Abstract
Background:
With the popularization of Enhanced Recovery After Surgery (ERAS), identifying patients with complications before discharging becomes important. This study aimed to explore the efficacy of C-reactive protein (CRP) in predicting infectious complications after gastrectomy.
Methods:
Patients with gastric cancer who underwent gastrectomy at Beijing Cancer Hospital from March 2017 to April 2018 were enrolled in the training set. Complications were prospectively registered. Receiver operating characteristic analysis was performed to assess the diagnostic accuracy of CRP via evaluating the area under the curve (AUC). Patients who had CRP tested on postoperative day (POD) 5 and accepted gastrectomy from April to December 2018 were included in the validation set to validate the cut-off value of CRP obtained from the training set.
Results:
A total of 350 patients were included (263 patients in the training set and 87 patients in the validation set). Out of these, 24 patients were diagnosed with infectious complications and 17 patients had anastomotic leakage in the training set. The CRP level on POD5 had superior diagnostic accuracy for infectious complications with an AUC of 0.81. The cut-off value of CRP on POD5 at 166.65 mg/L yielded 93% specificity and 97.2% negative predict value (NPV); For anastomotic leakage, the AUC of CRP on POD5 was 0.81. Using the cut-off value of CRP at 166.65 mg/L on POD5 achieved 92% specificity and 98.6% NPV. The optimal cut-off value (CRP 166.65 mg/L on POD5) was validated in the validation set. It achieved 97.5% specificity and 94.0% NPV for infectious complications, and 97.6% specificity and 96.4% NPV for anastomotic leakage.
Conclusion:
CRP is a reliable predictive marker for the diagnosis of inflammatory complications following gastric surgery. However, this study was based on preliminary data. The validity of this data needs confirmation by a larger number of cases.
Keywords: anastomotic leakage, complication, CRP, ERAS, predictive marker
Introduction
Gastric cancer is the second most common malignant tumor in China with high morbidity and mortality.1 Gastrectomy with lymph node dissection remains the main therapeutic method for most patients with gastric cancer.2 Despite the rapid development of surgical techniques, postoperative complications are still common. Among them, postoperative infectious complications (ICs), especially anastomotic leakage (AL), are known to prolong hospital stay, increase perioperative mortality, and have worse long-term oncological outcomes.3,4 Moreover, with the increasing popularity of fast track care, also known as enhanced recovery after surgery (ERAS), patients are often discharged on day 5–7 after gastrectomy. It is essential to identify patients with ICs or AL before discharging. In other words, precise screening of complications in the early postoperative phase is of fundamental importance in the ERAS program.5–7
C-reactive protein (CRP), an important systemic inflammatory biomarker, is extensively used in clinical settings for infection diagnosis.8,9 Many previous studies have discussed the correlation between serum CRP level and ICs in esophageal, pancreatic, and colorectal surgery.10–15 The diagnostic accuracy of CRP for AL has also been explored in patients who accepted the ERAS after bariatric surgery.16–18 However, despite their retrospective nature, the sample sizes are often too small to draw a conclusion. In addition, there are only a few studies that have touched on predicting the efficacy of CRP for ICs or AL after gastrectomy for gastric neoplasm, let alone furthering the validation of CRP. To this end, the aim of our study was to investigate the role of CRP as an early predictor of complications after gastrectomy in our prospectively maintained database and conduct internal validation to explore its possible role in the ERAS program.
Materials and methods
Study design
This study includes two parts, that is, the training set and the validation set. The first part was embedded in the APPEAL-GC study (Analysis of Parameters Predictive for Evident Anastomotic Leakage-Gastric Cancer) and shared the same data source. In this part, patients who underwent elective gastrectomy for gastric tumor in the Gastrointestinal Cancer Center, Ward I, Peking University Cancer Hospital and Institute from 1 April 2017 to 1 April 2018 were consecutively recruited. The data collection methods were in accordance with the APPEAL-II trial,19,20 a multi-centered European trial, but in gastric cancer population. The detailed methods of the APPEAL-GC study are reported elsewhere. For patients in the training set, we also prospectively collected their intra-abdominal drainage fluids for further analysis.
Patients who accepted gastrectomy in our ward and had serum CRP tested on postoperative day (POD) 5 from 24th April to 24th December 2018 were included for validation. This part was used to validate the cut-off value of CRP obtained from the training set.
The study was approved by the medical ethics committee of Peking University Cancer Hospital. All patients in this study have signed the written informed consent. There were no patients under the age of 16 in our study.
Clinical data registration
In the training set, the patients’ demographic data and clinical pathological characteristics were obtained from a prospectively maintained database. The registration of postoperative complications was prospectively conducted in the case report form, which followed the protocol of the APPEAL trial, and the severity of complications was scored using the Clavien–Dindo grading system.21 The white blood cell (WBC) count and CRP levels on POD1, POD3, and POD5 were retrospectively extracted from the electronic medical record system. In detail, the following data were included for analysis:
• Basic information: gender, age, body mass index (BMI), ASA (American Society of Anesthesiologists) score, diabetes, hypertension, preoperative treatment.
• Surgical information: operative approach.
• Pathological information: tumor location, Clinical TNM stage (cTNM) stage.
• Complication information: AL, abdominal abscess, pleural effusion, surgical site infection, infection without a definite cause, empyema, pneumonia.
• Laboratory tests information: WBC and CRP on POD1, 3, 5.
In the validation, the included patients’ data were retracted from the prospectively maintained database. The complication data were reviewed by surgeons and consultants weekly.
