Abstract
This study reports a new application area of difluoro enol silyl ethers, which can be easily obtained from trifluoromethyl ketones. The main focus has been directed to the electrophilic fluoroalkylation and arylation methods. The trifluoromethylthiolation of difluoro enol silyl ethers can be used for the construction of a novel trifluoromethylthio-α,α-difluoroketone (−COCF2SCF3) functionality. The −CF2SCF3 moiety has interesting properties due to the electron-withdrawing, albeit lipophilic, character of the SCF3 group, which can be combined with the high electrophilicity of the difluoroketone motif. The methodology could also be extended to difluoro homologation of the trifluoromethyl ketones using the Togni reagent. In addition, we presented a method for transition-metal-free arylation of difluoro enol silyl ethers based on hypervalent iodines.
Introduction
Organofluorine compounds have found many important applications in several areas of life sciences. The high metabolic stability, ability to modify the lipophilicity, acid–base properties, and overall reactivity/bioavailability of small molecules1 led to a widespread application of organofluorines especially in medicinal chemistry2 and agrochemistry.3 As a consequence, expanding the chemical space of new organofluorine compounds has attracted great attention in industrial and academic research.4 An important class of druglike molecules is based on α-fluorinated ketone motifs (Figure 1). Due to the strong electron-withdrawing character of di-, tri-, and perfluoro alkyl groups, the electrophilicity of the neighboring keto functionality is enhanced. As a consequence, nucleophilic functionalities of enzymes, such as the hydroxy group of serine or other residues, readily interact with the low lying π*(C=O) orbitals forming (covalently bound) ketal/hemiketal-type products.5 Thus, α-fluorinated ketones are important pharmacophores for serine proteases and related enzymes (Figure 1). For instance, small molecules with β-amino-α,α-difluoroketone motifs (Figure 1a) are selective inhibitors of human renin (protease regulating blood pressure).6 Certain types of α,α-difluoroketones (Figure 1b) interact with the hydroxy methylglutaryl binding domain of coenzyme A (HMG CoA) reductase and are thus efficient inhibitors of these enzymes.7 Phospholipase A2 (iPLA2) enzymes, which are involved in inflammatory disorders (such as arthritis and autoimmune diseases), can be efficiently inhibited by perfluoroethyl ketones (Figure 1c).8 Human neutrophil elastase (HNE) is a serine protease, which can also be involved in pathophysiological states (such as cystic fibrosis and chronic bronchitis) and inhibited by trifluoromethyl ketones (Figure 1d).9 The binding properties to HNE can be improved by variation of the fluorinated carbonyl activating group.10 Thus, perfluoroethyl ketones (Figure 1e) have also been considered as HNE inhibitors.9b,10,11 Application of the α-trifluoromethylthio ketones is less common in druglike molecules than the fluoro or fluoroalkyl analogues, which might be the consequence of synthetic limitations. This pharmacophore occurs, for example, in cefazaflur (Figure 1f), which is a cephalosporin antibiotic.12
Figure 1.
Bioactive small molecules with α-fluoro and related pharmacophores.
Difluoro enol silyl ethers are useful synthons for introduction of the α,α-difluoro carbonyl functional group.13 These compounds can be prepared from trifluoroketones (1) by Mg-mediated cleavage of one of the C–F bonds (Figure 2a).14 This reaction affords fairly stable difluoro enol silyl ethers (2), which are difficult to isolate, and therefore, derivatives of 2 are usually reacted with various electrophiles without purification. The standard applications13a involve aldol reactions,14a,15 Mannich reactions,16 protonation,14b halogenation,17 and arylation18 reactions (Figure 2b).
Figure 2.
Synthesis and electrophilic transformations of difluoro enol silyl ethers.
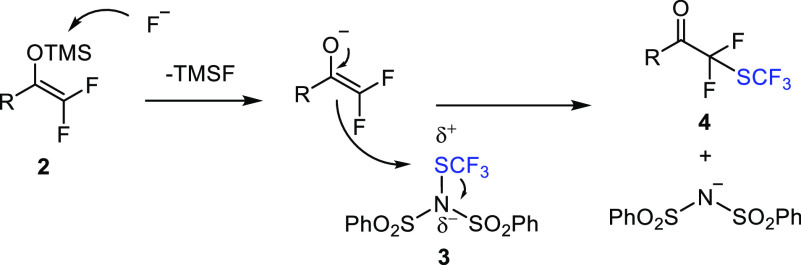
As a part of our organofluorine chemistry program,19 we sought to expand the reagent scope of difluoro enol silyl ethers (2) to reactions with new types of electrophiles. Our interest was 2-fold: (i) testing electrophilic reagents for the introduction of (S)CF3 groups and (ii) studying the application of hypervalent iodine-based electrophiles (Figure 2c) in C–C bond-forming reactions with 2.
Many recent efforts have been undertaken to find new methodologies for selective introduction of the SCF3 group.4b,20 This group frequently occurs in drug molecules (e.g., Figure 1f) because of its excellent pharmacochemical properties, such as the strong electron-withdrawing character and an exceptionally high Hansch lipophilicity parameter (π = 1.44).21 Although α-trifluoromethylthiolation of ketones is described in the literature,22 introduction of the SCF3 group in difluoro enol silyl ethers 2 has never been reported. We hypothesized (Figure 3) that reacting 2 with sufficiently reactive electrophilic SCF3 transfer reagents, such as benzenesulfonimide 3,23 a novel perfluoroalkyl (−COCF2SCF3) functionality could be constructed (4). In this way, the difluoro ketone group could be equipped with an electron-withdrawing but lipophilic group allowing, for example, extension of the pharmacochemical space of α-fluorinated ketones (Figure 1). In fact, very few synthetic methods have been reported for construction/introduction of the −CF2SCF3 functionality,24 while none of these literature methods were suitable for the preparation of −COCF2SCF3-containing molecules.
Figure 3.
Hypothesis of trifluoromethylthiolation of difluoro enol silyl ethers using N-trifluoromethylthiodibenzenesulfonimide and fluoride.
Results and Discussion
In line with our hypothesis (Figure 3), when N-trifluoromethylthiodibenzenesulfonimide 3(23) was reacted with difluoro enol silyl ether 2a (freshly prepared14 from trifluoroacetophenone 1a with Mg and TMSCl) in the presence of KF, −COCF2SCF3-functionalized product 4a was obtained (Table 1). The optimal conditions involved application of 2a, 3, and KF in equimolar amounts in acetonitrile at room temperature for 3 h. Under these conditions, 4a was obtained in 78% (NMR) yield along with 3% of the protonated analogue 5a (Table 1, entry 1). Deviation from these conditions led to lower yields and/or extensive formation of 5a.
Table 1. Deviation of Reaction Conditions for the Trifluoromethylthiolation of 2aa.
| entry | additive | yieldb of 4a (%) | yieldb of 5a (%) | recoveryb of 2a (%) |
|---|---|---|---|---|
| 1 | 1.0 equiv of KF | 78 | 3 | − |
| 2 | 1.0 equiv of CsF | 61 | 6 | − |
| 3 | 1.0 equiv of TBAF·3H2O | 15 | 45 | − |
| 4 | 1.0 equiv of TBAT | 41 | 3 | − |
| 5 | 10 mol % of DABCO | − | − | 70 |
| 6 | 10 mol % of FeCl2 | 8 | − | 78 |
| 7c | 1.0 equiv of KF | − | − | 76 |
| 8 | − | trace | − | 75 |
General procedure: 2a (0.1 mmol), 3 (0.1 mmol), and additive were stirred in MeCN (0.5 mL) at room temperature for 3 h.
19F NMR yield using PhCF3 as the internal standard.
DCM was used as a solvent instead of MeCN.
When KF was replaced by CsF, the yield slightly decreased (entry 2). Using TBAF as an activator instead of KF proved to be even less effective (entry 3). The reaction proceeded with poor yield (15%) and extensive formation of protonated side product 5a. Application of TBAT additive (entry 4) led to a higher yield (41%) than use of TBAF. However, the yield was still lower than with KF. Since tertiary amines have been successfully used as activators in aldol reactions of 2,15d we attempted to replace KF with DABCO. However, we could not detect formation of 4a in the presence of DABCO (entry 5). We have also attempted to use FeCl2 as an activator, which proved to be efficient in the analogue trifluoromethylation reaction (see below). However, the trifluoromethylthiolation reaction proceeded with only 8% yield (entry 6). When acetonitrile was replaced with dichloromethane, formation of product 4a was not observed (entry 7). A possible explanation is the poor solubility of KF in dichloromethane. In the absence of any activator, only traces of 4a were formed (entry 8).
With the optimized conditions in hand, we explored the substrate scope of the trifluoromethylthiolation of 2 (Table 2). As mentioned above, the reaction of 2a proceeds with high NMR yield (78%), but because of the volatility of 4a only 25% of the product could be isolated. The volatility of the products could not be decreased by hydration of the difluoro-keto groups. p-Phenyl-substituted ketone 4b formed smoothly and could be isolated in 78% yield. The reaction of naphthyl enol silyl ether 2c afforded 4c in 84% yield within 4 h. Reactions using substrates with electron-withdrawing substituents in the aromatic ring gave the corresponding products 4d,e with 67% and 62% yield, respectively. The difluoro enol silyl ether with an electron-withdrawing fluoro group also reacted smoothly, affording product 4f in 57% NMR yield. However, because of the volatility of 4f, it could be isolated only in 35% yield. Alkenyl difluoromethyl enolates could also be trifluoromethylthiolated with good yields, as 4g and 4h are obtained in 70% and 60% yields, respectively. We were also able to prepare the −CF2SCF3 analogue of the synthetic intermediate of the HNE inhibitor25 presented in Figure 1d. Compound 4i formed with 35% yield. The reaction could be scaled up to 1.0 mmol scale (4b) without significant change of the yield (71%).
Table 2. Substrate Scope of the Trifluoromethylthiolation Reaction of Difluoro Enol Silyl Ethersa.
General procedure: 2 (0.1 mmol), 3 (0.1 mmol), and KF (0.1 mmol) were stirred in MeCN (0.5 mL) at room temperature for 3 h.
19F NMR yield using PhCF3 as the internal standard.
Reaction conducted on 1.0 mmol scale.
