Abstract
Dengue viral (DENV) infection has a broad clinical spectrum ranging from classical febrile illness to life-threatening disease. Literature suggests that spectrum of illness could be due to differences in innate immune-responses; however, the knowledge is still at infancy. Amongst the various cells involved in innate immune responses, NK cells play a central role, particularly in anti-viral immunity. Thus in this study we have evaluated the role of NK-cells during acute-DENV infection and its influence on severity of disease, by analyzing activation, cytotoxic receptors, cytolytic granule contents and degranulation markers on NK-cells during different stages of infection.
Based on the clinical manifestations and severity of the disease, DENV patients were classified into patients with dengue without warning signs (DF), dengue with warning signs (DFWS) and severe dengue (SD) patients. During acute-DENV infection, though there was no alteration in frequency of NK-cells, significant increase in frequency of CD56bright subset in DF patients (p < 0.05) was observed, while it remained unaltered in SD patients. We also found that, CD56dim NK-cell subset of DF patients had elevated CD69 expression, granzyme B and intracellular IFN-γ levels compared to SD patients (p < 0.05). Amongst the NK-cell cytotoxicity receptor (NCR), NKp30 receptor was significantly elevated in DF patients (p < 0.05), however in SD patients it was comparable to healthy controls. This receptor is essential for dendritic cells-NK-cells crosstalk for initiating adaptive immune response. IL-15 is known to induce NKp30 expression, which was also seen to be elevated in DF patients (p < 0.05) but unaltered in SD patients. In SD patients, even post-6 days of infection i.e. during recovery phase, CD69 and NKp30 expression did not raise, suggesting impaired NK-cell response in these patients.
To summarize, our study reports, that efficient NK cell response during acute phase of DENV infection is crucial for preventing severity of the disease. This study helps in understanding the dynamics of NK cell response in immunopathogenesis of DENV infection; which is crucial for development of efficacious therapeutics as well as vaccine.
Keywords: Immunology, Microbiology, Public health, Virology, Hematological system, Infectious disease, NK cell response, Severe dengue viral infection, Immunopathogenesis
Immunology; Microbiology; Public health; Virology; Hematological system; Infectious disease; NK cell response; Severe dengue viral infection; Immunopathogenesis.
1. Introduction
Dengue viral (DENV)-infection is the most significant mosquito-borne viral illness affecting mankind and its public health threat is prominently expanding in tropical and subtropical countries like India [1]. It has large clinical spectrum, ranging from mild undifferentiated fever to severe life threatening disease. WHO classification based on the clinical manifestations, classifies dengue patients into dengue without warning signs (DF), dengue with warning signs (DFWS) and severe dengue (SD) patients [2]. Cumulative data have demonstrated that both the innate and adaptive immune responses participate in the control and pathogenesis of DENV disease [3]. Though much work has been devoted to understanding the adaptive immune response to DENV, the knowledge of innate immune response is still at its infancy. As many DENV-infected patients even remain asymptomatic post infection, a rapid initiation of the innate host defense may be the critical limiting step in the infection.
Amongst the various cells contributing to innate immune response, NK cells play a crucial role in regulating both innate and adaptive immune responses especially against viral infections [4]. These cells are characterized as CD56+CD3- cells and based on intensity of CD56 expression are further differentiated into CD56dim and CD56bright subtypes, which differ in terms of phenotype, effector function and tissue localization. CD56dim cells are cytotoxic in function while CD56bright cells are cytokine releasing cells. Both these cell subsets play important role in mounting an immune response against viral infections. NK cells can lyse target cells, through direct cell-cell contact by forming immune synapse and releasing perforin and granzymes; as well as by secreting immunoregulatory cytokines that augment the antigen-specific immune response [4, 5]. Equilibrium between signals from various activation and inhibitory receptors controls the function of these cells. These receptors recognize their ligands in tumor or virus-infected cells; and modulation of these markers contribute to NK cell mediated response [4].
Previous studies have reported, elevated NK cell cytotoxicity and modulation of NK cell receptors post-DENV infection [6], however, its contribution to the severity of DENV-disease still remains unclear. Thus in this study, we comprehensively evaluated NK cells during the progression of the DENV-infection and studied its correlation with severity of the disease. This study will contribute in understanding the role of NK cell response in immunopathogenesis of DENV infection; which is important for development of efficacious therapeutics as well as vaccine.
2. Materials and methods
2.1. Human ethics statement
This study was approved by Institutional Ethics committee (IEC) for Human subjects of National Institute of Immunohematology and Seth GS Medical College and KEM Hospital. Patients received information about research and provided written informed consent to participate in this study.
2.2. Study cohort
Patients with fever visiting the Department of Medicine, KEM Hospital during July 2016 to October 2017 were tested for panel of infections. Of these, 122 DENV patients who tested positive for both-specific IgG and IgM capture ELISA and dengue NS1 antigen test were included in the study. The day of fever was considered from the onset and not from the day of enrolment in the hospital. All these patients were negative for chikungunya, malaria and leptospirosis. In order to determine the severity of the disease, clinical manifestations and laboratory investigations viz. platelet counts, hematocrits, white cell counts of these patients were serially monitored.
Of these 122 patients, we could obtain 40 patient (16 DF; 11 DFWS; 13 SD) samples at 2 time points-day 2–6 (acute phase) and day >6 (recovery phase) of fever.
