrosis and cirrhosis, that is a critical risk factor for hepatocellular carcinoma (HCC) development. Its pathogenesis is intertwined with obesity and type 2 diabetes (T2D). However, the predisposition to develop MAFLD is severely influenced by environmental and inherited cues. The rs641738 variant close to MBOAT7 gene has been identified by a genome-wide association screening in heavy drinkers. Although this variant has been associated with the entire spectrum of MAFLD, these results have not been completely replicated and the debate is still opened. Thus, functional studies that unravel the biological mechanisms underlying the genetic association with fatty liver are required. This review aims to summarize the clinical and experimental findings regarding the rs641738 variation and MBOAT7 function, with the purpose to shed light to its role as novel player in MAFLD pathophysiology.
Keywords: MBOAT7, LPIAT1, MAFLD, NASH, Hyperinsulinemia, Insulin resistance, Phospholipids
1. Introduction
Nonalcoholic or more recently re-defined Metabolic associated fatty liver disease (MAFLD) is the most common chronic liver disorder worldwide, affecting more than one third of the general population (around 30% of adults in industrialized countries) [1, 2]. Thus, given its increasing proportion, it represents a primary health, social and economic concern [3]. MAFLD is defined by enhanced hepatic fat deposition that exceeds 5% of liver weight, in absence of alcohol abuse and it entails a wide spectrum of hepatic clinical conditions, spanning from uncomplicated steatosis to nonalcoholic steatohepatitis (NASH), defined by the presence of lobular inflammation and hepatocyte ballooning, to fibrosis, cirrhosis and, only in a minor percentage of cases, to hepatocellular carcinoma (HCC) [4].
MAFLD is epidemiologically related to obesity, type 2 diabetes (T2D) and metabolic syndrome (MetS) [5] and its pathogenesis is closely entangled with increased adiposity, insulin resistance (IR) and dyslipidemia [6]. Indeed, it is regarded as the hepatic manifestation of MetS and its prevalence increases along with body mass index (BMI), reaching 60–70% in obese patients [7]. The risk of progressive MAFLD is higher in patients with severe hyperinsulinemia, IR and T2D, which are identified as the strongest predictors of advanced fibrosis and cirrhosis. Therefore, the prevalence of MAFLD in T2D patients is estimated at 34–74% and it is even higher in T2D patients with obesity (almost 100%) [8]. As a consequence, unhealthy dietary habits, excessive caloric intake, high fructose consumption and poor physical exercise greatly contribute to this pathological condition [9].
Nonetheless, the inter-individual predisposition to develop MAFLD may be also attributable to inherited factors [10]. Single nucleotide polymorphisms (SNPs) in genes regulating hepatic lipid remodeling, among which Patatin-like Phospholipase Domain-containing 3 (PNPLA3), Transmembrane 6 Superfamily Member 2 (TM6SF2) and Membrane Bound O-acyltransferase Domain-containing 7 (MBOAT7), have been broadly associated with increased susceptibility to develop the entire spectrum of MAFLD from steatosis towards NASH and fibrosis [11]. The unraveling of the biological associations between fatty liver and inherited risk factors, and their interplay with environmental context are primary goals in the study of MAFLD pathophysiology. More deeply, the mechanism whereby the less explored MBOAT7 variations exert detrimental effects at hepatic level remains to be fully elucidated.
Currently, no therapeutic consensus exists for the treatment of MAFLD and lifestyle modifications, regular physical activity and sustained weight loss remain the cornerstone of approaches to patients with MAFLD, with the purpose to improve glycemic control, hepatic insulin sensitivity, liver enzymes and histology [12]. However, mounting evidence points out that the individual genetic background has to be considered to personalize genome-based therapeutic approaches [9].
Therefore, this review pursuits to highlight recent findings about the role of MBOAT7 regulation in hyperinsulinemia and IR as a novel example of the gene-environment interaction. These concepts pave the way to improve our knowledge about the mechanisms that predispose to steatosis during IR and emphasize the idea to target MBOAT7 for therapeutic strategies.
2. Insulin resistance and MAFLD pathogenesis
Although the precise mechanism of MAFLD pathogenesis is still under definition, current knowledge supports a model whereby the development of liver injury is multifactorial, commonly referred to as the ‘multi-hits’ hypothesis [13], [14], [15], [16]. In particular, IR is accountable for the ‘first hit’ that induces the rising of hepatic steatosis.
IR is defined as a pathological condition in which physiological or elevated insulin concentrations produce an attenuated biological response, mainly in terms of glucose homeostasis [17]. IR may be due to either impaired insulin receptor activity and/or expression or to a derangement in insulin response through the downstream signaling cascades, or less frequently to loss-of-function mutations in Insulin receptor (INSR) gene. In obese subjects, IR results in chronically elevated insulin concentrations (hyperinsulinemia), alterations in insulin secretion and clearance, increased glucose output and decreased hepatic glucose utilization [18]. To compensate the dampened insulin sensitivity, pancreatic β-cells over-produce insulin, eventually undergoing to exhaustion and leading to impaired glucose tolerance and T2D [19]. Furthermore, in IR context, insulin fails to inactivate gluconeogenesis [20].
In muscle and adipose tissue, IR determines an impairment of glucose uptake and promotes an enhanced activity of hormone-sensitive lipoprotein lipases, favoring lipid storage dismissal [21]. Therefore, in MAFLD patients, it is well supported that hepatic fat accumulation results from an increased efflux of non-esterified or free fatty acids (FFAs) from adipose tissue to the liver where they are stored into the hepatocytes as triglycerides (TAG), in order to protect cells from lipotoxicity [22]. In addition, hyperinsulinemia exacerbates fat depot formation, by inducing de novo lipogenesis from glucose through sterol regulatory element-binding protein-1c (SREBP-1c). Reduction in neutral lipid secretion through very low-density lipoproteins (VLDL) and in β-oxidation due to mitochondrial dysfunction are also involved in steatosis onset [23]. In turn, fatty liver per se may then precipitate hepatic IR promoting metabolic disturbances and cardiovascular damage [24]. Lipid metabolites, such as diacylglycerols (DAG), ceramides and long chain acyl-CoA have also been implicated in IR, affecting insulin signaling [25]. Thus, the composition of lipid species and the tight regulation of the phospholipid distribution in plasmatic and organelle membranes is extremely essential to maintain the physiological signal transduction, the adequate response to insulin and the exchange of metabolites from membranes to intracellular compartments [26, 27]. These notions suggest that alteration in intracellular lipid profiles may further contribute to fatty liver [28]. Indeed, growing evidence indicates that changes in polyunsaturated fatty acids (PUFAs) play a crucial role in MAFLD onset. In particular, it has been observed that arachidonic acid and eicosapentanoic acid-related species decreased in the liver of MAFLD patients, supporting a reduction of enzymatic activity of desaturases [28].
Modifications in lipid homeostasis play also a role in the cascade of events that may precipitate fatty liver to NASH. In particular, arachidonic acid is released from the phospholipids of the membranes by phospholipase A2, that is extremely enhanced in NASH patients. The utilization of arachidonic acid by cyclooxygenases may also contribute to its reduction observed in NASH patients and to its conversion into proinflammatory prostaglandins, thromboxanes, and leukotrienes [28]. Thus, lipids are intertwined with inflammatory pathways and cellular injuries in NASH [29].
