Abstract
Tumor-associated inflammation and immune responses are key components in the tumor microenvironment (TME) that regulate tumor growth, progression and metastasis. Tumor-associated myeloid cells (TAMCs) are a group of cells that play multiple key roles including induction of tumor-associated inflammation/angiogenesis and regulation of tumor-specific T cell responses. Thus, identification and characterization of key pathways that can regulate TAMCs are of critical importance for developing cancer immunotherapy. Recent studies suggest that CD200-CD200 receptor (CD200R) interaction may be important in regulating the TME via affecting TAMCs. In this chapter, we will give a brief overview of the CD200-CD200R axis, including the biology behind CD200-CD200R interaction and the role(s) it plays in tumor microenvironment and tumor growth, and activation/effector functions of T cells. We will also discuss CD200-CD200R’s role as potential checkpoint molecules for cancer immunotherapy. Further investigation of the CD200-CD200R pathway will not only advance our understanding of tumor pathogenesis and immunity, but also provide the rationale for CD200-CD200R-targeted immunotherapy of human cancer.
Keywords: CD200, CD200 receptor, Tumor microenvironment, Tumor immunity, Tumor associated myeloid cells (TAMCs), Tumor associated macrophages (TAMs), Myeloid derived suppressor cells (MDSCs), Regulatory T cells (Tregs), Dendritic cells (DCs), Cytotoxic T Lymphocytes (CTLs), Immunotherapy
1. Introduction
Tumor-associated inflammation and immune responses are major contributors in regulating tumor growth and progression and establishing a tumor microenvironment (TME) (1). Tumor-associated myeloid cells (TAMCs) are a group of cells that play key roles in inducing tumor-associated inflammation/angiogenesis(2, 3), activating tumor invasion/metastasis(4, 5) and regulating tumor-specific T cell responses(6). Therefore, to better understand cancer pathogenesis and pave the way for developing effective cancer immune therapy, identification and characterization of key pathways that regulate TAMCs in the TME is of critical importance. In this regard, accumulating evidence (7–9) suggests that CD200-CD200 receptor (CD200R) interaction may be important in regulating the TME. In the past decade, reports suggesting an association between CD200-CD200R pathway and prognosis in human cancer patients (10, 11) has caused an explosion of interest in these molecules and their interactions. Today, clinical trials of patients with advanced cancer are underway based on blockade of this pathway using antibodies (12). In this chapter, we will give a brief overview of the biological aspects of the CD200-CD200R axis, and its role in tumor microenvironment, tumor growth, T cell activation and effector functions and its potential role as “checkpoint molecules” for cancer immunotherapy.
2. The biology of CD200-CD200R axis
CD200 (also known as OX-2) is a member of the Ig super family (IgSF) of proteins and shares structural similarities with the B7 family of proteins (Figure 1). It contains two extracellular immunoglobulin domains and a small 19aa intracellular domain with no known signaling motif (13). CD200 is expressed in a variety of normal tissues including B and activated T lymphocytes (14–18). Recent studies have revealed that CD200 is also over-expressed in a variety of human cancer cells including human melanoma(19), ovarian cancer(20), myeloid leukemia(11), some B cell malignancies(10) and a majority of endocrine malignancies such as small cell lung carcinoma(21). CD200R, the cognate ligand for CD200, is also an IgSF protein (22). The expression pattern of mouse and human CD200R is similar, with strong expression in macrophages, neutrophils and mast cells (23). Unlike most of the IgSF receptors, CD200R lacks ITIM domains (24). However, its 67 AA cytoplasmic tail contains three tyrosine residues, and the third tyrosine residue is located within an NPXY motif, which is phosphorylated upon ligation of the CD200R(25). This leads to the recruitment and phosphorylation of Dok-2 and 1, which then bind to RasGAP and SHIP (25–27). In macrophages and mast cells, this cascade has been shown to inhibit the phosphorylation of ERK, P38 and JNK(26), and the activation of myeloid cells (28). CD200R signaling in macrophage appears to limit autoimmune inflammation in animal models of multiple sclerosis, arthritis(29), and lung injury caused by viral infection(30), as CD200-deficient mice exhibit hyper active macrophages with significant increases in disease severity. Notably, CD200R-deficient mice were more susceptible to arthritis, presumably due to enhanced macrophage functions (31). These findings suggest that CD200-CD200R pathway is mainly involved in regulating the functions of myeloid lineages of cells. Although CD200R expression is mainly found in macrophages and neutrophils, further research revealed lower levels of CD200R expression in dendritic cells (DC) and some subsets of T cells (23, 32, 33), suggesting additional functions for CD200R signaling in regulating these cell types. Some laboratories reported elevated cytotoxic T cell (CTL) responses in CD200−/− mice infected with influenza virus (30, 34), while other research suggested autoantigen specific T cell responses were normal (29, 31). To make matters more complicated, certain studies propose CD200 signaling is required for the induction of T cell tolerance (35, 36). Although these studies yielded conflicting results, they all confer CD200-CD200R signaling contributes to T cell response regulation.
