Abstract
Antibiotic resistance due to transferable resistance genes is one of the most important concerns in Klebsiella pneumoniae isolated from nosocomial infections. Eighty-eight K. pneumoniae isolates were confirmed through biochemical methods. In addition, antimicrobial susceptibility testing was performed using a disc-diffusion method. Extended-spectrum β-lactamase production among the isolates was screened using a double-disc synergism test, and the resistance genes were identified using PCR. The eight loci for multiple-locus variable number tandem repeat analysis (MLVA) genotyping were selected along with the primers. According to our findings, neomycin (5; 5.6%) and carbapenems (10; 11.3%) showed the most remarkable inhibitory effect but co-trimoxazole (46; 52.2%) was the least effective antibiotic against K. pneumoniae isolates. blaCTX-M-1, qnrA, qnrB, qnrS, intI, intII, aac3 and aac6 were detected in 30 (34%), 5 (5.6%), 29 (32.9%), 23 (26.1%), 88 (100%), 72 (81.8%), 26 (29.5%) and 28 (31.8%) of the 88 isolates, respectively. But none of the K. pneumoniae isolates expressed the intIII gene. Using MLVA, 23 MLVA types and eight clusters were identified. Extended-spectrum β-lactamase-producing K. pneumoniae isolates were classified into two clonal complexes. Effective strategies for infection control should be applied to monitor and control the spread of multidrug-resistant isolates by the resistance genes located on the mobile genetic elements.
Key words: Antibiotics resistance, carbapenem, ESBLs, K. pnemoniae, MLVA, multidrug-resistant, nosocomial infections
Introduction
Klebsiella species form a group of Gram-negative bacilli that are highly prevalent and can be found in different areas, including the human body and the environment. Klebsiella pneumoniae is one of the most important bacteria and is known as the third major cause of hospital-acquired infections, meningitis, pneumonia, bacteraemia, wound infections and urinary tract infections [[1], [2], [3], [4]]. Klebsiella pneumoniae producing extended-spectrum β-lactamases (ESBLs) show resistance to most antibiotics [5,6]. One member of the Class A ESBLs, the CTX-M, also showed a high-level resistance to cefotaxime, ceftriaxone, cefepime and cefpirome. CTX-M-type enzymes have been among widespread variants of the ESBL group; however, the incidence of ESBL TEM and SHV derivatives remained unchanged [7,8]. The ESBL-producing strains develop resistance to other antibiotics such as aminoglycosides, tetracyclines and sulfonamides, whereas bacteria that produce the CTX-M exhibit co-resistance to quinolones as well [8,9]. Quinolones enter bacteria directly through the cytoplasmic membrane and target type II topoisomerases, DNA gyrase and topoisomerase IV, and disrupt DNA replication [10]. QnrA, a plasmid-encoded quinolone resistance protein, is a member of the pentapeptide repeat protein family that protects topoisomerase IV and gyrase from quinolone binding [[11], [12], [13]]. The prevalence of ESBL- and Qnr-positive strains has been reported to be 4%–48% in different studies [[14], [15], [16]]. Plasmids that carry a qnrA determinant also transport other antibiotic resistance genes such as bla genes (β-lactamases) and aac (encodes an acetyltransferase) [10,17]. ACCs (Aminocyclopropane-1-Carboxylate Synthase) are a group of the most common aminoglycoside-modifying enzymes in Gram-negative bacteria, particularly K. pneumoniae; they are subdivided into four groups containing AAC(1), AAC(2ʹ), AAC(3) and ACC(6ʹ) [18]. Although integrons are not mobile genetic elements they can be transmitted by transposons, plasmids and chromosomes, and easily spread among Gram-negative bacteria. The role of integrons in the development of multidrug-resistant (MDR) strains is the issue that causes concern about the presence of integrons in bacteria [19, 20]. The emergence of MDR and extensively drug-resistant (XDR) bacteria, particularly K. pneumoniae, is of serious concern in the development of new therapies for bacterial infections. This study is designed to discover the patterns of resistance against commonly used antibiotics in different lineages of K. pneumoniae clinical isolates with special attention to fluoroquinolones, carbapenems, β-lactams and aminoglycosides.
Materials and methods
Bacterial isolates
A total of 88 non-duplicate clinical isolates of K. pneumoniae were selected from urine, blood and sputum samples that were collected from five Tehran hospitals in Iran, over a period of 1 year (2017–2018). Samples were taken from both inpatients and outpatients who were hospitalized in the infectious and burns wards. The following standard characteristics of clinical K. pneumoniae were used to identify all isolates by biochemical methods [21].
Antimicrobial susceptibility test
An antibiotic susceptibility test was carried out using the disc-diffusion method according to the Clinical and Laboratory Standard Institute guideline (CLSI) and the Committee on Antimicrobial Susceptibility Testing (there is no standard for neomycin in Enterobacteriaceae in the CLSI) [22]. All K. pneumoniae isolates were tested for their susceptibility profiles against the antimicrobial agents shown in Table 1. The prevalence of MDR and XDR in these isolates was also investigated. MDR was defined as acquired resistance to at least one agent in three or more antimicrobial categories. XDR was defined as bacterial isolates remain susceptible to only one or two antimicrobial categories [23,24].
Table 1.
