Abstract
Bisphenol A (BPA) is a contaminant in virtually all Americans. To examine BPA’s adverse effects, the FDA-NCTR, NIEHS, and 14 groups of academic scientists formed a consortium: CLARITY-BPA. The purpose of our study was to investigate the effects of a wide range of doses of BPA on fetal development of the NCTR CD-SD male rat urogenital sinus (UGS). Pregnant rats were administered BPA or positive control ethinylestradiol (EE2) daily, via oral gavage, from gestational day 6 through parturition. Tissues were collected on postnatal day 1 and the UGS was analyzed using computer-assisted 3-D reconstruction. Importantly, only low doses of BPA, as well as EE2, significantly changed birth weight and UGS morphology, including an increased size of the colliculus and decreased size of the urethra, consistent with prior reported BPA and EE2 effects. Our findings provide further evidence that BPA mediates nonmonotonic developmental effects on the fetal urogenital sinus.
Keywords: bisphenol-A, rodents, urogenital sinus, endocrine disruption, CLARITY-BPA
Introduction
Bisphenol-A (BPA) is a ubiquitous xenoestrogen that can leach from a wide range of products, including food and beverage containers, thermal receipts and medical equipment [1, 2]. The evidence indicates that mothers are exposed to BPA and that BPA can cross the placenta, potentially affecting human fetal development [3]. The current oral dose considered safe throughout lifespan by the Food and Drug Administration (FDA) is 50 μg/kg body weight/day. However, extensive research has shown adverse effects on the development of male rodent urogenital tracts exposed prenatally with BPA doses far below those considered safe by the FDA. There are also urogenital tract (bladder, urethra, prostate) abnormalities in adulthood due to BPA or estradiol exposure [4–8]. In developing and adult humans, BPA has been implicated in a wide range of health problems [9, 10]. The focus of CLARITY-BPA was on what would happen in the “low dose” range. Low-dose effects refer to biological changes that occur in the range of human exposures or at doses that are lower than those typically used in the U.S. EPA’s standard testing paradigm for evaluating reproductive and developmental toxicity.
Consistent with the developmental origins of health and disease (DOHaD) model, we have previously found statistically significant effects of low doses of estrogens on the UGS of fetal mice and rats [4, 11–17]. For example, we have found that BPA increases estrogen receptor alpha (ERα) gene expression (ESR1) in UGS mesenchyme in male mouse fetuses in both primary culture [15, 16] and in vivo, which was associated with changes in DNA methyltransferase [17]. An increase in androgen receptor gene expression caused by BPA was also found in these studies, and an increase in androgen receptor protein has been found in the adult male mouse prostate after fetal exposure to elevated estradiol or BPA during fetal life [11, 18, 19].
Low doses of BPA during development have been related to squamous metaplasia and abnormalities in basal cells that persisted in adulthood, such as multilayering and permanent expression of cytokeratin 10 in mice [20]. In addition, only low doses of BPA caused an increase in basal cells in rats that were from the collaborative program we were part of, suggesting an increase prostate cancer susceptibility and changes in adult prostate stem cell homeostasis [21]. In both of these studies the greatest effects were in the dorsolateral region of the prostate, consistent with all of our prior findings. Finally, fetal exposure to low, but not high, doses of BPA results in an increase in body weight and body fat, as well as disruption of hormones that regulate metabolic processes in adult mice [22, 23]. In mice, BPA has not been related to body weight at birth, although BPA has been reported to increase body weight at birth as well as later in life in a study using rats [24].
Due to the well-characterized estrogenic activity of BPA and over eight thousand published studies by independent academic investigators reporting a wide range of adverse health effects [25], several nations have banned the use of BPA in select products. However, in the USA, the FDA has deemed BPA to be safe at current levels of exposure. The basis for this difference is the absence of the application of the precautionary principle in the USA as opposed to countries in Europe. However, the Center for the Evaluation for Risks to Human Reproduction (CERHR) within the US National Toxicology Program (NTP) concluded in 2008 that there was “some concern” for effects of BPA on the urogenital system in males. The recommendation that further studies be conducted contributed to the decision by the National Institute of Environmental Health Sciences (NIEHS) to fund 14 different groups in a collaborative Consortium Linking Academic and Regulatory Insights on the Toxicity of BPA (CLARITY-BPA) to compare their results to those from the guideline studies conducted by the FDA’s NCTR scientists [26]. Unfortunately, the FDA has now refused to allow the NTP to issue an official final NTP summary report comparing the results of the core guideline FDA study and the results from studies by academic investigators. The second objective of CLARITY-BPA was to determine whether academic investigators could replicate their prior published results in animals produced by the FDA’s National Center for Toxicological Research (NCTR).
Traditional toxicological study methods used by industry and government regulatory agencies involve assessing chemical hazard by administering very high doses that are not relevant to exposures experienced by the general population. The use of only very high doses ignores the possibility of endocrine disruption by chemicals such as BPA. Endocrine disruptors and other hormones involve ligand/receptor mediated mechanisms that evolved to operate at extremely low doses along with multiple mechanisms that lead to very different outcomes when exposure occurs at high doses [27–29].
There is a marked difference in conclusions regarding BPA safety between academic scientists and both FDA and industry studies. This is due to the different approaches that are used: hypothesis driven academic studies versus experiments conducted for chemical risk assessments using guideline protocols developed in the last century [25, 28, 30, 31]. Resolving the discordant BPA literature was a major factor in funding CLARITY-BPA. This program involved a collaboration between the NTP, NIEHS and U.S. Food and Drug Administration’s (FDA’s) NCTR laboratory. The objective of the CLARITY-BPA project was to determine the effects of BPA in Sprague-Dawley derived (CD-SD) rats raised by the NCTR, with the NCTR conducting guideline studies and academic collaborator teams conducting NIEHS-funded state-of-the art research on animals/tissues provided to them by the NCTR. The purpose of CLARITY-BPA and detailed methods have been published [32, 33].
This study herein, was one of the 14 academic collaborator projects that was funded. Our study evaluated the effects of fetal BPA exposure on male urogenital sinus (UGS) morphology at birth using novel 3-D modeling techniques to take measurements coupled with immunocytochemistry. As with most of the other CLARITY-BPA academic studies that were funded by NIEHS, we had previously reported that exposure to BPA below the FDA safe dose, as well as exposure to estradiol, ethinylestradiol and diethylstilbestrol, resulted in malformation of the fetal urogenital tract in male mice as well as changes in androgen and estrogen receptors [4, 11, 14–16].
Pregnant CD-SD rats were administered BPA via oral gavage using a range of doses: from a low dose (2.5 μg/kg/day) relevant to human exposure up to a 10,000-fold higher dose that was within the toxicological dose range used in traditional chemical risk assessment studies, based on the assumption that dose-response relationships are monotonic [29]. We report here that for the animals selected and sent blind to us by NCTR staff, exposure of male rat fetuses to low, but not high, doses of BPA significantly increased body weight at birth and significantly altered the size and shape of estrogen target tissues within the fetal UGS, consistent with prior findings for BPA and other estrogens in rodents.
Materials and Methods
Animal Husbandry and Dosing
The post-natal day 1 (PND1) CD-SD rat pups needed for this study were obtained from litters generated for the CLARITY-BPA consortium program [32]. The study design details, including animal husbandry, diet, breeding, dosing, administration and timing have been fully described [33], therefore only methods relevant to our study are summarized here. Dams and pups remained undisturbed on the day of birth until terminal pup weights were taken, and pups were euthanized by decapitation on the day after birth. For consistency with NTP/FDA reports, we will refer here to the day after birth, when the pups were euthanized, as PND1. Animals were maintained by the NCTR under Good Laboratory Practice protocols in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AALAC), and all procedures were pre-approved by the NCTR Institutional Animal Care and Use Committee (IACUC).
The diet was soy- and alfalfa-free (5K96 verified casein diet 10 IF; Purina Mills), and Millipore-filtered water was provided in glass bottles with silicone stoppers; both were provided ad libitum. Diet samples and other study materials, including bedding, drinking water and cages, were monitored for BPA and other myco/phytoestrogens by liquid chromatography/mass spectrometry. All diet samples contained less than 5 ppb BPA and less than the protocol-specified maximum level of phyto/mycoestrogens. All other study materials tested were found to have BPA levels below analytical blank levels.
NCTR CD-SD female rats were randomly assigned to one of eight treatment groups: 0.3% carboxymethylcellulose (CMC) vehicle controls, 2.5, 25, 250, 2500, or 25000 μg/kg bw/day BPA, as well as 0.05 or 0.5 μg/kg bw/day EE2. These groups are referred to as: controls, BPA-2.5, BPA-25, BPA-250, BPA-2500, BPA-25000, EE2–0.05 AND EE2–0.5. Starting at gestational day 6 and continuing until the day of parturition, dams from all groups were administered a daily treatment dose via oral gavage using a modified Hamilton Microlab ML511C programmable 115V pump (Hamilton Co. Reno, NV).
Mating pairs were assigned randomly and consisted of 11–15 week-old males and 10–14 week-old females, while avoiding any sibling or cousin pairing. Matings occurred in five loads (replicate blocks) spaced 4 weeks apart. Pups used in this study were reported as evenly spread across loads 2, 3, 4 and 5, although only a few pups came from loads 4 and 5, and all BPA 2500 pups came from load 2 (Supplemental Materials, Table 5). We were sent 10 rat pups per treatment group (80 rats total), but there was a large body weight range of 3.7 to 9.6 grams. A total of 46 pups were chosen (5–6 pups per treatment group) for this study. The expected minimum of 6 pups was not met for two groups due to our body weight cutoff range of 5 to 8.1 g (in the BPA-2.5 group), and one BPA-2500 animal was not able to be digitally reconstructed. To eliminate the possibility of litter effects, one pup per litter was randomly selected by NCTR personnel and provided to us coded. We were thus blind to treatment group until the data were submitted to the NTP and locked into a data base. Some information regarding litter composition (such as sex ratio) was missing (Supplemental Materials, Table 5) apparently due to misclassification of the sex of some of the pups, although all pups provided to us were males.