Definition of complications
In this study, the ICs included AL, abdominal abscess, pleural effusion, surgical site infection, infection without a definite cause, empyema, and pneumonia. The diagnostic criteria are in accordance with the APPEAL trial. The major cause of ICs, that is, AL, was further specified and its diagnostic criteria was as follows: radiological changes were seen after surgery (with or without clinical intervention); color, turbidity, fecal matter, or other indicative changes occurred in the drainage fluid; the peri-anastomotic abscess and agnogenic intra-abdominal infection was also considered as a manifestation of AL.22
Statistical analysis
All statistical analyses were performed using SPSS 22.0. Quantitative variables following the Gaussian distribution were reported as mean and standard deviation, whereas non-Gaussian distribution variables were defined by median and range. Qualitative variables were reported as the number of cases and percentages. Differences in quantitative variables following a Gaussian distribution were tested using Student’s t-test; the Kruskal–Wallis or Mann–Whitney tests were used for variables following the non-Gaussian distribution. A comparison of qualitative variables was performed with the chi-square test. Receiver operating characteristic analysis (ROC) was performed to assess the diagnostic accuracy of CRP via evaluating the area under the curve (AUC). AUC was interpreted as follows: a test with an AUC of >0.5 indicated some ability of the test to discriminate between those with and without the outcome of interest, but tests with an AUC of ⩾0.8 were considered to show high-diagnostic accuracy, with those closest to 1 considered to be the most predictive. Cut-off values for CRP were selected based on those values that gave the best combination of high sensitivity and high specificity. Univariate and multivariate logistic regression analyses were performed to identify clinical risk factors for the development of ICs or AL. Selected variables for the multivariate analysis, and any variable whose univariate test p value was < 0.05, were considered as candidates for inclusion. In all cases, bilateral p < 0.05 was considered statistically significant.
Results
A total of 350 patients were enrolled in this study (263 patients in the training set and 87 patients in the validation set). The 263 patients in the training set comprised 209 males and 54 females with a median age of 62 (54–67) years, ranging from 27 to 83. The median BMI was 24.22–26 The important comorbidities for patients in the training set included diabetes (24/263) and hypertension (75/263). The 87 patients in the validation set comprised 61 males and 26 females with a median age of 62 (54–66) years, ranging from 27 to 82. The median BMI was 24.22–26 There were 9 patients with diabetes and 25 patients with hypertension in the validation set. Patients’ characteristics, ASA score, comorbidities, and surgical data are shown in Tables 1 and 2. For the training set, the postoperative complications were diagnosed in 81 patients within 30 PODs, and the complication rate was 30.79%. ICs were observed in 24 patients, including 17 patients who suffered from AL; the AL rate was 6.46%. Furthermore, 5 patients of those 24 patients were diagnosed with more than one ICs (Supplemental Table S1). Apart from one patient, all patients survived until 30 days post-operatively.
Table 1.
Patients’ characteristics, surgical information, and pathological information in training set (n = 263).
| Total (n = 263) | Postoperative complications (n = 81) | p value | Infectious complications (n = 24) | p value | Anastomotic leakage (n = 17) | p value | |
|---|---|---|---|---|---|---|---|
| Gender | 0.74 | 0.45 | 0.54 | ||||
| Male | 209 | 63 | 21 | 15 | |||
| Female | 54 | 18 | 3 | 2 | |||
| Age | 62 (54–67) | 64 (57–68) | 0.02 a | 66 (61–71) | 0.01 a | 66 (63–71) | 0.03 a |
| <65 | 163 | 41 | 0.01 | 9 | 0.01 | 6 | 0.02 |
| ⩾65 | 100 | 40 | 15 | 11 | |||
| BMI (kg/m2) | 24 (22–26) | 23.5 (21–25) | 0.42a | 24 (21–25) | 0.49a | 24.5 (21–26) | 0.87a |
| ASA score | 0.42 | 0.56 | 0.09 | ||||
| 1 | 11 | 5 | 2 | 2 | |||
| 2 | 235 | 70 | 20 | 13 | |||
| 3 | 16 | 6 | 2 | 2 | |||
| Miss | 1 | 0 | 0 | 0 | |||
| Diabetes | 1.00 | 1.00 | 0.96 | ||||
| No | 239 | 74 | 22 | 16 | |||
| Yes | 24 | 7 | 2 | 1 | |||
| Hypertension | 0.46 | 0.64 | 0.36 | ||||
| No | 188 | 55 | 16 | 10 | |||
| Yes | 75 | 26 | 8 | 7 | |||
| Preoperative treatment | 0.63 | 0.44 | 1.000 | ||||
| No | 204 | 61 | 17 | 13 | |||
| Yes | 59 | 20 | 7 | 4 | |||
| cTNM stage | 0.87 | 0.16 | 0.28 | ||||
| I | 63 | 18 | 2 | 1 | |||
| II | 51 | 14 | 4 | 3 | |||
| III | 123 | 40 | 16 | 11 | |||
| IV | 26 | 9 | 2 | 2 | |||
| Tumor location | <0.001 | 0.002 | <0.01 | ||||
| Upper gastric | 87 | 39 | 15 | 13 | |||
| Non-upper gastric | 176 | 42 | 9 | 4 | |||
| Tumor differentiation | 0.13 | 0.19 | 0.53 | ||||
| G1 | 10 | 4 | 1 | 0 | |||
| G2 | 124 | 46 | 16 | 11 | |||
| G3 | 110 | 27 | 6 | 5 | |||
| Unknown | 19 | 4 | 1 | 1 | |||
| Operative approach | 0.50 | 0.09 | 0.01 | ||||
| Open | 144 | 47 | 9 | 4 | |||
| Laparoscopic | 119 | 34 | 15 | 13 | |||
| Resection range | 0.003 | 0.04 | <0.01 | ||||
| Distal gastrectomy | 128 | 26 | 6 | 2 | |||
| Total gastrectomy | 119 | 50 | 18 | 15 | |||
| Combined resection | 6 | 2 | 0 | 0 | |||
| Others | 10 | 3 | 0 | 0 | |||
| Lymph node dissection | 1.00 | 0.96 | 1.00 | ||||
| D0 | 0 | 0 | 0 | 0 | |||
| D1+ | 17 | 5 | 1 | 1 | |||
| D2 | 246 | 76 | 23 | 16 |
Bold values here represent the p values 0.05, which were considered statistically significant.