Antibiotic resistance patterns of Klebsiella pneumoniae clinical isolates from five hospitals
| Antibiotics | Total samples |
ESBL-positive (30 isolates) | ESBL-negative (58 isolates) | p value∗ | ||
|---|---|---|---|---|---|---|
| Resistance | Intermediate | Sensitive | ||||
| Amoxicillin/clavulanic acid | 26 (29.5%) | 8 (9.0%) | 54 (61.3%) | 12 (40.0%) | 14 (24.1%) | 0.02 |
| Ceftazidime | 34 (38.6%) | 2 (2.2%) | 52 (59.0%) | 23 (76.6%) | 11 (18.9%) | < 0.001 |
| Cefotaxime | 37 (42.0%) | 1 (1.1%) | 50 (56.8%) | 22 (73.3%) | 15 (25.8%) | < 0.001 |
| Cefepime | 22 (25.0%) | 1 (1.1%) | 65 (73.8%) | 14 (46.6%) | 8 (13.7%) | < 0.001 |
| Ceftriaxone | 40 (45.4%) | 4 (4.5%) | 44 (50.0%) | 22 (73.3%) | 18 (31.0%) | < 0.001 |
| Aztreonam | 38 (43.1%) | 2 (2.2%) | 48 (54.5%) | 21 (70.0%) | 17 (29.3%) | < 0.001 |
| Ertapenem | 11 (12.5%) | 5 (5.6%) | 72 (81.8%) | 4 (13.3%) | 7 (12.0%) | 0.10 |
| Imipenem | 10 (11.3%) | 3 (3.4%) | 75 (85.2%) | 6 (20.0%) | 4 (6.8%) | 0.01 |
| Norfloxacin | 27 (30.6%) | 1 (1.1%) | 60 (68.1%) | 10 (33.3%) | 17 (29.3%) | 0.11 |
| Levofloxacin | 19 (21.5%) | 1 (1.1%) | 68 (77.2%) | 7 (23.3%) | 12 (20.6%) | 0.13 |
| Nalidixic acid | 33 (37.5%) | 11 (12.5%) | 44 (50.0%) | 13 (43.3%) | 20 (34.4%) | 0.06 |
| Ofloxacin | 23 (26.1%) | 5 (5.6%) | 60 (68.1%) | 9 (30.0%) | 14 (24.1%) | 0.06 |
| Ciprofloxacin | 23 (26.1%) | 1 (1.1%) | 64 (72.7%) | 9 (30.0%) | 14 (24.1%) | 0.05 |
| Amikacin | 18 (20.4%) | 2 (2.2%) | 68 (77.2%) | 13 (43.3%) | 5 (8.6%) | < 0.001 |
| Tetracycline | 25 (28.4%) | 1 (1.1%) | 62 (70.4%) | 9 (30.0%) | 16 (27.5%) | 0.12 |
| Gentamicin | 24 (27.2%) | 2 (2.2%) | 62 (70.4%) | 16 (53.3%) | 8 (13.7%) | < 0.001 |
| Tobramycin | 24 (27.2%) | 2 (2.2%) | 62 (70.4%) | 16 (53.3%) | 8 (13.7%) | < 0.001 |
| Streptomycin | 38 (43.1%) | 3 (3.4%) | 47 (53.4%) | 20 (66.6%) | 18 (31.0%) | < 0.001 |
| Kanamycin | 29 (32.9%) | 4 (4.5%) | 55 (62.5%) | 15 (50.0%) | 14 (24.1%) | < 0.001 |
| Neomycin | 5 (5.6%) | 5 (5.6%) | 78 (88.6%) | 2 (6.6%) | 3 (5.1%) | 0.15 |
| Co-trimoxazole | 46 (52.2%) | 0 (0.0%) | 42 (47.7%) | 18 (60.0%) | 28 (48.2%) | 0.10 |
Abbreviation: ESBL, extended-spectrum β-lactamase.
Value of p is for a comparison of resistance among ESBL-producers with that among non-producers.
Screening of ESBL phenotype
The phenotypic confirmatory test was performed using cefotaxime-clavulanic acid or ceftazidime-clavulanic acid as a two-disc synergism versus ceftazidime or cefotaxime alone. If the inhibition zone around the synergism discs was ≥5 mm compared with ceftazidime or cefotaxime alone, then the isolate was found to be ESBL-producing. Klebsiella pneumoniae ATCC 700603 and Escherichia coli ATCC 25922 were used as positive and negative controls, respectively [21].
Detection of resistance genes
Antibiotic resistance genes and integrons (Table 2) were detected using standard PCR amplification. To extract DNA, the DNA extraction kit (GeNet Bio Company, Daejeon, Korea; Cat. No, K-3000) was used according to the manufacturer's guidelines. PCR were carried out in a final volume of 25 μL by using 3 μL DNA solution, 12.5 μL of Master Mix (Qiagen HotStar Taq polymerase), 1 μL of 10 pmol of each primer and 7.5 μL of distilled water. Amplification reactions were performed on a thermal cycler (model) with the following PCR conditions: a denaturation step at 94°C for 5 min, followed by 30 cycles of denaturation at 94°C for 1 min, primer annealing at 51°C to 60°C for 40 s, and final extension step at 72°C for 5 min. Eventually, PCR products were detected using electrophoresis on a 1% agarose gel, visualized by safe stain and illuminated by UV light.
Table 2.
