Abstract
Talaromyces marneffei infection is an endemic opportunistic infection for immunodepression patients, especially HIV-positive patients. Our case describes an unendemic and HIV-negative patient who presented with fever, subcutaneous mass, osteolytic destruction of the skull and subcutaneous abscess penetrating the diseased skull. The growth of Talaromyces marneffei was identified by the culture of the frontal pus, sputum, blood and bone marrow. Due to severe nausea and vomiting during the use of amphotericin B, voriconazole was finally selected for treatment. Two weeks after intravenous infusion of voriconazole injection, the patient was given oral voriconazole tablets for 5 months. After the initial intravenous treatment of voriconazole, the patient developed increased dyspnea and required ventilator support with endotracheal intubation, and methylprednisolone was given intravenously for 5 days. All lesions absorbed and no obvious discomfort was found during the follow-up at the third month after discharge. At present, the patient has been followed up for more than 3 years without recurrence. The case aims to raise doctors’ awareness of this rare disease in non-endemic areas and HIV-negative patients.
Keywords: Talaromyces marneffei, T. marneffei, HIV-negative, osteolysis destruction
Introduction
Talaromyces marneffei is a dimorphic fungus which has been an important opportunistic infection in immunocompromised individuals, especially in human immunodeficiency virus (HIV)-positive individuals.1 In recent years, the number and proportion of T. marneffei infection have been increasing in HIV-negative patients with other immunocompromised conditions.2 Moreover, some studies have shown that neutralizing anti–interferon-γ autoantibodies (nAIGAs) may play an important role in the pathogenesis of HIV-negative T. marneffei infection and may be related to more serious refractory infections and relapses.3 We describe the case of an HIV-negative patient who presented with fever, pulmonary mass, skull osteolysis and frontal mass. Finally, the diagnosis of T. marneffei was confirmed by the culture of pus, sputum, blood and bone marrow. He developed acute respiratory failure after 3 days of antifungal therapy. With five days intravenous methylprednisolone, all symptoms improved gradually.
Case presentation
A 55-year-old Chinese man presented in 2016 with a 2-month history of cough and expectoration, increasing shortness of breath, headache and hoarseness for 1 week. He was a farmer who grew up in a small town in Zhejiang province and worked in Guangzhou for 2 years 20 years ago. He took unknown components for relief of his headache 2 months before admission and found forehead scalp mass 1 month before admission. He has no history of dirty sex and blood transfusions. In addition to these, there were no other diseases history.
Physical examination revealed a 3-cm ill-defined tough mass in the right forehead and he was febrile at 38.5°C. Examination of his respiratory tract found decreased breathing sounds in his lower left lung. Other systems were unremarkable. Oxygen saturation on pulse oximetry was 97% on air.
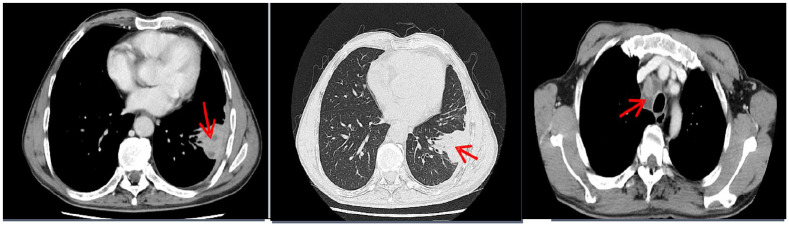
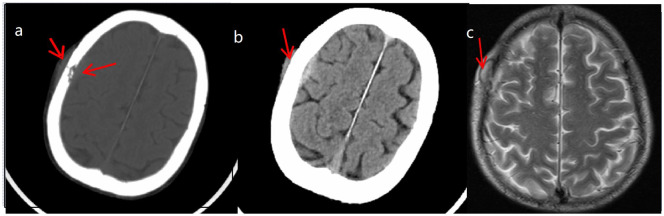
