Highlights
-
•
Childhood assaultive trauma exposure is linked to less cortical thickness.
-
•
Cortical thickness in prefrontal regions is inversely associated with aggression.
-
•
Prefrontal thickness mediated the link between trauma exposure and aggression.
Keywords: Stress exposure, Cycle of violence, Orbitofrontal cortex, Pericalcarine
Abstract
Although the link between childhood maltreatment and violence perpetration in adulthood (i.e., the “cycle of violence”) is well-documented, the neural mechanisms driving these processes remain relatively unknown. The objectives of this study were to investigate whether cortical thickness in adulthood varies as a function of childhood assaultive trauma exposure and whether such neurobiological markers of early trauma relate to the perpetration of aggression across the lifespan. In a sample of 138 ethnically-diverse men and women, whole-brain analysis of the cortical mantle revealed that individuals with exposure to assaultive trauma before age 13 had less cortical thickness in two clusters that survived multiple comparison correction: a region that peaked in the left lateral orbitofrontal cortex (OFC) and a region peaking in the right pericalcarine cortex. Diminished cortical thickness in the left OFC cluster was, in turn, associated with greater physical aggression, and mediation analysis revealed that reductions in cortical thickness in this left prefrontal region partially accounted for the association between exposure to childhood assaultive trauma and lifetime perpetration of aggression in adulthood. Findings extend previous investigations into the morphological correlates of early assaultive trauma by implicating reductions in cortical thickness as a potential mechanism linking early violence exposure to violence perpetration that extends into adulthood.
1. Introduction
Childhood adversity is consistently linked to a myriad of poor health outcomes and is considered one of the most potent predictors of psychosocial and neurobiological development (Hughes et al., 2016, Oh et al., 2018, Tottenham and Galván, 2016). The mechanisms driving the cascade of poor long-term outcomes resulting from childhood maltreatment, however, remain unclear. Although the psychological problems that manifest following early adversity are well-documented, research on the long-term neurobiological consequences of childhood maltreatment are comparatively understudied. Understanding the neurobiological sequelae of childhood maltreatment is crucial for identifying mechanisms that perpetuate the harmful effects of childhood adversity into adulthood and impair psychosocial functioning (Tyrka et al., 2013). To this end, the first objective of this study is to investigate whether brain structure in adulthood varies as a function of chronic exposure to childhood assaultive trauma.
A robust behavioral consequence of childhood adversity is engagement in aggressive behavior in adolescence and adulthood (Heleniak and McLaughlin, 2019, Wright et al., 2019). Research has demonstrated that individuals with early exposure to adversity, particularly adversity marked by violence, are more likely to perpetrate aggressive behavior against others later in life (Augsburger et al., 2019, Dodge et al., 1990, Maxfield and Widom, 1996). Recent work has sought to identify intermediate mechanisms that drive this “cycle of violence”. The deleterious impact of chronic stress exposure on neural health and development (Lupien et al., 2009, Shields and Slavich, 2017) is a mechanism that has received growing empirical support. Decades of neuroimaging research has documented the impact of childhood trauma on structural alterations in the brain, particularly in subcortical regions (for a recent meta-analysis, see Lim et al., 2014). Consistent with animal models, some studies have demonstrated that trauma exposure early in development is associated with cortical thinning in prefrontal regions that are important for regulating behavior, including the anterior cingulate cortex (ACC) and orbitofrontal cortex (OFC) (Dannlowski et al., 2012, Kelly et al., 2013, McLaughlin et al., 2014). It is posited that one mechanism linking stress to neural degeneration is chronic dysregulation in the hypothalamic-pituitary-adrenal (HPA) axis (Craig, 2007, McCrory et al., 2010), which can lead to neural degeneration in prefrontal glucocorticoid receptor-enriched brain regions (Heim et al., 2002, Lupien et al., 2016, Miller and Sadeh, 2014).
Although links between early stress and neural integrity have been clearly identified, very little research has examined how stress-related neurobiological alterations contribute to the cycle of violence. Gold et al. (2016) found that exposure to childhood physical and sexual abuse among 58 adolescents was related to thinner cortex in several prefrontal and temporal brain regions selected a priori as regions of interest, with only thinner cortex in the bilateral parahippocampal gyrus relating to higher levels of externalizing behavior problems. Another study of 51 adolescents by the same research group replicated the reductions in cortical thickness in prefrontal and temporal regions and extended these findings by showing that thickness in bilateral parahippocampal gyrus mediated the association between childhood abuse and antisocial behavior in adolescence (Busso et al., 2017). Taken together, extant findings provide initial empirical evidence that exposure to assaultive abuse in childhood predicts cortical thinning in prefrontal and temporal brain regions, though research directly linking these abuse-related neurobiological alterations with the perpetration of aggression is lacking.
Other significant gaps exist in the literature on the neurobiological sequelae of assaultive trauma in childhood that limit understanding of the long-term impact of these experiences. First, the vast majority of studies have used adolescent samples, which limits knowledge of how the neurobiological consequences of childhood abuse contribute to psychosocial problems in adulthood. An exception is a study by Corbo et al. (2014) in an adult sample of veterans that found that childhood interpersonal trauma was not associated with overall reductions in cortical thickness, though a trend emerged in posterior cingulate. A second limitation is that no studies have investigated whether childhood maltreatment-linked neural alterations relate specifically to aggression, but rather have focused on externalizing symptoms and antisocial behavior more broadly (e.g., Busso et al., 2017, Gold et al., 2016). Third, prior work has mostly taken an region of interest analytic approach, which limits knowledge of the breath of regions impacted by childhood maltreatment.
This study was designed to address these limitations by examining the long-term neurobiological sequelae of repeated childhood exposure to assaultive trauma. We focused on repeated exposure to assaultive trauma in childhood, because research indicates this type of trauma leads to chronic overactivation of the stress response system (Cross et al., 2017, Tarullo and Gunnar, 2006) and, in turn, has the most harmful effects on the developing cerebral cortex (Cowell et al., 2015, Tottenham and Galván, 2016). Further, exposure to assaultive forms of trauma, as opposed to non-violent experiences (e.g., natural disasters), is uniquely associated with the later perpetration of aggression, consistent with the “cycle of violence” model (Bynion et al., 2018, Dodge et al., 1990). First, we tested the hypothesis that exposure to assaultive traumas in childhood would be linked to thinner cortex in adulthood. Second, we hypothesized that cortical thickness in the brain regions associated with early assaultive trauma would also relate to the perpetration of aggressive behavior. Finally, to test the “cycle to violence” model, we investigated cortical thickness as a mechanism through which childhood exposure to trauma predicted lifetime aggressive behaviors.