Primers for cephalosporin-, aminoglycoside- and fluoroquinolone-resistance genes
| Genes | Primer sequence | Product size (bp) | Annealing temperature | References |
|---|---|---|---|---|
| blaCTX-M-1 | F: GACTATTCATGTTGTTGTTATTTC R: TTACAAACCGTTGGTGACG |
923 | 58 | [26] |
| aac(3)-II-cr | F: ATATCGCGATGCATACGCGG R: GACGGCCTCTAACCGGAAGG |
877 | 55 | [27] |
| aac(6′)-Ib | F:TTGCGATGCTCTATGAGTGGCTA R: CTCGAATGCCTGGCGTGTTT |
472 | 55 | [27] |
| qnrA | F:GATCGGCAAAGGTTAGGTCA R:ATTTCTCACGCCAGGATTTG |
627 | 53 | [21] |
| qnrB | F:GATCGTGAAAGCCAGAAAGG R:ACGATGCCTGGTAGTTGTCC |
562 | 53 | [21] |
| qnrS | F:AGTGATCTCACCTTCACCGC R: CAGGCTGCAATTTTGATACC |
550 | 53 | [21] |
| intI | F:CAGTGGACATAAGCCTGTTC R:CCCGAGGCATAGACTGTA |
160 | 55 | [21] |
| intII | F:TTGCGAGTATCCTAACCTG R:TTACCTGCACTGGATTAAGC |
789 | 55 | [21] |
| intIII | F: AGTGGGTGGCGAATGAGTG R: TGTTCTTGTATCGGCAGGTG |
600 | 60 | [19] |
Multiple-locus variable number tandem repeat analysis (MLVA) assay
For typing of all ESBL-producing K. pneumoniae isolates the MLVA, using loci A, D, E, H, I, J, K and L was used as defined previously [25]. The number of repeats can be easily conducted from the PCR product sizes by manual reading. To convert the product sizes into repeat numbers, a principle defined in a previous study was applied [25]. A dendrogram of genetic relationships was also produced using the unweighted pair group method with arithmetic averages (UPGMA) based on allelic profiles. In addition, the minimum spanning tree was created with a categorical ratio based on the allelic profiles of the K. pneumoniae isolates. Simpson's index of diversity was used to calculate the diversity of ESBL-producing isolates. This index has a range between 0 and 1, where scores of 1 or near to 1 indicate high diversities and 0 or close to 0 indicate low diversities.
Statistical analysis
The collected data were entered into a database using SPSS version 22 for K. pneumoniae isolates. The interpretation of the results was based on frequencies. Carmer's V test was exerted to investigate the relationship between age of patients and different antibiotic resistance rates. A value of p < 0.05 was considered statistically significant for association between all antibiotics, resistance genes and clinical isolates by the chi-square test.
Results
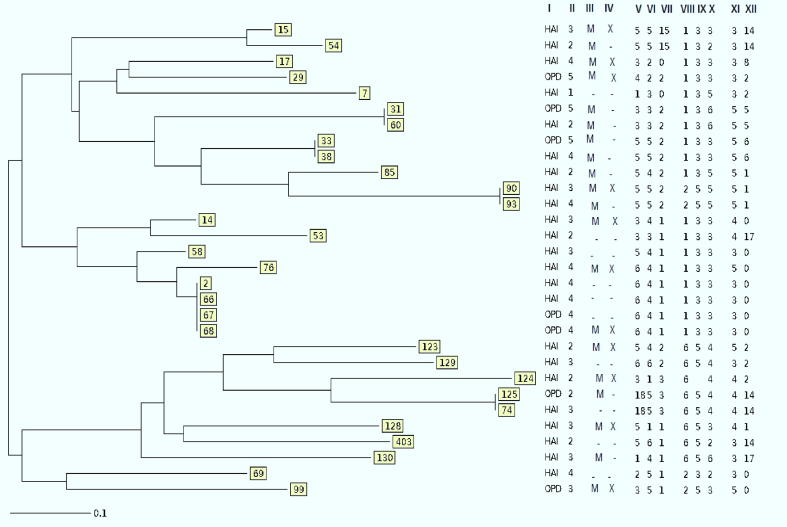
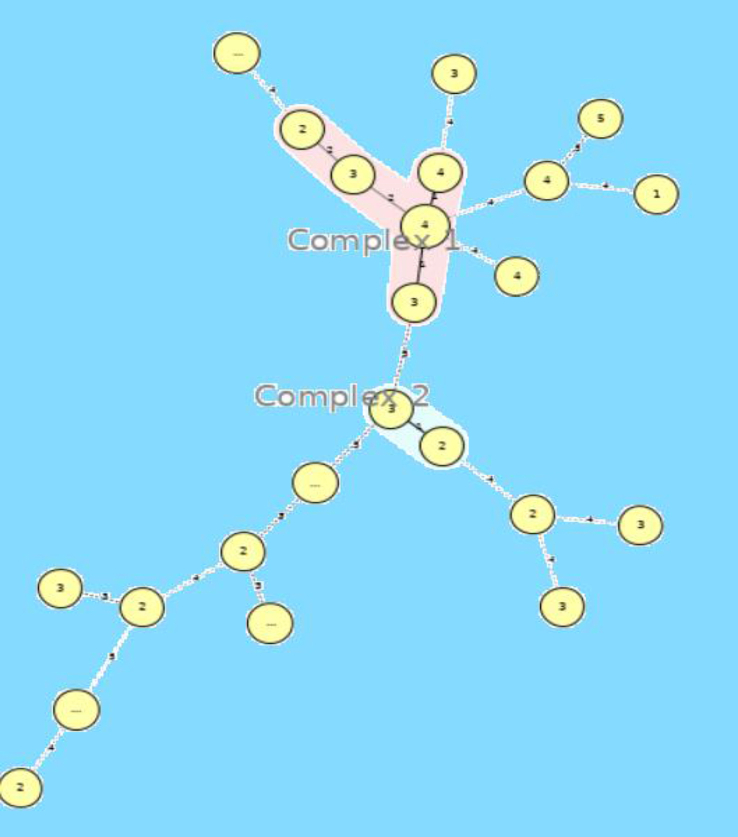
In all, 88 K. pneumoniae were isolated: 83 from urine (94.3%), four from sputum (4.5%) and one from blood (1.1%). Of all samples, 68 (77.2%) K. pneumoniae isolates were recovered from female individuals and the other 20 (22.7%) samples were isolated from male individuals. Twenty-eight (31.8%) isolates out of 88 samples were collected from outpatients and 60 (68.1%) isolates were from inpatients. In the present study, the age of patients from whom the samples were collected ranged from 1 to 92 (48.63 ± 27.54) years. Antibiotic susceptibility tests demonstrated that the highest antibiotic resistance in K. pneumoniae isolates was against co-trimoxazole, to which 46 (52.2%) isolates were resistant. The most effective antibiotic against K. pneumoniae isolates was neomycin. Imipenem (85.2%), ertapenem (81.8%), levofloxacin (77.2%), amikacin (77.2%), ciprofloxacin (72.7%), gentamicin (70.4%) and tetracycline (70.4%) were effective against more than 70.0% of the K. pneumoniae isolates (Table 1). The results also showed that imipenem remained more effective than ertapenem. A significant association was observed between different antibiotic resistance and the hospital from which the samples were collected. There was no relationship between different age of patients and different antibiotic resistance rates. Most resistant samples were isolated from hospital 4, but more samples that were susceptible to all antibiotics were isolated from hospital 2 (p ≤ 0.05). Among 88 K. pneumoniae isolates, 43 (48.8%) and 20 (22.7%) were MDR and XDR, respectively. The highest rate of MDR and XDR K. pneumoniae was isolated from hospital 4 whereas the isolates with the lowest resistance belonged to hospital 3. Data analysis showed a significant correlation between MDR and