Tissue Collection and Embedding
The hind end from the male PND1 pups were fixed in 10% neutral-buffered formalin for 24 hours and then shipped to the vom Saal Lab in 70% ethanol. The entire male lower urogenital tract, including the urogenital sinus (UGS), bladder, and associated accessory sex glands was collected and placed into RNAse-free ddH2O overnight until agar and paraffin-embedding the next day. Before agar-embedding, the top 1/3 of the bladder was removed to organize a tissue array (multiple UGS’ per block), dorsal side down. There were three histology cassettes with agar-embedded urogenital tissues. We used a standard overnight protocol in the Histology Core at the University of Wisconsin. In order to ensure that there were vehicle controls equally spread across these cassettes for immunohistochemistry, the identity of just the vehicle control samples were provided by the NTP prior to conducting the immunohistochemistry; this occurred after completion of the morphometric analyses, which were conducted blind.
Tissue Sectioning, Immunohistochemistry and Image Collection
Formalin-fixed, paraffin-embedded PND1 rat urogenital tracts (UGT) were serially sectioned in RNAse-free conditions, 5-μm thick. To identify the UGS lumen and other structures, every third slide was then stained for basal epithelial cell nuclear marker p63 ΔN (BioLegends, 619002, 1:500), using diaminobenzidine (DAB) as the chromogen. To assess putative estrogen regulated factors and prostatic proteins, immunohistochemistry was completed using 9 different antibodies focusing on the UGS. Antibodies used, dilutions and protocol highlights are summarized in Supplemental Materials, Table 1. Photographs were acquired with a 4x objective lens (N.A.=0.13) on an 80i microscope using the DS-Fi2 camera and NIS Elements software (Nikon Instruments, Melville, NY). All images of the p63 stained slides were imported into BioVis3D software (Montevideo, Uruguay), defining the distance between each image as 15 μm. BioVis3D was used to trace the UGS on every image in order to render it in three-dimensions (3-D) (Fig. 1).
Fig. 1.
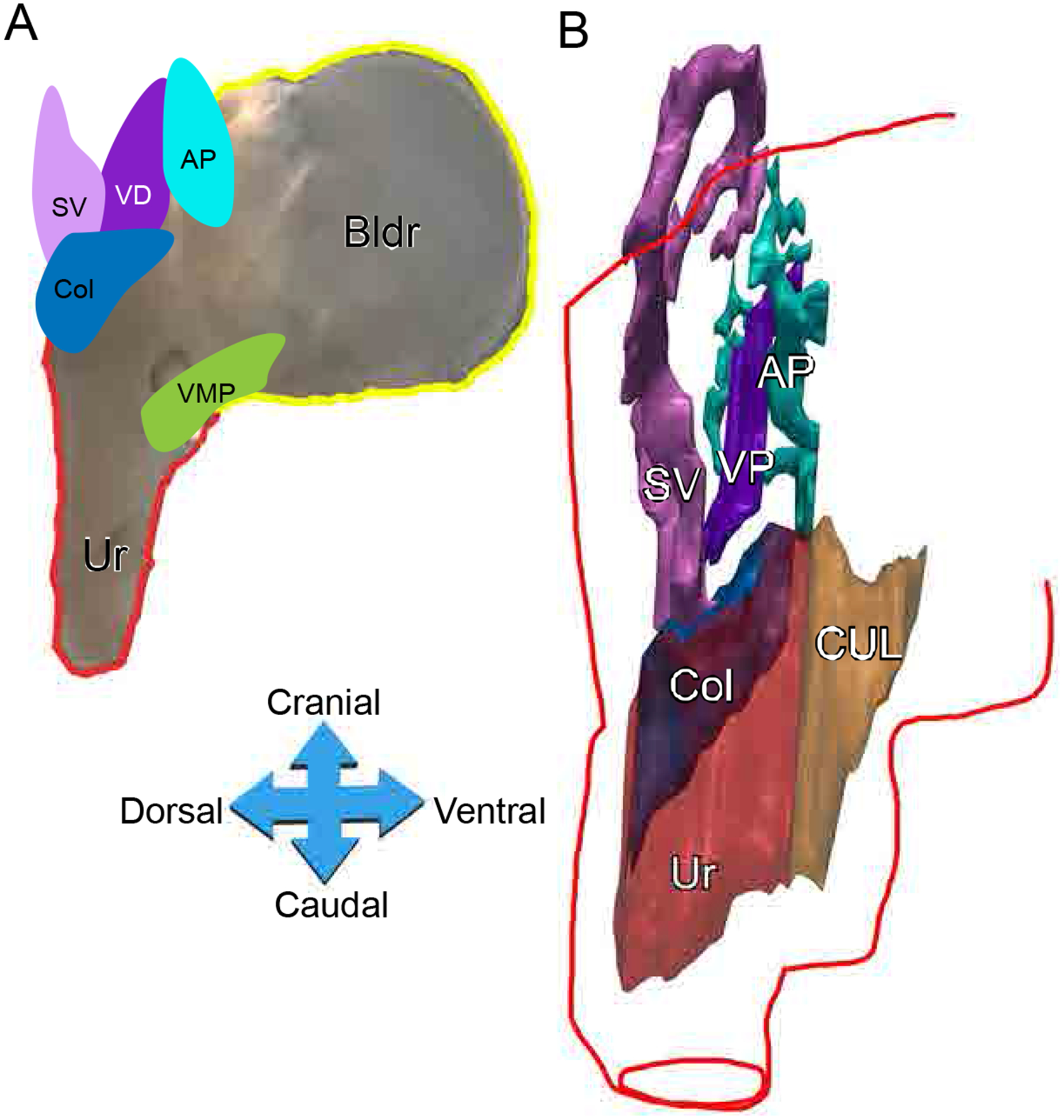
Anatomy and three-Dimensional (3-D) reconstruction of the rat urogenital sinus (UGS). Representative urogenital tract of PND1 pups (A). A 3D reconstructed model of the urogenital sinus. Post 3D reconstruction, a red outline indicating the outside stromal layer of the UGS was added to the image (B). Key: bladder (Bldr), urethra (Ur), colliculus (Col), seminal vesicles (SV), vas deferens (VD), anterior prostate (AP), cranial urethral lumen (CUL), ventral mesenchymal pad (VMP)
3-D Reconstruction Analysis Criteria and Morphometric Definitions
Traces of urogenital epithelial cells were made using p63-positivity in epithelial cells for each UGS 3-D reconstruction (Fig. 2). P63 has been used previously to detect seminal vesicles (SV), ventral prostate (VP), dorsolateral prostate (DLP), anterior prostate (AP) and urothelium [4]. Visualization of p63 positive structures are shown in Fig. 2. The UGS measurement criteria are described in Fig. 3 and Supplemental Materials, Table 2.
Fig 2.
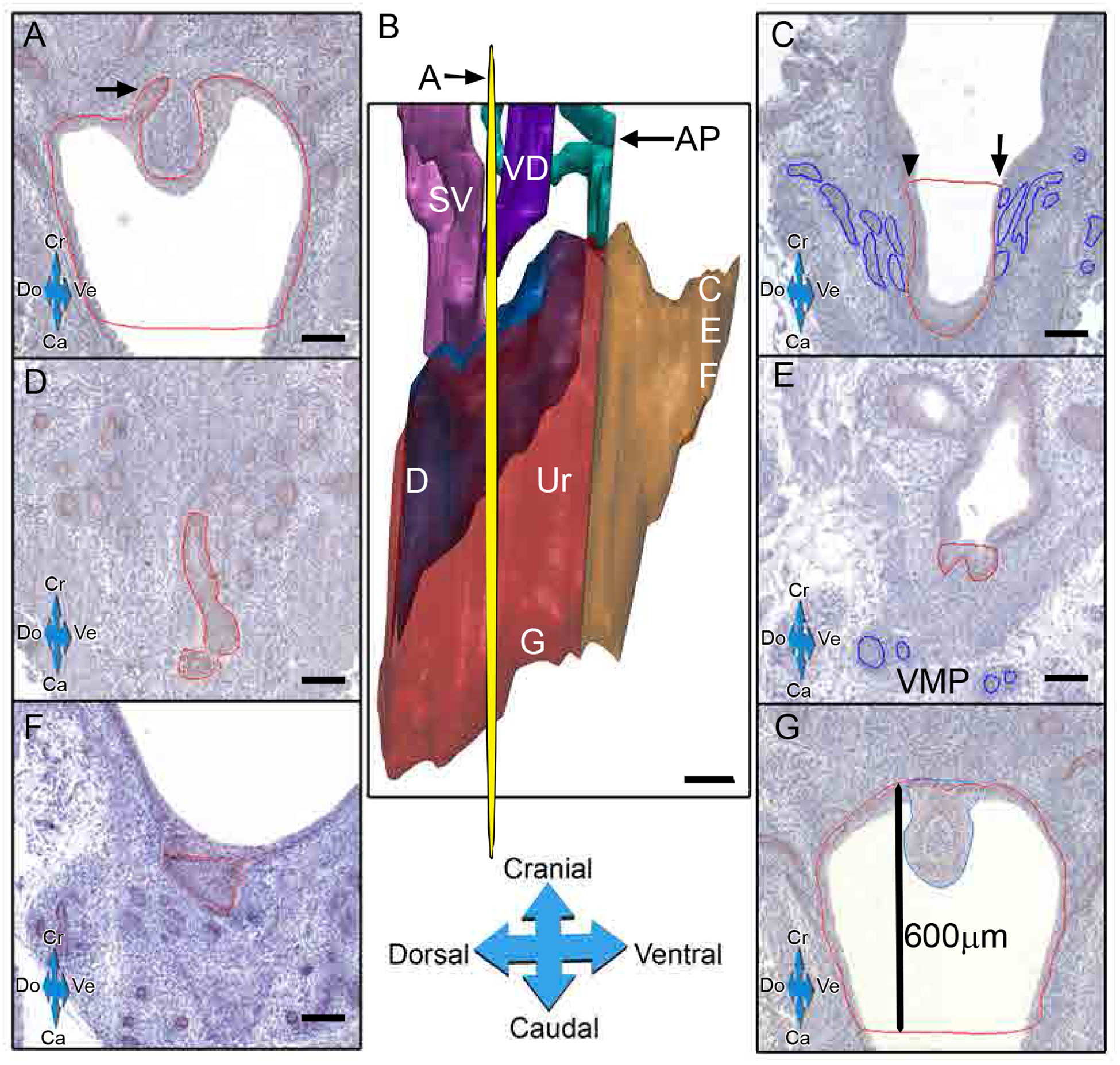
Tracing of epithelial structures for 3D-reconstruction. IHC for p63 (DAB-brown) was performed on rat pup rat urogenital sinuses (UGS) sections and traced for 3D reconstruction on post-natal day (PND)-1 UGSs. Red line indicates urothelium p63 positivity (arrow) trace cranially whenever possible (A). The yellow plane indicates the cross section A and corresponding sections, labeled C-G, are serial sections parallel to A (B). At the beginning of the bladder neck, the cranial urethral line (orange) was drawn across the lumen at the point where the UGE thickens (arrowhead) or where the most cranial ventral prostate (VP, blue) bud (arrow) (C). Dorsal UGS traces were made on sections when the first urethral p63-positive cells appear (D). Ventral traces were made when the last VP buds (blue) appeared prior to VMP (E). The most ventral portion of the urethra (area within orange line) was determined where p63-positive cells were last present (F). The caudal end of the urethra was determined by a fixed length (600μm) from the cranial portion of the colliculus (blue) (G).
