Abstract
Background:
Bronchoscopic lung volume reduction (BLVR) via valve implantation can be achieved by targeting severely hyperinflated and emphysematously destructed lung areas in patients with chronic obstructive lung disease. Lack of collateral ventilation (CV) is important for good outcomes with BLVR. CV can be measured using the catheter-based Chartis system. The aim of this study was to evaluate the correlation between total exhaled volume drained from the target lobe measured by Chartis and clinical outcomes after BLVR in CV-negative patients.
Methods:
From January 2016 to March 2019, 60 patients were included in this retrospective single-center analysis. Drained volume (TVol) measured by Chartis was recorded and compared with lung function and physical performance parameters. Outcome variables included the percentage change in lung function [forced expiratory volume in 1 s (FEV1), residual volume (RV), and inspiratory vital capacity (IVC)]. Secondary outcomes were the degree of target lobe volume reduction (TLVR), change in 6-min walk distance (6MWD), and change in chronic obstructive pulmonary disease (COPD) assessment test (CAT) score.
Results:
Drained volume correlated significantly with post-BLVR change in FEV1 (r = 0.663), IVC (r = 0.611), RV (r = −0.368), and TLVR (r = 0.635) (all p < 0.05). In a priori-defined patient subgroups based on drained volume [<100 ml (n = 19), 100−400 ml (n = 33), and >400 ml (n = 8)]; mean changes in FEV1 were 2.6%, 17.4%, and 51.3%; in RV were −3.9%, −10.6%, and −23.8%; in IVC were −4.0%, 10.6%, and 62.4%; and in TLVR were 525 ml (39%), 1375 ml (73%) and 1760 ml (100%), respectively. There were no significant correlations between absolute and percentage changes in 6MWD and the CAT score. Lung volume reduction was diagnosed in 32 (53%) cases.
Conclusion:
Drained volume measured by the Chartis system correlated with functional improvement in CV-negative patients undergoing BLVR.
The reviews of this paper are available via the supplemental material section.
Keywords: bronchoscopic lung volume reduction, Chartis, collateral ventilation, emphysema
Introduction
Chronic obstructive pulmonary disease (COPD) is a global epidemic with various clinical manifestations.1 Emphysema is characterized by major pathological changes in lung tissue. Bronchoscopic lung volume reduction (BLVR) with valves has been shown to decrease hyperinflation and increase lung function, exercise capacity, and quality of life in patients with emphysema.2–7
The success of BLVR is heavily dependent on the selection of patients without collateral ventilation (CV) to the supplied lobe.8 There are currently two diagnostic methods to assess CV. The first is visual analysis of high-resolution quantitative computer tomography (QCT), which facilitates more efficient patient selection. Internet cloud-based platforms such as StratX and VIDA Diagnostics are established suppliers for QCT analysis.9,10 Recently, semi-automatic single photon emission computed tomography (SPECT)/CT analysis has also been shown to successfully quantify target lobe volumes along with ventilation and perfusion.11 The second is the catheter-based Chartis System (Pulmonx, Inc., Redwood City, CA, USA), which compares expiratory flow with resistance pressure.12–14 The importance of CV in determining the outcome of BLVR was not well understood when early clinical trials were designed, meaning that only modest improvements were documented in the first prospective randomized controlled trial (RCT) of BLVR.15 The absence of CV is the most important prerequisite for treatment success when using unidirectional valves for lung volume reduction.
Although QCT fissure analysis is a less invasive method of determining the CV status of a patient, combining this with findings obtained using the catheter-based Chartis system results in a high level of accuracy for the detection of CV (almost 90%), particularly in cases where visual CV assessment remains unclear.16 However, there are currently no published studies looking at the relationship between individual Chartis measurements and clinical outcomes, such as change in lung function. Lung volume, as indicator of space and quantity (assessed primarily using CT imaging), is an important factor when considering therapy options for patients with emphysema and COPD. Patients with high-volume emphysema [residual volume (RV) >200%] are thought to benefit more from BLVR.17 Clinical observations suggest that the drained volume expressed in milliliters is related to the degree of hyperinflation, and, therefore, to the severity of the emphysema. This means that patients with high grades of the disease, in particular, show larger drained volumes and also gain a greater therapeutic benefit. The predictive value of drained volume has not yet been identified.
The aim of this study was to explore the correlation of total exhaled volume (TVol) drained from the target lobe measured by Chartis with clinical outcomes after BLVR, determined by changes in pulmonary function, physical performance, and target lobe volume reduction.
Methods
Study design and patient selection
This retrospective single-center study included patients who had a Chartis measurement prior to valve implantation at the department of interventional pneumology of the University Medicine Essen-Ruhrlandklinik between January 2016 and March 2019. Patients were excluded if they were not undergoing physical rehabilitation, if the target lobe for valve implantation was not directly measured with the Chartis system, if a therapy option other than endobronchial valves (EBV; e.g., coils) was used, or if the measurement was positive for CV.
Patients with forced expiratory volume in 1 s (FEV1) <40% predicted, and RV > 200% predicted were considered for BLVR treatment with EBV (Zephyr© Endobronchial Valve; Pulmonx, Inc.). Additional diagnostic methods in all patients included CV assessment with CT, QCT fissure analysis (StratX© software, Pulmonx, Inc.) and Chartis measurement, as well as perfusion scan and 6-min walk test (6MWT). Chartis measurements with low volumes of <50 ml and high volumes of >750 ml were considered for treatment if the curve pattern and QCT analysis were consistent with CV-negative properties. Treatment decision for each patient was made after multidisciplinary board discussion with all involved specialties including thoracic surgeons, interventional pulmonologists, and radiologists. Lung function testing and 6MWT were performed routinely at 3 and 6 months after BLVR, and follow-up CT scan was performed at 3 months post-procedure. The study protocol was approved by the research institute’s Committee on Human Research and all patients provided written, informed consent for BLVR treatment.