2. Methods
2.1. Participants
Participants were 140 ethnically diverse men and women recruited from the community using flyers and online postings. Interested individuals were eligible to participate if they were between the ages of 18–55 and fluent in the English language. Exclusion criteria included evidence of psychosis, an estimated IQ in the Intellectual Disability range, the presence of serious medical or neurological conditions (e.g. epilepsy), history of three or more head injuries with loss of consciousness, and the presence any MRI contraindications (e.g. metal implants, current pregnancy). All participants with complete interview, self-report, and MRI data were included in the analysis. Participants were removed who reported more than one previous head injury (n = 2) to reduce the potential impact of these events on cortical thickness. Thus, final analyses included 138 participants.
The sample was socioeconomically disadvantaged on average, as described in Table 1. For example, the median household income for the past year in the sample was $30,000. The majority of participants also came from communities with high rates of violent and non-violent crime (https://www.neighborhoodscout.com/de/wilmington/crime on 12/2/19) (Wilmington, DE Crime Rates, 2019). These characteristics suggest that the sample is at relatively higher risk for exposure to assaultive trauma than other community samples.
Table 1.
Sample Characteristics (N = 138).
| Age (M/SD) | 31.8/9.33 |
| Female (n, %) | 73/52.9% |
| Race/Ethnicity (n, %) | |
| Black/African-American | 47/34.1% |
| Caucasian/White | 71/51.4% |
| Hispanic | 22/15.9% |
| Currently Employed (n, %) | 88/63.7% |
| Past Year Household Income (M/SD) | $49,494/$48,698 |
2.2. Procedures
Written and oral consent was obtained from all individuals prior to participation. Participants completed a battery of self-report measures, a clinical interview, and a neuroimaging protocol across two study visits. The University Institutional Review Board approved all protocols and procedures (Protocol #’s: 1073423-17, 1361164-1).
2.3. Measures
2.3.1. Cortical thickness
Data were collected using a Siemens 3T Magnetom Prisma scanner with a 64-channel head coil. A T1-weighted multi-echo MPRAGE anatomical scan (resolution = 1 mm3, TR = 2530 ms, TEs = 1.69, 3.55, 5.41, 7.27 ms) was collected, which has the advantage of less distortion and higher contrast than standard MPRAGE sequences, resulting in more reliable cortical models (van der Kouwe et al., 2008). A T2-weighted variable flip-angle turbo spin-echo scan (resolution = 1 mm3, TR = 3200 ms, TE = 564 ms) was collected, which is used in FreeSurfer to better differentiate the gray-matter-dura boundary. The thickness of the cortical mantle at each vertex was estimated using FreeSurfer’s (v6) standard morphometric pipeline (Salat et al., 2004). Quality assurance was conducted using procedures similar to the steps outlined in the Qoala-T manual (Klapwijk et al., 2019). First, both T1 and T2 images were visually inspected for artifacts, motion, etc. Following FreeSurfer’s standard pipeline, at least two trained raters examined the data for errors, including the inclusion of dura or skull after brain extraction or errors in the pial or white matter surfaces. Data were spatially smoothed using a Gaussian kernel of 10-mm full-width at half maximum.
2.3.2. Exposure to assaultive trauma in childhood
The Detailed Trauma Screen from the SCID-5-RV Stressor Related Disorders module (First et al., 2015) was used to assess history of exposure to traumatic events. Approximately half of participants completed this measure via a clinical interview conducted by either a licensed clinical psychologist or trained doctoral students under the supervision of this psychologist (n = 73) and the remainder completed it via a self-report survey (n = 65).
Assaultive trauma was operationalized as direct exposure to threatened or actual physical violence, including assault, robbery, domestic violence, being threatened with a weapon, exposure to an active war zone, or physical or sexual abuse. Non-assaultive events were operationalized as exposure to life threatening danger that did not involve threatened or actual physical violence, such as terrifying medical events, natural disasters, motor vehicle accidents, or death of a loved one (Cisler et al., 2012, Sadeh et al., 2015). Exposure to repeated childhood assaultive trauma was defined as either (i) experiencing at least two types of assaultive traumatic events or (ii) one type of assaultive trauma experienced multiple times, before age 13. Individuals who experienced at least two assaultive traumas in childhood were compared to those with other patterns of trauma exposure (e.g., a single assaultive trauma in childhood or multiple assaultive traumas that occurred only after childhood), because this analytic approach ensures a conservative test of our hypotheses. More specifically, comparing individuals with multiple childhood assaultive exposures to individuals with other patterns and types of trauma exposure should make it harder to detect significant differences in cortical thickness as a function of assaultive trauma exposure if the number of exposures or developmental period of exposure does not play a role in this relationship.
2.3.3. Physical aggression
Physical aggression was assessed using the Risky, Impulsive, and Self-destructive behavior Questionnaire (RISQ; Sadeh and Baskin-Sommers, 2017). The RISQ is a 38-item self-report questionnaire that measures frequency of a range of risky and impulsive behaviors, including aggression. Participants reported how many times they engaged in each behavior in their lifetime and in the last month. Total aggression scores were created by summing five items (e.g., “gotten in a physical fight”, “threatened someone with a weapon, such as a knife or gun”) for past month and lifetime aggression. To reduce positive skewness, responses were categorized into 5 bins that constrained the range of possible responses at the high end of the distribution: 0, 1–10, 11–50, 51–100, >100 times (Sadeh and Baskin-Sommers, 2017) and a rank-based Blom normalization was applied.
2.4. Data analytic plan
We tested for and did not find differences in age [F(1,136) = 0.92, p = 0.34], educational attainment [X2 (5,138) = 9.69, p = 0.08], concussion history [X2 (1,138) = 0.50, p = 0.48], or income [F(1,128) = 3.01, p = 0.08] between individuals assessed via self-report and interview. Sex differences across the two samples did appear [X2 (1,138) = 8.08, p = 0.004], with more women than men in the self-report sample. Sex was controlled for in all subsequent analyses.