3. The rs641738 variant is considered a risk factor for MAFLD onset and progression
MAFLD has a strong inherited component and several genetic risk factors are recognized to influence its onset and severity, as broadly revealed by epidemiological, familial and twin studies [30], [31], [32]. Indeed, the individual susceptibility to develop MAFLD is striking diverse among subjects characterized by the same adiposity, supporting the notion that the genetic make-up contributes to the phenotypic variability of MAFLD. In particular, hepatic fat accumulation which is epidemiologically related to IR and MetS, has been indicated as the main driver of the progression to end-stage liver injuries in genetically predisposed individuals. Thus, the effect of each genetic variation on the spectrum of MAFLD is closely intertwined with their ability to induce fat accumulation [33].
In 2015, the first genome-wide association study (GWAS), regarding the inherited determinants of alcoholic cirrhosis in heavy drinkers, identified the common rs641738 C>T variant as a novel mediator of the susceptibility to develop hepatic damage [34, 35]. This naturally occurring variation is localized in the Membrane bound o-acyltransferase domain-containing 7 – Transmembrane channel-like 4 (MBOAT7-TMC4) locus on chromosome 19 [34]. Mancina and Dongiovanni further corroborated these findings, demonstrating that the rs641738 variant associates with steatosis severity and with the entire spectrum of liver damage related to MAFLD, including HCC [36], [37], [38]. Their observations were well supported since they analyzed the rs641738 distribution among two different independent cohorts, the Dallas Heart study (DHS) cohort and the Liver biopsy cross-sectional cohort (LBC). The former is a population-study that includes 3854 individuals of whom 2736 underwent to proton magnetic resonance spectroscopy to measure hepatic TAG content, whereas the latter entails 1149 European subjects, who underwent liver biopsy for suspected NASH or severe obesity. Patients carrying the T allele displayed an enhanced TAG content, higher prevalence of NASH and fibrosis compared to non-carriers [36]. This data has been even confirmed in a cohort of pediatric individuals in which MBOAT7 variation correlated with high circulating liver enzymes, mainly ALT levels and C-reactive protein (CRP) concentrations, and with increased total body fat percentage [39]. These results may introduce the concept that MBOAT7 might regulate not only hepatic fat accumulation but also the whole body adiposity [39, 40]. In 2018, Di Sessa and collaborators supported this evidence, revealing that T allele carriers were characterized by high ALT, more advanced steatosis and fibrosis in 1002 obese pediatric patients. Moreover, they found a combined effect of the rs641738, PNPLA3 I148M, and TM6SF2 E167K variants on pediatric MAFLD risk [41]. This combined effect has been previously investigated in a multicenter biopsy-based study by Krawczyk and colleagues, revealing that the co-presence of the three main risk variants correlates with more advanced liver injury in adult MAFLD patients, testified by a severe enhancement of circulating liver enzymes [42]. Thus, score-based strategies on the evaluation of polygenic determinants of MAFLD are considered highly predictive and they can be exploited to improve diagnostic accuracy and to guide treatment options [43, 44].
Notably, the rs641738 variant has been identified as a risk factor for the transition to early fibrosis in viral hepatitis B (HBV) and C (HCV), possibly representing a common modifier of liver damage [45, 46]. Moreover, it predisposes to HCC development, even in the absence of cirrhosis in 765 patients with MAFLD and in 1121 non-cirrhotic patients affected by HCV or alcoholic liver disease (ALD) [38]. Even more, rare loss-of-function variants in MBOAT7 have been found to be associated with HCC in NAFLD patients [47]. Nonetheless, it has been implicated in primary biliary cholangitis, exerting a positive effect on transplant free survival [48, 49].
However, the association between the rs641738 variant and liver injuries remains still controversial and not fully replicated, mainly due to the different sample size, clinical features and severity of the disease (i.e. obesity and T2D presence), and ethnicity of the cohorts enrolled in the studies or to the diverse assessment of hepatic steatosis [50], [51], [52], [53], [54]. In particular, the T allele frequency in different ethnicity is highly variable ranging from 0.44 in Europeans, 0.32 in African-Americans and 0.34 in Hispanics and displays even intra-ethnic variability (0.24 in East Asians compared to 0.53 in those of South Asian ancestry). For example, the loss of association between the rs641738 variant and hepatic steatosis assessed by ultrasonography has been observed in 831 Taiwanese children by Lin YC and coworkers [55]. Moreover, for complex diseases, an interplay between genetics and environmental factors exists and gene–environment interactions may amplify the phenotypic effects of the inherited variations [56]. Indeed, the associations between common variants and MAFLD may be unmasked by the increased adiposity, thus enhancing the genetic risk [56]. Thus, we could speculated that in cohort in which the obesity rate is low among NAFLD patients, the association between the rs641738 variant and NAFLD did not obtained a statistical significance [54].
Therefore, a large meta-analysis is required to deeply elucidate the role of this variation on the spectrum of MAFLD. Very recent results which have been obtained by considering data from 1047,265 participants, of whom 8303 had liver biopsies, across 42 studies, confirmed the positive correlation between the rs641738 variant and liver fat, ALT, histological severity of MAFLD, fibrosis and HCC in individuals of European descent [57]. In particular, it has been reported that in T allele carriers, the total risk of NAFLD, advanced fibrosis and HCC is attested at 20%, 30% and 40% more compared to non-carriers, respectively. Thus, the effect sizes reported for the MBOAT7 rs641738 variant is smaller compared to the ones of PNPLA3 I148M and TM6SF2 E167K. Likewise, functional studies are required to extensively explain the mechanisms through which the presence of the rs641738 variant may favor hepatic fat accumulation and progressive liver damage.
Although NAFLD pathogenesis is closely entangled with metabolic syndrome features among which atherogenic dyslipidemia and cardiovascular disease risk, it remains unclear whether the rs641738 variant may exert an impact on cardiovascular diseases [58, 59]. Indeed, while it is well described that the PNPLA3 I148M and the TM6SF2 E167K variants play a protective role against coronary artery disease (CAD), the rs641738 T allele seems to have a neutral effect, but this association remains poorly explored and further studies are necessary to validate these findings [60]. Moreover, the MBOAT7 rs8736 C>T variant located in the 3′ UTR has been associated with cardiovascular outcomes and TT carriers showed significantly reduced levels of PI (18:0;0–20:4;0) reinforcing the concept of a correlation between genetic factors and lipid species composition [61]. The main studies that explored the association between the rs641738 and liver damage have been listed in Table 1.
Table 1.
List of studies that explored the association between the rs641738 and liver damage.