We were able to extend the above methodology to trifluoromethylation of difluoro enol silyl ethers 2 (Table 3). Using Togni reagent4c6a (instead of 3), difluoro homologation of trifluoromethyl ketones (1) could be achieved. The trifluoromethylation of difluoro enol silyl ether 2a proceeded with poor NMR yield (35%) in the presence of KF as the mediator (Table 3). However, when catalytic amounts of DABCO or FeCl2 were used the yield could be improved to 43% and 62%, respectively. Application of Sc(OTf)3 led to formation of 7a with 2% yield. When the other type of Togni reagent4c (1-trifluoromethyl-3,3-dimethyl-1,2-benziodoxole, 6b) was used, the yield dropped to 20%. With Umemoto reagent 5-(trifluoromethyl)dibenzothiophenium tetrafluoroborate,26 only 1% of 7a was obtained. Considering these results, further reactions were carried out with 6a in the presence of FeCl2 catalyst.27 Similar to the SCF3 analog (4a), compound 7a was volatile, and therefore, the yield was not determined. However, perfluoroethyl compound 7b with a phenyl substituent on the aromatic ring could be isolated in 85% yield. The naphthyl analogue was obtained in 33% yield. However, 7e, with the electron-donating EtS group on the aromatic ring, formed in high yield (70%). In the presence of the electron-withdrawing fluoro substituent, the reactions still proceeded smoothly, affording 7f with 65% NMR yield. Product 7f was also volatile, and therefore, it was not isolated. Trifluoromethyl vinyl ketone 1h also underwent difluorohomologation via 2h, affording 7h in 20% yield. Similarly to the trifluoromethyltiolation (4i), the drug intermediate of HNE inhibitors (Figure 1d), 1i, could be converted to its perfluoroethyl analogue 7i in 33% yield.
Table 3. Substrate Scope of the Trifluoromethylation Reaction of Difluoro Enol Silyl Ethersa.
General procedure: 6a (0.1 mmol), 2 (0.1 mmol), and FeCl2 (0.01 mmol) were stirred in MeCN (0.5 mL) at room temperature for 2 h.
19F NMR yield using PhCF3 as the internal standard.
With trifluoromethylbenziodoxole 6b instead.
With Umemoto reagent instead of 6a.
Interestingly, the above trifluoromethylation and trifluoromethylthiolation reactions could also be performed in a sequence (Figure 4). Thus, 7b (obtained from 1b by trifluoromethylation) may undergo a subsequent trifluoromethylthiolation with 3 via enolate 2j to afford 4j in 86% yield. The interesting feature of 4j is that all three functional groups, which are widely used in organofluorine chemistry, are attached to the same carbon center.
Figure 4.
Trifluoromethylthiolation of 2j.
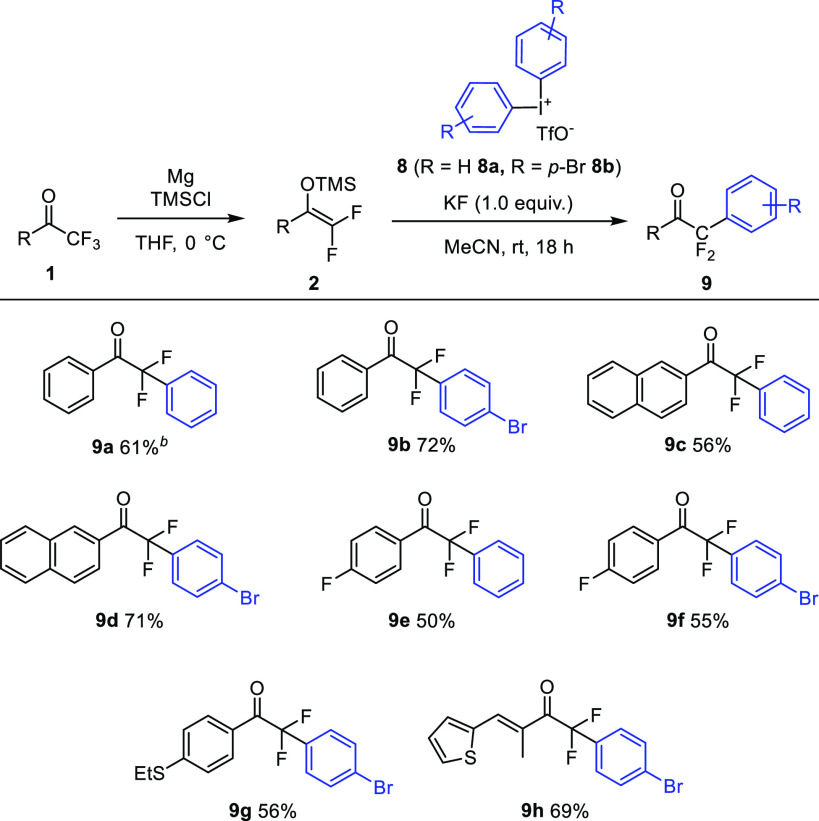
The above results with the Togni reagent 6a suggested that other hypervalent iodines28 can also be useful electrophiles for the functionalization of difluoro enol silyl ethers 2. Indeed, we have found that 2 reacted smoothly with diphenyl iodonium salt 8a (Table 4) in the presence of KF under conditions very similar to those of the above trifluoromethylthiolation reactions (Table 2). The reaction afforded 9a with 61% yield using KF as mediator, while formation of 9a was not observed with DABCO or FeCl2 as mediators (instead of KF). The bromo analogue 8b reacted with an even higher yield of 72%. The synthesis of aryl bromide products proceeded smoothly under the applied transition-metal-free conditions. Thus, both phenyl- and bromophenyl-containing difluoroketones 9c–9g could be easily obtained in 50–71% yields. Not only aryl ketones but also vinyl ketone 1h could be converted to bromophenyl derivative 9h (69%) via enolate 2h. We attempted to use alkynyl derivatives of hypervalent iodine reagents,29 but formation of the corresponding difluoroketone product was not observed.
Table 4. Substrate Scope of the Arylation Reaction of Difluoro Enol Silyl Ethersa.
General procedure: 8 (0.1 mmol), 2 (0.1 mmol), and KF (0.1 mmol) were stirred in MeCN (0.5 mL) at room temperature for 18 h.
When FeCl2 (10 mol %) or DABCO (10 mol %) was used instead, no 9a was detected.
Conclusions
In summary, we have presented a new method for the functionalization of difluoro enol silyl ethers with electrophilic alkyl fluoride and aryl-transfer reagents. Using trifluoromethylthiolation reagent 3, −COCF3 functionalities could be converted to −COCF2SCF3 groups. The electron-withdrawing but lipophilic −CF2SCF3 group is an interesting new modifier for the reactivity of the keto groups in bioactive compounds (Figure 1). We have also presented two extensions of the methodology using hypervalent iodine-based electrophiles. By use of Togni reagent 6a, difluoro homologation of the −COCF3 group can be performed. This way of construction of a perfluoroethyl group can be used as an alternative method for the introduction of −CF2CF3 moiety to ketones.30 In addition, we have shown that transition-metal-free arylation of difluoro enol silyl ethers can be performed using hypervalent iodines as aryl source. This method complements the reported transition-metal-catalyzed cross-coupling and other related arylation reactions to obtain −COCF2Ar functionality.18,31 Overall, the new methodology is suitable for the expansion of the synthetic space of difluoro ketone based compounds including bioactive molecules.
Experimental Section
General Information
The difluoro enol silyl ethers 2 were freshly prepared (and used without purification) from the corresponding trifluoroketones according to the literature procedures reported by the Olah/Prakash14b and the Uneyama14a,32 groups. Trifluoromethylthiolating reagent 3 was synthesized according to a procedure by Shen and co-workers.23 Trifluoromethylating reagent 6a (trifluoromethylbenziodoxolone) and 6b (trifluoromethylbenziodoxole) were synthesized according to the reported literature procedures.27,33 Anhydrous acetonitrile was purchased from Sigma-Aldrich and stored in an argon-filled glovebox. KF was dried by heating with a heat gun (550 °C) for about 2 min under vacuum and kept under Ar before use in the glovebox. All other chemicals, including diaryliodonium salts 8a and 8b, were obtained from commercial sources and used as received. 1H, 13C, and 19F NMR spectra were recorded in CDCl3 (internal standard: 7.26 ppm, 1H; 77.0 ppm, 13C) using 400 or 500 MHz spectrometers. High-resolution mass data (HRMS) were recorded on a Bruker microTOF ESI-TOF mass spectrometer. For column chromatography, silica gel (35–70 μm) was used. Unless otherwise stated, the reactions were conducted under Ar atmosphere.
(E)-1,1,1-Trifluoro-3-methyl-4-(naphthalen-2-yl)but-3-en-2-one (1g)
The trifluoromethylenone was synthesized according to a literature procedure34 using 2-naphthaldehyde. The product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate = 115/1) to afford ketone 1g as a pale yellow solid (1.12 g, 85% yield); mp 78.6–79.8 °C. 1H NMR (CDCl3, 400 MHz) δ 7.98 (s, 1H), 7.95–7.84 (m, 4H), 7.61–7.51 (m, 3H), 2.27 (s, 3H); 19F NMR (CDCl3, 377 MHz) δ −68.80; 13C{1H} NMR (CDCl3, 100 MHz) δ 182.3 (q, JCF = 33.1 Hz), 145.9 (q, JCF = 3.5 Hz), 133.6, 132.9, 132.1, 131.01, 130.95, 128.6, 128.4, 127.74, 127.66, 126.9, 126.8, 116.9 (q, JCF = 291.7 Hz), 13.5; HRMS (ESI) m/z [M + H]+ calcd for C15H12F3O 265.0835, found 265.0837.
