Abstract
Primary central nervous system lymphoma (PCNSL) is a rare group of extra-nodal non-Hodgkin lymphoma which is confined to the central nervous system or eyes. This article aims to present a brief profile of PCNSL diagnosis and treatment in immunocompetent patients. The authors retrieved information from the PubMed database up to September 2019. The annual incidence of PCNSL increased over the last four decades. The prognosis of PCNSL has improved mainly due to the introduction and wide-spread use of high-dose methotrexate, which is now the backbone of all first-line treatment polychemotherapy regimens. Gene expression profiling and next-generation sequencing analyses have revealed mutations that induce activation of nuclear factor-κB, B cell antigen receptor, and Janus kinases/signal transducer and activator of transcription proteins signal pathways. Some novel agents are investigated in the treatment of relapsed PCNSL including immunotherapy and targeted therapy. In particular, lenalidomide and ibrutinib have demonstrated durable efficiency. Treatment of PCNSL has evolved in the last 40 years and survival outcomes have improved in most patient groups, but there is still room to improve outcome by optimizing current chemotherapy and novel agents.
Keywords: Diagnosis, Primary central nervous system lymphoma, Treatment
Introduction
Primary central nervous system lymphoma (PCNSL) is a rare extra-nodal sub-type of non-Hodgkin lymphoma which is limited to the brain, spinal cord, leptomeninges, or eyes, without evidence of systemic involvement. It is often initially sensitive to both chemotherapy and radiation therapy but the survival is usually poor compared to those for systemic lymphomas outside the central nervous system (CNS).[1]
PCNSL accounts for 4% of newly diagnosed brain tumors and 4% to 6% of all extra-nodal lymphomas, with an incidence of 0.4 to 0.5/100,000 per year. PCNSL can occur in patients with immunosuppressive conditions (acquired immune deficiency syndrome, congenital immunodeficiency, post-transplant immunosuppression) as well as immunocompetent individuals. The incidence of PCNSL has increased over the past four decades, especially in older adult patients. PCNSL can occur at any stage of adulthood, with a median age at diagnosis of 65 years. Men are prominently affected compared to women.[2] Immunohistochemistry staining shows that the majority of PCNSL resembles the activated B cell (ABC) sub-type of diffuse large B-cell lymphoma (DLBCL). However, genomic profiling has provided insights into the pathogenesis of this disease in recent years. Mutations in the adaptor protein myeloid differentiation primary response 88 (MYD88) and B-cell antigen receptor-associated protein cluster of differentiation 79B (CD79B) are frequently observed in PCNSL.[3] The amplification of programmed cell death 1 (PD-1) gene and overexpression of PD-1 protein are associated with poor prognosis.[4] These new insights into the mutational and signal activation have helped to identify novel target drugs to improve the prognosis of patients with relapsed/refractory (r/r) PCNSL.
This review focuses on the clinical presentation, diagnosis, and management of PCNSL in immunocompetent patients.
Clinical Presentation and Diagnosis
PCNSL has no specific clinical manifestations. More than 80% of patients develop intracranial mass lesions and leptomeningeal involvement occurs in 11% to 20% of cases.[5] Eye involvement occurs in 15% to 25% of patients and primary intraocular lymphoma is a sub-type of PCNSL. Patients usually develop neurologic signs over weeks, including focal neurologic deficits (56%–70%), mental status and behavioral changes (32%–43%), symptoms of increased intracranial pressure (headaches, nausea, vomiting, papilledema, 32%–33%), and seizures (11%–14%), depending on the site of CNS involvement.[6,7]
Cranial contrast magnetic resonance imaging (MRI) with contrast injection is the preferred method for radiologic evaluation. About 60% to 70% of patients have a single-mass lesion that appears to be homogenously enhanced with mild edema with diffusion-weighted imaging restriction abnormalities.[8] Positron emission tomography-computed tomography (PET/CT) is also a useful tool for diagnosis, with high sensitivity (88%) and specificity (86%) that is widely used for the assessment of systemic disease.[9] Complete remission (CR) of interim PET/CT may predict progression-free survival (PFS), but it was not related to overall survival (OS) in PCNSL.[10,11]
Stereotactic biopsy guided by MRI is the preferred option for diagnosis, which can yield positive results in 90% of PCNSL cases; open biopsy procedures are rarely necessary. Steroid pre-treatment should be avoided before the biopsy. Atypical cells in the cerebrospinal fluid (CSF) cytology and abnormal B cell populations with restricted κ/λ expression are considered solid evidence of PCNSL.[12] Biomarkers from the CSF are used for PCNSL diagnosis. Bivariate elevated chemokine ligand 13 plus interleukin 10 (IL-10) is highly specific for the diagnosis of CNS lymphoma.[13] Data from the Peking Union Medical College Hospital reported sensitivity and specificity of 95% and 100%, respectively, for a CSF IL-10/IL-6 cut-off of 0.72.[14] Pentsova et al[15] confirmed the efficiency of liquid biopsy of CSF by next-generation sequencing (NGS) technology in CNS in 2016. Hattori et al[16] performed droplet digital polymerase chain reaction (ddPCR) in 14 consecutive PCNSL patients, reporting that ddPCR can sensitively detect the MYD88 L265P mutation in cell-free deoxyribonucleic acid serum samples. However, there remains no solid evidence to support liquid biopsy such as NGS or ddPCR as tools for monitoring for minimal residual diseases.[17,18]
Pathology
DLBCLs account for >90% of PCNSLs; the remainder comprises Burkitt lymphomas, low-grade lymphomas, or T-cell lymphomas (peripheral T-cell and anaplastic large T-cell lymphomas). PCNSL cells morphologically resemble centroblasts in light-scope imaging and B cell markers are strongly expressed. CD10 is detectable in only 10% to 20% of cases and interferon regulatory factor 4 (IRF4/MUM1) is strongly expressed in more than 90% of patients.[19] B-cell lymphoma 6 (BCL-6) and MYC overexpression are related to poor survival.[20,21] No specific chemokines have been identified to explain why PCNSL is restricted to the CNS.