| First author, year, reference | Country, Ancestry | Study Type | Sample size, features | Liver disease diagnosis | Associations |
|---|---|---|---|---|---|
| Buch et al., 2015 [35] | Caucasians | GWAS | Discovery: 712 cases and 1426 controls; Validation: 1148 cases and 922 controls |
Liver biopsy, ultrasound (US), MRI | Alcohol-related cirrhosis |
| Mancina and Dongiovanni et al., 2016 [36] | Multi-ethnic | Population-based (first stage); cases only (second stage) | 3854 participants from the DHS (first stage) 1149 cases from LBC (second stage) |
DHS: liver spectroscopy (n = 2736); LBC: liver biopsy |
Steatosis, NASH, Fibrosis stage |
| Luukkonen et al., 2016 [37] | Caucasians | Cases only | 125 cases | Liver biopsy | Steatosis, NASH, Fibrosis stage |
| Viitasalo et al., 2016 [39] | Caucasians | Population-based | 467 children | Not assessed | Plasma ALT levels |
| Krawczyk et al., 2016 [40] | Caucasians | Cases only | 84 obese individuals scheduled for bariatric surgery | Liver biopsy and MRI | Circulating TAG, total cholesterol, LDL, and serum glucose levels No evidence of association with steatosis |
| Thabet et al., 2016 [46] | Caucasians | Case-control | Discovery: 931 HCV cases; 270 controls Validation: 765 HCV cases; 75 HCV-related HCC cases |
Liver biopsy | Hepatic inflammation and fibrosis stage |
| Donati et al., 2017 [38] | Caucasians | Case-control | 765 non-cirrhotic MAFLD cases (HCC, n = 132); 1121 non-cirrhotic patients affected by ALD or HCV (HCC, n = 25) | Liver biopsy and US | Increased risk of MAFLD-HCC and alcohol-related or HCV-related HCC |
| Thabet et al., 2017 [45] | Multi-ethnic | Cases only | 1101 HBV cases | Liver biopsy | Hepatic inflammation and fibrosis stage |
| Krawczyk et al., 2017 [42] | Caucasians | Cases only | 515 MAFLD cases | Liver biopsy (n = 320) | Fibrosis stage |
| Di Sessa et al., 2018 [41] | Caucasians | Cases only | 1002 obese children | US and indirect measurement of liver fibrosis | Plasma ALT levels, steatosis and fibrosis |
| Krawczyk et al., 2018 [44] | Caucasians | Cases only | 63 MAFLD cases | Liver biopsy | MAFLD risk |
| Di Costanzo et al., 2018 [43] | Caucasians | Cases-control | 218 MAFLD cases and 227 controls | US | MAFLD presence and severity |
| Umano et al., 2018*[87] | Multi-ethnic | Cases only | 860 obese children | MRI (n = 490) | Steatosis and glucose metabolism (only in Caucasians) |
| Koo et al., 2018 [53] | Asians | Case-control | 416 cases and 109 controls | Liver biopsy | No evidence of association |
| Sookoian et al., 2018 [51] | Caucasians | Case-control | 372 cases and 262 controls | Liver biopsy | No evidence of association |
| Lin et al., 2018 [55] | Asians | Cases only | 831 obese children | US | No evidence of association |
| Basyte-Bacevice et al., 2019 [52] | Caucasians | Case-control | 462 cases with alcohol or HCV-related fibrosis and 550 controls | MRI | No evidence of association |
| Xia et al., 2019 [50] | Multi-ethnic | Meta-analysis | 20 studies, including 5415 cases and 17,896 controls | Mixed | No evidence of association |
| Freund et al., 2020 [49] | Caucasians | Cases only | 262 PSC cases | Not assessed | Liver transplant free survival |
| Anstee et al., 2020 [54] | Caucasians | GWAS | Discovery: 1483 cases and 17,781 controls; Validation: 559 cases and 945 controls |
Liver biopsy, US | No evidence of association with MAFLD |
| Teo et al., 2020 [57] | Multi-ethnic | Meta-analysis | 42 studies, including 1047,265 participants | Liver biopsy (n = 8303), MRI | Steatosis, MAFLD severity, fibrosis stage, HCC and plasma ALT levels |
These associations are referred to the rs626283 polymorphism in the MBOAT7 gene.
4. MBOAT7 function
MBOAT7 gene is the mammalian orthologue of mboa-7, that has been firstly identified by using a RNA interference–based genetic screen in Caenorhabditis elegans [62]. mboa-7 deletion mutants showed a decreased Eicosapentaenoic acid (EPA), that is the predominant PUFA in C. elegans, reduced phosphatidylinositol species (PI) [62] and impaired PI-3-phosphate (PI3P)-related events such as early endosome morphology and autophagy [63]. Thus, these findings revealed that mboa-7, is required for incorporation of PUFAs into PI.
MBOAT7 is also referred to as Lysophosphatidyl-inositol acyltransferase 1 (LPIAT1). MBOAT7 gene codifies for an enzyme, member of the “Lands’ Cycle” of phospholipid acyl-chain remodeling of the membranes, through sequential deacylation and reacylation reactions. It catalyzes a desaturation of the second acyl-chain of phospholipids and specifically transfers a PUFA, in form of acyl-CoA to lysoPI and other lysophospholipids, using as preferential substrate the arachidonoyl-CoA. Thus, it is a fine-tune regulator of the amount of free arachidonic acid, that, as mentioned above, is a potent trigger for hepatic inflammation and fibrosis, due to its conversion in eicosanoids [64]. Indeed, in neutrophils MBOAT7 activation has been found to be related to anti-inflammatory processes, by limiting the availability of free arachidonic acid for the synthesis of Leukotriene B4, that is a strong chemoattractant mediator [65].
It has been deeply investigated that Mboat7 participates to brain development in mice, since arachidonic acid is the most enriched PUFA in the brain and it is involved in multiple aspects of neuronal development and function. Mboat7 knock-out (KO) mice show almost no activity with arachidonoyl-CoA as an acyl donor and show reduced arachidonic acid contents in PI and PI phosphates (PIP, PIP2 and PI3P). Specifically, arachidonic acid-containing PI/PI phosphates play an important role in normal cortical lamination during brain development in mice. Indeed, Mboat7 KO mice die within a month and show atrophy of the cerebral cortex and hippocampus, disordered cortical lamination and neuronal processes and delayed neuronal migration in the cortex [63,66]. Notably, inactivating variants in MBOAT7 lead to intellectual disability accompanied by epilepsy and autistic features in patients [67,68]. In turn, up-regulation of MBOAT7 in intrauterine growth restriction (IUGR) neonates could represent an adaptive response to an adverse fetal environment [67].
PI are enabled to regulate membrane dynamics and signal transduction pathways, whereas PI phosphates are synthesized by PI kinases and phosphatases and play crucial roles in the regulation of a wide variety of cellular processes via specific interactions of PIP-binding proteins [69,70]. Among PI phosphates, PI 3-phosphate (PI3P) regulates vesicular trafficking pathways, including endocytosis, endosome-to-Golgi retrograde transport, autophagy and mTOR signaling [71]. Therefore, given its regulatory role in lipid composition of the membranes, we could speculate that MBOAT7 function might also influence signal transduction pathways and the dynamism of cell membranes, essential for membrane fusion and fission steps during endocytosis, exocytosis, cytokinesis and vesicle trafficking.
At a cellular level, the highest expression of MBOAT7 is found in circulating monocytes and lymphocytes, which it has been attested at 7-fold more than that of human hepatocytes, sinusoidal endothelial cells and HSCs. Conversely, its expression in cholangiocytes is very low [38, 49, 72]. MBOAT7 topological structure has been recently solved by Caddeo at al, which demonstrated that is a multi-spanning integral membrane protein with 6 transmembrane domains and the putative catalytic dyad in the lumen [73]. It is localized specifically in membrane fractions rich in phospholipids, such as the ER and the mitochondria-associated membranes (MAM), where arachidonic acid-selective acyl-CoA synthases are enriched [36]. MAM is the membrane bridging ER and mitochondria, which is involved in the biosynthesis and trafficking of lipids between the two organelles [74, 75] and in lipid droplets formation [76]. It interacts with the small subunit of serine palmitoyltransferase a (ssSPTa), that plays a role in fatty acid remodeling of PI, probably by facilitating MBOAT7 localization in MAM [77].