Blood samples of 50 age-matched healthy volunteers' without history of any febrile or other illnesses in the previous 3 months were obtained as controls.
2.3. Flow cytometry analysis
2.3.1. Immune cell enumeration
Lymphocyte subsets analysis enumerating NK cells, T cells and B cells was performed using dual platform; wherein absolute white blood cells (WBC) count and lymphocyte absolute count was determined using Sysmex XS-800i and lymphocyte subset analysis by flow cytometry using BD Multitest 6-color TBNK reagent. Cells were acquired on FACS Aria I and analysis was performed on FACS Diva and FlowJo software (BD Biosciences, San Jose, CA, USA).
2.3.2. NK cell phenotyping
NK cell receptors were analyzed after staining with appropriate antibody cocktail: anti-CD56-PC7 (N901), anti-NKp30-PE (Z25), anti-NKp44-PE (Z231), anti-NKp46-PE (BAB281), from Beckman Coulter; anti-CD69-FITC (FN50), anti-CD45-APC (J33), anti-CD3-PCP (UCHT1), anti-CD3-FITC (UCHT1), anti-CD3-APC (UCHT1), anti-CD57-FITC (NK-1), anti-Granzyme-B-FITC (GB11), anti-HLA-DR-PE (TU-36), anti-CD8-APC-Cy7 (SK-1), anti-CD107a-FITC (H4A3), anti-Perforin-PE (δ69) from Becton Dickinson; anti-NKG2D-APC (BAT221) from Miltenyi Biotec from R&D Systems.
2.3.3. Cytolytic markers and functional assays
For intracellular staining, cells were fixed and permeabilized with cytofix/cytoperm kit (Becton Dickinson) and stained with perforin-PE (Gδ9) and granzyme-B-FITC (GB11), as described.
To determine CD107a-FITC (H4A3) expression (degranulation marker), cells were stimulated with Phorbol-12- myristate-13-acetate (PMA, 0.15 μg/ml, Sigma Chem. Co., St. Louis, MO) and Ca2+ Ionophore (Ionomycin, 3 μg/ml, Sigma Chem. Co., St. Louis, MO) for 2 h and fixation/permeabilization was further performed to determine IFN-γ content (IFN-γ PE).
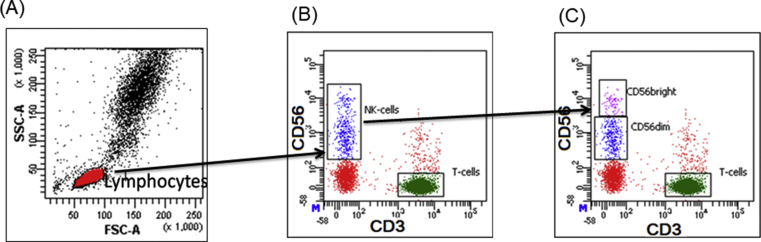
Lymphocytes were either gated on forward and side scatter parameters or CD45 expression for further analysis. NK cells were gated as CD56+CD3-, and were divided into two subsets CD3−CD56dim NK cell (CD56dim NK) and CD3−CD56bright NK cell (CD56bright NK) subsets were distinguished based on intensity of CD56 expression (Figure 1). Receptor expression was presented as the percentage of cells in a gated NK cells region. The threshold between negative and positive was defined by the fluorescence minus one (FMO) method. At least 20,000 lymphocytes were acquired on FACSAria fusion cytometer (Becton Dickinson) and was analyzed using FACS DIVA software.
Figure 1.
Gating strategy: (A) Lymphocytes were gated on forward CD45 expression (B) NK cells were gated as CD56+CD3-, (C) NK cells were divided into two subsets on the basis of CD56 surface intensity viz. CD56dim NK and CD56bright NK cell subsets.
2.3.4. Determination of cytokine levels
As per manufactures instructions, plasma levels of IL-10, IL-15, and IFN-γ in DENV patients and healthy controls were determined by flowcytometer using AIMPLEX kit (Aimplex Biosciences, Inc.).
2.4. Statistical analysis
Median and range for all the parameters was calculated. 2-way ANNOVA test and Mann-Whitney U test was used to compare data between the groups. Spearman correlation was performed to analyze the correlation between variables. Analyses were performed with GraphPad Prism (GraphPad Software, Inc. Version 5.0). Differences were considered statistically significant if the 2-tailed p value was ≤0.05.
3. Results
3.1. Clinical characteristics and laboratory findings
Total of 122 patients diagnosed with primary DENV infection were enrolled in the study. All these patients were in the acute phase of infection i.e. day 2–6 of fever. These patients were classified into dengue without warning signs (DF) (n = 58), dengue with warning signs (DFWS) (n = 38) and severe dengue (SD) (n = 26) as per the latest WHO classification. Patients with more than 2 organ involvement were considered to be severe [2] and they presented with varied clinical presentations including central nervous system (CNS) manifestations (seizures, encephalopathy), bleeding, fluid accumulation, acute respiratory distress syndrome (ARDS), and liver/kidney dysfunction. There were no significant statistical differences in age and sex between groups of patients analyzed.
The laboratory diagnosis results showed that the levels of WBC and creatinine were significantly higher in SD patients compared to DF patients while hemoglobin and hematoocrit levels were significantly low in SD patients. Though, AST and ALT were higher in SD patients and platelets were higher in DF patients, the difference was not statistically different (Table 1).
Table 1.