(E)-1,1,1-Trifluoro-3-methyl-4-(thiophene-2-yl)but-3-en-2-one (1h)
The trifluoromethylenone was synthesized according to a literature procedure34 using thiophene-2-carbaldehyde. The product was purified by silica gel column chromatography (eluent: petroleum ether/ethyl acetate = 100/1) to afford the desired ketone 1h as a yellow oil (1.00 g, 91% yield): 1H NMR (CDCl3, 400 MHz) δ 7.90 (s, 1H), 7.71 (d, J = 5.1 Hz, 1H), 7.48 (d, J = 3.7 Hz, 1H), 7.22 (dd, J = 5.1, 3.7 Hz, 1H), 2.25 (s, 3H); 19F NMR (CDCl3, 377 MHz) δ −68.64; 13C{1H} NMR (CDCl3, 100 MHz) δ 181.4 (q, JCF = 33.1 Hz), 138.4, 138.2 (q, JCF = 3.7 Hz), 135.2, 132.8, 128.0, 126.8, 117.0 (q, JCF = 291.8 Hz), 13.3; HRMS (ESI) m/z [M + H]+ calcd for C9H8F3OS 221.0242, found 221.0243.
((1-([1,1′-Biphenyl]-4-yl)-2,3,3,3-tetrafluoroprop-1-en-1-yl)oxy)trimethylsilane (2j)
The difluoro enol silyl ether 2j was synthesized according to the literature procedure.32 A round-bottom flask containing a stir bar was charged with magnesium turnings (0.74 mmol, 2.1 equiv) activated by heating under vacuum. The flask was cooled to −10 °C, and dry THF (1.4 mL) was added, followed by TMSCl (1.5 mmol, 4.2 equiv). To the mixture was added dropwise a solution of the ketone 7b (0.35 mmol, 1.0 equiv) in THF (0.4 mL). The reaction mixture was stirred at −10 °C for 3 h, and then the solvent and the excess of TMSCl were evaporated, and pentane was added to the residue. The mixture was filtered through a pad of Celite and washed with pentane. The solvent was removed under reduced pressure to obtain the yellow liquid 2j in 55% yield as a mixture of E/Z isomers (97:3 by 19F NMR): 1H NMR (CDCl3, 400 MHz) δ 7.68–7.59 (m, 6H), 7.49–7.44 (m, 2H), 7.41–7.36 (m, 1H), 0.13 (s, 9H); 19F NMR (CDCl3, 377 MHz) δ (E isomer, major) −65.54 (d, JFF = 10.4 Hz), −165.81 (q, JFF = 10.5 Hz); (Z isomer, minor) −63.80 (d, JFF = 13.2 Hz), −153.45 (q, JFF = 13.2 Hz); 13C{1H} NMR (CDCl3, 100 MHz) δ (E isomer) 142.9 (dq, JCF = 30.5, 2.6 Hz), 142.7, 140.1, 138.0 (dq, JCF = 241.6, 36.8 Hz), 131.7 (d, JCF = 5.1 Hz), 128.9, 128.5 (d, JCF = 5.7 Hz), 127.9, 127.1, 126.9, 120.4 (dd, JCF = 271.4, 37.4 Hz), 0.2.
General Procedure A: Trifluoromethylthiolation Reaction of Difluoro Enol Silyl Ethers
To a mixture of the difluoro enol silyl ether 2 (0.1 mmol), N-trifluoromethylthiodibenzenesulfonimide 3 (39.7 mg, 0.1 mmol, 1.0 equiv), and KF (5.8 mg, 0.1 mmol, 1.0 equiv) was added 0.5 mL of dry MeCN in a glovebox. Unless otherwise stated, the reaction mixtures were stirred at room temperature for 3 h. After evaporation, the product was isolated by silica gel column chromatography.
2,2-Difluoro-1-phenyl-2-((trifluoromethyl)thio)ethan-1-one (4a)
The title compound 4a was prepared according to the general procedure A. The NMR yield (78%) was determined by 19F NMR (with 1H decoupling, using 3s of delay time in 32 scans) with PhCF3 as the internal standard. The product was purified by silica gel column chromatography (eluent: pentane) to afford 4a as a volatile pale yellow oil (6.3 mg, 25% yield): 1H NMR (CDCl3, 400 MHz) δ 8.17–8.11 (m, 2H), 7.78–7.72 (m, 1H), 7.62–7.55 (m, 2H); 19F NMR (CDCl3, 377 MHz) δ −36.12 (t, JFF = 8.9 Hz), −70.49 (q, JFF = 8.9 Hz); 13C{1H} NMR (CDCl3, 100 MHz) δ 184.1 (t, JCF = 27.9 Hz), 135.8, 130.5 (t, JCF = 2.9 Hz), 129.3 (t, JCF = 2.9 Hz), 129.1, 128.3 (q, JCF = 309.0 Hz), 126.6 (t, JCF = 299.5 Hz); HRMS (ESI) m/z [M + H]+ calcd for C9H6F5OS 257.0054, found 257.0058.
1-([1,1′-Biphenyl]-4-yl)-2,2-difluoro-2-((trifluoromethyl) thio)ethan-1-one (4b)
The title compound 4b was prepared according to the general procedure A above and purified by silica gel column chromatography (eluent: pentane) to afford 4b as a white solid (26.0 mg, 78% yield): mp 49.6–50.6 °C; 1H NMR (CDCl3, 400 MHz) δ 8.20 (d, J = 8.7 Hz, 2H), 7.80–7.74 (m, 2H), 7.68–7.63 (m, 2H), 7.54–7.48 (m, 2H), 7.48–7.42 (m, 1H); 19F NMR (CDCl3, 377 MHz) δ −36.10 (t, JFF = 8.9 Hz), −70.28 (q, JFF = 8.9 Hz); 13C{1H} NMR (CDCl3, 100 MHz) δ 183.7 (t, JCF = 27.2 Hz), 148.5, 139.1, 131.1 (t, JCF = 2.8 Hz), 129.1, 129.0, 128.3 (q, JCF = 309.0 Hz), 127.8 (t, JCF = 2.9 Hz), 127.7, 127.4, 126.8 (t, JCF = 298.5 Hz); HRMS (ESI) m/z [M + H]+ calcd for C15H10F5OS 333.0367, found 333.0369.
Synthesis of 4b on 1.0 mmol Scale
To a mixture of the difluoro enol silyl ether 2b (0.30 g, 1.0 mmol), N-trifluoromethylthiodibenzenesulfonimide 3 (0.40 g, 0.1 mmol, 1.0 equiv), and KF (58 mg, 1.0 mmol, 1.0 equiv) was added 5.0 mL of dry MeCN in a glovebox. The reaction mixture was stirred at room temperature for 3 h. After evaporation, the product was isolated by silica gel column chromatography (eluent: pentane) to afford 4b as a white solid (0.24 g, 71% yield).
2,2-Difluoro-1-(naphthalen-2-yl)-2-((trifluoromethyl) thio)ethan-1-one (4c)
The title compound 4c was prepared according to the general procedure A above with a reaction time of 4 h and purified by silica gel column chromatography (eluent: pentane) to afford 4c as a white solid (25.6 mg, 84% yield): mp 63.1–64.1 °C; 1H NMR (CDCl3, 400 MHz) δ 8.71 (s, 1 H), 8.07 (d, J = 8.7 Hz, 1H), 8.02 (d, J = 8.1 Hz, 1H), 7.96 (d, J = 8.8 Hz, 1H), 7.91 (d, J = 8.2 Hz, 1H), 7.74–7.67 (m, 1 H), 7.66–7.59 (m, 1 H); 19F NMR (CDCl3, 377 MHz) δ −36.11 (t, JFF = 8.9 Hz), −69.48 (q, JFF = 8.9 Hz); 13C{1H} NMR (CDCl3, 100 MHz) δ 184.1 (t, JCF = 27.7 Hz), 136.6, 133.7 (t, JCF = 4.1 Hz), 132.2, 130.31, 130.25, 129.2, 128.4 (q, JCF = 308.7 Hz), 127.9, 127.5, 127.0 (t, JCF = 298.5 Hz), 126.4 (t, JCF = 2.8 Hz), 124.4; HRMS (ESI) m/z [M + H]+ calcd for C13H8F5OS 307.0211, found 307.0208.
2,2-Difluoro-1-(4-methoxyphenyl)-2-((trifluoromethyl)thio)ethan-1-one (4d)
The title compound 4d was prepared according to the general procedure A above with a reaction time of 18 h. Compound 4d was purified by silica gel column chromatography (eluent: pentane/EA = 150/1) to afford 4d as a yellow oil (19.2 mg, 67% yield): 1H NMR (CDCl3, 400 MHz) δ 8.12 (d, J = 9.1 Hz, 2H), 7.06–7.00 (m, 2H), 3.94 (s, 3H); 19F NMR (CDCl3, 377 MHz) δ −36.32 (t, JFF = 8.9 Hz), −69.54 (q, JFF = 8.9 Hz); 13C{1H} NMR (CDCl3, 100 MHz) δ 182.5 (t, JCF = 27.2 Hz), 165.7, 133.2 (t, JCF = 2.9 Hz), 128.4 (q, JCF = 308.7 Hz), 127.2 (t, JCF = 299.0 Hz), 121.9 (t, JCF = 3.0 Hz), 114.5, 55.7; HRMS (ESI) m/z [M + H]+ calcd for C10H8F5O2S 287.0160, found 287.0161.
2,2-Difluoro-1-(4-(ethylthio)phenyl)-2-((trifluoromethyl) thio)ethan-1-one (4e)
The title compound 4e was prepared according to the general procedure A above and purified by silica gel column chromatography (eluent: pentane) to afford 4e as a pale yellow solid (19.7 mg, 62% yield): mp 54.3–55.4 °C; 1H NMR (CDCl3, 400 MHz) δ 7.99 (d, J = 8.5 Hz, 2H), 7.33 (d, J = 8.7 Hz, 2H), 3.06 (q, J = 7.4 Hz, 2H), 1.41 (t, J = 7.4 Hz, 3H); 19F NMR (CDCl3, 377 MHz) δ −36.21 (t, JFF = 8.9 Hz), −70.02 (q, JFF = 8.9 Hz); 13C{1H} NMR (CDCl3, 100 MHz) δ 183.0 (t, JCF = 27.5 Hz), 149.7, 130.7 (t, JCF = 2.9 Hz), 128.4 (q, JCF = 308.7 Hz), 127.0 (t, JCF = 300.0 Hz), 125.8, 125.1 (t, JCF = 2.9 Hz), 25.6, 13.7; HRMS (ESI) m/z [M + H]+ calcd for C11H10F5OS2 317.0088, found 317.0096.