Mann–Whitney tests.
ASA, American Society of Anesthesiologists; BMI, Body mass index; cTNM, Clinical TNM Stage.
Table 2.
Patients’ characteristics, surgical information, and pathological information in validation set (n = 87).
| Total (n = 87) | Postoperative complications (n = 28) | p value | Infectious complications (n = 7) | p value | Anastomotic leakage (n = 5) | p value | |
|---|---|---|---|---|---|---|---|
| Gender | 0.62 | 1.00 | 1.00 | ||||
| Male | 61 | 21 | 5 | 4 | |||
| Female | 26 | 7 | 2 | 1 | |||
| Age | 62 (54–66) | 62 (54–66) | 0.69a | 64 (62–66) | 0.34a | 64 (58–65) | 0.64a |
| <65 | 62 | 21 | 0.63 | 5 | 1.00 | 4 | 1.00 |
| ⩾65 | 25 | 7 | 2 | 1 | |||
| BMI (kg/m2) | 24 (22–26) | 22 (20–24) | 0.01 a | 22 (20–24) | 0.09a | 21 (19–23) | 0.06a |
| ASA Score | 1.00 | 1.00 | 1.00 | ||||
| 1 | 6 | 2 | 0 | 0 | |||
| 2 | 77 | 24 | 7 | 5 | |||
| 3 | 3 | 1 | 0 | 0 | |||
| Miss | 1 | 1 | 0 | 0 | |||
| Diabetes | 0.07 | 1.00 | 1.00 | ||||
| No | 78 | 28 | 7 | 5 | |||
| Yes | 9 | 0 | 0 | 0 | |||
| Hypertension | 0.14 | 1.00 | 0.95 | ||||
| No | 62 | 23 | 5 | 3 | |||
| Yes | 25 | 5 | 2 | 2 | |||
| Preoperative treatment | 1.00 | 0.49 | 1.000 | ||||
| No | 54 | 17 | 3 | 3 | |||
| Yes | 33 | 11 | 4 | 2 | |||
| cTNM stage | 0.09 | 0.66 | 0.60 | ||||
| I | 9 | 3 | 0 | 0 | |||
| II | 12 | 2 | 0 | 0 | |||
| III | 27 | 5 | 2 | 1 | |||
| IV | 36 | 16 | 5 | 4 | |||
| Unknown | 3 | 2 | 0 | 0 | |||
| Tumor location | 0.12 | 0.50 | 1.00 | ||||
| Upper gastric | 38 | 9 | 2 | 2 | |||
| Non-upper gastric | 48 | 18 | 5 | 3 | |||
| Unknown | 1 | 1 | 0 | 0 | |||
| Tumor differentiation | 0.05 | 0.31 | 0.38 | ||||
| G1 | 2 | 2 | 0 | 0 | |||
| G2 | 21 | 3 | 0 | 0 | |||
| G3 | 53 | 19 | 5 | 5 | |||
| Unknown | 11 | 4 | 2 | 0 | |||
| Operative approach | 0.36 | 0.66 | 1.00 | ||||
| Open | 49 | 18 | 5 | 3 | |||
| Laparoscopic | 38 | 10 | 2 | 2 | |||
| Resection range | 0.27 | 0.27 | 0.55 | ||||
| Distal gastrectomy | 30 | 8 | 1 | 1 | |||
| Total gastrectomy | 46 | 14 | 6 | 4 | |||
| Combined resection | 0 | 0 | 0 | 0 | |||
| Others | 11 | 6 | 0 | 0 | |||
| Lymph node dissection | 0.11 | 1.00 | 1.00 | ||||
| D0 | 2 | 2 | 0 | 0 | |||
| D1+ | 6 | 1 | 0 | 0 | |||
| D2 | 79 | 25 | 7 | 5 |
Bold values here represent the p values 0.05, which were considered statistically significant.
Mann–Whitney tests.
ASA, American Society of Anesthesiologists; BMI, Body mass index; cTNM, Clinical TNM Stage.
In the training set, for patients with ICs, WBC count (determined on POD5) was significantly increased in contrast to patients without ICs (p = 0.018). The serum CRP levels were also higher in patients with ICs on both POD3 (p = 0.016) and POD5 (p = 0.017; Supplemental Table S2a, Figure 1). However, there were no significant differences in CRP levels between patients with and without AL on POD1, POD3, or POD5. By comparison, WBC count on POD5 was remarkably higher in patients with AL (p = 0.045) (Supplemental Table S2b, Figure 2).
Figure 1.