Clinical routine indexes in different dengue severity group.
| DF (n = 58) | DFWS (n = 38) | SD (n = 26) | P value |
|||
|---|---|---|---|---|---|---|
| DF-DFWS | DF-SD | DFWS-SD | ||||
| Median Age (Yrs) | 22 ± 10.6 | 25 ± 6.7 | 26 ± 9.9 | NS | NS | NS |
| WBC (X109) | 4.1 ± 2.2 | 3.9 ± 2.3 | 6.5 ± 3.6 | NS | 0.02∗ | 0.02∗ |
| Hb | 13.3 ± 2 | 13.3 ± 3.3 | 10.4 ± 3 | NS | 0.0002∗ | 0.02∗ |
| PLT (X109) | 106 ± 80 | 74 ± 53 | 76 ± 64 | NS | NS | NS |
| HCT | 40.88 ± 4.9 | 37.55 ± 6.9 | 33.23 ± 9.1 | NS | 0.005∗ | NS |
| ALT (U/L) | 174 ± 197 | 171 ± 184 | 1081 ± 1505 | NS | NS | NS |
| AST (U/L) | 96 ± 93 | 119 ± 74 | 511 ± 733 | NS | NS | NS |
| Creatinine (mg/dL) | 1.15 ± 0.2 | 1.27 ± 0.4 | 2.7 ± 2.1 | NS | 0.008∗ | NS |
Median ± SD of clinical routine indexes were presented and performed analysis using Mann–Whitney U test between two cohorts. The statistical significance is indicated by asterisks: ∗P < 0.05. NS = difference not significant. DF: dengue without warning signs; DFWS: dengue fever with symptoms; SD: severe dengue; WBC: White blood cells; Hb: hemoglobin; PLT: platelets; HCT: hematocrit; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase.
3.2. Increased frequency of CD56bright NK cells of DF patients during acute DENV infection
Using multicolour flowcytometry, we performed immunophenotyping for evaluating frequencies and absolute counts of lymphocyte subsets including, NK cells, T cells, T helper cells, T cytotoxic cells and B cells.
NK cell numbers and frequency in peripheral blood of in different groups of DENV patients did not differ significantly (Table 2). The frequency and absolute counts of other lymphocyte subsets (T cells, Tc cells, Th cells and B cells) were also comparable to different severity group (Table 2).
Table 2.
Lymphocyte subsets in different dengue severity group.
| DF (n = 58) |
DFWS (n = 38) |
SD (n = 26) |
P value | ||||
|---|---|---|---|---|---|---|---|
| % | Abs counts (/μl) | % | Abs counts (/μl) | % | Abs counts (/μl) | ||
| NK cells (CD56+CD3-) | 8 ± 7.1 | 91 ± 80.4 | 6 ± 7.1 | 53 ± 93.6 | 6 ± 4.1 | 132 ± 93.1 | NS |
| T cells (CD3+) | 68 ± 11.3 | 775 ± 489 | 69 ± 8.7 | 961 ± 643 | 68 ± 11.9 | 809 ± 773 | NS |
| Th cells (CD3+CD4+) | 33 ± 10.5 | 378 ± 314 | 37 ± 10 | 341 ± 418 | 34 ± 10.2 | 441 ± 436 | NS |
| Tc cells (CD3+CD8+) | 26 ± 8.2 | 297 ± 217 | 27 ± 8.9 | 519 ± 242 | 30 ± 13.2 | 419 ± 345 | NS |
| B cells (CD19+) | 18 ± 8.6 | 195 ± 235 | 15 ± 6.5 | 108 ± 205 | 16 ± 9.3 | 294 ± 172 | NS |
Median ± SD of clinical routine indexes were presented and performed analysis using Mann–Whitney U test between two cohorts. The statistical significance is indicated by asterisks: ∗P < 0.05. NS = difference not significant DF: dengue without warning signs; DFWS: dengue fever with symptoms; SD: severe dengue.
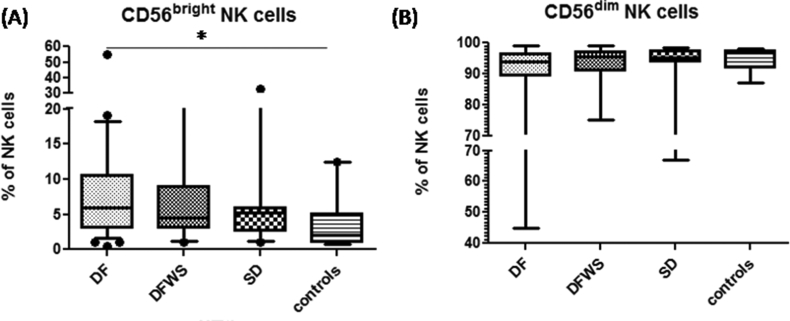
As compared with healthy controls (4 ± 6% of NK cells), DF patients had significantly elevated CD56brightNK cell subset frequency (6 ± 5.1% of NK cells) (p < 0.05); whereas it remained unaltered in DFWS (5 ± 8% of NK cells) and SD (5 ± 8.65% of NK cells) patients. Though CD56dim NK cell subset frequency was decreased in DF patients as compared to healthy subjects, the difference was not significant (p = 0.31) (Figure 2).
Figure 2.