2,2-Difluoro-1-(4-fluorophenyl)-2-((trifluoromethyl)thio)ethan-1-one (4f)
The title compound 4f was prepared according to the general procedure A. The NMR yield (57%) was determined by 19F NMR (with 1H decoupling, using 3s of delay time in 32 scans) with PhCF3 as the internal standard. The product was purified by silica gel column chromatography (eluent: pentane) to afford 4f as a volatile colorless oil (10.2 mg, 35% yield): 1H NMR (CDCl3, 400 MHz) δ 8.21–8.13 (m, 2H), 7.28–7.19 (m, 2H); 19F NMR (CDCl3, 377 MHz) δ −36.08 (t, JFF = 8.9 Hz), −70.35 (q, JFF = 8.9 Hz), 98.86 to –98.94 (m); 13C{1H} NMR (CDCl3, 100 MHz) δ 182.7 (t, JCF = 27.9 Hz), 167.3 (d, JCF = 260.6 Hz), 133.5 (dt, JCF = 10.1 Hz, JCF = 3.1 Hz), 128.2 (q, JCF = 308.3 Hz), 126.6 (t, JCF = 300.0 Hz), 125.7–125.6 (m), 116.7 (d, JCF = 22.2 Hz); HRMS (ESI) m/z [M + H]+ calcd for C9H5F6OS 274.9960, found 274.9963.
(E)-1,1-Difluoro-3-methyl-4-(naphthalen-2-yl)-1-((trifluoromethyl)thio)but-3-en-2-one (4g)
The title compound 4g was prepared according to the general procedure A above and purified by silica gel column chromatography (eluent: pentane) to afford 4g as a yellow solid (24.2 mg, 70% yield): mp 34.2–35.2 °C; 1H NMR (CDCl3, 400 MHz) δ 8.05 (s, 1H), 7.99 (s, 1H), 7.93–7.85 (m, 3H), 7.61–7.53 (m, 3H), 2.27 (s, 3H); 19F NMR (CDCl3, 377 MHz) δ −36.60 (t, JFF = 9.0 Hz), −67.13 (q, JFF = 9.0 Hz); 13C{1H} NMR (CDCl3, 100 MHz) δ 185.9 (t, JCF = 25.9 Hz), 147.1 (t, JCF = 5.6 Hz), 133.7, 132.9, 132.0, 131.3, 130.1 (t, JCF = 2.6 Hz), 128.7, 128.4 (q, JCF = 309.0 Hz), 128.4, 127.81, 127.75, 127.5 (t, JCF = 299.0 Hz), 126.9, 126.8, 13.7; HRMS (ESI) m/z [M + H]+ Calcd for C16H12F5OS 347.0524, found 347.0531.
(E)-1,1-Difluoro-3-methyl-4-(thiophen-2-yl)-1-((trifluoro- methyl)thio)but-3-en-2-one (4h)
The title compound 4h was prepared according to the general procedure A above and purified by silica gel column chromatography (eluent: pentane) to afford 4h as a yellow oil (18.0 mg, 60% yield): 1H NMR (CDCl3, 400 MHz) δ 8.07 (s, 1H), 7.74 (d, J = 5.1 Hz, 1H), 7.50 (d, J = 3.7 Hz, 1H), 7.23 (dd, J1 = 5.1 Hz, J2 = 3.8 Hz, 1H), 2.24 (s, 3H); 19F NMR (CDCl3, 377 MHz) δ −36.73 (t, JFF = 9.0 Hz), −66.70 (q, JFF = 9.0 Hz); 13C{1H} NMR (CDCl3, 100 MHz) δ 185.0 (t, JCF = 26.0 Hz), 139.3 (t, JCF = 6.0 Hz), 138.4, 135.7, 133.4, 128.5 (q, JCF = 309.0 Hz), 128.1, 127.9 (t, JCF = 300.5 Hz), 125.7 (t, JCF = 2.7 Hz), 13.5; HRMS (ESI) m/z [M + H]+ calcd for C10H8F5OS2 302.9931, found 302.9928.
N-(1,1-Difluoro-4-methyl-2-oxo-1-((trifluoromethyl)thio)pentan-3-yl)benzamide (4i)
The title compound 4i was prepared according to the general procedure A above with a reaction time of 18 h. Compound 4i was purified by silica gel column chromatography (eluent: pentane/ethyl acetate = 20/1 for the first round, pentane/Et2O = 20/1 for the second round) to afford 4i as a white solid (12.3 mg, 35% yield): mp 74.1–75.1 °C; 1H NMR (CDCl3, 400 MHz) δ 7.82–7.76 (m, 2H), 7.58–7.53 (m, 1H), 7.50–7.44 (m, 2H), 6.49 (d, J = 7.7 Hz, 1H), 5.36–5.29 (m, 1H), 2.50–2.40 (m, 1H), 1.13 (d, J = 6.8 Hz, 3H), 0.97 (d, J = 6.9 Hz, 3H); 19F NMR (CDCl3, 377 MHz) δ −35.50 (t, JFF = 8.9 Hz), −79.01 (ddq, J = 806.8, 231.6, 8.9 Hz); 13C{1H} NMR (CDCl3, 100 MHz) δ 194.4 (dd, JCF = 30.0, 26.1 Hz), 167.5, 133.2, 132.2, 128.8, 127.1, 127.9 (q, JCF = 309.3 Hz), 123.1 (t, JCF = 297.5 Hz), 58.6, 30.0, 20.0, 16.8; HRMS (ESI) m/z [M + Na]+ calcd for C14H14F5NO2SNa 378.0558, found 378.0561.
1-([1,1′-Biphenyl]-4-yl)-2,3,3,3-tetrafluoro-2-((trifluoromethyl)thio)propan-1-one (4j)
The title compound 4j was prepared according to the general procedure A above with a reaction time of 3 h. Compound 4j was purified by silica gel column chromatography (eluent: pentane) to afford 4j as a colorless oil (32.8 mg, 86% yield): 1H NMR (CDCl3, 400 MHz) δ 8.14 (dd, J = 8.6, 1.9 Hz, 2H), 7.78–7.72 (m, 2H), 7.67–7.62 (m, 2H), 7.53–7.42 (m, 3H); 19F NMR (CDCl3, 377 MHz) δ −35.16 (dq, JFF = 9.6, 4.8 Hz), −73.88 (dq, JFF = 9.7, 4.8 Hz), −146.80 to −147.02 (m); 13C{1H} NMR (CDCl3, 100 MHz) δ 187.4 (d, JCF = 24.2 Hz), 147.9, 139.1, 131.2 (d, JCF = 4.0 Hz), 131.0 (d, JCF = 7.8 Hz), 129.1, 128.9, 127.7 (q, JCF = 311.6 Hz), 127.4 (d, JCF = 1.2 Hz), 127.3, 120.5 (qd, JCF = 287.2, 29.9 Hz), 102.6 (dq, JCF = 260.0, 34.0 Hz). Despite repeated attempts, we were not able to obtain high-resolution mass data for 4j. The probable reason is the difficult ionization of the molecule bearing a −COCF(CF3)(SCF3) group.
General Procedure B: Trifluoromethylation of Difluoro Enol Silyl Ethers
To a mixture of trifluoromethylbenziodoxolone 6a (31.6 mg, 0.1 mmol, 1.0 equiv) and FeCl2 (1.3 mg, 0.01 mmol, 10 mol %) was added 0.5 mL of dry MeCN and a difluoro enol silyl ether 2 (0.1 mmol), in a glovebox. The reaction mixture was stirred at room temperature for 2 h. After evaporation, the product was isolated by silica gel column chromatography.
1-Phenyl-2,2,3,3,3-pentafluoropropan-1-one (7a)
The title compound 7a was prepared according to the general procedure B above. The NMR yield (62%) was determined by 19F NMR (with 1H decoupling, using 3s of delay time in 32 scans) with PhCF3 as the internal standard. Compound 7a was purified by silica gel column chromatography (eluent: pentane) to afford the volatile 7a with pentane: 1H NMR (CDCl3, 400 MHz) δ 8.09 (d, J = 8.6 Hz, 2H), 7.72 (t, J = 7.4 Hz, 1H), 7.55 (t, J = 8.0 Hz, 2H); 19F NMR (CDCl3, 377 MHz) δ −81.56, −115.53. The spectroscopic data is in agreement with the literature values.35
1-([1,1′-Biphenyl]-4-yl)-2,2,3,3,3-pentafluoropropan-1-one (7b)
The title compound 7b was prepared according to the general procedure B above and purified by silica gel column chromatography (eluent: pentane) to afford 7b as a colorless solid (27.0 mg, 85% yield): mp 42.6–42.6 °C; 1H NMR (CDCl3, 400 MHz) δ 8.17 (d, J = 8.5 Hz, 2H), 7.77 (d, J = 8.6 Hz, 2H), 7.65 (d, J = 7.1 Hz, 2H), 7.50 (t, J = 7.3 Hz, 2H), 7.45 (t, J = 7.2 Hz, 1H); 19F NMR (CDCl3, 377 MHz) δ −81.51, −115.48; 13C{1H} NMR (CDCl3, 126 MHz) δ 182.7 (t, JCF = 26.6 Hz), 148.2, 139.1, 130.8 (t, JCF = 3.3 Hz), 129.6, 129.1, 128.9, 127.6, 127.4, 118.0 (m), 108.8 (m). The spectroscopic data is in agreement with the literature values.36
1-(Naphthalen-2-yl)-2,2,3,3,3-pentafluoropropan-1-one (7c)
The title compound 7c was prepared according to the general procedure B above and purified by silica gel column chromatography (eluent: pentane) to afford 7c as a colorless oil (9.0 mg, 33% yield): 1H NMR (CDCl3, 400 MHz) δ 8.67 (s, 1H), 8.11–8.04 (m, 1H), 8.06–7.99 (m, 1H), 7.96 (d, J = 8.8 Hz, 1H), 7.91 (d, J = 7.7 Hz, 1H), 7.70 (ddd, J = 8.2, 6.9, 1.3 Hz, 1H), 7.62 (ddd, J = 8.1, 6.9, 1.2 Hz, 1H); 19F NMR (CDCl3, 377 MHz) δ −81.47, −114.82. 13C{1H} NMR (CDCl3, 126 MHz) δ 183.1 (t, JCF = 26.6 Hz), 136.4, 133.2 (t, JCF = 4.5 Hz), 132.2, 130.3, 130.1, 129.1, 128.3, 127.9, 127.4, 124.2, 118.9 (qt, JCF = 286.7, 33.8 Hz), 108.9 (tq, JCF = 269.5, 37.1 Hz). The spectroscopic data is in agreement with the literature values.37
1-(4-(Ethylthio)phenyl)-2,2,3,3,3-pentafluoropropan-1-one (7e)
The title compound 7e was prepared according to the general procedure B above and purified by silica gel column chromatography (eluent: petroleum ether to afford 7e as a yellow oil (19.9 mg, 70% yield). 1H NMR (CDCl3, 400 MHz) δ 7.97 (d, J = 8.7 Hz, 2H), 7.36–7.29 (m, 2H), 3.06 (q, J = 7.4 Hz, 2H), 1.41 (t, J = 7.4 Hz, 3H); 19F NMR (CDCl3, 377 MHz) δ −81.62, −115.44; 13C{1H} NMR (CDCl3, 100 MHz) δ 181.9 (t, JCF = 26.4 Hz), 149.2, 130.4 (t, JCF = 3.3 Hz), 127.1, 125.7, 118.0 (qt, JCF = 286.4 Hz, JCF = 33.8 Hz), 108.8 (tq, JCF = 268.0, 37.1 Hz), 25.6, 13.7; HRMS (ESI) m/z [M + H]+ Calcd for C11H10F5OS 285.0367, found 285.0366.