XDR isolates and the hospital that provided the samples (p ≤ 0.05). In this study, 30 (34%) isolates were phenotypically ESBL-producing. After PCR detection, the expression of blaCTX-M-1 genes was confirmed in all phenotypically positive ESBL isolates. The result of antibiotic resistance in K. pneumoniae isolates demonstrated that resistance to all antibiotics in ESBL-producing K. pneumoniae was higher than in non-ESBL K. pneumoniae (Table 1). Among the ESBL-positive isolates, 19 (63.33%) were MDR, and 11 (36.67%) isolates were detected as XDR (supplementary table). Therefore, there was a remarkable relationship between ESBL-positive isolates, MDR, and XDR isolates (P ≤ 0.05). Among the 88 isolates, 35 (39.7%) plasmid-mediated quinolone resistance-positive isolates were detected. The presence of qnrA, qnrB and qnrS genes were observed in 5 (5.6%), 29 (32.9%) and 23 (26.1%) of the isolates, respectively. Among these 35 plasmid-mediated quinolone resistance-positive isolates, 22 (81.48%) and 10 (37.03%) isolates were MDR and XDR, respectively. Data analysis showed a significant relationship between the presence of qnr genes and MDR and XDR isolates (p ≤ 0.05). Furthermore, a direct association between the expression of qnr genes and resistance to quinolone and fluoroquinolones was observed. Among qnr genes, the qnrB made the greatest contribution to resistance to quinolone and fluoroquinolones (p ≤ 0.05). The association of qnrS and qnrB detected in some isolates has raised the level of resistance to quinolone antibiotics, suggesting that the synergistic effects of qnrS with qnrB in isolates may increase defence against quinolones (see Supplementary material, Table S1). Overall, screening the isolates for the presence of the int genes revealed that all isolates (100%) contained the intI gene. Seventy-two (81.8%) of the 88 isolates possessed intII and intI, but intIII was not identified in any of the K. pneumoniae isolates. In addition, resistance to some antibiotics such as tetracycline, gentamycin, tobramycin, ciprofloxacin, aztreonam, ceftriaxone, cefotaxime and ceftazidime was significantly associated with the presence of class II integron (p ≤ 0.05). Furthermore, a remarkable relationship between the presence of integron class II and existence of ESBL, aac(6′)-Ib-cr, and aac(3)-II-cr was observed (p ≤ 0.05). A higher prevalence of class II integron was identified in inpatients than outpatients (p ≤ 0.05). Twenty-eight (31.8%) isolates were positive for the aac(6′)-Ib-cr gene, but 26 (29.5%) isolates harboured the aac(3)-II-cr gene. Among the total of 88 K. pneumoniae isolates, 26 (29.5%) possessed both aac(6′)-Ib-cr and aac(3)-II-cr genes. Statistical analysis revealed that not only were aac(6′)-Ib-cr and aac(3)-II-cr remarkably related to some aminoglycoside antibiotic resistance, including amikacin, gentamicin, tobramycin and streptomycin, but also a correlation between aac(6′)-Ib-cr and aac(3)-II-cr and blaCTX-M-1, qnrB and intII genes was found (p ≤ 0.05). In the present study, 11 (12.5%) K. pneumoniae isolates were found that harboured a complex of resistance genes together that were easily transferable (blaCTX-M-1/aac/qnr/int); all 11 isolates were MDR or XDR and high-level antibiotic resistance was observed in them. However, in our study, no significant difference was found between the rates of blaCTX-M-1, aac and qnr genes among hospitalized patients and outpatients (p > 0.05). Overall, among the variable-number of tandem repeat (VNTR) loci, ‘A’ locus was found as the most frequent VNTRs, containing seven different alleles. D and E loci contained only three different alleles and showed the lowest discriminatory power. The UPGMA dendrogram showed that 30 K. pneumoniae isolates were discriminated into 23 different MLVA types and eight clusters. Minimum spanning tree analysis of the 30 K. pneumoniae indicated that different isolates were co-circulating within the hospitals. These isolates fell into two different clonal complexes. These two clonal complexes consisted of isolates circulating among the three hospitals from the five hospitals under study, and 20 singletons (Fig. 1).