Chartis CV analysis
The Chartis system is based on an endobronchial catheter to access the most severely hyperinflated emphysematous lung areas identified with prior high-resolution CT scan and QCT lung report. With the use of this catheter, a balloon is inflated within a lobar bronchus to occlude the lumen and measure expiratory flow (ExpF), inspiratory pressure, and their ratio [expressed as resistance (Rndx)]. Declining flow and a concomitant rise in resistance indicates CV negativity. The TVol of the target lobe was measured and expressed next to the flow/resistance graph within the console. All measurements were performed with flexible bronchoscopy in spontaneously breathing patients. For local anesthesia and sedation, 10–20 ml of 1% lidocaine, 10–40 mg bolus doses of propofol, and up to 5 mg midazolam were applied.
Quantitative computed tomography
Optimal pre-BLVR CV assessment is a two-step process using the Chartis measurement and a QCT analysis based on a high-resolution CT scan, allowing evaluation of lobe volumes, the degree of emphysema, heterogeneity, and the interlobular fissure. Patients with a fissure integrity of <80% are usually not considered for LVR with valves. Another HR-CT was done to measure the volume of the targeted lobe after BLVR, and the QCT was prospectively analyzed to determine TLVR.
Outcome variables and data sources
We analyzed Chartis measurements for total volume drained from the target lobe and a CV-negative result was based on the time of flow until sustained resistance of ⩾10 cmH20 × s/ml. Primary outcomes were the relative (percentage) changes from baseline in FEV1, RV, and inspiratory vital capacity (IVC). Secondary outcomes were the absolute and percent change in distance on the 6MWT (6MWD), change in COPD assessment test (CAT) score, and volume reduction (VR) of the target lobe. We recorded the best-achieved value within an interval of 6 months after valve implantation. All parameters were obtained from the database of the hospital information system. Lung function parameters (FEV1, RV, IVC) were measured using body plethysmography according to the clinical routine. The 6MWT was performed according to a standard protocol, with information about minimum oxygen saturation, usage of walking aid devices, and supplemental oxygen in liters. The CAT score was assessed using a questionnaire. Partial atelectasis and full atelectasis were radiologically assessed using a chest radiograph and CT. A positive outcome was identified when full atelectasis was observed or when there was a rise of diaphragm or an ipsilateral mediastinal shift indicating successful volume reduction. With the post-BLVR QCT, both TLVR and the degree of atelectasis could be identified.
Statistical analysis
Correlations between the drained volume from the target lobe and lung function and TLVR variables were evaluated using the Pearson’s correlation coefficient (r). Multiple regression with backward elimination using a significance level of 0.10 was performed to identify variables predicting changes in the lung function parameters and TLVR. Variables included were TVol, pre-treatment lobar volume, upper versus lower lobes, and the baseline lung function values. Variables that were not statistically significant were then removed to build a final regression model. Baseline and follow-up values were compared using a paired t test. A subgroup analysis was performed in patient subgroups based on the volume drained from the target lobe (<100 ml, 100–400 ml, and >400 ml). These volume intervals were arbitrarily defined a priori based on clinical observation and experience. Statistical comparisons between groups were performed with one-way ANOVA for a 3-volume-interval group differentiation, and with Chi squared test. Statistical significance was defined as p < 0.05. All statistical analyses were performed using SPSS version 23 (IBM, New York, NY, USA).
Results
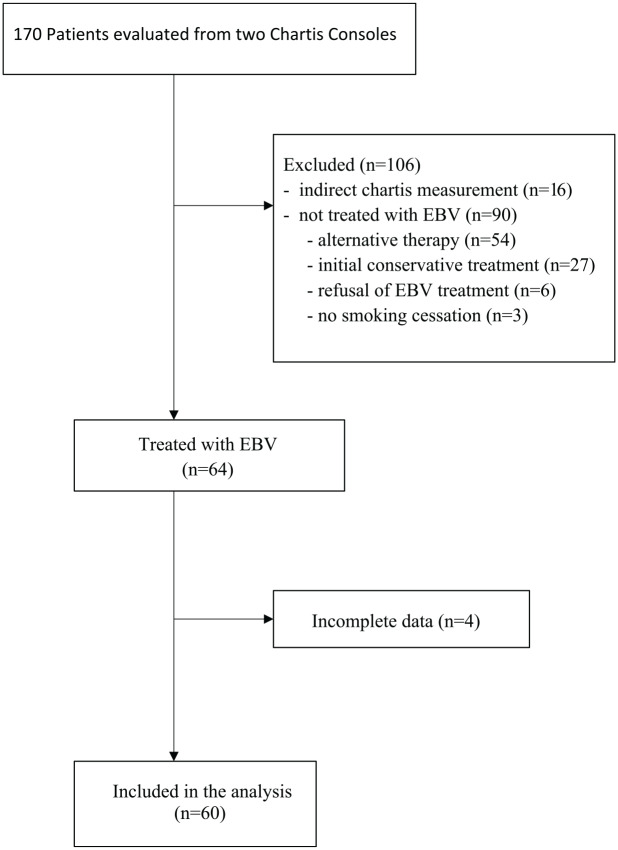
Of the 170 examined Chartis measurements, complete data sets were available for 60 patients (33 female and 27 male, mean age 61.5 years) (Figure 1). The most common reasons for patient exclusion were not performing EBV-treatment after Chartis measurement due to lung function and clinical disease severity that did not meet the criteria for BLVR indication (meaning that the decision was in favor of conservative or alternative treatment), denial of therapy because of continued nicotine abuse, or treatment refusal by the patient (n = 90). CV negativity was identified indirectly by performing Chartis measurement on the adjacent lobe in 16 cases. Follow-up data were missing for four cases. Drained volume was <100 ml in 19 patients, 100–400 ml in 33 patients, and >400 ml in eight patients.
Figure 1.
Patient selection flow chart.
EBV, endobronchial valves.