Fig. 3.
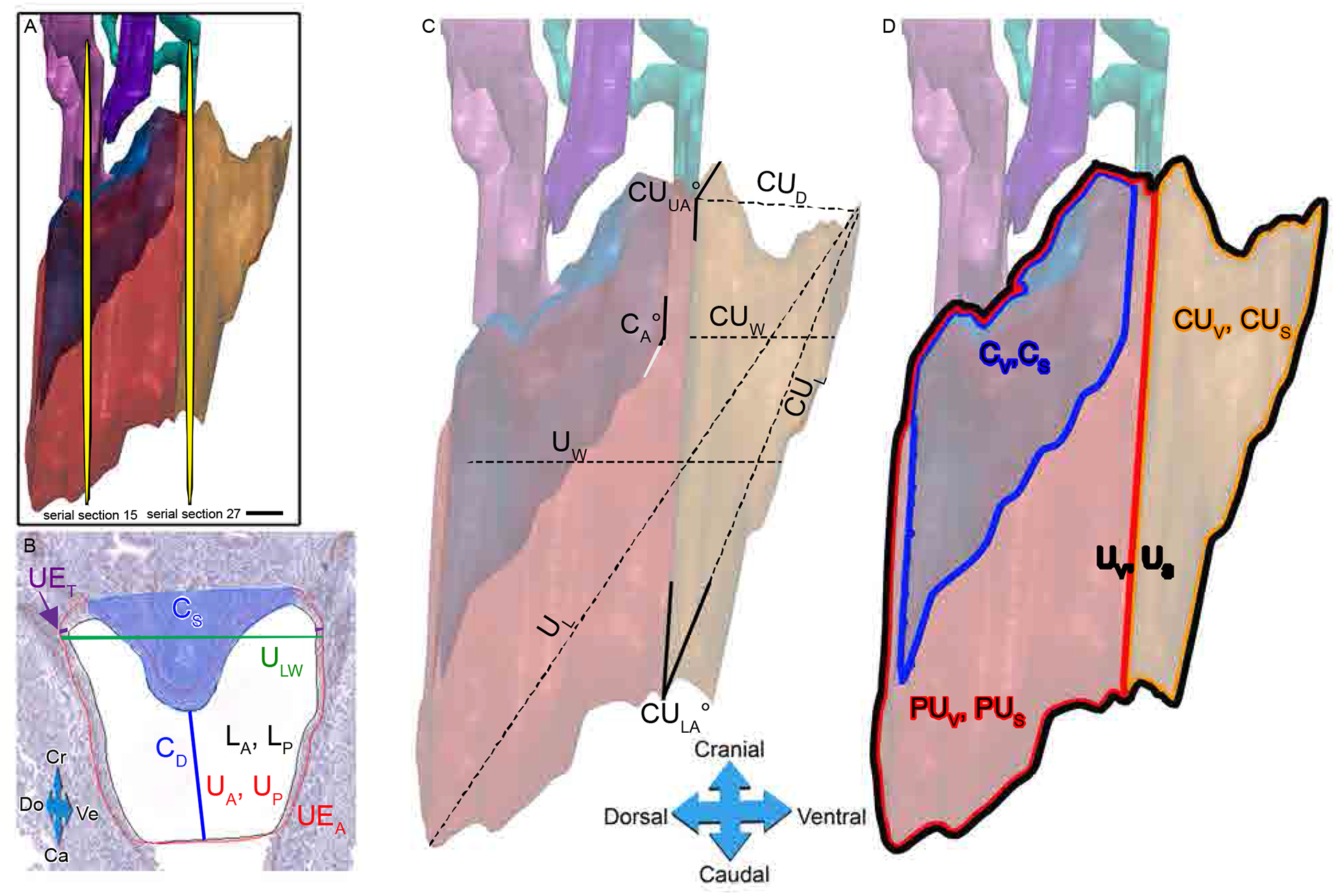
Determination of 3D urogenital sinus morphometric criteria. Morphometrics were based on measurements taken from either the section at the widest lateral width or the midway section of the urethra. The widest UGS section was chosen from the cranial view point of the 3D reconstruction, indicated as a yellow plane serial section 15. The midway section is halfway point between the emergence of AP in the urethra and the bladder: urethra junction, indicated as yellow plane serial section 27 (A). A black line indicates the inner lumen edge of p63-positive urethral epithelium. A red line indicates the p63-positive urethral epithelium. The shaded blue area indicates the colliculus (B). Several measurements were taken from the IHC image of the widest UGS section (B) as well as the midway section (image not shown), including the urethral lateral width (ULW), colliculus distance (CD), colliculus size (Cs), luminal area and perimeter within urothelium (LA and LP, respectively), urethral area and perimeter within p63-positive urothelium (UA and UP, respectively), urothelium thickness (UET) and urothelium area minus the lumen area (UEA) (B). Further morphometrics were calculated, including cranial urethral distance (CUD), cranial urethral upper and lower angles (CUUA°, CULA°, respectively), total urethra volume and surface area (UV, US, respectively), cranial urethra volume and surface area (CUV, CUS, respectively), dorsal to ventral width at the cranial urethra (CUW), dorsal to ventral urethra width (UW), caudal to cranial distance of the cranial urethra (CUL), volume and surface area of the prostatic urethra (PUV, PUS, respectively), volume and surface area of the colliculus (CV, CS, respectively), caudal to cranial length of the urethra (UL) and colliculus angle (CA°), marked as the angle the colliculus makes at the cranial urethra, was also measured (C).
Since typical measurements of growth (e.g. mass) are not attainable for the UGS, we evaluated multiple cellular and anatomical measurements to ascertain if changes in the UGS occurred due to maternal estrogenic chemical treatments (Fig. 3 & Supplemental Table 2 & 3). After 3D images were constructed, measurements were made in two anatomical areas to standardize measurements in size, shape, volume, and other quantitative sites. The first anatomical area was the midway section (i.e. halfway point) between where the AP ducts first connect to the urethral lumen and where the bladder first connects to the urethral lumen. The second anatomical area was the widest slice, which is the section with the widest lateral width of the urogenital epithelia (Fig. 3).
From the 3-D view of the entire region of the UGS (Fig. 3-A), the section from the entire urethra with the widest urethral lumen was chosen (serial section 15) to take the following morphometrics shown in Fig. 3-B: The urethral area (UA) and perimeter (UP) are drawn in red around the p63 positive urethral epithelium. The luminal area (LA) and perimeter (LP) are drawn in black inside the p63 positive urethral epithelium. The urothelium area (UEA) was calculated by subtracting the luminal area from the urethral area. To calculate the urothelium thickness (UET), two distances (shown in purple) were measured on both the left and right lateral sides of the urothelium at the widest point of the urethra and averaged. A line (CD) was drawn from the lowest point of the colliculus (shaded blue in Fig. 3-B) to the point identified in Fig 2-G that was 600 μm below the top of the colliculus. This was used as a proxy for the size of the colliculus (CS); colliculus size was calculated as the inverse of the CD line (1/ CD in μm × 1000; Fig. 3-B). The urethral lateral width (ULW) was measured across the widest, lateral part of the p63 positive urothelium in dark green. All of the same morphometrics were taken on the midway section (Fig. 3-A, serial section 27).
Additional morphometrics were taken on the entire 3D reconstructed urethra (Fig. 3-C, D). The measures of the total urethra (red + orange) included: urethral volume (UV) and surface area (US), dorsal to ventral urethral width (UW), caudal to cranial urethral length (UL). The separation of the cranial urethra and prostatic urethra was defined by where the anterior prostate buds emerged from the urethral epithelium. For the prostatic region of the urethra (red), the volume (PUV) and surface area (PUS) were measured, as well as for the urethra associated with the colliculus, both the volume (CV) and surface area (CS) were measured. The colliculus angle was measured as the angle the colliculus made with the cranial urethra (CA°). For the cranial region of the urethra, the following measurements were taken: the volume (CUV) and surface area (CUS), cranial urethra distance (CUD), which is the distance in μm from the most cranial point examined to where the anterior prostate emerged; the cranial urethra dorsal to ventral width (CUW); the cranial urethra caudal to cranial length (CUL); the cranial urethra lower angle (CULA), which is the angle made between the prostatic and cranial urethra on the caudal end; and finally, the cranial urethra upper angle (CUUA), which is the angle made between the prostatic and cranial urethra on the cranial end.
Data Decoding and Statistical Analysis
Prior to any data analysis, the raw, blinded morphometry and immunochemistry data files were uploaded to the NTP Chemical Effects in Biological Systems (CEBS) database. They were then “locked” down to prevent further editing after independently being verified that all expected data points were accounted for. Once all data were submitted from the academic investigators, the data set was decoded to provide information about the treatment groups so that statistical analyses could be performed.
The number of animals examined per group was based primarily on our prior findings in Timms et al. [4]. Morphometric measurements were analyzed using linear one-way analysis of variance (ANOVA) followed by Fisher’s Least Significant Difference (LSD) (SAS v9) test comparing only controls to each of the treatment groups; separate analyses were conducted for BPA and EE2 data so that their effects could be assessed independently. In addition, R Statistical software (R v2.14.2; RStudio v0.95.263) was used to compare the goodness of fit of just the BPA data to a linear or nonmonotonic function by addition of a quadratic term as previously described [23, 34]. Since each pup came from a separate litter, no correction for litter was required. As shown in Table 4 and 5 (Supplemental Materials), there were no differences in litter size or sex ratio based on treatment group (Supplemental Materials, Table 4, 5). The analysis of the morphometric data was conducted by dividing each variable measured by the animal’s body weight, since body weight can impact the size of internal organs. Graphs are box and whisker plots showing max, min, quartiles, medians (line), means (indicated by “+”. The data presented are non-transformed, although the p-values in some cases are based on reciprocal transformations to reduce heterogeneity of variance. In all graphs *p≤0.05 and **p≤0.01 were considered statistically significant and p≤0.1 as showing a trend.