Molecular Pathogenesis
PCNSL has unique genomic alterations, including the deletion of chromosome 6p21 harboring the human leukocyte antigen locus, recurrent 9p21 losses, and 9p24.1 copy number alterations and translocations that encode programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2).[3,22] In particular, deletions at the 6q21-23 region containing: (i) protein tyrosine phosphatase, receptor type, K, a protein tyrosine phosphatase involving in cell adhesion signaling; (ii) positive regulatory domain 1, a suppressor of tumor activity and regulator of B cell differentiation; and (iii) A20, also known as tumor necrosis factor-alpha-induced protein 3, which down-regulates nuclear factor-κB (NF-κB) signaling. Recurrent chromosomal losses at the 9p21 region, which encodes loci involved in cell cycle regulation including cyclin-dependent kinase inhibitor 2A. NGS has revealed additional gene mutations and PCNSL displays different gene expression profiles from those of other types of DLBCL. In 2018, Schmitz et al[23] and Chapuy et al[24] summarized the genetic sub-types of DLBCL, in which PCNSL was assigned to the MCD and C5 sub-types, with frequent MYD88, CD79b mutations accompanied by E-twenty-six variant transcription factor 6, PIM1 mutations and Bcl-2 gain. Several signal pathways are crucial in PCNSL molecular pathogenesis. MYD88L265Pis the most common mutation in PCNSLs. MYD88 encodes a signaling adaptor protein that induces activation of NF-κB and the Janus kinases/signal transducer and activator of transcription 3 (JAK/STAT3) pathway after stimulation of Toll-like receptors, interferon-β production, and IL-1/IL-18 receptors, this mutation is related to poor survival, which occurs in 40% to 100% of patients. CD79b is another common mutation, which occurs in more than 30% of cases and activates the NF-κB signaling pathway via the B cell antigen receptor (BCR) signaling pathway.[16,25–27] The BCR pathway transmits its signals to the CBM signalosome complex composed of caspase recruitment domain-containing protein 11, B-cell lymphoma/leukemia 10 and mucosa-associated lymphoid tissue lymphoma translocation protein 1. Balint and colleagues identified ataxia-telangiectasia mutated (ATM), tumor protein 53 (TP53), phosphatase and tensin homolog(PTEN), phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA), janus kinase 3 (JAK3), protein tyrosine phosphatase non-receptor type 1 (PTPN1), and KRAS mutations in PCNSL tumor cells by NGS and reported TP53 and ATM mutations to be negative prognostic factors.[25] These mutations were also found in CSF samples. Monitoring for the MYD88L265P mutation in CSF by ddPCR was shown to be as effective as MRI evaluation in 2018.[16] The JAK/STAT signaling pathway was activated by IL-4 and IL-10 in vitro studies.[27] JAK/STAT intracellular signaling pathway is up-regulated in the micro-environment of tumor vessels, which are correlated with tumor response and progression.
Prognostic Factors
Two prognostic score systems were developed more than 10 years ago. The International Extranodal Lymphoma Study Group (IELSG) reviewed 105 patients with PCNSL and proposed the IELSG score comprising five parameters: age >60 years, Eastern Cooperative Oncology Group status >1, elevated serum lactate dehydrogenase level, elevated CSF protein concentration, and involvement of deep regions of the brain. In the low-risk (0–1 factors), medium-risk (2–3 factors), and high-risk (4–5 factors) groups, the 2-year survival rates were 80%, 48%, and 15%, respectively.[28] The Memorial Sloan Kettering Cancer Center prognostic score uses two parameters: age <50 years and Karnofsky performance score ≥70.[29] CR after induction therapy was an independent factor for longer OS.
Induction Therapy
Treatment strategies for PCNSL have improved over the decades; however, no consensus on the optimal regimen has yet been established. High-dose methotrexate (HD-MTX) is the backbone of systemic therapy but the role of surgery, the optimal upfront combination regimen, and the role of radiation remain controversial.
Surgery and radiation
The role of surgery in PCNSL is generally restricted to stereotactic biopsy due to multifocal and diffusely infiltrative tumor growth. Moreover, surgical resection increases the risk of permanent neurologic deficits and delay chemotherapy. No survival benefit from sub-total or gross total resection has been observed. While experts agreed that open surgery should be restricted to selected patients, Weller challenged this opinion in 2012. Data from the German PCNSL Study Group-1 showed clinical outcome improvements in patients undergoing MRI-guided sub-total or gross total resection; however, the benefit may have been related to a bias in the basal physical status.[30]
PCNSL is sensitive to radiation therapy; therefore, whole-brain radiotherapy (WBRT) combined with corticosteroids was the standard regimen for initial treatment in the 1980s. Although the early overall response rate (ORR) reached 90%, the high relapse rate limited its use. Most patients relapsed within 1 year and the OS was only 10 to 17 months.[31] WBRT also significantly increased the risk of neurotoxicity and more than 25% of patients older than 65 years of age developed cognitive impairments that increased mortality.[32] Fine et al[33] reported a 6-month PFS of only 33% in patients more than 70 years of age treated with WBRT. Given the lack of durable responses to radiation and the risk of neurotoxicity associated with this therapy, WBRT alone is no longer recommended as an initial treatment for most patients with PCNSL. In some selected situations such as multi-chemotherapy intolerance or relapse after chemotherapy, WBRT remains a good option. WBRT is widely used as consolidation treatment. Some experts suggest that low-dose radiation can decrease neurotoxicity. Morris conducted a phase II single-institution study to assess whether reduced-dose WBRT for consolidation led to reduced neurotoxicity and durable disease control. PCNSLs were treated with rituximab, HD-MTX, vincristine, and procarbazine (R-MVP) followed by reduced-dose WBRT (23.4 Gy). No cognitive impairment was observed clinically in formal psychometric testing; the ORR was 78% and the median PFS was 7.7 years.[34]
Systemic chemotherapy
Many drugs cannot permeate the blood-brain-barrier (BBB); therefore, CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) used in the management of systemic lymphoma does not confer a survival benefit. Currently, systemic chemotherapy containing HD-MTX is widely used in the treatment of PCNSL [Table 1].
Table 1.
