Abstract
The treatment landscape for patients with advanced non-small cell lung cancer has evolved greatly with the advent of immune checkpoint inhibitors. However, many patients do not derive benefit from checkpoint blockade, developing either primary or secondary resistance, highlighting a need for alternative approaches to modulate immune function. In this review, we highlight the absence of a common definition of primary and secondary resistance and summarize their frequency and clinical characteristics. Furthermore, we provide an overview of the biomarkers and mechanisms of resistance involving the tumor, the tumor microenvironment and the host, and suggest treatment strategies to overcome these mechanisms and improve clinical outcomes.
Keywords: acquired resistance, immune checkpoint inhibitors, PD-L1, primary resistance
Introduction
Immune checkpoint inhibitors (ICIs) have altered the treatment landscape for advanced lung cancer since their initial approval in patients with pre-treated advanced non-small cell lung carcinoma (NSCLC). They are now standard of care, either in combination or as monotherapy, in advanced non-oncogene-driven NSCLC, extensive stage small cell carcinoma in combination with chemotherapy and as a consolidation therapy in unresectable stage III NSCLC. However, responses to ICI therapy are not ubiquitous, with many patients displaying primary, also known as innate, resistance to ICI monotherapy. In addition, a number of patients who derive an initial clinical benefit from ICI will subsequently experience systemic disease progression, exhibiting secondary or acquired resistance. In this paper, we review the definition and clinical characteristics of resistance, provide an overview on biomarkers of such resistance, as well as systemic treatment approaches in patients with NSCLC in the first-line setting and in patients who have progressed after prior exposure to immunotherapy. The role of treatment beyond progression for patients with slow progression and/or mixed treatment response with clinical benefit,1–3 and the use of local therapy (surgery, stereotactic body radiation therapy and radio-frequency ablation) for oligo-progression4 are beyond the scope of this article and has been discussed in other reviews.5–7
The immune response and ICI therapy
The generation of an anti-tumor immune response relies on a cyclical process of events elegantly described as the cancer immunity cycle.8 Initially, tumor cell death leads to the release of antigens, which are captured by dendritic cells (DCs) and antigen-presenting cells (APCs). Next, APCs present captured antigens via the major histocompatibility complex (MHC), leading to the priming and activation of naïve T cells, which traffic to and infiltrate the tumor. In the final step, activated cytotoxic CD8+ T cells and natural killer (NK) cells identify tumor cells and enact cytotoxic activity leading to cell death.
Negative regulators of T-cell activation exist as immune checkpoints, with programmed death 1 (PD-1) and cytotoxic T lymphocyte antigen (CTLA-4), the most studied pathways. Tumor cells exploit such inhibitory pathways to evade host immune surveillance.9 Interruption of these pathways with antibodies targeting PD-1/PD-L1 (pembrolizumab, nivolumab, atezolizumab, durvalumab), and CTLA-4 (ipilimumab, tremelimumab) work to facilitate host immune response against the tumor. The management of patients with advanced NSCLC with immune checkpoint blockade has been reviewed elsewhere.10,11
Resistance to ICIs
Resistance can be categorized as either primary (innate) or secondary (acquired) (Figure 1).12,13 However, defining such resistance has been challenging and no single accepted definition exists. Primary resistance has been defined as disease progression by RECIST criteria on first CT evaluation or death prior to first CT evaluation14 whereas in another paper, it was defined as those that fail to ever respond.13 It represents a major clinical problem in patients with advanced NSCLC with a frequency of 7–27% reported with first-line ICI with or without chemotherapy and 20–44% in the pre-treated setting with ICI monotherapy, assuming we take the definition for primary resistance as progressive disease (PD) as best response (Table 1).
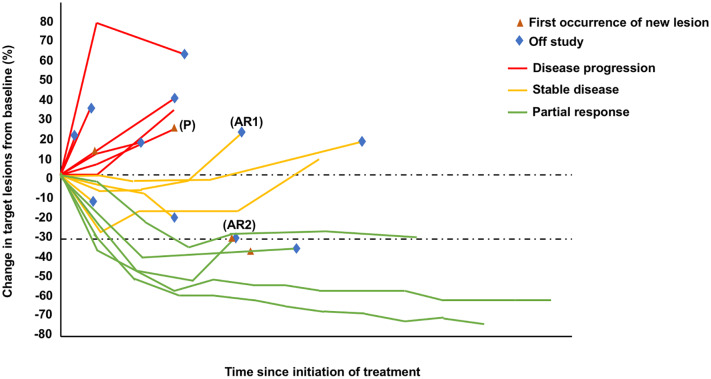
Figure 1.
A spider plot representing examples of resistance to immune checkpoint inhibition with (P) primary resistance, defined as best response being disease progression; (AR1) acquired resistance, defined as initial stable disease and subsequent disease progression; and (AR2) acquired resistance, defined as initial response and subsequent disease progression.
Table 1.
Frequency of primary resistance (disease progression as best response) in selected studies of immune checkpoint inhibitors with or without chemotherapy.
| Study | Treatment | ORR (%) | PD as best response (%) | |
|---|---|---|---|---|
| First line setting | ||||
| Monotherapy | KN02415 | Pembrolizumab | 44.8 | 22 |
| KN04216 | Pembrolizumab (TPS ⩾1%) | 27.3 | 21 | |
| CM02617 | Nivolumab | 26 | 27 | |
| Chemotherapy + ICI | KN18918 | Chemotherapy + Pembrolizumab | 47.6 | 8.8 |
| KN40719 | Chemotherapy + Pembrolizumab | 58.4 | 6.9 | |
| IMpower13020 | Chemotherapy + Atezolizumab | 49.2 | 11 | |
| IMpower13121 | Chemotherapy + Atezolizumab | 49 | Not reported | |
| IMpower15022 | Chemotherapy + Bevacizumab + Atezolizumab | 63.5 | 18 | |
| ICI + ICI | CM22723 | Ipilimumab + Nivolumab (TMB high) | 45.3 | 15.8 |
| MYSTIC24 | Durvalumab + Tremelimumab | 34.4 | Not reported | |
| Pre-treated setting | ||||
| Monotherapy | CM01725 | Nivolumab | 20 | 41 |
| CM05726 | Nivolumab | 19 | 44 | |
| KN01027 | Pembrolizumab (TPS ⩾1%) | 18 | 20–25 | |
| OAK28 | Atezolizumab | 14 | 44 | |
CM, CHECKMATE; ICI, immune checkpoint inhibitor; KN, KEYNOTE; ORR, overall response rate; PD, progressive disease; TPS, tumor proportion score.
Secondary resistance has been classified as disease progression after partial response (PR) or complete response (CR)13,29 or initial clinical benefit followed by the development of resistance.14
Defining resistance is complicated by the presence of atypical response patterns, such as pseudo-progression that has been reported with ICIs. Pseudo-progression has been defined as response to treatment after initial progression and was first observed in patients with melanoma treated with ipilimumab. The recognition of this uncommon entity, described in less than 10% of patients treated with ICIs, resulted in the development of the specific immune-related response criteria (irRC), as the original response evaluation criteria in solid tumors (RECIST) criteria was designed to assess response to conventional chemotherapy.30–33 These include immune-related RECIST (irRECIST) and, more recently, the consensus assessment guideline immunotherapy RECIST (iRECIST). Both share the need for confirmation of PD at least 4 weeks and up to 12 weeks (irRECIST) or 8 weeks (iRECIST) after an initial scan showing apparent progression. iRECIST describes initial progression as immune unconfirmed PD (iUPD), only becoming immune confirmed PD (iCPD) if there is further increase in target lesion measurement on follow up imaging studies.34 Recognizing pseudo-progression is important for several reasons: first, to avoid premature cessation of potentially effective treatment; second, not to continue a costly, potentially toxic ineffective therapy; and, finally, not to delay administering a new line of therapy. One must remember that pseudo-progression is a rare event in NSCLC and true progression is the most likely occurrence in the event of new lesions or growth of existing target lesions. Therefore, we may suggest that for most patients classical RECIST is most relevant to assess the presence of PD and therefore resistance. In a subgroup of patients who have clinical improvement or stability with asymptomatic and relatively slow progression on initial imaging one can follow guidelines as per irRECIST/iRECIST and confirm findings with a follow-up scan at least 4 weeks later. Progression as best response would constitute primary resistance as opposed to PD on first CT assessment, thus allowing for repeat assessment in those patients with possible pseudo-progression. Secondary resistance, as suggested above should include those patients who progress after initial clinical benefit.
The importance of achieving treatment response to ICI was underlined in a recent pooled analysis of four studies of nivolumab in patients with pre-treated NSCLC where survival was influenced by treatment response category. The median survival in patients who had achieved a CR/PR, stable disease (SD), and progression of disease (PD) at 6 months was not reached, 15.5 months, and 7.3 months, respectively. Importantly, and not surprisingly, survival was worse in patients with primary resistance (progression as best response) compared with patients with secondary resistance (disease progression after SD, CR, or PR). The 3-year overall survival (OS) rate for patients who progressed after CR/PR, SD, and PD as best response was 29%, 12%, and 3%, respectively.35 These results highlight the need to understand mechanisms of resistance and develop novel therapeutic approaches to overcome resistance.
Given the varying definitions of primary and secondary resistance, the frequencies of their occurrence and the differences in survival seen in patients displaying such resistance, efforts should be undertaken to standardize the definitions used in order to provide a consistent approach to the conduct, interpretation, and analysis of clinical studies. Analysis of trial datasets similar to Antonia et al.35 on the association between response status and survival may aid in clarifying the definition of primary and secondary resistance. This process, as highlighted above, is however complicated by atypical response patterns seen with ICIs.
