Abstract
Purpose:
Medications that target androgen receptor activity (e.g., finasteride) or smooth muscle contractility (e.g., doxazosin) do not resolve lower urinary tract symptoms (LUTS) indicative of lower urinary tract dysfunction (LUTD) for an important subgroup of men. Recently, fibrosis has been implicated as another pathobiology contributing to male LUTS, but no systematic studies have been conducted to assess fibrosis within the context of medical treatment. The purpose of this study was to determine whether fibrotic changes in the prostate transition zone (TZ) were associated with an increased risk for clinical progression among participants treated with doxazosin, finasteride, or finasteride+doxazosin in the Medical Therapy of Prostatic Symptoms (MTOPS) study.
Methods:
TZ biopsy tissues from men who did or did not experience clinical progression on placebo, doxazosin, finasteride, or combination therapy were assessed for collagen content and architectural changes using picrosirius red birefringence and CT-FIRE analysis. Correlations were made with annotated demographic and clinical data. Statistical analyses utilized Pearson correlation coefficients, ANOVA, and t-tests.
Results:
High levels of wavy, aligned prostate TZ collagen were significantly correlated with an increased risk for clinical progression among MTOPS trial participants treated with doxazosin+finasteride, particularly among those with high body mass index.
Conclusions:
Fibrotic changes in the prostate transition zone (TZ) is associated with an increased risk for clinical progression in men treated with doxazosin+finasteride. Anti-fibrotic therapeutics might provide a new treatment approach for men with LUTD who do not respond to current medical treatment approaches.
Keywords: Fibrosis, Doxazosin, Finasteride, LUTS, MTOPS
INTRODUCTION
Prevalence rates for lower urinary tract dysfunction (LUTD), often attributed to Benign Prostatic Hyperplasia (BPH) and manifest as lower urinary tract symptoms (LUTS), ranges from 50%–75% among men 50–80+ years of age.1 LUTD is a progressive disorder that manifests as urgency, nocturia, urinary frequency, weak urinary stream, and incomplete bladder emptying. Without effective treatment, LUTD can lead to bladder outlet obstruction and bladder dysfunction, acute urinary retention, and renal dysfunction.2 Medically-based approaches for treating LUTS include 5α-reductase inhibitors (e.g., finasteride), α-1-adrenergic-receptor antagonists (e.g., doxazosin), either singly or in combination.2, 3 More recently, phospho-diesterase type 5 (PDE5) inhibitors (e.g., taladafil) have shown promise for treating LUTS concomitant with erectile dysfunction.4 Surgical ablation is typically reserved for use in cases with limited or adverse response to medical treatment.4, 5
The Medical Therapy of Prostatic Symptoms (MTOPS) study was designed to evaluate whether therapy with the α-adrenergic-receptor antagonist, doxazosin, or the 5α-reductase inhibitor, finasteride, alone or in combination, would prevent or delay the progression of BPH.6, 7 The results of this study showed that the clinical progression of BPH at study’s end, as measured by a ≥4 pt. increase in AUASS, was evident for 36/786 (4.5%) of men given combination therapy, 65/768 (8.5%) given finasteride, 55/756 (7.3%) given doxazosin, and 97/737 (13.2%) given placebo.7 This suggests that, though largely effective, doxazosin and finasteride do not target all mechanisms contributing to LUTD.
Studies from our laboratories and others have suggested that aging- and inflammation-associated fibrosis can remodel the extracellular matrix to increase prostate tissue stiffness and reduce urethral flexibility, resulting in urinary flow obstruction.8–10 Peri-urethral transition zone (TZ) tissue fibrosis as a pathobiology contributing to LUTD is not therapeutically targeted by either doxazosin or finasteride. Therefore, we sought to test the hypothesis that fibrotic changes in the prostate TZ were associated with an increased risk for clinical progression among participants treated with doxazosin, finasteride, or finasteride+doxazosin (combination therapy). The results of this study showed that high levels of prostate TZ collagen were significantly correlated with an increased risk for clinical progression among MTOPS trial participants treated with doxazosin+finasteride, particularly among those with high body mass index. These data suggest that anti-fibrotic therapeutics might provide a new treatment approach for men with LUTD who do not respond to current medical treatment approaches.
MATERIALS AND METHODS
Clinical Specimens and Study Design.