Treatment regimens for newly-diagnosed PCNSL
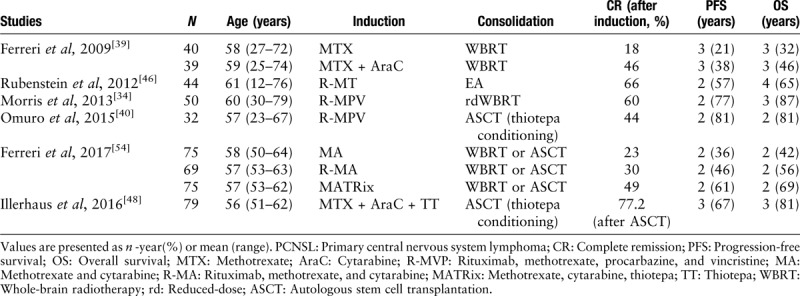
HD-MTX-based polychemotherapy
While HD-MTX is now considered the cornerstone of systemic chemotherapy for newly diagnosed PCNSL, the most effective dose has not yet been established.[35–37] Most experts and guidelines suggest the administration of more than 6 cycles at the minimal dose of 3.5 g/m2 as induction treatment and multi-drug regimens with HD-MTX increased the efficiency and are widely used; however, the best partners of MTX have not been determined. The IELSG20 randomized controlled trial evaluated the role of HD-MTX combined with cytarabine, reporting improved response rates (69% vs. 40%) and longer PFS (18 vs. 3 months) with the addition of cytarabine to MTX, despite a higher rate of hematologic toxicity (92% vs. 15%). The 3-year failure-free survival was 21% for the MTX group and 38% for the MTX plus cytarabine group (P = 0.01), with 3-year OS rates of 32% and 46%, respectively (P = 0.07). Although this was a relatively small population (79 patients in two groups), it was the first randomized trial of combination chemotherapy in PCNSL.[38] The IELSG32, a randomized study that included 227 patients, investigated the addition of rituximab and/or thiotepa to a regimen of MTX and cytarabine. The highest response rates were observed in patients administered HD-MTX/cytarabine/rituximab(MATRix, 86%) compared to the rates in patients administered HD-MTX/cytarabine/rituximab (73%) or HD-MTX/cytarabine alone (53%), with 2-year OS rates of 42% (95% confidence interval [CI] 36–48%), 56% (50–62%), and 69% (64–74%), respectively.[39] However, the high incidence of neurotoxicity with combined modality treatments that include WBRT is now widely recognized, especially in elderly patients. Two-phase 2, single-arm studies investigated the efficacy of MTX, procarbazine, and vincristine (MPV) followed by dose-reduced WBRT or autologous stem-cell transplantation (ASCT) in newly diagnosed PCNSL patients reported CR rates of 47% and 42% and median OS of 24 and >37 months, respectively.[34,40] In conclusion, the combination regimen improved the outcome accompanied by increased adverse effects; however, the toxicity limited their use in elderly patients.
Rituximab is a standard component of treatment for systemic B-cell lymphomas. Due to its high molecular weight, its concentration in CSF is only 0.1% to 1% of serum. The role of rituximab in the treatment of PCNSL has been under debate. A pilot study of 12 PCNSL patients investigated the efficiency of single-agent rituximab reported an ORR of 36%.[41] In 2014, Holdhoff reviewed the effect of adding rituximab to an HD-MTX regimen showing that rituximab improved the CR (P = 0.0145), PFS (P = 0.003), and OS (P = 0.01) rates.[42] The HOVON 105/ALLG NHL 24 trial, a recent randomized study of 200 patients, investigated the addition of rituximab to the MBVP (MTX, carmustine, teniposide, and prednisone) regimen, with all participators administered cytarabine or WBRT as consolidation therapy. The event-free survival rates at 1 year were 49% and 52% in the MBVP and R-MBVP groups (P = 0.99); however, only two cycles of intensive rituximab in the first weeks were administered in experiment group rather than sustained rituximab each cycle in routine practice.[43] We cannot decide the useless of rituximab according to this study. Intraventricular injection via an Ommaya reservoir is another method for rituximab administration. Rubenstein and colleagues investigated the safety and efficiency of intraventricular rituximab in 14 patients. The maximum tolerated dose was 25 mg and the CR rate was 42.8%. The common adverse effects were fever and hypertension.[44]
Consolidation Treatment
Consolidation treatment in patients with PCNSLs that responded to induction therapy can provide deeper remission for disease cure. The two strategies for consolidation include WBRT and high-dose chemotherapy followed by autologous stem cell support. For frail patients, maintenance with oral drugs is an alternative.
A large-scale random phase 3 study of HD-MTX induction followed by WBRT (45 Gy) for consolidation reported a higher frequency of long-term neurotoxicity for the WBRT group (49% vs. 26%) and no difference in OS (37.1 vs. 32.4 months, P = 0.71). The improvement in PFS was counteracted by the neurotoxicity-related deaths.[45] However, other studies reported contradictory results. A multi-center phase 2 study observed no significant neurocognitive decline after consolidative reduced-dose WBRT (23.4 Gy) and cytarabine in patients who had achieved a CR to induction chemotherapy including HD-MTX, with a PFS of 7.7 years.[34] For PCNSL, relapse and late neurotoxicity effects can occur many years after treatment; thus, longer follow-up is recommended to clarify the long-term oncologic outcomes. In summary, the value of consolidation WBRT and the optimal dose of radiotherapy remain controversial, especially in older patients; however, low-dose WBRT may be an alternative option.
Due to the long-term neurotoxicity, WBRT is omitted from treatment regimens and chemotherapy consolidation has increased in recent decades. The multi-center CALGB 50202 study treated patients with methotrexate, temozolomide (Temodar), and rituximab (MT-R), followed by consolidation with high-dose etoposide and cytarabine. The results showed an ORR of 72% with a median PFS of 48 months. However, the hematological toxicities should be noticed, as 81% of patients developed grade 4 thrombocytopenia. Although the authors concluded that patients >60 years of age did as well as younger patients, more concerns should be paid to older patients.[46] High-dose chemotherapy (HDC)/ASCT is widely used in hematological malignancies. Different conditioning regimens have led to varied outcomes: components of the BEAM regimen (carmustine, etoposide, cytarabine, and melphalan) cannot effectively penetrate the BBB; thus, thiotepa-based treatments have demonstrated better clinical results. In 2015, Omuro et al[40] investigated the efficiency of R-MVP (rituximab, methotrexate, procarbazine, and vincristine) induction followed by TBC (thiotepa, cyclophosphamide, busulfan) conditioning for consolidation in 32 patients, with excellent disease control rates (ORR 97% and 2-year PFS 79%) and no long-term neurotoxicity. Several following phase 2 trials confirmed the efficiency of thiotepa-based conditioning regimens, with high safety (4% treatment-related mortality).[47,48]
The role of HDC/ASCT and WBRT for consolidation remains controversial. Several retrospective studies have reported the efficacy of HDC-ASCT as a first-line treatment for PCNSL.[49,50] The IELSG32 study, a random phase 3 study, assigned 122 patients (1:1) to WBRT (45 Gy) and thiotepa-based HDC/ASCT and reported 2-year PFS rates of 80% and 69%, respectively, without significant difference between groups; however, long-term neurotoxicity was noted in the WBRT group.[51] In 2019, the ANOCEF-GOELAMS randomized phase II PRECIS study evaluated the efficiency and toxicity of WBRT and ASCT as first-line treatment in 140 younger patients (<60 years) assigned to receive WBRT or ASCT as consolidation therapy, with a conditioning regimen comprising TBC and a WBRT dose of 40 Gy. The 2-year PFS rates were 63% (95% confidence interval [CI], 49%–81%) and 87% (95% CI, 77%–98%) in the WBRT and ASCT arms, respectively, which tended to favor ASCT.[52] The main difference between these studies, besides the induction regimen, were patient characteristics, in which 14% of patients were older than 65 years in the IELSG32 study. In summary, HDC-ASCT is a promising consolidation strategy in PCNSL, especially in younger (<65 years) and fit patients.