Clinical characteristics of resistance to ICIs
Published reports on the clinical features of resistance to ICIs in advanced NSCLC have been sparse. In a study of patients (n = 93) with pre-treated advanced NSCLC who received ICI monotherapy, the authors defined primary resistance as disease progression on first radiologic evaluation or death prior to first CT evaluation, and reported it in 38.7% of patients.14 The characteristics associated with such resistance included never smokers or those who smoked fewer pack years, more involved sites, more prior therapies, and a lower mean albumin level.14 Factors associated with acquired resistance, defined as progression or death in patients after an initial clinical benefit were performance status and depth of response.14 In another study (n = 26), the median time to acquired resistance was 313 days with a 2-year survival rate from acquired resistance of 70% and there was a reported tendency for progression at lymph nodes sites.29
Biomarkers of resistance to ICI
Biomarkers for immune checkpoint inhibition that have been studied most are PD-L1 expression, tumor mutation burden (TMB) and T-cell infiltration.36 Currently the only approved predictive biomarker for immune checkpoint blockade is PD-L1 expression using immunohistochemistry (IHC) with higher levels of expression associated with improved outcomes.11 Conversely, a lower PD-L1 expression is associated with lower benefit,10,11 but despite this, advanced NSCLC patients with negative PD-L1 expression can still obtain benefit from the addition of anti-PD1 therapy over standard therapy.18,19,37 TMB, a potential predictive biomarker for ICI treatment, corresponds to somatic mutations detected by DNA sequencing. An increased number of nonsynonymous mutations results in higher neoantigen production and thus potentially increased immune recognition and response.38 Studies have reported higher TMB is associated with improved outcomes.23,39–42 The intra-tumoral heterogeneity (ITH) can also affect immune response. Patients with high neoantigen burden and low ITH treated with immune checkpoint blockade had improved OS compared with those with a high ITH.43 Decreased T-cell infiltration has been reported to be associated with a poorer prognosis44,45 and to be predictive of a decreased response to immune checkpoint blockade.46,47
Identifying biomarkers of ICI resistance is an emerging field and includes factors involving the tumor, the tumor microenvironment (TME), and the host (Figure 2). Examples related to ICI resistance in NSCLC are highlighted where possible, but biomarkers in other solid tumors are also discussed where applicable.
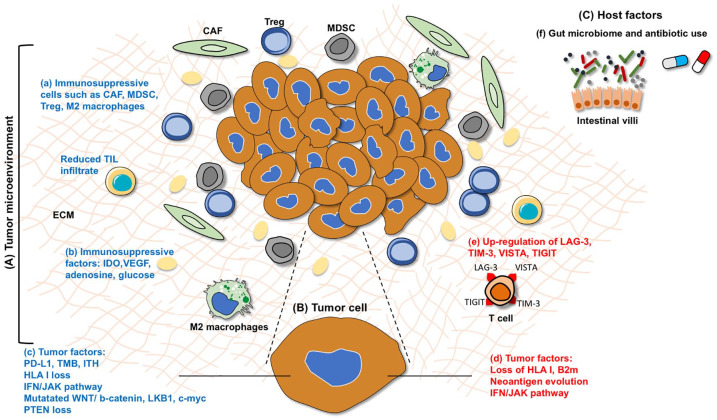
Figure 2.
Biomarkers of primary and acquired resistance occurring in the (A) tumor microenvironment (TME), (B) in the tumor, and (C) host factors. Within the TME, factors involved in primary resistance (blue font) includes the presence of (a) immunosuppressive cells including cancer associated fibroblasts (CAFs), myeloid derived suppressor cells (MDSCs), regulatory T cells (T reg), M2 macrophages, and reduced tumor infiltrating lymphocytes (TILs), (b) immune-suppressive molecules such as indoleamine 2,3-dioxygenase (IDO), adenosine, vascular endothelial growth factor (VEGF), glucose, and (c) tumoral factors such as reduced PD-L1 expression, tumor mutation burden (TMB), intra-tumoral heterogeneity (ITH), genetic loss of HLA class I, dysregulated IFN/JAK pathway, aberrant oncologic signaling pathways (PTEN loss, mutations in WNT/b-catenin, LKB1, c-myc). Biomarkers associated with acquired resistance (red font) include (d) loss of B2m and MHC-I, neoantigen evolution with loss of neoepitopes; the IFN/JAK escape pathway with loss of function JAK-1 and JAK-2 mutations and (e) the upregulation of other immune checkpoints such as T-cell immunoglobulin, mucin domain-3 protein (TIM-3), lymphocyte-activation gene 3 (LAG-3), B and T lymphocyte attenuator (BTLA), T-cell immunoreceptor tyrosine-based inhibition motif domain (TIGIT), and V-domain immunoglobulin-containing suppressor of T-cell activation (VISTA). Host factors affecting resistance includes (e) gut microbiome and antibiotic use.
Tumor factors
Tumor biomarkers associated with resistance can be generally classified into the following: tumor antigen presentation, IFN/JAK escape pathway, aberrant oncologic signaling pathways, immunosuppressive immune cells/molecules, and other immune checkpoints (Table 2).
Table 2.
Use of novel agents to overcome resistance.
| Target | Examples | Potential therapeutic approaches |
|---|---|---|
| Tumor antigen presentation | B2m, HLA, neoantigen loss | Radiotherapy, chemotherapy, epigenetic therapies, cancer vaccines, oncolytic viruses |
| IFN/JAK escape pathway | JAK-1, JAK-2 mutations | STING agonists, bispecific T-cells |
| Immunosuppressive immune cells/molecules | CAFs, MDSCs, Treg, macrophages IDO, adenosine, VEGF, glucose |
Gemcitabine, entinostat, ATRA, Targeting immuno-metabolism (glycolysis, adenosine, kynurenine pathways) VEGF inhibitors |
| Co-stimulatory signals | OX-40, 41BB, CD40, GITR | Combination therapy targeting OX-40, 41BB, CD40, GITR |
| Other immune checkpoint inhibitors | LAG-3, TIM-3, VISTA | Combination therapy targeting LAG-3, TIM-3 |
ATRA, all-trans retinoic acid; B2m, β2 microglobulin; CAF, cancer-associated fibroblast; GITR, glucocorticoid-induced tumor necrosis factor receptor-related protein; HLA, human leukocyte antigen; IDO, indoleamine-pyrrole 2,3-dioxygenase; IFN, interferon; JAK, janus kinase; LAG3, lymphocyte-activation gene 3; MDSC, myeloid-derived suppressor cell; STING, stimulator of interferon genes; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; Treg, regulatory T cell; VEGF, vascular endothelial growth factor; VISTA, V-domain Ig suppressor of T-cell activation.
Tumor antigen presentation
Disruptions in tumor antigen presentation such as alterations in antigen presentation pathways and neoantigen loss can contribute to both primary and acquired resistance.12 Defects in antigen presentation pathways can be mediated through HLA I loss or β2-microglobulin (β2-m) function. The loss of HLA class I antigens is associated with reduced tumor infiltrating T-lymphocytes48 and patients with a range of solid tumors and HLA I homozygosity treated with ICI had worse OS.49 In contrast, there was no association between HLA class I genotype and outcomes in patients with advanced NSCLC treated with PD-1/PD-L1 inhibitors.50 Further studies are required to elucidate the association between HLA I and outcomes with ICIs. Antigen presentation can be dysregulated in secondary resistance through HLA mutations or loss of heterozygosity.51,52
Another mechanism of acquired resistance disrupting antigen presentation is the loss of functional β2-m53 including a truncating mutation in β2-m.54 Acquired resistance in NSCLC has also been associated with the elimination of mutation-associated neoantigens. The loss of neoantigens can occur via the elimination of tumor subclones or deletion of truncal chromosomal regions.55
IFN-γ/JAK-STAT escape pathway
IFN-γ induces anti-tumor immune response via the activation of Janus kinase 1 or 2 (JAK-1, JAK-2). In melanoma, JAK1/2 loss of function mutations are associated with both primary and acquired resistance to ICI therapy due to an impaired response to IFN-γ stimulation.54,56,57 A small number of samples in the NSCLC TCGA cohort showed inactivating mutations in JAK258 which was associated with significantly reduced PD-L1 expression although the correlation to ICI treatment response is yet to be elucidated in this setting.