FreeSurfer’s QDEC application was used to examine whole-cortex associations between childhood assaultive trauma exposure (1 = childhood assaultive trauma present; 0 = not present) and thickness in the cortical mantle using general linear models. FreeSurfer’s QDEC (Query, Design, Estimate, Contrast) application permits group analysis of morphometry data derived from the FreeSurfer processing stream by performing general linear models. It was used to examine whole-cortex associations between childhood assaultive trauma exposure (1 = repeated childhood assaultive trauma present; 0 = not present) and thickness in the cortical mantle. FreeSurfer’s method for constructing and transforming the cerebral cortex involves a high resolution, surface-based averaging technique that aligns cortical folding patterns. The technical details of these procedures have been described elsewhere (Fischl et al., 2001, Reuter et al., 2012). Cortical thickness was calculated as the distance between the gray/white boundary to the gray/cerebrospinal fluid boundary at each vertex on the tessellated surface (Fischl and Dale, 2000).
To correct for multiple comparisons, we applied a standard correction procedure using the FreeSurfer analysis software. More specifically, cortical thickness results were compared to p-values associated with different cluster sizes and smoothing levels that were derived by FreeSurfer using Gaussian Monte Carlo simulations with 10,000 iterations. This Monte Carlo null-Z simulation approach is an iterative process that uses random image generation, individual voxel probability thresholding, and minimum cluster size thresholding. The frequency count of cluster sizes determines the probability of a false positive detection per image (Hagler et al., 2006). This multiple comparison correction took into account the number of comparisons conducted across both hemispheres using the Monte Carlo simulation with a vertex-wise threshold set at p < 0.01 and a cluster-based threshold of p < 0.05 (Hagler et al., 2006). Explanatory and dependent variables were examined for outliers and nonlinear distributions. Only cortical thickness clusters that survived correction for multiple comparisons and removal of univariate or bivariate outliers were considered significant and presented in the Results section.
Age, sex, education level (1 = <12; 2 = High School; 3 = Associates; 4 = Bachelors; 5 = Masters; 6 = Professional/Doc), body-mass index (BMI), and trauma assessment method (0 = interview, 1 = self-report) were entered as covariates in all analyses, given associations between these variables and trauma exposure, cortical thickness, and aggression in previous research (e.g., Busso et al., 2017, Gold et al., 2016, Miglin et al., 2019). Univariate general linear models were conducted test group differences in physical aggression that occurred in the past month and across the lifespan, controlling for age, sex, education level, BMI, concussion history (1 = concussion history; 0 = no concussion history), and trauma assessment method as covariates. Partial correlations were conducted to assess the links between cortical thickness in the identified cortical regions and past month and lifetime aggression, after accounting for covariates. These analyses were conducted in SPSS v26.
Next, supplementary analyses were conducted to test the specificity of the association between childhood assaultive trauma and cortical thickness in these regions. Specifically, thickness in each cluster was regressed on other trauma profiles to examine whether exposure to non-assaultive trauma in childhood or adult-only assaultive trauma also explained variance in the clusters that were associated with childhood assaultive trauma. We also examined a history of concussion, major depressive disorder, and alcohol use disorder as potential confounds by extracting each cluster and running a hierarchical linear regression analysis with the standard covariates entered in block 1 (age, sex, BMI, education level), additional potential confounds in block 2 (concussions, depression, alcohol use disorder), and childhood assaultive trauma in block 3.
Finally, we tested mediation analyses in Mplus v8.0 (Muthén and Muthén, 2017; ‘model indirect’ procedure, maximum likelihood estimator with standardized estimates) to test cortical thickness as a potential mediator of the association between childhood assaultive trauma and physical aggression. The indirect effect was tested using bias-corrected bootstrapping, with 10,000 iterations (Hayes and Scharkow, 2013). For the mediation analyses, we extracted total cortical thickness from an ROI based on the FreeSurfer Destrieux cortical parcellations that included the entirety of the identified cluster (described in more detail below). The mediation analysis was adjusted for age, sex, BMI, education level, and concussion history. There were no missing data.
3. Results
3.1. Prevalence of childhood adversity and links with aggression
The sample reported a range of lifetime trauma exposure (Min/Max = 0/6 events), with a little over a third of the sample (36.2%, n = 50) meeting criteria for inclusion in the repeated childhood assaultive trauma group. Among individuals with repeated childhood assaultive trauma, the most common forms were physical abuse (78.0%), sexual abuse (34.0%), and witnessing violence (14.0%). Comparatively, 10.2% (n = 9) of the remaining sample reported a history of non-assaultive trauma (e.g., natural disaster, terrifying medical experience) before age 13. This finding indicates that there was some degree of non-violent trauma exposure in the comparison group.
As expected, individuals with childhood assaultive trauma exposure reported more aggressive behaviors in the past month [F(1,130) = 14.80, p =<0.00, R2 = 0.13] and across their lifetime [F(1,130) = 22.51, p < 0.001, R2 = 0.37] than those in the comparison group. Importantly, repeated exposure to non-assaultive traumas prior to the age 13 was not associated with past month [F(1,80) = 0.51, p = 0.48, R2 = 0.05] or lifetime [F(1,80) = 0.55, p = 0.46, R2 = 0.30] physical aggression. No sex differences were observed in exposure to childhood assaultive trauma [X2 (1,138) = 1.59, p = 0.21] or in lifetime aggressive behaviors [F(1,136) = 2.75, p = 0.10, R2 = 0.02].
3.2. Childhood assaultive trauma relates to cortical thickness in adulthood
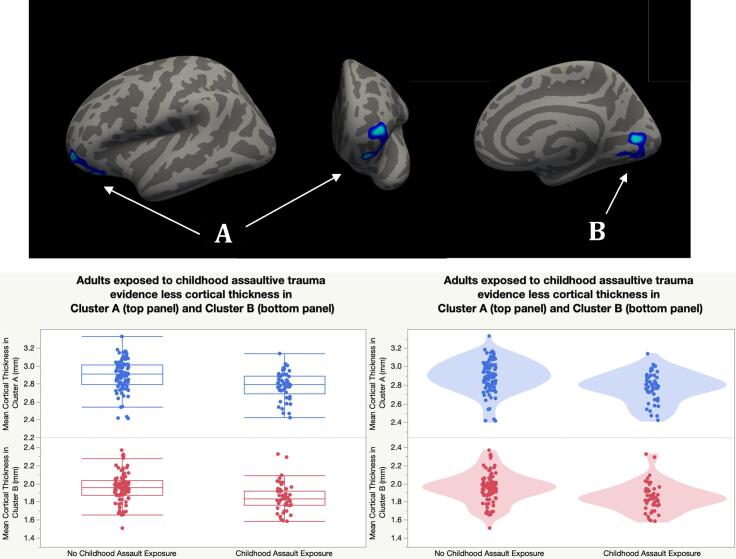
Vertex-wise analysis of the cortical mantle revealed that cortical thickness was related repeated childhood assaultive trauma exposure in two clusters that survived correction for multiple comparisons (see Fig. 1 and Table 2). In the left hemisphere, individuals with childhood assaultive trauma showed less thickness in a cluster that peaked in the lateral orbitofrontal cortex (OFC) and spanned regions of the rostral middle frontal gyrus (MFG) and orbital part of the inferior frontal gyrus (IFG). In the right hemisphere, individuals exposed to childhood assaultive trauma showed thinner cortex in a cluster that peaked in the pericalcarine region and included the lingual gyrus1.