Due to the high relapse rate of PCNSL, we recommend that fit patients should proceed to consolidation treatment, with consolidation strategies and regimens balanced for individual cases.
The maintenance strategies are often used in unfit or fragile patients. Elderly patients receiving induction regimen followed by temozolomide maintenance had the same outcomes as younger patients receiving a more intensive regimen, with a 2-year OS of 56% to 61%.[53] Low-dose lenalidomide (5–10 mg/day) for maintenance has been explored in a small population, with encouraging responses. Among 13 elder (≥70 years) patients with partial or complete response to MTX/rituximab-based induction, the median PFS has not been reached after 31.6 months of follow-up and no treatment-related death has been reported.[54] A similar result was found in relapsed patients administered maintenance lenalidomide.[55] Finally, a case report reported that nivolumab maintenance resulted in long-term remission of 18 months in a patient with multiple PCNSL relapses.[56]
Progress in Salvage Treatment
Despite advances in the understanding of the pathophysiologic processes and improvements in chemo-radio-treatment in recent decades, more than 20% of PCNSL patients are refractory to the first-line treatment and nearly half relapse within 10 to 18 months. Moreover, relapse has been observed in some patients for more than 5 years after treatment. The prognosis of primary refractory or relapsed PCNSL is very poor, with a median survival of only 2 months without the administration of an effective treatment. The choice of salvage treatment is dependent on patient age, performance status, previous treatments, and duration of response. Re-treatment is reasonable for patients with late relapse after HD-MTX regimens. Among 31 patients with relapsed PCNSL retreated with MTX (8.0 g/m2), the median time to the first relapse was 24.4 months after initiating MTX treatment. The ORR was 91% and the median survival from relapse was 61.9 months.[57] Among patients with disease resistant to MTX, switching to regimens without MTX is the most commonly used approach. Soussain et al[58] reported in 2008 that 43 patients treated with an EA (etoposide, cytarabine) regimen followed by TBC conditioning and ASCT had a median OS of 58.6 months. The relapsed patients who previously underwent ASCT can benefit from salvage regimens followed by a second ASCT.[59] WBRT is an effective salvage treatment for patients who have never received radiation therapy, with overall radiographic response rates of 74% to 79%; however, the duration is usually short, with an OS of 10 to 16 months.
Increased understanding of the molecular pathogenesis, epigenetics, and tumor microenvironment has revealed potential new targets for PCNSL, with therapies including immune-mediated medications, and kinase-targeting inhibitors gradually emerging.
Lenalidomide is an immunomodulatory drug with direct and indirect antineoplastic activity mediated through distinct immunomodulatory mechanisms such as cell-autonomous cytotoxicity effects by inhibition of IRF4 and MYC pro-survival signals to enhance antibody-dependent cell-mediated cytotoxicity. Lenalidomide has demonstrated significant anti-tumor effects in ABC sub-type DLBCL, with an ORR of about 30%.[60,61] A pioneering study of nine consecutive central nervous system lymphoma (CNSL) patients confirmed the efficacy of lenalidomide monotherapy, with an ORR of 50% at 25 mg and well-tolerated treatment.[62] The REVLRI trial, a phase 2 single-arm study, assessed the effect of eight 28-day cycles of R2 (rituximab, lenalidomide) in r/r PCNSLs. The responding patients received low-dose lenalidomide as maintenance. The results showed CR in 39.5% of patients, with a median PFS and OS of 7.8 and 17.7 months, respectively.[63] The response rate of the R2 regimen was lower than mono-agent lenalidomide in r/r PCNSL, and the reasons included: a high proportion of intraocular lymphoma in REVLRI study, the patient selection bias of previous small studies. The combination of pomalidomide and dexamethasone has demonstrated therapeutic activity, with an ORR of 43% in 21 patients and a PFS of 5.3 months.[64] Given the high frequency of mutations in the BCR and MYD88 pathways, the Bruton tyrosine kinase inhibitor ibrutinib with good CNS distribution appears to be a promising therapeutic option. The first report of ibrutinib efficiency in PCNSL appeared in 2017,[65] in which 14 refractory/relapsed PCNSL patients were administered single-agent ibrutinib. Seven of the patients showed a radiologic response. Lionakis et al[66] introduced the novel dose-adjusted temozolomide, etoposide, doxil, dexamethasone, ibrutinib, rituximab combined regimen. All 18 patients were heavily treated (previous regimen lines 1–6) and 85% were refractory patients. The CR rate was 84% but 7(39%) of the 18 patients developed invasive aspergillus infections. Further development of this regimen should focus on reducing the incidence of aspergillosis; for example, with the use of voriconazole prophylaxis. A small phase 2 study evaluating the efficiency of MTX and ibrutinib that enrolled 15 patients reported an ORR of 80% and no invasive aspergillus.[18] The latter regimen was better tolerated regarding both safety and financial issues. Immune-checkpoint inhibitors have been widely used in solid tumors and might represent another promising treatment approach. The recent discovery of the frequent 9p24.1/PD-L1/PD-L2 copy number alterations and consequent increased PD-L1 expression in PCNSL provided the rationale to evaluate the efficacy of PD-1 antibodies including nivolumab, a human IgG4 antibody that targets PD-1 and blocks its interactions with PD-L1. Nayak et al[56] reported long-term responses in a small retrospective study, in which five of six patients achieved objective responses following nivolumab treatment. Moreover, several prospective studies are further investigating the concept of immune evasion and PD-1 blockade in PCNSL, including evaluations of pembrolizumab (NCT02779101) and nivolumab (NCT02857426).
Chimeric antigen receptor (CAR) T-cell therapy, has recently been approved for the treatment of recurrent/refractory systemic DLBCL and CAR T-cells have been shown to penetrate the CNS. A 68-year-old woman with secondary CNSL achieved a durable remission after CAR T-cell therapy, demonstrating the potential of this therapy in PCNSL.[67] However, cytokine-related encephalopathy is a fatal complication of CAR T-cell therapy, which has restricted its use in PCNSL.