Laboratory studies showed a hemoglobin concentration of 122 g/dL, leukocyte count of 27,900/mm3, with a differential count of 87.3% neutrophils (24,400/mm3) and 6.7% lymphocytes (18,700/mm3), platelet count of 243,000/mm3, erythrocyte sedimentation rate of 78 mm/h, C-reactive protein (CRP) of 184.9 mm/L, plasma albumin of 28 g/L, serum procalcitonin of 0.611 ng/mL, the patient was HIV-negative (third-generation reagent, detection of enzyme-linked immunosorbent assay (ELISA) method and chemical luminescence method for serum HIV-1/HIV-2 antibody) and also negative for hepatitis B virus and syphilis pathogens. Levels of natural killer cells were 63.1% (11,800/mm3), CD4+ T-cells 23.6% (2785/mm3), CD8+ T-cells 35% (4130/mm3) and CD4+/8+ ratio 0.67. Pastorex aspergillus testing with patient’s serum, including galactomannan testing and fungi 1-3-β-D glucan testing, was negative. Acid-fast bacillus test of sputum smear was negative. Purified protein derivative (PPD) test was negative. Chest contrast-enhanced computed tomography (CT) scans revealed high-density shadow on left lower lobe, obvious enhancement and mediastinal lymph node enlargement with necrosis (Figure 1). Cranial CT (Figure 2(a) and (b)) and magnetic resonance imaging (MRI) (Figure 2(c)) showed bone destruction of the right frontal bone with local soft tissue shadow penetrating the damaged area. The main manifestations of bone damage are osteolytic destruction and bone loss (Figure 2). No osteolytic damage was found in the vertebrae on chest CT. Whether there was osteolytic damage in other parts was not further evaluated in this case. The endobronchial ultrasound-guided transbronchial needle aspiration and the CT-guided percutaneous needle aspiration all showed acute and chronic inflammation pathological change, but no tumor cell was found. At this point, the culture of blood, sputum, mediastinal lymph node puncture and lung puncture were all free of pathogenic bacteria.
Figure 1.
Chest contrast-enhanced CT (on admission) showed high-density shadow on left lower lobe, obvious enhancement and mediastinal lymph node enlargement with necrosis.
Figure 2.
(a, b) Cranial CT and (c) cranial MRI (on admission) absorption damage associated with soft tissue shadows on the right side of the frontal bone.
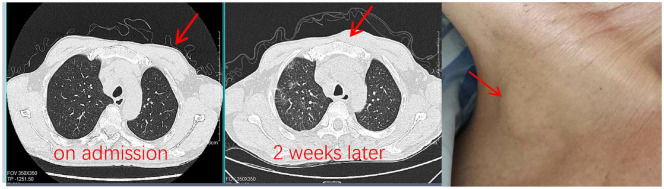
The patient was given meropenem intravenously after admission. But he was still feverish with the maximum temperature close to 40°C. Leukocyte count and CRP increased continuously, and hemoglobin concentration and plasma albumin decreased progressively. At the same time, new subcutaneous mass was found in the right neck and left chest wall (Figure 3).
Figure 3.
New masses appeared on his left chest wall and right neck.
On the 14th day of admission, bone marrow puncture and biopsy were performed. The smear results were negative, and the bone marrow culture was continued. The diagnosis was still unclear. On the 16th day of admission, when the patient’s frontal mass was found to have a sense of fluctuation, we took about 3 mL of pus (Figure 4) to the laboratory for smear and culture. Gram smears and smears for fungi and acid-fast bacilli were all negative.
Figure 4.
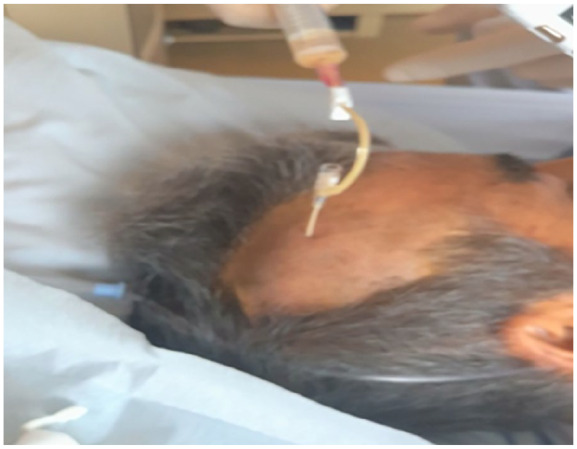
Percutaneous puncture of the frontal mass.