Fig.1. CD200-CD200R axis is considered to be a pair of checkpoint molecules that regulate tumor-specific immune responses.
CD200 and CD200R shares similar structures with other important immunoglobulin family members such as CD47-SIRPa, PD1-PD-L1 and CTLA4-B7.
3. CD200-CD200R interaction in tumor microenvironment and its impact on tumor growth and progression
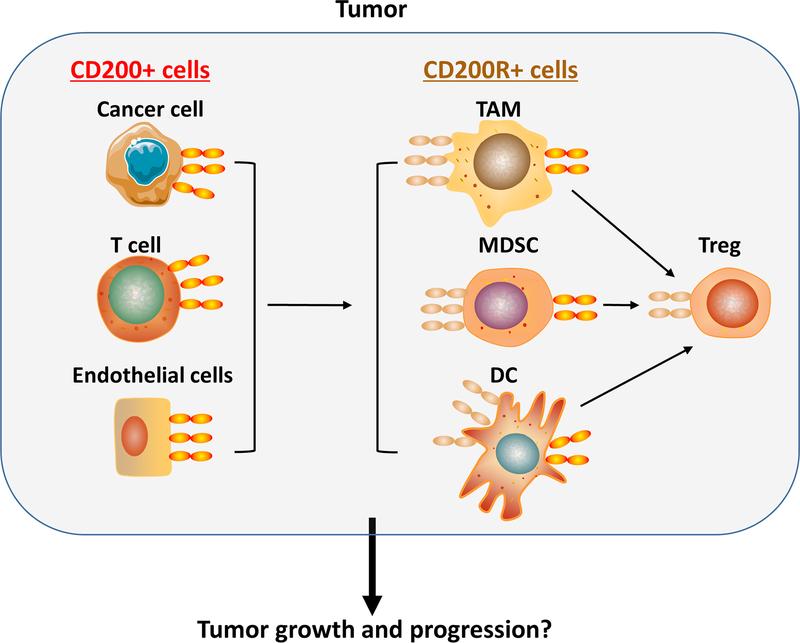
In the tumor microenvironment, a number of cell types express CD200 and/or CD200R (Figure 2). CD200 is over-expressed in cancer cells of a variety of human tumors including melanoma(19), ovarian cancer(20), some B cell malignancies(10) and many endocrine malignancies such as small cell lung carcinoma(21). Additionally, endothelial cells from tumor blood vessels and activated T, B and myeloid cells in the TME express significant levels of CD200. TAMCs, including tumor-associated macrophages (TAM), myeloid-derived suppressor cells (MDSC) and tumor-associated dendritic cells (TADCs), are the major lineages of cells expressing CD200R in the TME (7). Other immune cells such as Tregs also express significant levels of CD200R. The complicated interactions of CD200-CD200R among these cell types can significantly shape TME and affect tumor growth and progression (Figure 2). This can explain the confounding dilemma: Why do studies on the role of CD200 expression in tumors often lead to controversial results?
Fig.2. CD200-CD200R interaction in tumor microenvironment.
In the TME, CD200 is mainly expressed in some types of cancer cells, activated immune cells such as T cells, and endothelial cells, while CD200R is predominantly expressed in myeloid cells such as TAMs, MDSCs and DCs. Tregs also express significant levels of CD200R. Interactions among these cell types in TME are likely to determine the outcome of the tumor associated inflammation and immune response, and subsequently affect tumor growth and metastasis.
A human study in 2006 suggested that CD200 mRNA expression in myeloma cells is associated with decreased survival of patients (10). However, this result was later challenged by another report, which showed that loss of CD200 protein expression on myeloma cells is correlated with a clinically more aggressive disease, characterized by expression of a 70-gene signature (37). CD200 expression in acute myeloid leukemia (AML) is associated with poor prognosis (11). However, a more recent study demonstrated that CD200 expression in chronic lymphocytic leukemia (CLL) is actually associated with better prognosis (38). Similarly, CD200 is associated with tumor grading and metastasis in bladder cancer (39), while in breast cancer, CD200 is mainly present in patients with early-stage breast cancer, which does not favor nodal metastasis (40). Thus, it appears that tumor CD200 plays differential roles in in human cancer depending on the tumor type.