MTOPS participants were randomized and double-masked to one of the following 4 treatment groups: (1) [finasteride] placebo and [doxazosin] placebo; (2) active doxazosin and [finasteride] placebo; (3) active finasteride and [doxazosin] placebo; (4) active finasteride and doxazosin.6 The study included a prostate biopsy sub-study that collected six core tissue samples from 37% of trial participants at baseline, year one, and year five (or end of study).6 These included core samples from each of the left and right TZ, and one core sample from each of the left and right peripheral zones.6 From the year five/end of study cohort, the NIDDK Central Repository identified left and right TZ biopsies for 29 [doxazosin] placebo+ [finasteride] placebo-treated, 17 doxazosin + [finasteride] placebo-treated; 21 finasteride + [doxazosin] placebo-treated, and 11 finasteride+ doxazosin-treated men who demonstrated clinical progression of BPH/LUTS as defined by a ≥4 point increase in AUASS, as well as an equivalent number of age-matched men who did not demonstrate clinical progression. In order to minimize the overall impact of our study on the NIDDK Central Repository MTOPS specimen collection, we obtained ten each age-matched left and right TZ specimens from patients who did or did not demonstrate clinical progression at the end of the study from each of the 4 treatment groups (Table 1A). Of the total 160 biopsy specimens procured, 142 (for glandular tissue assessment) and 150 (for stromal tissue assessment) were available (Table 1B). Data annotated to the biopsy specimens included in the final analysis were participant age, height in inches, weight in pounds, ethnicity, body mass index, and prostate volume (whole gland, transition zone, or peripheral zone).
Table 1 –
Biopsy Substudy Participants by Treatment and Clinical Outcome
| Treatment |
||||
|---|---|---|---|---|
| Placebo + Placebo | Doxazosin + Placebo | Finasteride + Placebo | Doxazosin + Finasteride | |
| CLINICAL OUTCOME | ||||
| A. Entire Biopsy | ||||
| Non-Progression | 10 | 10 | 10 | 10 |
| Progression | 10 | 10 | 10 | 10 |
| B. Glandular Component | ||||
| Non-Progression | 9 | 8 | 10 | 9 |
| Progression | 10 | 8 | 9 | 8 |
| Stromal Component | ||||
| Non-Progression | 9 | 8 | 10 | 9 |
| Progression | 10 | 10 | 9 | 10 |
Tissue Processing, Imaging, and Analysis.
Five µm sections were taken from each of the 80 paired left and right TZ formalin fixed, paraffin-embedded biopsies and fixed onto positively charged microscope slides. Photomicroscopy was accomplished using a Nikon Eclipse E600 microscope with circular polarized light filters and a Nikon DSFi2 camera.
Picrosirius red staining and analysis:
Tissue sections were stained with picrosirius red (PSR) and images were acquired using a 10x objective under brightfield and circular polarized filters, as birefringence under polarized light is highly specific for collagen.11 Up to quadruplicate images were used for analysis, and all images were normalized for a white (brightfield) or black (polarized) background using the color correct plugin for ImageJ.12 Collagen birefringence was quantified using macros in ImageJ.11 Pixel counts were summed across left and right TZ biopsies and normalized to the sum of the total pixels provided from the thresholded brightfield image using the triangle method in ImageJ. Glandular versus stromal analyses were performed separately on images that either had greater than two epithelial ducts (glandular) or less than three epithelial ducts (stromal).
Curvelet Transform-Fiber Extraction (CT-FIRE) analysis:
The color corrected birefringent images were converted to 8 bit gray scale and batch analyzed in CT-FIRE for fiber length, width, density, straightness, angle and alignment as previously described.13, 14
Statistical Analysis.
PSR staining analysis and CT-FIRE data was tested for statistical significance using two-way ANOVA and Fisher’s least significant difference (LSD) post-hoc tests as well as t-tests, when necessary. Annotated attributes were evaluated for association to collagen content using Pearson correlation coefficients when the attribute was continuous and a t-test or ANOVA when the attribute was categorical (p-values <0.05 were considered significant). All analyses were performed using Graphpad Prism (La Jolla, CA) or SAS statistical software version 9.4 (SAS Institute Inc., Cary, NC).
RESULTS
Altered Collagen Structure in TZ Prostate Tissues Correlates with Clinical Progression. Alterations in collagen structure, including fiber linearity and organization, have been associated with pathological conditions such as cancer and impaired tissue regeneration.13, 15–17 Therefore, these parameters were assessed in TZ biopsies to identify similarities or differences between the four treatment groups. TZ biopsies from men who demonstrated clinical progression demonstrated significantly less straight (i.e. wavy) tissue collagen fibers than those from men who did not progress (p<0.001) (Figure 1A). TZ biopsy collagen fiber alignment (Figure 1B) and angle (Figure 1C) were significantly (p<0.0001) increased in biopsies from men who experienced clinical progression. Collagen fiber width was significantly (p<0.05) decreased in biopsies from men in the progression compared to non-progression groups (Figure 1D). TZ biopsy tissues from participants who did not demonstrate clinical progression had significantly less dense, thicker, straighter and more misaligned fibers (upper panel) than those from men who demonstrated clinical progression, who had denser, thinner, wavier, and better aligned fibers (lower panel) (Figure 1E). Collagen metrics are summarized in Table 2.