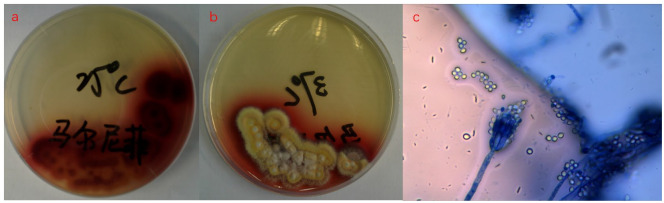
T. marneffei was isolated from pus after 4 days of pus culture. On the same day, T. marneffei was isolated from bone marrow culture, blood culture and sputum culture. Figure 5(a) shows fungal culture of pus yielded mycelia at 25°C with production of red pigment; Figure 5(b) shows yeast phase at 37°C with decreased production of red pigment; and Figure 5(c) shows sausage and broom dendrites were observed after Gram staining at 25°C under the microscope (400×).
Figure 5.
(a) Fungal culture of pus yielded mycelia at 25°C with production of red pigment; (b) yeast phase at 37°C with decreased production of red pigment; and (c) sausage and broom dendrites were observed after Gram staining at 25°C under the microscope (400×).
Due to severe nausea and vomiting, the patient could not tolerate intravenous injection of amphotericin B. On the 22nd day of admission, he was started on intravenous voriconazole 400 mg twice a day, changed to 200 mg twice a day the next day. After 3 days of antifungal treatment, the patient’s temperature decreased to the normal range, his subcutaneous mass decreased significantly, but acute respiratory distress occurred and the oxygen index was less than 200. We excluded possible complications, including pulmonary embolism, acute heart failure and pneumothorax. Intubation and ventilator ventilation were performed immediately. Chest CT scan showed bilateral diffuse exudative lesions and left lower lung mass was significantly absorbed when compared with admission. We continued to use voriconazole and intravenous injection of 40 mg methylprednisolone once a day for 5 days. The patient stopped the ventilator and removed the endotracheal tube on the fourth day after intubation. After 2 weeks of injection treatment, he was discharged with oral voriconazole 200 mg twice daily for 5 months. During the follow-up of third month after discharge, the lesions were completely absorbed. Considering the bone destruction of the patient, we suggested that the patient continue to take drugs for 2 months. At present, the drug treatment has been stopped for more than 3.5 years, and he has no discomfort. The telephone follow-up is continuing.
Discussion
T. marneffei (previously named Penicillium marneffei) is an endemic organism in Southeast Asia and Southern China. It is a dimorphic fungus exhibiting a mycelial form at 25°C and yeast form at 37°C. The mycelial form produces a diffusible red pigment in culture.1,4 The yeast cells reproduce by fission rather than by budding, which serves as an aid for diagnosis.4 T. marneffei infection is a common but serious opportunistic fungal infection in Acquired Immune Deficiency Syndrome (AIDS) patients. The way humans get T. marneffei is unclear. Some subspecies of Bamboo rats have been identified as organism reservoirs, but how humans get the organism from rats or other ways is still unidentified.5 Contact with T. marneffei endemic region is a key risk factor.6 From Zheng et al.’s7 report, we know that most patients present with the onset of symptoms 6–12 months after leaving the endemic areas; the longest incubation period we saw from PubMed literature is 10 years. In our case, the patient worked in Guangdong for 2 years 20 years ago. We think he may have contracted the pathogen at that stage. According to the current report, he may have the longest incubation period. The pathogenesis is not clear. Most experts believe that this may be related to the decline of immunity. Some studies have shown that nAIGAs may play an important role in the pathogenesis of HIV-negative T. marneffei infection and may be related to more serious refractory infections and relapses.3 Immunodeficiency due to anti-IFN-γ autoantibodies is an emerging adult-onset immunodeficiency syndrome first described in 2004.8,9 The association between anti-IFN-γ autoantibodies and T. marneffei infection was first described among eight Chinese patients living in Hong Kong by Chan et al.2 T. marneffei infections in patients with anti-IFN-γ autoantibodies usually manifest as fever of unknown origin, cervical lymphadenitis and/or mild symptomatic infection with positive serology.10 The patient in our case did not find any diseases or factors leading to decreased immunity. Due to limited testing, no anti-IFN-γ autoantibodies testing was carried out. At the time of hospitalization, we may not be able to rule out whether there are potential or hidden diseases (such as cancers or HIV infection window period), or whether there is immune deficiency syndrome caused by anti-IFN-γ autoantibodies. It has been nearly 4 years since the onset of the disease in March 2016. The patient has no relapse and the HIV antibody is negative repeatedly checked by ELISA method and chemical luminescence method. The latest visit time is 30 April 2020. The patient had no symptoms and continued to engage in agricultural physical labor 6 months after discharge. We can exclude the possibility of tumor and HIV infection now. Since no anti-IFN-γ autoantibodies were detected, the antibody-related immunodeficiency syndrome cannot be completely ruled out. We reviewed the literature and found that a 46-year-old woman who had positive anti-IFN-γ autoantibodies found a recurrence in 6 years follow-up after treatment.11 We will continue to follow-up the patient, and if there is any test condition, we will carry out anti-IFN-γ autoantibodies test as early as possible.