Aberrant oncologic signaling pathways
Dysregulation in oncologic signaling pathways can impair the immune response by altering the TME, resulting in resistance to ICI.59
Upregulation of β-catenin signaling is associated with reduced T cell infiltration60 and a “cold” non-inflamed tumor.61 A gain of function alteration in c-MYC is associated with decreased T cell activation and infiltration.59 Loss of phosphatase and tensin homolog (PTEN), a negative regulator or the PI3K/Akt/mTOR pathway, is linked to decreased tumor T cell infiltration and resistance to anti-PD1 therapy.62–64 Mutations in the tumor suppressor LKB1 with or without KRAS mutations are associated with an immunosuppressive TME and resistance to ICI.42,65 Improved outcomes have been reported in patients in patients with NSCLC with TP53 and or KRAS mutations treated with ICI.66–68 In patients with NSCLC harboring EGFR mutations, treatment with pembrolizumab in the first-line setting was ineffective.69 In addition, in the pre-treated setting, the effect of ICI monotherapy appears blunted in EGFR mutated NSCLC70 with a similar OS to docetaxel, whereas PD-1/PD-L1 inhibition was superior to docetaxel in wild-type EGFR NSCLC.71 These observations are explained in part by the fact constitutive EGFR activation leads to IFN-γ independent PD-L1 expression and increased levels of immunosuppressive cytokines.72
Hypoxic TME
Hypoxia and acidosis from tumor glycolytic metabolism have immunosuppressive effects on the TME,73 resulting in reduced CD8+ T-cell activity, upregulation of Treg, and macrophage switch from an inflammatory M1 phenotype to immunosuppressive M2.74–76 Studies of lung cancer cell lines have reported hypoxia-induced resistance to cytotoxic T lymphocyte mediated lysis77 and, more recently, tumor-associated macrophages (TAMs) were reported to enhance tumor hypoxia in NSCLC and modulate the activity of immune checkpoint inhibition.78
Immunosuppressive immune cells/molecules
Vascular endothelial growth factor (VEGF) is associated with an immunosuppressive TME and resistance to immunotherapy79–81 by inhibiting DC maturation, decreasing T-cell tumor infiltration, and increasing MDSCs and Treg.82–84 Retrospective analysis shows a high ORR achieved with the combination of docetaxel and the VEGF receptor 2 inhibitor, ramucirumab, in patients with prior exposure to nivolumab.85 Indoleamine 2,3-dioxygenase 1 (IDO1) catabolizes tryptophan to kynurenine and has been associated with suppression of T effector cell function and induction of Treg activation and antigen-specific immune tolerance, leading to ICI resistance.86 Increased ratio of kynurenine: tryptophan is associated with shorter survival in NSCLC and early progression on anti-PD1 therapy.86,87
Immune checkpoints
Upregulation of other immune checkpoints such as T-cell immunoglobulin and mucin domain-3 protein (TIM-3), lymphocyte-activation gene 3 (LAG-3), B and T lymphocyte attenuator (BTLA), T-cell immunoreceptor tyrosine-based inhibition motif domain (TIGIT), and/or V-domain immunoglobulin-containing suppressor of T-cell activation (VISTA) has been seen in several solid-organ malignancies. In NSCLC TIM-3 upregulation was seen in patients exhibiting secondary resistance to ICI.88–92
Host factors
A link between the gut microbiome and response to immunotherapy has been reported. Sarcomas (MCA205) in mice fed a germ-free diet failed to respond to CTLA-4 blockade and upon administration of Bacteroides fragilis, anti-tumor response was restored.93 Gut microbiome diversity and enrichment of certain bacterial species such Bifidobacterium, Akkermansia, and Faecalibacterium has been associated with sensitivity to immune checkpoint blockade in patients with NSCLC, urothelial cancer, renal cell carcinoma (RCC) and melanoma whereas Bacteroidales species has been associated with decreased response.94–96
The importance of such diversity may explain the negative effects of antibiotics on ICI treatment response seen in patients with melanoma, RCC, and NSCLC.97–99 The mechanism by which the microbiome influences response to ICI is yet to be fully elucidated but it is possible the microbiota influences anti-tumor immunity through gut metabolites facilitating T helper response and maturation of DCs.93,96,100
Treatment approaches to overcome resistance
To reduce the rate of primary resistance, therapeutic strategies include combining ICI with chemotherapy and/or novel agents. In patients with progression after exposure to ICI, approaches include cessation of ICI and switching to chemotherapy, addition of chemotherapy to ICI, or the addition of a novel agent to ICI. Selected ongoing trials of combination treatment in patients who are immunotherapy naïve and with prior immunotherapy exposure are summarized in Table 3.
Table 3.
Selected studies of ICIs combined with novel agents to overcome resistance.
| Potential treatment approaches | Study phase | ICI therapy status (naïve or prior) | Treatment | Cancer type | ClinicalTrials.gov identifier |
|---|---|---|---|---|---|
| Tumor antigen presentation | |||||
| ICI + Oncolytic viruses | I/II | Naïve/prior | Durvalumab + Pexa-Vec versus
Durvalumab + Tremelimumab + Pexa-Vec |
Colorectal | NCT03206073 |
| II | Naïve | Pembrolizumab + Pelareorep | Pancreatic | NCT03723915 | |
| I/II | Naïve/prior | Pembrolizumab + CVA21 | NSCLC | NCT02824965 | |
| I | Naïve/prior | Pembrolizumab + CVA21 | NSCLC, bladder, prostate, melanoma | NCT02043665 | |
| II | Naïve | Pembrolizumab + DNX-2401 | Glioblastoma, gliosarcoma | NCT02798406 | |
| I/II | Naïve | Nivolumab + Intra-pleural Talimogene Laherparepvec | Advanced solid tumors with malignant effusion | NCT03597009 | |
| II | Prior | Pembrolizumab + Talimogene Laherparepvec | Melanoma | NCT02965716 | |
| Ib | Naïve/prior | Atezolizumab + Talimogene Laherparepvec | TNBC, colorectal | NCT03256344 | |
| II | Naïve | Pembrolizumab + ADV/HSV-tk + SBRT | TNBC, NSCLC | NCT03004183 | |
| ICI + Targeted therapy | I | Naïve | Pembrolizumab + Afatinib | NSCLC | NCT02364609 |
| II | Naïve | Pembrolizumab + Afatinib | Lung (SCC) | NCT03157089 | |
| I | Naïve/prior | Ipilimumab or Nivolumab + Erlotinib or Crizotinib | NSCLC | NCT01998126 | |
| I/II | Naïve/prior | Nivolumab + Nimotuzumab | NSCLC | NCT02947386 | |
| II | Naïve | Nivolumab + EGF816 or INC280 | NSCLC | NCT02323126 | |
| I | Naïve | Pembrolizumab + Binimetinib | NSCLC | NCT03991819 | |
| II | Prior | Atezolizumab + Cobimetinib | NSCLC | NCT03600701 | |
| I/II | Naïve/prior | Durvalumab + Tremelimumab, +Selumetinib | NSCLC | NCT03581487 | |
| I | Naïve/prior | Pembrolizumab + Trametinib | NSCLC | NCT03299088 | |
| I/II | Naïve/prior | Pembrolizumab + Trametinib | NSCLC | NCT03225664 | |
| III | Naïve | Maintenance Pembrolizumab + Olaparib or pemetrexed (after induction Pembrolizumab/Platinum/Pemetrexed | NSCLC | NCT03976323 | |
| I | Naïve/prior | Durvalumab + AZD5363 + Olaparib | Solid tumors | NCT03772561 | |
| ICI + Cancer vaccines | I/II | Naïve/prior | Nivolumab or Pembrolizumab + CIMAvax | NSCLC, HNSCC | NCT02955290 |
| I | Naïve | Pembrolizumab + NEO-PV-01 | NSCLC (non-squamous) | NCT03380871 | |
| I/II | Naïve/prior | Pembrolizumab + Galinpepimut-S | Solid tumors | NCT03761914 | |
| Ib | Naïve | Pembrolizumab + PVX-410 | TNBC (HLA-A2+) | NCT03362060 | |
| ICI + Chemotherapy | Ib | Naïve | Pembrolizumab + Liposomal Doxorubicin | Breast (endocrine resistant) | NCT03591276 |
| II | Naïve/prior | Atezolizumab + Vinorelbine | NSCLC | NCT03801304 | |
| ICI + Radiotherapy | I | Naïve | Ipilimumab + Nivolumab + RT | NSCLC | NCT04013542 |
| II | Naïve | Anti-PD1 + Radiotherapy | Melanoma | NCT04017897 | |
| ICI + ACT | I | Prior | FT500 versus
FT500 + ICI (nivolumab, pembrolizumab, atezolizumab) |
Advanced solid tumors, lymphoma | NCT03841110 |
| I | Naïve | Nivolumab + Cyclophosphamide + Fludarabine + TIL + IL-2 | NSCLC | NCT03215810 | |
| II | Naïve/prior | GSK3377794 versus
Pembrolizumab + GSK3377794 |
NSCLC | NCT03709706 | |
| II | Naïve | Anti-PD-1 + D-CIK | Advanced solid tumors | NCT02886897 | |
| ICI + HDAC inhibitors | I/II | Naïve/prior | Pembrolizumab + Entinostat | NSCLC, melanoma, colorectal (MSS) | NCT02437136 |
| I/II | Naïve/prior | Pembrolizumab + Vorinostat | NSCLC | NCT02638090 | |
| IFN/JAK escape pathway | |||||
| ICI + Sting agonists | I | Naïve/prior | GSK3745417 versus
Pembrolizumab + GSK3745417 |
Advanced solid tumors | NCT03843359 |
| I | Naïve/prior | PDR001 + MIW815 | Advanced solid tumors, lymphomas | NCT03172936 | |
| I | Naïve/prior | MIW815 ± Ipilimumab | Advanced solid tumors, lymphomas | NCT02675439 | |
| ICI + JAK inhibitor | II | Naïve | Pembrolizumab + Itacitinib | NSCLC | NCT03425006 |
| ICI + PI3Ki | Ib/II | Prior | Pembrolizumab + idelalisib | NSCLC | NCT03257722 |
| Immunosuppressive immune cells/molecules | |||||
| ICI + VEGF inhibitor | I/II | Naïve/prior | Nivolumab + Ipilimumab + Nintedanib | NSCLC | NCT03377023 |
| II | Naïve/prior | Nivolumab + Ramucirumab | NSCLC | NCT03527108 | |
| I/II | Naïve | Pembrolizumab + Lenvatinib | NSCLC, RCC, endometrial, urothelial, HNSCC | NCT02501096 | |