Figure 1.
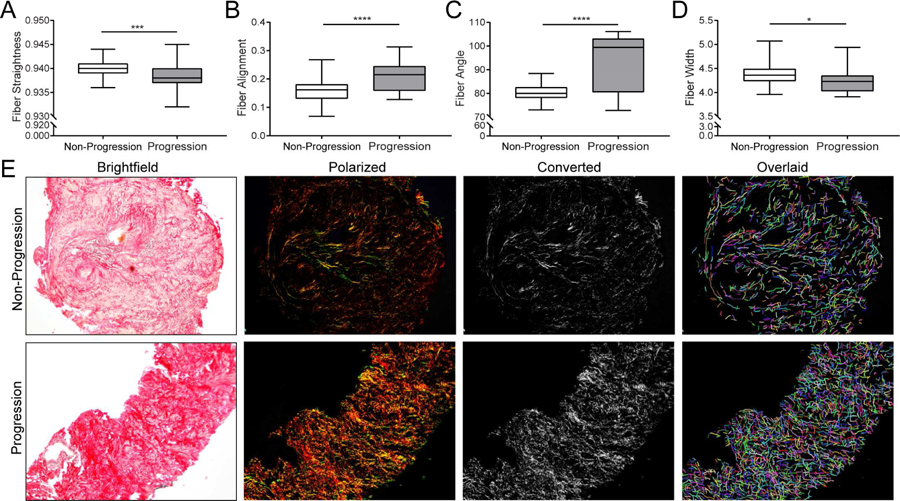
Collagen Fibril Structure of Transition Zone Biopsy Specimens. Box and whisker plots indicating lower extreme, lower quartile, median (line), mean (+), upper quartile and upper extremes. A. Boxplot of data for collagen fiber linearity, or straightness, for which TZ biopsies were significantly less linear (i.e. wavy) for participants who experienced clinical progression than non-progression (p<0.001). Assessment of collagen fiber linearity or straightness was accomplished by measuring the distance of a straight line drawn between fiber end points divided by the distance of a line drawn along the entire fiber. Scores = 1.0 indicated perfect linearity, or fiber straightness, whereas scores <1.0 indicated fiber curvature. B. Boxplot of data for collagen fiber alignment indicates TZ biopsies from patients who experienced clinical progression had significantly increased fiber alignment than those who did not (p<0.0001). Collagen fiber alignment quantifies how the fibers are aligned with each other by measuring the similarity of all fiber angles. Scores = 1.0 indicated perfect fiber alignment with each other, whereas scores <1.0 indicated fibers that are less aligned with each other. C. Boxplot of data for collagen fiber angle indicates those that biopsies from men who experienced clinical progression had significantly larger angles than from those who did not (p<0.0001). Assessment of collagen fiber angle was accomplished by measuring the angle made between a straight line drawn between fiber end points and a horizontal line drawn across the fiber. D. Boxplot of data for collagen fiber width showing patients that biopsies from men who experienced clinical progression had thinner fibers than from those who did not (p<0.05). E. Representative picrosirius red stained brightfield, circular polarized, converted grayscale and CT-FIRE overlaid pseudo-colored fibers of core biopsies demonstrating collagen fiber alignment was more dense, thin, wavy and organized in TZ biopsy tissue from men treated with doxazosin + finasteride who experienced clinical progression (lower panel) compared to those who did not (upper panel).