Special Considerations for Older Patients With PCNSL
More than half of PCNSL patients are older than 65 years of age and are ineligible for intensive chemotherapy and ASCT. Age is an independent poor predictor of PCNSL and survival in the elderly population has not changed in the last 40 years (6 months in the 1970s vs. 7 months in the 2010s, P = 0.10).[68]
A systematic review assessed clinical data from 783 elderly patients with PCNSL (>65 years) from 20 eligible studies. After a median follow-up of 40 months, HD-MTX-based therapy improved survival (hazard ratio [HR] 0.70, 95% CI 0.53–0.93) but no differences were observed between HD-MTX plus oral chemotherapy and more aggressive HD-MTX-based therapies (HR 1.39, 95% CI 0.90–2.15). Radiotherapy improved survival but was correlated with an increased risk of neurological side-effects.[69] Temozolomide and procarbazine are widely used in elder patients and several phase II studies have demonstrated the efficiency of MPV regimens, with good safety. Adding rituximab to MVP regimens can induce higher response rates.[70–72] A retrospective study from the European Society for Blood and Marrow Transplantation evaluated the role of ASCT in elder patients. In that study, 52 patients treated with a thiotepa conditioning regimen plus ASCT had 2-year PFS and OS of 62% and 70.8%, respectively, with a treatment-related mortality rate of 3.8%.[49] HDT-ASCT with thiotepa-based conditioning regimes may be feasible and effective in fit elderly PCNSL patients. Considering the late neurotoxicity in elder patients, WBRT was gradually omitted from induction and consolidation treatments.
Conclusions
Significant progress in diagnosis and treatment has been achieved in recent decades. The optimal treatment strategy has yet to be established; however, HD-MTX-based chemotherapy is currently considered the backbone of induction therapy for newly diagnosed PCNSL, although relapse is common. New technologies and biological studies will provide details about PCNSL genomics and potential biomarkers of diagnosis and prognosis. Further clinical studies will provide more evidence of optimal doses or combinations of induction chemotherapy, consolidation strategies, and salvage regimens. The limited resources for PCNSL patients must be allocated thoughtfully to answer emerging questions. Ibrutinib and lenalidomide have demonstrated promising results and additional small molecule medications and other novel agents will continue to change the disease landscape.
Conflicts of interest
None.
Footnotes
How to cite this article: Zhang Y, Zhou DB. Primary central nervous system lymphoma: status and advances in diagnosis, molecular pathogenesis, and treatment. Chin Med J 2020;133:1462–1469. doi: 10.1097/CM9.0000000000000844
References
- 1.Rubenstein JL, Gupta NK, Mannis GN, LaMarre AK, Treseler P. How I treat CNS lymphomas. Blood 2013; 122:2318–2330. doi: 10.1182/blood-2013-06-453084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villano JL, Koshy M, Shaikh H, Dolecek TA, McCarthy BJ. Age, gender, and racial differences in incidence and survival in primary CNS lymphoma. Br J Cancer 2011; 105:1414–1418. doi: 10.1038/bjc.2011.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapuy B, Roemer MGM, Stewart C, Tan Y, Abo RP, Zhang L, et al. Targetable genetic features of primary testicular and primary central nervous system lymphomas. Blood 2016; 127:869–881. doi: 10.1182/blood-2015-10-673236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sethi TK, Kovach AE, Grover NS, Huang L-C, Lee LA, Rubinstein SM, et al. Clinicopathologic correlates of MYD88 L265P mutation and programmed cell death (PD-1) pathway in primary central nervous system lymphoma. Leuk Lymphoma 2019; 60:2880–2889. doi: 10.1080/10428194.2019.1620942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bataille B, Delwail V, Menet E, Vandermarcq P, Ingrand P, Wager M, et al. Primary intracerebral malignant lymphoma: report of 248 cases. J Neurosurg 2000; 92:261–266. doi: 10.3171/jns.2000.92.2.0261. [DOI] [PubMed] [Google Scholar]
- 6.Grommes C, DeAngelis LM. Primary CNS lymphoma. J Clin Oncol 2017; 35:2410–2418. doi: 10.1200/JCO.2017.72.7602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tienan Z, Shujie W, Wei Z, Jian L, Bing H, Minghui D, et al. Clinical characteristics and outcome of patients with primary central nervous system lymphoma (in Chinese). Chin J Hematol 2015; 36:849–852. doi: 10.3760/cma.j.issn.0253-2727.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Küker W, Nägele T, Korfel A, Heckl S, Thiel E, Bamberg M, et al. Primary central nervous system lymphomas (PCNSL): MRI features at presentation in 100 patients. J Neurooncol 2005; 72:169–177. doi: 10.1007/s11060-004-3390-7. [DOI] [PubMed] [Google Scholar]
- 9.Zou Y, Tong J, Leng H, Jiang J, Pan M, Chen Z. Diagnostic value of using 18F-FDG PET and PET/CT in immunocompetent patients with primary central nervous system lymphoma: a systematic review and meta-analysis. Oncotarget 2017; 8:41518–41528. doi: 10.18632/oncotarget.17456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jo JC, Yoon DH, Kim S, Lee K, Kang EH, Park JS, et al. Interim 18 F-FGD PET/CT may not predict the outcome in primary central nervous system lymphoma patients treated with sequential treatment with methotrexate and cytarabine. Ann Hematol 2017; 96:1509–1515. doi: 10.1007/s00277-017-3068-9. [DOI] [PubMed] [Google Scholar]