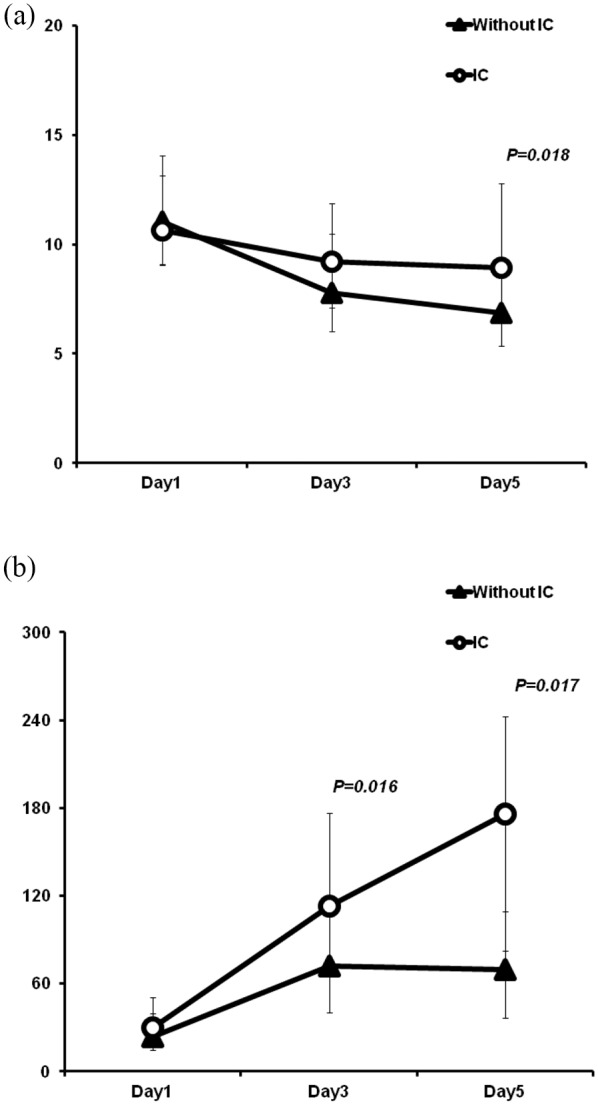
Postoperative chronological changes in WBC (a) and CRP level (b) between patients with or without infectious complications.
CRP, C-reactive protein; IC, infectious complication; WBC, white blood cell.
Figure 2.
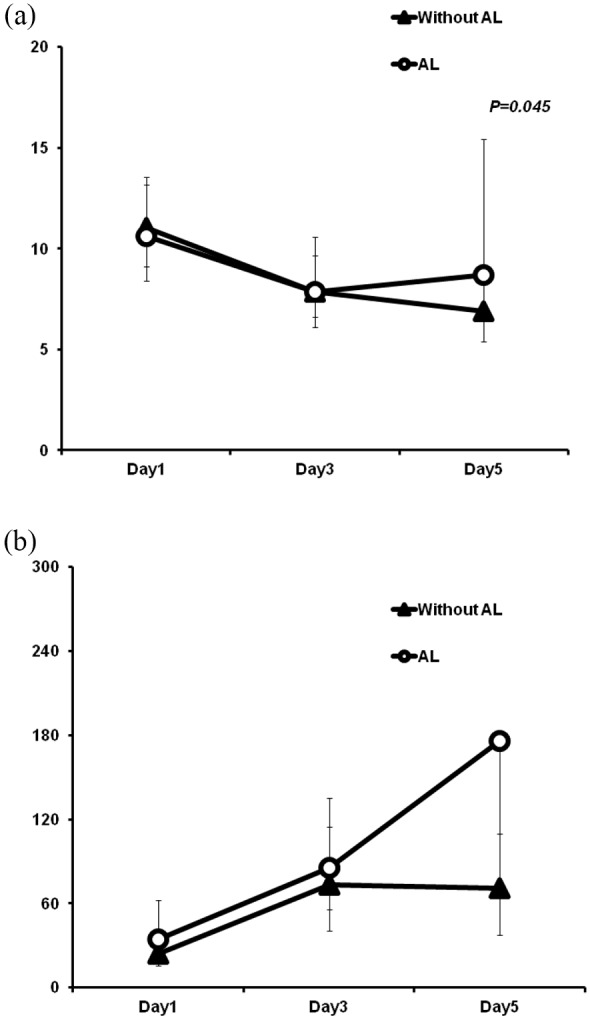
Postoperative chronological changes in WBC (a) and CRP level (b) between patients with or without anastomotic leakage.
AL, anastomotic leakage; CRP, C-reactive protein; WBC, white blood cell.
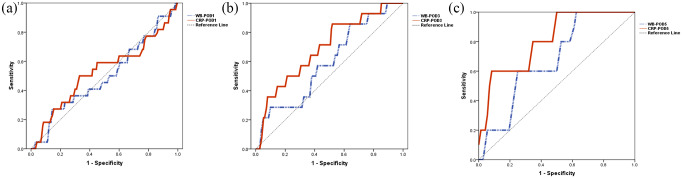
ROC analysis was applied for the detection of ICs (Table 3, Figure 3). In the training set, on POD1, AUC of the WBC count was 0.497 (p = 0.964). On POD3 and POD5, the AUCs of the WBC count were 0.594 (p = 0.244) and 0.678 (p = 0.186), respectively. However, both of their optimal cut-off values were below the upper limit of the normal range (10 × 109/L). The corresponding AUCs of CRP on POD1 and POD3 were 0.530 (p = 0.646) and 0.693 (p = 0.017). On POD5, the AUC of CRP was 0.811 (p = 0.021), the optimal cut-off value was 166.65 mg/L, which achieved 60% sensitivity, 93% specificity, 97.2% negative predictive value (NPV), and 37.5% positive predictive value (PPV). Based on the results from the training set, the optimal cut-off value (CRP 166.65 mg/L on POD5) was further validated in the validation set. It could achieve 29% sensitivity, 98% specificity, 94.0% NPV, and 50.0% PPV. The AUC was 0.857 (p = 0.002; Supplemental Table S3). In detail, among the validation set of 87 patients, there were 83 patients whose CRP value on POD5 was below the cut-off value. Of those, five patients developed ICs.
Table 3.