Baseline mean FEV1 was 0.7 ± 0.2 l (25.7 ± 5.9% predicted), mean RV was 5.9 ± 1.4 l (274.0 ± 54.8% predicted), mean IVC was 2.4 ± 0.7 l (67.3 ± 15.6% predicted), and mean 6MWD was 292 ± 76 m (Table 1).
Table 1.
Demographic and clinical characteristics at baseline.
| Variables | Patients (n = 60) |
|---|---|
| Female/male, n (%) | 33/27 (55/45) |
| Age, years | 61.5 ± 6.1 |
| Weight, kg | 67.4 ± 14.9 |
| Height, cm | 170.4 ± 8.4 |
| Body mass index, kg/m2 | 23.1 ± 4.2 |
| Smoking history, pack-years | 42.6 ± 20.4 |
| FEV1 | |
| Liters | 0.73 ± 0.18 |
| % predicted | 25.7 ± 5.92 |
| RV | |
| Liters | 5.9 ± 1.37 |
| % predicted | 274 ± 54.8 |
| IVC | |
| Liters | 2.37 ± 0.74 |
| % predicted | 67.3 ± 15.6 |
| TLV, milliliters | 1714 ± 502.7 |
| 6MWD, meters | 292 ± 76 |
| CAT score (n = 21) | 25 ± 5 |
Values are mean ± standard deviation, or number of patients (%).
6MWD, 6-min walking distance; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; IVC, inspiratory vital capacity; RV, residual volume; TLV, target lobe volume.
The CAT score was recorded in 21 (mean score 25 ± 5). All 60 patients had complete occlusion of one lobe with EBV. The left upper lobe (LUL) was treated in the majority of cases (n = 36), and the highest mean volume was drained from the right upper lobe (RUL; 281 ml). Generally, the upper lobes yielded a higher drained volume (mean 258 ml) than the lower lobes (mean 95 ml); measurement duration did not differ between the different lobes. Target lobes and drained volumes are summarized in Table 2.
Table 2.
Target lobes (n = 60).
| Lobe treated | n (%) | Volume drained (ml) | Min/max (ml) | Measurement length (s) | Min/max (s) |
|---|---|---|---|---|---|
| Upper lobes | 44 (73) | 258 ± 223 | 18/967 | 156 ± 71 | 51/395 |
| Lower lobes | 16 (27) | 95 ± 76 | 19/283 | 149 ± 88 | 48/341 |
| Left upper lobe | 36 (60) | 249 ± 207 | 18/967 | 153 ± 65 | 51/293 |
| Left lower lobe | 6 (10) | 111 ± 98.6 | 19/283 | 141 ± 96 | 59/282 |
| Right upper lobe | 8 (13) | 281 ± 294 | 35/726 | 168 ± 95 | 72/395 |
| Right middle lobe | 1 (2) | 119 | 119 | 246 | 246 |
| Right lower lobe | 9 (15) | 78 ± 69 | 20/218 | 144 ± 91 | 48/341 |
| Total | 60 (100) | 212 ± 206 | 18/967 | 154 ± 75 | 48/395 |
Values are mean ± standard deviation, number of patients (%), minimum and maximum.
A mean of 3.8 valves per lobe were implanted, and 32 patients (53%) had confirmed volume reduction in the chest radiograph and CT. Within 6 months after treatment, pneumothorax had occurred in nine cases (15%). Although a pneumothorax can be a severe complication after valve implantation requiring intensive monitoring, it is also predictive for a successful treatment response.18 No cases of valve displacement or migration occurred. There were significant improvements in FEV1, RV, and IVC between baseline and follow up (all p < 0.001), but 6MWD and CAT score did not change significantly (Table 3).
Table 3.
Changes in clinical parameters.
| Variables | Pre-implant (baseline) | Post-implant (⩽6 months) | p value |
|---|---|---|---|
| FEV1 (n = 60) | |||
| Value in liters (95% CI) | 0.73 (0.68, 0.78) | 0.84 (0.79, 0.89) | |
| % change (95% CI of the mean) | – | +17.2 (11.1, 23.4) | <0.001 |
| RV (n = 60) | |||
| Value in liters (95% CI) | 5.9 (5.54, 6.3) | 5.18 (4.86, 5.5) | |
| % change (95% CI of the mean) | – | –10.0 (–13.9, –6.2) | <0.001 |
| IVC (n = 60) | |||
| Value in liters (95% CI) | 2.37 (2.18, 2.56) | 2.5 (2.35, 2.7) | |
| % change (95% CI of the mean) | – | +12.9 (2.0, 23.8) | <0.001 |
| TLV (n = 60) | |||
| Value in milliliters (95% CI) | 1722 (1562.5, 1883.3) | 507.2 (314.3, 700.1) | |
| % change (95% CI of the mean) | – | –68.6 (56.9, 80.3) | <0.001 |
| 6MWD (n = 60) | |||
| Value in meters (95% CI) | 292.8 (272.7, 312.8) | 313.3 (291.2, 335.4) | |
| % change (95% CI of the mean) | – | +8.7 (1.7, 15.8) | n.s. |
| Change in meters (95% CI) | 20.5 (–1.9, 42.8) | n.s. | |
| CAT score (n = 21) | |||
| Points (95% CI) | 25.2 (22.9, 27.5) | 23.7 (20.9, 26.5) | |
| Change in points (95% CI) | – | –1.57 (–3.7, 0.5) | n.s. |
6MWD, 6-min walking distance; CAT, COPD assessment test; CI, confidence interval; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; IVC, inspiratory vital capacity; n.s., not statistically significant; RV, residual volume.