Fig. 1.
Dendrogram showing the cluster analysis of 30 extended-spectrum β-lactamase (ESBL) -positive Klebsiella pneumoniae isolates based on the multiple-locus variable number tandem repeat analysis (MLVA) profile. I = HAI (hospital-acquired infections) and OPD (community-acquired infections), II = numbers of different hospitals, III = M (multidrug resistance), IV = X (extreme drug resistance), V to XII = different alleles of variable number of tandem repeat markers.
Discussion
In this study, antibiotic resistance patterns showed that these bacteria have high resistance to cephalosporins and aztreonam, which can be attributed to the production of ESBLs in these bacteria. Also, most of the resistant isolates were collected from hospital 4 (n = 15), whereas wild isolates mainly came from hospital 2 (n = 1). This may be a result of patient recruitment, because the patient samples in hospital 4 were mainly from inpatients, while those in hospital 2 were mainly from outpatients. Similar to our findings, Bina et al. reported that the highest and lowest resistance rates in their study were related to piperacillin (60.6%) and imipenem (13.9%), respectively [26]. The results of a study by Kiaei et al. also showed that resistance to cephalosporins was high, and imipenem had the best inhibitory effect on K. pneumoniae. Furthermore, they reported that other antibiotics, such as fosfomycin, ertapenem and gentamicin, had a good effect on isolates, with 8%, 9% and 8% resistance, respectively [24]. In a previous study in Iran, the rate of resistance of K. pneumoniae isolates to the antibiotic imipenem was 7.2%, which is less than the resistance obtained from the present study; it can be concluded that resistance to carbapenems is increasing in Iran [27]. The prevalence of carbapenem-resistant K. pneumoniae in the hospitals included in our study was lower than that reported in China; however, the rate of MDR strains in the Chinese study was 46.2%, which is close to our finding [28]. Our results also showed that carbapenems and neomycin together had the best performance compared with other antibiotics on isolated bacteria, although 11.3% resistance to imipenem and 12.5% resistance to ertapenem was observed, which can be indicative of the increased prevalence of resistance to carbapenems in K. pneumoniae in Tehran hospitals compared with previous studies [29,30]. Due to the good performance of carbapenems in controlling the MDR bacteria, spreading resistance to these antibiotics can increase the prevalence of XDR K. pneumoniae isolates, which increases the cost and duration of treatment. Also, the emergence of XDR K. pneumoniae isolates has been observed in different parts of the world [31,32]. Nevertheless, carbapenems have been significantly effective on K. pneumoniae isolates in various regions of the world as reported in the studies by Zhang et al. and Marsh et al. [33,34]. The results of the present study showed that 34% of our strains were ESBL producers, which is much lower than other reports from different countries. A study by Taati et al. showed that 60.7% of the clinical isolates of K. pneumoniae produced ESBL, which is higher than the results of the present study, and confirms that resistance can vary even in different cities of a country [35]. In India, the rate of ESBL-positive K. pneumoniae was reported to be 73.28% in 2010 [36]. Aljanaby et al. from Iraq reported that 81.39% of the K. pneumoniae isolates were ESBL producers [37]. However, some studies showed a similar prevalence of ESBL genes in K. pneumoniae isolates such as the study conducted by Zhang et al. [33]. This discrepancy between the reported frequencies of ESBL isolates in different regions of the world could be dependent on several factors, including the drug abuse pattern, differences in virulence strains in terms of their degrees, antimicrobial stewardship programme, geographical diversity and the practices applied for control of infection. All ESBL-positive isolates also had a blaCTX-M-1 gene, indicating that the presence of this resistance gene is the most effective factor in representing the ESBL phenotype. Our results and other studies [21,38] have shown that the rate of resistance to antibiotics in ESBL-producing K. pneumoniae is higher than in ESBL-negative isolates. These bacteria have high potential to gain resistance to other antibiotic families and the treatment of infections caused by them is very difficult. The prevalences of qnrA, qnrB and qnrS in our clinical isolates were 5.6%, 32.9% and 26.1%, respectively. Before screening was introduced, the qnr plasmid-borne mechanism was a major concern for increasing antibiotic resistance because the placement of other genes on these plasmids facilitates their transfer from one isolate to another. Similar to our finding, Kiaei et al. reported that qnrB was the dominant gene among the qnr genes and the prevalences of qnrB and qnrS were 30% and 6.7%, respectively [24]. Furthermore, the study that was performed by Benaicha et al. showed that qnrB (11.86%) was the most frequent gene among the qnr genes and the prevalence of qnrS was reported to be 5.93% [39]. It is noteworthy that in this study, 100% of isolates contained intI, and 