Results
Body Weight.
After decoding the submitted blinded data by the NTP, we were able to group and analyze the 3-D reconstructed PND1 male rat urethras as well as other data, such as the body weight of the pups. The CD-SD rat strain has been subjected to selective breeding for increased body weight and large litter size by Charles River Laboratories since 1950, and a breeding stock was purchased by the NCTR in 1972 [30, 35]. Although the CLARITY-BPA study was designed to reduce inter-institutional experimental variation (e.g. dosing, housing, animal housing, tissue collection, etc.), there was a large range of body weights (3.7 g – 9.6 g) in this study (Fig. 4-A), and some of the groups had higher variance in body weight than the other groups (Fig. 4-B). Nonetheless, we observed that body weights were significantly lighter in the gavaged vehicle control group than in the lower dose BPA treatment groups (2.5, 25, 250 μg/kg/day), as well as in comparison to both EE2 treated groups (0.05 and 0.5 μg/kg/day) for PND1 pups (Fig. 4-B). Interestingly, in contrast to the low-dose BPA effects, fetal exposure to the highest doses (BPA-2500 and BPA-25000) did not significantly alter body weight of male pups on PND1. Addition of a quadratic term in the R-statistic showed a significantly better fit (p≤0.05) for a nonmonotonic relationship between BPA dose and body weight relative to the linear analysis.
Fig. 4.
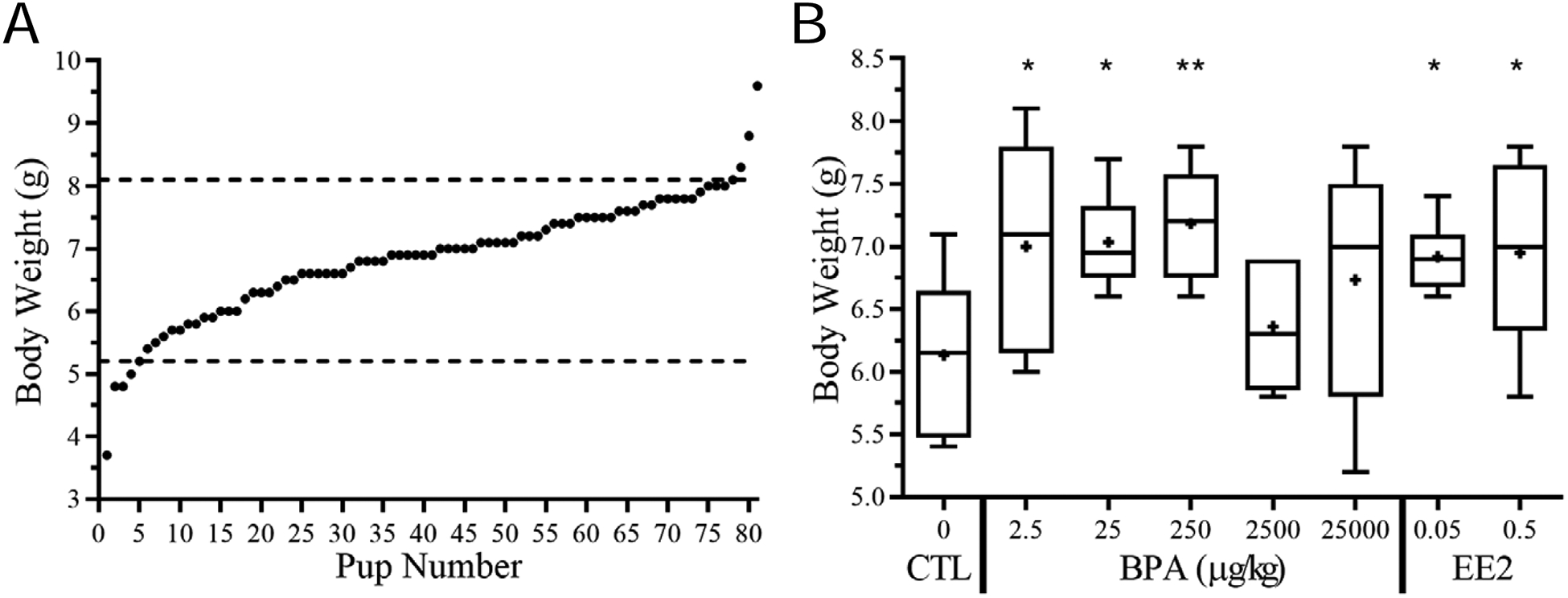
BPA exposure leads to changes in body weights in rat postnatal day one (PND1) pups. Range of body weights for all pups in CLARITY-BPA PND1 study; Mean=6.854g, SD=0.954, CV=13.9%, Range=3.7–9.6g (A). Due to high variability in pup weight, cutoffs were made (dashed lines). Distribution of PND1 pup body weight by treatment (B).
Litter size ranged from 11–18 pups, with 50% of the litters having 14–15 pups. While regression analysis revealed that litter size was significantly (R2≤0.05) related to pup body weight, as is expected in any given population (Supplemental Fig. 1), this did not account for the lighter vehicle controls, since litter size did not vary as a function of treatment. On the day of birth (dams’ gestation day ranged from GD20-GD22), the BPA 25 and 250 exposed dams were significantly (p≤0.05) heavier than control dams, while the mean weight of all pups from the litters from which our pups were selected was not significantly different from vehicle controls for any dose group (Supplemental Materials, Table 4, 5).
Effect of Treatment on Colliculus Angle and Size
BPA significantly altered the angle of the colliculus (CA°), which is the angle the colliculus makes with the cranial urethra (Fig. 5-A), for the BPA-2.5, 25, 250, and 25000 groups as well as the EE2–0.05 group; there was a trend (p=0.07) for a difference for EE2–0.5 treatment group (Fig. 5-B). Colliculus size (CS) measured on the UGS widest luminal section (Fig. 5-C) was significantly increased by both the BPA-250 and EE2–0.5 doses (p≤0.05) (Fig. 5-D).
Fig. 5.
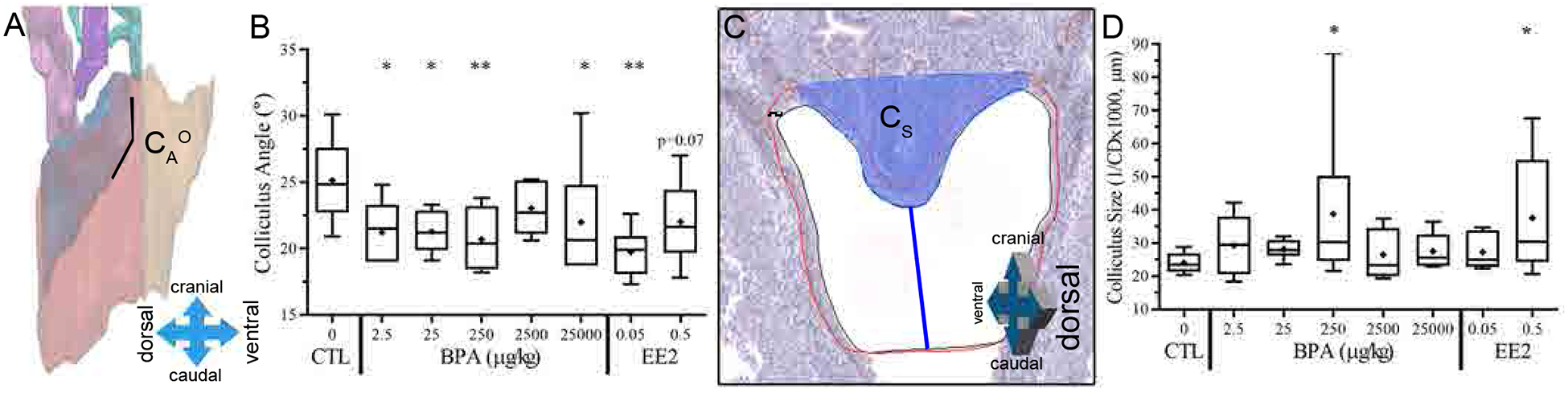
Colliculus measurements in PND1 rat pups. Colliculus angle (CA°) was defined as the angle the colliculus makes at the juncture with the cranial urethra (A) and analyzed by treatment (B). The colliculus size (CS), shaded blue was determined by measuring the colliculus distance (CD) between the lowest caudal point of the colliculus and the lowest caudal point drawn on the urethra and taking the reciprocal (C) and analyzed by treatment (D).
Dorsal-Ventral and Cranial-Caudal Length of the Urethra
We examined UGS morphometrics based on the entire 3-D reconstruction of the sections using the BioVis3D software (Fig. 6-A). The dorsal-ventral width of the entire urethra was significantly smaller for the BPA-25 and EE2–0.05 treatment groups (p≤0.05), while the of EE2–0.05 group tended to be smaller relative to vehicle controls (p=0.07) (Fig. 6-B). The cranial-caudal length of the total UGS urethra (UL) was also significantly smaller (Fig. 6-C) in the BPA-2.5 group and EE2–0.05 group compared to the vehicle controls (p≤0.05). This shortening of the urethral lumen is also seen in the cranial portion of the urethral lumen (yellow), which is cranial to where the anterior prostate buds emerge, replicating prior findings [4]. Compared to vehicle controls, the cranial urethral lumen width (CUW; Fig. 6-D) was significantly smaller in the BPA-25 group (p≤0.05) and the cranial urethral length (CUL; Fig. 6-E) was significantly smaller in the BPA-2.5 group (p≤0.01), and tended to be smaller in the BPA-250 group (p=0.06) relative to vehicle controls.
Fig. 6.