Table 2 –
Collagen Metrics in MTOPS Core Biopsies
| Placebo |
Doxazosin |
Finasteride |
Doxazosin+Finasteride |
|||||
|---|---|---|---|---|---|---|---|---|
| Non-Progression | Progression | Non-Progression | Progression | Non-Progression | Progression | Non-Progression | Progression | |
| Fiber Length | 35.14 (0.39) | 35.38 (0.67) | 34.96 (0.32) | 34.74 (0.65) | 35.16 (0.49) | 35.04 (0.61) | 35.07 (0.37) | 35.80 (0.56) |
| Fiber Width | 4.44 (0.09) | 4.15 (0.05) | 4.37 (0.07) | 4.28 (0.11) | 4.42 (0.11) | 4.28 (0.09) | 4.34 (0.05) | 4.29 (0.08) |
| Fiber Density | 32.17 (2.41) | 29.78 (2.18) | 28.23 (1.56) | 32.73 (2.41) | 33.61 (2.29) | 29.81 (2.27) | 28.06 (1.61) | 35.52 (2.44) |
| Fiber Straightness | 0.940 (0.001) | 0.939 (0.001) | 0.940 (0.000) | 0.939 (0.001) | 0.940 (0.001) | 0.938 (0.001) | 0.940 (0.001) | 0.937 (0.001) |
| Fiber Angle | 81.73 (1.59) | 94.05 (3.86) | 79.92 (0.56) | 92.98 (3.48) | 79.61 (0.73) | 94.01 (4.19) | 79.02 (1.16) | 95.01 (3.48) |
| Fiber Alignment | 0.15 (0.02) | 0.22 (0.02) | 0.15 (0.01) | 0.20 (0.02) | 0.16 (0.01) | 0.21 (0.02) | 0.18 (0.01) | 0.21 (0.02) |
Values are mean (SE), bold values p<0.05 Response vs Progression by ANOVA with Fisher’s LSD Post Hoc
High Collagen Content in Prostate TZ Tissues Correlates with Clinical Progression in the Combination Treatment Group. PSR staining of MTOPS specimens shows lower levels of collagen in biopsies from men who did not progress during the course of the trial (upper panels) compared to those who exhibited clinical progression (lower panels) (Figure 2A). The average TZ biopsy collagen content ranged from 21.9–27.6% in the [doxazoxin] placebo + [finasteride] placebo, doxazosin + [finasteride] placebo, and finasteride + [doxazosin] placebo treatment groups in both the progression and non-progression cohorts. However, the average collagen content of TZ tissues in the doxazosin+finasteride clinical progression group (average 32.5%, SD=9.4%, gray boxes) was significantly higher than all the clinical non-progression groups (white boxes), as well as the finasteride + [doxazosin] placebo progression group (p<0.05). Importantly, within the doxazosin+finasteride treatment group, those biopsies from men who exhibited clinical progression exhibited significantly (p<0.01) more collagen content than those from men who did not clinically progress (average 21.9%, SD=5.8%) (Figure 2B).
Figure 2.
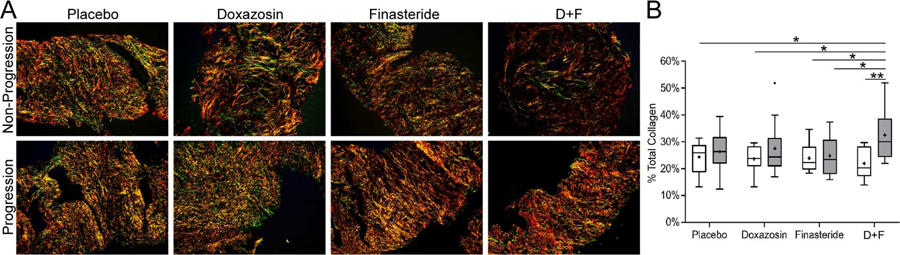
Total Collagen Content of Study Population Transition Zone Biopsy Specimens. A. Picrosirius red staining of MTOPS specimens taken under circular polarized light, clinically responded (upper panels) and clinically progressed (lower panels). B. Box and whisker plots of total collagen content averaged from entire left and right TZ biopsy specimens. Gray boxes, collagen content of biopsy tissues from men who demonstrated clinical progression; white boxes, from those who did not; N = 9 – 10/group. TZ biopsy specimens from men treated with doxazosin+finsteride who experienced clinical progression demonstrated significantly higher total collagen content than from men who did not demonstrate clinical progression after any treatment (p<0.01) or who demonstrated clinical progression after finasteride + placebo treatment (p<0.05).
We next assessed the collagen content in TZ tissues that were predominantly glandular or stromal. Stromal tissues demonstrated an average collagen content (28.5%, SD=9.9%) significantly (p<0.0001) higher than that of glandular tissues (21.5%, SD=7.4%) (Figure 3A). Moreover, the average biopsy collagen content of both glandular and stromal tissues was significantly (p<0.05, p<0.01, respectively) higher for trial participants who demonstrated clinical progression (23.6%, SD=7.7% and 31.6, SD=11.1%, gray boxes) compared to non-progression (19.4%, SD=6.6% and 25.3%, SD=7.3%, white boxes) (Figure 3B). Therefore, high levels of collagen localized within glandular and stromal TZ tissues was significantly associated with risk for clinical progression.
Figure 3.