Receiver operating characteristic analysis for the diagnosis of infectious complication.
| POD | AUC | p value | 95% CI | Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|---|---|
| WBC count (×109/L) | |||||||||
| 1 | 0.497 | 0.964 | 0.363–0.631 | 14.265 | 27 | 86 | 17.6 | 91.5 | |
| 3 | 0.594 | 0.244 | 0.445–0.743 | 6.820 | 86 | 37 | 11.2 | 96.5 | |
| 5 | 0.678 | 0.186 | 0.478–0.878 | 6.225 | 100 | 39 | 10.2 | 100 | |
| CRP (mg/L) | |||||||||
| 1 | 0.530 | 0.646 | 0.387–0.672 | 46.550 | 27 | 83 | 14.6 | 91.2 | |
| 3 | 0.693 | 0.017 | 0.552–0.835 | 69.450 | 86 | 49 | 13.5 | 97.3 | |
| 5 | 0.811 | 0.021 | 0.636–0.986 | 166.650 | 60 | 93 | 37.5 | 97.1 | |
Bold values here represent the p values 0.05, which were considered statistically significant.
AUC, area under the curve; CI, confidence interval; CRP, C-reactive protein; NPV, negative predict value; POD, postoperative day; PPV, positive predict value; WBC, white blood cell.
Figure 3.
ROC curves for the diagnostic accuracy of WBC and CRP on (a) POD1, (b) POD3, and (c) POD5 in predicting infectious complications.
CRP, C-reactive protein; POD, postoperative day; ROC, receiver operating characteristic; WBC, white blood cell.
ROC analysis for the diagnosis of AL was also implemented in the training set (Table 4, Supplemental Figure S1). Using ROC analysis for AL, the AUC of the WBC count on POD1 was 0.475 (p = 0.566). The AUCs of the WBC count on POD3 and POD5 were 0.457 (p = 0.667) and 0.676 (p = 0.305), respectively. As for ICs, their optimal cut-off values failed to meet a level upon the upper limit of the normal range. In contrast, the AUC of CRP level on POD1 and POD3 were 0.565 (p = 0.385) and 0.559 (p = 0.549), respectively. On POD5, the AUC of CRP level was 0.806 (p = 0.073); a cut-off value at 166.650 mg/L achieved 67% sensitivity, 92.0% specificity, 98.6% NPV, and 25.0% PPV. This cut-off value was also applied to the validation set, which achieved 40% sensitivity, 98% specificity, 96.4% NPV, and 50.0% PPV. The AUC was 0.866 (p = 0.006; Supplemental Table S3). Specifically, CRP level on POD5 of 83 patients in the validation set was lower than the cut-off value, and AL occurred in three of those patients.
Table 4.
Receiver operating characteristic analysis for the diagnosis of anastomotic leakage.
| POD | AUC | p value | 95% CI | Cut-off value | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|---|---|---|---|
| WBC count (×109/L) | |||||||||
| 1 | 0.457 | 0.566 | 0.301–0.612 | 14.265 | 25 | 85 | 11.8 | 93.6 | |
| 3 | 0.457 | 0.667 | 0.301–0.614 | 6.820 | 78 | 35 | 6.5 | 96.5 | |
| 5 | 0.676 | 0.305 | 0.497–0.854 | 6.585 | 100 | 49 | 7.3 | 100 | |
| CRP (mg/L) | |||||||||
| 1 | 0.565 | 0.385 | 0.393–0.737 | 33.400 | 56 | 69 | 12.3 | 95.3 | |
| 3 | 0.559 | 0.549 | 0.392–0.727 | 69.450 | 78 | 47 | 7.9 | 97.3 | |
| 5 | 0.806 | 0.073 | 0.552–1.000 | 166.650 | 67 | 92 | 25.0 | 98.6 | |
AUC, area under the curve; CI, confidence interval; CRP, C-reactive protein; NPV, negative predict value; POD, postoperative day; PPV, positive predict value; WBC, white blood cell.
Furthermore, based on data from the 263 patients in the training set, the univariate analysis showed that age, tumor location, resection range, WBC count on POD3 (p = 0.047), and CRP level on POD3 (p = 0.025) and POD5 (p = 0.003) were correlated to developing ICs. Then, these three systemic infectious markers were separately adjusted according to age and tumor location by using multivariate analysis. The outcome proved that a CRP level greater than 69.450 mg/L on POD3 (p = 0.047) and greater than 166.65 mg/L on POD5 (p = 0.003) were both independent risk factors for ICs (Table 5). The distribution of age, tumor location, operative approach, resection range, and CRP level on POD1 (p = 0.046) and POD5 (p = 0.015) were significantly different between patients with and without AL on univariate analysis; however, only CRP level on POD5 greater than 166.650 mg/L (p = 0.025) was identified as an independent risk factor for AL after multivariate analysis (Table 6).
Table 5.
Univariate and multivariate analysis of WBC and CRP level for infectious complication.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| WBC-POD1 | 0.229 | |||
| ⩽14.265 | Reference | |||
| >14.265 | 1.838 (0.682–4.955) | |||
| WBC-POD3 | 0.047 | |||
| ⩽6.820 | Reference | Reference | 0.079 | |
| >6.820 | 3.611 (1.018–12.810) | 3.203 (0.872–11.759) | ||
| WBC-POD5 | 0.103 | |||
| ⩽6.225 | Reference | |||
| >6.225 | 5.769 (0.704–47.309) | |||
| CRP-POD1 | 0.267 | |||
| ⩽46.550 | Reference | |||
| >46.550 | 1.768 (0.646–4.838) | |||
| CRP-POD3 | 0.025 | 0.047 | ||
| ⩽69.450 | Reference | Reference | ||
| >69.450 | 5.766 (1.248–26.645) | 4.803 (1.019–22.627) | ||
| CRP-POD5 | 0.003 | 0.003 | ||
| ⩽166.650 | Reference | Reference | ||
| >166.650 | 20.700 (2.784–153.918) | 20.700 (2.784–153.918) | ||
Bold values here represent the p values 0.05, which were considered statistically significant.