81.8% possessed intII, but intIII was not found in any of the isolates. The prevalences of intI (46%) and intII (40%) genes were lower in our previous study [40], but the reported significant associations between resistance to many of the tested antibiotics and the presence of integrons were similar to the present study [40]. In another study it was reported that the frequency of class I, II and III integrons among 37 MDR K. pneumoniae were 27 (72.9%), 19 (51.3%) and 3 (8.1%), respectively, which is different from our findings [19]. The high prevalence of integrons in K. pneumoniae especially among hospitalized patients suggests the role of integrons as mobile genetic elements in antibiotic resistance. In this research, 31.8% and 29.5% of K. pneumoniae isolates represented aac(6′)-Ib-cr and aac(3)-II-cr, respectively. In Iran, a study on certain clinical populations reported a high prevalence of aac(6′)-Ib-cr (74%) and aac(3)-II-cr (73%) [41]. These results were comparable to those obtained by El-Badawy et al. who studied the prevalence of aac(6′)-Ib-cr and aac(3)-II-cr among clinical K. pneumoniae isolates, and found that 88% and 58% of K. pneumoniae isolates causing infection were aac(6′)-Ib-cr and aac(3)-II-cr-positive [42]. Low prevalence rates for acc(6′)-Ib and acc(3′)-II among K. pneumoniae isolates were also reported by Liang et al., which is similar to our findings [43]. The present study also determined that the tested aminoglycoside-resistant K. pneumoniae isolates were positive for aac(6′)-Ib-cr and aac(3)-II-cr, which has been previously observed [42,43]. MLVA analysis of this study showed 23 MLVA types among 30 Klebsiella isolates. This genetic diversity has also been described in previous studies [25,44]. As shown in Fig. 2, most of the isolates were categorized into singleton isolates. This genetic heterogeneity can be a serious challenge to the infection control processes, in particular the circulation of the same genotypes among isolates of hospital and community infections (31, 60 and 33; 38 and 2; 66, 67, 68 and 125; and 74 isolates) as shown in the UPGMA dendrogram, there is not only evidence for gene transfer among different hospitals, but also there is the risk of transmission of these resistance genes to community isolates. Furthermore, the location of resistance genes in mobile genetic elements such as integrons and plasmids proposed horizontal transmission of these resistance elements. In this study, only the ESBL-positive isolates were MLVA typed and therefore the discriminatory power of this MLVA scheme as Simpson's index of diversity (0.84) is less than expected. However, the selection of five different hospitals has improved the discriminatory power. The results showed that use of drugs has high limitations. ESBL-producing strains have the potential to acquire resistance to other antibiotic classes, including fluoroquinolones and aminoglycosides. One of the main causes of antibiotic resistance is mobile genetic elements such as integrons that spread easily among bacteria and can produce MDR bacteria with multiple resistance genes. However, low resistance to imipenem and ertapenem can indicate the need to control the spread of resistance to these antibiotics, because with the development of resistance to carbapenems, the treatment of Gram-negative bacteria that produce β-lactams will be a major problem.
Fig. 2.
Minimum spanning tree representation of the multiple-locus variable number tandem repeat analysis (MLVA) clustering of the 30 ESBL-positive Klebsiella pneumoniae clinical isolates isolated from patients in five hospitals of Tehran. Each circle represents a unique genotype, numbers under the circles represent the number of hospitals. The colour halo around the circles corresponds to CCs. Circles without a colour halo represent singletons. Numbers on the lines show the number of loci that are different between two MLVA profiles.
Funding
None.
Conflicts of interest
We declare no conflicts of interests.
Acknowledgements
The authors thank the Iran University of Medical Sciences for supporting this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nmni.2020.100693.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Spagnolo A.M., Orlando P., Panatto D., Perdelli F., Cristina M.L. An overview of carbapenem-resistant Klebsiella pneumoniae: epidemiology and control measures. Rev Med Microbiol. 2014;25:7–14. [Google Scholar]
- 2.Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bahramian A., Shariati A., Azimi T., Sharahi J.Y., Bostanghadiri N., Gachkar L. First report of New Delhi metallo-β-lactamase-6 (NDM-6) among Klebsiella pneumoniae ST147 strains isolated from dialysis patients in Iran. Infection, Genet Evol. 2019;69:142–145. doi: 10.1016/j.meegid.2019.01.030. [DOI] [PubMed] [Google Scholar]
- 4.Murphy C.N., Clegg S. Klebsiella pneumoniae and type 3 fimbriae: nosocomial infection, regulation and biofilm formation. Future Microbiol. 2012;7:991–1002. doi: 10.2217/fmb.12.74. [DOI] [PubMed] [Google Scholar]