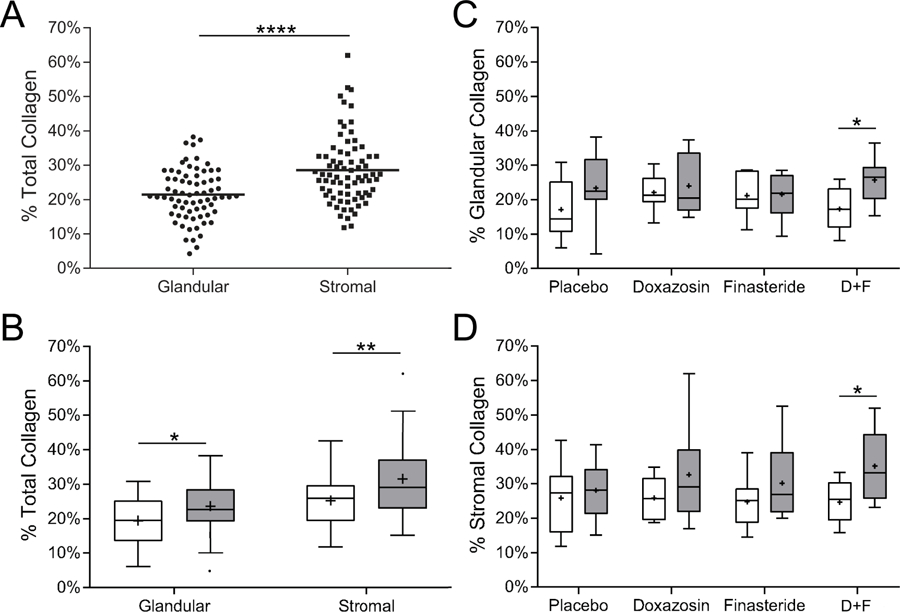
Collagen Content of Glandular or Stromal Components of Transition Zone Biopsy Specimens. If present, glandular (>2 epithelial ducts/field) or stromal (≤2 epithelial ducts/field) components of TZ biopsy specimens were evaluated for collagen content. A. Dot plot of percent total collagen content of glandular components of 36 biopsy specimens from 35 trial participants who exhibited clinical progression and 35 who did not (mean collagen, 21.5%); and of stromal components of biopsies from 39 men who exhibited clinical progression (mean collagen, 28.5%) and 36 who did not (groups as delineated in Table 1). The percent total collagen content of biopsies exhibiting stromal histology was significantly higher (p<0.0001) than those exhibiting glandular histology. B. Box and whisker plots of total collagen content averaged from glandular and stromal components of left and right TZ biopsy specimens from trial participants exhibiting clinical progression (gray boxes) or not (white boxes). Lower extreme, lower quartile, median (line), mean (+), upper quartile and upper extremes are indicated. The total collagen content of TZ biopsy specimens exhibiting glandular or stromal histology from trial participants demonstrating clinical progression was significantly higher (glandular, p<0.05 and stromal, p<0.01) than those who did not. C, D. Box and whisker plots of the total collagen content of glandular components (C) and stromal components (D) of TZ biopsy specimens from trial participants in each treatment group who demonstrated progression (gray boxes) or not (white boxes). The collagen content of biopsy specimens from the doxazosin+finsteride treatment group was significantly higher for men who demonstrated clinical progression than those who did not in both the glandular and stromal tissues (p<0.05).
A more stratified evaluation by treatment revealed that the average collagen content of predominantly glandular epithelial TZ tissues was significantly (p<0.05) higher for trial participants who demonstrated clinical progression (25.7%, SD=6.6%) compared to those who did not (17.3%, SD=6.2%) (Figure 3C). Similarly, the average collagen content of predominantly stromal TZ tissues was significantly (p<0.05) higher for trial participants who demonstrated clinical progression (35.1%, SD=10.3%) compared to those who did not (24.6%, SD=6.0%) (Figure 3D). Therefore, clinical progression among participants receiving doxazosin+finasteride combination treatment was positively associated with high TZ collagen levels regardless of whether tissue composition was predominantly glandular epithelial or stromal.
High Transition Zone Collagen Content Correlates with High Body Mass Index. Analysis of participant demographic and selected annotated clinical data showed that TZ collagen content was not associated with participant age, height, or ethnicity. However, high participant body mass index (BMI) was significantly (p<0.05) associated with high TZ collagen content. Prostate TZ volume and body weight were positively correlated with high collagen content and were significant only at p=0.070 and p=0.072, respectively. These data are summarized in Table 3.