CI, confidence interval; CRP, C-reactive protein; OR, odds ratio; POD, postoperative day; WBC, white blood cell.
Table 6.
Univariate and multivariate analysis of WBC and CRP level for anastomotic leakage.
| Univariate analysis |
Multivariate analysis |
|||
|---|---|---|---|---|
| OR (95% CI) | p value | Adjusted OR (95% CI) | p value | |
| WBC-POD1 | 0.480 | |||
| ⩽14.265 | Reference | |||
| >14.265 | 1.521 (0.474–4.879) | |||
| WBC-POD3 | 0.237 | |||
| ⩽6.820 | Reference | |||
| >6.820 | 2.207 (0.594–8.205) | |||
| WBC-POD5 | 0.094 | |||
| ⩽6.585 | Reference | |||
| >6.585 | 6.222 (0.733–52.787) | |||
| CRP-POD1 | 0.046 | – | ||
| ⩽33.400 | Reference | – | ||
| >33.400 | 2.853 (1.018–7.997) | − | ||
| CRP-POD3 | 0.160 | |||
| ⩽69.450 | Reference | |||
| >69.450 | 3.159 (0.636–15.685) | |||
| CRP-POD5 | 0.015 | 0.028 | ||
| ⩽166.650 | Reference | Reference | ||
| >166.650 | 23.333 (1.838–296.192) | 20.000 (1.374–291.067) | ||
Bold values here represent the p values <0.05, which were considered statistically significant.CI, confidence interval; CRP, C-reactive protein; OR, odds ratio; POD, postoperative day; WBC, white blood cell.
Discussion
In our study, we analyzed the diagnostic value of the systematic inflammatory parameters in a prospectively maintained database. We found that CRP on POD5 is a very good candidate for ICs screening, which was also confirmed in our further internal validation. Our data suggest that a CRP level lower than 166.650 mg/L can be used as a screening marker in the ERAS program for a discharge decision making.
In recent years, there are a series of studies attempting to establish a cut-off value for the systematic inflammatory parameters for postoperative complications, such as AL or intra-abdominal abscesses. One common finding of those studies is that the predictive value of WBC count is limited, which was also confirmed in our study. On top of that, in general, these studies can be categorized into two types: one is to predict the complications with an abnormal CRP level in the early PODs, and the other is to find those patients who are free of complications before discharge with a relatively “acceptable” CRP level.
To summarize those studies, it seems that predicting complications with CRP tests is unsatisfactory in the early PODs. Kim et al. reported a cut-off value of 83 mg/L on POD1 after gastrectomy to predict ICs, while the sensitivity was only 40% and the AUC was 0.56.23 Similar data were also reported in other types of gastrointestinal surgery,10,15 which indicate a poor diagnostic ability of CRP in the early PODs after gastrointestinal surgery. This is in accordance with the clinical experience that abnormal CRP level is very common in the early days after surgery. Giaccaglia et al. suggested to avoid the measurement of the systematic inflammatory markers such as CPR, procalcitonin (PCT), or WBC on POD1, considering a possible physiological fluctuation by transient bacterial contamination during the operation or preparation of intestinal anastomosis.22 Different from the data on POD1, the diagnostic efficiency of CRP on POD3 seems better in this study. Our results revealed that the optimal cut-off value of CRP on POD3 had the best sensitivity for both ICs (86%) and AL (78%). However, the poor PPVs (13.5% for ICs, 7.9% for AL) limited its application in clinical.
In contrast to predicting the high-risk patients of complications, some studies investigated the potential of CRP in excluding those low-risk patients in the recovery progress, which is extremely useful in terms of the ERAS program. A meta-analysis that included 7 studies with a total of 2483 patients has concluded that CRP was a useful negative predictive parameter for AL after colorectal surgery.24 It was also reported that on the third day after minimally invasive esophagectomy, the cut-off value of CRP at 181 mg/L achieved 74% specificity and 94% NPV. The authors concluded that such results indicated that CRP on POD3 could be used for early oral diet advancement within an ERAS program.11,25 Our study shows that the cut-off value of CRP at 69.450 mg/L on POD3 yielded over 97% NPV for both ICs and AL, but its specificity was under 50%. By contrast, on POD5, the cut-off value of CRP at 166.650 mg/L achieved the best balance of specificity and NPV for both ICs and AL (both >90%), which was further confirmed in the validation set. Furthermore, the AUCs of CRP on POD5 for ICs and AL were also highest (both >0.8). The reason for the remarkably enhanced diagnostic capacity of CRP may be that, on the fifth day after surgery, the mixing effects such as surgical trauma, blood loss, or absorption of necrotic tissues that are known to increase CRP levels were usually well controlled.17 Based on over 90% specificity and an equally high NPV, CRP can be used as an indicator for safe discharge in the ERAS program. For example, if the CRP level does not exceed 166.650 mg/L [which is much higher than the normal level (<8 mg/L)], patients can be allowed to discharge on POD5.
In fact, the clinical utility of CRP on POD5 is evident. Garcia-Granero et al. published a study with 250 patients after colorectal operation and likewise found that CRP on POD5 was an eligible marker for early discharge, as its specificity was 83% and NPV was 98%.26 Benoit et al. suggested that a CRP before POD5 of <100 mg/L was reassuring and may permit the patient to leave hospital in safe conditions.26,27 In addition, the PREDICS (Procalcitonin Reveals Early Dehiscence in Colorectal Surgery) study also showed that CRP predicts safe patient discharge after colorectal surgery.28 However, in the field of gastric surgery, to the best of our knowledge, there were few studies exploring the role of CRP in safe discharge during early postoperative phase, at least in terms of gastric cancer surgery. Our study proved that CRP on POD5 was a reliable biomarker for screening out patients without ICs or AL. This was the most valuable finding of this study and important within the ERAS programs in order to ensure a safe and early discharge.