In animal studies, CD200 expression was found in cancer stem cells of basal cell carcinoma and associated with tumor initiation capacity (41) or positively correlated with the metastatic capacity in squamous cell carcinoma (42). However, these tumor types do not overexpress CD200, and it remains unclear if expression of CD200 on cancer stem cells is responsible for their capacity in tumor initiation and metastasis. In CD200−/− mice, scientists observed reduced carcinogen-induced tumor development (43). In CD200R-deficient mice, decreased growth and metastasis of CD200-positive EMT6 tumors was observed (44). However, another work showed 4THM breast tumors exhibit accelerated growth and metastasis in CD200R−/− mice compared to WT mice (45). In a recent study (9), we found that CD200R-deficient mice exhibit accelerated growth only in CD200-positive B16 tumors (no difference was observed in CD200-negative B16 tumor growth). Strikingly, CD200R-deficient mice receiving CD200-positive B16 cells intravenously exhibited massive tumor growth in multiple organs including liver, lung, kidney and peritoneal cavity, while the growth of the same tumors in wild type mice was limited. CD200-positive tumors grown in CD200R-deficient mice contained higher numbers of CD11b+Ly6C+ myeloid cells and exhibited increased expression of VEGF and HIF-1α genes with increased angiogenesis. Based on these results, we hypothesize that CD200 expressed on tumor cells mainly interacts with CD200R-positive myeloid cells which inhibits tumor cell expansion within TME (Figure 3). This model may explain why tumors exhibit accelerated or reduced growth in the absence of CD200-CD200R interaction. Expansion of M2 macrophages and MDSCs will enhance tumor-associated inflammation/angiogenesis (2, 3), leading to tumor invasion/metastasis(4, 5) while also regulating tumor-specific T cell responses(6). All these events culminate in enhanced tumor growth. In contrast, expansion of M1 macrophages will lead to tumor growth inhibition due to their direct anti-tumor effects which include induction of tumor-specific T cell responses (46). Although this hypothetical model remains to be tested in more tumor models, current data available in literature does suggest that CD200-CD200R pathway differentially regulates tumor growth and progression in different tumor models, based on the imbalance of inflammation and immunity of various TMEs (33).
Fig.3. Tumor expressed CD200 inhibits the expansion of myeloid cells in TME.
Cancer cells are known to recruit myeloid cells to the tumor microenvironment through the secretion of myeloid cell growth factors such as M-CSF-1. When the CD200-CD200R axis is intact (left panel), tumor expressed CD200 can inhibit myeloid cell expansion via interaction with CD200R. In the absence of CD200-CD200R interaction (right panel), significant expansion of myeloid cells occur. However, the types of myeloid cells that expand may depend on available factors driving myeloid cell differentiation in the tumor microenvironment.
In addition to cancer cell expressed CD200, endothelial cells from tumor vessels express high levels of CD200 (47). Presently, the significance of endothelial CD200 in tumor growth and progression remains unclear. It is suggested that endothelial cell CD200 is important for immune cell-endothelial cell interactions (48) and suppresses immune cell functions (47). It is possible that endothelial CD200 may also affect the recruitment of CD200R-positive myeloid cells into tumors, thereby affecting tumor growth and progression. Today we know that some CD200R-positive myeloid cells also express CD200 upon activation (49). The significance of the CD200-CD200R interactions among these cell types remains to be determined.
4. CD200-CD200R interaction in regulating activation and effector functions of tumor-specific T cells
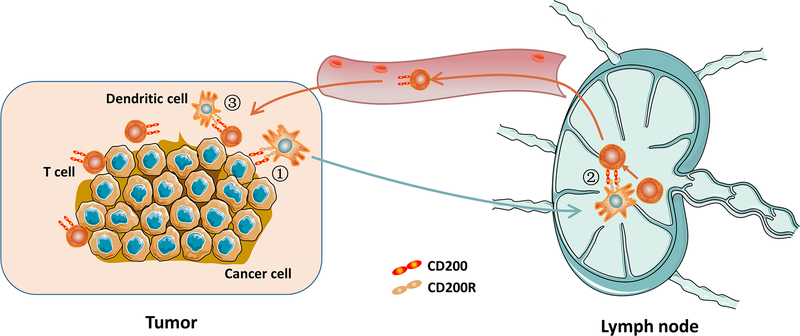
Dendritic cells play key roles in induction of T cell responses including anti-tumor T cell responses. Since CD200R is expressed in DC, some types of cancer cells constitutively express CD200, and activated T cells upregulate CD200, it is expected that CD200-CD200R interaction plays a role in induction of anti-tumor T cell responses. Figure 4 outlines the possible checkpoints where CD200-CD200R interaction may regulate DC induction of an anti-tumor T cell response. First, in TME, CD200-positive tumor cells or their debris are captured by DCs. This affects uptake of tumor antigen by DCs and subsequently DC differentiation. This process may also affect DC expansion in TME. At this time, no data concerning how CD200-CD200R interaction affects DC uptake of antigen is available. Second, after tumor antigen capture, DCs migrate to lymph nodes and present tumor antigens to T cells. After activation, T cells upregulate CD200, which in turn may affect DC function through CD200R and thereby influence T cell activation. In this regard, Xiong et al. recently showed that tumor-derived vaccines containing CD200 indeed inhibit T cell activation (50). Third, activated T cells (CD200-positive) infiltrate TME, where they are reactivated by DCs. CD200 on T cells may interfere with T cell reactivation by DC through interaction with CD200R and thereby affect T cell effector functions. Whether CD200R in tumor associated DC regulates their function remains to be investigated. However, in a subset of DC (plasmacytoid DC), CD200R signaling did induce Indoleamine 2,3-Dioxygenase (IDO), which initiated the immunosuppressive pathway of Tryptophan (51). In the tumor microenvironment, pDC are normally rare, therefore the general significance of this observation remains to be determined.