The lung function parameter that correlated best with drained volume from the Chartis measurement was change in FEV1 from baseline to follow up (r = 0.663), followed by change in IVC (r = 0.611); change in RV was also significantly correlated with drained volume (r = –0.368). Measurement duration also correlated significantly with the drained volume (r = 0.53) but showed only low, non-significant correlation with the outcome variables. A post-BLVR QCT for the assessment of the target lobe volume (TLV) could be done in 33 cases. The TLVR correlated significantly with the drained volume [(ml) r = 0.635, (%) r = 0.608] (Table 4, Figure 2). Baseline values for FEV1, RV, and the target lobe volume also correlated significantly with drained volume. The heterogeneity of the emphysema that could be evaluated with high-resolution CT, and the StratX lung report was not associated with drained volume in this investigation.
Table 4.
Correlation coefficients.
| Variable | Correlation with drained volume (r) | p value | |
|---|---|---|---|
| Baseline parameters | |||
| TLV | (ml) | 0.449 | <0.05 |
| FEV1 | (ml) | –0.266 | <0.05 |
| RV | (ml) | 0.529 | <0.001 |
| IVC | (ml) | –0.135 | n.s. |
| 6MWD | (m) | –0.087 | n.s. |
| Change from baseline to follow up | |||
| ΔFEV1 | (%) | 0.663 | <0.001 |
| ΔRV | (%) | –0.368 | <0.05 |
| ΔIVC | (%) | 0.611 | <0.001 |
| TLVR | (ml) | 0.635 | <0.001 |
| TLVR | (%) | 0.608 | <0.001 |
| Δ6MWD | (m) | 0.128 | n.s. |
| Δ6MWD | (%) | 0.115 | n.s. |
Correlation coefficient measured with Pearson correlation (n = 60).
6MWD, 6-min walking distance; CAT, COPD assessment test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; IVC, inspiratory vital capacity; n.s., not statistically significant; RV, residual volume; TLV, target lobe volume; TLVR, target lobe volume reduction.
Figure 2.
Scatter plots showing correlation between changes in lung function from baseline to follow up and drained volume.
FEV1, forced expiratory volume in 1 s; IVC, inspiratory vital capacity; RV, residual volume; TLVR, target lobe volume reduction.
Changes in all lung function, the degree of TLVR, and physical performance variables varied widely between patient subgroups based on drained volume, being lowest in those with smaller drained volumes (Table 5). The regression model showed that the drained volume (TVol) and the corresponding baseline lung function value were significantly associated with all changes in lung function parameters and the TLVR (p < 0.001). The higher the TVol of the Chartis measurement, the greater the improvement in lung function, as shown by an increase in FEV1 and IVC, and a decrease in RV. Pre-treatment lobar volume was also significantly associated with changes in RV and IVC. Upper lobe volume, despite the higher volume being drained, was not significantly associated with a change in outcome (Supplemental Tables S1–S5).
Table 5.
Group differentiation for three thresholds of drained volume.
| Variables | Drained volume (TVol), ml | |||
|---|---|---|---|---|
| <100 (n = 19) | 100–400 (n = 33) | >400 (n = 8) | p value | |
| ΔFEV1 (%) | 2.59 | 17.37 | 51.3 | <0.001 |
| ΔRV (%) | –3.92 | –10.6 | –23.8 | 0.007 |
| ΔIVC (%) | –3.97 | 10.6 | 62.4 | <0.001 |
| Δ6MWD (%) | –3.34 | 15.17 | 8.56 | n.s. |
| ΔFEV1 >100 ml† , n (%) | 3 (16) | 17 (52) | 8 (100) | <0.001* |
| ΔRV > 310 ml† , n (%) | 10 (53) | 20 (61) | 8 (100) | <0.001* |
| 6MWD > 25 m† , n (%) | 5 (26) | 17 (52) | 3 (38) | n.s.* |
| <100 (n = 7) | 100–400 (n = 11) | >400 (n = 3) | ||
| CAT ⩾ 2†, n (%) | 2 (29) | 5 (45) | 1 (33) | n.s.* |
| <100 (n = 8) | 100–400 (n = 21) | >400 (n = 4) | ||
| TLVR (ml) | 525 | 1375 | 1760 | <0.001 |
| TLVR (%) | 39 | 73 | 100 | <0.001 |
| Full atelectasis (n) | 0 (0) | 12 (57) | 4 (100) | <0.001 |
One-way ANOVA for 3-volume-interval.
Chi squared test.
Minimal clinically important difference.
Δ, mean change from baseline to follow up.
6MWD, 6-min walking distance; CAT, COPD assessment test (mean value); COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; IVC, inspiratory vital capacity; n.s., not statistically significant; RV, residual volume; TLVR, target lobe volume reduction.
Regarding the minimal clinically important difference (MCID) for lung function after intervention, we recorded for thresholds of >100 ml for ΔFEV1 and >310 ml for ΔRV, along with >25 m for the 6MWD and ⩾2 points for the CAT score.19–22 All members of the subgroup with a drained volume of >400 ml achieved the MCID for both parameters, whereas 16% of the <100 ml subgroup and 52% of the 100–400 ml subgroup had a ΔFEV1 >100 ml, and 53% (<100 ml) and 61% (100–400 ml) had ΔRV > 310 ml.
Discussion
This study evaluated the value of drained volume using the Chartis measurement for predicting clinical outcome after BLVR in CV-negative patients. There were good correlations between drained volume, lung function, and the TLVR. Those with higher drained volumes showed better clinical improvements in FEV1, RV, and IVC, along with a higher degree of the TLVR.
Careful patient selection is essential in endoscopic lung volume reduction with interventional valve therapy. It is already known that baseline lung function parameters predict the outcome of therapy, and many emphysema care centers usually consider BLVR for patients with FEV1 <40% predicted and RV > 200% predicted.17,23 The degree of hyperinflation indicated by the RV predicts outcome because patients with greater hyperinflation (and hence greater RV) achieve a larger lung volume reduction and also gain more benefit.24,25 It seems clinically obvious that more extensive hyperinflation means that the Chartis measurement shows a higher volume being drained from the target lobe, thus implying better clinical improvements. Other than the QCT-analysis, which can be used to identify hyperinflated lung areas, the Chartis measurement displays the actual pathologic condition of lung volumes, airways, and airflow. The demonstration in this study that TVol significantly correlated with RV and target lobe volume (TLV) at baseline, and with changes in FEV1, RV, IVC from baseline to follow up, provides evidence to support this. The positive correlation with the QCT-analyzed TLVR further substantiates this relationship. Even though there was a significant correlation between TVol and both outcome parameters and TVol and TLV at baseline, there was no correlation between TLV and outcome parameters. This demonstrates, in our observation, that TLV does not have a predictive value.