| III | Naïve | Pembrolizumab+ Platinum chemotherapy + Pemetrexed ± Lenvatinib | NSCLC (non-squamous) | NCT03829319 | |
| II | Naïve | Atezolizumab + Bevacizumab | NSCLC | NCT04099836 | |
| I | Naïve | Nivolumab or Pembrolizumab + Vorolanib | HCC, gastric, GEJ | NCT03511222 | |
| ICI + IDO inhibitor | II | Naïve | Pembrolizumab ± Epacadostat | NSCLC | NCT03322540 |
| II | Naïve | Pembrolizumab + Platinum doublet + Epacadostat | NSCLC | NCT03322566 | |
| I/II | Naïve | Pembrolizumab + IO102 ± Platinum doublet | NSCLC | NCT03562871 | |
| II | Naïve | Nivolumab ± BMS986205 | HNSCC | NCT03854032 | |
| I/II | Naïve | Pembrolizumab, Nivolumab or Ipilimumab + Indoximod | Melanoma | NCT02073123 | |
| ICI + Adenosine receptor antagonist | I/Ib | Naïve/prior | PBF509 versus
PBF509+PDR001 |
NSCLC | NCT02403193 |
| I | Naïve/prior | AB928 + chemotherapy versus
AB928 + Pembrolizumab + chemotherapy versus AB122 |
NSCLC | NCT03846310 | |
| I/Ib | Naïve/prior | CPI-444 Atezolizumab + CPI-444 |
Advanced solid tumors | NCT02655822 | |
| I | Naïve/prior | MK-3814 Pembrolizumab + MK-3814 |
Advanced solid tumors | NCT03099161 | |
| ICI + CD73 inhibitor | I | Naïve/prior | NZV930 versus
PDR001 + NZV930 |
Advanced solid tumors | NCT035490002 |
| I/II | Naïve/prior | BMS-986179 Nivolumab + BMS-986179 Nivolumab + BMS-986179 + rHuPH20 |
Advanced solid tumors | NCT02754141 | |
| I | Naïve/prior | Oleclumab versus
Durvalumab + Oleclumab |
Solid tumors | NCT02503774 | |
| I | Naïve | Durvalumab + Oleclumab Durvalumab + Oleclumab + Chemotherapy |
NSCLC | NCT03819465 | |
| ICI + RANKL inhibitor | II | Naïve | Nivolumab + Denosumab | NSCLC | NCT03669523 |
| ICI + CD39 inhibitor | I | Naïve/prior | TTX030 versus
Pembrolizumab + TTX030 versus Chemotherapy + TTX030 |
Advanced solid tumors, lymphoma | NCT03884556 |
| ICI + anti-IL-1β | III | Naïve | Platinum doublet + Pembrolizumab ± Canakinumab | NSCLC | NCT03631199 |
| Co-stimulatory signals | |||||
| ICI + r-interleukin | I | Naïve/prior | rIL-15 + Nivolumab versus
rIL-15 + Ipilimumab versus rIL-15 + Nivolumab + Ipilimumab |
Advanced solid tumors | NCT03388632 |
| I | Naïve | Pembrolizumab + rIL-12 | Advanced solid tumors | NCT03030378 | |
| PD-L1x4-1BB bispecific antibody | I | Naïve/prior | INBRX-105 | Solid tumors, lymphoma | NCT03809624 |
| I | Naïve/prior | ES101 | Solid tumors | NCT04009460 | |
| ICI + Anti-ICOS | I/II | Naïve/prior | KY1044
versus, Atezolizumab + KY1044 |
Advanced solid tumors | NCT03829501 |
| II | Naïve/prior | Tremelimumab + GSK3359609 | Advanced solid tumors | NCT03693612 | |
| ICI + Anti-GITR | I/II | Naïve/prior | Ipilimumab + Nivolumab + BMS-986156 ± SBRT | Advanced solid tumors | NCT04021043 |
| I/Ib | Naïve/prior | GWN323 versus
PDR001 + GWN323 |
Advanced solid tumors, lymphoma | NCT02740270 | |
| ICI + Microbiota | I | Naïve/prior | Anti-PD-1/PD-L1 + MET4 | Advanced solid tumors | NCT03686202 |
| I | – | Anti-PD1/PDL1 + Fecal microbial transplantation | Melanoma | NCT03772899 | |
| I | Naïve | Nivolumab + Ipilimumab ± CBM588 | RCC | NCT03829111 | |
| II | Naïve | Pembrolizumab + Fecal transplant | Prostate | NCT04116775 | |
| ICI + TLR9 agonist | I | Naïve/prior | Nivolumab + DV281 | NSCLC | NCT03326752 |
| I/II | Naïve/prior | Pembrolizumab + intra-tumoral AST-008 | Advanced solid tumors | NCT03684785 | |
| Co-inhibitory or other immune checkpoints | |||||
| Anti-PD(L)1 + Anti-CTLA4 | III | Naïve | Nivolumab versus
Nivolumab + Ipilimumab versus Nivolumab + Platinum doublet versus Platinum doublet |
NSCLC | NCT02477826 |
| III | Naïve | Nivolumab + Ipilimumab Carboplatin doublet |
NSCLC | NCT03351361 | |
| III | Naïve | REGN2810 + ipilimumab versus
REGN2810 + platinum doublet + Ipilimumab versus Pembrolizumab |
NSCLC | NCT03515629 | |
| III | Naïve | Durvalumab + Tremelimumab versus
Chemotherapy |
NSCLC | NCT02542293 | |
| III | Naïve | Pembrolizumab ± Ipilimumab | NSCLC | NCT03302234 | |
| III | Naïve | Nivolumab + chemotherapy versus
Nivolumab + Ipilimumab versus Chemotherapy |
NSCLC | NCT02864251 | |
| II | Naïve | Nivolumab + Ipilimumab + Temozolomide | Colorectal (MSS, MGMT promoter methylated) | NCT03832621 | |
| ICI + LAG-3 inhibitor | II | Naïve/prior | Pembrolizumab + Eftilagimod Alpha | NSCLC HNSCC |
NCT03625323 |
| I/II | Naïve/prior | LAG525 ± PDR001 | Advanced solid tumors | NCT02460224 | |
| I | Naïve/prior | BI 754111 + BI 754091 | Advanced solid tumors | NCT03156114 | |
| II | Prior | Nivolumab + Relatlimab | Colorectal (MSI-H) | NCT03607890 | |
| II | Naïve | Nivolumab + Relatlimab | Colorectal (MSS) | NCT03642067 | |
| II | Naïve | Nivolumab + Relatlimab | Melanoma | NCT03743766 | |
| ICI + TIM-3 inhibitor | II | Naïve | TSR-042 + TSR-022 | HCC | NCT03680508 |
| I | Naïve/prior | TSR-022 TSR-022 + nivolumab TSR-022 + TSR-042 TSR-022 + TSR-042 + TSR-033 |
Advanced solid tumors | NCT02817633 | |
| I/II | Naïve/prior | Tislelizumab + BGB-A425 | Advanced solid tumors | NCT03744468 | |
| I | Naïve/prior | RO7121661 (bispecific antibody) | Advanced solid tumors | NCT03708328 | |
| ICI + anti-TGIT | I | Naïve/prior | AB122 AB122 + AB154 |
Advanced solid tumors | NCT03628677 |
ACT, adoptive cell therapy; AML, acute myelogenous leukemia; CTLA-4, cytotoxic T-lymphocyte-associated protein 4; CVA21, coxsackie virus 21; GEJ, gastro-esophageal junction; GITR, glucocorticoid-induced tumor necrosis factor receptor-related protein; HCC, hepatocellular carcinoma; HDAC, histone deacetylase; HNSCC, head and neck squamous cell carcinoma; ICI, immune checkpoint inhibitor; ICOS, inducible co-stimulator; IDO, indoleamine-pyrrole 2,3-dioxygenase; IFN, interferon; JAK, janus kinase; LAG3, lymphocyte-activation gene 3; MET-4, microbial ecosystem therapeutics; MGMT, methylguanine-DNA methyltransferase; MSI-H, microsatellite instability high; MSS, microsatellite stable; NSCLC, non-small cell lung carcinoma; PI3Ki, phosphatidylinositol-3-kinase inhibitor; RCC, renal cell carcinoma; rIL-12, recombinant interleukin-12; rIL-15, recombinant interleukin 15; STING, stimulator of interferon genes; TGIT, T-cell immunoreceptor with Ig and ITIM domains; TIL, tumor-infiltrating lymphocyte; TIM-3, T-cell immunoglobulin and mucin-domain containing-3; TLR, Toll-like receptor; TNBC, triple negative breast cancer; VEGF, vascular endothelial growth factor.
Addition of chemotherapy to ICI
In the first-line setting, pembrolizumab monotherapy is superior to chemotherapy in NSCLC with a PD-L1 expression of ⩾1%, but progression as best response is seen in about 21–22% of patients (Table 1).15,16 Addition of doublet chemotherapy to pembrolizumab in this setting reduces the rate of such primary resistance to 6.9–8.8%, as reported in KEYNOTE 189 and 407.18,19 The benefit of chemotherapy in such a combination is due to induction of immunogenic cell death and modulation of immune response8 and in the first-line advanced setting is associated with improved outcomes compared with chemotherapy alone.18,19,22
The benefit of adding chemotherapy to patients progressing on an ICI is being examined. For example, a phase II study [ClinicalTrials.gov identifier: NCT03083808] is enrolling patients with prior platinum-based chemotherapy and prior PD-1 or PD-L1 inhibitor as their most recent treatment who have had at least a 3-month PFS on this therapy. Patients will be treated with pembrolizumab combined with either gemcitabine, docetaxel, or pemetrexed.
An ECOG-ACRIN phase III study will also examine the effect of adding chemotherapy following pembrolizumab failure. Patients with advanced non-squamous NSCLC with PD-L1 expression of at least 1% will be treated with pembrolizumab and, upon progression, will switch to chemotherapy. In the second arm, patients will be treated with first-line pembrolizumab and at the time of disease progression, chemotherapy will be added to pembrolizumab, and in the third arm, acting as control, patients will receive chemotherapy and pembrolizumab [ClinicalTrials.gov identifier: NCT03793179].
Switching to chemotherapy with or without an anti-angiogenic agent
In the setting where patients have progressed on an ICI, cessation of therapy and switching to chemotherapy, either to a platinum doublet if ICI monotherapy was given in the first-line setting, or docetaxel with or without an anti-angiogenic agent if an ICI and a platinum doublet was administered previously. Retrospective studies have suggested improved response rates with cytotoxic chemotherapy in patients following progression after ICI treatment (Table 4). Schvartsman et al. reported an overall response rate (ORR) of 39% with single-agent chemotherapy in patients who have received prior platinum chemotherapy and PD-1/PD-L1 inhibitor.101 In a Korean study, patients progressing on first-line PD-1/PD-L1 inhibitor were treated with a platinum doublet or single-agent chemotherapy, with a reported ORR of 66.7% and 46.9%, respectively.102 Responses seen with first-line platinum doublet are typically 27–32% as reported in the control arms of KEYNOTE 024 and KEYNOTE 042.15,16
Table 4.