Comparison of (A) CD56brightNK cells and (B) CD56dimNK cell frequencies amongst the different groups of DENV patients classified based on severity. Box-and-whiskers graph. The box extends from the 10th to the 90th percentile and the line at the middle is the median. Mann-Whitney U test was used to evaluate differences between NK cell frequencies in different groups. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. DF: dengue without warning signs; DFWS: dengue fever with symptoms; SD: severe dengue.
3.3. Differential activation of CD56bright and CD56dim NK cells during acute DENV infection is influenced by severity of DENV infection
Activation of NK cells and T cells was evaluated based on expression of CD69 (early activation marker) and HLA-DR (late activation marker) on these cells.
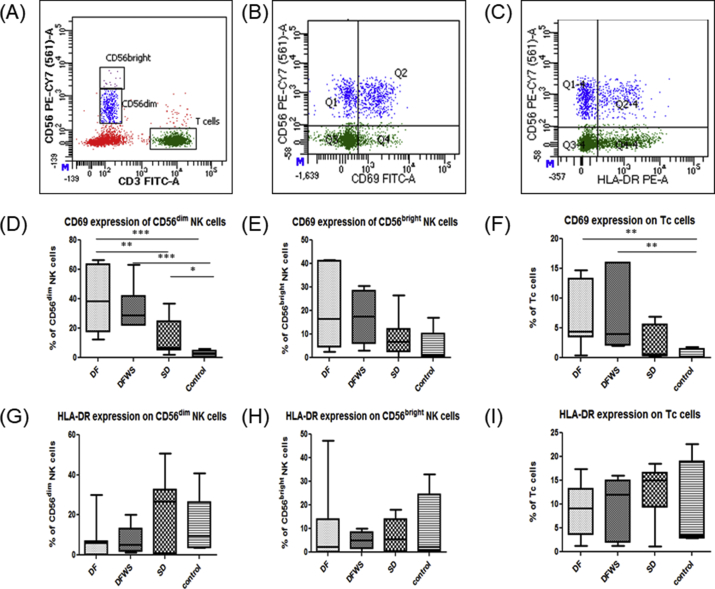
In all DENV patients, irrespective of severity, frequency of CD69 expressing CD56dim NK cells was increased significantly compared to healthy controls (p < 0.01), however, frequency of CD69 expressing CD56bright NK cells did not differ significantly. DF patients had significantly elevated frequency of CD69 expressing CD56dim NK cells compared to SD patients (p < 0.05) (Figure 3D,E).
Figure 3.
Phenotype analysis of activation receptors on peripheral CD56dim NK, CD56bright NK cells and Tc cells. Representative gating FACs plots are shows (A) Gating strategy for CD56bright and CD56dim cells based on intensity of CD56 expression (B) gating for CD69 + NK cells (C) gating for HLA-DR + NK cells. Rest of the graphs are box-and-whiskers graph. The box extends from the 10th to the 90th percentile and the line at the middle is the median. CD69 expression on (D) CD56dim NK cells (E) CD56bright NK cells (F) Tc cells. HLA-DR expression on (G) CD56dim NK cells (H) CD56bright NK cells (I) Tc cells. Mann-Whitney U test was used to evaluate differences between activation markers in different groups. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. DF: dengue without warning signs; DFWS: dengue fever with symptoms; SD: severe dengue.
Though compared to healthy controls, DENV patients had elevated CD69 expression on Tc cells (p < 0.01), it did not differ with severity (Figure 3F).
HLA-DR expression on NK cells as well as on Tc cells irrespective of severity was comparable to healthy controls during acute DENV infection (Figure 3G, H, I).
3.4. Expression of NKp30 expression elevated in mild patients
Equilibrium between the signals from activating and inhibitory NK cell surface receptors controls its function. Thus to explore association of NK cell receptor (NKR) expression pattern with severity of DENV infection, comprehensive immunophenotyping of NK cell subsets was performed which included NKG2D, NKp30, NKp44, NKp46.
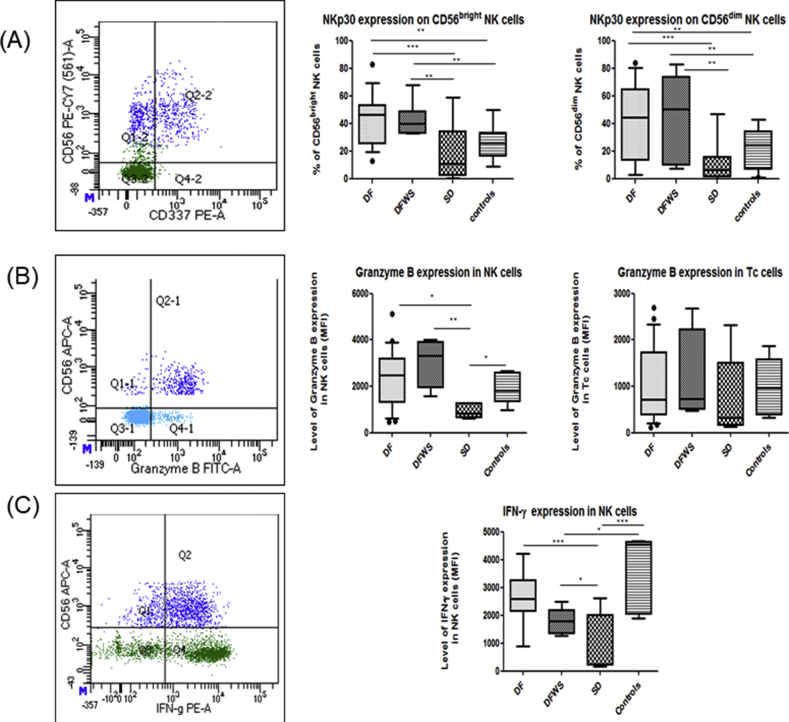
Frequency of NKp30 (CD337) expressing NK cells was significantly elevated in DF and DFWS patients compared to healthy controls and SD patients (p < 0.05) (Figure 4A).