1-(4-Fluorophenyl)-2,2,3,3,3-pentafluoropropan-1-one (7f)
The title compound 7f was prepared according to the general procedure B above. The NMR yield (65%) was determined by 19F NMR (with 1H decoupling, using 3s of delay time in 32 scans) with PhCF3 as the internal standard. The perfluoroethylketone was further purified by silica gel column chromatography (eluent: pentane) to afford the volatile 7f with traces of pentane: 1H NMR (CDCl3, 400 MHz) δ 8.18–8.09 (m, 2H), 7.27–7.19 (m, 2H); 19F NMR (CDCl3, 377 MHz) δ −81.71, −99.22 to −100.72 (m), −115.58. 13C{1H} NMR (CDCl3, 100 MHz) δ 181.6 (t, JCF = 27.0 Hz), 167.1 (d, JCF = 260.1 Hz), 133.2 (dt, JCF = 10.0, 3.4 Hz), 127.4 (d, JCF = 3.0 Hz), 118.0 (qt, JCF = 286.7, 33.7 Hz), 116.54 (d, JCF = 22.2 Hz), 108.7 (tq, JCF = 268.7, 37.3 Hz). The spectroscopic data is in agreement with the literature values.35
(E)-2-Methyl-1-(thiophene-2-yl)-4,4,5,5,5-pentafluoro-pent-1-en-3-one (7h)
The title compound 7h was prepared according to the general procedure B above and purified by silica gel column chromatography (eluent: first run pentane; second run: pentane/Et2O/triethylamine = 60/1/2) to afford 7h as a yellow oil (5.5 mg, 20% yield): 1H NMR (CDCl3, 400 MHz) δ 7.99 (s, 1H), 7.71 (d, J = 5.0 Hz, 1H), 7.48 (d, J = 3.6 Hz, 1H), 7.26–7.19 (m, 1H), 2.25 (s, 3H); 19F NMR (CDCl3, 377 MHz) δ −81.65, −112.52; 13C{1H} NMR (CDCl3, 126 MHz) δ 183.6 (t, JCF = 24.9 Hz), 138.4, 138.3 (t, JCF = 6.5 Hz), 135.3, 133.0, 128.0, 127.9, 118.2 (m), 109.1 (m), 13.4. HRMS (ESI) m/z [M + H]+ calcd for C10H8F5OS 271.0211, found 271.0213.
N-(5,5,6,6,6-Pentafluoro-2-methyl-4-oxohexan-3-yl)benzamide (7i)
The title compound 7i was prepared according to the general procedure B above and purified by silica gel column chromatography (eluent: pentane/ethyl acetate = 20/1) to afford 7i as a yellow solid (10.5 mg, 33% yield): mp 90.9–92.5 °C; 1H NMR (CDCl3, 400 MHz) δ 7.83–7.76 (m, 2H), 7.61–7.51 (m, 1H), 7.47 (t, J = 7.5 Hz, 2H), 6.49 (d, J = 6.4 Hz, 1H), 5.38 (ddd, J = 8.7, 4.2, 1.4 Hz, 1H), 2.45 (dq, J = 11.1, 6.8 Hz, 1H), 1.14 (d, J = 6.8 Hz, 3H), 0.94 (d, J = 6.9 Hz, 3H); 19F NMR (CDCl3, 377 MHz) δ −81.52, −121.43 (dd, JFF = 638.6, 296.0 Hz); 13C{1H} NMR (CDCl3, 100 MHz) δ 194.1 (dd, JCF = 28.7, 25.3 Hz), 167.3, 133.3, 132.2, 128.8, 127.1, 117.7 (m), 108.2 (m), 59.3, 29.7, 20.0, 16.4; HRMS (ESI) m/z [M + Na]+ calcd for C14H14F5NO2Na 346.0837, found 346.0843. The spectroscopic data is in agreement with the literature values.38
General Procedure C: Arylation Reaction of Difluoro Enol Silyl Ethers
To a mixture of the difluoro enol silyl ether 2 (0.1 mmol), diaryliodonium salt 8 (0.1 mmol, 1.0 equiv), and KF (5.8 mg, 0.1 mmol, 1.0 equiv) was added 0.5 mL of dry MeCN in a glovebox. The reaction was stirred at room temperature for 18 h. After evaporation, the product was isolated by silica gel column chromatography.
2,2-Difluoro-1,2-diphenylethan-1-one (9a)
The title compound 9a was prepared according to the general procedure C above and purified by silica gel column chromatography (eluent: pentane/Et2O = 200/1) to afford 9a as a pale yellow oil (14.2 mg, 61% yield). 1H NMR (CDCl3, 400 MHz) δ 8.06–8.00 (m, 2H), 7.65–7.55 (m, 3H), 7.52–7.42 (m, 5H); 19F NMR (CDCl3, 377 MHz) δ −97.53; 13C{1H} NMR (CDCl3, 100 MHz) δ 189.0 (t, JCF = 31.0 Hz), 134.2, 133.1 (t, JCF = 25.0 Hz), 132.2, 130.9 (t, JCF = 1.9 Hz), 130.3 (t, JCF = 2.9 Hz), 128.8, 128.6, 125.6 (t, JCF = 6.0 Hz), 116.9 (t, JCF = 253.2 Hz); HRMS (ESI) m/z [M + Na]+ calcd for C14H10F2ONa 255.0592, found 255.0593. The spectroscopic data is in agreement with the literature values.39
2,2-Difluoro-2-(4-bromophenyl)-1-phenylethan-1-one (9b)
The title compound 9b was prepared according to the general procedure C above and purified by silica gel column chromatography (eluent: pentane/EA = 100/1) to afford 9b as a yellow oil (22.4 mg, 72% yield): 1H NMR (CDCl3, 400 MHz) δ 8.06–8.00 (m, 2H), 7.64–7.57 (m, 3H), 7.51–7.43 (m, 4H); 19F NMR (CDCl3, 377 MHz) δ −97.56; 13C{1H} NMR (CDCl3, 100 MHz) δ 188.5 (t, JCF = 31.3 Hz), 134.4, 132.1 (t, JCF = 25.5 Hz), 132.1, 131.9 (t, JCF = 1.5 Hz), 130.2 (t, JCF = 3.0 Hz), 128.7, 127.4 (t, JCF = 6.0 Hz), 125.6 (t, JCF = 2.2 Hz), 116.7 (t, JCF = 254.2 Hz); HRMS (ESI) m/z [M + Na]+ calcd for C14H979BrF2ONa 332.9697, found 332.9693. The spectroscopic data is in agreement with the literature values.40
2,2-Difluoro-1-(naphthalen-2-yl)-2-phenylethan-1-one (9c)
The title compound 9c was prepared according to the general procedure C above and purified by silica gel column chromatography (eluent: pentane/Et2O/Et3N = 40/1/2 for two rounds) to afford 9c as a pale yellow solid (15.7 mg, 56% yield): mp 71.6–72.7 °C; 1H NMR (CDCl3, 400 MHz) δ 8.62 (s, 1H), 8.05 (dd, J = 8.8, 1.5 Hz, 1H), 7.94 (d, J = 8.1 Hz, 1H), 7.87 (t, J = 8.0 Hz, 2H), 7.70–7.66 (m, 2H), 7.65–7.60 (m, 1H), 7.58–7.52 (m, 1H), 7.51–7.45 (m, 3H); 19F NMR (CDCl3, 377 MHz) δ −96.92; 13C{1H} NMR (CDCl3, 100 MHz) δ 188.9 (t, JCF = 30.9 Hz), 135.9, 133.3 (t, JCF = 25.0 Hz), 133.0 (t, JCF = 3.9 Hz), 132.2, 130.9 (t, JCF = 1.7 Hz), 130.0, 129.4, 129.3, 128.8, 128.5, 127.7, 127.0, 125.7 (t, JCF = 6.0 Hz), 125.0 (t, JCF = 2.1 Hz), 117.0 (t, JCF = 253.3 Hz); HRMS (ESI) m/z [M + Na]+ calcd for C18H12F2ONa 305.0748, found 305.0747. The spectroscopic data are in agreement with the literature values.40
2-(4-Bromophenyl)-2,2-difluoro-1-(naphthalen-2-yl)ethan-1-one (9d)
The title compound 9d was prepared according to the general procedure C above and purified by silica gel column chromatography (eluent: pentane/EA = 200/1) to afford 9d as a white solid (25.6 mg, 71% yield): mp 80.7–81.7 °C; 1H NMR (CDCl3, 400 MHz) δ 8.62 (s, 1H), 8.04 (d, J = 8.8 Hz, 1H), 7.95 (d, J = 8.1 Hz, 1H), 7.88 (t, J = 8.5 Hz, 2H), 7.67–7.59 (m, 3H), 7.59–7.50 (m, 3H); 19F NMR (CDCl3, 377 MHz) δ −96.91; 13C{1H} NMR (CDCl3, 100 MHz) δ 188.4 (t, JCF = 31.2 Hz), 135.9, 133.0 (t, JCF = 4.1 Hz), 132.3 (t, JCF = 25.48 Hz), 132.2, 132.1, 130.0, 129.5, 129.1 (t, JCF = 1.6 Hz), 128.6, 127.8, 127.4 (t, JCF = 6.0 Hz), 127.1, 125.6 (t, JCF = 2.2 Hz), 124.8 (t, JCF = 2.1 Hz), 116.9 (t, JCF = 254.2 Hz); HRMS (ESI) m/z [M + Na]+ calcd for C18H1179BrF2ONa 382.9854, found 382.9853.