Studies of subsequent chemotherapy with or without anti-angiogenic agent in patients with prior immune checkpoint inhibitor therapy.
| Study | N | Prior treatment | Treatment | ORR | Survival |
|---|---|---|---|---|---|
| Schvartsman et al.101 | 28 | Platinum chemotherapy, PD-1/PD-L1 inhibitor | Single-agent chemotherapy | 39% | 4.7 m (PFS) |
| Grigg et al.103 | 38 | PD-1/PD-L1 inhibitor | Platinum doublet ± bevacizumab Ramucirumab + docetaxel Single-agent chemotherapy |
25% | 3.8 m (TTP) |
| Leger et al.104 | 67 | PD-1/PD-L1 inhibitor | Single-agent chemotherapy | 27% | NR |
| Park et al.102 | 24 49 |
PD-1/PD-L1 inhibitor | Platinum doublet monotherapy |
66.7% 46.9% |
4.5 m (PFS) 3.8 m (PFS) |
| Grohe et al. 105 | 22 | Platinum chemo, pembrolizumab/ nivolumab | Nintedanib + docetaxel | 58% | 5.5 m (PFS) |
| Corral et al.106 | 11 | Platinum chemotherapy, PD-1/PD-L1 inhibitor | Nintedanib + docetaxel | 36.5% | NR |
| Capelletto et al.107 | 16 | Chemotherapy, PD-1/PD-L1 inhibitor | Nintedanib + docetaxel | NR | 5.84 m (PFS) |
| Molife et al.108 | 265 | Chemotherapy, PD-1/PD-L1 inhibitor | Ramucirumab + chemotherapy | NR | 26.5 m (OS) |
m, months; NR, not reported; ORR, overall response rate; OS, overall survival; PFS, progression-free survival; TTP, time to progression.
In patients who have received a prior platinum doublet and an ICI, the ORR with docetaxel combined with nintedanib was reported to be 36.5%106 and 58%105 (Table 4). These results compare favorably with the docetaxel arm in CHECKMATE 057, CHECKMATE 017, and KEYNOTE 010, with an ORR of 9–12% and in studies of docetaxel combined with nintedanib (LUME-Lung 1), or ramucirumab (REVEL) with ORR of 4.4% and 23%, respectively.109,110 The biological basis to explain the efficacy of combination docetaxel with an anti-angiogenic agent is unknown but possible explanations include the inhibition of the immunosuppressive VEGF pathway which may alter the TME to an immune-permissive state, leading to leading to anti-tumor immunity.83 However, given the retrospective nature and small sample size in the majority of these studies, prospective studies on the role of combining chemotherapy with an anti-angiogenic agent should be performed.
Addition of a novel agent to an ICI
Combining an ICI with a novel agent in the first-line or subsequent therapy setting is an area of intense research interest. Potential therapeutic strategies to overcome resistance and increase sensitivity to immunotherapy include targeting the tumor antigen presentation pathway, the IFN/JAK escape pathway, immunosuppressive immune cells/molecules, co-inhibitory or other immune checkpoints and co-stimulatory signals (Table 2). Examples for each approach will be discussed and selected ongoing studies are summarized in Table 3.
Targeting tumor antigen presentation pathway
Approaches to improve tumor antigenicity include combining an ICI with modalities such as chemotherapy, epigenetic therapies, radiotherapy (RT), cancer vaccines, or oncolytic viruses.
Cytotoxic chemotherapy increases the efficacy of ICI by inducing immunogenic cell death and modulating immune response8 as mentioned previously. Epigenetic mechanisms play a role in immunosuppression111 and DNA methyltransferase and histone deacetylase (HDAC) inhibitors can induce an immunostimulatory response by inhibiting Treg, MDSCs, and upregulating antigen presentation and cytokine production.112 A phase II study of pembrolizumab plus the HDAC inhibitor entinostat, in patients with advanced NSCLC with prior progression on PD-1/PD-L1 inhibitor (ENCORE-601), reported a response rate of only 11%.113 Although the prespecified ORR target was not reached, insight gained through biomarker studies may aid in patient selection for future studies.113,114
An alternative strategy is to combine RT with an ICI. RT induces immunogenic cell death and increases tumor antigen presentation. A phase II study (PEMBRO-RT) compared pembrolizumab with or without single-site-directed RT.115 A non-significant improvement in outcomes was seen and further studies are required to evaluate its potential benefit. Cancer vaccines such as DC vaccines, peptide vaccines, and neoantigen vaccines, can improve antigen presentation and recognition, increase tumor antigen-specific CTLs and enhance tumor T-cell infiltration, respectively, thus restoring anti-tumor immunity.116 A phase I study of a personalized neoantigen vaccine (NEO-PV-01) plus nivolumab in PD-1/PD-L1 naïve NSCLC reported a response rate of 25%.117 Oncolytic viruses can selectively infect tumor cells, induce tumor cell lysis, leading to systemic anti-tumor immunity.118 In fact, Talimogene laherparepvec (T-VEC) is the first FDA-approved virotherapeutic approach in the treatment of patients with unresectable melanoma.119 In a phase I study (KEYNOTE-200), patients with advanced NSCLC were treated with coxsackievirus 21, an oncolytic virus, plus pembrolizumab. The overall response was 23% in ICI naïve patients.120
Targeting the IFN/JAK escape pathway
The stimulator of interferon genes (STING) pathway plays an important role in adaptive anti-tumor response and represents an attractive immuno-therapeutic target. Pre-clinical models resistant to ICI were re-sensitized when combined with STING agonists.121 In a phase I study where patients were treated with intra-tumoral MK-1454, a STING agonist, as monotherapy or in combination with pembrolizumab, the ORR was 0% and 25%, respectively,122 suggesting combination therapy may be the optimal approach.
Oncologic signaling pathways
Combining a PD-1/PD-L1 inhibitor with molecular targeted agents improves anti-tumor activity in BRAF mutant melanoma123–125 and has been shown to be a successful treatment approach in patients with advanced RCC.126,127 However, early phase studies in oncogene-driven NSCLC treated with ICI and an EGFR or ALK TKI, highlighted increased and unexpected toxicities, and reported response rates were lower than observed with single-agent targeted therapy.128,129 Further evaluation of the optimal sequence, schedule, and dosing of such combinations will be required.59,72.
Immunosuppressive immune cells/molecules
The combination of VEGF inhibitors and ICI can negate an immune-suppressive TME and reverse resistance to immunotherapy.82,83,130 For example, bevacizumab plus atezolizumab and chemotherapy is associated with an improvement in PFS and OS in patients with advanced NSCLC.22 In the pre-treated setting sitravatinib (MGCD516), a tyrosine kinase inhibitor targeting VEGFR2, PDGFRA, KIT, Tyro, AXL, and MER may restore or enhance the activity of immune checkpoint blockade in NSCLC patients with immunotherapy resistance.131 A phase II study of sitravatinib plus nivolumab in NSCLC was reported to show a response rate of 16% in patients who have progressed following prior ICI.132 In a phase II study of patients with prior anti-PD-1/PDL1 therapy, the combination of pembrolizumab and lenvatinib, a VEGFR/FGFR/PDGFRα, RET, and KIT inhibitor, reported an ORR of 33.3%.133 Another approach to overcome resistance is by targeting macrophages. The CD47-SIRPα axis signals the macrophage to ignore cells in which CD47 is expressed and tumors upregulate CD47 to evade immune response.134 ALX148 is an antibody that binds and blocks CD47, resulting in enhanced macrophage phagocytosis and an increased ratio of inflammatory M1 TAMs to immunosuppressive M2 TAMs.135 In a phase I study of patients with advanced solid tumors treated with ALX148 and pembrolizumab, the disease control rate (DCR) in NSCLC patients with or without prior ICI was 17%.136
Increasing co-stimulatory signals
T-cell activation can be augmented by agonists stimulating targets such as OX40, 4-1BB, glucocorticoid-induced TNFR-related protein (GITR), and inducible T-cell co-stimulator (ICOS). Co-stimulation induces cytotoxic T-cell proliferation, increased survival and effector function.137 In a phase I study of patients with solid tumors treated with single-agent TRX518, a GITR agonist, no responses were observed but subsequent pre-clinical work showed the addition of PD-1 blockade overcame anti-GITR resistance and induced tumor regression,138 and thus providing a rationale for combining with an ICI (Table 3). Early phase studies of GITR agents such as MK-1248 and MK-4166 have reported responses when combined a PD-1 inhibitor.139
Co-inhibitory or other immune checkpoints
The effectiveness of two ICIs in advanced NSCLC was shown in CHECKMATE 227 with ipilimumab plus nivolumab resulting in an improvement in PFS in patients with a high TMB23 and prolonged OS in patients regardless of PD-L1 status.140 In contrast, the combination durvalumab and tremelimumab in the MYSTIC study did not meet the primary endpoint for OS versus chemotherapy.24
Other checkpoints have also been studied. Inhibition of LAG-3 restores T effector cells activity and reduces the activity regulatory T cells, enhancing the anti-tumor activity of PD-1 inhibition.141 The combination of anti-PD-1 and anti-LAG-3 therapy has been reported to increase anti-tumor activity compared with anti-PD-1 alone in melanoma patients who have progressed on anti-PD-1 therapy.142 In a phase I/II study of pre-treated patients with advanced solid tumors treated with a LAG-3 inhibitor (LAG-525) and PD-1 inhibitor (PDR001), durable responses were observed in three out of eight patients with mesothelioma and two out of five patients with triple negative breast cancer, but no responses were seen in patients with NSCLC.143 In a phase I study, NSCLC patients with prior anti-PD-1/PD-L1 treatment received TSR-022 (TIM-3 inhibitor) in combination with TSR-042 (PD-1 inhibitor), with a response rate of 13% reported.144
Such ICI combinations in patients who have progressed on ICI monotherapy may help to target the changing TME seen during treatment with anti-PD1 therapy with on treatment biopsy assessment showing upregulation of related checkpoint genes PDCD1 (PD-1), CD284 (PD-L1), CTLA-4, and LAG3 among others.145 An adaptive approach may be required with alternating combinations utilized dependent on biopsy assessment in view of these dynamic changes.
Future approaches and conclusion
ICI therapy is associated with durable responses in a minority of patients with many displaying primary resistance, while secondary resistance to therapy subsequently occurs in a significant proportion. Here we have reviewed some of the main drivers behind such resistance and potential therapeutic strategies to overcome them.