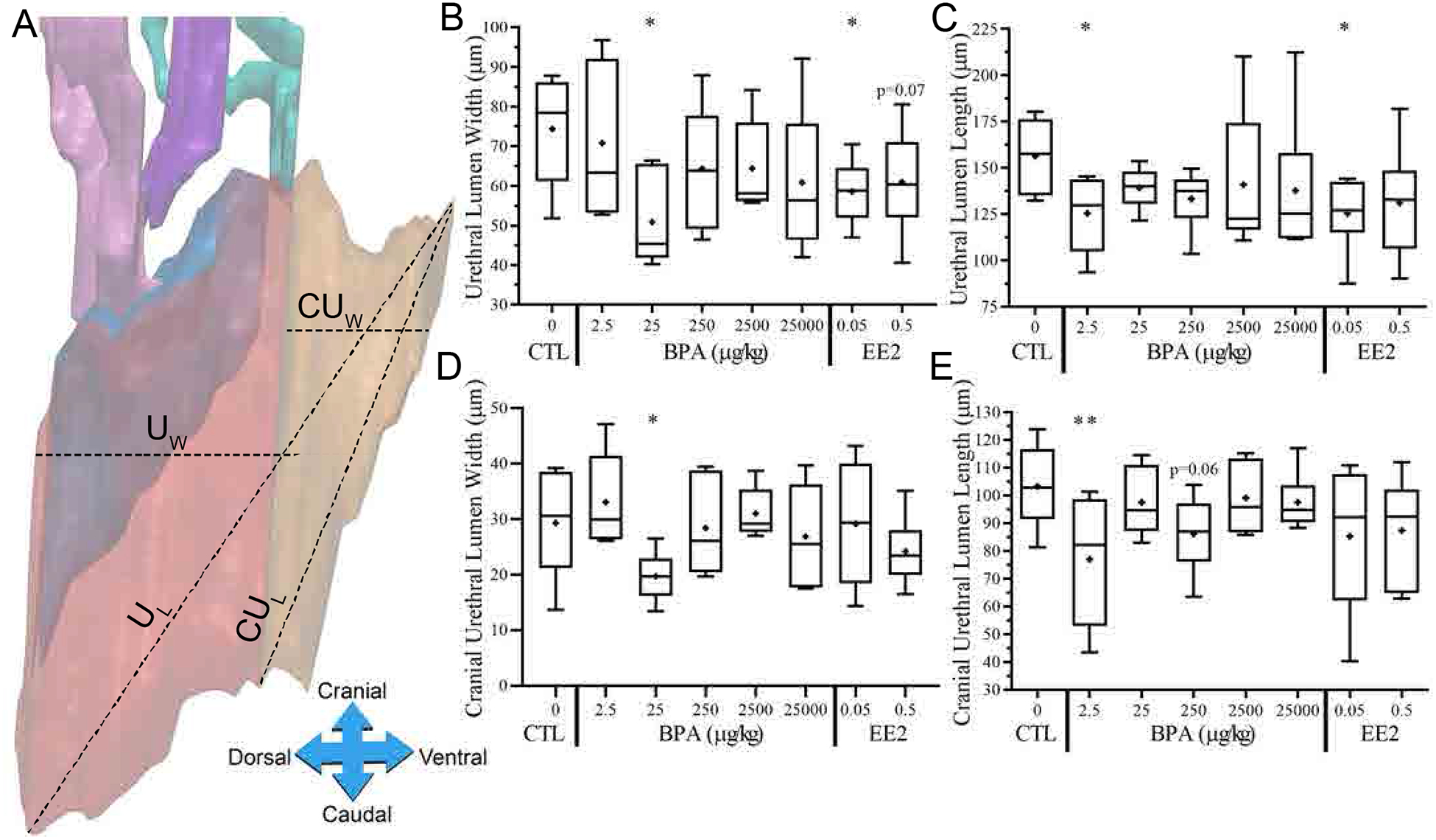
Low dose BPA induces changes in UGS urethral size. The total width of the urethral lumen (UW), which includes the cranial portion, was measured from dorsal to ventral ends (A) and analyzed by treatment (B). The total length of the urethral lumen (UL) was also measured caudally to cranially (A) and analyzed by treatment (C). Cranial portions of the urethral lumens (yellow shaded) were measured for width (CUW) and length (CUL) (A) and analyzed by treatment (D, E).
Urothelium Thickness Overall 3D Profiles.
The thickness of the urothelium based on the widest section (Fig. 7-A) was significantly smaller for the BPA-25, BPA-250 and BPA-2500 groups (p≤0.05, Fig. 7-C) compared to vehicle controls, although urothelium thickness for the widest slice was not significantly affected by either dose of EE2. Urothelium thickness measured on the halfway slice (Fig. 7-B) was also found to be significantly smaller (p≤0.05) for the BPA-250 treated group compared to vehicle controls (Fig. 7D). This finding of a statistically significant difference at this one dose at this point in the urethra will need to be replicated.
Fig. 7.
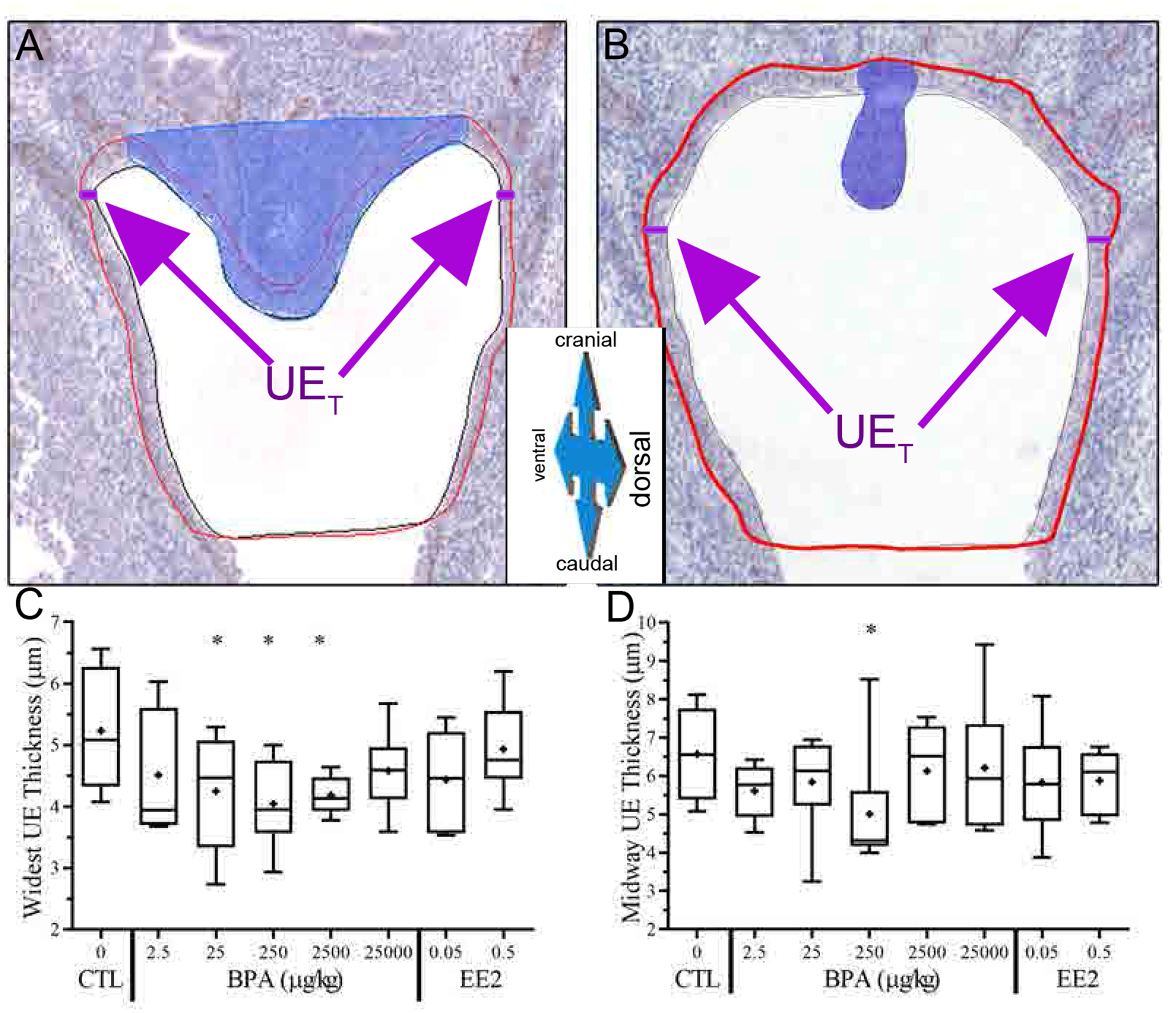
BPA decreases urogenital sinus epithelium thickness. To calculate the urothelium thickness (UET), two distances were measured on both the left and right lateral sides of the urothelium at the widest point of the urethra and averaged (see text for details; A, B). Drawn red line indicates area of urothelium, as defined by tissue positive for p63 protein via IHC. At the widest section (C) and midway section (D), the urothelium thickness was analyzed by treatment.
Colliculus shape
Examples from each treatment group exhibit high variability in urethral shape and size, particularly noticeable in the cranial urethra and colliculus profiles as defined by 3-D reconstruction software (BioVis3D) (Supplemental Fig. 2). This variability is also prominent within treatment groups (data not shown).
Immunohistochemistry
Assessment of all antibodies for estrogen and prostatic pathways yielded staining consistent with the localization of each protein. An example of this staining pattern for each antibody tested is presented in Supplemental Fig. 3–11. Quantitative assessment of all proteins was inconclusive due to the high variability within and between each treatment group.
Discussion
This CLARITY-BPA trial was the first of its kind to enlist collaborations between 3 types of organizations (NIH, FDA, Academia). The study involved blinded sample analyses, and centralized animal/treatment provision by the NCTR. One of the major goals of the CLARITY-BPA program was to determine whether academic investigators would be able to replicate their prior significant findings with low doses of BPA, relevant to exposures experienced by the general population. This means that the investigators had previously published findings using other methods of administration of BPA and other animal models, most known to have a greater sensitivity to estrogenic chemicals and drugs relative to the NCTR CD-SD rat [25, 30].
Increase in Colliculus Size
We found that BPA-2.5, BPA-25, BPA-250 and BPA-25000 altered the morphology of the colliculus (the shape of the colliculus in relation to the urethra referred to as colliculus angle (CAο)) relative to vehicle controls (Fig 5-B), and that BPA-250 significantly increased the size of the colliculus (Fig. 5-D). The colliculus angle effect of BPA was consistent with a significant effect of the EE2–0.05 dose and a tendency (p=0.07) for an effect with the EE2–0.5 dose, suggesting an estrogenic mode of action identified in prior experiments.