Although there was a general benefit from valve placement in our study population, our results showed greater benefits and lung function improvements in the subgroup of patients with the greatest TVol. In contrast, we did not observe any correlation between drained volume and either 6MWD or CAT score. The 6MWD has previously been shown to correlate with baseline lung function parameters,26,27 but changes in 6MWD do not appear to correlate with changes in lung function.28 It is possible that our inability to detect improvements in these parameters in the subgroup of patients with drained volume >400 ml was due to the small number of patients in this subgroup. Another possible explanation could be that our study population might have a very severe manifestation of emphysema with highly impaired baseline FEV1 and RV compared with previous EBV trials, meaning that physical performance was not improved even after treatment.29 In retrospective analysis, Darwiche et al. and Trudzinski et al. showed that EBV treatment can be safely performed in patients with very low FEV1. Although improvements in lung function were recorded in these studies, there was no significant change in 6MWD.30,31 Quality of life was determined using the CAT. Complete pre- and post-intervention data were available for only 21/60 patients (35%). CAT scores did not change significantly from baseline after BLVR, and there was no correlation with drained volume. The nine cases of pneumothorax occurring within 6 months after EBV treatment in our study were distributed equally between subgroups, and there was no relationship between the drained volume and the risk of post-treatment pneumothorax.
As noted above, the Chartis System is a safe method to improve accuracy for patient identification and therapy decision making as part of the combined diagnostics for collateral ventilation. It was first described as an endobronchial CV assessment (ECVA) by Aljuri and Freitag in 2008 and later developed as a commercial diagnostic tool.32,33 Chartis is used on the most emphysematous transformed lobe identified via prior CT scanning and QCT report and provides qualitative evidence of collateral ventilation by recording expiratory flow and inspiratory pressure. In addition, it quantitatively detects the total volume being drained from the target lobe and the time of this measuring maneuver is recorded. The functional mechanisms of this device have been studied previously, and it has been shown to be a safe and efficient qualitative diagnostic tool.14,32,34 Use of Chartis does not increase the risks associated with valve placement. The focus of the current study was mainly on drained volume. However, there is a place for additional investigation of CV-negative patients based on the highly individual curve shapes with varying rates of pressure, measurement length, and drained volume, and the interaction between factors.
The diagnostic value of Chartis is strongly dependent on the examiner’s skills and experience. Herzog and colleagues described four different phenotypes with associated curve patterns: CV-positive, low-flow, low-plateau, and CV-negative.35 Different thresholds for pressure (Rndx) and volume (TVol) were used to define the different phenotypes. A CV-negative result has been defined as drained volume of 50–750 ml. Volumes below 50 ml were designated as a low-flow phenotype almost only present in the lower lobes with a time range up to 30 s. Volumes above 750 ml indicate a CV-positive result. Gesierich et al. also suggested that drained volumes of <50 ml or flow times of <1 min are associated with a collapse phenomenon or the so-called delayed collapse phenomenon. These have to be distinguished from a CV-negative result.36 In our study, we also classified volumes below 50 ml and above 750 ml as CV-negative if the curve pattern was consistent with the previously described properties of CV-negative measurements, and the (Q)CT analysis indicated no CV. Figure 3 shows examples of typical curve patterns that were measured outside of the discussed range. Measured volumes of <50 ml were evenly distributed to the upper lobes (UL) and lower lobes (LL) (TVol < 50 ml, n = 14; 7 UL, 7 LL). There were two cases of volumes >750 ml (967 ml and 857 ml), both measured in the left upper lobe.
Figure 3.
Chartis curve patterns. The upper orange and blue curves show the expiratory flow and inspiratory pressure. The lower curve represents the corresponding resistance. Examples for <50 ml in (a) and >750 ml (b).
TVol, drained volume.
Anesthesia technique has been shown to have an impact on the Chartis maneuver.37 Although there was no difference in the treatment outcome, more measurements were required to identify CV-status and measurements under conscious sedation took significantly longer than those performed under general anesthesia. Furthermore, the mean airflow volume (TVol) was higher during the conscious sedation procedures. Because we performed all measurements under conscious sedation, measurements were probably longer and generated higher drained volumes compared with measurements performed under general anesthesia. This should not confound our findings because measurements were done under conscious sedation (rather than a mix of anesthesia types). However, we hypothesize that the findings should be similar for both anesthesia techniques because the drained volume depends mostly on the degree of hyperinflation, but this needs to be verified by data from measurements under general anesthesia.