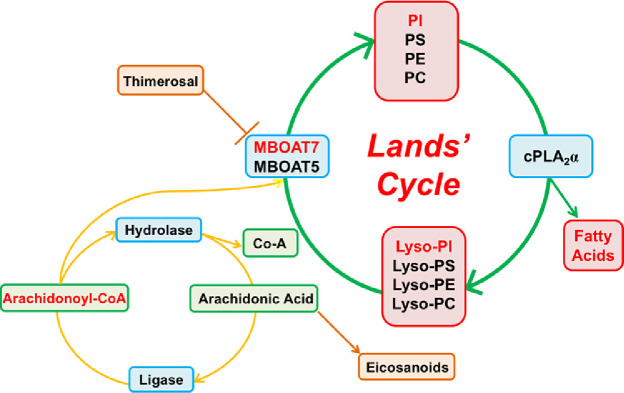
Another enzyme of the MBOAT family, MBOAT5 (mammalian orthologue of mboa-6 [78]), also named Lysophosphatidylcholine acyltransferase 3 (LPCAT3), has similar enzymatic activity to MBOAT7. It is ubiquitously expressed, especially in liver, testis, kidney, pancreas and adipose tissue, where it participates in the acyl-chain remodeling of Phosphatidylcholines (PC), Phosphatidylserine (PS) and Phosphatidylethanolamines (PE) [79]. Expression of LPCAT3 mRNA is controlled by liver X receptors (LXR) and is induced during adipogenesis [80]. Mboat5 KO mice are neonatally lethal due to an extensive TAG accumulation in enterocytes since TAG are not assembled into lipoproteins. Mice with specific deletion of hepatic Mboat5 display a decreased arachidonic acid-containing PC, PS and PE in the liver and an increased risk of hepatic steatosis onset, due to altered lipid kinetics within hepatocytes, and inflammation [81, 82]. Conversely, the induction of Mboat5 ameliorates saturated free fatty acid-induced ER stress, lowering also blood glucose and insulin levels in Lepob/ob mice [80, 83]. The acyltransferase activities of both MBOAT5 and MBOAT7 is susceptible to inhibition by Thimerosal, a thiol-reactive reagent widely used as a preservative in several biological and drug product [65] (Fig. 1). This organomercury compound is the only described reagent that exert a direct effect on MBOAT5 and MBOAT7.
Fig. 1.
Phosphatidylcholines (PC), Phosphatidylserine (PS), Phosphatidylethanolamines (PE) and Phosphatidylinositols (PI) are the main components of the membranes. Phospholipase A2 (cPLA2) releases free arachidonic acid from the sn-2 position of phospholipids, generating the corresponding lysophospholipids and free arachidonic acid. The free arachidonic acid produced may become the precursor of proinflammatory mediators (Eicosanoids) or it may be returned to the phospholipid pool through the activation of an acyl-CoA ligase and either MBOAT7 (that uses lyso-PI) or MBOAT5 (that uses lyso-PC, lyso-PS, and lyso-PE). Thimerosal, an MBOAT unspecific inhibitor, increases the production of Eicosanoids (Prostaglandins and Leukotrienes). Modified by Gijon et al. [65].
5. Pathogenic effect of the rs641738 variant and functional studies
Functional studies aim to decipher the mechanisms through which inherited risk variants may induce the development of fatty liver and its progression to more severe forms. In the past years, Sookoian and coworkers showed that the analysis of the expression of quantitative trait loci (eQTLs) revealed a correlation between the rs641738 variant and tissue-specific MBOAT7 expression in liver and fat [51]. Moreover, Mancina and Dongiovanni have clearly elucidated that the underlying mechanisms behind the association between the rs641738 variant and liver damage is related to the hampered hepatic gene and protein expression of MBOAT7 and not of TMC4, determining changes in PI species, as further confirmed by Luukkonen et al. [36, 37]. Consistently with an impaired hepatic MBOAT7 enzymatic activity, patients carrying the T risk allele display changes in plasma and hepatic PI species, decreasing specifically PI enriched in omega-3 PUFA and arachidonic acid [36, 37]. In particular, these patients are characterized by lower concentrations of arachidonoyl-PI/total and in turn, by higher levels of oleyl-PI/total PI linoleoyl-Pl/total PI ratios (PI containing saturated and monounsaturated fatty acid chains) [36, 37].
The rs641738 T allele seems to be associated with reduced MBOAT7 expression in human hepatocytes and in immune cells, but not in HSCs. Moreover, the polarization of monocytes to both M1 and M2 macrophages is related to MBOAT7 down-regulation, supporting its role in triggering inflammation [72, 84].
The observed reduction of both mRNA and protein synthesis in T allele carriers seems to be due to the linkage disequilibrium between the rs641738 variant and those in the 3′UTR of MBOAT7 (eg, rs8736 C>T, R2 > 0.95 in the CEU population from the 1000 genomes project), which may influence mRNA stability/translation [51]. In severely obese patients, indeed, it has been demonstrated that the rs641738 variation is in strong linkage disequilibrium with the rs8736 polymorphism (R2 = 0.98), that show a more close association with MAFLD due to a stronger MBOAT7 impairment [38]. However, these observations have been not replicated by Sookoian and colleagues, which demonstrated that MBOAT7 is down-regulated in MAFLD patients even independently of the presence of the rs641738 polymorphism [51].
This evidence have been further corroborated by an our very recent paper [72], in which we pointed out that hepatic MBOAT7 down-regulation is a maladaptive response to inherited or diet-induced hyperinsulinemia and it causes intracellular fat accumulation in clinical samples, in in vivo models of MAFLD and in genetically edited HepG2 cells (MBOAT7−/−). Indeed, in overweight adults, MBOAT7 is hampered in presence of hyperinsulinemia and severe liver damage, independently of the genetic background. This data has been confirmed in experimental models of MAFLD, in which the reduction of MBOAT7 expression is greater during obesity and hyperinsulinemia. Specifically, MBOAT7 is physiologically down-regulated during fasting-feeding cycles in liver, adipose tissue and in intestine and during hyperinsulinemia in vivo, in dependence of insulin signaling activation [85]. Conversely, MBOAT5 was up-regulated during hyperinsulinemia, suggesting that MBOAT7, but not MBOAT5, down-regulation may be involved in hepatic fat accumulation in metabolic disorders [72]. A possible link between MBOAT7 and IR has been provided even by Helsley and colleagues [86], who confirmed a dramatic MBOAT7 suppression during obesity and IR and who correlated its adipose tissue expression with indices of insulin sensitivity. In keeping with these findings, Umano and coworkers found an association between lower degree of whole-body insulin sensitivity and MBOAT7 in obese children [87]. Furthermore, it has been demonstrated in Mendelian randomization studies that the ability of a genetic variant to induce IR is attributable to its impact on the severity of liver damage [33, 88].
In addition, we revealed that MBOAT7 is causally involved in the pathogenesis of fatty liver. Indeed, acute silencing of hepatic MBOAT7 to levels similar to those observed in carriers of the rs641738 variant induces hepatic fat accumulation, rapidly leading to steatosis development. Moreover, hepatocytes acquire a cell-autonomous property to accumulate giant lipid droplets when MBOAT7 expression is impaired. According to this notion, we highlighted that MBOAT7−/- hepatocytes accumulate saturated phospholipids, mainly PI, which may be delivered to saturated and mono-unsaturated DAG and TAG synthesis, exacerbating fat deposition (Fig. 2). In line with this data, MBOAT7−/− cells display an induction of lipogenic program and a reduced ability to respond to insulin stimulation through Akt signalling, maybe due to alterations in lipid composition of the membranes. Helsley et al., supported this hypothesis referring that a reduction of MBOAT7 expression exacerbates hepatic IR in high fat diet (HFD)-fed mice and promotes a severe hyperinsulinemia in the fasted state [86]. It has been demonstrated that enhanced saturation of phospholipids may favor oxidative stress and impair signal transductions [89]. For instance, the exposure of 3T3-L1 adipocytes to a challenge of saturated fatty acids may affect insulin sensitivity by hampering membrane lateral diffusion [90]. The reduced membrane fluidity influences also the effectiveness of insulin-independent glucose transporters (GLUTs), impairing glucose uptake [89].