Fig.4. CD200-CD200R interaction and DC induction of anti-tumor T cell responses.
The following are the checkpoints where CD200-CD200R may play a role during the process: 1) CD200R-positive DCs migrate to TME, where they pick up dead CD200-positive tumor cells or their debris; 2) DCs loaded with tumor antigen meet T cells in lymph nodes and activate them (upregulate CD200); and 3) CD200-positive T cells infiltrate tumors where DCs reactivate them.
Based on the model proposed in Figure 4, CD200-CD200R should play a regulatory role in DC-mediated activation of T cells. However, findings regarding the role of CD200-CD200R in T cell activation and effector function are often controversial. In vitro co-culture experiments using allogeneic lymphocytes and CD200-positive cancer cells such as melanoma cells suggest that blockade of CD200-CD200R interaction increases IFN-γ production by T cells (19, 52–54). In vivo mouse studies demonstrated that in some tumor models, CD200 signal, derived from either tumor cells or host cells, inhibits anti-tumor immune responses (35, 55–57). However, we found CD200-positive tumors grown in wild type mice contained more IFN-γ/TNF-α secreting tumor infiltrating T cells (7–9). On the other hand, we found that CD200-positive B16 tumors grown in CD200R-deficient mice contained much less infiltrated T cells (7). The discrepancy in results, suggests that the role of CD200 in tumor immunity may differ based on tumor types (33). The current understanding of the role of CD200R signaling in tumor immunity is very limited. Based on the model provided in Figure 2, we suggest that CD200R is predominantly expressed on DC and a group of “immune suppressors” in the TME. We anticipate that signaling from these CD200R expressing cells will affect T cell response and T cell effector functions. Eventually, these opposing pro- or anti-signals will determine the specific T cell response that develops in a particular TME.
We previously tested whether CD200-positive tumors are susceptible to T cell adoptive transfer therapy. P1CTL cells that recognize tumor antigen P1A were adoptively transferred into mice bearing CD200-positive or CD200-negative J558 tumors. Strikingly, we found that established CD200-positive tumors were often completely rejected by adoptively transferred CTLs, without tumor recurrence. In contrast, CD200-negative tumors were initially rejected by adoptively transferred CTLs, but the majority of tumors recurred due to tumor antigen mutation. Tumor expression of CD200 significantly inhibited suppressive activity and IL-10 production by tumor-associated myeloid cells. As a result, more CTLs accumulated in tumor beds and exhibited a greater capacity to produce IFN-γ in CD200-positive tumors compared to CD200-negative tumors (7, 8). Based on these results, we propose a cellular model to explain the mechanism by which CD200-positive tumors respond better to CTL therapy (Figure 5). In this model, tumor associated myeloid cells serve as tumor growth enhancers. In the absence of CD200-CD200R interaction, tumor cells do not inhibit myeloid cell expansion and function. The freely expanding myeloid cells (when CD200-CD200R expression is absent or inhibited) help establish tumors. In contrast, in the presence of CD200-CD200R interaction, the expansion and functions of myeloid cells are inhibited, thereby failing to help mutated tumor cells in establishing tumors.
Fig.5. A proposed mechanism of how tumor expressed CD200 controls tumor evasion of T cell therapy.
Adoptively transferred tumor-specific T cells can destroy both CD200+ and CD200- cancer cells while they fail to eliminate cancer cells that mutate tumor antigen. However, in the presence of tumor CD200 (lower panel), mutated cancer cells cannot grow back to tumor due to lack of help from myeloid cells, leading to tumor rejection. In the absence of tumor CD200 (upper panel), expanded myeloid cells can help mutated tumor cells regrow into a tumor, leading to tumor recurrence.
5. Is targeting CD200-CD200R feasible for cancer immunotherapy?
Since the CD200-CD200R pathway regulates immune cell functions and shares similarities with other checkpoint molecules, there is a broad interest in manipulating this pathway for cancer therapy. Currently, CD200 blockade is a proposed immunotherapeutic option for CD200-positive human cancers. This therapeutic strategy is based on studies performed in a hu-SCID model, where established tumors are rejected by adoptively transferred peripheral blood mononuclear cells upon CD200 blockade (58–60). In a phase I study, Samalizumab (an anti-human CD200 Ab) was injected into 23 patients with advanced chronic lymphocytic leukemia (CLL) and 3 patients with multiple myeloma (MM). While the treatment was ineffective in the 3 MM patients, reduced CD200 expression was observed in CLL cells of treated patients. Notably, they observed antibody-mediated depletion of CD200 expressing CD4+ effector T cells (12). Since CD200 is broadly expressed in normal tissues, targeting CD200 is difficult and may have potential side effects. Thus, developing non-depleting CD200 antibodies is necessary for CD200 blockade therapy. As an alternative approach, targeting CD200R should be more feasible for treating human cancer due to its limited expression pattern in normal tissues and abundant presence in TME of essentially all types of solid tumors. To determine if enhancing CD200R signaling could affect tumor growth, we tested the efficacy of an agonistic anti-CD200R mAb (OX110)(23, 30) in treating lung metastasis of CD200-negative melanoma (8). We found that OX110 treatment significantly inhibited tumor foci formation in the lungs. Consistent with this study, Pilch et al. recently showed that co-injection of TLR7 agonist and anti-CD200R antibody reshaped TME and induced anti-tumor myeloid cells in the mouse CT26 colon tumor model (61). Thus, targeting CD200R rather than CD200 should be a feasible approach for human cancer therapy. Furthermore, the broad expression of CD200 in some types of blood cancer has inspired novel T cell therapies (an indirect use of the CD200-CD200R axis). For instance, the Greenberg group has designed CD19 CAR-T cells that express CD200R whose intracellular domain is replaced with a CD28 signaling motif (62). Adoptive transfer of these CD200R manipulated, CD19 targeted CAR-T cells resulted in significant clearance of leukemic cells in treated animals. In the future, this strategy can also be utilized to treat human solid tumors that overexpress CD200.