Figure 4.
Comparison of NKp30, Granzyme B and intracellular IFN-γ expression on NK cells amongst the different groups of DENV patients classified based on severity. Representative gating FACs plots are shown on the left hand side. Dark blue color indicates NK cells; Light blue color indicates Th cells; Green color indicates T cells. Box-and-whiskers graph are shown. The box extends from the 10th to the 90th percentile and the line at the middle is the median. (A) NKp30 expression on CD56dim NK cells and CD56bright NK cells, (B) Granzyme B expression on NK cells and Tc cells, (C) Intracellular IFN-γ expression in NK cells. Mann-Whitney U test was used to evaluate differences between activation markers in different groups. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001. DF: dengue without warning signs; DFWS: dengue fever with symptoms; SD: severe dengue.
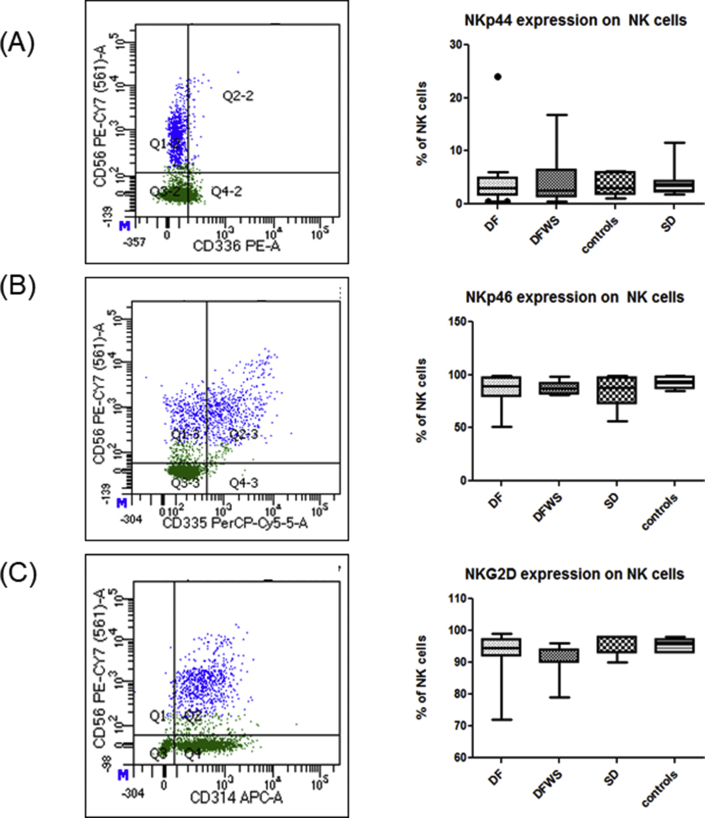
Expression of NKG2D, NKp44 and NKp46 on NK cells of DENV patients was comparable to healthy controls and also was not influenced by severity of DENV-infection (Figure 5).
Figure 5.
Comparison of NKp44, NKp46 and NKG2D expression on NK cells amongst the different groups of DENV patients classified based on severity. Representative gating FACs plots are shown on the left hand side. Dark blue color indicates NK cells; Green color indicates T cells. On the right hand side. Box-and-whiskers graph are shown. The box extends from the 10th to the 90th percentile and the line at the middle is the median. (A) NKp44 expression, (B) NKp46 expression, (C) NKG2D expression. Mann-Whitney U test was used to evaluate differences between activation markers in different groups. ∗P < 0.05, ∗∗P < 0.01, ∗∗∗P < 0.001.
3.5. Impaired NK cell function in severe dengue patients
NK cell function in DENV patients was evaluated by measuring CD107a, (degranulation marker) expression on stimulated NK cells, expression levels of cytolytic granules (perforin and granzymes B) and IFN- γ production.
As the majority of NK cells (>90%) express perforin, granzyme B and IFN-γ, we used the mean fluorescence intensity (MFI) as a measure of protein content per cell to determine if DENV infection modulated granule proteins in NK cells.
Granzyme B and IFN-γ expression in NK cells was significantly elevated in DF patients compared to SD patients (median 2482 vs. 835; 2530 vs. 240 respectively) (p < 0.05). Interestingly, granzyme B expression in Tc cells was comparable to healthy controls and did not differ with severity (Figure 4 B, C).
Compared to healthy controls, DENV patients had significantly elevated Perforin (p < 0.05) and also CD107a expression on stimulated NK cells (p < 0.05); however, it did not correlate with severity.