There are some limitations in our study. One is the retrospective nature of this investigation from a single emphysema care center. The Chartis measurement, especially under conscious sedation, is a method with variable results depending on the examiner and individual difficulty of the maneuver. Although we only evaluated distinct CV-negative curve patterns, we cannot exclude a bias related to the operator’s experience because a range of examiners do the measurements on a daily basis. Generally, there are patients who do not tolerate Chartis measurement, meaning that increased bronchoconstriction, mucus secretion, or coughing can lead to incorrect data recordings. In 12/60 cases in our study, the measurement for the targeted lobe had to be repeated to get a distinct CV-negative curve pattern. We found no connection between the number of measurements per lobe and the drained volume, and the 12 cases were distributed across all volume groups (4 in <100 ml, 6 in 100–400 ml, and 2 in >400 ml). Moreover, it can be technically difficult to measure the lower lobes because the inflated balloon might occlude a segment, especially the B6 bronchus, so that recordings here might be incorrect as well.38 We could not collect quality of life data in all cases, so changes in quality of life data may not be representative. Due to reasons such as incompatible CT-resolution, missing HR-CT slices, or presence of postprocedural pneumothorax, we analyzed only 33 post-BLVR QCTs to determine the TLVR. A complete examination would have been desirable but given that there was a highly statistically significant correlation with drained volume, this outcome can be considered as representative. No cases of valve displacement or migration after EBV treatment were observed. In six cases, there was a history of prior unsuccessful EBV treatment where re-implantation was necessary, and our data include outcome values after re-implantation. In terms of Chartis measurements per lobe, we have a discrepancy towards the LUL (n = 36). In general, we observed that the LUL was treated in the majority of cases in our center, but this included indirect CV measurement for the left lower lobe (LLL). There was also indirect CV measurement of the right lung, which also contributes to the high LUL number. In addition, there were individual cases of emphysema, especially of the right middle lobe (RML), where a missing fissure integrity or a certain emphysema pattern was known. In these cases there was a pre-Chartis decision favoring alternative therapy options (e.g., surgery). In summary, further prospective, multicenter studies are needed to confirm the results observed in this investigation. Finally, this study was conducted in an emphysema care center with high numbers of COPD patients and experience. Therefore, it shows only possible, but not generalizable, results. In terms of clinical implementation, we consider it necessary that the volume of the Chartis measurement should be completely finished to get the drained volume of the targeted lobe. This is particularly essential in patients with a slow decline of flow because these patients tend to yield higher drained volumes and significantly improve after BLVR. Additional data on TVol should then be added, and assessed together with the usual pre-treatment diagnostic procedures. This could aid the decision-making process for individual cases that are not clearly within the range of the defined patient selection guidelines, or where a case is close to a cutoff value. Moreover, when diagnostic tools such as QCT are unclear, or do not match symptom burden but clinical severity requires treatment, the Chartis measurement is helpful for further therapy planning.
In conclusion, there is a clinically meaningful and statistically significant correlation between the drained volume from the Chartis measurement and changes in lung function (FEV1, RV, and IVC) after BLVR with valves. Thus, the Chartis System not only provides qualitative diagnostic information about whether valve implantation is feasible, but also allows prediction of post-procedural lung function improvements in CV-negative patients.
Supplemental Material
Supplemental material, Author_Response_1 for Predictive value of Chartis measurement for lung function improvements in bronchoscopic lung volume reduction by Johannes Wienker, Rüdiger Karpf-Wissel, Faustina Funke, Christian Taube, Julia Wälscher, Jane Winantea, Sandra Maier, Khaled Mardanzai and Kaid Darwiche in Therapeutic Advances in Respiratory Disease
Supplemental material, Regression_Model_Supplementary for Predictive value of Chartis measurement for lung function improvements in bronchoscopic lung volume reduction by Johannes Wienker, Rüdiger Karpf-Wissel, Faustina Funke, Christian Taube, Julia Wälscher, Jane Winantea, Sandra Maier, Khaled Mardanzai and Kaid Darwiche in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Predictive value of Chartis measurement for lung function improvements in bronchoscopic lung volume reduction by Johannes Wienker, Rüdiger Karpf-Wissel, Faustina Funke, Christian Taube, Julia Wälscher, Jane Winantea, Sandra Maier, Khaled Mardanzai and Kaid Darwiche in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Predictive value of Chartis measurement for lung function improvements in bronchoscopic lung volume reduction by Johannes Wienker, Rüdiger Karpf-Wissel, Faustina Funke, Christian Taube, Julia Wälscher, Jane Winantea, Sandra Maier, Khaled Mardanzai and Kaid Darwiche in Therapeutic Advances in Respiratory Disease
Acknowledgments
The authors would like to thank Birte Schwarz and Jennifer Thälker for their friendly assistance in data collection and management and Sarah Dietz-Terjung for statistical advice. English language editing assistance was provided by Nicola Ryan, independent medical writer. We acknowledge support by the Open Access Publication Fund of the University of Duisburg-Essen.
Footnotes
Author contribution(s): Johannes Wienker: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing-original draft; Writing-review & editing.
Rüdiger Karpf-Wissel: Conceptualization; Formal analysis; Methodology; Writing-review & editing.
Faustina Funke: Data curation; Investigation; Writing-review & editing.
Christian Taube: Data curation; Investigation; Writing-review & editing.
Julia Wälscher: Data curation; Investigation; Writing-review & editing.
Jane Winantea: Data curation; Investigation; Writing-review & editing.
Sandra Maier: Investigation; Writing-review & editing.
Khaled Mardanzai: Investigation; Writing-review & editing.
Kaid Darwiche: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Writing-original draft; Writing-review & editing.
Conflict of interest statement: KD has received travel grants, speaker fees or research grants from Storz, Olympus, Boston Scientific, Nuvaira, PulmonX, Broncus Medical, ERBE, bess, Boehringer, Novartis and Sysmex. RKW has received speaker fees from PulmonX, PneumRx, Olympus, Leufen, Novartis and travel grants from PneumRx. JuW has received travel grants and speaker fees from Boehringer and Roche. All other authors have no potential conflict of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Pulmonx, Inc. provided the post-BLVR QCT analysis.
ORCID iD: Johannes Wienker  https://orcid.org/0000-0002-5841-8181
https://orcid.org/0000-0002-5841-8181
Supplemental material: The reviews of this paper are available via the supplemental material section.
Contributor Information
Johannes Wienker, Department of Pneumology, Division of Interventional Pneumology, University Medicine Essen-Ruhrlandklinik, Tüschener Weg 40, Essen, NRW 45239, Germany.
Rüdiger Karpf-Wissel, Department of Pneumology, University Medicine Essen-Ruhrlandklinik, Essen, Nordrhein-Westfalen, Germany.
Faustina Funke, Department of Pneumology, University Medicine Essen-Ruhrlandklinik, Essen, Nordrhein-Westfalen, Germany.