Table 3 –
Correlations Between Total Collagen Content and MTOPS Clinical Data
| Age | r = 0.005 (p=0.964) |
| Height (in) | r = −0.047 (p=0.684) |
| Weight (lbs) | r = 0.203 (p=0.072) |
| Body Mass Index | r = 0.257 (p=0.022) |
| Whole Prostate Volume | r = −0.184 (p=0.105) |
| TZ Prostate Volume | r = −0.206 (p=0.070) |
Pearson correlation coefficients (continuous variables) ANOVA or t-test (categorical variables)
DISCUSSION
Medical and surgical interventions do not achieve symptom relief for all men with LUTD or may not provide a durable response.4, 5 This suggests that other underlying pathobiologies, either incipient or emergent over time, contribute to LUTD that are not addressed by current interventional approaches.
Previous studies have shown that collagen accumulation consistent with fibrosis is significantly higher in the prostatic TZ, particularly the peri-urethral region, in tissues from men reporting moderate/high AUASI scores compared to those reporting no or mild symptoms.8, 10, 11 However, no studies to-date have examined whether TZ fibrosis is associated with an increased risk for clinical progression among men undergoing the most common forms of medical treatment for LUTS, e.g., doxazosin, finasteride, or doxazosin+finasteride (combination) therapy. To address this knowledge gap, we examined the MTOPS tissue specimens and annotated data to test the hypothesis that fibrotic changes in the prostate TZ may be associated with an increased risk for clinical progression among participants treated with doxazosin, finasteride, or finasteride+doxazosin (combination) therapy.
Our findings show significant alterations in collagen architecture (i.e. fiber metrics) in TZ biopsy specimens from men who exhibited clinical progression compared to those who did not. Such alterations, including differences in collagen fibril width and cross-linking, have been noted in tissues from individuals with idiopathic pulmonary fibrosis18 and other pathological conditions such as cancer and impaired tissue regeneration.13, 15–17 For example, normal ovarian stroma has less dense, straighter, and disorganized collagen fibers, while high grade ovarian cancer stroma has more dense, wavy and organized fibers.17 Indeed, collagen organization in TZ biopsy specimens from MTOPS participants who did not demonstrate clinical progression were morphologically similar to that of normal ovarian stroma, whereas those from men who demonstrated clinical progression were more similar to that of ovarian cancer stroma and idiopathic pulmonary fibrosis. A lack of data regarding the precise anatomical orientation of the MTOPS TZ biopsies, e.g., the angle of the biopsy needle and depth of penetration, is a limitation of the current study, since variability in this regard could impact the interpretation of collagen fibril length and orientation.6
A key finding from our study is that the collagen content of TZ biopsy tissues was significantly higher in TZ biopsy tissues from men who experienced clinical progression while undergoing treatment with doxazosin+finasteride. This finding is highly suggestive that peri-urethral fibrosis, which is not targeted by doxazosin or finasteride, may have increased the risk for clinical progression in these individuals. Moreover, the average (mean) percent collagen content was higher for all treatment groups among participants who demonstrated clinical progression compared to those who did not, though these differences did not reach statistical significance. This may be due to the low number of participants in each group (n≤10). Alternatively, the participants treated with doxazosin may have benefitted from treatment with finasteride (and vice versa) if treatment cross-over had been permitted during the trial.
The finding that collagen deposition was localized to stroma from stromal and glandular TZ tissues is consistent with reports that collagen-secreting stromal cell types, including fibrocytes, resident fibroblasts, activated fibroblasts, smooth muscle, pericytes, myofibroblasts or others, are observed in the human prostate.19–24 This suggests that the high levels of collagen observed in TZ biopsies from men who demonstrated clinical progression are likely deposited by stromal elements in both predominantly glandular as well as stromal biopsies. This is consistent with a previous report that collagen content was inversely proportional to glandular content in peri-urethral tissues from men with LUTD.8 That report also showed that prostate tissue stiffness, indicative of fibrosis, was significantly higher in tissues with a collagen content of ≥32%, a value which is remarkably similar to that reported here (31.6%) for collagen content in predominantly stromal biopsies from men who exhibited clinical progression. Additionally, finding higher TZ volume with higher collagen content (p=0.07, Table 3) could reflect either TZ stromal expansion or concurrence of epithelial and stromal expansion, the latter suggesting concurrence of BPH and fibrosis contributing to LUTD.25
The observed positive correlation observed between high collagen content and high body mass index is perhaps not surprising given the wealth of data associating metabolic syndrome with inflammation, which promotes fibrosis and collagen accumulation, and with LUTD.26, 27 A recent study reported that multivariate logistic regression analysis of prostate tissue from 80 consecutive patients who underwent retropubic radical prostatectomy for prostate cancer showed that inflammation and metabolic syndrome were the only independent predictors of peri-urethral fibrosis.28 The link between metabolic syndrome, peri-urethral fibrosis, and LUTD may be exceptionally strong as it also emerged from the analysis of the relatively small number of samples examined in the present study.