2,2-Difluoro-1-(4-fluorophenyl)-2-phenylethan-1-one (9e)
The title compound 9e was prepared according to the general procedure C above and purified by silica gel column chromatography (eluent: pentane/Et2O = 200/1) to afford 9e as a yellow oil (12.5 mg, 50% yield): 1H NMR (CDCl3, 400 MHz) δ 8.14–8.03 (m, 2H), 7.65–7.55 (m, 2H), 7.53–7.42 (m, 3H), 7.12 (t, J = 8.6 Hz, 2H); 19F NMR (CDCl3, 377 MHz) δ −97.46, −102.19 to −102.30 (m); 13C{1H} NMR (CDCl3, 100 MHz) δ 187.4 (t, JCF = 31.4 Hz), 166.3 (d, JCF = 257.7 Hz), 133.2 (dt, JCF = 9.6, 3.2 Hz), 132.9 (t, JCF = 25.2 Hz), 131.0 (t, JCF = 1.8 Hz), 128.9, 125.6 (t, JCF = 6.0 Hz), 116.0 (d, JCF = 21.8 Hz), 128.5, 116.9 (d, JCF = 253.2 Hz); HRMS (ESI) m/z [M + Na]+ calcd for C14H9F3ONa 273.0498, found 273.0506.
2-(4-Bromophenyl)-2,2-difluoro-1-(4-fluorophenyl)ethan- 1-one (9f)
The title compound 9f was prepared according to the general procedure C above and purified by silica gel column chromatography (eluent: pentane/Et2O = 200/1) to afford 9f as a colorless oil (18.2 mg, 55% yield): 1H NMR (CDCl3, 400 MHz) δ 8.14–8.03 (m, 2H), 7.61 (d, J = 8.8 Hz, 2H), 7.46 (d, J = 8.8 Hz, 2H), 7.19–7.10 (m, 2H); 19F NMR (CDCl3, 377 MHz) δ −97.45, −101.78 to −101.69 (m); 13C{1H} NMR (CDCl3, 100 MHz) δ 187.0 (t, JCF = 31.69 Hz), 166.4 (d, JCF = 258.2 Hz), 133.2 (dt, JCF = 9.7, 3.2 Hz), 132.1, 131.9 (t, JCF = 25.3 Hz), 128.2, 127.4 (t, JCF = 6.0 Hz), 125.7 (t, JCF = 2.2 Hz), 116.7 (t, JCF = 253.9 Hz), 116.1 (d, JCF = 22.0 Hz); HRMS (ESI) m/z [M + Na]+ calcd for C14H879BrF3ONa 350.9603, found 350.9607.
2-(4-Bromophenyl)-1-(4-(ethylthio)phenyl)-2,2-difluoroethan-1-one (9g)
The title compound 9g was prepared according to the general procedure C above and purified by silica gel column chromatography (eluent: pentane/Et2O/Et3N = 60/1/2 for the first round, and pentane/Et2O = 200/1 for the second round) to afford 9g as a pale yellow solid (20.9 mg, 56% yield): mp 50.9–52.1 °C; 1H NMR (CDCl3, 400 MHz) δ 7.92 (d, J = 8.7 Hz, 2H), 7.60 (d, J = 8.7 Hz, 2H), 7.46 (d, J = 8.7 Hz, 2H), 7.28–7.24 (m, 2H), 3.02 (q, J = 7.4 Hz, 2H), 1.38 (t, J = 7.4 Hz, 3H); 19F NMR (CDCl3, 377 MHz) δ −97.36; 13C{1H} NMR (CDCl3, 100 MHz) δ 187.3 (t, JCF = 31.0 Hz), 147.4, 132.2 (t, JCF = 25.6 Hz), 132.0, 130.6 (t, JCF = 3.1 Hz), 128.0, 127.4 (t, JCF = 6.0 Hz), 125.7, 125.5 (t, JCF = 2.2 Hz), 116.7 (t, JCF = 254.0 Hz), 25.7, 13.8; HRMS (ESI) m/z [M + Na]+ Calcd for C16H1379BrF2OSNa 392.9731, found 392.9727.
(E)-1-(4-Bromophenyl)-1,1-difluoro-3-methyl-4-(thiophene-2-yl)but-3-en-2-one (9h)
The title compound 9h was prepared according to the general procedure C above and purified by silica gel column chromatography (eluent: pentane to pentane/Et2O = 300/1) to afford 9h as a yellow oil (24.5 mg, 69% yield): 1H NMR (CDCl3, 400 MHz) δ 7.91 (s, 1H), 7.65–7.57 (m, 3H), 7.47–7.42 (m, 2H), 7.34 (d, J = 3.7 Hz, 1H), 7.16 (dd, J1 = 5.1, J2 = 3.7 Hz, 1H), 2.21 (s, 3H); 19F NMR (CDCl3, 377 MHz) δ −94.74; 13C{1H} NMR (CDCl3, 100 MHz) δ 189.2 (t, JCF = 29.6 Hz), 138.7, 137.2 (t, JCF = 5.6 Hz), 134.2, 133.0 (t, JCF = 25.6 Hz), 132.0, 131.8, 128.2, 127.7, 127.1 (t, JCF = 6.0 Hz), 125.3 (t, JCF = 2.2 Hz), 116.9 (t, JCF = 254.4 Hz), 13.9; HRMS (ESI) m/z [M + Na]+ calcd for C15H1179BrF2OSNa 378.9574, found 378.9570.
Acknowledgments
We thank the Knut och Alice Wallenbergs Foundation (Dnr: 2018.0066) and the Swedish Research Council (VR) for financial support and Carl Tryggers Foundation (CTS 17:458) for a postdoctoral fellowship to X.J. The ERASMUS stipend for D.B. is gratefully acknowledged.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.joc.0c01030.
NMR spectra of all the products (PDF)
Author Contributions
‡ X.J. and D.M. contributed equally.
The authors declare no competing financial interest.
Supplementary Material
References
- a Müller K.; Faeh C.; Diederich F. Fluorine in Pharmaceuticals: Looking Beyond Intuition. Science 2007, 317, 1881. 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]; b O’Hagan D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308. 10.1039/B711844A. [DOI] [PubMed] [Google Scholar]; c Johnson B. M.; Shu Y.-Z.; Zhuo X.; Meanwell N. A. Metabolic and Pharmaceutical Aspects of Fluorinated Compounds. J. Med. Chem. 2020, 10.1021/acs.jmedchem.9b01877. [DOI] [PubMed] [Google Scholar]
- a Zhou Y.; Wang J.; Gu Z.; Wang S.; Zhu W.; Aceña J. L.; Soloshonok V. A.; Izawa K.; Liu H. Next Generation of Fluorine-Containing Pharmaceuticals, Compounds Currently in Phase II–III Clinical Trials of Major Pharmaceutical Companies: New Structural Trends and Therapeutic Areas. Chem. Rev. 2016, 116, 422. 10.1021/acs.chemrev.5b00392. [DOI] [PubMed] [Google Scholar]; b Mei H.; Han J.; Fustero S.; Medio-Simon M.; Sedgwick D. M.; Santi C.; Ruzziconi R.; Soloshonok V. A. Fluorine-Containing Drugs Approved by the FDA in 2018. Chem. - Eur. J. 2019, 25, 11797. 10.1002/chem.201901840. [DOI] [PubMed] [Google Scholar]; c Preshlock S.; Tredwell M.; Gouverneur V. 18F-Labeling of Arenes and Heteroarenes for Applications in Positron Emission Tomography. Chem. Rev. 2016, 116, 719. 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- a Leroux F.; Jeschke P.; Schlosser M. α-Fluorinated Ethers, Thioethers, and Amines: Anomerically Biased Species. Chem. Rev. 2005, 105, 827. 10.1021/cr040075b. [DOI] [PubMed] [Google Scholar]; b Jeschke P. The Unique Role of Fluorine in the Design of Active Ingredients for Modern Crop Protection. ChemBioChem 2004, 5, 570. 10.1002/cbic.200300833. [DOI] [PubMed] [Google Scholar]
- a Liang T.; Neumann C. N.; Ritter T. Introduction of Fluorine and Fluorine-Containing Functional Groups. Angew. Chem., Int. Ed. 2013, 52, 8214. 10.1002/anie.201206566. [DOI] [PubMed] [Google Scholar]; b Xu X.-H.; Matsuzaki K.; Shibata N. Synthetic Methods for Compounds Having CF3–S Units on Carbon by Trifluoromethylation, Trifluoromethylthiolation, Triflylation, and Related Reactions. Chem. Rev. 2015, 115, 731. 10.1021/cr500193b. [DOI] [PubMed] [Google Scholar]; c Charpentier J.; Früh N.; Togni A. Electrophilic Trifluoromethylation Using Hypervalent Iodine Reagents. Chem. Rev. 2015, 115, 650. 10.1021/cr500223h. [DOI] [PubMed] [Google Scholar]; d Zhu Y.; Han J.; Wang J.; Shibata N.; Sodeoka M.; Soloshonok V. A.; Coelho J. A. S.; Toste F. D. Modern Approaches for Asymmetric Construction of Carbon–Fluorine Quaternary Stereogenic Centers: Synthetic Challenges and Pharmaceutical Needs. Chem. Rev. 2018, 118, 3887. 10.1021/acs.chemrev.7b00778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Bégué J.-P.; Bonnet-Delpon D., Inhibition of Enzymes by Fluorinated Compounds. In Bioorganic and Medicinal Chemistry of Fluorine; Wiley, 2008; pp 223. [Google Scholar]; b Gelb M. H.; Svaren J. P.; Abeles R. H. Fluoro ketone inhibitors of hydrolytic enzymes. Biochemistry 1985, 24, 1813. 10.1021/bi00329a001. [DOI] [PubMed] [Google Scholar]
- Schirlin D.; Tarnus C.; Baltzer S.; Rémy J. M. MDL 74147, a novel selective and soluble inhibitor of human renin. Synthesis, structure-activity relationship, species and protease selectivities. Bioorg. Med. Chem. Lett. 1992, 2, 651. 10.1016/S0960-894X(00)80383-2. [DOI] [Google Scholar]
- Dreyer G. B.; Metcalf B. W. α,α-Difluoroketone inhibitors of HMG CoA reductase. Tetrahedron Lett. 1988, 29, 6885. 10.1016/S0040-4039(00)88466-X. [DOI] [Google Scholar]