Christian Taube, Department of Pneumology, University Medicine Essen-Ruhrlandklinik, Essen, Nordrhein-Westfalen, Germany.
Julia Wälscher, Department of Pneumology, University Medicine Essen-Ruhrlandklinik, Essen, Nordrhein-Westfalen, Germany.
Jane Winantea, Department of Pneumology, University Medicine Essen-Ruhrlandklinik, Essen, Nordrhein-Westfalen, Germany.
Sandra Maier, Department of Diagnostic and Interventional Radiology and Neuroradiology, University Hospital Essen, Essen, Nordrhein-Westfalen, Germany.
Khaled Mardanzai, Department of Thoracic Surgery, University Medicine Essen-Ruhrlandklinik, Essen, Nordrhein-Westfalen, Germany.
Kaid Darwiche, Department of Pneumology, University Medicine Essen-Ruhrlandklinik, Essen, Nordrhein-Westfalen, Germany.
References
- 1. López-Campos JL, Tan W, Soriano JB. Global burden of COPD. Respirology 2016; 21: 14–23. [DOI] [PubMed] [Google Scholar]
- 2. Kemp SV, Slebos DJ, Kirk A, et al. A multicenter randomized controlled trial of zephyr endobronchial valve treatment in heterogeneous emphysema (TRANSFORM). Am J Respir Crit Care Med 2017; 196: 1535–1543. [DOI] [PubMed] [Google Scholar]
- 3. Davey C, Zoumot Z, Jordan S, et al. Bronchoscopic lung volume reduction with endobronchial valves for patients with heterogeneous emphysema and intact interlobar fissures (the BeLieVeR-HIFi study): a randomised controlled trial. Lancet 2015; 386: 1066–1073. [DOI] [PubMed] [Google Scholar]
- 4. Valipour A, Slebos DJ, Herth F, et al. Endobronchial valve therapy in patients with homogeneous emphysema. Results from the IMPACT study. Am J Respir Crit Care Med 2016; 194: 1073–1082. [DOI] [PubMed] [Google Scholar]
- 5. Klooster K, ten Hacken NHT, Hartman JE, et al. Endobronchial valves for emphysema without interlobar collateral ventilation. N Engl J Med 2015; 373: 2325–2335. [DOI] [PubMed] [Google Scholar]
- 6. Darwiche K, Aigner C. Clinical management of lung volume reduction in end stage emphysema patients. J Thorac Dis 2018; 10: S2732–S2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gompelmann D, Heinhold T, Rötting M, et al. Long-term follow up after endoscopic valve therapy in patients with severe emphysema. Ther Adv Respir Dis. Epub ahead of print 2 August 2019. DOI: 10.1177/1753466619866101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Slebos DJ, Shah PL, Herth FJF, et al. Endobronchial valves for endoscopic lung volume reduction: best practice recommendations from expert panel on endoscopic lung volume reduction. Respiration 2017; 93: 138–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fiorelli A, Poggi C, Anile M, et al. Visual analysis versus quantitative CT analysis of interlobar fissure integrity in selecting emphysematous patients for endobronchial valve treatment. Interact Cardiovasc Thorac Surg. Epub ahead of print 28 December 2018. DOI: 10.1093/icvts/ivy340. [DOI] [PubMed] [Google Scholar]
- 10. de Oliveira HG, de Oliveira SM, Rambo RR, et al. Fissure integrity and volume reduction in emphysema: a retrospective study. Respiration 2016; 91: 471–479. [DOI] [PubMed] [Google Scholar]
- 11. Kristiansen JF, Perch M, Iversen M, et al. Lobar quantification by ventilation/perfusion SPECT/CT in patients with severe emphysema undergoing lung volume reduction with endobronchial valves. Respiration 2019; 98: 230–238. [DOI] [PubMed] [Google Scholar]
- 12. Gompelmann D, Eberhardt R, Slebos DJ, et al. Diagnostic performance comparison of the Chartis system and high-resolution computerized tomography fissure analysis for planning endoscopic lung volume reduction. Respirology 2014; 19: 524–530. [DOI] [PubMed] [Google Scholar]
- 13. Schuhmann M, Raffy P, Yin Y, et al. Computed tomography predictors of response to endobronchial valve lung reduction treatment. Comparison with Chartis. Am J Respir Crit Care Med 2015; 191: 767–774. [DOI] [PubMed] [Google Scholar]
- 14. Herth FJF, Eberhardt R, Gompelmann D, et al. Radiological and clinical outcomes of using Chartis™ to plan endobronchial valve treatment. Eur Respir J 2013; 41: 302–308. [DOI] [PubMed] [Google Scholar]
- 15. Sciurba FC, Ernst A, Herth FJF, et al. A randomized study of endobronchial valves for advanced emphysema. N Engl J Med 2010; 363: 1233–1244. [DOI] [PubMed] [Google Scholar]
- 16. Koster TD, van Rikxoort EM, Huebner RH, et al. Predicting lung volume reduction after endobronchial valve therapy is maximized using a combination of diagnostic tools. Respiration 2016; 92: 150–157. [DOI] [PubMed] [Google Scholar]
- 17. Herth FJF, Slebos DJ, Criner GJ, et al. Endoscopic lung volume reduction: an expert panel recommendation - update 2017. Respiration 2017; 94: 380–388. [DOI] [PubMed] [Google Scholar]
- 18. Gompelmann D, Herth FJF, Slebos DJ, et al. Pneumothorax following endobronchial valve therapy and its impact on clinical outcomes in severe emphysema. Respiration 2014; 87: 485–491. [DOI] [PubMed] [Google Scholar]
- 19. Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2005; 2: 111–124. [DOI] [PubMed] [Google Scholar]
- 20. Hartman JE, ten Hacken NHT, Klooster K, et al. The minimal important difference for residual volume in patients with severe emphysema. Eur Respir J 2012; 40: 1137–1141. [DOI] [PubMed] [Google Scholar]