Based on the data presented here, a new paradigm for the alleviation of male LUTS emerges. Clearly, doxazosin or finasteride treatment, alone or in combination can be very effective for the management of LUTS. Surgery is an option for men who fail treatment. However, surgical ablation of prostate tissue is invasive, subject to co-morbidities, and may not produce a durable response. The current study shows that fibrotic changes in the prostate TZ may be associated with an increased risk for clinical progression among men treated with doxazosin, finasteride, or finasteride+doxazosin (combination) therapy. This finding suggests that anti-fibrotics might offer another medical treatment option that could be employed prior to surgical intervention (Figure 4).
Figure 4.
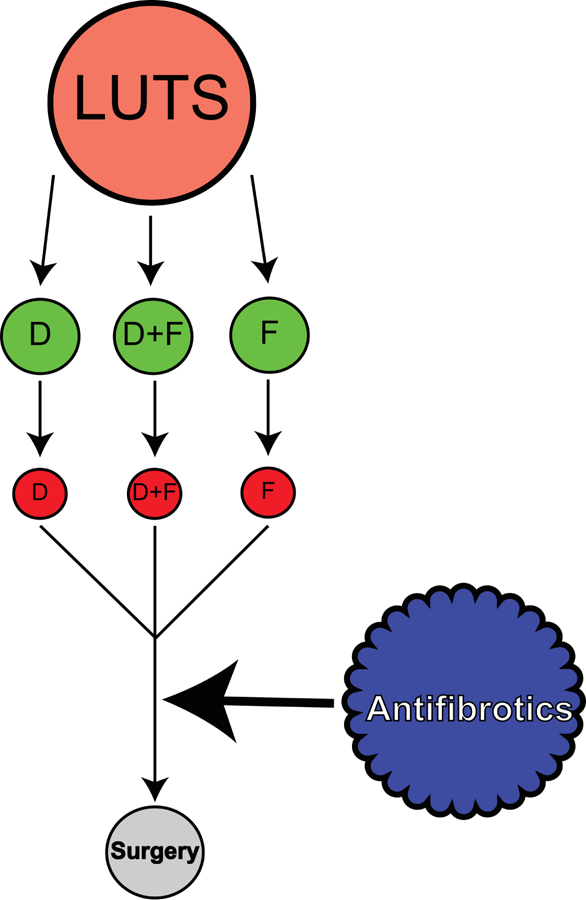
Paradigm for Therapeutic Treatment of LUTS.
Medical approaches for the alleviation of LUTS include doxazosin (D) or finasteride (F), either alone or in combination (D+F). These approaches are often clinically effective (denoted as green circles). However, men who demonstrate high peri-urethral collagen content may be at risk for therapy failure (red circles) and might benefit from treatment with anti-fibrotics. In the event that anti-fibrotic treatment does not sufficiently alleviate symptoms, patients can go on to receive surgical intervention.
CONCLUSIONS
High levels of TZ collagen are significantly correlated with an increased risk for clinical progression among MTOPS trial participants treated with doxazosin+finasteride, particularly among those with high body mass index. These data suggest that anti-fibrotic therapeutics might provide a new treatment approach for men with LUTD who do not respond to current medical treatment approaches.
ACKNOWLEDGEMENTS
We would like to thank Mike Guill from the NIDDK Central Repository for his assistance with acquiring and understanding data annotated to the biopsy specimens for this study. A special thanks to Dr. Reginald Bruskewitz for his clinical insight and review of this manuscript. This work was supported by award NIH/NIDDK U54 DK104310 (W.A.R., J.A.M.) and ES001332 (W.A.R)
LITERATURE CITED
- 1.Egan KB: The Epidemiology of Benign Prostatic Hyperplasia Associated with Lower Urinary Tract Symptoms: Prevalence and Incident Rates. Urol Clin North Am, 43: 289, 2016 [DOI] [PubMed] [Google Scholar]
- 2.McVary: A Review of Combination Therapy in Patients with Benign Prostatic Hyperplasia. 2007 [DOI] [PubMed]
- 3.Laborde EE, McVary KT: Medical management of lower urinary tract symptoms. Rev Urol, 11: S19, 2009 [PMC free article] [PubMed] [Google Scholar]
- 4.Kim EH, Larson JA, Andriole GL: Management of Benign Prostatic Hyperplasia. Annu Rev Med, 67: 137, 2016 [DOI] [PubMed] [Google Scholar]
- 5.Blankstein U, Van Asseldonk B, Elterman DS: BPH update: medical versus interventional management. Can J Urol, 23: 10, 2016 [PubMed] [Google Scholar]
- 6.Bautista OM, Kusek JW, Nyberg LM et al. : Study design of the Medical Therapy of Prostatic Symptoms (MTOPS) trial. Control Clin Trials, 24: 224, 2003 [DOI] [PubMed] [Google Scholar]
- 7.McConnell JD, Roehrborn CG, Bautista OM et al. : The long-term effect of doxazosin, finasteride, and combination therapy on the clinical progression of benign prostatic hyperplasia. N Engl J Med, 349: 2387, 2003 [DOI] [PubMed] [Google Scholar]
- 8.Ma J, Gharaee-Kermani M, Kunju L et al. : Prostatic fibrosis is associated with lower urinary tract symptoms. J Urol, 188: 1375, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gharaee-Kermani M, Rodriguez-Nieves JA, Mehra R et al. : Obesity-induced diabetes and lower urinary tract fibrosis promote urinary voiding dysfunction in a mouse model. Prostate, 2013 [DOI] [PMC free article] [PubMed]