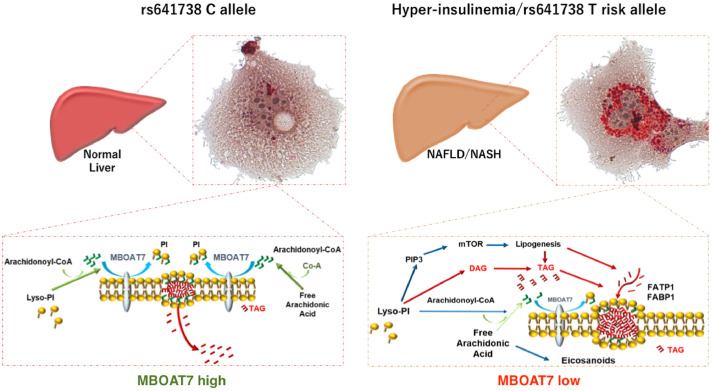
Fig. 2.
During fasting or in rs641738 C allele carriers (left panel), MBOAT7 is highly expressed and it localizes into the membranes, where it conjugates arachidonoyl-CoA to the second acyl-chain of Lyso-PI, thus guaranteeing their physiological fluidity and the dynamism, that allows the exchange of the metabolites from the membranes to intracellular compartments. During hyperinsulinemia or in carriers of the T risk allele (right panel), MBOAT7 is reduced, favoring the increase of saturated PI, which are accumulated and delivered to TAG synthesis. This process requires the up-regulation of FATP1 and associates in vitro with enhanced de novo lipogenesis. Modified from Meroni et al., [72].
Notwithstanding, the process of fat deposition even requires the contribution of another player namely fatty acid transporter (FATP1) and the consequent induction of FFA uptake. Indeed, as MBOAT7 is also expressed in adipose tissue, we can hypothesize that MBOAT7 down-regulation may promote lipolysis and FFAs release into the circulation, as we revealed in MBOAT7 acutely silenced mice and in T allele carriers [72]. According to this notion, FATP1 genetic deletion rescued the intracellular fat accumulation and the increased lipogenesis observed in MBOAT7−/− hepatocytes. In addition, FATP1 expression which is inversely related to that of MBOAT7, is independently associated with the presence of T2D, lipogenesis and inflammation in obese patients with MAFLD [72].
The causative role of MBOAT7 in fatty liver onset has been independently confirmed even by Helsley [86], who elegantly evidenced that Mboat7 loss, but not Tmc4, is sufficient to promote the progression of NAFLD in the setting of HFD, and then by Tanaka and collaborators, who exploited hepatocyte-specific MBOAT7 KO mice and MBOAT7-depleted human hepatic cells [91]. These authors revealed that MBOAT7 KO mice fed HFD spontaneously develop steatosis, and hepatic fibrosis and that MBOAT7 deficiency favors the formation of lipid droplets in cultured hepatic cells and in liver spheroids as a consequence of higher TAG synthesis fueled by a non-canonical pathway, that directly entails the shunting of PI to TAG synthesis [91]. Notwithstanding, MBOAT7 depletion in 3D-spheroids composed by hepatocytes and HSCs, induced cytokines release, fibrogenic markers expression and collagen deposition [91], due to the accumulation of the MBOAT7 substrate LPI lipids [86]. Indeed, circulating saturated LPI were found to be significantly elevated in patients affected by advanced fibrosis compared to healthy individuals. In turn, LPI administration may promote hepatic inflammation and fibrosis in MBOAT7 deficient mice, but not in their wild-type littermates [86]. Notably, this data has been further corroborated by Fondevila et al., who revealed that the increased circulating LPI levels in obese NASH patients, are related to the hepatic over-expression of the G protein-coupled receptor 55 (GPR55), a putative cannabinoid receptor [92]. Moreover, LPI administration in mice and in cultured cells induced lipogenic genes and HSCs activation, in a GPR55-dependent manner. Indeed, GPR55 deficiency ameliorated hepatic injuries in mice fed HF, methionine choline deficient (MCD) diets or injected with carbon tetrachloride (CCl4). Taken together, these observations point out that the restoration of MBOAT7 activity or a reduction of its effectors may constitute possible therapeutic strategy to improve liver damage in NAFLD patients [93], [94], [95].
6. Conclusion
Several lines of evidence indicate that MBOAT7 may represent a modifier of liver damage in both genetically or diet-induced MAFLD. Indeed, it has been made clear that the possible mechanisms behind the biological association between the rs641738 variant and liver damage is due to the hampered hepatic gene and protein expression of MBOAT7 which induces changes in PI composition pattern, favoring in turn TAG synthesis. However, irrespectively of the genetic background, hyperinsulinemia, a typical feature of metabolic syndrome and of post-prandial state, contributes to MBOAT7 impairment, favoring hepatic fat accumulation. These novel observations may introduce the new concept that MBOAT7 dysfunction may participate to the cascade of events that may precipitate chronic hyperinsulinemia to steatosis development (Fig. 3). Moreover, MBOAT7 impairment may facilitate the progression towards steatohepatitis and fibrosis, profoundly affecting lipid composition of the membranes and altering lipid mediator profiles. Therefore, this, surely, constitutes a new example of gene-environment interaction, which should be deeply explored in future studies.
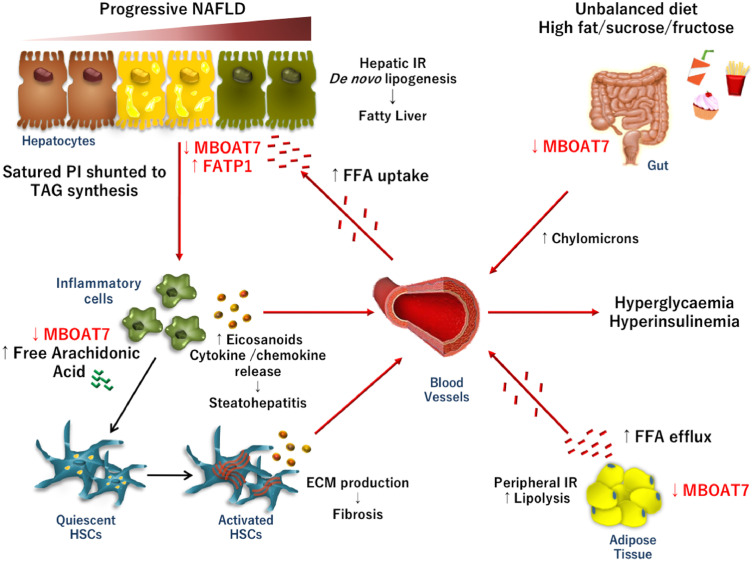
Fig. 3.