- Nikolaou A.; Kokotou M. G.; Vasilakaki S.; Kokotos G. Small-molecule inhibitors as potential therapeutics and as tools to understand the role of phospholipases A2. Biochim. Biophys. Acta, Mol. Cell Biol. Lipids 2019, 1864, 941. 10.1016/j.bbalip.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Veale C. A.; Bernstein P. R.; Bohnert C. M.; Brown F. J.; Bryant C.; Damewood J. R.; Earley R.; Feeney S. W.; Edwards P. D.; Gomes B.; Hulsizer J. M.; Kosmider B. J.; Krell R. D.; Moore G.; Salcedo T. W.; Shaw A.; Silberstein D. S.; Steelman G. B.; Stein M.; Strimpler A.; Thomas R. M.; Vacek E. P.; Williams J. C.; Wolanin D. J.; Woolson S. Orally Active Trifluoromethyl Ketone Inhibitors of Human Leukocyte Elastase. J. Med. Chem. 1997, 40, 3173. 10.1021/jm970250z. [DOI] [PubMed] [Google Scholar]; b Edwards P. D.; Andisik D. W.; Bryant C. A.; Ewing B.; Gomes B.; Lewis J. J.; Rakiewicz D.; Steelman G.; Strimpler A.; Trainor D. A.; Tuthill P. A.; Mauger R. C.; Veale C. A.; Wildonger R. A.; Williams J. C.; Wolanin D. J.; Zottola M. Discovery and Biological Activity of Orally Active Peptidyl Trifluoromethyl Ketone Inhibitors of Human Neutrophil Elastase. J. Med. Chem. 1997, 40, 1876. 10.1021/jm960819g. [DOI] [PubMed] [Google Scholar]
- Cregge R. J.; Durham S. L.; Farr R. A.; Gallion S. L.; Hare C. M.; Hoffman R. V.; Janusz M. J.; Kim H.-O.; Koehl J. R.; Mehdi S.; Metz W. A.; Peet N. P.; Pelton J. T.; Schreuder H. A.; Sunder S.; Tardif C. Inhibition of Human Neutrophil Elastase. 4. Design, Synthesis, X-ray Crystallographic Analysis, and Structure–Activity Relationships for a Series of P2-Modified, Orally Active Peptidyl Pentafluoroethyl Ketones. J. Med. Chem. 1998, 41, 2461. 10.1021/jm970812e. [DOI] [PubMed] [Google Scholar]
- Angelastro M. R.; Baugh L. E.; Bey P.; Burkhart J. P.; Chen T.-M.; Durham S. L.; Hare C. M.; Huber E. W.; Janusz M. J. Inhibition of Human Neutrophil Elastase with Peptidyl Electrophilic Ketones. 2. Orally Active PG-Val-Pro-Val Pentafluoroethyl Ketones. J. Med. Chem. 1994, 37, 4538. 10.1021/jm00052a013. [DOI] [PubMed] [Google Scholar]
- Counts G. W.; Gregory D.; Zeleznik D.; Turck M. Cefazaflur, a New Parenteral Cephalosporin: In Vitro Studies. Antimicrob. Agents Chemother. 1977, 11, 708. 10.1128/AAC.11.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Hu X.-S.; Yu J.-S.; Zhou J. Catalytic selective mono- and difluoroalkylation using fluorinated silyl enol ethers. Chem. Commun. 2019, 55, 13638. 10.1039/C9CC07677H. [DOI] [PubMed] [Google Scholar]; b Decostanzi M.; Campagne J. M.; Leclerc E. Fluorinated enol ethers: their synthesis and reactivity. Org. Biomol. Chem. 2015, 13, 7351. 10.1039/C5OB00855G. [DOI] [PubMed] [Google Scholar]
- a Amii H.; Kobayashi T.; Hatamoto Y.; Uneyama K. Mg0-promoted selective C–F bond cleavage of trifluoromethyl ketones: a convenient method for the synthesis of 2,2-difluoro enol silanes. Chem. Commun. 1999, 1323. 10.1039/a903681d. [DOI] [Google Scholar]; b Surya Prakash G. K.; Hu J.; Olah G. A. Facile preparation of di- and monofluoromethyl ketones from trifluoromethyl ketones via fluorinated enol silyl ethers. J. Fluorine Chem. 2001, 112, 355. 10.1016/S0022-1139(01)00535-8. [DOI] [Google Scholar]
- a Lefebvre O.; Brigaud T.; Portella C. Mixed Organofluorine–Organosilicon Chemistry. 13. One-Pot Synthesis of Difluoroaldols from Acylsilanes and Trifluoromethyltrimethylsilane. Application to the Synthesis of a Difluoro Analogue of Egomaketone. J. Org. Chem. 2001, 66, 1941. 10.1021/jo001549j. [DOI] [PubMed] [Google Scholar]; b Yuan Z.-L.; Wei Y.; Shi M. Reaction of aldimines and difluoroenoxysilane, an unexpected protocol for the synthesis of 2,2-difluoro-3-hydroxy-1-ones. Tetrahedron 2010, 66, 7361. 10.1016/j.tet.2010.07.034. [DOI] [Google Scholar]; c Liu Y.-L.; Zhou J. Organocatalytic asymmetric synthesis of 3-difluoroalkyl 3-hydroxyoxindoles. Chem. Commun. 2012, 48, 1919. 10.1039/c2cc17140f. [DOI] [PubMed] [Google Scholar]; d Yu J.-S.; Liu Y.-L.; Tang J.; Wang X.; Zhou J. Highly Efficient “On Water” Catalyst-Free Nucleophilic Addition Reactions Using Difluoroenoxysilanes: Dramatic Fluorine Effects. Angew. Chem., Int. Ed. 2014, 53, 9512. 10.1002/anie.201404432. [DOI] [PubMed] [Google Scholar]
- a Jonet S.; Cherouvrier F.; Brigaud T.; Portella C. Mild Synthesis of β-Amino-α,α-difluoro Ketones from Acylsilanes and Trifluoromethyltrimethylsilane in a One-Pot Imino Aldol Reaction. Eur. J. Org. Chem. 2005, 2005, 4304. 10.1002/ejoc.200500106. [DOI] [Google Scholar]; b Yu J.-S.; Zhou J. A highly efficient Mukaiyama–Mannich reaction of N-Boc isatin ketimines and other active cyclic ketimines using difluoroenol silyl ethers catalyzed by Ph3PAuOTf. Org. Biomol. Chem. 2015, 13, 10968. 10.1039/C5OB01895A. [DOI] [PubMed] [Google Scholar]
- a Prakash G. K. S.; Hu J.; Alauddin M. M.; Conti P. S.; Olah G. A. A general method of halogenation for synthesis of α-halodifluoromethyl ketones and [18F]-labeled trifluoromethyl ketones. J. Fluorine Chem. 2003, 121, 239. 10.1016/S0022-1139(03)00039-3. [DOI] [Google Scholar]; b Qiu Z.-M.; Burton D. J. Synthesis of.alpha.,.alpha.-Difluoro-Functionalized Ketones. J. Org. Chem. 1995, 60, 5570. 10.1021/jo00122a044. [DOI] [Google Scholar]
- a Guo Y.; Shreeve J. n. M. Effect of fluorine on palladium-catalyzed cross-coupling reactions of aryl bromides with trifluoromethyl aryl ketones via difluoroenol silyl or monofluoroenol silyl ethers. Chem. Commun. 2007, 3583. 10.1039/b705137a. [DOI] [PubMed] [Google Scholar]; b Wu Y.-b.; Lu G.-p.; Zhou B.-j.; Bu M.-j.; Wan L.; Cai C. Visible-light-initiated difluoromethylation of arene diazonium tetrafluoroborates. Chem. Commun. 2016, 52, 5965. 10.1039/C6CC00177G. [DOI] [PubMed] [Google Scholar]; c Uneyama K.; Tanaka H.; Kobayashi S.; Shioyama M.; Amii H. Oxidative Cross-Coupling of β,β-Difluoroenol Silyl Ethers with Nucleophiles: A Dipole-Inversion Method to Difluoroketones. Org. Lett. 2004, 6, 2733. 10.1021/ol049055m. [DOI] [PubMed] [Google Scholar]
- a Janson P. G.; Ghoneim I.; Ilchenko N. O.; Szabó K. J. Electrophilic Trifluoromethylation by Copper-Catalyzed Addition of CF3-Transfer Reagents to Alkenes and Alkynes. Org. Lett. 2012, 14, 2882. 10.1021/ol3011419. [DOI] [PubMed] [Google Scholar]; b Ilchenko N. O.; Tasch B. O. A.; Szabó K. J. Mild Silver-Mediated Geminal Difluorination of Styrenes using an Air- and Moisture-Stable Fluoroiodane Reagent. Angew. Chem., Int. Ed. 2014, 53, 12897. 10.1002/anie.201408812. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Yuan W.; Eriksson L.; Szabó K. J. Rhodium-Catalyzed Geminal Oxyfluorination and Oxytrifluoro-Methylation of Diazocarbonyl Compounds. Angew. Chem., Int. Ed. 2016, 55, 8410. 10.1002/anie.201602137. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Lübcke M.; Bezhan D.; Szabo K. J. Trifluoromethylthiolation-Arylation of Diazocarbonyl Compounds by Modified Hooz Multicomponent Coupling. Chem. Sci. 2019, 10, 5990. 10.1039/C9SC00829B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Shao X.; Xu C.; Lu L.; Shen Q. Shelf-Stable Electrophilic Reagents for Trifluoromethylthiolation. Acc. Chem. Res. 2015, 48, 1227. 10.1021/acs.accounts.5b00047. [DOI] [PubMed] [Google Scholar]; b Tlili A.; Billard T. Formation of C-SCF3 Bonds through Direct Trifluoromethylthiolation. Angew. Chem., Int. Ed. 2013, 52, 6818. 10.1002/anie.201301438. [DOI] [PubMed] [Google Scholar]; c Rossi S.; Puglisi A.; Raimondi L.; Benaglia M. Synthesis of Alpha-trifluoromethylthio Carbonyl Compounds: A Survey of the Methods for the Direct Introduction of the SCF3 Group on to Organic Molecules. ChemCatChem 2018, 10, 2717. 10.1002/cctc.201800170. [DOI] [Google Scholar]