Fig. 1.
Individuals with Exposure to Repeated Childhood Assaultive Trauma Evidence Less Cortical Thickness in Adulthood. Note: (A) left lateral OFC/rMFG/pars orbitalis, (B) right pericalcarine/lingual gyrus. Analyses were adjusted for age, sex, education level, BMI, and assessment method. All clusters survived Monte Carlo Simulation correction for multiple comparisons across both hemispheres (p < 0.05).
Table 2.
Significant cortical thickness clusters associated with repeated childhood assaultive trauma.
| Cluster No. | Cluster | Peak F-Value | Peak MNI (x,y,z) | CWP [90% CI] | No. of Vertices | Cluster Size (mm2) |
|---|---|---|---|---|---|---|
| 1 | LH lateral OFC/rMFG/pars orbitalis | −3.85 | −20.0, 33.5, −13.2 | 0.03 [0.03, 0.03] | 1409 | 898.23 |
| 2 | RH pericalcarine/lingual gyrus | −3.44 | 16.7, −75.6, 9.1 | 0.002 [0.001, 0.002] | 1566 | 1340.74 |
Note: N = 138. Covariates included age, sex, education level, trauma assessment method, and BMI. All clusters survived Monte Carlo Simulation correction for multiple comparisons across both hemispheres (p < 0.05). CWP = clusterwise p-value. 90% CI = 90% confidence interval for CWP. LH = Left Hemisphere. RH = Right Hemisphere. OFC = orbitofrontal cortex. rMFG = rostral middle frontal gyrus.
Supplementary analyses to test the specificity of the association between repeated childhood assaultive trauma and cortical thickness in these regions were conducted next. These analyses revealed that cortical thickness in the clusters were not associated with exposure to non-assaultive trauma in childhood (ps > 0.05) or adult-only assaultive trauma exposure (ps > 0.05).
Next, we examined a history of concussion (25.4% of sample), major depressive disorder (41.3% of sample), and alcohol use disorder (34.1% of sample) to rule these out as potential confounds. Individuals with childhood assaultive trauma continued to evidence less thickness in the left prefrontal and right pericalcarine clusters than individuals in the comparison group with these variables entered in the model (ps < 0.01).
3.3. Thickness clusters associated with childhood assaultive trauma and aggression
We examined whether mean thickness in the clusters identified above was also correlated with aggressive behavior. Consistent with hypotheses, cortical thickness in the left OFC/IFG/rMFG cluster was associated with physical aggression (r = −0.30, p < 0.001), such that less cortical thickness in this cluster was linked to greater aggressive behavior. In contrast, cortical thickness in the right pericalcarine cluster was not associated with aggressive behavior (r = −0.08, p = 0.39).
3.4. Cortical thickness as a mediator between childhood assaultive trauma and aggression
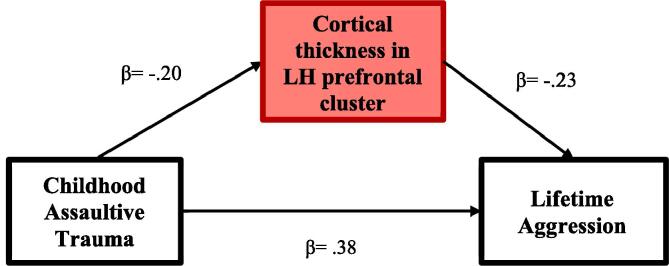
Based on the significant association between the left lateral OFC cluster and aggression, we conducted a mediation analysis to test whether thickness in this region explained the association between childhood assaultive trauma and aggressive behavior. To reduce bias in the mediation analysis, we created an ROI from FreeSurfer Destrieux cortical parcellations that included the entirety of the identified cluster. Specifically, we merged the fronto-marginal gyrus (of Wernicke) and sulcus, orbital gyri, and orbital sulci labels (within each participant’s space) to create a single ROI. Thickness was extracted from this ROI and used in the mediation analysis.
In order to test the “cycle of violence” hypothesis, we used mediation analysis to examine whether cortical thickness in the left frontal region served as an intermediate mechanism linking childhood assaultive trauma and physical aggression.2 The mediation analysis was adjusted for age, sex, BMI, education level, concussion history, and trauma assessment method. Consistent with the cycle of violence model, a significant indirect effect emerged from childhood assaultive trauma to physical aggression via thickness in the identified ROI (β = 0.05, p = 0.04, 95% CI [0.01, 0.09]). The direct effects from childhood assaultive trauma to thickness (β = −0.20, p = 0.01, 95% CI [−0.35, −0.07]) and from thickness to aggression (β = −0.23, p = 0.001, 95% CI [−0.36, −0.11]) were also significant. Finally, the direct path from childhood assaultive trauma to aggression remained significant when cortical thickness in the left prefrontal cluster was entered in the model (β = 0.39, p < 0.001, 95% CI [0.24, 0.51]), suggesting thickness in this region partially mediated these associations. In total, the mediation model explained 24.0% of the variance in cortical thickness in the left prefrontal cluster (p = 0.001) and 24.5% of the variance in lifetime physical aggression (p < 0.001). Standardized results are reported in Fig. 2.
Fig. 2.
Cortical thickness in the left lateral OFC mediates the association between childhood assaultive trauma and physical aggression. Note: Standardized results reported. Models are adjusted for age, sex, education level, BMI, concussion history, and assessment method as covariates. LH = left hemisphere.