Currently, multiple studies examining the combination of immunotherapeutic agents with cytotoxic chemotherapy, radiation, or molecular targeted agents are underway, with the aim of reducing resistance and providing long-lasting disease control. In addition, combining immunotherapeutic agents with ICI is an area of intense research, with agents targeting the IFN/JAK escape pathway, immunosuppressive immune cells and molecules, co-inhibitory/immune checkpoints, and co-stimulatory signals (Table 3). With the rapid pace of immunotherapy drug development and the burgeoning number and often duplicate combination studies,146 to increase the chances of success, rationally designed clinical trials of combination agents becomes imperative and should be based on robust pre-clinical data, together with the use of pharmacodynamic biomarkers and novel innovative endpoints.147
It should be noted that much of our current knowledge on resistance mechanisms and its biomarkers is derived from melanoma studies, and the ability to apply this in the NSCLC setting is uncertain with further studies specific to lung cancer required. Such studies will ideally incorporate a standardized definition of primary and secondary ICI resistance as suggested in this review to allow accurate categorization of response and they will need to overcome the problem of sample accessibility to allow longitudinal tumor assessments in order to accurately depict on treatment changes underlying resistance.
To date, precision medicine has been applied successfully in oncogene-driven NSCLC.148 To enable personalized cancer immunotherapy, advances in immune-diagnostics and biomarker development are ongoing together with major efforts to increase our understanding of the mechanisms of response and resistance to ICIs.64,80,149–151
Footnotes
Conflict of interest statement: RAS has received honoraria from Astra-Zeneca, BMS, Boehringer Ingelheim, Celgene, Lilly, Merck, Novartis, Pfizer, Roche, Taiho, Takeda, and Yuhan; and research funding from Astra-Zeneca and Boehringer Ingelheim. RJW reports no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Contributor Information
Robert J. Walsh, Department of Haematology–Oncology, National University Cancer Institute Singapore, Singapore
Ross A. Soo, Department of Haematology–Oncology, National University Cancer Institute Singapore, National University Health System, 1E Kent Ridge Road, NUHS Tower Block Level 7, Singapore, 119228, Singapore.
References
- 1. Kazandjian D, Keegan P, Suzman DL, et al. Characterization of outcomes in patients with metastatic non-small cell lung cancer treated with programmed cell death protein 1 inhibitors past RECIST version 1.1-defined disease progression in clinical trials. Semin Oncol 2017; 44: 3–7. [DOI] [PubMed] [Google Scholar]
- 2. Ricciuti B, Genova C, Bassanelli M, et al. Safety and efficacy of nivolumab in patients with advanced non-small-cell lung cancer treated beyond progression. Clin Lung Cancer 2019; 20: 178–185.e172. [DOI] [PubMed] [Google Scholar]
- 3. Gandara DR, von Pawel J, Mazieres J, et al. Atezolizumab treatment beyond progression in advanced NSCLC: results from the randomized, phase III OAK study. J Thorac Oncol 2018; 13: 1906–1918. [DOI] [PubMed] [Google Scholar]
- 4. Kim C, Hoang CD, Kesarwala AH, et al. Role of local ablative therapy in patients with oligometastatic and oligoprogressive non-small cell lung cancer. J Thorac Oncol 2017; 12: 179–193. [DOI] [PubMed] [Google Scholar]
- 5. Ning MS, Gomez DR, Heymach JV, et al. Stereotactic ablative body radiation for oligometastatic and oligoprogressive disease. Transl Lung Cancer Res 2019; 8: 97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Laurie SA, Banerji S, Blais N, et al. Canadian consensus: oligoprogressive, pseudoprogressive, and oligometastatic non-small-cell lung cancer. Curr Oncol 2019; 26: e81–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tumati V, Iyengar P. The current state of oligometastatic and oligoprogressive non-small cell lung cancer. J Thorac Dis 2018; 10: S2537–S2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Daniel S, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013; 39: 1–10. [DOI] [PubMed] [Google Scholar]
- 9. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012; 12: 252–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Low JL, Walsh RJ, Ang Y, et al. The evolving immuno-oncology landscape in advanced lung cancer: first-line treatment of non-small cell lung cancer. Ther Adv Med Oncol 2019; 11: 1758835919870360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Doroshow DB, Sanmamed MF, Hastings K, et al. Immunotherapy in non-small cell lung cancer: facts and hopes. Clin Cancer Res 2019; 25: 4592–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Syn NL, Teng MWL, Mok TSK, et al. De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol 2017; 18: e731–e741. [DOI] [PubMed] [Google Scholar]
- 13. Jenkins RW, Barbie DA, Flaherty KT. Mechanisms of resistance to immune checkpoint inhibitors. Br J Cancer 2018; 118: 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shah S, Wood K, Labadie B, et al. Clinical and molecular features of innate and acquired resistance to anti-PD-1/PD-L1 therapy in lung cancer. Oncotarget 2017; 9: 4375–4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016; 375: 1823–1833. [DOI] [PubMed] [Google Scholar]
- 16. Mok TSK, Wu Y-L, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet 2019; 393: 1819–1830. [DOI] [PubMed] [Google Scholar]
- 17. Carbone DP, Reck M, Paz-Ares L, et al. First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 2017; 376: 2415–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gandhi L, Rodríguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med 2018; 378: 2078–2092. [DOI] [PubMed] [Google Scholar]
- 19. Paz-Ares L, Luft A, Vicente D, et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med 2018; 379: 2040–2051. [DOI] [PubMed] [Google Scholar]
- 20. West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2019; 20: 924–937. [DOI] [PubMed] [Google Scholar]
- 21. Socinski MA, Koynov KD, Berard H, et al. LBA65 IMpower131: progression-free survival (PFS) and overall survival (OS) analysis of a randomised phase III study of atezolizumab + carboplatin + paclitaxel or nab-paclitaxel vs carboplatin + nab-paclitaxel in 1L advanced squamous NSCLC. Ann Oncol 2018; 29(Suppl. 8): mdy424-077. [Google Scholar]
- 22. Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med 2018; 378: 2288–2301. [DOI] [PubMed] [Google Scholar]
- 23. Hellmann MD, Ciuleanu T-E, Pluzanski A, et al. Nivolumab plus ipilimumab in lung cancer with a high tumor mutational burden. N Engl J Med 2018; 378: 2093–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rizvi NA, Chul Cho B, Reinmuth N, et al. LBA6 Durvalumab with or without tremelimumab vs platinum-based chemotherapy as first-line treatment for metastatic non-small cell lung cancer: MYSTIC. Ann Oncol 2018; 29(Suppl. 10): mdy511-005. [Google Scholar]
- 25. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med 2015; 373: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med 2015; 373: 1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016; 387: 1540–1550. [DOI] [PubMed] [Google Scholar]
- 28. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet 2017; 389: 255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gettinger SN, Wurtz A, Goldberg SB, et al. Clinical features and management of acquired resistance to PD-1 axis inhibitors in 26 patients with advanced non-small cell lung cancer. J Thorac Oncol 2018; 13: 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chiou VL, Burotto M. Pseudoprogression and immune-related response in solid tumors. J Clin Oncol 2015; 33: 3541–3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hodi FS, Ballinger M, Lyons B, et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 2018; 36: 850–858. [DOI] [PubMed] [Google Scholar]
- 32. Tazdait M, Mezquita L, Lahmar J, et al. Patterns of responses in metastatic NSCLC during PD-1 or PDL-1 inhibitor therapy: comparison of RECIST 1.1, irRECIST and iRECIST criteria. Eur J Cancer 2018; 88: 38–47. [DOI] [PubMed] [Google Scholar]
- 33. Nishino M, Giobbie-Hurder A, Gargano M, et al. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res 2013; 19: 3936–3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Borcoman E, Nandikolla A, Long G, et al. Patterns of response and progression to immunotherapy. Am Soc Clin Oncol Educ Book 2018; 38: 169–178. [DOI] [PubMed] [Google Scholar]
- 35. Antonia SJ, Borghaei H, Ramalingam SS, et al. Four-year survival with nivolumab in patients with previously treated advanced non-small-cell lung cancer: a pooled analysis. Lancet Oncol 2019; 20: 1395–1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Topalian SL, Taube JM, Anders RA, et al. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer 2016; 16: 275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ 2018; 362: k3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Rizvi NA, Hellmann MD, Snyder A, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015; 348: 124–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gandara DR, Paul SM, Kowanetz M, et al. Blood-based tumor mutational burden as a predictor of clinical benefit in non-small-cell lung cancer patients treated with atezolizumab. Nat Med 2018; 24: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 40. Kim ES, Velcheti V, Mekhail T, et al. LBA55 primary efficacy results from B-F1RST, a prospective phase II trial evaluating blood-based tumour mutational burden (bTMB) as a predictive biomarker for atezolizumab (atezo) in 1L non-small cell lung cancer (NSCLC). Ann Oncol 2018; 29(Suppl. 8): mdy424-067. [Google Scholar]