- 5.Martin R.M., Bachman M.A. Colonization, infection, and the accessory genome of Klebsiella pneumoniae. Front Cell Infect Microbiol. 2018;8:4. doi: 10.3389/fcimb.2018.00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mazzariol A., Bazaj A., Cornaglia G. Multi-drug-resistant Gram-negative bacteria causing urinary tract infections: a review. J Chemother. 2017;29(Suppl. 1):2–9. doi: 10.1080/1120009X.2017.1380395. [DOI] [PubMed] [Google Scholar]
- 7.Bonnet R. Growing group of extended-spectrum β-lactamases: the CTX-M enzymes. Antimicrob Agents Chemother. 2004;48:1–14. doi: 10.1128/AAC.48.1.1-14.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cantón R., Coque T.M. The CTX-M β-lactamase pandemic. Curr Opin Microbiol. 2006;9:466–475. doi: 10.1016/j.mib.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 9.Morosini M.-I., García-Castillo M., Coque T.M., Valverde A., Novais An, Loza E. Antibiotic coresistance in extended-spectrum-β-lactamase-producing Enterobacteriaceae and in vitro activity of tigecycline. Antimicrob Agents Chemother. 2006;50:2695–2699. doi: 10.1128/AAC.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordmann P., Poirel L. Emergence of plasmid-mediated resistance to quinolones in Enterobacteriaceae. J Antimicrob Chemother. 2005;56:463–469. doi: 10.1093/jac/dki245. [DOI] [PubMed] [Google Scholar]
- 11.Bateman A., Murzin A.G., Teichmann S.A. Structure and distribution of pentapeptide repeats in bacteria. Protein Sci. 1998;7:1477–1480. doi: 10.1002/pro.5560070625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacoby G.A., Walsh K.E., Mills D.M., Walker V.J., Oh H., Robicsek A. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob Agents Chemother. 2006;50:1178–1182. doi: 10.1128/AAC.50.4.1178-1182.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hata M., Suzuki M., Matsumoto M., Takahashi M., Sato K., Ibe S. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob Agents Chemother. 2005;49:801–803. doi: 10.1128/AAC.49.2.801-803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mammeri H., Van De Loo M., Poirel L., Martinez-Martinez L., Nordmann P. Emergence of plasmid-mediated quinolone resistance in Escherichia coli in Europe. Antimicrob Agents Chemother. 2005;49:71–76. doi: 10.1128/AAC.49.1.71-76.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poirel L., Van De Loo M., Mammeri H., Nordmann P. Association of plasmid-mediated quinolone resistance with extended-spectrum β-lactamase VEB-1. Antimicrob Agents Chemother. 2005;49:3091–3094. doi: 10.1128/AAC.49.7.3091-3094.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robicsek A., Sahm D., Strahilevitz J., Jacoby G., Hooper D. Broader distribution of plasmid-mediated quinolone resistance in the United States. Antimicrob Agents Chemother. 2005;49:3001–3003. doi: 10.1128/AAC.49.7.3001-3003.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X.-Z. Quinolone resistance in bacteria: emphasis on plasmid-mediated mechanisms. Int J Antimicrob Agents. 2005;25:453–463. doi: 10.1016/j.ijantimicag.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 18.Mustafa M.S., Abdullah R.M. Antimicrobial susceptibility and molecular detection of acc(6ʹ)-Ib and acc(6ʹ)-II genes among Klebsiella pneumoniae isolates collected from patients. J Pharmaceut Sci Res. 2018;10:1048–1052. [Google Scholar]
- 19.Rizk D.E., El-Mahdy A.M. Emergence of class 1 to 3 integrons among members of Enterobacteriaceae in Egypt. Microb Pathog. 2017;112:50–56. doi: 10.1016/j.micpath.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 20.Jazayeri Moghadas A., Kalantari F., Sarfi M., Shahhoseini S., Mirkalantari S. Evaluation of Virulence Factors and Antibiotic Resistance Patterns in Clinical Urine Isolates of Klebsiella pneumoniae in Semnan, Iran. J Microbiol. 2018;11(7):e63637. doi: 10.5812/jjm.63637. Jundishapur. [DOI] [Google Scholar]
- 21.Hadizadeh M., Norouzi A., Taghadosi R., Mohebi S., Mohammadi M., Hasanzade A. Prevalence of qnr, intI, and intII genes in extended-spectrum β-lactamase (ESBL)-producing Escherichia coli isolated from clinical samples in Iran. Trop J Pharmaceut Res. 2017;16:141–147. [Google Scholar]
- 22.CLSI C . CLSI; Wayne, PA: 2018. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. M100-S24 January. [Google Scholar]
- 23.Mirkalantari S., Moghadas A.J. Diversity determination of CTX-M1 producing Klebsiella pneumoniae using multilocus variable-number tandem repeat analysis, semnan, Iran. Jundishapur J Microbiol. 2018;11(7) [Google Scholar]
- 24.Kiaei S., Moradi M., Nave H.H., Hashemizadeh Z., Taati-Moghadam M., Kalantar-Neyestanaki D. Emergence of co-existence of bla NDM with rmtC and qnrB genes in clinical carbapenem-resistant Klebsiella pneumoniae isolates in burning center from southeast of Iran. Folia Microbiol. 2019;64:55–62. doi: 10.1007/s12223-018-0630-3. [DOI] [PubMed] [Google Scholar]
- 25.Ranjbar R., Memariani H., Sorouri R., Memariani M. Distribution of virulence genes and genotyping of CTX-M-15-producing Klebsiella pneumoniae isolated from patients with community-acquired urinary tract infection (CA-UTI) Microb Pathog. 2016;100:244–249. doi: 10.1016/j.micpath.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 26.Bina M., Pournajaf A., Mirkalantari S., Talebi M., Irajian G. Detection of the Klebsiella pneumoniae carbapenemase (KPC) in K. pneumoniae isolated from the clinical samples by the phenotypic and genotypic methods. Iran J Pathol. 2015;10:199. [PMC free article] [PubMed] [Google Scholar]