3.6. NK cells activation and phenotype during progression of DENV disease
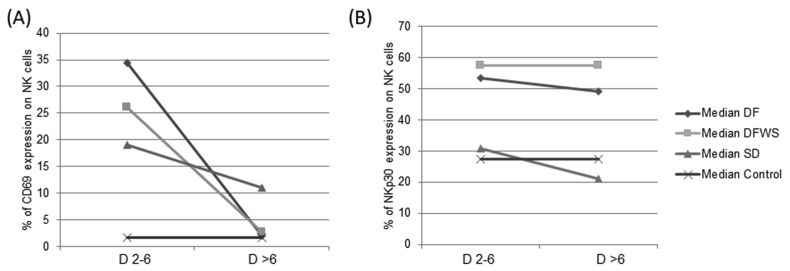
To understand the activation of NK cells during the progression of the disease, CD69 expression on NK cells was evaluated in 40 DENV patients (16 DF; 11 DFWS; 13 SD), during acute phase (day 2–6 of fever) and recovery phase (day >6 of fever).
During acute phase CD69 expression on NK cells was significantly elevated in DENV-infected patients and DF patients had elevated expression compared to DFWS and SD patients. With progression of the disease, NK cell activation in DF and DFWS patients was normalized, however, in SD patients even during recovery phase (>D6) it remained significantly elevated compared to healthy controls but was still lower than that observed during acute phase (Day 2–6 of fever) in DF and DFWS patients (Figure 6A).
Figure 6.
Paired analysis of (A) CD69 and (B) NKp30 expression on NK cells in different DENV patients group during acute phase (day 2–6 of fever) and recovery phase (day >6 of fever) DF: dengue without warning signs; DFWS: dengue fever with symptoms; SD: severe dengue.
Since NKp30 expression on CD56bright NK cells as well as CD56dim NK cells was significantly elevated in DF patients during acute phase, we evaluated this marker on total NK cells during acute as well as recovery phase in DENV patients. During acute as well as recovery phase, NKp30 expression was significantly elevated on NK cells of DF and DFWS patients compared to SD patients and healthy controls. Even post 6 days of infection, NKp30 expression on NK cells of SD patients did not increase and remained comparable to healthy controls (Figure 6B).
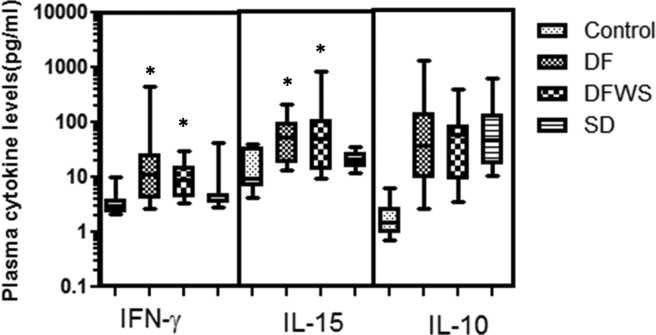
3.7. Elevated IL-15 and IFN-γ levels in mild patients
During the acute DENV-infection, compared to SD patients, DF patients had significantly elevated IL-15 and IFN-γ levels, whereas IL-10 levels did not differ with severity (Figure 7).
Figure 7.
Comparison of IFN-γ, IL-15 and IL-10 amongst the different groups of DENV patients classified based on severity. Box-and-whiskers graph. The box extends from the 10th to the 90th percentile and the line at the middle is the median. Mann-Whitney U test was used to evaluate differences between activation markers in different groups. ∗P < 0.05. DF: dengue without warning signs; DFWS: dengue fever with symptoms; SD: severe dengue; control: healthy control.
4. Discussion
DENV infection has a broad clinical spectrum ranging from undifferentiated DENV fever to life threatening severe disease. Although much is known about this disease, the reason for this variability is largely unknown. The fact that many DENV infected patients even remain asymptomatic post infection, a prompt response of the innate host defense is thought to be the critical limiting step in the infection. The disease outcome not only depends on the virus but also on the host genetics and prior immunity [7, 8, 9]. Moreover, there is increasing evidence from experimental systems of a major role for NK cells in limiting the severity of viral infections [10]. However, data from human infections is limited. Detecting subsets of immune cells is one beneficial way to understand immune function, which may assist in clinical diagnoses of diseases and provide evidence for disease pathogenesis, course, and prognosis. There is evidence that during acute phase of DENV infection, NK cells are activated and undergo repertoire modulation [6, 11], and recently Keawvichit (2017) reported that the patterns of tissue homing molecules of CD56bright and CD56dim subsets of NK cells are different and suggested these might play a critical role in the immune response against acute DENV infection [12]. Thus, to understand if these two major NK cell subsets influence the severity of DENV-disease, in this study, we comprehensively evaluated CD56bright and CD56dim subsets of NK cells and correlated it with severity of the disease during acute infection and progression of disease.
Though few group have shown an increased percentage of NK cells in DENV-infection [13], in our cohort, NK cell numbers and frequency in peripheral blood of DENV patients did not vary significantly. These variations in the findings might be attributed to the difference in the phase of the disease, patient genetic variations and/or different virus genotypes associated with the geographical incidence and age of the patients included in the study.
In our cohort of patients, expansion of CD56brightNK cells was observed in mild DENV patients but not in severe patients. CD56bright NK cells are numerically in the minority in peripheral blood but are abundant cytokine producers and are only weakly cytotoxic. These subset of NK cells are important in both early immune responses and in shaping of the adaptive response (IFN-γ) as well as playing a role of regulatory NK cells (IL-10) and thus are thought to have immunoregulatory properties [14]. Interferon (IFN)-γ secreted by NK cells has shown potent antiviral effects against DENV infection in early phases [15]. In our cohort, during the acute phase, DF patients had significantly elevated IFN-γ levels compared to SD patients, however, IL-10 level did not differ with severity. Thus, the expansion of CD56bright NK cells in mild patients may be contributing to antiviral effects against DENV infection in mild DENV patients which needs to be evaluated further.