4. Discussion
This study sought to identify neurobiological correlates of childhood exposure to assaultive forms of trauma. Furthermore, we tested whether identified neurobiological markers associated with childhood assaultive trauma account for the link between early violence exposure and aggressive tendencies later in life. Whole-brain analysis of the cortical mantle revealed that individuals with repeated exposure to assaultive trauma in childhood had less thickness in a cluster in the left hemisphere that peaked in the lateral OFC and spanned regions of the rostral MFG and the orbital part of IFG. In addition, individuals with childhood assaultive trauma histories also evidenced a thinner cortex in an occipital cluster of the right hemisphere that peaked in the pericalcarine region and included the lingual gyrus. Notably, thickness in a ROI-derived left prefrontal cluster mediated the association between early violence exposure and the perpetration of physical violence across the lifespan. These findings extend previous investigations into the morphological correlates of early assaultive trauma by showing that reductions in cortical thickness associated with these experiences link early exposure to violence with aggression perpetration in adulthood, thus advancing our knowledge of potential neurobiological mechanisms that may perpetuate the cycle of violence.
4.1. Childhood assaultive trauma and cortical thickness
We identified one cluster in the left prefrontal cortex in which less thickness was associated with assaultive trauma in childhood. Adults who experienced assaultive trauma in childhood evidenced thinner cortex in the OFC/IFG/rMFG cluster relative to those who did not. Previous work has found that the cortex in OFC is thinner among individuals who experienced childhood maltreatment (Kelly et al., 2013, McLaughlin et al., 2014), and the present findings extend the literature by demonstrating that similar associations are observable among adult samples. Thinner cortex in this region also replicates previous findings in adult samples with chronic stress exposure (Corbo et al., 2014) and persistent post-traumatic stress symptoms (Franz et al., 2019). In the right hemisphere, individuals with childhood assaultive trauma exposure also had thinner cortex in a cluster found in the occipital cortex. Interestingly, other studies have also reported structural reductions in the occipital cortex among maltreated children (McLaughlin et al., 2014) and adults with a history of childhood abuse (Tomoda et al., 2009). Further, these structural findings are in line with results from functional-based MRI studies Specifically, studies have shown that adults with and without a history of childhood maltreatment evidence divergent activation patterns in both the prefrontal and occipital cortices (Frewen et al., 2017, Seghete et al., 2017, Zhong et al., 2020). Thus, the findings of the present study fit within the greater neuroimaging literature documenting links between childhood adversity and neural adaptations within these cortices.
It is notable that supplementary analyses indicated that our findings appear to be relatively specific to childhood assaultive trauma. Specifically, cortical thickness in these clusters was not related to exposure to non-assaultive trauma in childhood (e.g., natural disasters, serious accidents, life-threatening medical experiences). This specificity for assaultive trauma converges with models suggesting that particularly severe or sustained HPA-axis activation leads to alterations in structures with high concentrations of glucocorticoid receptors, including PFC (McEwen, 2012). Indeed, previous research suggests that childhood assaultive traumas are associated with a greater risk of HPA-axis alterations and future mental health problems than non-assaultive traumas (Cisler et al., 2012, Heim et al., 2002). Our findings coincide with previous work showing structural changes to prefrontal cortical regions as a function of childhood violence exposure using youth samples (e.g., Busso et al., 2017), and extends this work by demonstrating that the effects of early adversity on brain structure persists into adulthood.
Similarly, we found that thickness in the identified clusters did not vary for individuals who first experienced assaultive trauma as an adult, suggesting that the developmental window during which exposure occurs modulates the impact of these events. In particular, childhood may represent a sensitive period during which exposure to assaultive trauma has a more profound impact on the developing cortex, particularly in prefrontal structures that are still maturing and rich in cortisol receptors, and thus more sensitive to cortisol elevations (Tottenham and Galván, 2016). This idea is consistent with proposals that prefrontal systems are particularly vulnerable to the effects of early life stress exposure given that maturational development in these areas extends into early adulthood (Hart and Rubia, 2012, Hanson et al., 2010).
4.2. Diminished cortical thickness and physical aggression
A second aim of the present study was to examine whether the regions identified above, in which thickness was related to childhood assaultive trauma, were also relevant for understanding aggressive behaviors. The motivation for this analysis was to further our understanding of the “cycle of violence” (Maxfield and Widom, 1996) by identifying potential intermediate neurobiological mechanisms linking early violence exposure with perpetration later in life. For example, OFC, IFG, and rostral MFG are heavily implicated in top-down regulatory processes, including emotion regulation, decision-making, and inhibition, that are crucial for regulating aggressive behaviors (Bechara et al., 2000, Blair and Lee, 2013). Although the relevance of occipital cortices for aggression is less clear, emerging evidence has linked activity in the visual cortex to engagement in violent and antisocial behaviors (Bertsch et al., 2013, Charpentier et al., 2016). For example, visual perception is crucial for facial expression processing, which is necessary for developing empathy. In addition, reduced cortical thickness in the right pericalcarine cortex has been linked to associated psychological processes, such as sensation seeking and engagement in risky behaviors (Holmes et al., 2016, Miglin et al., 2019). Thus, visual processing may indirectly impact the likelihood to display aggressive tendencies (Charpentier et al., 2016, Horan et al., 2014). Together, the present results are consistent with literature linking both frontal and occipital cortices to behaviors marked by impulse control and decision-making disruptions.
Consistent with our hypotheses regarding developmental models of violence, cortical thickness in the OFC/IFG/rMFG region linked to childhood assaultive trauma was also associated with lifetime aggression in the expected direction. Furthermore, thinner cortex in this prefrontal region emerged as a significant mediator of the relationship between childhood assaultive trauma and aggression. Thus, our findings indicate that the impact of assaultive trauma in childhood on thickness in regions of the PFC may serve as an intermediate mechanism which increases risk for the perpetration of aggression in adulthood. Although associations between childhood adversity, morphology, and externalizing symptoms have been documented in youth samples (e.g., Gold et al., 2016), the present study provides the first evidence that the neurobiological sequelae of exposure to childhood assaultive trauma relate to aggression that persists into adulthood.
Given the cross-sectional nature of the data, the temporal ordering of the variables in mediation analyses was based on developmental theories on the cycle of violence. Without longitudinal data it is impossible to rule out other possible relationships among the study variables. For example, there may be heritable vulnerabilities that place individuals at heightened risk for both exposure to assaultive trauma and diminished cortical thickness in the regions we identified, which could potentiate aggressive behavior. This would be in line with epigenetic research highlighting the role of shared genetic factors in the cycle of violence phenomenon (Caspi et al., 2002, Craig, 2007). Thus, prospective neuroimaging studies that incorporate genetic factors are needed to clarify the causal relationships between these variables.