- 11.Birsen R, Blanc E, Willems L, Burroni B, Legoff M, Le Ray E, et al. Prognostic value of early 18F-FDG PET scanning evaluation in immunocompetent primary CNS lymphoma patients. Oncotarget 2018; 9:16822–16831. doi: 10.18632/oncotarget.24706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roy S, Josephson SA, Fridlyand J, Karch J, Kadoch C, Karrim J, et al. Protein biomarker identification in the CSF of patients with CNS lymphoma. J Clin Oncol 2008; 26:96–105. doi: 10.1200/JCO.2007.12.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubenstein JL, Wong VS, Kadoch C, Gao HX, Barajas R, Chen L, et al. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood 2013; 121:4740–4748. doi: 10.1182/blood-2013-01-476333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song Y, Zhang W, Zhang L, Wu W, Zhang Y, Han X, et al. Cerebrospinal fluid IL-10 and IL-10/IL-6 as accurate diagnostic biomarkers for primary central nervous system large B-cell lymphoma. Sci Rep 2016; 6:38671.doi: 10.1038/srep38671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pentsova EI, Shah RH, Tang J, Boire A, You D, Briggs S, et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol 2016; 34:2404–2415. doi: 10.1200/JCO.2016.66.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hattori K, Sakata-Yanagimoto M, Suehara Y, Yokoyama Y, Kato T, Kurita N, et al. Clinical significance of disease-specific MYD88 mutations in circulating DNA in primary central nervous system lymphoma. Cancer Sci 2018; 109:225–230. doi: 10.1111/cas.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiemcke-Jiwa LS, Minnema MC, Radersma-van Loon JH, Jiwa NM, de Boer M, Leguit RJ, et al. The use of droplet digital PCR in liquid biopsies: a highly sensitive technique for MYD88 p.(L265P) detection in cerebrospinal fluid. Hematol Oncol 2018; 36:429–435. doi: 10.1002/hon.2489. [DOI] [PubMed] [Google Scholar]
- 18.Grommes C, Tang SS, Wolfe J, Kaley TJ, Daras M, Pentsova EI, et al. Phase 1b trial of an ibrutinib-based combination therapy in recurrent/refractory CNS lymphoma. Blood 2019; 133:436–445. doi: 10.1182/blood-2018-09-875732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horn H, Ziepert M, Wartenberg M, Staiger AM, Barth TFE, Bernd HW, et al. Different biological risk factors in young poor-prognosis and elderly patients with diffuse large B-cell lymphoma. Leukemia 2015; 29:1564–1570. doi: 10.1038/leu.2015.43. [DOI] [PubMed] [Google Scholar]
- 20.Kim S, Nam SJ, Kwon D, Kim H, Lee E, Kim TM, et al. MYC and BCL2 overexpression is associated with a higher class of Memorial Sloan-Kettering Cancer Center prognostic model and poor clinical outcome in primary diffuse large B-cell lymphoma of the central nervous system. BMC Cancer 2016; 16:1–11. doi: 10.1186/s12885-016-2397-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lossos C, Bayraktar S, Weinzierl E, Younes SF, Hosein PJ, Tibshirani RJ, et al. LMO2 and BCL6 are associated with improved survival in primary central nervous system lymphoma. Br J Haematol 2014; 165:640–648. doi: 10.1111/bjh.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Aguilar A, Idbaih A, Boisselier B, Habbita N, Rossetto M, Laurenge A, et al. Recurrent mutations of MYD88 and TBL1XR1 in primary central nervous system lymphomas. Clin Cancer Res 2012; 18:5203–5211. doi: 10.1158/1078-0432.CCR-12-0845. [DOI] [PubMed] [Google Scholar]
- 23.Schmitz R, Wright GW, Huang DW, Johnson CA, Phelan JD, Wang JQ, et al. Genetics and pathogenesis of diffuse large B-cell lymphoma. N Engl J Med 2018; 378:1396–1407. doi: 10.1056/NEJMoa1801445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chapuy B, Stewart C, Dunford AJ, Kim J, Kamburov A, Redd RA, et al. Molecular subtypes of diffuse large B cell lymphoma are associated with distinct pathogenic mechanisms and outcomes. Nat Med 2018; 24:679–690. doi: 10.1038/s41591-018-0016-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Balint MT, Jelicic J, Mihaljevic B, Kostic J, Stanic B, Balint B, et al. Gene mutation profiles in primary diffuse large B cell lymphoma of central nervous system: next generation sequencing analyses. Int J Mol Sci 2016; 17:1–13. doi: 10.3390/ijms17050683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takano S, Hattori K, Ishikawa E, Narita Y, Iwadate Y, Yamaguchi F, et al. MyD88 mutation in elderly predicts poor prognosis in primary central nervous system lymphoma: multi-institutional analysis. World Neurosurg 2018; 112:e69–e73. doi: 10.1016/j.wneu.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 27.Sung CO, Kim SC, Karnan S, Karube K, Shin HJ, Nam DH, et al. Genomic profiling combined with gene expression profiling in primary central nervous system lymphoma. Blood 2011; 117:1291–1300. doi: 10.1182/blood-2010-07-297861. [DOI] [PubMed] [Google Scholar]
- 28.Ferreri AJM, Blay J-Y, Reni M, Pasini F, Spina M, Ambrosetti A, et al. Prognostic scoring system for primary CNS lymphomas: the international extranodal lymphoma study group experience. J Clin Oncol 2003; 21:266–272. doi: 10.1200/JCO.2003.09.139. [DOI] [PubMed] [Google Scholar]
- 29.Abrey LE, Ben-Porat L, Panageas KS, Yahalom J, Berkey B, Curran W, et al. Primary central nervous system lymphoma: the memorial Sloan-Kettering cancer center prognostic model. J Clin Oncol 2006; 24:5711–5715. doi: 10.1200/JCO.2006.08.2941. [DOI] [PubMed] [Google Scholar]
- 30.Weller M, Martus P, Roth P, Thiel E, Korfel A. Surgery for primary CNS lymphoma? Challenging a paradigm. Neuro Oncol 2012; 14:1481–1484. doi: 10.1093/neuonc/nos159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nelson DF, Martz KL, Bonner H, Nelson JS, Newall J, Kerman HD, et al. Non-Hodgkin's lymphoma of the brain: can high dose, large volume radiation therapy improve survival? Report on a prospective trial by the radiation therapy oncology group (RTOG): RTOG 8315. Int J Radiat Oncol 1992; 23:9–17. doi: 10.1016/0360-3016(92)90538-S. [DOI] [PubMed] [Google Scholar]
- 32.Abrey LE, DeAngelis LM, Yahalom J. Long-term survival in primary CNS lymphoma. J Clin Oncol 1998; 16:859–863. doi: 10.1200/JCO.1998.16.3.859. [DOI] [PubMed] [Google Scholar]
- 33.Fine HA. Primary central nervous system lymphoma. Ann Intern Med 1993; 119:1093.doi: 10.7326/0003-4819-119-11-199312010-00007. [DOI] [PubMed] [Google Scholar]