6. Concluding Remarks and Future Perspective
Although CD200 and CD200R are considered to be a pair of checkpoint molecules that potentially regulate immune responses and immunotherapy, there are considerable differences between the CD200-CD200R axis and other important checkpoint molecules such as PD-1-PD-L1. For instance, tumor infiltrating T cells, especially CD8+ T cells do not normally express CD200R, while tumor-associated myeloid cells are the main cell types that express CD200R. Thus, CD200-CD200R pathway does not directly regulate T cells. It actively affects and regulates the functions of myeloid cells in the TME, thereby indirectly gauging the activation level and effector functions of T cells in the respective environment. At this stage, the signaling events mediated by CD200R in these cell types and their biological effects are not very clear. Further studies on the basic biology of the CD200-CD200R axis in the TME are necessary.
Because of the complicated cellular interactions that may be regulated by CD200-CD200R in the tumor microenvironment (Figure 2), in-depth studies using genetic mouse models are needed to figure out the role and functions of these cellular interactions in different tumor types. Among such interactions, the role of CD200R signaling in Tregs is a complete mystery. We speculate that CD200-CD200R signaling in TME regulates the homeostasis and functions of Tregs. Similarly, studying the roles of CD200R signaling in tumor associated DC (Figure 4) is of paramount importance for developing CD200R-based cancer immunotherapy.
For future CD200-CD200R targeted cancer therapy, careful studies are needed to evaluate what types of cancer patients may benefit. Since CD200-CD200R differs from other checkpoint molecules, blockade of this “checkpoint” may not unleash anti-tumor immune responses, depending on the tumor microenvironment. We predict that in tumors where M1 type macrophages are dominant, CD200 blockade will be beneficial. Additionally, since CD200 is more broadly expressed in normal tissues, targeting CD200R rather than CD200 is a more feasible approach to therapy of human cancer. Finally, for tumor types that overexpress CD200, CD200R signaling can be manipulated to transduce a positive signal and utilized in T cell therapy, as exemplified by Greenberg et al. (62).
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. 2008. Cancer-related inflammation. Nature 454: 436–44 [DOI] [PubMed] [Google Scholar]
- 2.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. 2004. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6: 409–21 [DOI] [PubMed] [Google Scholar]
- 3.Murdoch C, Muthana M, Coffelt SB, Lewis CE. 2008. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 8: 618–31 [DOI] [PubMed] [Google Scholar]
- 4.Qian BZ, Pollard JW. 2010. Macrophage diversity enhances tumor progression and metastasis. Cell 141: 39–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, Schioppa T, Porta C, Allavena P, Sica A. 2006. Role of tumor-associated macrophages in tumor progression and invasion. Cancer Metastasis Rev 25: 315–22 [DOI] [PubMed] [Google Scholar]
- 6.Gabrilovich DI, Nagaraj S. 2009. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9: 162–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang L, Liu JQ, Talebian F, El-Omrani HY, Khattabi M, Yu L, Bai XF. 2010. Tumor expression of CD200 inhibits IL-10 production by tumor-associated myeloid cells and prevents tumor immune evasion of CTL therapy. Eur J Immunol 40: 2569–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talebian F, Liu JQ, Liu Z, Khattabi M, He Y, Ganju R, Bai XF. 2012. Melanoma cell expression of CD200 inhibits tumor formation and lung metastasis via inhibition of myeloid cell functions. PLoS One 7: e31442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu JQ, Talebian F, Wu L, Liu Z, Li MS, Wu L, Zhu J, Markowitz J, Carson WE 3rd, Basu S, Bai XF. 2016. A Critical Role for CD200R Signaling in Limiting the Growth and Metastasis of CD200+ Melanoma. J Immunol 197: 1489–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moreaux J, Hose D, Reme T, Jourdan E, Hundemer M, Legouffe E, Moine P, Bourin P, Moos M, Corre J, Mohler T, De Vos J, Rossi JF, Goldschmidt H, Klein B. 2006. CD200 is a new prognostic factor in multiple myeloma. Blood 108: 4194–7 [DOI] [PubMed] [Google Scholar]