Unhealthy dietary habits, excessive caloric intake, high fructose consumption and poor physical exercise are widely recognized as risk factors of IR development. During diet-induced or pathological hyperinsulinemia, MBOAT7 is hampered in intestine, adipose tissue and in liver. In adipose tissue, peripheral IR, induces MBOAT7 down-regulation and lipolysis, favoring an exaggerated free fatty acid (FFA) release into the bloodstream. As a consequence, FFAs uptake increases in hepatocytes, even due to the up-regulation of the fatty acid transporter FATP1. Then, FFAs are stored in intracellular lipid droplets as triglycerides (TAG). Moreover, high insulin concentrations hamper hepatic gene and protein expression of MBOAT7, determining changes in PI composition pattern, favoring in turn, TAG synthesis and de novo lipogenesis. These events may precipitate hepatic fat deposition and fatty liver onset. In turn, MBOAT7 down-regulation per se may be causally implicated in fatty liver and in IR exasperation. The impairment in MBOAT7 function may also facilitate the switch from simple steatosis to steatohepatitis and fibrosis, affecting lipid composition of the membranes of inflammatory cells and altering lipid mediator profiles. Thus, the increase amount of free arachidonic acid and its conversion in pro-inflammatory mediators triggers immune cell activation. Cytokine release and hyperinsulinemia may then stimulate HSCs to produce ECM, perpetuating fibrogenic processes.
7. Outstanding questions
Nowadays, TAG accumulated in lipid droplets are not considered as just ‘innocent bystander’, but real culprits of the cellular injuries [96]. Indeed, emerging evidence pinpoints the role of lipid droplets in the multiple processes that leading to steatohepatitis [96, 97]. They are enormously dynamic, modifying their location, size, lipid and protein composition in response to environmental stimuli. Thus, they are engaged not only in energy expenditure but also in signaling pathways, acting as hubs that integrate metabolic and inflammatory processes [97]. The deep understanding of the biological implication of changes in lipid composition may be useful to narrow the gap in the knowledge of MAFLD pathogenesis.
We are aware that in the complex architecture of MAFLD, several other contributors may participate to fatty liver onset. We are also conscious that further studies are required to better explain how IR may lead to liver damage. Thus, it should be useful to better address the impact of the rs641738 variation on hepatic disorders and the mechanisms entailing MBOAT7 down-regulation in IR-induced liver injuries. In the future, researches aimed to identify therapeutic approaches that influence MBOAT7 activity might represent a novel targeted strategy in the management of patients with MAFLD and in particular in those with diabetes. Furthermore, the effects of rs641738 variant and MBOAT7 alterations on MetS components, circulating lipids and cardiovascular risk remain uncharted.
8. Search strategy and selection criteria
Data for this Review were identified by searches of MEDLINE and PubMed, and references from relevant articles using the search terms “LPIAT1”, “MBOAT7”, “MAFLD”, “NAFLD”,“NASH” and “rs641738”. Reports from meetings were not included. Only articles published in English between 2000 and 2020 were included.
Declaration of Competing Interest
The authors declare no conflict of interest.
Acknowledgement/Funding statement
The study was supported by the Ricerca Corrente Fondazione IRCCS Cà Granda and Ricerca Finalizzata Ministero della Salute RF-2013–02358319. The funders had no role in design, data collection, data analysis, interpretation or writing of the paper.
References
- 1.Eslam M., Sanyal A.J., George J. MAfld: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014. doi: 10.1053/j.gastro.2019.11.312. e1. [DOI] [PubMed] [Google Scholar]
- 2.Eslam M. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73(1):202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 3.Younossi Z.M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 4.Wong R.J. Nonalcoholic steatohepatitis is the second leading etiology of liver disease among adults awaiting liver transplantation in the United States. Gastroenterology. 2015;148(3):547–555. doi: 10.1053/j.gastro.2014.11.039. [DOI] [PubMed] [Google Scholar]
- 5.Marchesini G. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50(8):1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 6.Byrne C.D., Targher G. NAFLD: a multisystem disease. J Hepatol. 2015;62(1 Suppl):S47–S64. doi: 10.1016/j.jhep.2014.12.012. [DOI] [PubMed] [Google Scholar]
- 7.Angulo P. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397. doi: 10.1053/j.gastro.2015.04.043. e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dongiovanni P. The role of insulin resistance in nonalcoholic steatohepatitis and liver disease development–a potential therapeutic target? Expert Rev Gastroenterol Hepatol. 2016;10(2):229–242. doi: 10.1586/17474124.2016.1110018. [DOI] [PubMed] [Google Scholar]
- 9.Meroni, M., et al., Nutrition and genetics in NAFLD: the perfect binomium. 2020;21(8). [DOI] [PMC free article] [PubMed]
- 10.Dongiovanni P., Valenti L. Genetics of nonalcoholic fatty liver disease. Metab Clin Exp. 2016;65(8):1026–1037. doi: 10.1016/j.metabol.2015.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Dongiovanni, P. and M. Meroni, miRNA signature in NAFLD: a turning point for a non-invasive diagnosis. 2018.19(12). [DOI] [PMC free article] [PubMed]
- 12.Dongiovanni P., Valenti L. A nutrigenomic approach to non-alcoholic fatty liver disease. Int J Mol Sci. 2017;18(7):1534. doi: 10.3390/ijms18071534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Estep J.M. Expression of cytokine signaling genes in morbidly obese patients with non-alcoholic steatohepatitis and hepatic fibrosis. Obes Surg. 2009;19(5):617–624. doi: 10.1007/s11695-009-9814-x. [DOI] [PubMed] [Google Scholar]
- 14.Malhi H., Gores G.J. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28(4):360–369. doi: 10.1055/s-0028-1091980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miele L. Hepatic mitochondrial beta-oxidation in patients with nonalcoholic steatohepatitis assessed by 13C-octanoate breath test. Am J Gastroenterol. 2003;98(10):2335–2336. doi: 10.1111/j.1572-0241.2003.07725.x. [DOI] [PubMed] [Google Scholar]
- 16.Charlton M. Apolipoprotein synthesis in nonalcoholic steatohepatitis. Hepatology. 2002;35(4):898–904. doi: 10.1053/jhep.2002.32527. [DOI] [PubMed] [Google Scholar]
- 17.Cefalu W.T. Insulin resistance: cellular and clinical concepts. Exp Biol Med (Maywood) 2001;226(1):13–26. doi: 10.1177/153537020122600103. [DOI] [PubMed] [Google Scholar]