Specifically, we previously reported that in CD-1 mice (the model animal used by the National Toxicology Program), as a result of feeding pregnant females a 10-μg/kg/day dose of BPA or a hundred-fold lower dose (0.1 μg/kg/day) of either EE2 or DES, that the colliculus in male fetuses was both enlarged and malformed, consistent with the altered colliculus angle data reported here [4]. In a previous experiment, the remnant of the Mullerian ducts in males, which persists as the utricle in the male prostate, was significantly enlarged in male CD-1 mouse fetuses by maternal exposure to a high (200 μg/kg/day) dose of DES [4]. Our finding of a change in the morphology (angle) of the colliculus at the highest dose tested (the only “high dose” effect that was statistically significant) is consistent with other findings regarding changes in the colliculus in response to a high dose of DES in CD-1 mice [36]. The colliculus region of the prostate is where the ejaculatory ducts enter the urethra, and is also where the merged Mullerian (paramesonephric) ducts end as a blind utricular pouch. This portion of the Mullerian ducts differentiates in female fetuses into the cranial portion of the vagina and is thus highly sensitive to estrogen.
It was recognized decades ago that the size of the utricle and colliculus were highly variable in men. Since the utricle is presumptive vaginal tissue, this variability in utricular and colliculus size was hypothesized to be due to differences in fetal exposure to estrogen [37]. Glenister [38] also identified that the utricle was the region with the most pronounced hyperplasia/metaplasia relative to other regions of the prostate, which is consistent with this tissue being the most sensitive to elevated estrogen from drugs and estrogenic environmental chemicals [4], as well as elevated endogenous estradiol [11, 12].
Reduced Urethral Lumen Size
We also confirmed in the present study (Fig. 6) our prior reports that fetal exposure to elevated levels of estradiol [11, 12], and the estrogenic drugs, DES and EE2, as well as BPA [4], resulted in narrowing of the urethra lumen relative to vehicle controls. In the present experiment, we found a decrease (p≤0.05) in dorsal-ventral width of the urethra (UW, and CUW, BPA-25 group, Fig. 6-B, D) and shortening of the length of the urethra (UL and CUL, BPA-2.5 group, Fig. 6-C, E). The EE2–0.05 dose also reduced the urethral length and width measurement (p≤0.05, Fig 6-B, C), and tended to be smaller for the EE2–0.5 dose (Fig. 6-B, C), adding to the numerous studies indicating that these effects were mediated by an estrogenic mode of action. In addition, we have reported that in adult male mice, estradiol exposure results in obstructive voiding disorder and other urogenital abnormalities [7]; and similar bladder/urethra/prostate and voiding abnormalities occurred due to administration of BPA to adult mice [39]. While our studies have focused on urogenital abnormalities, including prostate hyperplasia, Prins and colleagues have conducted numerous studies relating developmental exposure of BPA to subsequent prostate carcinogenesis, including in NCTR CD-SD rats as part of CLARITY-BPA [21, 40].
Reduction in Urothelium Thickness
A novel finding in the present study, which we had not previously examined, was that there was a decrease in the thickness of the urothelium in the BPA-25, BPA-250 and BPA-2500 groups (Fig. 7-C). However, no significant effects on urothelium thickness were observed for either EE2 dose, suggesting that this effect may not have involved an estrogenic mode of action. Since BPA has been reported to act via multiple mechanisms [10], future research is required to determine the mechanisms mediating this effect.
Increase in Body Weight at Birth
We observed a significant increase in body weight on PND1 in the BPA-2.5, BPA-25 and BPA-250 groups, as well as in response to both doses of EE2, in comparison to the vehicle controls (Fig. 4-B). This finding has to be viewed from the perspective that the NCTR CD-SD rat was subjected to decades of continuous selective breeding for rapid growth rate, increased body weight, and large litter size [30]. Another issue is that in a prior large study examining a wide range of doses of EE2 [41], the NTP did not observe a significant increase in body weight at birth in response to maternal administration of EE2 doses within the range used in the present experiment. However, in this present experiment the EE2, which is orally active, was mixed into the feed rather than administered by intragastric gavage. Thus, an essential variable that differed between the present study and the prior study by the NTP on EE2 was that from GD 6 through the day of parturition, the pregnant females in the present study were picked up, restrained and administered chemicals by having a gavage needle put down their throat. These procedures, especially gavage administration of chemicals, are stressful and may lead to adverse effects, reviewed in [42]. It is also important to note that in the NTP/FDA core CLARITY-BPA portion of this collaborative study, significant differences in body weight based on treatment were not reported, although the core study was conducted on animals from different litters [43, 44].
While most studies involving prenatal exposure to BPA have not reported that body weight at birth is increased, a prior study reported that BPA significantly increased body weight at birth in male Sprague-Dawley rats when administered via drinking water at approximately 70 μg/kg/day, which is within the range of doses that increased body weight in the present experiment [24]. Thus, our finding of increased body weight at birth due to low-dose BPA and EE2 exposure is not an isolated finding. It is important to note that rats at parturition have urogenital tracts that are approximately equivalent to gestation week 17 human fetuses, which is prior to the major growth that occurs in human fetuses in the third trimester [45, 46].
In contrast to the effects of BPA on fetal growth, there is significant evidence that exposure to BPA is a factor in subsequent obesity, type 2 diabetes, and all other aspects of metabolic syndrome [47]. Another issue is that, particularly at high doses, BPA administered by intragastric gavage would likely irritate the stomach/intestinal mucosa, since BPA is a known irritant, which could alter absorption and other aspects of pharmacokinetics [48].
A problem we encountered was higher than expected variability not only in pup body weights (Fig. 4-A), but some groups had greater variability than other groups. This heterogeneity of variance was reflected in the immunohistochemistry experiments (Supplemental Materials, Fig. 3–11), which reduced the likelihood of observing significant effects.
The Finding of Nonmonotonic Dose-Response (NMDR) Relationships
A critical finding herein is that, consistent with many prior studies [reviewed in: [29]], significant effects occurred in response to exposure to low doses of BPA that were within the range of exposure of the general public [49–51]. However, these effects were not observed in response to the highest BPA-25000 dose. This is important because the only suggestion of an effect at the highest BPA-25000 dose was on the colliculus angle. This indicates a change in colliculus morphology (Fig. 5-B), which is consistent with the idea that high dose-DES has a greater inhibitory effect on Mullerian duct regression than does a very low dose of DES [4]. For all other outcomes that we examined, we found significant effects in the low dose range of BPA (2.5, 25 and 250 μg/kg/day), which are considered by the FDA to be BPA doses below the Lowest Observed Adverse Effect Level (LOAEL) [10].
The BPA-2.5 group was found to show the greatest effects of exposure by a number of other academic investigators, as well as in the core study conducted by the NCTR [Reviewed in: [52, 53]]. Chemicals commonly encountered in food, water, air and household products, such as BPA, are not found at mg/kg levels of exposure, with the exception of occupational exposures, which for workers in plants using BPA can lead to BPA levels in their urine 1000-fold higher than the general population [54, 55]. These very high mg/kg/day doses are commonly referred to as toxicologically relevant doses, since they produce internal serum concentrations of BPA that are far higher than are found in biomonitoring studies involving the general public [51, 56]. This finding demonstrates the concept that saturating receptors (e.g. estrogen receptors) with ligand (e.g. estrogenic drugs, BPA) down regulates endogenous receptors and function, and leads to different sets of genes being activated or suppressed [15, 57]. This supports the concept that low (or physiologic) doses are needed to see relevant effects for exposures that are encountered by the general public. Simultaneously, the use of only a few very high doses supports the prediction that BPA is “safe” at low, environmentally relevant exposures [29, 58].
A dose-response curve is nonmonotonic when the slope of the curve changes sign somewhere within the range of doses examined. We have published statistical methods to assess nonmonotonicity [23, 34]. Nonmonotonic dose-responses are very common, almost universal, in the action of endocrine-active hormones and chemicals [29]. To determine the shape of the dose-response curve there should be 5 or more doses examined. NMDR curves have profound implications for the typical use of just a few very high doses to estimate a threshold below which exposures are deemed safe, established using safety factors and the assumption that the dose-response curve is monotonic, which is the current approach in chemical risk assessments in the USA and elsewhere. The magnitude of the error that can occur by ignoring the possibility of a NMDR curve has been discussed [28].
Nonmonnotonic dose-response mechanisms for endocrine disrupting chemicals thus present a unique challenge to the methods used by the FDA to assess BPA safety (for details and additional references see [29]: 1. Low-dose upregulation of receptors and high dose inhibition is referred to as receptor up-regulation and down-regulation. A common misunderstanding is that maximum response occurs only at maximal hormone receptor occupancy (receptor saturation). In actual physiological dose-response, maximal responses occur at low receptor occupancy, while a high concentration of a ligand can suppress responses. 2. Activation of expression of regulated genes can be maximal at low doses, while expression of the regulated gene can be suppressed at high doses. Since entirely different genes are activated at different doses, there is a unique array of genes expressed at each dose. For example, BPA binds to and stimulates transcriptional activity of estrogen receptors at much lower doses than are required for BPA to bind to androgen receptors, whose transcriptional activity is suppressed by BPA, leading to very different effects at low and high doses. The extensive literature supporting this invalidates the rejection by the FDA of findings that are significant at only one or a few doses (which is the case for our findings) that are separated by 10-fold differences in dose, since a 10-fold changes in hormone concentration can lead to dramatic differences (significant up-regulation vs. down-regulation) in gene-expression [15, 28, 57]. 3. Negative feedback systems are critical for maintaining homeostasis, and endogenous neural and pituitary hormone levels are subject to inhibition as a function of increasing dose of exogenous hormonally active chemicals. If the exogenous chemical is a selective estrogen reeceptor modulator (SERM) that does not fully replicate the affinity and actions of the endogenous hormone not being produced (which is the case for BPA), the suppression of endogenous hormones as the concentration of an endocrine disruptor increases can lead to unexpected outcomes. 4. A chemical can induce enzymes that lead to an increase in an endogenous hormone; for example, BPA stimulates aromatase (CYP19A1) expression leading to an increase in intracellular estradiol in prostate mesenchyme [59]. Other enzymes involved in metabolism may be activated or inhibited at different doses, leading to changes in clearance and a reduction in response as dose changes, resulting in a NMDR. Other mechanisms mediating NMDRs and more detailed explanations are described in [29]. Additional reviews about nonmonotonic dose-response curves for endocrine disrupting chemicals and their implications for chemical risk assessments have been published [27, 58], including a review issued as a position paper for the Endocrine Society [60].