- 27.Moradi M., Norouzi A., Taatimoghadam M. Prevalence of bla-CTX-M, bla-SHV, and bla-TEM genes and comparison of antibiotic resistance pattern in extended-spectrum β-lactamase producing and non-producing groups of Klebsiella pneumoniae isolated from clinical samples in Kerman hospitals. J Fasa Univ Med Sci. 2016;6:120–128. [Google Scholar]
- 28.Yan J., Pu S., Jia X., Xu X., Yang S., Shi J. Multidrug resistance mechanisms of carbapenem resistant Klebsiella pneumoniae strains isolated in Chongqing, China. Ann Lab Med. 2017;37:398–407. doi: 10.3343/alm.2017.37.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahcheraghi F., Nobari S., Rahmati Ghezelgeh F., Nasiri S., Owlia P., Nikbin V.S. First report of New Delhi metallo-β-lactamase-1-producing Klebsiella pneumoniae in Iran. Microb Drug Resist. 2013;19:30–36. doi: 10.1089/mdr.2012.0078. [DOI] [PubMed] [Google Scholar]
- 30.Nobari S., Shahcheraghi F., Rahmati Ghezelgeh F., Valizadeh B. Molecular characterization of carbapenem-resistant strains of Klebsiella pneumoniae isolated from Iranian patients: first identification of blaKPC gene in Iran. Microb Drug Resist. 2014;20:285–293. doi: 10.1089/mdr.2013.0074. [DOI] [PubMed] [Google Scholar]
- 31.Li L., Yu T., Ma Y., Yang Z., Wang W., Song X. The genetic structures of an extensively drug resistant (XDR) Klebsiella pneumoniae and its plasmids. Front Cell Infect Microbiol. 2019;8:446. doi: 10.3389/fcimb.2018.00446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galani I., Anagnostoulis G., Chatzikonstantinou M., Petrikkos G., Souli M. Emergence of Klebsiella pneumoniae co-producing OXA-48, CTX-M-15, and ArmA in Greece. Clin Microbiol Infect. 2016;22:898–899. doi: 10.1016/j.cmi.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Zhang J., Zhou K., Zheng B., Zhao L., Shen P., Ji J. High prevalence of ESBL-producing Klebsiella pneumoniae causing community-onset infections in China. Front Microbiol. 2016;7:1830. doi: 10.3389/fmicb.2016.01830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marsh J.W., Krauland M.G., Nelson J.S., Schlackman J.L., Brooks A.M., Pasculle A.W. Genomic epidemiology of an endoscope-associated outbreak of Klebsiella pneumoniae carbapenemase (KPC)-producing K. pneumoniae. PLoS One. 2015;10(12) doi: 10.1371/journal.pone.0144310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taati Moghadam M., Hossieni Nave H., Mohebi S., Norouzi A. The evaluation of connection between integrons class I and II and ESBL-producing and non-ESBL Klebsiella pneumoniae isolated from clinical samples, Kerman. Iran J Med Microbiol. 2016;10:1–9. [Google Scholar]
- 36.Abhilash K., Veeraraghavan B., Abraham O. Epidemiology and outcome of bacteremia caused by extended spectrum β-lactamase (ESBL)-producing Escherichia coli and Klebsiella spp. in a tertiary care teaching hospital in south India. J Assoc Physicians India. 2010;58(Suppl. l):13–17. [PubMed] [Google Scholar]
- 37.Aljanaby A.A.J., Alhasnawi H. Research article phenotypic and molecular characterization of multidrug resistant Klebsiella pneumoniae isolated from different clinical sources in Al-Najaf Province-Iraq. Pak J Biol Sci. 2017;20:217–232. doi: 10.3923/pjbs.2017.217.232. [DOI] [PubMed] [Google Scholar]
- 38.Mohebi S., Hossieni Nave H., Norouzi A., Kandehkar Gharaman M., Taati Moghadam M. Detection of extended spectrum β lactamases on class I integron in Escherichia coli isolated from clinical samples. J Mazandaran Univ Med Sci. 2016;26:66–76. [Google Scholar]
- 39.Benaicha H., Barrijal S., Ezzakkioui F., Elmalki F. Prevalence of PMQR genes in E. coli and Klebsiella spp. isolated from north-west of Morocco. J Glob Antimicrob Resist. 2017;10:321–325. doi: 10.1016/j.jgar.2017.05.024. [DOI] [PubMed] [Google Scholar]
- 40.Mirkalantari S., Momeni N., Mirnejad R., Bineshian F. Assessment of the prevalence of Class I and II integrons in Klebsiella pneumoniae isolates from patients referred to the hospitals of Semnan, Iran. J Appl Biotechnol Rep. 2017;4:719–722. [Google Scholar]
- 41.Lotfollahi L., Samadi N., Hosainzadegan H., Qomi M.A. Prevalence of aac (3ʹ)-IIa and aac (6ʹ)-Ib genes incidence involved in aminoglycoside resistance in Klebsiella pneumoniae isolated from clinical samples in Urmia Hospitals, Iran. Am J Pharmaceut Res. 2015;5:326–334. [Google Scholar]
- 42.El-Badawy M.F., Tawakol W.M., El-Far S.W., Maghrabi I.A., Al-Ghamdi S.A., Mansy M.S. Molecular identification of aminoglycoside-modifying enzymes and plasmid-mediated quinolone resistance genes among Klebsiella pneumoniae clinical isolates recovered from Egyptian patients. Int J Microbiol. 2017;2017:8050432. doi: 10.1155/2017/8050432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang C., Xing B., Yang X., Fu Y., Feng Y., Zhang Y. Molecular epidemiology of aminoglycosides resistance on Klebsiella pneumoniae in a hospital in China. Int J Clin Exp Med. 2015;8:1381. [PMC free article] [PubMed] [Google Scholar]
- 44.Derakhshan S., Peerayeh S.N., Bakhshi B. Genotyping and characterization of CTX-M-15-producing Klebsiella pneumoniae isolated from an Iranian hospital. J Chemother. 2016;28:289–296. doi: 10.1179/1973947815Y.0000000002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.