- 11.Tonks A, Hills R, White P, Rosie B, Mills KI, Burnett AK, Darley RL. 2007. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia 21: 566–8 [DOI] [PubMed] [Google Scholar]
- 12.Mahadevan D, Lanasa MC, Farber C, Pandey M, Whelden M, Faas SJ, Ulery T, Kukreja A, Li L, Bedrosian CL, Zhang X, Heffner LT. 2019. Phase I study of samalizumab in chronic lymphocytic leukemia and multiple myeloma: blockade of the immune checkpoint CD200. J Immunother Cancer 7: 227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barclay AN, Clark MJ, McCaughan GW. 1986. Neuronal/lymphoid membrane glycoprotein MRC OX-2 is a member of the immunoglobulin superfamily with a light-chain-like structure. Biochem Soc Symp 51: 149–57 [PubMed] [Google Scholar]
- 14.Koning N, Swaab DF, Hoek RM, Huitinga I. 2009. Distribution of the immune inhibitory molecules CD200 and CD200R in the normal central nervous system and multiple sclerosis lesions suggests neuron-glia and glia-glia interactions. J Neuropathol Exp Neurol 68: 159–67 [DOI] [PubMed] [Google Scholar]
- 15.Ragheb R, Abrahams S, Beecroft R, Hu J, Ni J, Ramakrishna V, Yu G, Gorczynski RM. 1999. Preparation and functional properties of monoclonal antibodies to human, mouse and rat OX-2. Immunol Lett 68: 311–5 [DOI] [PubMed] [Google Scholar]
- 16.Dick AD, Broderick C, Forrester JV, Wright GJ. 2001. Distribution of OX2 antigen and OX2 receptor within retina. Invest Ophthalmol Vis Sci 42: 170–6 [PubMed] [Google Scholar]
- 17.Rosenblum MD, Olasz EB, Yancey KB, Woodliff JE, Lazarova Z, Gerber KA, Truitt RL. 2004. Expression of CD200 on epithelial cells of the murine hair follicle: a role in tissue-specific immune tolerance? J Invest Dermatol 123: 880–7 [DOI] [PubMed] [Google Scholar]
- 18.Wright GJ, Jones M, Puklavec MJ, Brown MH, Barclay AN. 2001. The unusual distribution of the neuronal/lymphoid cell surface CD200 (OX2) glycoprotein is conserved in humans. Immunology 102: 173–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petermann KB, Rozenberg GI, Zedek D, Groben P, McKinnon K, Buehler C, Kim WY, Shields JM, Penland S, Bear JE, Thomas NE, Serody JS, Sharpless NE. 2007. CD200 is induced by ERK and is a potential therapeutic target in melanoma. J Clin Invest 117: 3922–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moreaux J, Veyrune JL, Reme T, De Vos J, Klein B. 2008. CD200: a putative therapeutic target in cancer. Biochem Biophys Res Commun 366: 117–22 [DOI] [PubMed] [Google Scholar]
- 21.Love JE, Thompson K, Kilgore MR, Westerhoff M, Murphy CE, Papanicolau-Sengos A, McCormick KA, Shankaran V, Vandeven N, Miller F, Blom A, Nghiem PT, Kussick SJ. 2017. CD200 Expression in Neuroendocrine Neoplasms. Am J Clin Pathol 148: 236–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barclay AN, Wright GJ, Brooke G, Brown MH. 2002. CD200 and membrane protein interactions in the control of myeloid cells. Trends Immunol 23: 285–90 [DOI] [PubMed] [Google Scholar]
- 23.Wright GJ, Cherwinski H, Foster-Cuevas M, Brooke G, Puklavec MJ, Bigler M, Song Y, Jenmalm M, Gorman D, McClanahan T, Liu MR, Brown MH, Sedgwick JD, Phillips JH, Barclay AN. 2003. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J Immunol 171: 3034–46 [DOI] [PubMed] [Google Scholar]
- 24.Zhang S, Cherwinski H, Sedgwick JD, Phillips JH. 2004. Molecular mechanisms of CD200 inhibition of mast cell activation. J Immunol 173: 6786–93 [DOI] [PubMed] [Google Scholar]
- 25.Mihrshahi R, Barclay AN, Brown MH. 2009. Essential roles for Dok2 and RasGAP in CD200 receptor-mediated regulation of human myeloid cells. J Immunol 183: 4879–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Minas K, Liversidge J. 2006. Is the CD200/CD200 receptor interaction more than just a myeloid cell inhibitory signal? Crit Rev Immunol 26: 213–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mihrshahi R, Brown MH. 2010. Downstream of tyrosine kinase 1 and 2 play opposing roles in CD200 receptor signaling. J Immunol 185: 7216–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jenmalm MC, Cherwinski H, Bowman EP, Phillips JH, Sedgwick JD. 2006. Regulation of myeloid cell function through the CD200 receptor. J Immunol 176: 191–9 [DOI] [PubMed] [Google Scholar]