While nonmonotonic dose-response relationships are a common feature of hormones, hormonal drugs and endocrine disrupting chemicals such as BPA [29], the FDA has consistently rejected as “not biologically meaningful” low-dose findings that were not observed at high doses, and statistically significant results from CLARITY-BPA have been disregarded [61, 62]. The support of only a “monotonic” dose response is not plausible, because other selective estrogen receptor modulators, such as Tamoxifen, prescribed to millions of patients, is well known to have different effects at low vs. high doses. Tamoxifen at a low dose can stimulate breast cancer cell proliferation (referred to as Tamoxifen flare), while a high dose has the desired therapeutic inhibitory effect on metastatic estrogen receptor positive breast cancer [29]. The lack of consensus in evaluating endocrine disrupting chemicals in a consistent, biologically relevant manner (i.e. expectation of a nonmonotonic dose response), continues to prevent the field from moving forward, ultimately jeopardizing the health of humans and other organisms.
Limitations in CLARITY-BPA
A number of contentious decisions by the FDA in the design, execution and interpretation of the CLARITY-BPA experiment have been discussed in prior publications [25, 52]. One critical issue was the lack of an unhandled, non-gavaged control group. A previous pre-CLARITY study demonstrated oral gavage procedures created significantly different relative to the unhandled control group [63]. Thus, in interpreting CLARITY-BPA results, one needs to consider that the study involved potential stress-BPA interactions with subsequent biochemical, anatomical and behavioral effects.
Conclusions
Analysis of 35 morphometric measurements from 3D reconstructions of the UGS (Supplemental Table 3) revealed several significant or trending changes that were consistent with prior published findings, including changes in colliculus angle and an increase in colliculus size, a decrease in urethral lumen length and width due to low, but not high doses of BPA, similar to EE2. These changes all indicate that the urethra is smaller, associated with changes in colliculus shape, when exposed to low doses of BPA and EE2. We also observed a significant increase in body weight in male pups prenatally exposed to the 2.5, 25 and 250 μg/kg/day BPA doses as well as the two EE2 doses, but not the highest BPA dose. The observed increase in body weight at birth is interesting because NCTR CD-SD rats were selectively bred for rapid growth and susceptibility to obesity, which may be exacerbated by exposure to low doses of BPA and EE2 during fetal life.
Taken together, these data suggest that BPA at lower doses (physiologically relevant), but not the highest dose, mediates its effects on the fetal UGS via estrogenic pathways. A major limitation of our study was the high amount of variation observed within experimental groups. Unfortunately, this variation interfered with our ability to detect differences in protein levels using immunohistochemistry techniques, despite our attempts at optimizing each antibody (Supplemental Table 1).
Supplementary Material
Acknowledgments
Funding was provided by NIEHS grant ES020952 to FvS and WAR.
References
- [1].Hormann AM, vom Saal FS, Nagel SC, Stahlhut RW, Moyer CL, Ellersieck MR, Welshons WV, Toutain PL, Taylor JA, Holding thermal receipt paper and eating food after using hand sanitizer results in high serum bioactive and urine total levels of bisphenol A (BPA), PLoS One 9(10) (2014) e110509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, Huttner K, Hauser R, Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants, Environ Health Perspect 117(4) (2009) 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Gerona RR, Pan J, Zota AR, Schwartz JM, Friesen M, Taylor JA, Hunt PA, Woodruff TJ, Direct measurement of Bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: a cross-sectional study, Environ Health 15 (2016) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Timms BG, Howdeshell KL, Barton L, Bradley S, Richter CA, vom Saal FS, Estrogenic chemicals in plastic and oral contraceptives disrupt development of the mouse prostate and urethra, Proc. Natl. Acad. Sci 102 (2005) 7014–7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ho SM, Tang WY, Belmonte de Frausto J, Prins GS, Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4, Cancer Res 66(11) (2006) 5624–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Nicholson TM, Nguyen JL, Leverson GE, Taylor JA, Vom Saal FS, Wood RW, Ricke WA, The endocrine disruptor Bisphenol-A is implicated in urinary voiding dysfunction in male mice, Am J Physiol Renal Physiol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Nicholson TM, Ricke EA, Marker PC, Miano JM, Mayer RD, Timms BG, vom Saal FS, Wood RW, Ricke WA, Testosterone and 17beta-estradiol induce glandular prostatic growth, bladder outlet obstruction, and voiding dysfunction in male mice, Endocrinology 153(11) (2012) 5556–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].vom Saal FS, Cooke PS, Buchanan DL, Palanza P, Thayer KA, Nagel SC, Parmigiani S, Welshons WV, A physiologically based approach to the study of bisphenol A and other estrogenic chemicals on the size of reproductive organs, daily sperm production, and behavior, Toxicol. Ind. Health 14(1–2) (1998) 239–260. [DOI] [PubMed] [Google Scholar]
- [9].Rochester JR, Bisphenol A and human health: A review of the literature, Reprod Toxicol 42 (2013) 132–55. [DOI] [PubMed] [Google Scholar]
- [10].Vandenberg LN, Ehrlich S, Belcher SM, Ben-Jonathan N, Dolinoy DC, Hugo ES, Hunt PA, Newbold RR, Rubin BS, Saili KS, Soto AM, Wang H-S, vom Saal FS, Low Dose Effects of Bisphenol A: An Integrated Review of In Vitro, Laboratory Animal and Epidemiology Studies, Endocrine Disruption 1:1, e25078(http://www.tandfonline.com/toc/kend20/1/1?nav=tocList) (2013). [Google Scholar]
- [11].vom Saal FS, Timms BG, Montano MM, Palanza P, Thayer KA, Nagel SC, Dhar MD, Ganjam VK, Parmigiani S, Welshons WV, Prostate enlargement in mice due to fetal exposure to low doses of estradiol or diethylstilbestrol and opposite effects at high doses, Proc Nat Acad Sci 94(5) (1997) 20562061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Timms BG, Petersen SL, vom Saal FS, Prostate gland growth during development is stimulated in both male and female rat fetuses by intrauterine proximity to female fetuses, J. Urol 161 (1999) 1694–1701. [PubMed] [Google Scholar]
- [13].Welshons WV, Nagel SC, Thayer KA, Judy BM, Vom Saal FS, Low-dose bioactivity of xenoestrogens in animals: fetal exposure to low doses of methoxychlor and other xenoestrogens increases adult prostate size in mice, Toxicol Ind Health 15(1–2) (1999) 12–25. [DOI] [PubMed] [Google Scholar]
- [14].Thayer KA, Ruhlen RL, Howdeshell KL, Buchanan DL, Cooke PS, Preziosi D, Welshons WV, Haseman J, vom Saal FS, Altered prostate growth and daily sperm production in male mice exposed prenatally to subclinical doses of 17alpha-ethinyl oestradiol, Hum Reprod 16(5) (2001) 988–996. [DOI] [PubMed] [Google Scholar]
- [15].Taylor JA, Richter CA, Suzuki A, Watanabe H, Iguchi T, Coser KR, Shioda T, vom Saal FS, Dose-Related Estrogen Effects on Gene Expression in Fetal Mouse Prostate Mesenchymal Cells, PLoS One 7(10) (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Richter CA, Taylor JA, Ruhlen RR, Welshons WV, vom Saal FS, Estradiol and bisphenol A stimulate androgen receptor and estrogen receptor gene expression in fetal mouse prostate cells, Environ. Health Perspect 115 (2007) 902–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Bhandari RK, Taylor JA, Sommerfeld-Sager J, Tillitt DE, Ricke WA, vom Saal FS, Estrogen receptor 1 expression and methylation of Esr1 promoter in mouse fetal prostate mesenchymal cells induced by gestational exposure to bisphenol A or ethinylestradiol, Environ Epigenet 5(3) (2019) dvz012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Nonneman DJ, Ganjam VK, Welshons WV, Vom Saal FS, Intrauterine position effects on steroid metabolism and steroid receptors of reproductive organs in male mice, Biol Reprod 47(5) (1992) 723–729. [DOI] [PubMed] [Google Scholar]
- [19].Gupta C, Reproductive malformation of the male offspring following maternal exposure to estrogenic chemicals, Proc Soc Exp Biol Med 224(2) (2000) 61–68. [DOI] [PubMed] [Google Scholar]
- [20].Ogura Y, Ishii K, Kanda H, Kanai M, Arima K, Wang Y, Sugimura Y, Bisphenol A induces permanent squamous change in mouse prostatic epithelium, Differentiation; research in biological diversity 75(8) (2007) 745–756. [DOI] [PubMed] [Google Scholar]
- [21].Prins GS, Hu WY, Xie L, Shi GB, Hu DP, Birch L, Bosland MC, Evaluation of Bisphenol A (BPA) Exposures on Prostate Stem Cell Homeostasis and Prostate Cancer Risk in the NCTR-Sprague-Dawley Rat: An NIEHS/FDA CLARITY-BPA Consortium Study, Environ Health Perspect 126(11) (2018) 117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS, Exposure to bisphenol A advances puberty, Nature 401 (1999) 763–764. [DOI] [PubMed] [Google Scholar]
- [23].Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, Welshons WV, Besch-Williford CL, Palanza P, Parmigiani S, Vom Saal FS, Taylor JA, Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation, Reprod Toxicol 42 (2013) 256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Somm E, Schwitzgebel VM, Toulotte A, Cederroth CR, Combescure C, Nef S, Aubert ML, Huppi PS, Perinatal exposure to bisphenol a alters early adipogenesis in the rat, Environ Health Perspect 117(10) (2009) 1549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].vom Saal FS, Flaws in design, execution and interpretation limit CLARITY-BPA’s value for risk assessments of bisphenol A, Basic Clin Pharmacol Toxicol (2019) 1–12. [DOI] [PubMed] [Google Scholar]
- [26].NTP-CERHR, NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. http://ntp.niehs.nih.gov/ntp/ohat/bisphenol/Bisphenol.pdf, National Toxicology Program-Center for the Evaluation of Risks to Human Reproduction, NIH Publication No. 08–5994, September 2008. Accessed OCTOBER 7, 2019, 2008. [Google Scholar]