Few previous reports suggests that NK cell activation has detrimental role during DENV infection [16], while some reports suggests NK cell activation is beneficial for recovery [11]. To understand the activation of different NK cell subsets and its influence on severity of disease, CD69 expression on CD56bright and CD56dim NK ells was evaluated in these acute DENV patients. Interestingly it was observed that though CD56bright NK cells were expanded in mild patients during acute DENV infection, CD56dim NK cells, and not CD56bright NK cells had upregulated CD69 expression. CD69 is an early activation marker and it mediates NK cell cytotoxicity as well as it regulates other NK-cell functions [16]. In-vitro experiments performed by Olofsson et al (2014) for comparing migration and cytotoxicity in resting and activated NK cells revealed that activated NK cells showed significantly more dynamic migration behaviour and more efficient cytotoxicity compared to resting NK cells [17]. Compared to SD patients, DF patients had significantly elevated CD69 expression on CD56dim NK cells, which indicate more efficient response of NK cells against the DENV. Interestingly, though CD69 expression on Tc cells was also elevated during DENV infection, it did not differ with severity, indicating that it is exclusively NK cells activation during the acute phase of infection contributes in restraining the severity of the disease. Also during progression of the disease, in the recovery phase, NK cell activation in mild patients was normalized whereas in severe patients it remained elevated. However, even post-6 days of DENV infection, CD69 expression on NK cells of severe patients was lower than that seen during acute phase in mild patients. An increase in CD69 represents an immunoreactive phenotype and the expression is accompanied by an enhanced cytotoxicity against various target cells. Thus, increased CD69 expression on NK cells might indicate good prognosis and the organ involvement and severe clinical manifestations in SD patients could be attributed to the impaired NK cell activation.
Upon activation of NK cells in response to infection, NK cells employ three main strategies namely, the production of cytokines, the secretion of cytolytic granules and the use of death receptor-mediated cytolysis [18]. Production of IFN-γ is an important effector function of activated NK cells. During acute infection, significantly elevated intracellular IFN-γ expression in NK cells of DF patients was observed compared to DFWS and SD patients. In support of this, the level of granzyme B expression in DF patients was also elevated in DF patients compared to severe groups. Granzyme B belongs to a family of serine proteases promoting cytotoxic lymphocytes mediated eradication of intracellular pathogens via the induction of cell death and elevated Granzyme B expression in NK cells is a signature of immune activation [19]. Thus, this overall highlights the enhanced effector function of NK cells in DF patients.
Killing of virus-infected cells is also orchestrated by the balance between the signals derived from inhibitory/activating receptors [4, 20]. The existence of a direct protein-protein interaction between recombinant DENV soluble envelope E protein and NKp44 (but not NKp30 or NKp46) that could be involved in the triggering of cytolysis has been reported [21]. However, in our cohort, NKp44 expression was comparable to the healthy controls. Interestingly, it was observed that NKp30 (NK cell cytotoxicity receptor) was significantly elevated in DF patients whereas in SD patients remained unaltered. Even post-6 days of infection, SD patients had NKp30 expression comparable to healthy controls. NKp30 is considered as surrogate marker of NK cell functions in humans that regulates the dendritic and NK cell cross-talk, and mediates the release of Th1 cytokines [22, 23]. These NK cell–dendritic cell interactions are essential for both NK cell-mediated apoptosis as well as maturation of dendritic cells [24, 25]. During DENV infection, dendritic cells have dual role as both targets of DENV replication and mediators of innate and adaptive immunity. The cross-talk between NK cells and dendritic cells during DENV infection is vital for both clearances of infected dendritic cells as well as initiating adaptive immune response. So, NKp30 is a triggering receptor downstream of adhesion and plays an important role in NK cell activation, degranulation and cytotoxicity. Thus, failure to elevate NKp30 expression in SD patients during acute DENV-infection causes impaired NK cell function in these patients, contributing to severe disease. Cytokine IL-15, which is known to upregulate NKp30 expression on NK cells [26], was also significantly elevated in DF patients compared to severe patients. Recently, IL-15 was reported to up-regulate NKp30 expression and recovers NK cell function in acute myeloid leukemia (AML) patients supporting the use of IL-15 as a platform for NK- based therapies for AML patients. Thus, further in-vitro studies to understand modulation of NKp30 expression post-DENV infection and effect of IL-15 may contribute in designing future immunotherapeutic strategies for severe DENV-disease.
In conclusion, our study reports efficient NK cell response during acute phase of DENV infection is crucial for preventing severity of disease. Though there are various studies in in-vitro models and few in human populations, suggesting role of NK cells during DENV infection; to the best of our knowledge, this is the first study revealing the association between NK cell response and severity of DENV disease during the progression of disease. Inevitably, our results contribute in understanding the dynamics of NK cell response in immunopathogenesis of DENV infection and provide a platform for future pathogenesis-oriented studies. These studies will certainly contribute in development of efficacious therapeutics as well as vaccines against DENV infection.