- 34.Morris PG, Correa DD, Yahalom J, Raizer JJ, Schiff D, Grant B, et al. Rituximab, methotrexate, procarbazine, and vincristine followed by consolidation reduced-dose whole-brain radiotherapy and cytarabine in newly diagnosed primary CNS lymphoma: final results and long-term outcome. J Clin Oncol 2013; 31:3971–3979. doi: 10.1200/JCO.2013.50.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Skarin AT, Zuckerman KS, Pitman SW, Rosenthal DS, Moloney W, Frei E, et al. High-dose methotrexate with folinic acid in the treatment of advanced non-Hodgkin lymphoma including CNS involvement. Blood 1977; 50:1039–1047. [PubMed] [Google Scholar]
- 36.Khan RB, Shi W, Thaler HT, DeAngelis LM, Abrey LE. Is intrathecal methotrexate necessary in the treatment of primary CNS lymphoma? J Neurooncol 2002; 58:175–178. doi: 10.1023/a:1016077907952. [DOI] [PubMed] [Google Scholar]
- 37.Batchelor T, Carson K, O’Neill A, Grossman SA, Alavi J, New P, et al. Treatment of primary CNS lymphoma with methotrexate and deferred radiotherapy: a report of NABTT 96-07. J Clin Oncol 2003; 21:1044–1049. doi: 10.1200/JCO.2003.03.036. [DOI] [PubMed] [Google Scholar]
- 38.Ferreri AJ, Reni M, Foppoli M, Martelli M, Pangalis GA, Frezzato M, et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: a randomised phase 2 trial. Lancet 2009; 374:1512–1520. doi: 10.1016/S0140-6736(09)61416-1. [DOI] [PubMed] [Google Scholar]
- 39.Ferreri AJ, Cwynarski K, Pulczynski E, Ponzoni M, Deckert M, Politi LS, et al. Chemoimmunotherapy with methotrexate, cytarabine, thiotepa, and rituximab (MATRix regimen) in patients with primary CNS lymphoma: results of the first randomisation of the International Extranodal Lymphoma Study Group-32 (IELSG32) phase 2 trial. Lancet Haematol 2016; 3:e217–e227. doi: 10.1016/S2352-3026(16)00036-3. [DOI] [PubMed] [Google Scholar]
- 40.Omuro A, Correa DD, DeAngelis LM, Moskowitz CH, Matasar MJ, Kaley TJ, et al. R-MPV followed by high-dose chemotherapy with TBC and autologous stem-cell transplant for newly diagnosed primary CNS lymphoma. Blood 2015; 125:1403–1410. doi: 10.1182/blood-2014-10-604561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Batchelor TT, Grossman SA, Mikkelsen T, Ye X, Desideri S, Lesser GJ. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology 2011; 76:929–930. doi: 10.1212/WNL.0b013e31820f2d94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holdhoff M, Ambady P, Abdelaziz A, Sarai G, Bonekamp D, Blakeley J, et al. High-dose methotrexate with or without rituximab in newly diagnosed primary CNS lymphoma. Neurology 2014; 83:235–239. doi: 10.1212/WNL.0000000000000593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bromberg JEC, Issa S, Bakunina K, Minnema MC, Seute T, Durian M, et al. Rituximab in patients with primary CNS lymphoma (HOVON 105/ALLG NHL 24): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 2019; 20:216–228. doi: 10.1016/S1470-2045(18)30747-2. [DOI] [PubMed] [Google Scholar]
- 44.Rubenstein JL, Fridlyand J, Abrey L, Shen A, Karch J, Wang E, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 2007; 25:1350–1356. doi: 10.1200/JCO.2006.09.7311. [DOI] [PubMed] [Google Scholar]
- 45.Thiel E, Korfel A, Martus P, Kanz L, Griesinger F, Rauch M, et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): a phase 3, randomised, non-inferiority trial. Lancet Oncol 2010; 11:1036–1047. doi: 10.1016/S1470-2045(10)70229-1. [DOI] [PubMed] [Google Scholar]
- 46.Rubenstein JL, Hsi ED, Johnson JL, Jung SH, Nakashima MO, Grant B, et al. Intensive chemotherapy and immunotherapy in patients with newly diagnosed primary CNS lymphoma: CALGB 50202 (Alliance 50202). J Clin Oncol 2013; 31:3061–3068. doi: 10.1200/JCO.2012.46.9957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Illerhaus G, Kasenda B, Ihorst G, Egerer G, Lamprecht M, Keller U, et al. High-dose chemotherapy with autologous haemopoietic stem cell transplantation for newly diagnosed primary CNS lymphoma: a prospective, single-arm, phase 2 trial. Lancet Haematol 2016; 3:e388–e397. doi: 10.1016/S2352-3026(16)30050-3. [DOI] [PubMed] [Google Scholar]
- 48.DeFilipp Z, Li S, El-Jawahri A, Armand P, Nayak L, Wang N, et al. High-dose chemotherapy with thiotepa, busulfan, and cyclophosphamide and autologous stem cell transplantation for patients with primary central nervous system lymphoma in first complete remission. Cancer 2017; 123:3073–3079. doi: 10.1002/cncr.30695. [DOI] [PubMed] [Google Scholar]
- 49.Schorb E, Fox CP, Fritsch K, Isbell L, Neubauer A, Tzalavras A, et al. High-dose thiotepa-based chemotherapy with autologous stem cell support in elderly patients with primary central nervous system lymphoma: a European retrospective study. Bone Marrow Transplant 2017; 52:1113–1119. doi: 10.1038/bmt.2017.23. [DOI] [PubMed] [Google Scholar]
- 50.Kassam S, Chernucha E, O’Neill A, Hemmaway C, Cummins T, Montoto S, et al. High-dose chemotherapy and autologous stem cell transplantation for primary central nervous system lymphoma: a multi-centre retrospective analysis from the United Kingdom. Bone Marrow Transplant 2017; 52:1268–1272. doi: 10.1038/bmt.2017.101. [DOI] [PubMed] [Google Scholar]
- 51.Ferreri AJ, Cwynarski K, Pulczynski E, Hemmaway C, Cummins T, Montoto S, et al. Whole-brain radiotherapy or autologous stem-cell transplantation as consolidation strategies after high-dose methotrexate-based chemoimmunotherapy in patients with primary CNS lymphoma: results of the second randomisation of the International Extranodal L. Lancet Haematol 2017; 4:e510–e523. doi: 10.1016/S2352-3026(17)30174-6. [DOI] [PubMed] [Google Scholar]
- 52.Houillier C, Taillandier L, Dureau S, Lamy T, Laadhari M, Chinot O, et al. Radiotherapy or autologous stem-cell transplantation for primary CNS lymphoma in patients 60 years of age and younger: results of the intergroup ANOCEF-GOELAMS randomized phase II PRECIS study. J Clin Oncol 2019; 37:823–833. doi: 10.1200/JCO.18.00306. [DOI] [PubMed] [Google Scholar]