- 18.Thomas D.D., Corkey B.E. Hyperinsulinemia: an early indicator of metabolic dysfunction. J Endocr Soc. 2019;3(9):1727–1747. doi: 10.1210/js.2019-00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grancini V. Contribution of beta-cell dysfunction and insulin resistance to cirrhosis-associated diabetes: role of severity of liver disease. J Hepatol. 2015;63(6):1484–1490. doi: 10.1016/j.jhep.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Matsumoto M. Impaired regulation of hepatic glucose production in mice lacking the forkhead transcription factor Foxo1 in liver. Cell Metab. 2007;6(3):208–216. doi: 10.1016/j.cmet.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 21.Longo M. Adipose Tissue Dysfunction as Determinant of Obesity-Associated Metabolic Complications. Int J Mol Sci. 2019;20(9) doi: 10.3390/ijms20092358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bugianesi E. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48(4):634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 23.Fabbrini E. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134(2):424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Korenblat K.M. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134(5):1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ozcan U. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 26.Olzmann J.A., Carvalho P. Dynamics and functions of lipid droplets. Nat Rev Mol Cell Biol. 2019;20(3):137–155. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casares D., Escriba P.V. Membrane lipid composition: effect on membrane and organelle structure. Funct Compartmentalization Ther Avenues. 2019;20(9) doi: 10.3390/ijms20092167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Puri P. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46(4):1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- 29.Di Marzo V. Arachidonic acid and eicosanoids as targets and effectors in second messenger interactions. Prostaglandins Leukot Essent Fatty Acids. 1995;53(4):239–254. doi: 10.1016/0952-3278(95)90123-x. [DOI] [PubMed] [Google Scholar]
- 30.Sookoian S., Pirola C.J. Genetics of Nonalcoholic Fatty Liver Disease: From Pathogenesis to Therapeutics. Semin Liver Dis. 2019;39(2):124–140. doi: 10.1055/s-0039-1679920. [DOI] [PubMed] [Google Scholar]
- 31.Dongiovanni P., Anstee Q.M., Valenti L. Genetic predisposition in NAFLD and NASH: impact on severity of liver disease and response to treatment. Curr Pharm Des. 2013;19(29):5219–5238. doi: 10.2174/13816128113199990381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dongiovanni P., Romeo S., Valenti L. Genetic Factors in the Pathogenesis of Nonalcoholic Fatty Liver and Steatohepatitis. Biomed Res Int. 2015;2015 doi: 10.1155/2015/460190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dongiovanni, P., et al., Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. 2018.283(4): p. 356–370. [DOI] [PMC free article] [PubMed]
- 34.Stickel F. The genetics of alcohol dependence and alcohol-related liver disease. J Hepatol. 2017;66(1):195–211. doi: 10.1016/j.jhep.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 35.Buch, S., F. Stickel, and E. Trepo, A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. 2015.47(12): p. 1443–8. [DOI] [PubMed]
- 36.Mancina R.M. The MBOAT7-TMC4 variant rs641738 increases risk of nonalcoholic fatty liver disease in individuals of European descent. Gastroenterology. 2016;150(5):1219–1230. doi: 10.1053/j.gastro.2016.01.032. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Luukkonen P.K. The MBOAT7 variant rs641738 alters hepatic phosphatidylinositols and increases severity of non-alcoholic fatty liver disease in humans. J Hepatol. 2016;65(6):1263–1265. doi: 10.1016/j.jhep.2016.07.045. [DOI] [PubMed] [Google Scholar]
- 38.Donati B. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci Rep. 2017;7(1):4492. doi: 10.1038/s41598-017-04991-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Viitasalo A. Association of MBOAT7 gene variant with plasma ALT levels in children: the PANIC study. Pediatr Res. 2016;80(5):651–655. doi: 10.1038/pr.2016.139. [DOI] [PubMed] [Google Scholar]
- 40.Krawczyk M. PNPLA3 p.I148M variant is associated with greater reduction of liver fat content after bariatric surgery. Surg Obes Relat Dis. 2016;12(10):1838–1846. doi: 10.1016/j.soard.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 41.Di Sessa A. The membrane-bound O-Acyltransferase7 rs641738 variant in pediatric nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2018;67(1):69–74. doi: 10.1097/MPG.0000000000001979. [DOI] [PubMed] [Google Scholar]
- 42.Krawczyk M. Combined effects of the PNPLA3 rs738409, TM6SF2 rs58542926, and MBOAT7 rs641738 variants on NAFLD severity: a multicenter biopsy-based study. J Lipid Res. 2017;58(1):247–255. doi: 10.1194/jlr.P067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Di Costanzo, A., et al., Evaluation of polygenic determinants of non-alcoholic fatty liver disease (NAFLD) by a candidate genes resequencing strategy. 2018.8(1): p. 3702. [DOI] [PMC free article] [PubMed]
- 44.Krawczyk M. Could inherited predisposition drive non-obese fatty liver disease? Results from German tertiary referral centers. J Hum Genet. 2018;63(5):621–626. doi: 10.1038/s10038-018-0420-4. [DOI] [PubMed] [Google Scholar]
- 45.Thabet K. The membrane-bound O-acyltransferase domain-containing 7 variant rs641738 increases inflammation and fibrosis in chronic hepatitis B. Hepatology. 2017;65(6):1840–1850. doi: 10.1002/hep.29064. [DOI] [PubMed] [Google Scholar]
- 46.Thabet K. MBOAT7 rs641738 increases risk of liver inflammation and transition to fibrosis in chronic hepatitis C. Nat Commun. 2016;7:12757. doi: 10.1038/ncomms12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pelusi, S. and G. Baselli, Rare pathogenic variants predispose to hepatocellular carcinoma in nonalcoholic fatty liver disease. 2019.9(1): p. 3682. [DOI] [PMC free article] [PubMed]
- 48.Rahal H.K., Tabibian J.H. The MBOAT7 rs641738 variant in primary sclerosing cholangitis: A novel biomarker for prognostication. Clin Res Hepatol Gastroenterol. 2020 doi: 10.1016/j.clinre.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 49.Freund C. The MBOAT7 rs641738 variant is associated with an improved outcome in primary sclerosing cholangitis. Clin Res Hepatol Gastroenterol. 2020 doi: 10.1016/j.clinre.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 50.Xia Y. Meta-analysis of the association between MBOAT7 rs641738, TM6SF2 rs58542926 and nonalcoholic fatty liver disease susceptibility. Clin Res Hepatol Gastroenterol. 2019;43(5):533–541. doi: 10.1016/j.clinre.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 51.Sookoian S. Lack of evidence supporting a role of TMC4-rs641738 missense variant-MBOAT7- intergenic downstream variant-in the Susceptibility to Nonalcoholic Fatty Liver Disease. Sci Rep. 2018;8(1):5097. doi: 10.1038/s41598-018-23453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Basyte-Bacevice, V., et al., TM6SF2 and MBOAT7 Gene Variants in Liver Fibrosis and Cirrhosis. 2019.20(6). [DOI] [PMC free article] [PubMed]
- 53.Koo, B.K., S.K. Joo, and D. Kim, Additive effects of PNPLA3 and TM6SF2 on the histological severity of non-alcoholic fatty liver disease. 2018.33(6): p. 1277–1285. [DOI] [PubMed]
- 54.Anstee Q.M. Genome-wide association study of non-alcoholic fatty liver and steatohepatitis in a histologically-characterised cohort. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.04.003. [DOI] [PubMed] [Google Scholar]
- 55.Lin, Y.C., et al., Genetic determinants of hepatic steatosis and serum cytokeratin-18 fragment levels in Taiwanese children. 2018.38(7): p. 1300–1307. [DOI] [PubMed]
- 56.Stender, S. and J. Kozlitina, Adiposity amplifies the genetic risk of fatty liver disease conferred by multiple loci. 2017.49(6): p. 842–847. [DOI] [PMC free article] [PubMed]
- 57.Teo K. rs641738C>T near MBOAT7 is positively associated with liver fat, ALT, and histological severity of NAFLD: a meta-analysis. medRxiv. 2020 [Google Scholar]
- 58.Sookoian, S. and C.J. Pirola, Review article: shared disease mechanisms between non-alcoholic fatty liver disease and metabolic syndrome - translating knowledge from systems biology to the bedside. 2019.49(5): p. 516–527. [DOI] [PubMed]
- 59.Eslam, M. and J. George, Genetic contributions to NAFLD: leveraging shared genetics to uncover systems biology. 2020.17(1): p. 40–52. [DOI] [PubMed]
- 60.Simons N. PNPLA3, TM6SF2, and MBOAT7 genotypes and coronary artery disease. Gastroenterology. 2017;152(4):912–913. doi: 10.1053/j.gastro.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 61.Tabassum, R., J.T. Rämö, and P. Ripatti, Genetic architecture of human plasma lipidome and its link to cardiovascular disease. 2019.10(1): p. 4329. [DOI] [PMC free article] [PubMed]