However, there were several limitations to the present study. First, although postoperative complications were prospectively registered, the laboratory data were retrospectively collected, which resulted in an incomplete CRP and WBC count data. Second, although the internal validation was performed, our study was carried out in a single center, and the data was preliminary. The results need to be further validated in multi-center research with a higher sample volume. This is certainly one of our on-going research topics. Finally, only two inflammatory markers were chosen for analysis; postoperative parameters such as PCT and serum album were not included. As a result, the PPVs of CRP were generally low, particularly for predicting AL. Using animal models and basic medical research, inflammatory cytokines, collagen-related enzymes, and interleukins were demonstrated to participate in the mechanism of AL development. Integrating serum CRP with those parameters to construct a predictive model might achieve a better clinical applicability.
Conclusion
Serum CRP was a useful negative screening marker for ICs after gastric surgery among patients within the ERAS program. Because of a high negative predictive efficacy, patients after gastrectomy on POD5 would be allowed to discharge if their CRP levels were under the cut-off value.
Supplemental Material
Supplemental material, Supplementary_material for Clinical predictive efficacy of C-reactive protein for diagnosing infectious complications after gastric surgery by Jinyao Shi, Zhouqiao Wu, Qi Wang, Yan Zhang, Fei Shan, Shiyang Hou, Xiangji Ying, Longtao Huangfu, Ziyu Li and Jiafu Ji in Therapeutic Advances in Gastroenterology
Supplemental material, TAG936542_Supplemental_Figure_CLN for Clinical predictive efficacy of C-reactive protein for diagnosing infectious complications after gastric surgery by Jinyao Shi, Zhouqiao Wu, Qi Wang, Yan Zhang, Fei Shan, Shiyang Hou, Xiangji Ying, Longtao Huangfu, Ziyu Li and Jiafu Ji in Therapeutic Advances in Gastroenterology
Footnotes
Author contributions: Jinyao Shi and Zhouqiao Wu contributed to data analysis and manuscript writing. Qi Wang, Yan Zhang, Fei Shan, Shiyang Hou, and Xiangji Ying contributed to database maintenance and help analyse data. Jiafu Ji and Ziyu Li designed this study, reviewed and revised the paper and submitted the final manuscript. All authors approved the final manuscript and its submission.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Data availability statement: All datasets generated for this study are included in the manuscript/supplementary files.
Ethics statement: The current study is approved by the Peking University Cancer Hospital Ethics Committee (2016YJZ32).
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Key Technology Research and Development Program of the Ministry of Science and Technology of China (D171100006517004), Peking University Medicine Fund of Fostering Young Scholars’ Scientific & Technological Innovation (BMU2018PYB013) and Bethune Charitable Foundation (to Z. Wu).
Informed consent: All patients in this study have signed the written informed consent.
ORCID iD: Zhouqiao Wu  https://orcid.org/0000-0001-9222-181X
https://orcid.org/0000-0001-9222-181X
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Jinyao Shi, Gastrointestinal Cancer Center, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, P.R. China.
Zhouqiao Wu, Gastrointestinal Cancer Center, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, P.R. China.
Qi Wang, Gastrointestinal Cancer Center, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, P.R. China.
Yan Zhang, Gastrointestinal Cancer Center, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, P.R. China.
Fei Shan, Gastrointestinal Cancer Center, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, P.R. China.
Shiyang Hou, Gastrointestinal Cancer Center, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, P.R. China.
Xiangji Ying, Gastrointestinal Cancer Center, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, P.R. China.
Longtao Huangfu, Gastrointestinal Cancer Center, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, Beijing, P.R. China.
Ziyu Li, Gastrointestinal Cancer Center, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, No. 52 Fu-Cheng Road, Hai-Dian District, Beijing 100142, P.R. China.
Jiafu Ji, Gastrointestinal Cancer Center, Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education), Peking University Cancer Hospital and Institute, No. 52 Fu-Cheng Road, Hai-Dian District, Beijing 100142, P.R. China.