- 53.Pulczynski EJ, Kuittinen O, Erlanson M, Hagberg H, Fosså A, Eriksson M, et al. Successful change of treatment strategy in elderly patients with primary central nervous system lymphoma by de-escalating induction and introducing temozolomide maintenance: results from a phase II study by The Nordic Lymphoma Group. Haematologica 2015; 100:534–540. doi: 10.3324/haematol.2014.108472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jamil MO, Mehta A. Diffuse large B-cell lymphoma: prognostic markers and their impact on therapy. Expert Rev Hematol 2016; 9:471–477. doi: 10.1586/17474086.2016.1146584. [DOI] [PubMed] [Google Scholar]
- 55.Vu K, Mannis G, Hwang J, Geng H, Rubenstein JL. Low-dose lenalidomide maintenance after induction therapy in older patients with primary central nervous system lymphoma. Br J Haematol 2019; 186:180–183. doi: 10.1586/17474086.2016.1146584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nayak L, Iwamoto FM, Lacasce A, Mukundan S, Roemer MGM, Chapuy B, et al. PD-1 blockade with nivolumab in relapsed/refractory primary central nervous system and testicular lymphoma. Blood 2017; 129:3071–3073. doi: 10.1182/blood-2017-01-764209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Plotkin SR, Betensky RA, Hochberg FH, Grossman SA, Lesser GJ, Nabors LB, et al. Treatment of relapsed central nervous system lymphoma with high-dose methotrexate. Clin Cancer Res 2004; 10:5643–5646. doi: 10.1158/1078-0432.CCR-04-0159. [DOI] [PubMed] [Google Scholar]
- 58.Soussain C, Hoang-Xuan K, Taillandier L, Fourme E, Choquet S, Witz F, et al. Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. J Clin Oncol 2008; 26:2512–2518. doi: 10.1200/JCO.2007.13.5533. [DOI] [PubMed] [Google Scholar]
- 59.Kasenda B, Schorb E, Fritsch K, Hader C, Finke J, Illerhaus G. Primary CNS lymphoma–radiation-free salvage therapy by second autologous stem cell transplantation. Biol Blood Marrow Transplant 2011; 17:281–283. doi: 10.1016/j.bbmt.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 60.Czuczman MS, Trněný M, Davies A, Rule S, Linton KM, Wagner-Johnston N, et al. A phase 2/3 multicenter, randomized, open-label study to compare the efficacy and safety of lenalidomide versus investigator's choice in patients with relapsed or refractory diffuse large B-cell lymphoma. Clin Cancer Res 2017; 23:4127–4137. doi: 10.1158/1078-0432.CCR-16-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Witzig TE, Vose JM, Zinzani PL, Reeder CB, Buckstein R, Polikoff JA, et al. An international phase II trial of single-agent lenalidomide for relapsed or refractory aggressive B-cell non-Hodgkin's lymphoma. Ann Oncol 2011; 22:1622–1627. doi: 10.1093/annonc/mdq626. [DOI] [PubMed] [Google Scholar]
- 62.Houillier C, Choquet S, Touitou V, Martin-Duverneuil N, Navarro S, Mokhtari K, et al. Lenalidomide monotherapy as salvage treatment for recurrent primary CNS lymphoma. Neurology 2015; 84:325–326. doi: 10.1212/WNL.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 63.Ghesquieres H, Chevrier M, Laadhari M, Chinot O, Choquet S, Moluçon-Chabrot C, et al. Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective “proof of concept” phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA). Ann Oncol 2019; 30:621–628. doi: 10.1093/annonc/mdz032. [DOI] [PubMed] [Google Scholar]
- 64.Tun HW, Johnston PB, DeAngelis LM, Atherton PJ, Pederson LD, Koenig PA, et al. Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood 2018; 132:2240–2248. doi: 10.1182/blood-2018-02-835496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chamoun K, Choquet S, Boyle E, Houillier C, Larrieu-Ciron D, Al Jijakli A, et al. Ibrutinib monotherapy in relapsed/refractory CNS lymphoma: a retrospective case series. Neurology 2017; 88:101–102. doi: 10.1212/WNL.0000000000003420. [DOI] [PubMed] [Google Scholar]
- 66.Lionakis MS, Dunleavy K, Roschewski M, Widemann BC, Butman JA, Schmitz R, et al. Inhibition of B cell receptor signaling by ibrutinib in primary CNS lymphoma. Cancer Cell 2017; 31:833–843.e5. doi: 10.1016/j.ccell.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Löw S, Han CH, Batchelor TT. Primary central nervous system lymphoma. Ther Adv Neurol Disord 2018; 11:1756286418793562.doi: 10.1177/1756286418793562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mendez JS, Ostrom QT, Gittleman H, Kruchko C, DeAngelis LM, Barnholtz-Sloan JS, et al. The elderly left behind-changes in survival trends of primary central nervous system lymphoma over the past 4 decades. Neuro Oncol 2018; 20:687–694. doi: 10.1093/neuonc/nox187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kasenda B, Ferreri AJM, Marturano E, Forst D, Bromberg J, Ghesquieres H, et al. First-line treatment and outcome of elderly patients with primary central nervous system lymphoma (PCNSL)-a systematic review and individual patient data meta-analysis. Ann Oncol 2015; 26:1305–1313. doi: 10.1093/annonc/mdv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Omuro A, Chinot O, Taillandier L, Ghesquieres H, Soussain C, Delwail V, et al. Methotrexate and temozolomide versus methotrexate, procarbazine, vincristine, and cytarabine for primary CNS lymphoma in an elderly population: an intergroup ANOCEF-GOELAMS randomised phase 2 trial. Lancet Haematol 2015; 2:e251–e259. doi: 10.1016/S2352-3026(15)00074-5. [DOI] [PubMed] [Google Scholar]
- 71.Fritsch K, Kasenda B, Hader C, Nikkhah G, Prinz M, Haug V, et al. Immunochemotherapy with rituximab, methotrexate, procarbazine, and lomustine for primary CNS lymphoma (PCNSL) in the elderly. Ann Oncol 2011; 22:2080–2085. doi: 10.1093/annonc/mdq712. [DOI] [PubMed] [Google Scholar]
- 72.Fritsch K, Kasenda B, Schorb E, Hau P, Bloehdorn J, Möhle R, et al. High-dose methotrexate-based immuno-chemotherapy for elderly primary CNS lymphoma patients (PRIMAIN study). Leukemia 2017; 31:846–852. doi: 10.1038/leu.2016.334. [DOI] [PMC free article] [PubMed] [Google Scholar]