The common clinical features included fever, weight loss, anemia, generalized lymphadenopathy, hepatosplenomegaly, pulmonary involvement, mucosal ulcers and skin lesions.12,13 Osteolytic destruction caused by infection with T. marneffei is extremely rare.14 In Qiu et al.’s13 report, osteolytic destruction of T. marneffei infection mainly occurs in HIV-negative patients, often involving multiple parts of the skeleton, showing osteolytic destruction and bone loss. Bone changes often suggest that the disease is more serious, the recurrence rate is higher, the need for longer courses of antifungal treatment and poor prognosis. In our case, the patient has most of the above clinical manifestations. Particularly, osteolytic destruction of the skull and subcutaneous abscess penetrating the diseased skull have not been reported in other cases. Unfortunately, in the course of diagnosis and treatment of the disease, we have neglected to evaluate whether other parts of the skeleton are involved.
Whether it is clinical manifestations, imaging manifestations or plasma antigen detection, only fungal culture is the gold standard for diagnosis, which generally takes 3–7 days to grow. Zheng et al.’s7 study showed the delay of the diagnosis for P. marneffei and the absence of antifungal treatment independently predicted the early mortality of the patients. An early diagnosis and a standardized antifungal treatment are most important to improve the prognosis. Amphotericin B is the first-line treatment for T. marneffei infection. But our patient was unable to tolerate amphotericin B treatment due to severe nausea and vomiting. We chose voriconazole for the treatment because the literature review showed it is an effective and convenient drug for intravenous and oral sequential therapy.15 Later treatment process confirmed that our treatment options are effective and feasible. Bone destruction, subcutaneous mass and lung changes were completely absorbed in the third month after discharge. The patients continue to take voriconazole tablets for 2 months. However, the treatment cycle for antifungal drugs in these patients has not been clearly reported in the literature and is still in the exploration stage.
It is worth mentioning that after 3 days of antifungal treatment, the patient was observed a sharp increase in inflammatory response, but the original lesions improved. After 5 days of glucocorticoid use, diffuse exudative lesions were absorbed. We speculate that this change might be related to fungal death and release of inflammatory mediators under effective antifungal therapy. But there is no similar literature support. We hope there will be more relevant literature and research in the future.
Throughout the whole diagnosis and treatment process, we found that there were omissions. The initial blood culture, sputum culture and puncture culture were all negative. This may be related to the lack of effective communication between clinicians and microbiologists, resulting in the use of only common microbial culture methods, ignoring the possibility of special bacterial growth.
Conclusion
For patients with recurrent fever, lung mass and bone destruction, in addition to tumor, the possibility of T. marneffei and other special infectious diseases should be considered, and timely communication with colleagues in the microbiological laboratory should be made in order to select a more effective microbiological culture method. The case aims to raise doctors’ awareness of this rare disease in non-endemic areas and HIV-negative patients.
Acknowledgments
The author thanks the patient for giving his consent for publication of this case and also thanks the support of colleagues in the microbiology lab and ICU.
Footnotes
Declaration of conflicting interests: The author declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported by the Natural Science Project of Department of Education of Zhejiang Province (Grant No. Y201738604).