4.3. Strengths and limitations
The present study has several strengths, including a diverse representation of various forms of childhood adversity, application of whole-cortex analysis to identify childhood trauma-related cortical alterations, and the examination of the impact of childhood violence exposure on neurobiological health and related psychosocial problems in adulthood. While research with youth samples is useful in demonstrating concurrent associations between childhood adversity, cortical thickness, and externalizing problems, our findings add to the literature by testing whether the neural and behavioral impact of childhood violence exposure persists into adulthood.
Results of this study should be interpreted in light of its methodological limitations. First, exposure to childhood assaultive trauma was retrospectively assessed in a sample of adults which may be prone to retrospective recall bias. However, research examining the validity of retrospective reports of childhood adversity suggests that, although this approach is prone to measurement bias, recall of serious and easily operationalized events can be considered valid and usable in research (Hardt and Rutter, 2004). Further, we used two assessment methods of trauma exposure (self-report vs. interview). Although assessment method was included as a covariate in all analyses, this may be considered a limitation of the study design. Further, this study employed a cross-sectional design, which limits our interpretation related to directionality of effects. However, we conducted supplementary analyses to confirm that the clusters associated with childhood assaultive trauma exposure could not be accounted for by more recent traumatic experiences (adult-only assaultive trauma) or other types of childhood trauma (non-assaultive trauma), which strengthens the interpretation that our findings are specific to childhood assaultive trauma. Nonetheless, the extent to which cross-sectional data can be used to understand temporal mechanisms is limited.
Future studies using longitudinal designs should be used to extend the findings of this study and further test mediation models implicating cortical thickness as an intermediate mechanism linking childhood adversity and aggression. Third, while the current study focused on repeated assaultive traumas that occurred prior to the age of 13, our analyses did not account for the specific timing and extent of chronicity of assaultive traumatic experiences. Recent work has highlighted the importance of timing when examining the effects of chronic stress on the developing cortex and functional connectivity, given the degree of neuroplasticity throughout development (Gee, 2016, Tottenham and Galván, 2016). Moreover, it is well-established that there are sensitive periods of neural development in which the impact of childhood stress exposure may be most detrimental (Andersen et al., 2008, Pechtel et al., 2014, Teicher and Samson, 2016). Thus, more investigation needs to be conducted to better understand how exposure to assaultive trauma within smaller periods of development (e.g. infancy, toddlerhood, middle childhood) impacts psychosocial functioning later in life. Despite these limitations, this study provides new insights into the neurobiological mechanisms that may link early violence exposure with the aggressive behavior later in life – knowledge that is fundamental to mapping the etiological mechanisms perpetuating the cycle of violence.
5. Conclusion
Taken together, results from this study show that chronic exposure to childhood assaultive trauma is linked to less cortical thickness in regions of the occipital and prefrontal cortices in adulthood, a finding that replicated previous work with youth samples. Furthermore, cortical thickness in prefrontal regions (particularly, around the left OFC) may serve as an intermediate mechanism by which childhood trauma confers risk for later aggression, a phenomenon well-documented in the literature (i.e., “cycle of violence”; Dodge et al., 1990, Maxfield and Widom, 1996).
Funding
This research was supported in part by National Institutes of General Medical Sciences [2P20GM103653-06-6527] and Mental Health [1F31MH120936-01A1, 1R01MH116228-01A1, L30 MH117662, L30 MH117623].
CRediT authorship contribution statement
Nadia Bounoua: Conceptualization, Formal analysis, Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration, Funding acquisition. Rickie Miglin: Investigation, Data curation, Writing - original draft, Writing - review & editing, Visualization, Project administration. Jeffrey M. Spielberg: Methodology, Resources, Writing - original draft, Writing - review & editing, Visualization, Supervision, Funding acquisition. Naomi Sadeh: Conceptualization, Methodology, Formal analysis, Investigation, Resources, Data curation, Writing - original draft, Writing - review & editing, Visualization, Supervision, Project administration, Funding acquisition.
Footnotes
To estimate the reproducibility of our findings, we conducted a post-hoc sensitivity analysis using G*Power (Faul et al., 2007) for multiple linear regression analyses with six predictors (consistent with the QDEC analysis). Results indicated the minimum detectable effect size was estimated to range from a Cohen’s d of 0.64 at 80% power to 0.73 at 90% power. Our neuroimaging results produced effect sizes ranging from a Cohen’s d of 0.70 to 0.72. These findings suggest our sample size was sufficient to detect these effects.
A post-hoc Monte-Carlo power analysis for indirect effects was conducted using an application provided by Schoemann, Boulton & Short (2017) (available online: https://schoemanna.shinyapps.io/mc_power_med/). Results showed that, given our sample size of N = 138, power for a one mediator model was 0.60.