- 21. Holland AE, Hill CJ, Rasekaba T, et al. Updating the minimal important difference for six-minute walk distance in patients with chronic obstructive pulmonary disease. Arch Phys Med Rehabil 2010; 91: 221–225. [DOI] [PubMed] [Google Scholar]
- 22. Kon SSC, Canavan JL, Jones SE, et al. Minimum clinically important difference for the COPD Assessment Test: a prospective analysis. Lancet Respir Med 2014; 2: 195–203. [DOI] [PubMed] [Google Scholar]
- 23. Shah PL, Herth FJF. Current status of bronchoscopic lung volume reduction with endobronchial valves. Thorax 2014; 69: 280–286. [DOI] [PubMed] [Google Scholar]
- 24. Sciurba FC, Criner GJ, Strange C, et al. Effect of endobronchial coils vs usual care on exercise tolerance in patients with severe emphysema: the RENEW randomized clinical trial. JAMA 2016; 315: 2178–2189. [DOI] [PubMed] [Google Scholar]
- 25. Gompelmann D, Hofbauer T, Gerovasili V, et al. Predictors of clinical outcome in emphysema patients with atelectasis following endoscopic valve therapy: a retrospective study. Respirology 2016; 21: 1255–1261. [DOI] [PubMed] [Google Scholar]
- 26. Agrawal MB, Awad NT. Correlation between six minute walk test and spirometry in chronic pulmonary disease. J Clin Diagn Res 2015; 9: OC01–04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chen H, Liang BM, Tang YJ, et al. Relationship between 6-minute walk test and pulmonary function test in stable chronic obstructive pulmonary disease with different severities. Chin Med J 2012; 125: 3053–3058. [PubMed] [Google Scholar]
- 28. Karanth MS, Awad NT. Six minute walk test: a tool for predicting mortality in chronic pulmonary diseases. J Clin Diagn Res 2017; 11: OC34–OC38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Low SW, Lee JZ, Desai H, et al. Endobronchial valves therapy for advanced emphysema: a meta-analysis of randomized trials. J Bronchology Interv Pulmonol 2019; 26: 81–89. [DOI] [PubMed] [Google Scholar]
- 30. Darwiche K, Karpf-Wissel R, Eisenmann S, et al. Bronchoscopic lung volume reduction with endobronchial valves in Low-FEV1 patients. Respiration 2016; 92: 414–419. [DOI] [PubMed] [Google Scholar]
- 31. Trudzinski FC, Höink AJ, Leppert D, et al. Endoscopic lung volume reduction using endobronchial valves in patients with severe emphysema and very low FEV1. Respiration 2016; 92: 258–265. [DOI] [PubMed] [Google Scholar]
- 32. Aljuri N, Freitag L. Validation and pilot clinical study of a new bronchoscopic method to measure collateral ventilation before endobronchial lung volume reduction. J Appl Physiol 2009; 106: 774–783. [DOI] [PubMed] [Google Scholar]
- 33. Mantri S, Macaraeg C, Shetty S, et al. Technical advances: measurement of collateral flow in the lung with a dedicated endobronchial catheter system. J Bronchology Interv Pulmonol 2009; 16: 141–144. [DOI] [PubMed] [Google Scholar]
- 34. Gompelmann D, Eberhardt R, Michaud G, et al. Predicting atelectasis by assessment of collateral ventilation prior to endobronchial lung volume reduction: a feasibility study. Respiration 2010; 80: 419–425. [DOI] [PubMed] [Google Scholar]
- 35. Herzog D, Thomsen C, Poellinger A, et al. Outcomes of endobronchial valve treatment based on the precise criteria of an endobronchial catheter for detection of collateral ventilation under spontaneous breathing. Respiration 2016; 91: 69–78. [DOI] [PubMed] [Google Scholar]
- 36. Gesierich W, Samitas K, Reichenberger F, et al. Collapse phenomenon during Chartis collateral ventilation assessment. Eur Respir J 2016; 47: 1657–1667. [DOI] [PubMed] [Google Scholar]
- 37. Welling JBA, Hartman JE, Ten Hacken NHT, et al. Chartis measurement of collateral ventilation: conscious sedation versus general anesthesia - a retrospective comparison. Respiration 2018; 96: 480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Koster TD, Slebos DJ. The fissure: interlobar collateral ventilation and implications for endoscopic therapy in emphysema. Int J Chron Obstruct Pulmon Dis 2016; 11: 765–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Author_Response_1 for Predictive value of Chartis measurement for lung function improvements in bronchoscopic lung volume reduction by Johannes Wienker, Rüdiger Karpf-Wissel, Faustina Funke, Christian Taube, Julia Wälscher, Jane Winantea, Sandra Maier, Khaled Mardanzai and Kaid Darwiche in Therapeutic Advances in Respiratory Disease
Supplemental material, Regression_Model_Supplementary for Predictive value of Chartis measurement for lung function improvements in bronchoscopic lung volume reduction by Johannes Wienker, Rüdiger Karpf-Wissel, Faustina Funke, Christian Taube, Julia Wälscher, Jane Winantea, Sandra Maier, Khaled Mardanzai and Kaid Darwiche in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_1_v.1 for Predictive value of Chartis measurement for lung function improvements in bronchoscopic lung volume reduction by Johannes Wienker, Rüdiger Karpf-Wissel, Faustina Funke, Christian Taube, Julia Wälscher, Jane Winantea, Sandra Maier, Khaled Mardanzai and Kaid Darwiche in Therapeutic Advances in Respiratory Disease
Supplemental material, Reviewer_2_v.1 for Predictive value of Chartis measurement for lung function improvements in bronchoscopic lung volume reduction by Johannes Wienker, Rüdiger Karpf-Wissel, Faustina Funke, Christian Taube, Julia Wälscher, Jane Winantea, Sandra Maier, Khaled Mardanzai and Kaid Darwiche in Therapeutic Advances in Respiratory Disease