Declarations
Author contribution statement
Snehal Shabrish, Manisha Madkaikar: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Niteen Karnik: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Vishal Gupta, Priya Bhate: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Science Engineering research board (SERB) – Department of science and technology (DST) [YSS/2015/002029] and Indian Council of Medical Research (ICMR).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Hasan S., Jamdar S.F., Alalowi M., Al Ageel Al Beaiji S.M. Dengue virus: a global human threat: review of literature. J. Int. Soc. Prev. Community Dent. 2016;6:1–6. doi: 10.4103/2231-0762.175416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rezeki Hadinegoro S.S. Paediatrics and International Child Health the revised WHO dengue case classification: does the system need to be modified? Paediatr. Int. Child Health. 2013;32:33–38. doi: 10.1179/2046904712Z.00000000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uno N., Ross T.M. Dengue virus and the host innate immune response. Emerg. Microb. Infect. 2018;7:167–177. doi: 10.1038/s41426-018-0168-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cooper M.A., Fehniger T.A., Caligiuri M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–640. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 5.Biron C.A. Initial and innate responses to viral infections–pattern setting in immunity or disease. Curr. Opin. Microbiol. 1999;2:374–381. doi: 10.1016/s1369-5274(99)80066-6. [DOI] [PubMed] [Google Scholar]
- 6.Petitdemange C. Longitudinal analysis of natural killer cells in dengue virus-infected patients in Comparison to chikungunya and chikungunya/dengue virus-infected patients. PLoS Neglected Trop. Dis. 2016;10 doi: 10.1371/journal.pntd.0004499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hapuarachchi H.C. Clinical outcome and genetic differences within a monophyletic dengue virus type 2 population. PloS One. 2015;10 doi: 10.1371/journal.pone.0121696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffey L.L. Human genetic determinants of dengue virus susceptibility. Microb. Infect. 2009;11:143–156. doi: 10.1016/j.micinf.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Gibbons R.V. Analysis of repeat hospital admissions for dengue to estimate the frequency of third or fourth dengue infections resulting in admissions and dengue hemorrhagic fever, and serotype sequences. Am. J. Trop. Med. Hyg. 2007;77:910–913. [PubMed] [Google Scholar]
- 10.Arase H., Lanier L.L. Virus-driven evolution of natural killer cell receptors. Microb. Infect. 2002;4:1505–1512. doi: 10.1016/s1286-4579(02)00033-3. [DOI] [PubMed] [Google Scholar]
- 11.Azeredo E.L. NK cells, displaying early activation, cytotoxicity and adhesion molecules, are associated with mild dengue disease. Clin. Exp. Immunol. 2006;143:345–356. doi: 10.1111/j.1365-2249.2006.02996.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keawvichit R. Differences in activation and tissue homing markers of natural killer cell subsets during acute dengue infection. Immunology. 2018;153:455–465. doi: 10.1111/imm.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gandini M. Dengue virus induces NK cell activation through TRAIL expression during infection. Mediat. Inflamm. 2017:5649214. doi: 10.1155/2017/5649214. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poli A. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology. 2009;126:458–465. doi: 10.1111/j.1365-2567.2008.03027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suwannasaen D., Romphruk A., Leelayuwat C., Lertmemongkolchai G. Bystander T cells in human immune responses to dengue antigens. BMC Immunol. 2010;11:47. doi: 10.1186/1471-2172-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green S. Early CD69 expression on peripheral blood lymphocytes from children with dengue hemorrhagic fever. J. Infect. Dis. 1999;180:1429–1435. doi: 10.1086/315072. [DOI] [PubMed] [Google Scholar]
- 17.Olofsson P.E. Distinct migration and contact dynamics of resting and IL-2-activated human natural killer cells. Front. Immunol. 2014;5:80. doi: 10.3389/fimmu.2014.00080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S.-H., Miyagi T., Biron C.A. Keeping NK cells in highly regulated antiviral warfare. Trends Immunol. 2007;28:252–259. doi: 10.1016/j.it.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Mellor-Heineke S. Elevated granzyme B in cytotoxic lymphocytes is a signature of immune activation in hemophagocytic lymphohistiocytosis. Front. Immunol. 2013;4:72. doi: 10.3389/fimmu.2013.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marras F., Bozzano F., De Maria A. Involvement of activating NK cell receptors and their modulation in pathogen immunity. J. Biomed. Biotechnol. 2011;2011:152430. doi: 10.1155/2011/152430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hershkovitz O. NKp44 receptor mediates interaction of the envelope glycoproteins from the West Nile and dengue viruses with NK cells. J. Immunol. (Baltimore, Md.: 1950) 2009;183:2610–2621. doi: 10.4049/jimmunol.0802806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferlazzo G. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borg C. NK cell activation by dendritic cells (DCs) requires the formation of a synapse leading to IL-12 polarization in DCs. Blood. 2004;104:3267–3275. doi: 10.1182/blood-2004-01-0380. [DOI] [PubMed] [Google Scholar]
- 24.Pende D. Identification and molecular characterization of NKp30, a novel triggering receptor involved in natural cytotoxicity mediated by human natural killer cells. J. Exp. Med. 1999;190:1505–1516. doi: 10.1084/jem.190.10.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moretta L. Human NK cells and their receptors. Microb. Infect. 2002;4:1539–1544. doi: 10.1016/s1286-4579(02)00037-0. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Correa B. In vitro culture with interleukin-15 leads to expression of activating receptors and recovery of natural killer cell function in acute myeloid leukemia patients. Front. Immunol. 2017;8:931. doi: 10.3389/fimmu.2017.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]