References
- Andersen S.L., Tomada A., Vincow E.S., Valente E., Polcari A., Teicher M.H. Preliminary evidence for sensitive periods in the effect of childhood sexual abuse on regional brain development. J. Neuropsychiatry Clin. Neurosci. 2008;20(3):292–301. doi: 10.1176/appi.neuropsych.20.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augsburger M., Basler K., Maercker A. Is there a female cycle of violence after exposure to childhood maltreatment? A meta-analysis. Psychol. Med. 2019;1–11 doi: 10.1017/S0033291719000680. [DOI] [PubMed] [Google Scholar]
- Bechara A., Damasio H., Damasio A.R. Emotion, decision making and the orbitofrontal cortex. Cereb. Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- Bertsch K., Grothe M., Prehn K., Vohs K., Berger C., Hauenstein K., Herpertz S.C. Brain volumes differ between diagnostic groups of violent criminal offenders. Eur. Arch. Psychiatry Clin. Neurosci. 2013;263(7):593–606. doi: 10.1007/s00406-013-0391-6. [DOI] [PubMed] [Google Scholar]
- Blair R.J.R., Lee T.M. The social cognitive neuroscience of aggression, violence, and psychopathy. Soc. Neurosci. 2013;8(2):108–111. doi: 10.1080/17470919.2012.757869. [DOI] [PubMed] [Google Scholar]
- Busso D.S., McLaughlin K.A., Sheridan M.A. Dimensions of adversity, physiological reactivity, and externalizing psychopathology in adolescence: deprivation and threat. Psychosom. Med. 2017;79(2):162. doi: 10.1097/PSY.0000000000000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bynion T.M., Cloutier R., Blumenthal H., Mischel E.R., Rojas S.M., Leen-Feldner E.W. Violent interpersonal trauma predicts aggressive thoughts and behaviors towards self and others: findings from the National Comorbidity Survey-Adolescent Supplement. Soc. Psychiatry Psychiatr. Epidemiol. 2018;53(12):1361–1370. doi: 10.1007/s00127-018-1607-x. [DOI] [PubMed] [Google Scholar]
- Caspi A., McClay J., Moffitt T.E., Mill J., Martin J., Craig I.W., Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297(5582):851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Charpentier J., Dzemidzic M., West J., Oberlin B.G., Eiler W.J., II, Saykin A.J., Kareken D.A. Externalizing personality traits, empathy, and gray matter volume in healthy young drinkers. Psychiatry Research: Neuroimaging. 2016;248:64–72. doi: 10.1016/j.pscychresns.2016.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cisler J.M., Begle A.M., Amstadter A.B., Resnick H.S., Danielson C.K., Saunders B.E., Kilpatrick D.G. Exposure to interpersonal violence and risk for PTSD, depression, delinquency, and binge drinking among adolescents: Data from the NSA-R. J. Trauma. Stress. 2012;25(1):33–40. doi: 10.1002/jts.21672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbo V., Salat D.H., Amick M.M., Leritz E.C., Milberg W.P., McGlinchey R.E. Reduced cortical thickness in veterans exposed to early life trauma. Psychiatry Research: Neuroimaging. 2014;223(2):53–60. doi: 10.1016/j.pscychresns.2014.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowell R.A., Cicchetti D., Rogosch F.A., Toth S.L. Childhood maltreatment and its effect on neurocognitive functioning: timing and chronicity matter. Dev. Psychopathol. 2015;27(2):521–533. doi: 10.1017/S0954579415000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig I.W. The importance of stress and genetic variation in human aggression. BioEssays. 2007;29(3):227–236. doi: 10.1002/bies.20538. [DOI] [PubMed] [Google Scholar]
- Cross D., Fani N., Powers A., Bradley B. Neurobiological development in the context of childhood trauma. Clin. Psychol.: Sci. Practice. 2017;24(2):111–124. doi: 10.1111/cpsp.12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U., Stuhrmann A., Beutelmann V., Zwanzger P., Lenzen T., Grotegerd D., Lindner C. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol. Psychiatry. 2012;71(4):286–293. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- Dodge K.A., Bates J.E., Pettit G.S. Mechanisms in the cycle of violence. Science. 1990;250(4988):1678–1683. doi: 10.1126/science.2270481. [DOI] [PubMed] [Google Scholar]
- First, M.B., Williams, J.B.W., Karg, R.S., Spitzer, R.L., 2015. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association, 1–94.
- Fischl B., Dale A.M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc. Natl. Acad. Sci. 2000;97(20):11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B., Liu A., Dale A.M. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans. Med. Imaging. 2001;20(1):70–80. doi: 10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Franz C.E., Hatton S.N., Hauger R.L., Kredlow M.A., Dale A.M., Eyler L., McKenzie R.E. Posttraumatic stress symptom persistence across 24 years: association with brain structures. Brain Imaging Behav. 2019:1–13. doi: 10.1007/s11682-019-00059-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frewen P., Thornley E., Rabellino D., Lanius R. Neuroimaging the traumatized self: fMRI reveals altered response in cortical midline structures and occipital cortex during visual and verbal self-and other-referential processing in women with PTSD. Eur. J. Psychotraumatol. 2017;8(1):1314164. doi: 10.1080/20008198.2017.1314164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee, D.G., 2016. Sensitive periods of emotion regulation: Influences of parental care on frontoamygdala circuitry and plasticity. In H.J.V. Rutherford, L.C. Mayes (Eds.), Maternal brain plasticity: Preclinical and human research and implications for intervention. New Directions for Child and Adolescent Development, 153, pp. 87–110. [DOI] [PubMed]
- Gold A.L., Sheridan M.A., Peverill M., Busso D.S., Lambert H.K., Alves S., McLaughlin K.A. Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. J. Child Psychol. Psychiatry. 2016;57(10):1154–1164. doi: 10.1111/jcpp.12630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler D.J., Jr, Saygin A.P., Sereno M.I. Smoothing and cluster thresholding for cortical surface-based group analysis of fMRI data. Neuroimage. 2006;33(4):1093–1103. doi: 10.1016/j.neuroimage.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R.F., Sawyer G.K., Begle A.M., Hubel G.S. The impact of crime victimization on quality of life. J. Traumatic Stress. 2010;23(2):189–197. doi: 10.1002/jts.20508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J., Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J. Child Psychol. Psychiatry. 2004;45(2):260–273. doi: 10.1111/j.1469-7610.2004.00218.x. [DOI] [PubMed] [Google Scholar]
- Hart H., Rubia K. Neuroimaging of child abuse: a critical review. Front. Hum. Neurosci. 2012;6:52. doi: 10.3389/fnhum.2012.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A.F., Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: does method really matter? Psychol. Sci. 2013;24(10):1918–1927. doi: 10.1177/0956797613480187. [DOI] [PubMed] [Google Scholar]
- Heim C., Newport D.J., Wagner D., Wilcox M.M., Miller A.H., Nemeroff C.B. The role of early adverse experience and adulthood stress in the prediction of neuroendocrine stress reactivity in women: a multiple regression analysis. Depression Anxiety. 2002;15(3):117–125. doi: 10.1002/da.10015. [DOI] [PubMed] [Google Scholar]
- Heleniak C., McLaughlin K.A. Social-cognitive mechanisms in the cycle of violence: cognitive and affective theory of mind, and externalizing psychopathology in children and adolescents. Dev. Psychopathol. 2019;1–16 doi: 10.1017/S0954579419000725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A.J., Hollinshead M.O., Roffman J.L., Smoller J.W., Buckner R.L. Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. J. Neurosci. 2016;36(14):4038–4049. doi: 10.1523/JNEUROSCI.3206-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Iacoboni M., Cross K.A., Korb A., Lee J., Nori P., Green M.F. Self-reported empathy and neural activity during action imitation and observation in schizophrenia. NeuroImage: Clinical. 2014;5:100–108. doi: 10.1016/j.nicl.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes K., Hardcastle K., Bellis M.A. The impact of adverse childhood experiences on health: a systematic review and meta-analysis. Injury Prevention. 2016;22(Suppl. 2):105. [Google Scholar]
- Kelly P.A., Viding E., Wallace G.L., Schaer M., De Brito S.A., Robustelli B., McCrory E.J. Cortical thickness, surface area, and gyrification abnormalities in children exposed to maltreatment: neural markers of vulnerability? Biol. Psychiatry. 2013;74(11):845–852. doi: 10.1016/j.biopsych.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Klapwijk E.T., Van De Kamp F., Van Der Meulen M., Peters S., Wierenga L.M. Qoala-T: a supervised-learning tool for quality control of FreeSurfer segmented MRI data. Neuroimage. 2019;189:116–129. doi: 10.1016/j.neuroimage.2019.01.014. [DOI] [PubMed] [Google Scholar]
- Lim L., Radua J., Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am. J. Psychiatry. 2014;171(8):854–863. doi: 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- Lupien, S.J., Ouellet-Morin, I., Herba, C.M., Juster, R., McEwen, B.S., 2016. From vulnerability to neurotoxicity: a developmental approach to the effects of stress on the brain and behavior. In Epigenetics and neuroendocrinology, Springer, Cham, pp. 3–48. https://doi.org/10.1007/978-3-319-24493-8_1.