- a Hansch C.; Leo A.; Taft R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165. 10.1021/cr00002a004. [DOI] [Google Scholar]; b Hansch C.; Leo A.; Unger S. H.; Kim K. H.; Nikaitani D.; Lien E. J. Aromatic substituent constants for structure-activity correlations. J. Med. Chem. 1973, 16, 1207. 10.1021/jm00269a003. [DOI] [PubMed] [Google Scholar]
- a Alazet S.; Zimmer L.; Billard T. Electrophilic Trifluoromethylthiolation of Carbonyl Compounds. Chem. - Eur. J. 2014, 20, 8589. 10.1002/chem.201403409. [DOI] [PubMed] [Google Scholar]; b Arimori S.; Takada M.; Shibata N. Trifluoromethylthiolation of Allylsilanes and Silyl Enol Ethers with Trifluoromethanesulfonyl Hypervalent Iodonium Ylide under Copper Catalysis. Org. Lett. 2015, 17, 1063. 10.1021/acs.orglett.5b00057. [DOI] [PubMed] [Google Scholar]
- Zhang P.; Li M.; Xue X.-S.; Xu C.; Zhao Q.; Liu Y.; Wang H.; Guo Y.; Lu L.; Shen Q. N-Trifluoromethylthio-dibenzenesulfonimide: A Shelf-Stable, Broadly Applicable Electrophilic Trifluoromethylthiolating Reagent. J. Org. Chem. 2016, 81, 7486. 10.1021/acs.joc.6b01178. [DOI] [PubMed] [Google Scholar]
- a Harris J. F.; Stacey F. W. The Free Radical Addition of Trifluoromethanethiol to Fluoroölefins. J. Am. Chem. Soc. 1961, 83, 840. 10.1021/ja01465a026. [DOI] [Google Scholar]; b Harris J. F. The Free-radical Addition of Trifluoromethanesulfenyl Chloride to Haloölefins. J. Am. Chem. Soc. 1962, 84, 3148. 10.1021/ja00875a022. [DOI] [Google Scholar]; c Dear R. E. A.; Gilbert E. E. Telomerization of bis(trifluoromethyl)disulfide with polyfluoro-olefins. J. Fluorine Chem. 1974, 4, 107. 10.1016/S0022-1139(00)81727-3. [DOI] [Google Scholar]
- Bernstein P. R.; Andisik D.; Bradley P. K.; Bryant C. B.; Ceccarelli C.; Damewood J. R.; Earley R.; Edwards P. D.; Feeney S. Nonpeptidic Inhibitors of Human Leukocyte Elastase. 3. Design, Synthesis, X-ray Crystallographic Analysis, and Structure-Activity Relationships for a Series of Orally Active 3-Amino-6-phenyl-2-pyridinyl Trifluoromethyl Ketones. J. Med. Chem. 1994, 37, 3313. 10.1021/jm00046a016. [DOI] [PubMed] [Google Scholar]
- Umemoto T.; Ishihara S. Power-variable electrophilic trifluoromethylating agents. S-, Se-, and Te-(trifluoromethyl)dibenzothio-, -seleno-, and -tellurophenium salt system. J. Am. Chem. Soc. 1993, 115, 2156. 10.1021/ja00059a009. [DOI] [Google Scholar]
- Parsons A. T.; Senecal T. D.; Buchwald S. L. Iron(II)-Catalyzed Trifluoromethylation of Potassium Vinyltrifluoroborates. Angew. Chem., Int. Ed. 2012, 51, 2947. 10.1002/anie.201108267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Olofsson B.; Marek I.; Rappoport Z.. The Chemistry of Hypervalent Halogen Compounds. Wiley: 2018. [Google Scholar]; b Yoshimura A.; Zhdankin V. V. Advances in Synthetic Applications of Hypervalent Iodine Compounds. Chem. Rev. 2016, 116, 3328. 10.1021/acs.chemrev.5b00547. [DOI] [PubMed] [Google Scholar]; c Merritt E. A.; Olofsson B. Diaryliodonium Salts: A Journey from Obscurity to Fame. Angew. Chem., Int. Ed. 2009, 48, 9052. 10.1002/anie.200904689. [DOI] [PubMed] [Google Scholar]
- a Nicolai S.; Piemontesi C.; Waser J. A Palladium-Catalyzed Aminoalkynylation Strategy towards Bicyclic Heterocycles: Synthesis of (±)-Trachelanthamidine. Angew. Chem., Int. Ed. 2011, 50, 4680. 10.1002/anie.201100718. [DOI] [PubMed] [Google Scholar]; b Dixon L. I.; Carroll M. A.; Gregson T. J.; Ellames G. J.; Harrington R. W.; Clegg W. Synthesis and Reactivity of Aryl(alkynyl)iodonium Salts. Eur. J. Org. Chem. 2013, 2013, 2334. 10.1002/ejoc.201300092. [DOI] [Google Scholar]
- a Panferova L. I.; Miloserdov F. M.; Lishchynskyi A.; Martínez Belmonte M.; Benet-Buchholz J.; Grushin V. V. Well-Defined CuC2F5 Complexes and Pentafluoroethylation of Acid Chlorides. Angew. Chem., Int. Ed. 2015, 54, 5218. 10.1002/anie.201500341. [DOI] [PubMed] [Google Scholar]; b Kokotos C. G.; Baskakis C.; Kokotos G. Synthesis of Medicinally Interesting Polyfluoro Ketones via Perfluoroalkyl Lithium Reagents. J. Org. Chem. 2008, 73, 8623. 10.1021/jo801469x. [DOI] [PubMed] [Google Scholar]
- a Ge S.; Chaładaj W.; Hartwig J. F. Pd-Catalyzed α-Arylation of α,α-Difluoroketones with Aryl Bromides and Chlorides. A Route to Difluoromethylarenes. J. Am. Chem. Soc. 2014, 136, 4149. 10.1021/ja501117v. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Huang X.; Zhang Y.; Zhang C.; Zhang L.; Xu Y.; Kong L.; Wang Z.-X.; Peng B. The ortho-Difluoroalkylation of Aryliodanes with Enol Silyl Ethers: Rearrangement Enabled by a Fluorine Effect. Angew. Chem., Int. Ed. 2019, 58, 5956. 10.1002/anie.201900745. [DOI] [PubMed] [Google Scholar]
- Nakamura Y.; Ozeki Y.; Uneyama K. Reductive modification of difluoromethylene moiety in pentafluoropropionyl group. J. Fluorine Chem. 2008, 129, 274. 10.1016/j.jfluchem.2007.12.003. [DOI] [Google Scholar]
- Eisenberger P.; Gischig S.; Togni A. Novel 10-I-3 Hypervalent Iodine-Based Compounds for Electrophilic Trifluoromethylation. Chem. - Eur. J. 2006, 12, 2579. 10.1002/chem.200501052. [DOI] [PubMed] [Google Scholar]
- Davies A. T.; Pickett P. M.; Slawin A. M. Z.; Smith A. D. Asymmetric Synthesis of Tri- and Tetrasubstituted Trifluoromethyl Dihydropyranones from α-Aroyloxyaldehydes via NHC Redox Catalysis. ACS Catal. 2014, 4, 2696. 10.1021/cs500667g. [DOI] [Google Scholar]
- Gassman P. G.; O’Reilly N. J. Nucleophilic addition of the pentafluoroethyl group to aldehydes, ketones, and esters. J. Org. Chem. 1987, 52, 2481. 10.1021/jo00388a025. [DOI] [Google Scholar]
- Ichitsuka T.; Fujita T.; Ichikawa J. Nickel-Catalyzed Allylic C(sp3)–F Bond Activation of Trifluoromethyl Groups via β-Fluorine Elimination: Synthesis of Difluoro-1,4-dienes. ACS Catal. 2015, 5, 5947. 10.1021/acscatal.5b01463. [DOI] [Google Scholar]
- Ichitsuka T.; Fujita T.; Arita T.; Ichikawa J. Double C−F Bond Activation through β-Fluorine Elimination: Nickel-Mediated [3 + 2] Cycloaddition of 2-Trifluoromethyl-1-alkenes with Alkynes. Angew. Chem., Int. Ed. 2014, 53, 7564. 10.1002/anie.201402695. [DOI] [PubMed] [Google Scholar]
- Curran T. T. Implementation of the Dakin-West reaction for the preparation of an α-amino-pentafluoroethyl ketone. J. Fluorine Chem. 1995, 74, 107. 10.1016/0022-1139(95)03251-8. [DOI] [Google Scholar]
- Hsieh M.-T.; Lee K.-H.; Kuo S.-C.; Lin H.-C. Lewis acid-mediated defluorinative [3 + 2] cycloaddition/aromatization cascade of 2,2-difluoroethanol systems with nitriles. Adv. Synth. Catal. 2018, 360, 1605. 10.1002/adsc.201701581. [DOI] [Google Scholar]
- Guo C.; Wang R.-W.; Qing F.-L. Palladium catalyzed direct α-arylation of α,α-difluoroketones with aryl bromides. J. Fluorine Chem. 2012, 143, 135. 10.1016/j.jfluchem.2012.05.001. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.