- 62.Lee H.C. Caenorhabditis elegans mboa-7, a member of the MBOAT family, is required for selective incorporation of polyunsaturated fatty acids into phosphatidylinositol. Mol Biol Cell. 2008;19(3):1174–1184. doi: 10.1091/mbc.E07-09-0893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee H.C. LPIAT1 regulates arachidonic acid content in phosphatidylinositol and is required for cortical lamination in mice. Mol Biol Cell. 2012;23(24):4689–4700. doi: 10.1091/mbc.E12-09-0673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zarini S. Lysophospholipid acyltransferases and eicosanoid biosynthesis in zebrafish myeloid cells. Prostaglandins Other Lipid Mediat. 2014;113-115:52–61. doi: 10.1016/j.prostaglandins.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gijon M.A. Lysophospholipid acyltransferases and arachidonate recycling in human neutrophils. J Biol Chem. 2008;283(44):30235–30245. doi: 10.1074/jbc.M806194200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vogel P. Congenital hydrocephalus in genetically engineered mice. Vet Pathol. 2012;49(1):166–181. doi: 10.1177/0300985811415708. [DOI] [PubMed] [Google Scholar]
- 67.Ruis-Gonzalez M.D. Alterations of protein expression in serum of infants with intrauterine growth restriction and different gestational ages. J Proteomics. 2015;119:169–182. doi: 10.1016/j.jprot.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 68.Johansen A. Mutations in MBOAT7, encoding lysophosphatidylinositol acyltransferase I, lead to intellectual disability accompanied by epilepsy and autistic features. Am J Hum Genet. 2016;99(4):912–916. doi: 10.1016/j.ajhg.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Di Paolo G., De Camilli P. Phosphoinositides in cell regulation and membrane dynamics. Nature. 2006;443(7112):651–657. doi: 10.1038/nature05185. [DOI] [PubMed] [Google Scholar]
- 70.Sasaki T. Mammalian phosphoinositide kinases and phosphatases. Prog Lipid Res. 2009;48(6):307–343. doi: 10.1016/j.plipres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 71.Backer J.M. The regulation and function of Class III PI3Ks: novel roles for Vps34. Biochem J. 2008;410(1):1–17. doi: 10.1042/BJ20071427. [DOI] [PubMed] [Google Scholar]
- 72.Meroni M. Mboat7 down-regulation by hyper-insulinemia induces fat accumulation in hepatocytes. EBioMedicine. 2020;52 doi: 10.1016/j.ebiom.2020.102658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Caddeo A. MBOAT7 is anchored to endomembranes by six transmembrane domains. J Struct Biol. 2019;206(3):349–360. doi: 10.1016/j.jsb.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 74.Hayashi-Nishino M. A subdomain of the endoplasmic reticulum forms a cradle for autophagosome formation. Nat Cell Biol. 2009;11(12):1433–1437. doi: 10.1038/ncb1991. [DOI] [PubMed] [Google Scholar]
- 75.de Brito O.M., Scorrano L. An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. Embo J. 2010;29(16):2715–2723. doi: 10.1038/emboj.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Horl G. Sequential synthesis and methylation of phosphatidylethanolamine promote lipid droplet biosynthesis and stability in tissue culture and in vivo. J Biol Chem. 2011;286(19):17338–17350. doi: 10.1074/jbc.M111.234534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hirata Y. Identification of small subunit of serine palmitoyltransferase a as a lysophosphatidylinositol acyltransferase 1-interacting protein. Genes Cells. 2013;18(5):397–409. doi: 10.1111/gtc.12046. [DOI] [PubMed] [Google Scholar]
- 78.Matsuda S. Member of the membrane-bound O-acyltransferase (MBOAT) family encodes a lysophospholipid acyltransferase with broad substrate specificity. Genes Cells. 2008;13(8):879–888. doi: 10.1111/j.1365-2443.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- 79.Hashidate-Yoshida T. Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. Elife. 2015;4 doi: 10.7554/eLife.06328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Demeure O. Regulation of LPCAT3 by LXR. Gene. 2011;470(1-2):7–11. doi: 10.1016/j.gene.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 81.Kabir I. Small intestine but not liver lysophosphatidylcholine acyltransferase 3 (Lpcat3) deficiency has a dominant effect on plasma lipid metabolism. J Biol Chem. 2016;291(14):7651–7660. doi: 10.1074/jbc.M115.697011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rong X. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. Elife. 2015;4 doi: 10.7554/eLife.06557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cash J.G., Hui D.Y. Liver-specific overexpression of LPCAT3 reduces postprandial hyperglycemia and improves lipoprotein metabolic profile in mice. Nutr Diabetes. 2016;6:e206. doi: 10.1038/nutd.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Takemasu S. Lysophosphatidylinositol-acyltrasferase-1 (LPIAT1) is involved in cytosolic Ca(2+) oscillations in macrophages. Genes Cells. 2019 doi: 10.1111/gtc.12681. [DOI] [PubMed] [Google Scholar]
- 85.Pajvani U.B., Accili D. The new biology of diabetes. Diabetologia. 2015;58(11):2459–2468. doi: 10.1007/s00125-015-3722-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Helsley, R.N., et al., Obesity-linked suppression of membrane-bound O-acyltransferase 7 (MBOAT7) drives non-alcoholic fatty liver disease. 2019.8. [DOI] [PMC free article] [PubMed]
- 87.Umano G.R. The rs626283 variant in the MBOAT7 gene is associated with insulin resistance and fatty liver in caucasian obese youth. Am J Gastroenterol. 2018;113(3):376–383. doi: 10.1038/ajg.2018.1. [DOI] [PubMed] [Google Scholar]
- 88.Parisinos C.A. Genome-wide and Mendelian randomisation studies of liver MRI yield insights into the pathogenesis of steatohepatitis. J Hepatol. 2020 doi: 10.1016/j.jhep.2020.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weijers R.N. Lipid composition of cell membranes and its relevance in type 2 diabetes mellitus. Curr Diabetes Rev. 2012;8(5):390–400. doi: 10.2174/157339912802083531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grunfeld C., Baird K.L., Kahn C.R. Maintenance of 3T3-L1 cells in culture media containing saturated fatty acids decreases insulin binding and insulin action. Biochem Biophys Res Commun. 1981;103(1):219–226. doi: 10.1016/0006-291x(81)91682-x. [DOI] [PubMed] [Google Scholar]
- 91.Tanaka, Y., et al., LPIAT1/MBOAT7 depletion increases triglyceride synthesis fueled by high phosphatidylinositol turnover. 2020. [DOI] [PMC free article] [PubMed]
- 92.Fondevila, M.F., et al., The L-α-lysophosphatidylinositol/GPR55 system induces the development of non-alcoholic steatosis and steatohepatitis. 2020.
- 93.Romeo S., Sanyal A., Valenti L. Leveraging human genetics to identify potential new treatments for fatty liver disease. Cell Metab. 2020;31(1):35–45. doi: 10.1016/j.cmet.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 94.Sookoian, S., C.J. Pirola, and L. Valenti, Genetic pathways in nonalcoholic fatty liver disease: Insights from systems biology. 2020. [DOI] [PMC free article] [PubMed]
- 95.Eslam M., George J. Genetic Insights for Drug Development in NAFLD. Trends Pharmacol Sci. 2019;40(7):506–516. doi: 10.1016/j.tips.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 96.Neuschwander-Tetri B.A. Hepatic lipotoxicity and the pathogenesis of nonalcoholic steatohepatitis: the central role of nontriglyceride fatty acid metabolites. Hepatology. 2010;52(2):774–788. doi: 10.1002/hep.23719. [DOI] [PubMed] [Google Scholar]
- 97.Jarc E., Petan T. A twist of FATe: Lipid droplets and inflammatory lipid mediators. Biochimie. 2020;169:69–87. doi: 10.1016/j.biochi.2019.11.016. [DOI] [PubMed] [Google Scholar]