References
- 1. Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–132. [DOI] [PubMed] [Google Scholar]
- 2. Kim KM, An JY, Kim HI, et al. Major early complications following open, laparoscopic and robotic gastrectomy. Br J Surg 2012; 99: 1681–1687. [DOI] [PubMed] [Google Scholar]
- 3. Tokunaga M, Tanizawa Y, Bando E, et al. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol 2013; 20: 1575–1583. [DOI] [PubMed] [Google Scholar]
- 4. Krarup PM, Nordholm-Carstensen A, Jorgensen LN, et al. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg 2014; 259: 930–938. [DOI] [PubMed] [Google Scholar]
- 5. Wilmore DW, Kehlet H. Management of patients in fast track surgery. BMJ 2001; 322: 473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teeuwen PHE, Bleichrodt RP, Strik C, et al. Enhanced recovery after surgery (ERAS) versus conventional postoperative care in colorectal surgery. J Gastrointest Surg 2010; 14: 88–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spanjersberg WR, Reurings J, Keus F, et al. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev 2011; 2: CD007635. [DOI] [PubMed] [Google Scholar]
- 8. Du Clos TW. Function of C-reactive protein. Ann Med 2000; 32: 274–278. [DOI] [PubMed] [Google Scholar]
- 9. Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest 2003; 111: 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giardino A, Spolverato G, Regi P, et al. C-reactive protein and procalcitonin as predictors of postoperative inflammatory complications after pancreatic surgery. J Gastrointest Surg 2016; 20: 1482–1492. [DOI] [PubMed] [Google Scholar]
- 11. Miki Y, Toyokawa T, Kubo N, et al. C-reactive protein indicates early stage of postoperative infectious complications in patients following minimally invasive esophagectomy. World J Surg 2017; 41: 796–803. [DOI] [PubMed] [Google Scholar]
- 12. Gordon AC, Cross AJ, Foo EW, et al. C-reactive protein is a useful negative predictor of anastomotic leak in oesophago-gastric resection. ANZ J Surg 2018; 88: 223–227. [DOI] [PubMed] [Google Scholar]
- 13. Warschkow R, Tarantino I, Ukegjini K, et al. Diagnostic study and meta-analysis of C-reactive protein as a predictor of postoperative inflammatory complications after gastroesophageal cancer surgery. Langenbecks Arch Surg 2012; 397: 727–736. [DOI] [PubMed] [Google Scholar]
- 14. Hoeboer SH, Groeneveld ABJ, Engels N, et al. Rising C-reactive protein and procalcitonin levels precede early complications after esophagectomy. J Gastrointest Surg 2015; 19: 613–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muñoz JL, Alvarez MO, Cuquerella V, et al. Procalcitonin and C-reactive protein as early markers of anastomotic leak after laparoscopic colorectal surgery within an enhanced recovery after surgery (ERAS) program. Surg Endosc 2018; 32: 4003–4010. [DOI] [PubMed] [Google Scholar]
- 16. Ruiz-Tovar J, Muñoz JL, Gonzalez J, et al. C-reactive protein, fibrinogen, and procalcitonin levels as early markers of staple line leak after laparoscopic sleeve gastrectomy in morbidly obese patients within an enhanced recovery after surgery (ERAS) program. Surg Endosc 2017; 31: 5283–5288. [DOI] [PubMed] [Google Scholar]
- 17. Muñoz JL, Ruiz-Tovar J, Miranda E, et al. C-reactive protein and procalcitonin as early markers of septic complications after laparoscopic sleeve gastrectomy in morbidly obese patients within an enhanced recovery after surgery program. J Am Coll Surg 2016; 222: 831–837. [DOI] [PubMed] [Google Scholar]
- 18. Frask A, Orłowski M, Dowgiałło-Wnukiewicz N, et al. Clinical evaluation of C-reactive protein and procalcitonin for the early detection of postoperative complications after laparoscopic sleeve gastrectomy. Wideochir Inne Tech Maloinwazyjne 2017; 12: 160–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Komen N, Slieker J, Willemsen P, et al. ; APPEAL Study Group. Acute phase proteins in drain fluid: a new screening tool for colorectal anastomotic leakage? The APPEAL study: analysis of parameters predictive for evident anastomotic leakage. Am J Surg 2014; 208: 317–323. [DOI] [PubMed] [Google Scholar]
- 20. Komen N, Slieker J, Willemsen P, et al. Polymerase chain reaction for enterococcus faecalis in drain fluid: the first screening test for symptomatic colorectal anastomotic leakage. The appeal-study: analysis of parameters predictive for evident anastomotic leakage. Int J Colorectal Dis 2014; 29: 15–21. [DOI] [PubMed] [Google Scholar]
- 21. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004; 240: 205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Giaccaglia V, Salvi PF, Cunsolo GV, et al. Procalcitonin, as an early biomarker of colorectal anastomotic leak, facilitates enhanced recovery after surgery. J Crit Care 2014; 29: 528–532. [DOI] [PubMed] [Google Scholar]
- 23. Kim EY, Yim HW, Park CH, et al. C-reactive protein can be an early predictor of postoperative complications after gastrectomy for gastric cancer. Surg Endosc 2017; 31: 445–454. [DOI] [PubMed] [Google Scholar]
- 24. Singh PP, Zeng ISL, Srinivasa S, et al. Systematic review and meta-analysis of use of serum C-reactive protein levels to predict anastomotic leak after colorectal surgery. Br J Surg 2014; 101: 339–346. [DOI] [PubMed] [Google Scholar]
- 25. Asti E, Bonitta G, Melloni M, et al. Utility of C-reactive protein as predictive biomarker of anastomotic leak after minimally invasive esophagectomy. Langenbecks Arch Surg 2018; 403: 235–244. [DOI] [PubMed] [Google Scholar]
- 26. Garcia-Granero A, Frasson M, Flor-Lorente B, et al. Procalcitonin and C-reactive protein as early predictors of anastomotic leak in colorectal surgery: a prospective observational study. Dis Colon Rectum 2013; 56: 475–483. [DOI] [PubMed] [Google Scholar]
- 27. Benoit O, Faron M, Margot N, et al. C-reactive protein values after colorectal resection: can we discharge a patient with a C-reactive protein value >100? A retrospective cohort study. Dis Colon Rectum 2019; 62: 88–96. [DOI] [PubMed] [Google Scholar]
- 28. Giaccaglia V, Salvi PF, Antonelli MS, et al. Procalcitonin reveals early dehiscence in colorectal surgery: the PREDICS study. Ann Surg 2016; 263: 967–972. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_material for Clinical predictive efficacy of C-reactive protein for diagnosing infectious complications after gastric surgery by Jinyao Shi, Zhouqiao Wu, Qi Wang, Yan Zhang, Fei Shan, Shiyang Hou, Xiangji Ying, Longtao Huangfu, Ziyu Li and Jiafu Ji in Therapeutic Advances in Gastroenterology
Supplemental material, TAG936542_Supplemental_Figure_CLN for Clinical predictive efficacy of C-reactive protein for diagnosing infectious complications after gastric surgery by Jinyao Shi, Zhouqiao Wu, Qi Wang, Yan Zhang, Fei Shan, Shiyang Hou, Xiangji Ying, Longtao Huangfu, Ziyu Li and Jiafu Ji in Therapeutic Advances in Gastroenterology