- 10.Hao L, Greer T, Page D et al. : In-Depth Characterization and Validation of Human Urine Metabolomes Reveal Novel Metabolic Signatures of Lower Urinary Tract Symptoms. Sci Rep, 6: 30869, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauman TM, Nicholson TM, Abler LL et al. : Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. PLoS One, 9: e109102, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schneider CA, Rasband WS, Eliceiri KW: NIH Image to ImageJ: 25 years of image analysis. Nat Methods, 9: 671, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bredfeldt JS, Liu Y, Pehlke CA et al. : Computational segmentation of collagen fibers from second-harmonic generation images of breast cancer. J Biomed Opt, 19: 16007, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Y, Keikhosravi A, Mehta GS et al. : Methods for Quantifying Fibrillar Collagen Alignment. Methods Mol Biol, 1627: 429, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Venter C, Niesler CU: Cellular alignment and fusion: Quantifying the effect of macrophages and fibroblasts on myoblast terminal differentiation. Exp Cell Res, 370: 542, 2018 [DOI] [PubMed] [Google Scholar]
- 16.Kiss N, Haluszka D, Lorincz K et al. : Quantitative Analysis on Ex Vivo Nonlinear Microscopy Images of Basal Cell Carcinoma Samples in Comparison to Healthy Skin. Pathol Oncol Res, 2018 [DOI] [PubMed]
- 17.Tilbury KB, Campbell KR, Eliceiri KW et al. : Stromal alterations in ovarian cancers via wavelength dependent Second Harmonic Generation microscopy and optical scattering. BMC Cancer, 17: 102, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones MG, Andriotis OG, Roberts JJ et al. : Nanoscale dysregulation of collagen structure-function disrupts mechano-homeostasis and mediates pulmonary fibrosis. Elife, 7, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gharaee-Kermani M, Mehra R, Robinson DR et al. : Complex Cellular Composition of Solitary Fibrous Tumor of the Prostate. Am J Pathol, 2014 [DOI] [PMC free article] [PubMed]
- 20.Gharaee-Kermani M, Kasina S, Moore BB et al. : CXC-Type Chemokines Promote Myofibroblast Phenoconversion and Prostatic Fibrosis. PLoS ONE, 7: e49278, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Begley LA, Kasina S, MacDonald J et al. : The inflammatory microenvironment of the aging prostate facilitates cellular proliferation and hypertrophy. Cytokine, 43: 194, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schauer IG, Ressler SJ, Tuxhorn JA et al. : Elevated Epithelial Expression of Interleukin-8 Correlates with Myofibroblast Reactive Stroma in Benign Prostatic Hyperplasia. Urology, 72: 205, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hinz B: Formation and function of the myofibroblast during tissue repair. J Invest Dermatol, 127: 526, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Begley L, Monteleon C, Shah RB et al. : CXCL12 overexpression and secretion by aging fibroblasts enhance human prostate epithelial proliferation in vitro. Aging Cell, 4: 291, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Rodriguez-Nieves JA, Macoska JA: Prostatic fibrosis, lower urinary tract symptoms, and BPH. Nat Rev Urol, 10: 546, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sarma AV, Parsons JK, McVary K et al. : Diabetes and Benign Prostatic Hyperplasia/Lower Urinary Tract Symptoms—What do We Know? The Journal of Urology, 182: S32, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Michel MC, Mehlburger L, Schumacher H et al. : Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J Urol, 163: 1725, 2000 [PubMed] [Google Scholar]
- 28.Cantiello F, Cicione A, Salonia A et al. : Periurethral fibrosis secondary to prostatic inflammation causing lower urinary tract symptoms: a prospective cohort study. Urology, 81: 1018, 2013 [DOI] [PubMed] [Google Scholar]