- 41. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med 2017; 377: 2500–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hellmann MD, Nathanson T, Rizvi H, et al. Genomic features of response to combination immunotherapy in patients with advanced non-small-cell lung cancer. Cancer Cell 2018; 33: 843–852.e844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McGranahan N, Furness AJS, Rosenthal R, et al. Clonal neoantigens elicit T cell immunoreactivity and sensitivity to immune checkpoint blockade. Science 2016; 351: 1463–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schalper KA, Brown J, Carvajal-Hausdorf D, et al. Objective measurement and clinical significance of TILs in non-small cell lung cancer. J Natl Cancer Inst 2015; 107: dju435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Soo RA, Chen Z, Yan Teng RS, et al. Prognostic significance of immune cells in non-small cell lung cancer: meta-analysis. Oncotarget 2018; 9: 24801–24820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Herbst RS, Soria J-C, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014; 515: 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014; 515: 568–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Perea F, Sánchez-Palencia A, Gómez-Morales M, et al. HLA class I loss and PD-L1 expression in lung cancer: impact on T-cell infiltration and immune escape. Oncotarget 2017; 9: 4120–4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chowell D, Morris LGT, Grigg CM, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 2018; 359: 582–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Negrao MV, Lam VK, Reuben A, et al. PD-L1 expression, tumor mutational burden, and cancer gene mutations are stronger predictors of benefit from immune checkpoint blockade than HLA class I genotype in non-small cell lung cancer. J Thorac Oncol 2019; 14: 1021–1031. [DOI] [PubMed] [Google Scholar]
- 51. Shukla SA, Rooney MS, Rajasagi M, et al. Comprehensive analysis of cancer-associated somatic mutations in class I HLA genes. Nat Biotechnol 2015; 33: 1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. McGranahan N, Rosenthal R, Hiley CT, et al. Allele-specific HLA loss and immune escape in lung cancer evolution. Cell 2017; 171: 1259–1271.e1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Restifo NP, Marincola FM, Kawakami Y, et al. Loss of functional beta 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst 1996; 88: 100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016; 375: 819–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Anagnostou V, Smith KN, Forde PM, et al. Evolution of neoantigen landscape during immune checkpoint blockade in non-small cell lung cancer. Cancer Discov 2017; 7: 264–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shin DS, Zaretsky JM, Escuin-Ordinas H, et al. Primary resistance to PD-1 blockade mediated by JAK1/2 mutations. Cancer Discov 2017; 7: 188–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gao J, Shi LZ, Zhao H, et al. Loss of IFN-γ pathway genes in tumor cells as a mechanism of resistance to anti-CTLA-4 therapy. Cell 2016; 167: 397–404.e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li SD, Ma M, Li H, et al. Cancer gene profiling in non-small cell lung cancers reveals activating mutations in JAK2 and JAK3 with therapeutic implications. Genome Med 2017; 9: 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Spranger S, Gajewski TF. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat Rev Cancer 2018; 18: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 2015; 523: 231. [DOI] [PubMed] [Google Scholar]
- 61. Luke JJ, Bao R, Sweis RF, et al. WNT/β-catenin pathway activation correlates with immune exclusion across human cancers. Clin Cancer Res 2019; 25: 3074–3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Peng W, Chen JQ, Liu C, et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov 2016; 6: 202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. George S, Miao D, Demetri GD, et al. Loss of PTEN is associated with resistance to anti-PD-1 checkpoint blockade therapy in metastatic uterine leiomyosarcoma. Immunity 2017; 46: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Roh W, Chen P-L, Reuben A, et al. Integrated molecular analysis of tumor biopsies on sequential CTLA-4 and PD-1 blockade reveals markers of response and resistance. Sci Transl Med 2017; 9: eaah3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Koyama S, Akbay EA, Li YY, et al. STK11/LKB1 deficiency promotes neutrophil recruitment and proinflammatory cytokine production to suppress T-cell activity in the lung tumor microenvironment. Cancer Res 2016; 76: 999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Biton J, Mansuet-Lupo A, Pécuchet N, et al. TP53, STK11, and EGFR mutations predict tumor immune profile and the response to anti-PD-1 in lung adenocarcinoma. Clin Cancer Res 2018; 24: 5710–5723. [DOI] [PubMed] [Google Scholar]
- 67. Dong Z-Y, Zhong W-Z, Zhang X-C, et al. Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res 2017; 23: 3012–3024. [DOI] [PubMed] [Google Scholar]
- 68. Assoun S, Theou-Anton N, Nguenang M, et al. Association of TP53 mutations with response and longer survival under immune checkpoint inhibitors in advanced non-small-cell lung cancer. Lung Cancer 2019; 132: 65–71. [DOI] [PubMed] [Google Scholar]
- 69. Lisberg A, Cummings A, Goldman JW, et al. A phase II study of pembrolizumab in EGFR-mutant, PD-L1+, tyrosine kinase inhibitor naïve patients with advanced NSCLC. J Thorac Oncol 2018; 13: 1138–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Gainor JF, Shaw AT, Sequist LV, et al. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res 2016; 22: 4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lee CK, Man J, Lord S, et al. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol 2018; 4: 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Soo RA, Lim SM, Syn NL, et al. Immune checkpoint inhibitors in epidermal growth factor receptor mutant non-small cell lung cancer: current controversies and future directions. Lung Cancer 2018; 115: 12–20. [DOI] [PubMed] [Google Scholar]
- 73. Riera-Domingo C, Audigé A, Granja S, et al. Immunity, hypoxia, and metabolism–the Ménage à Trois of cancer: implications for immunotherapy. Physiol Rev 2020; 100: 1–102. [DOI] [PubMed] [Google Scholar]
- 74. Damgaci S, Ibrahim-Hashim A, Enriquez-Navas PM, et al. Hypoxia and acidosis: immune suppressors and therapeutic targets. Immunology 2018; 154: 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Huber V, Camisaschi C, Berzi A, et al. Cancer acidity: an ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol 2017; 43: 74–89. [DOI] [PubMed] [Google Scholar]
- 76. Erra Díaz F, Dantas E, Geffner J. Unravelling the interplay between extracellular acidosis and immune cells. Mediators Inflamm 2018; 2018: 1218297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Noman MZ, Janji B, Berchem G, et al. Hypoxia-induced autophagy. Autophagy 2012; 8: 704–706. [DOI] [PubMed] [Google Scholar]
- 78. Jeong H, Kim S, Hong BJ, et al. Tumor-associated macrophages enhance tumor hypoxia and aerobic glycolysis. Cancer Res 2019; 79: 795–806. [DOI] [PubMed] [Google Scholar]
- 79. Yuan J, Zhou J, Dong Z, et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab. Cancer Immunol Res 2014; 2: 127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Chen P-L, Roh W, Reuben A, et al. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov 2016; 6: 827–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Sabatino M, Kim-Schulze S, Panelli MC, et al. Serum vascular endothelial growth factor and fibronectin predict clinical response to high-dose interleukin-2 therapy. J Clin Oncol 2009; 27: 2645–2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Wang J, Chen J, Guo Y, et al. Strategies targeting angiogenesis in advanced non-small cell lung cancer. Oncotarget 2017; 8: 53854–53872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen DS, Hurwitz H. Combinations of bevacizumab with cancer immunotherapy. Cancer J 2018; 24: 193–204. [DOI] [PubMed] [Google Scholar]
- 84. Voron T, Colussi O, Marcheteau E, et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J Exp Med 2015; 212: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shiono A, Kaira K, Mouri A, et al. Improved efficacy of ramucirumab plus docetaxel after nivolumab failure in previously treated non-small cell lung cancer patients. Thorac Cancer 2019; 10: 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Brochez L, Chevolet I, Kruse V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur J Cancer 2017; 76: 167–182. [DOI] [PubMed] [Google Scholar]
- 87. Botticelli A, Cerbelli B, Lionetto L, et al. Can IDO activity predict primary resistance to anti-PD-1 treatment in NSCLC? J Transl Med 2018; 16: 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Shayan G, Srivastava R, Li J, et al. Adaptive resistance to anti-PD1 therapy by Tim-3 upregulation is mediated by the PI3K-Akt pathway in head and neck cancer. Oncoimmunology 2016; 6: e1261779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Huang R-Y, Francois A, McGray AR, et al. Compensatory upregulation of PD-1, LAG-3, and CTLA-4 limits the efficacy of single-agent checkpoint blockade in metastatic ovarian cancer. Oncoimmunology 2016; 6: e1249561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016; 7: 10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Gao J, Ward JF, Pettaway CA, et al. VISTA is an inhibitory immune checkpoint that is increased after ipilimumab therapy in patients with prostate cancer. Nat Med 2017; 23: 551–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Thommen DS, Schreiner J, Müller P, et al. Progression of lung cancer is associated with increased dysfunction of T cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res 2015; 3: 1344–1355. [DOI] [PubMed] [Google Scholar]
- 93. Vétizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by CTLA-4 blockade relies on the gut microbiota. Science 2015; 350: 1079–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 2018; 359: 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 2015; 350: 1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018; 359: 91–97. [DOI] [PubMed] [Google Scholar]
- 97. Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol 2018; 29: 1437–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Pinato DJ, Howlett S, Ottaviani D, et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol 2019; 5: 1774–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Elkrief A, El Raichani L, Richard C, et al. Antibiotics are associated with decreased progression-free survival of advanced melanoma patients treated with immune checkpoint inhibitors. Oncoimmunology 2019; 8: e1568812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Frankel AE, Coughlin LA, Kim J, et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia 2017; 19: 848–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schvartsman G, Peng SA, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer 2017; 112: 90–95. [DOI] [PubMed] [Google Scholar]
- 102. Park SE, Lee SH, Ahn JS, et al. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol 2018; 13: 106–111. [DOI] [PubMed] [Google Scholar]
- 103. Grigg C, Reuland BD, Sacher AG, et al. Clinical outcomes of patients with non-small cell lung cancer (NSCLC) receiving chemotherapy after immune checkpoint blockade. J Clin Oncol 2017; 35: 9082. [Google Scholar]