- Lupien S.J., McEwen B.S., Gunnar M.R., Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci. 2009;10(6):434. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- Maxfield M.G., Widom C.S. The cycle of violence: Revisited 6 years later. Arch. Pediatr. Adolesc. Med. 1996;150(4):390–395. doi: 10.1001/archpedi.1996.02170290056009. [DOI] [PubMed] [Google Scholar]
- McCrory E., De Brito S.A., Viding E. Research review: the neurobiology and genetics of maltreatment and adversity. J. Child Psychol. Psychiatry. 2010;51(10):1079–1095. doi: 10.1111/j.1469-7610.2010.02271.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Brain on stress: how the social environment gets under the skin. Proc. Natl. Acad. Sci. 2012;109(Suppl. 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin K.A., Sheridan M.A., Winter W., Fox N.A., Zeanah C.H., Nelson C.A. Widespread reductions in cortical thickness following severe early-life deprivation: a neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biol. Psychiatry. 2014;76(8):629–638. doi: 10.1016/j.biopsych.2013.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglin R., Bounoua N., Goodling S., Sheehan A., Spielberg J.M., Sadeh N. Cortical thickness links impulsive personality traits and risky behavior. Brain Sci. 2019;9(12):373. doi: 10.3390/brainsci9120373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.W., Sadeh N. Traumatic stress, oxidative stress and post-traumatic stress disorder: neurodegeneration and the accelerated-aging hypothesis. Mol. Psychiatry. 2014;19(11):1156. doi: 10.1038/mp.2014.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén, L.K., Muthén, B.O., 2017. Mplus, Version 8; Muthen & Muthen: Los Angeles, CA, USA, 2017. Google Scholar.
- Oh D.L., Jerman P., Marques S.S., Koita K., Boparai S.K.P., Harris N.B., Bucci M. Systematic review of pediatric health outcomes associated with childhood adversity. BMC Pediatrics. 2018;18(1):83. doi: 10.1186/s12887-018-1037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P., Lyons-Ruth K., Anderson C.M., Teicher M.H. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage. 2014;97:236–244. doi: 10.1016/j.neuroimage.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter M., Schmansky N.J., Rosas H.D., Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402–1418. doi: 10.1016/j.neuroimage.2012.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadeh N., Baskin-Sommers A. Risky, impulsive, and self-destructive behavior questionnaire (RISQ): a validation study. Assessment. 2017;24(8):1080–1094. doi: 10.1177/1073191116640356. [DOI] [PubMed] [Google Scholar]
- Sadeh N., Miller M.W., Wolf E.J., Harkness K.L. Negative emotionality and disconstraint influence PTSD symptom course via exposure to new major adverse life events. J. Anxiety Disord. 2015;31:20–27. doi: 10.1016/j.janxdis.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat D.H., Buckner R.L., Snyder A.Z., Greve D.N., Desikan R.S., Busa E., Fischl B. Thinning of the cerebral cortex in aging. Cereb. Cortex. 2004;14(7):721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Seghete K.L.M., Kaiser R.H., DePrince A.P., Banich M.T. General and emotion-specific alterations to cognitive control in women with a history of childhood abuse. Neuroimage: Clinical. 2017;16:151–164. doi: 10.1016/j.nicl.2017.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields, G.S., Slavich, G.M., 2017. Lifetime stress exposure and health: a review of contemporary assessment methods and biological mechanisms. Social Personality Pychology Compass, 11(8). https://doi.org/10.1111/spc3.12335. [DOI] [PMC free article] [PubMed]
- Tarullo A.R., Gunnar M.R. Child maltreatment and the developing HPA axis. Horm. Behav. 2006;50(4):632–639. doi: 10.1016/j.yhbeh.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Teicher M.H., Samson J.A. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J. Child Psychol. Psychiatry. 2016;57(3):241–266. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomoda A., Navalta C.P., Polcari A., Sadato N., Teicher M.H. Childhood sexual abuse is associated with reduced gray matter volume in visual cortex of young women. Biol. Psychiatry. 2009;66(7):642–648. doi: 10.1016/j.biopsych.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N., Galván A. Stress and the adolescent brain: amygdala-prefrontal cortex circuitry and ventral striatum as developmental targets. Neurosci. Biobehav. Rev. 2016;70:217–227. doi: 10.1016/j.neubiorev.2016.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka A.R., Burgers D.E., Philip N.S., Price L.H., Carpenter L.L. The neurobiological correlates of childhood adversity and implications for treatment. Acta Psychiatr. Scand. 2013;128(6):434–447. doi: 10.1111/acps.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kouwe A.J., Benner T., Salat D.H., Fischl B. Brain morphometry with multiecho MPRAGE. Neuroimage. 2008;40(2):559–569. doi: 10.1016/j.neuroimage.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmington, DE Crime Rates. Available online: (accessed on 2 December 2019).
- Wright N., Hill J., Pickles A., Sharp H. Callous-unemotional traits, low cortisol reactivity and physical aggression in children: findings from the Wirral Child Health and Development Study. Transl. Psychiatry. 2019;9(1):79. doi: 10.1038/s41398-019-0406-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X., Ming Q., Dong D., Sun X., Cheng C., Xiong G., Yao S. Childhood maltreatment experience influences neural response to psychosocial stress in adults: an fMRI study. Front. Psychol. 2020;10:2961. doi: 10.3389/fpsyg.2019.02961. [DOI] [PMC free article] [PubMed] [Google Scholar]