- 29.Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. 2000. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290: 1768–71 [DOI] [PubMed] [Google Scholar]
- 30.Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. 2008. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol 9: 1074–83 [DOI] [PubMed] [Google Scholar]
- 31.Simelyte E, Alzabin S, Boudakov I, Williams R. 2010. CD200R1 regulates the severity of arthritis but has minimal impact on the adaptive immune response. Clin Exp Immunol 162: 163–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rijkers ES, de Ruiter T, Baridi A, Veninga H, Hoek RM, Meyaard L. 2008. The inhibitory CD200R is differentially expressed on human and mouse T and B lymphocytes. Mol Immunol 45: 1126–35 [DOI] [PubMed] [Google Scholar]
- 33.Rygiel TP, Meyaard L. 2012. CD200R signaling in tumor tolerance and inflammation: A tricky balance. Curr Opin Immunol 24: 233–8 [DOI] [PubMed] [Google Scholar]
- 34.Rygiel TP, Rijkers ES, de Ruiter T, Stolte EH, van der Valk M, Rimmelzwaan GF, Boon L, van Loon AM, Coenjaerts FE, Hoek RM, Tesselaar K, Meyaard L. 2009. Lack of CD200 enhances pathological T cell responses during influenza infection. J Immunol 183: 1990–6 [DOI] [PubMed] [Google Scholar]
- 35.Rygiel TP, Karnam G, Goverse G, van der Marel AP, Greuter MJ, van Schaarenburg RA, Visser WF, Brenkman AB, Molenaar R, Hoek RM, Mebius RE, Meyaard L. 2011. CD200-CD200R signaling suppresses anti-tumor responses independently of CD200 expression on the tumor. Oncogene [DOI] [PubMed] [Google Scholar]
- 36.Gorczynski RM, Chen Z, He W, Khatri I, Sun Y, Yu K, Boudakov I. 2009. Expression of a CD200 transgene is necessary for induction but not maintenance of tolerance to cardiac and skin allografts. J Immunol 183: 1560–8 [DOI] [PubMed] [Google Scholar]
- 37.Alapat D, Coviello-Malle J, Owens R, Qu P, Barlogie B, Shaughnessy JD, Lorsbach RB. 2012. Diagnostic usefulness and prognostic impact of CD200 expression in lymphoid malignancies and plasma cell myeloma. Am J Clin Pathol 137: 93–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Arena G, Valvano L, Vitale C, Coscia M, Statuto T, Bellesi S, Lamorte D, Musto P, Laurenti L, D’Auria F. 2019. CD200 and prognosis in chronic lymphocytic leukemia: Conflicting results. Leuk Res 83: 106169. [DOI] [PubMed] [Google Scholar]
- 39.Rexin P, Tauchert A, Hanze J, Heers H, Schmidt A, Hofmann R, Hegele A. 2018. The Immune Checkpoint Molecule CD200 Is Associated with Tumor Grading and Metastasis in Bladder Cancer. Anticancer Res 38: 2749–54 [DOI] [PubMed] [Google Scholar]
- 40.Clark DA, Dhesy-Thind S, Ellis P, Ramsay J. 2014. The CD200-tolerance signaling molecule associated with pregnancy success is present in patients with early-stage breast cancer but does not favor nodal metastasis. Am J Reprod Immunol 72: 435–9 [DOI] [PubMed] [Google Scholar]
- 41.Colmont CS, Benketah A, Reed SH, Hawk NV, Telford WG, Ohyama M, Udey MC, Yee CL, Vogel JC, Patel GK. 2013. CD200-expressing human basal cell carcinoma cells initiate tumor growth. Proc Natl Acad Sci U S A 110: 1434–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stumpfova M, Ratner D, Desciak EB, Eliezri YD, Owens DM. 2010. The immunosuppressive surface ligand CD200 augments the metastatic capacity of squamous cell carcinoma. Cancer Res 70: 2962–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rygiel TP, Karnam G, Goverse G, van der Marel AP, Greuter MJ, van Schaarenburg RA, Visser WF, Brenkman AB, Molenaar R, Hoek RM, Mebius RE, Meyaard L. 2012. CD200-CD200R signaling suppresses anti-tumor responses independently of CD200 expression on the tumor. Oncogene 31: 2979–88 [DOI] [PubMed] [Google Scholar]
- 44.Podnos A, Clark DA, Erin N, Yu K, Gorczynski RM. 2012. Further evidence for a role of tumor CD200 expression in breast cancer metastasis: decreased metastasis in CD200R1KO mice or using CD200-silenced EMT6. Breast Cancer Res Treat 136: 117–27 [DOI] [PubMed] [Google Scholar]
- 45.Erin N, Podnos A, Tanriover G, Duymus O, Cote E, Khatri I, Gorczynski RM. 2015. Bidirectional effect of CD200 on breast cancer development and metastasis, with ultimate outcome determined by tumor aggressiveness and a cancer-induced inflammatory response. Oncogene 34: 3860–70 [DOI] [PubMed] [Google Scholar]