- 104. Leger PD, Rothschild S, Castellanos E, et al. Response to salvage chemotherapy following exposure to immune checkpoint inhibitors in patients with non-small cell lung cancer. J Clin Oncol 2017; 35: 9084. [Google Scholar]
- 105. Grohe C, Gleiber W, Haas S, et al. 119O Efficacy and safety of nintedanib + docetaxel in lung adenocarcinoma patients (pts) following treatment with immune checkpoint inhibitors (ICIs): first results of the ongoing non-interventional study (NIS) VARGADO. Ann Oncol 2019; 30. [Google Scholar]
- 106. Corral J, Majem M, Rodríguez-Abreu D, et al. Efficacy of nintedanib and docetaxel in patients with advanced lung adenocarcinoma treated with first-line chemotherapy and second-line immunotherapy in the nintedanib NPU program. Clin Transl Oncol 2019; 21: 1270–1279. [DOI] [PubMed] [Google Scholar]
- 107. Capelletto E, Osman G, Morabito A, et al. NSCLC survival expectancy for patients treated with docetaxel/nintedanib in the SENECA trial and previous immunotherapy. WCLC 2019. Abstract. [Google Scholar]
- 108. Molife C, Hess LM, Cui ZL, et al. Sequential therapy with ramucirumab and/or checkpoint inhibitors for non-small-cell lung cancer in routine practice. Future Oncol 2019; 15: 2915–2931. [DOI] [PubMed] [Google Scholar]
- 109. Reck M, Kaiser R, Mellemgaard A, et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non-small-cell lung cancer (LUME-Lung 1): a phase 3, double-blind, randomised controlled trial. Lancet Oncol 2014; 15: 143–155. [DOI] [PubMed] [Google Scholar]
- 110. Garon EB, Ciuleanu T-E, Arrieta O, et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second-line treatment of stage IV non-small-cell lung cancer after disease progression on platinum-based therapy (REVEL): a multicentre, double-blind, randomised phase 3 trial. Lancet 2014; 384: 665–673. [DOI] [PubMed] [Google Scholar]
- 111. Mazzone R, Zwergel C, Artico M, et al. The emerging role of epigenetics in human autoimmune disorders. Clin Epigenetics 2019; 11: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Orillion A, Hashimoto A, Damayanti N, et al. Entinostat neutralizes myeloid-derived suppressor cells and enhances the antitumor effect of PD-1 inhibition in murine models of lung and renal cell carcinoma. Clin Cancer Res 2017; 23: 5187–5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Hellmann M, Jänne P, Opyrchal M, et al. OA05.01 efficacy/safety of entinostat (ENT) and pembrolizumab (PEMBRO) in NSCLC patients previously treated with anti-PD-(L)1 therapy. J Thorac Oncol 2018; 13: S330. [Google Scholar]
- 114. Ramalingam SS, Hellmann MD, Awad MM, et al. Abstract CT078: tumor mutational burden (TMB) as a biomarker for clinical benefit from dual immune checkpoint blockade with nivolumab (nivo) + ipilimumab (ipi) in first-line (1L) non-small cell lung cancer (NSCLC): identification of TMB cutoff from CheckMate 568. Cancer Res 2018; 78: CT078. [Google Scholar]
- 115. Theelen WSME, Peulen HMU, Lalezari F, et al. Effect of pembrolizumab after stereotactic body radiotherapy vs pembrolizumab alone on tumor response in patients with advanced non-small cell lung cancer: results of the PEMBRO-RT phase 2 randomized clinical trial. JAMA Oncol 2019; 5: 1276–1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. van Willigen WW, Bloemendal M, Gerritsen WR, et al. Dendritic cell cancer therapy: vaccinating the right patient at the right time. Front Immunol 2018; 9: 2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Ott PA, Govindan R, Naing A, et al. 1127O A personal neoantigen vaccine, NEO-PV-01, with anti-PD1 induces broad de novo anti-tumor immunity in patients with metastatic melanoma, NSCLC, and bladder cancer. Ann Oncol 2018; 29(Suppl. 8): mdy288. [Google Scholar]
- 118. Kaufman HL, Kohlhapp FJ, Zloza A. Oncolytic viruses: a new class of immunotherapy drugs. Nat Rev Drug Discov 2015; 14: 642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Pol J, Kroemer G, Galluzzi L. First oncolytic virus approved for melanoma immunotherapy. Oncoimmunology 2015; 5: e1115641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Rudin CM, Pandha HS, Gupta S, et al. LBA40 Phase Ib KEYNOTE-200: a study of an intravenously delivered oncolytic virus, coxsackievirus A21 in combination with pembrolizumab in advanced NSCLC and bladder cancer patients. Ann Oncol 2018; 29(Suppl. 8): mdy424-050. [Google Scholar]
- 121. Flood BA, Higgs EF, Li S, et al. STING pathway agonism as a cancer therapeutic. Immunol Rev 2019; 290: 24–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Harrington KJ, Brody J, Ingham M, et al. LBA15 Preliminary results of the first-in-human (FIH) study of MK-1454, an agonist of stimulator of interferon genes (STING), as monotherapy or in combination with pembrolizumab (pembro) in patients with advanced solid tumors or lymphomas. Ann Oncol 2018; 29(Suppl. 8): mdy424-015. [Google Scholar]
- 123. Ribas A, Lawrence D, Atkinson V, et al. Combined BRAF and MEK inhibition with PD-1 blockade immunotherapy in BRAF-mutant melanoma. Nat Med 2019; 25: 936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Ascierto PA, Ferrucci PF, Fisher R, et al. Dabrafenib, trametinib and pembrolizumab or placebo in BRAF-mutant melanoma. Nat Med 2019; 25: 941–946. [DOI] [PubMed] [Google Scholar]
- 125. Sullivan RJ, Hamid O, Gonzalez R, et al. Atezolizumab plus cobimetinib and vemurafenib in BRAF-mutated melanoma patients. Nat Med 2019; 25: 929–935. [DOI] [PubMed] [Google Scholar]
- 126. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1116–1127. [DOI] [PubMed] [Google Scholar]
- 127. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019; 380: 1103–1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Yang JC-H, Gadgeel SM, Sequist LV, et al. Pembrolizumab in combination with erlotinib or gefitinib as first-line therapy for advanced NSCLC with sensitizing EGFR mutation. J Thorac Oncol 2019; 14: 553–559. [DOI] [PubMed] [Google Scholar]
- 129. Gettinger S, Hellmann MD, Chow LQM, et al. Nivolumab plus erlotinib in patients with EGFR-mutant advanced NSCLC. J Thorac Oncol 2018; 13: 1363–1372. [DOI] [PubMed] [Google Scholar]
- 130. Fukumura D, Kloepper J, Amoozgar Z, et al. Enhancing cancer immunotherapy using antiangiogenics: opportunities and challenges. Nat Rev Clin Oncol 2018; 15: 325–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Du W, Huang H, Sorrelle N, et al. Sitravatinib potentiates immune checkpoint blockade in refractory cancer models. JCI Insight 2018; 3: e124184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. He K. Preliminary biomarker analysis of sitravatinib in combination with nivolumab in NSCLC patients progressing on prior checkpoint inhibitor. 33rd Annual meeting & pre-conference programs of the Society for Immunotherapy of Cancer (SITC 2018). J ImmunoTher Cancer 2018; 6: 114.30400835 [Google Scholar]
- 133. Brose MS, Vogelzang NJ, DiSimone C, et al. A phase Ib/II trial of lenvatinib plus pembrolizumab in non-small cell lung cancer. J Clin Oncol 2019; 37: 16. [Google Scholar]
- 134. Weiskopf K. Cancer immunotherapy targeting the CD47/SIRPα axis. Eur J Cancer 2017; 76: 100–109. [DOI] [PubMed] [Google Scholar]
- 135. Kauder SE, Kuo TC, Harrabi O, et al. ALX148 blocks CD47 and enhances innate and adaptive antitumor immunity with a favorable safety profile. PLoS One 2018; 13: e0201832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Chow LQM, Gainor JF, Lakhani NJ, et al. A phase I study of ALX148, a CD47 blocker, in combination with established anticancer antibodies in patients with advanced malignancy. J Clin Oncol 2019; 37: 2514. [Google Scholar]
- 137. Sanmamed MF, Pastor F, Rodriguez A, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol 2015; 42: 640–655. [DOI] [PubMed] [Google Scholar]
- 138. Zappasodi R, Sirard C, Li Y, et al. Rational design of anti-GITR-based combination immunotherapy. Nat Med 2019; 25: 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Papadopoulos KP, Autio KA, Golan T, et al. Phase 1 study of MK-4166, an anti-human glucocorticoid-induced tumor necrosis factor receptor (GITR) antibody, as monotherapy or with pembrolizumab (pembro) in patients (pts) with advanced solid tumors. J Clin Oncol 2019; 37: 9509. [Google Scholar]
- 140. Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019; 381: 2020–2031. [DOI] [PubMed] [Google Scholar]
- 141. Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 2016; 44: 989–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Ascierto PA, Bono P, Bhatia S, et al. LBA18 Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma who progressed during prior anti-PD-1/PD-L1 therapy (mel prior IO) in all-comer and biomarker-enriched populations. Ann Oncol 2017; 28(Suppl. 5). [Google Scholar]
- 143. Hong DS, Schoffski P, Calvo A, et al. Phase I/II study of LAG525 ± spartalizumab (PDR001) in patients (pts) with advanced malignancies. J Clin Oncol 2018; 36: 3012. [Google Scholar]
- 144. Davar D. A phase 1 study of TSR-022, an anti-TIM-3 monoclonal antibody, in combination with TSR-042 (anti-PD-1) in patients with colorectal cancer and post-PD-1 NSCLC and melanoma. 33rd Annual meeting & pre-conference programs of the Society for Immunotherapy of Cancer (SITC 2018) J ImmunoTher Cancer 2018; 6: 114.30400835 [Google Scholar]
- 145. Riaz N, Havel JJ, Makarov V, et al. Tumor and microenvironment evolution during immunotherapy with nivolumab. Cell 2017; 171: 934–949.e916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Tang J, Shalabi A, Hubbard-Lucey VM. Comprehensive analysis of the clinical immuno-oncology landscape. Ann Oncol 2017; 29: 84–91. [DOI] [PubMed] [Google Scholar]
- 147. Smoragiewicz M, Bogaerts J, Calvo E, et al. Design and conduct of early clinical studies of immunotherapy agent combinations: recommendations from the task force on methodology for the development of innovative cancer therapies. Ann Oncol 2018; 29: 2175–2182. [DOI] [PubMed] [Google Scholar]
- 148. Shin SH, Bode AM, Dong Z. Addressing the challenges of applying precision oncology. NPJ Precis Oncol 2017; 1: 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Kim JM, Chen DS. Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann Oncol 2016; 27: 1492–1504. [DOI] [PubMed] [Google Scholar]
- 150. Jerby-Arnon L, Shah P, Cuoco MS, et al. A cancer cell program promotes T cell exclusion and resistance to checkpoint blockade. Cell 2018; 175: 984–997.e924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Hugo W, Zaretsky JM, Sun L, et al. Genomic and transcriptomic features of response to anti-PD-1 therapy in metastatic melanoma. Cell 2016; 165: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]