- [27].Welshons WV, Nagel SC, vom Saal FS, Large effects from small exposures. III. Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure, Endocrinol. 147(6 Suppl) (2006) S56–S69. [DOI] [PubMed] [Google Scholar]
- [28].Welshons WV, Thayer KA, Judy BM, Taylor JA, Curran EM, vom Saal FS, Large effects from small exposures. I. Mechanisms for endocrine-disrupting chemicals with estrogenic activity, Environ Health Perspect 111(8) (2003) 994–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR Jr., Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP, Hormones and endocrine-disrupting chemicals: low-dose effects and nonmonotonic dose responses, Endocr Rev 33(3) (2012) 378–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].vom Saal FS, Hughes C, An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment, Environ. Health Perspect 113 (2005) 926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Myers JP, vom Saal FS, Akingbemi BT, Arizono K, Belcher S, Colborn T, Chahoud I, Crain DA, Farabollini F, Guillette LJ Jr., Hassold T, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Laufer H, Marcus M, McLachlan JA, Nadal A, Oehlmann J, Olea N, Palanza P, Parmigiani S, Rubin BS, Schoenfelder G, Sonnenschein C, Soto AM, Talsness CE, Taylor JA, Vandenberg LN, Vandenbergh JG, Vogel S, Watson CS, Welshons WV, Zoeller RT, Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A, Environ Health Perspect 117(3) (2009) 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schug TT, Heindel JJ, Camacho L, Delclos KB, Howard P, Johnson AF, Aungst J, Keefe D, Newbold R, Walker NJ, Thomas Zoeller R, Bucher JR, A new approach to synergize academic and guideline-compliant research: the CLARITY-BPA research program, Reprod Toxicol 40 (2013) 35–40. [DOI] [PubMed] [Google Scholar]
- [33].Heindel JJ, Newbold RR, Bucher JR, Camacho L, Delclos KB, Lewis SM, Vanlandingham M, Churchwell MI, Twaddle NC, McLellen M, Chidambaram M, Bryant M, Woodling K, Gamboa da Costa G, Ferguson SA, Flaws J, Howard PC, Walker NJ, Zoeller RT, Fostel J, Favaro C, Schug TT, NIEHS/FDA CLARITY-BPA research program update, Reprod Toxicol 58 (2015) 33–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Do RP, Stahlhut RW, Ponzi D, Vom Saal FS, Taylor JA, Non-monotonic dose effects of in utero exposure to di(2-ethylhexyl) phthalate (DEHP) on testicular and serum testosterone and anogenital distance in male mouse fetuses, Reprod Toxicol 34(4) (2012) 614–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Latendresse JR, Bucci TJ, Olson G, Mellick P, Weis CC, Thorn B, Newbold RR, Delclos KB, Genistein and ethinyl estradiol dietary exposure in multigenerational and chronic studies induce similar proliferative lesions in mammary gland of male Sprague-Dawley rats, Reprod Toxicol 28(3) (2009) 342–353. [DOI] [PubMed] [Google Scholar]
- [36].McLachlan JA, Newbold RR, Bullock B, Reproductive tract lesions in male mice exposed prenatally to diethylstilbestrol, Science 190(4218) (1975) 991–2. [DOI] [PubMed] [Google Scholar]
- [37].Zuckerman S, The endocrine control of the prostate, Proc. Royal Soc. Med 29 (1936) 1557–1567. [PMC free article] [PubMed] [Google Scholar]
- [38].Glenister TW, The development of the utricle and of the so-called “middle” or “median” lobe of the human prostate, J. Anat. (Lond) 96 (1962) 443–455. [PMC free article] [PubMed] [Google Scholar]
- [39].Nicholson TM, Nguyen JL, Leverson GE, Taylor JA, Vom Saal FS, Wood RW, Ricke WA, Endocrine disruptor bisphenol A is implicated in urinary voiding dysfunction in male mice, Am J Physiol Renal Physiol 315(5) (2018) F1208–F1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Prins GS, Patisaul HB, Belcher SM, Vandenberg LN, CLARITY-BPA academic laboratory studies identify consistent low-dose Bisphenol A effects on multiple organ systems, Basic Clin Pharmacol Toxicol 125 Suppl 3 (2019) 14–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].NTP, National Toxicology Program,N.T.(2010).Multigenerational reproductive toxicology study of ethinylestradiol (CASNo.57-63-6) in Sprague-Dawley rats. Natl. Toxicol. Program Tech. ReportSer 1–312. https://ntp.niehs.nih.gov/ntp/htdocs/lt_rpts/tr547.pdf. Accessed: August 9, 2018, (2010). [PubMed] [Google Scholar]
- [42].Vandenberg LN, Welshons WV, vom Saal FS, Toutain PL, Myers JP, Should oral gavage be abandoned in toxicity testing of endocrine disruptors?, Environ Health 13(1) (2014) 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].NTP, NTP Research Report on the CLARITY-BPA Core Study: A Perinatal and Chronic Extended-Dose-Range Study of Bisphenol A in Rats National Toxicology Program Research Report 9, September, 2018. Accessed: July 29, 2019., 2018. [PubMed] [Google Scholar]
- [44].N’TP, NTP Research Report on the CLARITY-BPA Core Study: A Perinatal and Chronic Extended-Dose-Range Study of Bisphenol A in Rats National Toxicology Program Research Report 9, September, 2018. Accessed: July 29, 2019., 2018. [PubMed] [Google Scholar]
- [45].Cunha GR, Vezina CM, Isaacson D, Ricke WA, Timms BG, Cao M, Franco O, Baskin LS, Development of the human prostate, Differentiation 103 (2018) 24–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Cunha GR, Sinclair A, Ricke WA, Robboy SJ, Cao M, Baskin LS, Reproductive tract biology: Of mice and men, Differentiation 110 (2019) 49–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, Vandenberg LN, Vom Saal FS, Metabolism disrupting chemicals and metabolic disorders, Reprod Toxicol 68 (2017) 3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].EPA, Integrated Risk Information System (IRIS), U.S. Environmental Protection Agency, Chemical Assessment Summary, Bisphenol A; CASRN 80-05-7. https://www.google.com/search?source=hp&ei=5A2HWouXNMSizwK_srGoBg&q=EPA+reference+dose+for+bisphenol+A+fact+sheet&oq=EPA+reference+dose+for+bisphenol+A+fact+sheet&gs_l=psy-ab.3…961.14892.0.15293.46.44.0.0.0.0.403.4402.35j7j1j0j1.44.0.…0…1.1.64.psy-ab..2.39.3956.0..0j35i39k1j0i131k1j0i131i46k1j46i131k1j0i20i264k1j0i131i20i264k1j0i22i30k1j33i160k1j33i21k1.0.3_j4gsgJuHg. Accessed: March 9, 2018, 1988. [Google Scholar]
- [49].vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ Jr., Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT, Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure, Reprod Toxicol 24(2) (2007) 131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Stahlhut RW, Welshons WV, Swan SH, Bisphenol A data in NHANES suggest longer than expected half-life, substantial nonfood exposure, or both, Environ Health Perspect 117(5) (2009) 784–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G, Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A, Environ Health Perspect 118(8) (2010) 1055–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Prins GS, Patisaul HB, Belcher SM, Vandenberg LN, CLARITY-BPA Academic Laboratory Studies Consistently Identify Low-Dose Bisphenol A Effects on Multiple Organ Systems Basic & clinical pharmacology & toxicology online (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Vandenberg LN, Hunt PA, Gore AC, Endocrine disruptors and the future of toxicology testing - lessons from CLARITY-BPA, Nat Rev Endocrinol 15(6) (2019) 366–374. [DOI] [PubMed] [Google Scholar]
- [54].Hines CJ, Christianson AL, Jackson MV, Ye X, Pretty JR, Arnold JE, Calafat AM, An Evaluation of the Relationship among Urine, Air, and Hand Measures of Exposure to Bisphenol A (BPA) in US Manufacturing Workers, Ann Work Expo Health (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hines CJ, Jackson MV, Deddens JA, Clark JC, Ye X, Christianson AL, Meadows JW, Calafat AM, Urinary Bisphenol A (BPA) Concentrations among Workers in Industries that Manufacture and Use BPA in the USA, Annals of Work Exposures and Health 61(2) (2017) 164–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].vom Saal FS, Welshons WV, Evidence that bisphenol A (BPA) can be accurately measured without contamination in human serum and urine, and that BPA causes numerous hazards from multiple routes of exposure, Mol Cell Endocrinol 398(1–2) (2014) 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Coser KR, Chesnes J, Hur J, Ray S, Isselbacher KJ, Shioda T, Global analysis of ligand sensitivity of estrogen inducible and suppressible genes in MCF7/BUS breast cancer cells by DNA microarray, Proc. Natl. Acad. Sci. U. S. A 100 (2003) 13994–13999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].vom Saal FS, Sheehan DM, Challenging risk assessment, Forum for Applied Research and Public Policy 13 (1998) 11–18. [Google Scholar]
- [59].Arase S, Ishii K, Igarashi K, Aisaki K, Yoshio Y, Matsushima A, Shimohigashi Y, Arima K, Kanno J, Sugimura Y, Endocrine disrupter bisphenol a increases in situ estrogen production in the mouse urogenital sinus, Biol Reprod 84(4) (2011) 734–742. [DOI] [PubMed] [Google Scholar]
- [60].Zoeller RT, Brown TR, Doan LL, Gore AC, Skakkebaek NE, Soto AM, Woodruff TJ, vom Saal FS, Endocrine-disrupting chemicals and public health protection: a statement of principles from the endocrine society, Endocrinology 153(9) (2012) 4097–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].FDA, Statement from Stephen Ostroff M.D., Deputy Commissioner for Foods and Veterinary Medicine, on National Toxicology Program draft report on Bisphenol A, February 23, https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm598100.htm, Accessed: November 6, 2018, 2018.
- [62].FDA, Bisphenol A (BPA): Toxicology and pharmacokinetic data to inform on-going safety assessments. FDA Grand Rounds, Delclos KB, September 13, 2018. https://collaboration.fda.gov/p105t8mt6u1/?launcher=false&fcsContent=true&pbMode=normal, Accessed: September 15, 2018, 2018. [Google Scholar]
- [63].Cao J, Rebuli ME, Rogers J, Todd KL, Leyrer SM, Ferguson SA, Patisaul HB, Prenatal bisphenol A exposure alters sex-specific estrogen receptor expression in the neonatal rat hypothalamus and amygdala, Toxicol Sci 133(1) (2013) 157–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