Ethical approval: Ethical approval to report this case was obtained from the Human Research Ethics Committee of the Second Affiliated Hospital of Zhejiang University Medical College (Approval No. 2019-043).
Informed consent for publication: Written informed consent was obtained from the patient for publication of this case and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
ORCID iD: Youfei Chen  https://orcid.org/0000-0003-3253-2573
https://orcid.org/0000-0003-3253-2573
References
- 1. Deng Z, Connor D. Progressive disseminated penicilliosis cause by Penicillium marneffei. Report of eight cases and differentiation of the causative organism from Histoplasma capsulatum. Am J Clin Pathol 1985; 84: 323–327. [DOI] [PubMed] [Google Scholar]
- 2. Chan JF, Lau SK, Yuen KY, et al. Talaromyces (Penicillium) marneffei infection in non-HIV-infected patients. Emerg Microbes Infect 2016; 5(3): e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zeng W, Qiu Y, Tang S, et al. Characterization of anti-interferon-γ antibodies in HIV-negative patients infected with disseminated Talaromyces marneffei and Cryptococcosis. Open Forum Infect Dis 2019; 6(10): ofz208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chaiwun B, Khunamornpong S, Sirivanichai C, et al. Lymphadenopathy due to Penicillium marneffei infection: diagnosis by fine needle aspiration cytology. Mod Pathol 2002; 15(9): 939–943. [DOI] [PubMed] [Google Scholar]
- 5. Deng Z, Ribas J, Gibson D, et al. Infection caused by Penicillium marneffei in China and Southeast Asia:review of eighteen published cases and report of four more Chinese cases. Rev infect 1988; 10: 640–652. [DOI] [PubMed] [Google Scholar]
- 6. Zeng W, Qiu Y, Lu D, et al. A retrospective analysis of 7 human immunodeficiency virus-negative infants infected by Penicillium marneffei. Medicine 2015; 94(34): e1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zheng J, Gui X, Cao Q, et al. A clinical study of acquired immunodeficiency syndrome associated Penicillium marneffei infection from a non-endemic area in China. PloS One 2015; 10(6): e0130376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hoflich C, Sabat R, Rosseau S, et al. Naturally occurring anti-IFN-gamma autoantibody and severe infections with Mycobacterium cheloneae and Burkholderia cocovenenans. Blood 2004; 103: 673–375. [DOI] [PubMed] [Google Scholar]
- 9. Doffinger R, Helbert MR, Barcenas-Morales G, et al. Autoantibodies to interferon-gamma in a patient with selective susceptibility to mycobacterial infection and organ-specific autoimmunity. Clin Infect Dis 2004; 38: e10–e14. [DOI] [PubMed] [Google Scholar]
- 10. Tang BS, Chan JF, Chen M, et al. Disseminated penicilliosis, recurrent bacteremic nontyphoidal salmonellosis, and burkholderiosis associated with acquired immunodeficiency due to autoantibody against gamma interferon. Clin Vaccine Immunol 2010; 17: 1132–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Liang X, Si L, Li Y, et al. Talaromyces marneffei infection relapse presenting as osteolytic destruction followed by suspected nontuberculous mycobacterium infection during 6 years of follow-up: a case update. Int J Infect Dis 2020; 93: 208–210. [DOI] [PubMed] [Google Scholar]
- 12. Chitasombat M, Supparatpinyo K. Penicillium marneffei infection in immunocompromised host. Curr Fungal Infect Rep 2013; 7: 44–50. [Google Scholar]
- 13. Qiu Y, Zhang J, Liu G, et al. Retrospective analysis of 14 cases of disseminated Penicillium marneffei infection with osteolytic lesions. BMC Infect Dis 2015; 15: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu GN, Huang JS, Zhang JQ, et al. A penicillium marneffei infection within an osteolytic lesion in a HIV-negative patient. Int J Infect 2014; 23: 1–3. [DOI] [PubMed] [Google Scholar]
- 15. Cao C, Xi L, Chaturvedi V. Talaromycosis (Penicilliosis) due to Talaromyces (Penicillium) marneffei: insights into the clinical trends of a major fungal disease 60 years after the discovery of the pathogen. Mycopathologia 2019; 184: 709–720. [DOI] [PubMed] [Google Scholar]