- 46.Mantovani A, Marchesi F, Malesci A, Laghi L, Allavena P. 2017. Tumour-associated macrophages as treatment targets in oncology. Nat Rev Clin Oncol 14: 399–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Belkin DA, Mitsui H, Wang CQ, Gonzalez J, Zhang S, Shah KR, Coats I, Suarez-Farinas M, Krueger JG, Felsen D, Carucci JA. 2013. CD200 upregulation in vascular endothelium surrounding cutaneous squamous cell carcinoma. JAMA Dermatol 149: 178–86 [DOI] [PubMed] [Google Scholar]
- 48.Ko YC, Chien HF, Jiang-Shieh YF, Chang CY, Pai MH, Huang JP, Chen HM, Wu CH. 2009. Endothelial CD200 is heterogeneously distributed, regulated and involved in immune cell-endothelium interactions. J Anat 214: 183–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Singh A, Falk MK, Hviid TV, Sorensen TL. 2013. Increased expression of CD200 on circulating CD11b+ monocytes in patients with neovascular age-related macular degeneration. Ophthalmology 120: 1029–37 [DOI] [PubMed] [Google Scholar]
- 50.Xiong Z, Ampudia-Mesias E, Shaver R, Horbinski CM, Moertel CL, Olin MR. 2016. Tumor-derived vaccines containing CD200 inhibit immune activation: implications for immunotherapy. Immunotherapy 8: 1059–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fallarino F, Asselin-Paturel C, Vacca C, Bianchi R, Gizzi S, Fioretti MC, Trinchieri G, Grohmann U, Puccetti P. 2004. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J Immunol 173: 3748–54 [DOI] [PubMed] [Google Scholar]
- 52.Siva A, Xin H, Qin F, Oltean D, Bowdish KS, Kretz-Rommel A. 2007. Immune modulation by melanoma and ovarian tumor cells through expression of the immunosuppressive molecule CD200. Cancer Immunol Immunother [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McWhirter JR, Kretz-Rommel A, Saven A, Maruyama T, Potter KN, Mockridge CI, Ravey EP, Qin F, Bowdish KS. 2006. Antibodies selected from combinatorial libraries block a tumor antigen that plays a key role in immunomodulation. Proc Natl Acad Sci U S A 103: 1041–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wong KK, Khatri I, Shaha S, Spaner DE, Gorczynski RM. 2010. The role of CD200 in immunity to B cell lymphoma. J Leukoc Biol 88: 361–72 [DOI] [PubMed] [Google Scholar]
- 55.Gorczynski RM, Chen Z, Hu J, Kai Y, Lei J. 2001. Evidence of a role for CD200 in regulation of immune rejection of leukaemic tumour cells in C57BL/6 mice. Clin Exp Immunol 126: 220–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gorczynski RM, Clark DA, Erin N, Khatri I. 2011. Role of CD200 expression in regulation of metastasis of EMT6 tumor cells in mice. Breast Cancer Res Treat 130: 49–60 [DOI] [PubMed] [Google Scholar]
- 57.Gorczynski RM, Chen Z, Diao J, Khatri I, Wong K, Yu K, Behnke J. 2010. Breast cancer cell CD200 expression regulates immune response to EMT6 tumor cells in mice. Breast Cancer Res Treat 123: 405–15 [DOI] [PubMed] [Google Scholar]
- 58.Kretz-Rommel A, Qin F, Dakappagari N, Ravey EP, McWhirter J, Oltean D, Frederickson S, Maruyama T, Wild MA, Nolan MJ, Wu D, Springhorn J, Bowdish KS. 2007. CD200 expression on tumor cells suppresses antitumor immunity: new approaches to cancer immunotherapy. J Immunol 178: 5595–605 [DOI] [PubMed] [Google Scholar]
- 59.Kretz-Rommel A, Qin F, Dakappagari N, Cofiell R, Faas SJ, Bowdish KS. 2008. Blockade of CD200 in the presence or absence of antibody effector function: implications for anti-CD200 therapy. J Immunol 180: 699–705 [DOI] [PubMed] [Google Scholar]
- 60.Kretz-Rommel A, Bowdish KS. 2008. Rationale for anti-CD200 immunotherapy in B-CLL and other hematologic malignancies: new concepts in blocking immune suppression. Expert Opin Biol Ther 8: 5–15 [DOI] [PubMed] [Google Scholar]
- 61.Pilch Z, Tonecka K, Braniewska A, Sas Z, Skorzynski M, Boon L, Golab J, Meyaard L, Rygiel TP. 2018. Antitumor Activity of TLR7 Is Potentiated by CD200R Antibody Leading to Changes in the Tumor Microenvironment. Cancer Immunol Res 6: 930–40 [DOI] [PubMed] [Google Scholar]
- 62.Oda SK, Daman AW, Garcia NM, Wagener F, Schmitt TM, Tan X, Chapuis AG, Greenberg PD. 2017. A CD200R-CD28 fusion protein appropriates an inhibitory signal to enhance T-cell function and therapy of murine leukemia. Blood 130: 2410–9 [DOI] [PMC free article] [PubMed] [Google Scholar]